Abstract
A simple total synthesis of herqulines B and C is reported, modeled on the reductive biosynthesis reported previously by other researchers. Commencing from tyrosine, these alkaloids were fashioned through a dimerization, macrocyclization, and four consecutive reductions. Emerging from these studies are strategic insights on the synthesis of these strained alkaloids, as well as mild conditions for the exhaustive reduction of diketopiperizines.
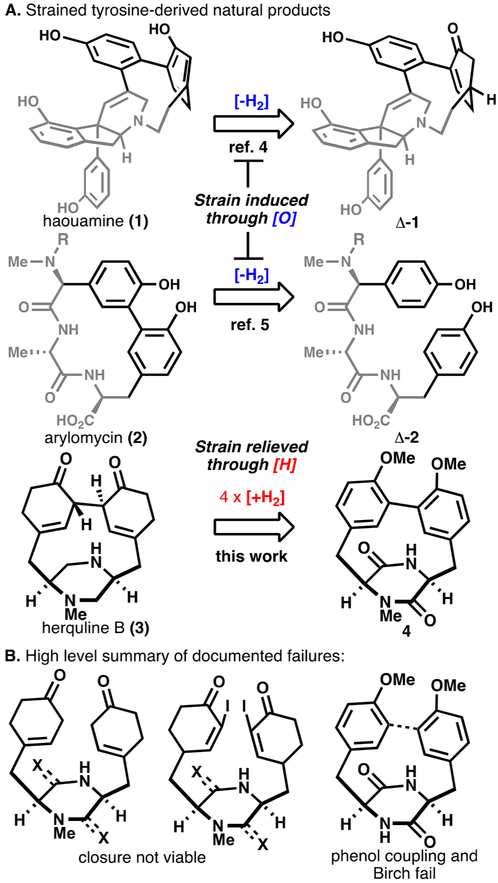
From morphine to vancomycin, tyrosine-based natural products comprise some of the most fascinating and biologically significant structures ever isolated.1 As a particularly redox-active amino acid, most biosyntheses of isolates originating from tyrosine involve key oxidative manipulations of the phenol moiety, leading to remarkable diversity.2 Early studies from Barton3 cemented the logic of how oxidized phenols prefer to react, and decades of biosynthetic studies have revealed the enzymatic machinery responsible for initiating such events.2 Our own studies in the area of tyrosine-based natural products have focused on peculiar frameworks notable for their strained cyclophane cores (Figure 1A), such as haouamine (1) and arylomycin (2). In both instances, strain was introduced through oxidative events; with the former relying on a late-stage unsaturation4 and the latter on a specific Cu-based oxidant.5 The herquline family, first isolated by Ōmura in 1979 from Penicillium herquei,6 of which herquline B (3) is a representative member,7 was of particular appeal due to its extensively reduced framework. A landmark study by the Tang group8 recently revealed its unusual reductive biosynthesis, while the Wood group demystified the stereochemistry of 3 in their elegant synthesis.9 The difficulty of assembling such structures is well documented and is described in detail below. This Communication outlines a concise entry into the herquline family that strategically mirrors the biosynthesis by employing a carefully choreographed sequence of chemical reductions from a simple tyrosine-based starting material.
Figure 1.
(A) Strained tyrosine-derived natural products are generally prepared through oxidation events with herquline as a notable outlier. (B) Summary of selected failed routes.
Despite the apparent simplicity of its 2-D depiction and the direct nature of the approach described below, the journey toward the herqulines was far from straightforward. Indeed, this small, strained alkaloid has confounded several others as documented by the impressive collection of approaches found in the dissertation literature.10 Structurally, the herqulines contain an unsymmetrical basic diamine wrapped within a bowl-shaped macrocycle containing two β,γ-unsaturated enones. From a strategic standpoint, several others10 have recounted efforts to these structures based on unsuccessful phenol-oxidative coupling, iodoenone-reductive coupling, and Birch reduction of a biaryl macrocycle or linear precursor (Figure 1B). Such knowledge influenced our first forays, and thus, numerous pathways were explored before returning to the current approach outlined herein. From a tactical standpoint, it is worth mentioning that, in accordance with most total synthesis endeavors,11 puzzling stability features of each intermediate dictated a specific sequence of events leading to the final route.
The synthesis of 3 commenced (Scheme 1) from known tyrosine-derived building blocks 4 and 5.12 Amide bond formation mediated by HATU delivered dipeptide 6 which was subjected to standard diketopiperazine (DKP) ring-forming conditions (HCO2H followed by heat)13 to furnish 7 in 70% overall yield from 4/5. The ensuing Pd-catalyzed macrocyclization to 8 was accomplished by adopting a procedure initially reported by Hutton14 and further improved by Schindler.15 In order to increase the yield further and perform the reaction on a larger scale (4.6 g), increasing the concentration (20 mM) proved helpful.
Scheme 1. Total Synthesis of Herqulines B (3) and C (14)a.
aReagents and conditions: (1) HATU (1.2 equiv), DIPEA (1.5 equiv), DMF, rt, overnight. (2) Formic acid, then sBuOH:PhMe (3:1), 105 °C (70% yield over two steps). (3) Pd(dppf)Cl2 (20 mol %), B2Pin2 (4.0 equiv), K2CO3 (6.0 equiv), DMSO:H2O (100:1, 20 mM), 90 °C, overnight (60% yield). (4) Li(0) (15 equiv), NH3, F3CCH2OH (8.0 equiv), THF, −78 °C, 1 h (77% yield). (5) [Ir(COE)2Cl]2 (20 mol %), Et2SiH2 (10 equiv), PhMe, 2 h reflux (86% yield). (6) PTSA (0.5 equiv), ethylene glycol (15 equiv), benzene, Dean–Stark trap, reflux. (7) Li(0) (15 equiv), NH3, tBuOH/THF (1:10), −78 °C, 2 h; THF/MeOH/1 N HCl (3:1); DBU, PhMe, 30 min, rt (28% over 3 steps). HATU = 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate; DIPEA = N,N-diisopropylethylamine; DMF = N,N-dimethylformamide; PhMe = toluene; dppf = 1,1’-bis(diphenylphosphino)ferrocene; DMSO = dimethyl sulfoxide; sBuOH = sec-butyl alcohol; THF = tetrahydrofuran; COE = cyclooctene; PTSA = p-toluenesulfonic acid; DBU = 1,8-diazobicyclo(5.4.0)undec-7-ene; LAH = lithium aluminum hydride; DIBAL = diisobutylaluminum hydride.
To convert 8 to the herquline core, no less than four reductions needed to be achieved. The precise order of events that appears in Scheme 1 was thus dictated empirically as a function of intermediate stability and reactivity. The first reduction was achieved using a venerable Birch reduction16 to deliver enol ether 9 in 77% yield. The proton source (TFE) was essential to deliver the regiochemical outcome observed to deliver the 1,2/4,5 unsaturation (vs the isomeric 3,4/1,6 diene that was observed when employing a range of other alcoholic proton sources). At this juncture, the mechanistic basis of this empirical observation is unclear. The substituents on the DKP also appear to play a role in governing selectivity as the N,N-dimethyl congener delivered exclusively the wrong selectivity. The next two reductions required chemoselective excision of the DKP oxygens, a maneuver historically accomplished using DIBAL or LAH.17 Exposure of 9 to either of these strong reducing agents led to monoreduction of the tertiary amide or decomposition, respectively. Ultimately, Cheng and Brookhart’s Ir-based reduction protocol18 provided a mild and reliable means to accomplish both reductions, thereby delivering 10 in 86% yield. To the best of our knowledge, this is the first use of such conditions to achieve exhaustive DKP reduction and will likely be useful in other contexts. The complete stereochemistry and structural assignments thus far were confirmed by X-ray crystallography of enone 11 after mild acidic hydrolysis.
The final reduction of 10 to access the herqulines proved extremely challenging, in accord with reports from others. All Birch reduction conditions screened on this substrate either cleanly returned 10 or led to reduction of the homobenzylic positions (4,5 and/or 1,2) before reducing the final aromatic ring. Such a result is not surprising given that Birch reductions of biaryls16 without overreduction of one of the arenes is rare. It was reasoned that introducing two additional sp3 carbon centers would both alleviate strain and prevent overreduction from occurring. Thus, exposure to ethylene glycol in the presence of PTSA led to clean conversion to ketal 12 which was taken forward in crude form to a final Birch reduction. Again, the proton source (TFE provided no observed product, whereas tBuOH was clean) proved pivotal in delivering reduced macrocycle 13. This ketal-enol ether containing structure was then hydrolyzed with 1 N HCl to deliver herquline C (14) and B (3) as an isolated 5:1 mixture. It should be noted that the crude reaction mixture was enriched in 14 but over time we noticed that it slowly converted to (−)-3.19 To complete the synthesis of 3, the mixture of isomers was simply treated with DBU in toluene for 30 min (28% overall yield from 10). In contrast to prior reports,8,9 3 appears to be thermodynamically stable when using the above conditions as no change was observed even at 90 °C with excess DBU after 10 h. It is worth emphasizing that our initial target was 3 (not 14) and that Wood et al.’s inaugural synthesis9 allowed for rapid assignment of the 3/14 mixture.
A number of strategic and tactical challenges needed to be overcome to access the strained, reduced cyclophane architecture of the herqulines. The peculiar selectivity of late-stage Birch reductions in highly complex settings as a function of subtle structural changes and differing proton sources is notable. The chemoselective complete reduction of DKPs using an Ir/silane system is a useful observation that enabled this route. The syntheses reported here are another reminder of the role of careful experimentation to overcome tactical hurdles in the pursuit of a strategically concise (7–8 steps from iodotyrosine building blocks) pathway.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Professor John L. Wood for sharing a copy of ref 9 prior to publication. Financial support for this work was provided by NIH (GM-118176). We thank Dr. D.-H. Huang and Dr. L. Pasternack for NMR spectroscopic assistance, Prof. A. L. Rheingold and Dr. C. E. Moore for X-ray crystallographic analysis, and Dr. Jason Chen for helpful discussions.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.8b13029.
Experimental procedures, analytical data (1H NMR, 13CNMR, MS) for all new compounds (PDF)
Crystallographic data for compound 11 (CIF)
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Walsh C; Tang Y Natural Product Biosynthesis: Chemical Logic and Enzymatic Machinery; Royal Society of Chemistry: London, 2017. [Google Scholar]; (b) Hesse M Alkaloids; Wiley-VCH: Zurich, 2002. [Google Scholar]
- (2).Tang M; Zou Y; Watanabe K; Walsh CT; Tang Y Oxidative Cyclization in Natural Product Biosynthesis. Chem. Rev. 2017, 117, 5226–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Barton DHR Some Recollections of Gap Jumping; American Chemical Society: Washington, D.C., 1991. [Google Scholar]
- (4).Burns NZ; Krylova I; Hannoush RN; Baran PS Scalable Total Synthesis and Biological Evaluation of Haouamine A and Its Atropisomer. J. Am. Chem. Soc. 2009, 131, 9172–9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Peters DS; Romesberg FE; Baran PS Scalable Access to Arylomycins via C–H Functionalization Logic. J. Am. Chem. Soc. 2018, 140, 2072–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Ōmura S; Hirano A; Iwai Y; Masuma R Herquline, A New Alkaloid Produced by Penicillium herquei. Fermentation, Isolation, and Properties. J. Antibiot. 1979, 32, 786–790. [DOI] [PubMed] [Google Scholar]
- (7).Enomoto Y; Shiomi K; Hayashi M; Masuma R; Kawakubo T; Tomosawa K; Iwai Y; Ōmura S Herquline B, a New Platelet Aggregation Inhibitor Produced by Penicillium herquei Fg-372. J. Antibiot. 1996, 49, 50–53. [DOI] [PubMed] [Google Scholar]
- (8).Yu X; Liu F; Zou Y; Tang M; Hang L; Houk KN; Tang Y Biosynthesis of Strained Piperazine Alkaloids: Uncovering the Precise Pathway of Herquline A. J. Am. Chem. Soc. 2016, 138, 13529–13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Cox JB; Kimishima A; Wood JL Total Synthesis of Herquline B and C. J. Am. Chem. Soc. 2018, DOI: 10.1021/jacs.8b10212. [DOI] [PubMed] [Google Scholar]
- (10).(a) Kim GT Ph.D. Thesis, Korea Advanced Institute of Science and Technology, November 1997. [Google Scholar]; (b) Hart JM Ph.D. Thesis, University of Leeds, June 2004. [Google Scholar]; (c) Stawski PS Ph.D. Thesis, Ludwig-Maximilians-Universität München, December 2012. [Google Scholar]; (d) Yang H Ph.D Thesis, University of Birmingham, August 2015. [Google Scholar]; (e) For conference proceedings describing progress toward the herqulines, see: Kawai N; Atsumi T; Arai N; Kuwajima I Nippon Kagakkai, Koen Yokoshu 2003, 83, 777. [Google Scholar]
- (11).(a) Sierra MA; de la Torre MC Dead Ends and Detours: Direct Ways to Successful Total Synthesis; Wiley-VCH: Zurich, 2004. [Google Scholar]; (b) Sierra SA; de la Torre MC; Cossio FP More Dead Ends and Detours: En Route to Successful Total Synthesis; Wiley-VCH: Zurich, 2013. [Google Scholar]
- (12).See the Supporting Information for preparation of known building blocks.
- (13).Suzuki K; Sasaki Y; Endo N; Mihara Y Acetic Acid-Catalyzed Diketopiperazine Synthesis. Chem. Pharm. Bull. 1981, 29, 233–237. [Google Scholar]
- (14).Cochrane JR; White JM; Wille U; Hutton CA Total Synthesis of Mycocyclosin. Org. Lett. 2012, 14, 2402–2405. [DOI] [PubMed] [Google Scholar]
- (15).Zhu X; McAtee CC; Schindler CS Scalable Synthesis of Mycocyclosin. Org. Lett. 2018, 20, 2862–2866. [DOI] [PubMed] [Google Scholar]
- (16).Rabideau PW; Marcinow Z Birch Reduction of Aromatic Compounds In Organic Reactions; Denmark SE, Ed.; Wiley & Sons: Hoboken, 1992. [Google Scholar]
- (17).Nakamura D; Kakiuchi K; Koga K; Shirai R Design and Synthesis of Novel C2-Symmetric Chiral Piperazines and an Application to Asymmetric Acylation of ∂-Symmetric 1,2-Diols. Org. Lett. 2006, 8, 6139–6142. [DOI] [PubMed] [Google Scholar]
- (18).Cheng C; Brookhart M Iridium-Catalyzed Reduction of Secondary Amides to Secondary Amines and Imines by Diethylsilane. J. Am. Chem. Soc. 2012, 134, 11304–11307. [DOI] [PubMed] [Google Scholar]
- (19).The gradual conversion of 14 into 3 was also observed upon routine domestic shipping according to Prof. Tang Yi
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.