Abstract
BACKGROUND
Hospitalized patients who are colonized with methicillin-resistant Staphylococcus aureus (MRSA) are at high risk for infection after discharge.
METHODS
We conducted a multicenter, randomized, controlled trial of postdischarge hygiene education, as compared with education plus decolonization, in patients colonized with MRSA (carriers). Decolonization involved chlorhexidine mouthwash, baths or showers with chlorhexidine, and nasal mupirocin for 5 days twice per month for 6 months. Participants were followed for 1 year. The primary outcome was MRSA infection as defined according to Centers for Disease Control and Prevention (CDC) criteria. Secondary outcomes included MRSA infection determined on the basis of clinical judgment, infection from any cause, and infection-related hospitalization. All analyses were performed with the use of proportional-hazards models in the per-protocol population (all participants who underwent randomization, met the inclusion criteria, and survived beyond the recruitment hospitalization) and as-treated population (participants stratified according to adherence).
RESULTS
In the per-protocol population, MRSA infection occurred in 98 of 1063 participants (9.2%) in the education group and in 67 of 1058 (6.3%) in the decolonization group; 84.8% of the MRSA infections led to hospitalization. Infection from any cause occurred in 23.7% of the participants in the education group and 19.6% of those in the decolonization group; 85.8% of the infections led to hospitalization. The hazard of MRSA infection was significantly lower in the decolonization group than in the education group (hazard ratio, 0.70; 95% confidence interval [CI], 0.52 to 0.96; P=0.03; number needed to treat to prevent one infection, 30; 95% CI, 18 to 230); this lower hazard led to a lower risk of hospitalization due to MRSA infection (hazard ratio, 0.71; 95% CI, 0.51 to 0.99). The decolonization group had lower likelihoods of clinically judged infection from any cause (hazard ratio, 0.83; 95% CI, 0.70 to 0.99) and infection-related hospitalization (hazard ratio, 0.76; 95% CI, 0.62 to 0.93); treatment effects for secondary out-comes should be interpreted with caution owing to a lack of prespecified adjustment for multiple comparisons. In as-treated analyses, participants in the decolonization group who adhered fully to the regimen had 44% fewer MRSA infections than the education group (hazard ratio, 0.56; 95% CI, 0.36 to 0.86) and had 40% fewer infections from any cause (hazard ratio, 0.60; 95% CI, 0.46 to 0.78). Side effects (all mild) occurred in 4.2% of the participants.
CONCLUSIONS
Postdischarge MRSA decolonization with chlorhexidine and mupirocin led to a 30% lower risk of MRSA infection than education alone. (Funded by the AHRQ Healthcare-Associated Infections Program and others; ClinicalTrials.gov number, NCT01209234.)
Methicillin-resistant staphylococcus aureus (MRSA) causes more than 80,000 invasive infections in the United States annually.1 It is the most common cause of skin, soft-tissue, and procedure-related infections.2 Rates of invasive MRSA infection are highest within 6 months after hospital discharge and do not normalize for 1 year.1,3,4
Approaches to MRSA have included education about both hygiene and environmental cleaning as well as decolonization with nasal mupirocin and chlorhexidine antiseptic baths to reduce carriage and prevent infection.5,6 Decolonization has reduced the risks of surgical-site infection, recurrent skin infection, and infection in the intensive care unit (ICU).7–10 Our goal was to evaluate whether, after hospital discharge, decolonization plus hygiene education was superior to education alone in reducing the likelihood of MRSA infection among patients colonized with MRSA (carriers).
METHODS
TRIAL DESIGN AND INTERVENTION
We conducted the Project CLEAR (Changing Lives by Eradicating Antibiotic Resistance) Trial as a multicenter, two-group, unblinded, randomized, controlled trial to compare the effect of hygiene education with that of education plus decolonization on the likelihood of postdischarge infection among MRSA carriers. This trial was approved by the institutional review board of the University of California Irvine. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol, available with the full text of this article at NEJM.org.
Participants were randomly assigned, in a 1:1 ratio, to the education group or the decolonization group. Randomization was performed with a randomized block design stratified according to Hispanic ethnic group and nursing home residence. In the education group, participants received and reviewed an educational binder (provided in English and Spanish) about MRSA and how it is spread and about recommendations for personal hygiene, laundry, and house-hold cleaning (Appendix A in the Supplementary Appendix, available at NEJM.org). In the decolonization group, participants received and reviewed the identical educational binder and also underwent decolonization for 5 days twice monthly for a period of 6 months after hospital discharge (Appendix B in the Supplementary Appendix). The decolonization intervention involved the use of 4% rinse-off chlorhexidine for daily bathing or showering, 0.12% chlorhexidine mouthwash twice daily, and 2% nasal mupirocin twice daily. All products were purchased with grant funds and were provided free of charge to the participants.
RECRUITMENT AND ELIGIBILITY CRITERIA
Recruitment involved written informed consent provided between January 10, 2011, and January 2, 2014, during inpatient admissions in 17 hospitals and 7 nursing homes in Southern California (Table S1 in the Supplementary Appendix). Eligibility requirements included an age of 18 years or older, hospitalization within the previous 30 days, positive testing for MRSA during the enrollment hospitalization or within the 30 days before or afterward, and the ability to bathe or shower (alone or assisted by a caregiver). Key exclusion criteria were hospice care and allergy to the decolonization products at recruitment. California mandates MRSA screening at hospital admission in high-risk patients: those undergoing hemodialysis, those who had a recent hospitalization (within the preceding 30 days), those who were undergoing imminent surgery, those who were admitted to the ICU, and those who were transferred from a nursing home.
FOLLOW-UP
Participants were followed for 12 months after discharge. In-person visits at home or in a research clinic occurred at recruitment and at months 1, 3, 6, and 9. An exit interview was conducted at 12 months. The trial had a fixed end date of June 30, 2014. Participants who were enrolled after July 1, 2013, had a truncated follow-up and had their data administratively censored at that time. Loss to follow-up was defined as the inability of trial staff to contact participants for 3 months, at which point the participant was removed from the trial as of the date of last contact. Participants received escalating compensation for completing follow-up visits ($25, $30, $35, and $50).
All participants were contacted monthly and requested to report any hospitalizations or clinic visits for infection. After trial closure, medical records from reported visits were requested, double-redacted for protected health information and trial-group assignment, and reviewed for trial outcomes. Records from enrollment hospitalizations were requested and reviewed for characteristics of the participants and the presence or absence of MRSA infection at the enrollment hospitalization. Records were requested up to five times, with five additional attempts to address incomplete records.
TRIAL OUTCOMES
Redacted medical records from enrollment hospitalizations and all reported subsequent medical visits were reviewed in a blinded fashion, with the use of standardized forms, by two physicians with expertise in infectious diseases (five of the authors) for coexisting conditions, antibiotic agents, and infection outcomes. If consensus was not reached, discordant outcomes were adjudicated by a third physician with expertise in infectious diseases.
The primary outcome was MRSA infection according to medical-record documentation of disease-specific infection criteria (according to 2013 guidelines) from the Centers for Disease Control and Prevention (CDC) in a time-to-event analysis.11 A priori secondary outcomes included MRSA infection defined in a time-to-event analysis according to the clinical judgment of two reviewers with expertise in infectious diseases who were unaware of the trial-group assignments, infection from any cause according to disease-specific CDC criteria in a time-to-event analysis, infection from any cause according to infectious disease clinical judgment in a time-to-event analysis, hospitalization due to infection, and new carriage of a MRSA strain that was resistant to mupirocin (evaluated by Etest, bioMérieux)12 or that had an elevated minimum inhibitory concentration (MIC) of chlorhexidine (≥8 μg per milliliter) on microbroth dilution.13,14 All outcomes were assessed on the basis of the first event per participant.
DATA COLLECTION
Surveys of health conditions, health care utilization, and household cleaning and bathing habits were administered during recruitment and all follow-up visits. Swabs of both nares, the throat, skin (axilla and groin), and any wounds were taken, but the results are not reported here. At each visit, participants in the decolonization group reported adherence to the intervention, and staff assessed the remaining product. Potential discrepancies were broached with the participant to obtain affirmation of actual adherence. Adherence was assessed as full (no missed doses), partial (some missed doses), and non-adherence (no doses used).
STATISTICAL ANALYSIS
The characteristics of the participants and outcomes were described by frequency and type according to trial group. Outcomes were summarized with the use of Kaplan–Meier estimates of infection-free distributions across the follow-up period and analyzed with the use of unadjusted Cox proportional-hazard models (per-protocol primary analysis) for the postdischarge trial population (all the participants who underwent randomization, met inclusion criteria, and survived beyond the recruitment hospitalization); outcomes were also analyzed according to the as-treated adherence strata (fully adherent, partially adherent, and nonadherent participant-time). In the as-treated analyses, information about participant adherence during at-risk periods before each visit was updated with the use of the adherence assessment at that visit.
The assumption of proportional hazards was assessed by means of residual diagnostic tests and formal hypothesis tests. P values are provided only for the primary outcome. Because the statistical analysis plan did not include a provision for correction for multiple comparisons when tests for prespecified secondary outcomes or post hoc exploratory outcomes were conducted, those results are reported as point estimates with 95% confidence intervals. The widths of the confidence intervals were not adjusted for multiple comparisons, so intervals should not be used to infer definitive treatment effects within subgroups or for secondary outcomes.
In post hoc exploratory analyses, we used adjusted Cox proportional-hazard models to address potential residual imbalances in the characteristics of the participants between the two groups after randomization. The characteristics of the participants were entered into the model if they were associated with outcomes at a P value of less than 0.20 in bivariate analyses. Characteristics included demographic data; educational level; insurance type; presence of coexisting conditions, devices, or wounds at enrollment; hospitalization or residence in a nursing home in the year before enrollment; ICU admission or surgery during enrollment hospitalization; need for assistance with bathing; frequency of bathing; and randomization strata. Adjusted models also accounted for two time-dependent covariates: receipt of anti-MRSA antibiotics and adherence to the intervention. The number needed to treat was calculated with the use of rates that accounted for participant-time that incorporated censoring due to loss to follow-up, withdrawal from the trial, or the end of the trial.15 Full details of the trial design and analytic approach are provided in the protocol and in the Supplementary Appendix.
RESULTS
PARTICIPANTS
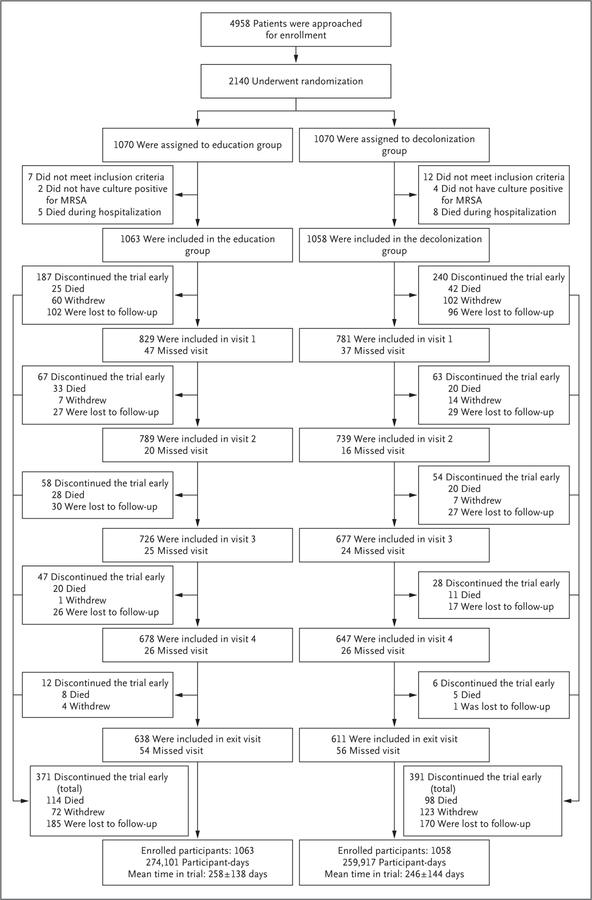
Figure 1 shows the randomization and follow-up of 2140 participants, of whom 19 were excluded after randomization because they did not meet inclusion criteria (6 participants did not have a positive MRSA test, and 13 died during the enrollment hospitalization). The characteristics of the final 2121 enrolled participants (per-protocol population) are provided in Table 1, and in Tables S2 through S4 in the Supplementary Appendix.
Figure 1. Randomization and Follow-up of the Participants.
This flow chart describes the recruitment and the four follow-up visits (at 1, 3, 6, and 9 months) for the 1-year period after hospital discharge. Recruitment occurred during hospitalization, and 19 participants were excluded from the postdischarge trial population because they did not meet inclusion criteria, leaving 2121 participants in the per-protocol population (1063 participants in the education group and 1058 in the decolonization group). Early exit from the trial was provided between each visit and included active withdrawal from the trial, loss to follow-up, and death. Active withdrawal represented situations in which participants indicated their desire to withdraw from the trial. Loss to follow-up was defined as the inability to contact the participant for 3 months, at which point the participant was removed from the trial at the time of last contact. Visits indicate both participants who successfully completed the visit and those who remained in the trial but missed that visit. The mean (±SD) time in the trial (in days) is shown for each group. All deaths were considered by the investigators to be unrelated to side effects from decolonization products. Summary boxes are provided at the bottom of the figure. MRSA denotes methicillin-resistant Staphylococcus aureus.
Table 1.
Characteristics of the Participants at Recruitment Hospitalization.*
| Characteristic | Education Group (N = 1063) | Decolonization Group (N = 1058) | P Value† |
|---|---|---|---|
| Age — yr | 56±17 | 56±17 | 0.78 |
| Male sex — no. (%) | 583 (54.8) | 565 (53.4) | 0.51 |
| Coexisting conditions‡ | |||
| Diabetes — no./total no. (%) | 424/1062 (39.9) | 462/1056 (43.8) | 0.08 |
| Chronic obstructive pulmonary disease — no./total no. (%) | 212/1055 (20.1) | 203/1045 (19.4) | 0.70 |
| Congestive heart failure — no./total no. (%) | 145/1055 (13.7) | 149/1045 (14.3) | 0.73 |
| Cancer — no./total no. (%) | 153/1055 (14.5) | 161/1045 (15.4) | 0.56 |
| Renal disease — no./total no. (%) | 140/1062 (13.2) | 134/1056 (12.7) | 0.74 |
| Charlson Comorbidity Index score§ | 1.7±1.6 | 1.7±1.6 | 0.49 |
| Bathe daily or every other day — no./total no. (%)¶ | 926/1037 (89.3) | 927/1034 (89.7) | 0.73 |
| Bathing assistance needed — no./total no. (%)¶ | 200/1025 (19.5) | 224/1013 (22.1) | 0.15 |
| MRSA source at enrollment — no. (%) | 0.79 | ||
| Nares‖ | 580 (54.6) | 602 (56.9) | |
| Wound | 320 (30.1) | 305 (28.8) | |
| Respiratory | 44 (4.1) | 45 (4.3) | |
| Blood | 43 (4.0) | 31 (2.9) | |
| Other | 76 (7.1) | 75 (7.1) | |
| Recruitment hospitalization** | |||
| Hospitalized in previous yr — no./total no. (%)‡ | 595/1046 (56.9) | 598/1041 (57.4) | 0.80 |
| Nursing home stay in previous yr — no./total no. (%)‡ | 165/1043 (15.8) | 168/1040 (16.2) | 0.84 |
| ICU stay — no./total no. (%) | 188/1055 (17.8) | 206/1045 (19.7) | 0.27 |
| Surgery — no./total no. (%) | 392/1055 (37.2) | 399/1045 (38.2) | 0.63 |
| MRSA infection — no./total no. (%)†† | 447/1055 (42.4) | 438/1045 (41.9) | 0.83 |
| Wound at hospital discharge — no./total no. (%) | 587/1055 (55.6) | 588/1045 (56.3) | 0.77 |
| Medical device at hospital discharge — no./total no. (%)‡‡ | 320/1055 (30.3) | 307/1045 (29.4) | 0.63 |
| Discharged to nursing home — no. (%) | 120 (11.3) | 116 (11.0) | 0.81 |
Plus–minus values are means ±SD. There were no significant differences between the two groups. Selected descriptive data are shown. For a full descriptive list of characteristics, see Table S2 in the Supplementary Appendix. ICU denotes intensive care unit.
Student’s t-test was performed for continuous variables, chi-square test for proportions, and Fisher’s exact test for proportions if the numerator was 5 or less.
Data reflect a positive response to either a survey question or chart review. Not all participants responded to every question, and not all enrollment charts were received from recruiting hospitals despite a signed release request, so data were missing for 21 participants.
Scores on the Charlson Comorbidity Index range from 0 to 10, with higher scores indicating more coexisting illness.
Data reflect respondents to the survey question among all the participants. Not all the participants responded to every question.
By law, California requires hospitals to screen five groups of patients for MRSA on hospital admission (patients who are transferred from a nursing home, who have been hospitalized in the past 30 days, who are undergoing hemodialysis, who are undergoing imminent surgery, and who are admitted to an ICU).
Data reflect chart review from the received medical records. Not all recruiting hospitals released participants’ medical records to the trial despite a signed release request, so records were missing for 21 participants.
Assessment of infection was based on criteria of the Centers for Disease Control and Prevention (CDC). Information regarding infection types is provided in Table S3 in the Supplementary Appendix.
Information about medical device types is provided in Table S4 in the Supplementary
According to the randomization strata, His-panic participants made up 31.9% of the education group (339 participants) and 32.0% of the decolonization group (339), and nursing home residents made up 11.3% of the education group (120) and 11.0% of the decolonization group (116). In a comparison of the education group with the decolonization group across the 1-year follow-up, early exit from the trial occurred in 34.9% of the participants (371 participants) and 37.0% (391), respectively (P = 0.32); withdrawal from the trial in 6.8% (72) and 11.6% (123), respectively (P<0.001); loss to follow-up in 17.4% (185) and 16.1% (170), respectively (P = 0.41); and death in 10.7% (114) and 9.3% (98), respectively (P = 0.26). The characteristics of the participants who withdrew from the trial or were lost to follow-up and of the participants in the decolonization group according to adherence category are shown in Table S5 in the Supplementary Appendix.
OUTCOMES
A total of 8395 full-text medical records were requested, and 8067 (96.1%) were received and redacted. Charts underwent duplicate blinded review (16,134 reviews) by physicians with expertise in infectious diseases at a rate of approximately 800 charts per month for 20 months. Of the 2121 enrollment admission records, 2100 (99.0%) were received. Of the 6271 subsequent inpatient and outpatient records, 5967 (95.2%) were received for outcome assessment. The over-all rate of reported hospitalizations per 365 days of follow-up was 1.97 in the education group and 1.75 in the decolonization group.
Regarding the primary outcome in the perprotocol analysis, 98 participants (9.2%) in the education group had a MRSA infection, as compared with 67 (6.3%) in the decolonization group (Table 2). This corresponded to an estimated MRSA infection rate in the education group of 0.139 infections per participant-year, as compared with 0.098 infections per participant-year in the decolonization group. Among first MRSA infections per participant, skin and soft-tissue infections and pneumonia were common. Across both groups, 84.8% (140 of 165) of the MRSA infections resulted in hospitalization, at a rate of 0.117 hospitalizations per participant-year in the education group and 0.083 per participant-year in the decolonization group. Bacteremia occurred in 28.5% (47 of 165) of all MRSA infections; the MRSA bacteremia rate was 0.040 events per participant-year in the education group and 0.028 per participant-year in the decolonization group. Findings were similar when MRSA infection was determined according to the clinical judgment of physicians with expertise in infectious diseases and according to CDC criteria (Table 2). All the MRSA infections were treated with an antibiotic, but the receipt of an antibiotic was not sufficient to render a decision of a MRSA infection.
Table 2.
MRSA Infection Outcomes (First Infection per Person) per 365 Days of Follow-up, According to Trial Group.*
| Variable | MRSA Infection, According to CDC Criteria† | MRSA Infection, According to Clinical Criteria | Any Infection, According to CDC Criteria | Any Infection, According to Clinical Criteria | ||||
|---|---|---|---|---|---|---|---|---|
| Education | Decolonization | Education | Decolonization | Education | Decolonization | Education | Decolonization | |
| All Participants | ||||||||
| Infection — no. of participants (no. of events/participant-yr) | ||||||||
| Any infection | 98 (0.139) | 67 (0.098) | 98 (0.139) | 68 (0.100) | 252 (0.407) | 207 (0.338) | 298 (0.498) | 246 (0.414) |
| Skin or soft-tissue infection | 34 (0.048) | 32 (0.047) | 35 (0.050) | 32 (0.047) | 80 (0.129) | 59 (0.096) | 97 (0.162) | 82 (0.138) |
| Pneumonia | 18 (0.026) | 9 (0.013) | 20 (0.028) | 10 (0.015) | 39 (0.063) | 25 (0.041) | 45 (0.075) | 34 (0.057) |
| Primary bloodstream or vascular infection | 11 (0.016) | 10 (0.015) | 12 (0.017) | 11 (0.016) | 20 (0.032) | 14 (0.023) | 20 (0.033) | 14 (0.024) |
| Bone or joint infection | 13 (0.019) | 9 (0.013) | 12 (0.017) | 8 (0.012) | 20 (0.032) | 22 (0.036) | 0.18 (0.030) | 17 (0.029) |
| Surgical-site infection | 13 (0.019) | 2 (0.003) | 13 (0.018) | 2 (0.003) | 20 (0.032) | 8 (0.013) | 22 (0.037) | 9 (0.015) |
| Urinary tract infection | 3 (0.004) | 2 (0.003) | 1 (0.001) | 1 (0.002) | 38 (0.061) | 46 (0.075) | 52 (0.087) | 56 (0.094) |
| Abdominal infection | 1 (0.001) | 2 (0.003) | 1 (0.001) | 2 (0.003) | 20 (0.032) | 21 (0.034) | 26 (0.044) | 18 (0.030) |
| Other infection | 5 (0.007) | 1 (0.002) | 4 (0.006) | 2 (0.003) | 15 (0.024) | 12 (0.020) | 18 (0.030) | 16 (0.027) |
| Infection involving bacteremia | 28 (0.040) | 19 (0.028) | 27 (0.038) | 18 (0.026) | 46 (0.074) | 37 (0.060) | 46 (0.077) | 33 (0.056) |
| Infection leading in hospitalization | 83 (0.117) | 57 (0.083) | 82 (0.115) | 56 (0.082) | 225 (0.356) | 169 (0.269) | 259 (0.420) | 199 (0.325) |
| Time to infection — days | 111±91 | 117±93 | 116±94 | 117±95 | 103±87 | 110±91 | 107±91 | 113±94 |
| Adherent Participants in Decolonization Group‡ | ||||||||
| Infection — no. of participants (no. of events/participant-yr) | ||||||||
| Any infection | 42 (0.085) | 42 (0.088) | 118 (0.272) | 142 (0.338) | ||||
| Skin or soft-tissue infection | 22 (0.045) | 22 (0.046) | 40 (0.092) | 54 (0.129) | ||||
| Pneumonia | 5 (0.010) | 5 (0.011) | 11 (0.025) | 16 (0.038) | ||||
| Primary bloodstream or vascular infection | 5 (0.010) | 6 (0.013) | 8 (0.019) | 8 (0.019) | ||||
| Bone or joint infection | 5 (0.010) | 4 (0.008) | 14 (0.032) | 11 (0.026) | ||||
| Surgical-site infection | 2 (0.004) | 2 (0.004) | 6 (0.014) | 7 (0.017) | ||||
| Urinary tract infection | 0 | 0 | 22 (0.051) | 27 (0.064) | ||||
| Abdominal infection | 2 (0.004) | 2 (0.004) | 12 (0.028) | 11 (0.026) | ||||
| Other infection | 1 (0.002) | 1 (0.002) | 5 (0.012) | 8 (0.019) | ||||
| Infection involving bacteremia | 9 (0.019) | 8 (0.017) | 19 (0.045) | 16 (0.039) | ||||
| Infection leading to hospitalization | 36 (0.075) | 34 (0.071) | 98 (0.226) | 115 (0.274) | ||||
| Time to infection — days | 122±93 | 125±96 | 119±89 | 123±94 | ||||
Participant-day denominators were censored by the specified outcome. Dates of infection onset based on CDC criteria may differ from those based on clinical judgment.
This was the primary outcome.
A total of 546 participants were considered to have adhered fully to the decolonization intervention.
In the analysis of infection from any cause according to CDC criteria, 23.7% of the participants in the education group (252 participants) had an infection, as compared with 19.6% of those in the decolonization group (207), which corresponded to an estimated rate of 0.407 infections per participant-year in the education group and 0.338 per participant-year in the decolonization group (Table 2). Skin and soft-tissue infections and pneumonia remained the most common infection types.
Pathogens were identified in 67.7% of the infections (Table S6 in the Supplementary Appendix). Participants in the decolonization intervention had a lower rate of infections due to grampositive pathogens or without cultured pathogens than those in the education group. There was a higher rate of gram-negative infection among the CDC-defined all-cause infections when participants in the decolonization intervention were compared with those in the education group, but this was not seen among clinically defined infections.
Across the two trial groups, infection from any cause led to hospitalization in 85.8% of the participants (394 of 459), and bacteremia occurred in 18.1% (83 of 459). The observed rate of hospitalization due to infection from any cause was 0.356 events per participant-year in the education group and 0.269 per participant-year in the decolonization group. The rate of bacteremia among participants with infection from any cause was 0.074 events per participant-year in the education group and 0.060 per participant-year in the decolonization group. Findings were similar when infection from any cause was determined according to clinical judgment (Table 2).
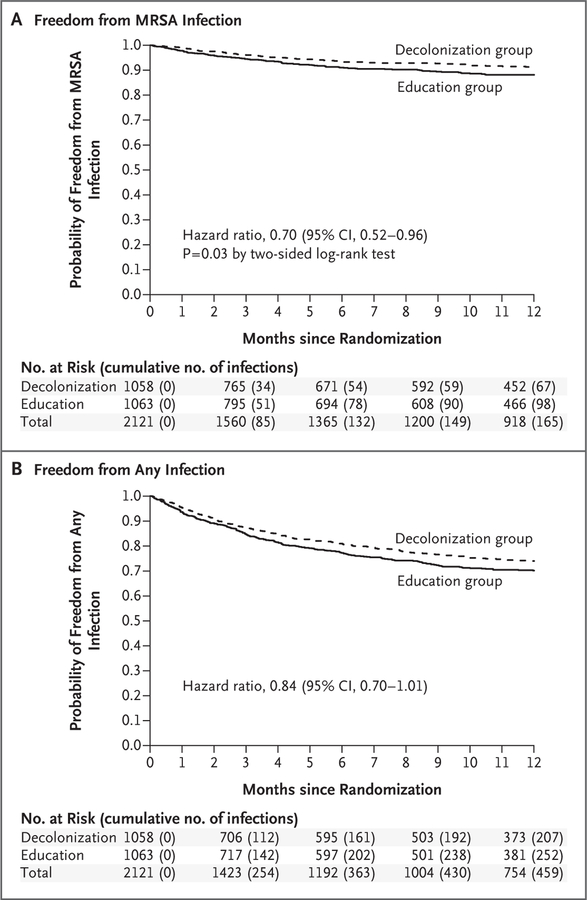
Estimates of the per-protocol treatment effects are shown in Table 3. No significant departures from proportional hazards were observed. In the main unadjusted analysis, the hazard of MRSA infection according to the CDC criteria (the primary outcome) was significantly lower in the decolonization group than in the education group (hazard ratio, 0.70; 95% confidence interval [CI], 0.52 to 0.96; P= 0.03). This lower hazard of MRSA infection led to a 29% lower risk of hospitalization due to CDC-defined MRSA infection in the decolonization group than in the education group (hazard ratio, 0.71; 95% CI, 0.51 to 0.99). The effect was nearly identical for cases and hospitalizations involving clinically defined MRSA infection. Kaplan–Meier curves showing the infection-free time for the primary outcome of CDC-defined MRSA infection and the secondary out-come of infection from any cause show that the curves remained separated even after the intervention ended in month 6 (Fig. 2, and Table S7 in the Supplementary Appendix). Adjusted models showed greater MRSA infection effects that were significant (Table 3). A total of 10 participants (0.9%) in the education group and in 3 (0.3%) in the decolonization group died from MRSA infection. Results of sensitivity analyses conducted regarding death and early withdrawal from the trial are provided in Table S8 in the Supplementary Appendix.
Table 3.
Effect of Decolonization Plus Education, as Compared with Education Alone, According to Cox Proportional-Hazard Models.*
| Variable | MRSA Infection, According to CDC Criteria | MRSA Infection, According to Clinical Criteria | Any Infection, According to CDC Criteria | Any Infection, According to Clinical Criteria |
|---|---|---|---|---|
| Per-protocol analysis | ||||
| Unadjusted hazard ratio (95% CI) | 0.70 (0.52–0.96)† | 0.71 (0.52–0.97) | 0.84 (0.70–1.01) | 0.83 (0.70–0.99) |
| Adjusted hazard ratio (95% CI)‡ | 0.61 (0.44–0.85) | 0.61 (0.43–0.84) | 0.80 (0.66–0.98) | 0.81 (0.68–0.97) |
| As-treated analysis§ | ||||
| Unadjusted hazard ratio (95% CI) | ||||
| Nonadherent | 1.31 (0.72–2.38) | 1.09 (0.57–2.10) | 1.68 (1.19–2.36) | 1.53 (1.11–2.13) |
| Partially adherent | 0.64 (0.40–1.00) | 0.72 (0.47–1.11) | 0.86 (0.67–1.11) | 0.92 (0.74–1.16) |
| Fully adherent | 0.56 (0.36–0.86) | 0.53 (0.34–0.83) | 0.60 (0.46–0.78) | 0.58 (0.45–0.74) |
| Adjusted hazard ratio (95% CI)¶ | ||||
| Nonadherent | 0.78 (0.36–1.71) | 0.72 (0.37–1.41) | 0.780 (0.51–1.26) | 0.76 (0.40–1.45) |
| Partially adherent | 0.75 (0.59–0.95) | 0.69 (0.54–0.88) | 0.78 (0.64–0.97) | 0.76 (0.63–0.92) |
| Fully adherent | 0.72 (0.57–0.92) | 0.66 (0.51–0.84) | 0.75 (0.60–0.94) | 0.72 (0.58–0.88) |
The per-protocol population included all the participants (2121) who underwent randomization, met the inclusion criteria, and survived beyond the recruitment hospitalization. The unadjusted analyses included all these participants. The adjusted models included the 1901 participants who provided data for all the baseline characteristics shown in Table S2 in the Supplementary Appendix.
A P value is provided only for the primary outcome (P = 0.03). Because the statistical analysis plan did not include a provision for correcting for multiple comparisons when tests for prespecified secondary outcomes or post hoc exploratory outcomes were conducted, these results are reported as point estimates with 95% confidence intervals. The widths of these confidence intervals were not adjusted for multiple comparisons, so intervals should not be used to infer definitive treatment effects within subgroups or for secondary outcomes.
Models evaluating the outcomes of MRSA infection according to CDC criteria and any infection according to clinical criteria were adjusted for randomization strata, sex, primary insurance type, diabetes, renal disease, liver disease, cancer, cerebrovascular disease, hospitalization within 12 months before enrollment hospitalization, medical device on discharge from enrollment hospitalization, bathing frequency, need for bathing assistance, and anti-MRSA antibiotics as time-varying covariates on the basis of variables associated with outcomes at a P value of less than 0.20 in bivariate analyses. Models evaluating the outcome of MRSA infection according to clinical criteria and any infection according to CDC criteria were adjusted for the same variables with the addition of age. Resistance to mupirocin did not significantly modify the effect of the trial group.
The as-treated analysis assessed the effect on trial outcomes on the basis of the participant’s level of adherence to the use of decolonization products as compared with the education group. Among the participants in the decolonization group, 65.6% of the participant-time involved full adherence (no missed doses); 19.6%, partial adherence (some missed doses); and 14.8%, nonadherence (no doses used). The comparator for each adherence subgroup was the overall education group.
As-treated models for all outcomes were adjusted for randomization strata, sex, primary insurance type, diabetes, renal disease, liver disease, hospitalization within 12 months before enrollment hospitalization, medical device on discharge from enrollment hospitalization, bathing frequency, and need for bathing assistance on the basis of variables associated with outcomes at a P value of less than 0.20 in bivariate analyses.
Figure 2. Kaplan–Meier Curves for Freedom from MRSA Infection and Infection from Any Cause, Assessed According to CDC Criteria.
Cases of MRSA infection and infection from any cause were assessed according to criteria of the Centers for Disease Control and Prevention come) was significantly greater in the decolonization group than in the education group. The curves remained separated even though decolonizatio stopped at 6 months. Details regarding the numbers of patients at risk for infection and those with infection at the specific time points are provided in Table S7 in the Supplementary Appendix.
The hazard of infection from any cause according to clinical judgment was lower in the decolonization group than in the education group (hazard ratio, 0.83; 95% CI, 0.70 to 0.99); similarly, the hazard of infection from any cause according to CDC criteria was lower in the decolonization group (hazard ratio, 0.84; 95% CI, 0.70 to 1.01) (Fig. 2B and Table 3). The risk of hospitalization due to infection from any cause was lower in the decolonization group than in the education group (hazard ratio, 0.76; 95% CI, 0.62 to 0.93). The results of the adjusted analyses were similar to those of the unadjusted analyses (Table 3). Deaths due to any infection occurred in 25 participants (2.3%) in the education group and 17 (1.6%) in the decolonization group.
EFFECT OF ADHERENCE
In as-treated analyses, 65.6% of the participant-time in the decolonization group involved full adherence; 19.6%, partial adherence; and 14.8%, nonadherence. Participants were highly consistent in adherence across the follow-up time. Increasing adherence was associated with increasingly lower rates of infection in both the adjusted and unadjusted models (Table 3). In comparisons of the adherence-category subgroups in the decolonization group with the education group overall, the likelihood of CDC-defined MRSA infection decreased 36% and 44%, respectively, as adherence increased from partial adherence (hazard ratio, 0.64; 95% CI, 0.40 to 1.00) to full adherence (hazard ratio, 0.56; 95% CI, 0.36 to 0.86). Similar effects were seen with regard to CDC-defined infection from any cause, which was 40% lower among fully adherent participants than among the participants in the education group (hazard ratio, 0.60; 95% CI, 0.46 to 0.78). Nonadherence was associated with a higher likelihood of infection from any cause than was observed among participants in the education group.
NUMBER NEEDED TO TREAT
Overall, the estimated number needed to treat to prevent a MRSA infection was 30 (95% CI, 18 to 230) and to prevent an associated hospitalization, 34 (95% CI, 20 to 336). The number needed to treat to prevent any infection was 26 (95% CI, 13 to 212) and to prevent an associated hospitalization, 28 (95% CI, 21 to 270). Among the participants who adhered fully to the intervention (all of whom were in the decolonization group), the number needed to treat to prevent a MRSA infection was 26 (95% CI, 18 to 83) and to prevent an associated hospitalization, 27 (95% CI, 20 to 46). The number needed to treat to prevent any infection was 11 (95% CI, 8 to 21) and to prevent an associated hospitalization, 12 (95% CI, 8 to 23).
ADVERSE EVENTS
Adverse events that were associated with the topical decolonization intervention were mild and uncommon, occurring in 44 participants (4.2%) (Table S9 in the Supplementary Appendix). Local irritation occurred with mupirocin in 1.1% of the participants (12 of 1058), with chlorhexidine bathing in 2.3% (24), and with chlorhexidine mouthwash in 1.1% (12). In those respective categories, 33% (4 of 12), 29% (7 of 24), and 50% (6 of 12) of the participants chose to continue using the product (overall, 39% of the participants with side effects).
A total of 12.6% of the 1591 participants with postrecruitment MRSA strains had high-level resistance to mupirocin (9.4% [150 participants]) or low-level resistance to mupirocin (3.1% [50]). A total of 1.9% of the participants were newly found to have a mupirocin-resistant strain at subsequent visits (1.9% [16 of 826 participants] in the education group and 2.0% [15 of 765] in the decolonization group, P = 0.97). A total of 1.5% of the participants in each group were newly found to have high-level mupirocin-resistant strains (1.6% [13 of 826 participants] in the education group and 1.4% [11 of 765] in the decolonization group, P = 0.82) when only sensitive strains were detected at recruitment. Chlorhexidine MICs of 8 μg or more per milliliter were rare (occurring in 2 participants overall [0.1%]). Both patients were in the intervention group, and both isolates had an MIC of 8 μg per milliliter and were negative for the qac A/B gene).
DISCUSSION
Infection-prevention campaigns have reduced the risks of health care–associated infections in hospitals, leaving the majority of preventable infections to the postdischarge setting.16 MRSA carriers are an appealing population target because of their higher risks of infection and postdischarge rehospitalization and the common practice of screening selected inpatients for MRSA colonization.1,17–19 In the CLEAR trial, topical decolonization led to lower risks of infections and readmissions than hygiene education alone among patients after the transition from hospital to home and other care settings. With a number needed to treat between 25 and 30 to prevent infection and hospitalization, this intervention is relevant to 1.8 million MRSA carriers (5% of inpatients) who are discharged from hospitals each year.16
Although decolonization has successfully prevented disease during temporary high-risk circumstances (e.g., recurrent skin infections, ICU care, and arthroplasty and cardiac surgery),6–10,19–22 a single 5-day decolonization regimen produced short-lived MRSA clearance in half the carriers.23–26 In contrast, twice-monthly decolonization provided protection for many months after discharge. The protective benefit continued after decolonization. In addition, this regimen was effective despite the greater variability in application with home bathing and showering than has occurred in previous inpatient trials that evaluated nursing-assisted chlorhexidine bathing and mupirocin application.8,9,22 This trial also showed that 4% rinse-off chlorhexidine was effective in a postdischarge population that typically takes showers or baths and is unlikely to use a 2% leave-on chlorhexidine product.8,9,22
Not surprisingly, participants who adhered fully to the decolonization intervention had rates of MRSA infection and infection from any cause that were at least 40% lower than the rates among participants in the education group, with a number needed to treat of 12 to prevent infection-related hospitalization. This finding probably is attributable to both the decolonization effect and the likelihood that these participants were more adherent to other prescribed treatments and health-promotion behavior than participants in the education group. Participants who fully adhered to the intervention had fewer coexisting conditions, had fewer devices, required less bathing assistance, and were more likely to have MRSA infection (rather than asymptomatic colonization) at the time of enrollment than either participants in the education group or participants in the decolonization group who had lower levels of adherence. These differences represent an important practical distinction. To the extent that physicians can identify patients who are able to adhere to an intervention, those patients would derive greater benefit from the recommendation to decolonize. Nonadherence was common among nursing home residents, which raises questions about research barriers in that care setting.
Decolonization appeared to affect the risks of skin and soft-tissue infections, surgical-site infections, pneumonia, and bacteremia, although sample-size constraints necessitate cautious speculation. Decolonization also appeared to reduce the rate of gram-positive pathogens and infections without a cultured pathogen. The higher rate of gram-negative pathogens in the decolonization group than in the education group was seen among the CDC-defined all-cause infections but not among the clinically defined infections and requires further substantiation. These observations are based on relatively small numbers; larger studies have shown that chlorhexidine can reduce the incidence of gram-negative infections and bacteriuria.27–30
The design of this trial did not permit us to determine the effect of hygiene education alone. Both trial groups received in-person visits and reminders about the importance of MRSA-prevention activities. In addition, the free product overcame financial disparities that could become evident with post-trial adoption of the decolonization intervention.
Some participants (<5%) in the decolonization group had mild side effects; among those participants, nearly 40% opted to continue using the agent. Resistance to chlorhexidine and mupirocin was not differentially engendered in the two groups. We defined an elevated chlorhexidine MIC as at least 8 μg per milliliter, although 4% chlorhexidine applies 40,000 μg per milliliter to the skin.
This trial is likely to be generalizable because it was inclusive. For example, the enrollment of participants with late-stage cancer contributed to the 10% anticipated mortality and the approximate 25% rate of withdrawal and loss to follow-up. These rates are similar to other post-discharge trials with shorter durations of followup than the durations in our trial.31–33 It is unknown whether the participants who withdrew or were lost to follow-up had different infection rates or intervention benefits. They were more educated and less likely to be Hispanic than those who did not withdraw or were not lost to follow-up, but the percentages of participants with coexisting conditions were similar.
Limitations of this trial include the unblended intervention, although outcomes were assessed in a blinded fashion. The trial also had substantial attrition over the 1-year follow-up, and adherence was based on reports by the participants, with spot checks of remaining product, both of which may not reflect actual use. In addition, nearly all infections led to hospitalization, which suggests that milder infections escaped detection. Most outpatient and nursing home records had insufficient documentation for the event to be deemed infection according to the CDC or clinical criteria. Thus, it remains unknown whether the observed 30% lower risk of MRSA infection or the observed 17% lower risk of infection from any cause with decolonization than with education alone would apply to less severe infections that did not lead to hospitalization. Finally, although resistance to chlorhexidine and mupirocin did not emerge during the trial, the development of resistance may take time, beyond the follow-up period of this trial.
In conclusion, inpatients with MRSA-positive cultures who had been randomly assigned to undergo decolonization with topical chlorhexidine and mupirocin for 6 months after discharge had lower risks of MRSA infection, infection from any cause, and hospitalization over the 1 year after discharge than those who had been randomly assigned to receive hygiene education only.
Supplementary Material
Acknowledgments
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC), the National Institutes of Health (NIH), or the Agency for Healthcare Research and Quality (AHRQ).
Supported by a grant (R01HS019388, to Dr. Huang) from the AHRQ Healthcare-Associated Infections Program and by the University of California Irvine Institute for Clinical and Translational Science, which was funded by a grant from the NIH Clinical and Translational Sciences Award program (UL1 TR000153).
Dr. Huang reports conducting clinical studies in which participating nursing homes and hospitals received donated products from Stryker (Sage Products), Mölnlycke, 3M, Clorox, Xttrium Laboratories, and Medline; Ms. Singh, Dr. Park, and Mr. Chang, conducting clinical studies in which participating nursing homes and hospitals received donated products from Stryker (Sage Products), 3M, Clorox, Xttrium Laboratories, and Medline; Dr. McKinnell, receiving grant support and consulting fees from Achaogen and Theravance Biopharma, grant support, consulting fees, and lecture fees from Allergan, consulting fees from Cempra, Melinta Therapeutics, Menarini Group, and Thermo Fisher Scientific, and fees for serving as a research investigator from Science 37, conducting clinical studies in which participating nursing homes and hospitals received donated products from Stryker (Sage Products), 3M, Clorox, Xttrium Laboratories, and Medline, and serving as cofounder of Expert Stewardship; Ms. Gombosev, conducting clinical studies in which participating nursing homes and hospitals received donated products from Stryker (Sage Products), Mölnlycke, 3M, and Clorox; Dr. Rashid, conducting clinical studies in which participating nursing homes and hospitals received donated products from Stryker (Sage Products), Clorox, and Medline; Dr. Bolaris, conducting clinical studies in which participating nursing homes received donated products from 3M and Clorox; Dr. Robinson, serving as cofounder of Expert Stewardship; Dr. Amin, receiving consulting fees from Paratek Pharmaceuticals; Dr. Septimus, conducting clinical studies in which participating hospitals received donated product from Stryker (Sage Products), Mölnlycke, and Medline; Dr. Weinstein, conducting clinical studies in which participating nursing homes and hospitals received donated products from Stryker (Sage Products) and Mölnlycke; Dr. Hayden, conducting clinical studies in which participating nursing homes and hospitals received donated product from Stryker (Sage Products), Mölnlycke, and Medline and donated laboratory services from OpGen and receiving grant support and conducting clinical studies in which participating nursing homes and hospitals received donated product from Clorox; and Dr. Miller, receiving grant support from Gilead Sciences, Merck, Abbott, Cepheid, Genentech, Atox Bio, and Paratek Pharmaceuticals, grant support and fees for serving on an advisory board from Achaogen and grant support, consulting fees, and fees for serving on an advisory board from Tetraphase and conducting clinical studies in which participating nursing homes and hospitals received donated products from Stryker (Sage Products), 3M, Clorox, Xttrium Laboratories, and Medline. No other potential conflict of interest relevant to this article was reported.
We thank the trial participants and their families; the trial staff (Lauren Akahoshi, Jenna Alcaron, Isabel Alegria, Stephanie Arredondo-Glacet, Elizabeth Arreola, Donald Bayley, Nelly Beltran, Barbara Bodenhoefer, Stefan Boghossian, Diane Capobianco, Claudia Cervantes, Sarah Chevallier, Heather Clayton, Ramiro Correa, Nathalia Cressey, Tiffany Dam, Rupak Datta, Jennifer Do, Phillip Duffy, Tabitha Dutciuc, Marlene Estevez, Margarita Flores, Lauren Heim, Sandra Ibarra, Usme Khusbu, Bryn Launer, Cameron Lee, Brian Lewis, Karen Lolans, Andrea Marcantonio, Donna Matsudairas, Lisa [Angie] McErlain, Maria Mejia, Job Mendez, Angela Mendoza, Melanie Meton, Nicole Mohajer, Jennifer Nam, Ann Nguyen, Hanna Owens, Jalpa Patel, Lena Portillo, Sean Prendergast, Katy Precaido, Belinda Prado, Deborah Prunean, Victor Quan, Courtney Reynolds, Diana Romero, Aubrianne Rose, Maureen Schroeder, Grace Tagudar, Cynthia Toyoshima, Ivonne Turner, Qixin Wang, Emily Wawro, and Jun Zozobrado); two physicians who actively supported and facilitated recruitment (David Petreccia and Anjali Vora); the members of our community advisory board (Patricia Cantero, Ph.D., and Jeanine Thomas); the members of our data and safety monitoring board (Honghu Liu, David Beenhouwer, and George Sakoulas); and the staff of the many hospitals and nursing homes that supported the recruitment of participants in the trial.
APPENDIX
The authors’ full names and academic degrees are as follows: Susan S. Huang, M.D., M.P.H, Raveena Singh, M.A., James A. McKinnell, M.D., Steven Park, M.D., Ph.D., Adrijana Gombosev, M.S., Samantha J. Eells, M.P.H., Daniel L. Gillen, Ph.D., Diane Kim, B.S., Syma Rashid, M.D., Raul Macias-Gil, M.D., Michael A. Bolaris, M.D., Thomas Tjoa, M.P.H., M.S., Chenghua Cao, M.P.H., Suzie S. Hong, M.S., Jennifer Lequieu, B.S., Eric Cui, B.S., Justin Chang, B.S., Jiayi He, M.S., Kaye Evans, B.A., Ellena Peterson, Ph.D., Gail Simpson, M.D., Philip Robinson, M.D., Chester Choi, M.D., Charles C. Bailey, Jr., M.D., James D. Leo, M.D., Alpesh Amin, M.D., Donald Goldmann, M.D., John A. Jernigan, M.D., Richard Platt, M.D., Edward Septimus, M.D., Robert A. Weinstein, M.D., Mary K. Hayden, M.D., and Loren G. Miller, M.D., M.P.H.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med 2013;173:1970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14. [DOI] [PubMed] [Google Scholar]
- 3.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 2001;344:11–6. [DOI] [PubMed] [Google Scholar]
- 4.Huang SS, Hinrichsen VL, Datta R, et al. Methicillin-resistant Staphylococcus aureus infection and hospitalization in high-risk patients in the year following detection. PLoS One 2011;6(9):e24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Methicillin-resistant Staphylococcus aureus: information for patients Atlanta: Centers for Disease Control and Prevention, 2016. (https://www.cdc.gov/mrsa/healthcare/patient/index.html). [Google Scholar]
- 6.Septimus EJ, Schweizer ML. Decolonization in prevention of health care-associated infections. Clin Microbiol Rev 2016; 29:201–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bode LGM, Kluytmans JAJW, Wertheim HFL, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010;362:9–17. [DOI] [PubMed] [Google Scholar]
- 8.Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013;368:2255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med 2013;368:533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011;52(3):e18–e55. [DOI] [PubMed] [Google Scholar]
- 11.CDC/NHSN protocol clarifications: Identifying healthcare-associated infections (HAI) in NHSN Atlanta: Centers for Disease Control and Prevention, 2013. (https://www.cdc.gov/nhsn/pdfs/validation/2013/pscmanual_july2013.pdf). [Google Scholar]
- 12.Hayden MK, Lolans K, Haffenreffer K, et al. Chlorhexidine and mupirocin susceptibility of methicillin-resistant Staphylococcus aureus isolates in the REDUCE-MRSA trial. J Clin Microbiol 2016;54:2735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard 8th ed. Wayne, PA: Clinical and Laboratory Standards Institute, 2009. [Google Scholar]
- 14.Morrissey I, Oggioni MR, Knight D, et al. Evaluation of epidemiological cutoff values indicates that biocide resistant subpopulations are uncommon in natural isolates of clinically-relevant microorganisms. PLoS One 2014;9(1):e86669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999;319:1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 2007;122:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein EY, Mojica N, Jiang W, et al. Trends in methicillin-resistant Staphylococcus aureus hospitalizations in the United States, 2010–2014. Clin Infect Dis 2017;65:1921–3. [DOI] [PubMed] [Google Scholar]
- 18.Duffy J, Dumyati G, Bulens S, et al. Community-onset invasive methicillin-resistant Staphylococcus aureus infections following hospital discharge. Am J Infect Control 2013;41:782–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarvis WR, Schlosser J, Chinn RY, Tweeten S, Jackson M. National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at US health care facilities, 2006. Am J Infect Control 2007;35:631–7. [DOI] [PubMed] [Google Scholar]
- 20.Perl TM, Cullen JJ, Wenzel RP, et al. Intranasal mupirocin to prevent post-operative Staphylococcus aureus infections. N Engl J Med 2002;346:1871–7. [DOI] [PubMed] [Google Scholar]
- 21.Schweizer ML, Chiang HY, Septimus E, et al. Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip, or knee surgery. JAMA 2015;313:2162–71. [DOI] [PubMed] [Google Scholar]
- 22.Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet 2013;381:1099–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wertheim HFL, Verveer J, Boelens HAM, van Belkum A, Verbrugh HA, Vos MC. Effect of mupirocin treatment on nasal, pharyngeal, and perineal carriage of Staphylococcus aureus in healthy adults. Antimicrob Agents Chemother 2005;49: 1465–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Immerman I, Ramos NL, Katz GM, Hutzler LH, Phillips MS, Bosco JA. The persistence of Staphylococcus aureus decolonization after mupirocin and topical chlorhexidine: implications for patients requiring multiple or delayed procedures. J Arthroplasty 2012;27:870–6. [DOI] [PubMed] [Google Scholar]
- 25.Mody L, Kauffman CA, McNeil SA, Galecki AT, Bradley SF. Mupirocin-based decolonization of Staphylococcus aureus carriers in residents of 2 long-term care facilities: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2003;37:1467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wendt C, Schinke S, Württemberger M, Oberdorfer K, Bock-Hensley O, von Baum H. Value of whole-body washing with chlorhexidine for the eradication of methicillin-resistant Staphylococcus aureus: a randomized, placebo-controlled, double-blind clinical trial. Infect Control Hosp Epidemiol 2007;28:1036–43. [DOI] [PubMed] [Google Scholar]
- 27.Hayden MK, Lin MY, Lolans K, et al. Prevention of colonization and infection by Klebsiella pneumoniae carbapenemaseproducing enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis 2015; 60:1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin MY, Lolans K, Blom DW, et al. The effectiveness of routine daily chlorhexidine gluconate bathing in reducing Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae skin burden among long-term acute care hospital patients. Infect Control Hosp Epidemiol 2014;35:440–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassir N, Thomas G, Hraiech S, et al. Chlorhexidine daily bathing: impact on health care-associated infections caused by gram-negative bacteria. Am J Infect Control 2015;43:640–3. [DOI] [PubMed] [Google Scholar]
- 30.Huang SS, Septimus E, Hayden MK, et al. Effect of body surface decolonisation on bacteriuria and candiduria in intensive care units: an analysis of a cluster-randomised trial. Lancet Infect Dis 2016;16:70–9. [DOI] [PubMed] [Google Scholar]
- 31.Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013;368:513–23. [DOI] [PubMed] [Google Scholar]
- 32.Prasad Shrestha M, Scott RM, Man Joshi D, et al. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med 2007;356:895–903. [DOI] [PubMed] [Google Scholar]
- 33.Michaels JA, Brazier JE, Campbell WB, MacIntyre JB, Palfreyman SJ, Ratcliffe J. Randomized clinical trial comparing surgery with conservative treatment for uncomplicated varicose veins. Br J Surg 2006; 93:175–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.