Abstract
Background
Schizophrenia is a disabling serious mental illness that can be chronic. Haloperidol, one of the first generation of antipsychotic drugs, is effective in the treatment of schizophrenia but can have adverse side effects. The effects of stopping haloperidol in people with schizophrenia who are stable on their prescription are not well researched in the context of systematic reviews.
Objectives
To review the effects of haloperidol discontinuation in people with schizophrenia who are stable on haloperidol.
Search methods
On 20 February 2015, 24 May 2017, and 12 January 2019, we searched the Cochrane Schizophrenia Group’s Study‐Based Register of Trials including trial registers.
Selection criteria
We included clinical trials randomising adults with schizophrenia or related disorders who were receiving haloperidol, and were stable. We included trials that randomised such participants to either continue their current treatment with haloperidol or discontinue their haloperidol treatment. We included trials that met our selection criteria and reported usable data.
Data collection and analysis
We independently checked all records retrieved from the search and obtained full reports of relevant records for closer inspection. We extracted data from included studies independently. All usable data were dichotomous, and we calculated relative risks (RR) and their 95% confidence intervals (95% CI) using a fixed‐effect model. We assessed risk of bias within the included studies and used GRADE to create a 'Summary of findings' table.
Main results
We included five randomised controlled trials (RCTs) with 232 participants comparing haloperidol discontinuation with haloperidol continuation. Discontinuation was achieved in all five studies by replacing haloperidol with placebo. The trials' size ranged between 23 and 87 participants. The methods of randomisation, allocation concealment and blinding were poorly reported.
Participants allocated to discontinuing haloperidol treatment were more likely to show no improvement in global state compared with those in the haloperidol continuation group (n = 49; 1 RCT; RR 2.06, 95% CI 1.33 to 3.20; very low quality evidence: our confidence in the effect estimate is limited due to relevant methodological shortcomings of included trials). Those who continued haloperidol treatment were less likely to experience a relapse compared to people who discontinued taking haloperidol (n = 165; 4 RCTs; RR 1.80, 95% CI 1.18 to 2.74; very low quality evidence). Satisfaction with treatment (measured as numbers leaving the study early) was similar between groups (n = 43; 1 RCT; RR 0.13, 95% CI 0.01 to 2.28; very low quality evidence).
No usable mental state, general functioning, general behaviour or adverse effect data were reported by any of the trials.
Authors' conclusions
This review provides limited evidence derived from small, short‐term studies. The longest study was for one year, making it difficult to generalise the results to a life‐long disorder. Very low quality evidence shows that discontinuation of haloperidol is associated with an increased risk of relapse and a reduction in the risk of 'global state improvement'. However, participant satisfaction with haloperidol treatment was not different from participant satisfaction with haloperidol discontinuation as measured by leaving the studies early. Due to the very low quality of these results, firm conclusions cannot be made. In addition, the available studies did not report usable data regarding the adverse effects of haloperidol treatment.
Considering that haloperidol is one of the most widely used antipsychotic drugs, it was surprising that only a small number of studies into the benefit and harm of haloperidol discontinuation were available. Moreover, the available studies did not report on outcomes that are important to clinicians and to people with schizophrenia, particularly adverse effects and social outcomes. Better designed trials are warranted.
Plain language summary
What happens if haloperidol treatment is discontinued for people with schizophrenia
Review question
What are the effects of discontinuing the antipsychotic haloperidol in people with schizophrenia who are stable on haloperidol treatment.
Background
Schizophrenia involves a breakdown in the relationships between thought, emotion, and behaviour, leading to thought disorder, faulty perception, inappropriate actions and feelings, and social withdrawal. Haloperidol is one of the first drugs to be used in the treatment of schizophrenia. Haloperidol is known to be effective for treating the psychotic symptoms (such as delusions and hallucinations) of schizophrenia; it can also, however, have unpleasant side effects. The effects of stopping or withdrawing haloperidol treatment are not well researched.
Searching for evidence
We ran electronic searches of Cochrane Schizophrenia's trial register, most recently on 24 January 2019, for trials that randomised people with schizophrenia and are stable on haloperidol treatment to either discontinue taking haloperidol or to continue taking haloperidol. The review authors found and checked 54 records.
Evidence found
Five trials met the review requirements and provided usable data.
The evidence currently available from randomised controlled trials is of very low quality and suggests that haloperidol discontinuation is associated with poorer outcomes for people with schizophrenia in terms of their overall symptom improvement (global state). It also shows that people discontinuing haloperidol treatment are more likely to experience relapse (reoccurrence of symptoms) during the first year post treatment. There were no trials providing evidence on what happens after the first year. The number of participants leaving the study early (which can be an indication of satisfaction with treatment) was similar between the two treatments.
The very low quality of the currently available evidence is due to methodological shortcomings of the included trials. This lowers our confidence in the reliability of results, and hampers their generalisability to real‐world situations.
Conclusions
It is disappointing that only a small number of studies were identified, and the size and quality of these studies were limited. The evidence available does not enable us to fully answer important questions regarding the effects of discontinuing haloperidol treatment. In particular there is no evidence available related to the side effects of haloperidol. New trials of better quality are needed.
Summary of findings
Summary of findings for the main comparison. Haloperidol discontinuation compared to haloperidol continuation for schizophrenia.
| Haloperidol discontinuation compared to haloperidol continuation for schizophrenia | ||||||
| Patient or population: schizophrenia Setting: inpatient and outpatient Intervention: haloperidol discontinualtion Comparison: haloperidol continualtion | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with haloperidol continuation | Risk with haloperidol discontinuation | |||||
| Global state: clinically important change ‒ not improved (CGI) follow‐up: 90 days | Study population | RR 2.06 (1.33 to 3.20) | 49 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1, 2 | A single short term study. Placebo was compared to haloperidol tablets and haloperidol liquid. The two haloperidol groups were combined for this analysis. | |
| 42 per 100 | 20 per 100 (6 to 68) | |||||
| Global state: relapse: ‒ follow‐up: range 90 days to 1 year | Study population | RR 1.80 (1.18 to 2.74) | 165 (4 RCTs) | ⊝⊝⊝ VERY LOW 1, 3,4 | 1 short‐term, 1 medium‐term and 2 long‐term studies. The longest study was for 1 year. 3 studies used oral haloperidol and 1 used long‐acting intramuscular haloperidol. | |
| 36 per 100 | 68 per 100 (45 to 100) | |||||
| Mental state: clinically important change in general mental state | No data were available for these important outcomes. | |||||
| General functioning: clinically important change in general functioning including working ability | ||||||
| General behaviour: clinically important change in general behaviour | ||||||
| Quality of life: clinically important change in quality of life | ||||||
| Satisfaction with treatment: leaving the study early follow‐up: 1 years | Study population | RR 0.13 (0.01 to 2.28) | 43 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 ,3,5 | ||
| 19 per 100 | 3 per 100 (0 to 21) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by 2 levels due to very serious risk of bias: randomisation is implied
2 Downgraded by 1 level due to imprecision: data from one trial, small sample size and wide confidence interval
3Downgraded by 1 level due to serious risk of bias: random assignment and allocation concealment unclear
4 Downgraded by 1 level due to inconsistency: high heterogenity I² > 70%
5 Downgraded by 1 level due to serious risk of attrition bias: high attrition rate
Background
Description of the condition
Schizophrenia is often a chronic and disabling psychiatric disorder. It afflicts approximately 1% of the world‐wide population with little gender differences (Berger 2003). The median incidence of schizophrenia has been estimated to be 15.2/100,000 persons (McGrath 2008). The typical manifestations of schizophrenia are 'positive' symptoms such as fixed beliefs that are not amenable to change in light of conflicting evidence (delusions) and perceptions without cause (hallucinations); and 'negative' symptoms such as apathy and lack of drive, disorganisation of behaviour and thought, and catatonic symptoms such as mannerisms and bizarre posturing (Carpenter 1994). The degree of suffering and disability is considerable, with 80% to 90% of affected people not working (Marvaha 2004), and up to 10% dying by suicide (Tsuang 1978).
Description of the intervention
Haloperidol is one of the most frequently used antipsychotic medications (Lohse 2009). It is a first‐generation ('typical', 'conventional') antipsychotic drug with very high antidopaminergic activity. Its mean elimination half‐life has been reported to range from 15 to 37 hours and its bioavailability is 60% to 70% (Kudo 1999). Haloperidol is highly effective in treating schizophrenia, but the downside is that it is associated with severe extrapyramidal side effects. The most predominant among these extrapyramidal side effects are dystonia, parkinsonian‐like syndrome, and tardive dyskinesia. Other side effects include anticholinergic effects (e.g. constipation, dry mouth, blurred vision, and urinary hesitancy), sexual dysfunction, elevations in serum prolactin, and sedation — and there could even be shown a relationship with sudden death. Therefore, clinicians and people with schizophrenia often face a trade‐off between protection against psychotic episodes and adverse effects.
Haloperidol is effective in treating the acute phases of schizophrenia (Irving 2006). However, it remains unclear how long haloperidol treatment should continue after the acute phase of the illness subsides. The intervention studied in this review is the discontinuation of haloperidol in people with schizophrenia who have already responded to haloperidol treatment.
How the intervention might work
Haloperidol is one of the butyrophenone family of antipsychotic (neuroleptic) drugs (López‐Munoz 2009). It is thought that haloperidol prevents the occurrence of delusions and hallucinations by blocking the dopamine D2 receptors in the meso‐cortico‐limbic system. A similar antidopaminergic activity in the dorsolateral striatum may contribute to the adverse extrapyramidal effects that are associated with haloperidol treatment (Xiberas 2001).
Why it is important to do this review
Although schizophrenia is generally thought to be a lifelong disorder requiring long‐term pharmacological treatment (Essali 1993), the course of schizophrenia varies, and may follow one of four patterns (Shepherd 1989).
13% may have a single episode with no subsequent impairment.
30% may have several episodes with no or minimal impairment.
10% may suffer impairment following the first episode with occasional exacerbation of symptoms and no return to normality.
47% show impairment increasing after each exacerbation.
Long‐term antipsychotic treatment has been the norm for people diagnosed with schizophrenia. However, this antipsychotic maintenance treatment has been questioned (Moncrieff 2015). Predicting the need for antipsychotic maintenance treatment would improve the management of schizophrenia. Medication cessation studies may help identify the characteristics of those patients who will have a single episode and not require maintenance drug treatment, those who will follow a relapsing course and may benefit from intermittent treatment, and those who require indefinite maintenance drug treatment. In this review, we aimed to investigate the quantitative effects of stopping haloperidol for people stable on this drug by reviewing available trial‐based evidence.
This is one of a series of Cochrane Reviews on the effects of haloperidol (Table 2).
1. Cochrane schizophrenia reviews relevant to haloperidol.
| Comparison | Reference |
| Aripiprazole versus haloperidol for people with schizophrenia and schizophrenia‐like psychoses | Bhattacharjee 2016 |
| Depot haloperidol decanoate for schizophrenia | Quraishi 1999 |
| Haloperidol (route of administration) for people with schizophrenia | Hanafi 2017 |
| Haloperidol discontinuation for schizophrenia [Protocol of this review] | Essali 2014 |
| Haloperidol dose for the acute phase of schizophrenia | Donnelly 2013 |
| Haloperidol for long‐term aggression in psychosis | Khushu 2016 |
| Haloperidol for psychosis‐induced aggression or agitation (rapid tranquillisation) | Ostinelli 2017 |
| Haloperidol plus promethazine for psychosis‐induced aggression | Huf 2016 |
| Haloperidol versus chlorpromazine for schizophrenia | Leucht 2008 |
| Haloperidol versus first‐generation antipsychotics for the treatment of schizophrenia and other psychotic disorders | Dold 2015 |
| Haloperidol versus low‐potency first‐generation antipsychotic drugs for schizophrenia | Tardy 2014 |
| Haloperidol versus placebo for schizophrenia | Adams 2013 |
| Haloperidol versus risperidone for schizophrenia | Ray 2017 |
Objectives
To review the effects of haloperidol discontinuation in people with schizophrenia who are stable on haloperidol.
Methods
Criteria for considering studies for this review
Types of studies
We included in this review all relevant randomised controlled trials (RCTs). If a trial was described as 'double blind' but implied randomisation, we included it in a sensitivity analysis (Sensitivity analysis). If inclusion did not result in a substantive difference, it remained in the analyses. If inclusion did result in important clinically significant but not necessarily statistically significant differences, we would not have added the data from a lower quality study to the results of the better trials, but would have presented such data within a subcategory.
We would have excluded quasi‐randomised studies, such as those allocating by alternate days of the week. Where people were given additional treatments, we only included data if the adjunct treatment was evenly distributed between groups and it was only the haloperidol that was randomised.
Types of participants
Adults, however defined, with schizophrenia or related disorders, including schizophreniform disorder, schizoaffective disorder and delusional disorder by any means of diagnosis, who were stable on haloperidol (given orally or by intramuscular injection).
We are interested in making sure that information is as relevant to the current care of people with schizophrenia as possible so proposed, if possible, to clearly highlight:
the current clinical state (acute, early post‐acute, partial remission, remission);
the stage (prodromal, first episode, early illness, persistent); and
whether the studies primarily focused on people with particular problems (for example, negative symptoms, treatment‐resistant illnesses).
Types of interventions
Discontinuation of haloperidol treatment, however this was done in the trials, e.g. gradually, abruptly or under cover of placebo.
Continuation of haloperidol treatment at any dose or mode of administration (oral or by injection).
Types of outcome measures
We divided all outcomes into short term (up to three months), medium term (over three and up to six months) and long term (over six months).
Primary outcomes
1. Global state
1.1 Clinically important change ‒ as defined by each study 1.2 Relapse ‒ as defined by each study
Secondary outcomes
1. Death ‒ suicide and natural causes
2. Global state
2.1 Average endpoint global state score 2.2 Average change in global state scores
3. Service outcomes
3.1 Hospitalisation 3.2 Inability to be discharged from hospital
4. Mental state (with particular reference to the positive and negative symptoms of schizophrenia)
4.1 Clinically important change in general mental state 4.2 Average endpoint general mental state score 4.3 Average change in general mental state scores 4.4 Clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia, depression, mania) 4.5 Average endpoint specific symptom score 4.6 Average change in specific symptom scores
5. General functioning
5.1 Clinically important change in general functioning including working ability 5.2 Average endpoint general functioning score 5.3 Average change in general functioning scores 5.4 Clinically important change in specific aspects of functioning, such as social or life skills 5.5 Average endpoint specific aspects of functioning, such as social or life skills 5.6 Average change in specific aspects of functioning, such as social or life skills
6. Behaviour
6.1 Clinically important change in general behaviour 6.2 Average endpoint general behaviour score 6.3 Average change in general behaviour scores 6.4 Clinically important change in specific aspects of behaviour 6.5 Average endpoint specific aspects of behaviour 6.6 Average change in specific aspects of behaviour
7. Adverse effects ‒ general and specific (important adverse effects included movement disorders, weight gain, fits and blood reactions leading to therapy discontinuation)
7.1 Clinically important general adverse effects 7.2 Average endpoint general adverse effect score 7.3 Average change in general adverse effect scores 7.4 Clinically important specific adverse effects 7.5 Average endpoint specific adverse effects 7.6 Average change in specific adverse effects
8. Satisfaction with treatment (including subjective well‐being and family burden)
8.1 Leaving the studies early 8.2 Recipient of care not satisfied with treatment 8.3 Recipient of care average satisfaction score 8.4 Recipient of care average change in satisfaction scores 8.5 Carer not satisfied with treatment 8.6 Carer average satisfaction score 8.7 Carer average change in satisfaction scores
9. Quality of life
9.1 Clinically important change in quality of life 9.2 Average endpoint quality of life score 9.3 Average change in quality of life scores 9.4 Clinically important change in specific aspects of quality of life 9.5 Average endpoint specific aspects of quality of life 9.6 Average change in specific aspects of quality of life
10. Economic outcomes
10.1 Direct costs 10.2 Indirect costs
11. Cognitive functioning
11.1 Clinically important change in cognitive functioning 11.2 Average endpoint cognitive functioning score 11.3 Average change in cognitive functioning scores 11.4 Clinically important change in specific aspects of cognitive functioning 11.5 Average endpoint specific aspects of cognitive functioning 11.6 Average change in specific aspects of cognitive functioning
12. 'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2011); and used GRADEpro GDT to export data from our review to create a 'Summary of findings' table. These tables provide outcome‐specific information concerning the overall certainty of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rate as important to patient care and decision making.
We selected the following main outcomes for inclusion in the 'Summary of findings' table*.
Global state: clinically important change (any time frame)
Global state: relapse as defined by each study (any time frame)
Mental state: clinically important change in general mental state (any time frame)
General functioning: clinically important change in general functioning including working ability (any time frame)
General behaviour: clinically important change in general behaviour (any time frame)
Quality of life: clinically important change in quality of life (any time frame)
Satisfaction with treatment: leaving the study early
Search methods for identification of studies
Electronic searches
Cochrane Schizophrenia Group's Study‐Based Register of Trials
On 20 February 2015, 24 May 2017, and 24 January 2019, the Information Specialist searched the register using the following search strategy:
(*Haloperidol* AND *Discontinuation*) in Intervention Field of STUDY
In such study‐based registers, searching the major concept retrieves all the synonyms and relevant studies because all the studies have already been organised based on their interventions and linked to the relevant topics (Shokraneh 2017).
This register is compiled by systematic searches of major resources (CENTRAL, MEDLINE, Embase, CINAHL, AMED, BIOSIS, ClinicalTrials.Gov, PsycINFO, PubMed, World Health Organization (WHO) ICTRP) and their monthly updates, ProQuest Dissertations and Theses A&I and its quarterly update, Chinese databases (CBM, CNKI, and Wanfang) and their annual updates, handsearches, grey literature, and conference proceedings (see Group's website). There is no language, date, document type, or publication status limitations for inclusion of records into the register.
For previous search term text, please see Appendix 1.
Searching other resources
1. Reference searching
We inspected the reference lists of all included studies for further relevant studies.
2. Personal contact
We would have contacted, when necessary, the first author of each included study for information regarding unpublished trials.
Data collection and analysis
Selection of studies
All seven review authors independently scrutinised the abstracts of retrieved studies. Where disputes arose, we acquired the full reports for more detailed scrutiny. All seven authors independently inspected the full reports of the abstracts meeting the review criteria in order to ensure reliable selection. Where it was not possible to resolve disagreement by discussion, one author (AE) acted as the final arbiter and we attempted to contact the authors of the study for clarification.
Data extraction and management
1. Extraction
Four review authors (KT, SA, AA, MRDA) extracted data from all included studies. In addition, to ensure reliability, two review authors (MEM, NA) independently extracted data from all included studies. We discussed all disagreement, documented decisions and contacted the authors of studies for clarification when necessary. With any remaining problems, review author AE helped clarify issues and we documented these final decisions.
We extracted data presented only in graphs and figures wherever possible, but included such data only if two authors independently reached the same result. We attempted to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary. For multicentre studies, where possible we would have extracted data relevant to each component centre separately.
2. Management
2.1. Forms
We extracted data onto standard, simple forms.
2.2. Scale‐derived data
We would have included continuous data from rating scales only if:
the psychometric properties of the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000); and
the measuring instrument had not been written or modified by one of the trialists for that particular trial.
Ideally the measuring instrument should either be a self‐report or completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly, and we would have included the relevant information in the 'Description of studies' section.
2.3. Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and difficult‐to‐measure conditions such as schizophrenia. We decided to primarily use endpoint data, and only use change data if the former were not available. We would have combined endpoint and change data in the analysis as we prefer to use mean differences (MDs) rather than standardised mean differences (SMDs) (Deeks 2011).
2.4. Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to all data before inclusion.
Standard deviations (SDs) and means are reported in the paper or obtainable from the authors.
When a scale starts from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution (Altman 1996).
If a scale started from a positive value (such as the Positive and Negative Syndrome Scale), which can have values from 30 to 210) (Kay 1986), we would have modified the calculation described above to take the scale starting point into account. In these cases skew is present if 2 SD > (S − S min), where S is the mean score and 'S min' is the minimum score.
Endpoint scores on scales often have a finite start and end point and these rules can be applied. Skewed data pose less of a problem when looking at means if the sample size is large (> 200) and we would have entered these into the syntheses. We would have presented skewed endpoint data from studies of less than 200 participants as 'other data' within the Data and analyses section rather than enter such data into statistical analyses.
When continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. We would have presented and entered change data into analyses.
2.5. Common measure
To facilitate comparison between trials, we intended to convert variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6. Conversion of continuous to binary
Where possible, we made every effort to convert outcome measures to dichotomous data. This can be achieved by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale or the Positive and Negative Syndrome Scale (Kay 1986; Overall 1962), this could be considered as a clinically significant response (Leucht 2005a; Leucht 2005b). If data based on these thresholds were not available, we would have used the primary cut‐off presented by the original authors.
2.7. Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for haloperidol discontinuation.
Assessment of risk of bias in included studies
Review authors (KT, SA, AA, MRDA, MEM and NA) worked independently to assess risk of bias using criteria described in the Cochrane Handbook for Systemic Reviews of Interventions (Higgins 2011a). This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
We resolved any disagreement by consensus, with the involvement of an arbiter (AE). Where inadequate details of randomisation and other characteristics of trials were provided, we were to contact authors of the studies in order to obtain further information. We were to report non‐concurrence in quality assessment, and disputes were resolved by discussion.
We have noted the level of risk of bias in both the text of the review and in a 'Summary of findings' table.
Measures of treatment effect
1. Binary data
For binary outcomes, we calculated standard estimation of the risk ratios (RRs) and their 95% confidence intervals (95% CIs). It has been shown that RR is more intuitive than odds ratios (ORs) (Boissel 1999), and that ORs tend to be interpreted as RRs by clinicians (Deeks 2000). The 'number needed to treat for an additional beneficial outcome' (NNTB) or 'number needed to treat for an additional harmful outcome' (NNTH) statistics with their 95% CIs are intuitively attractive to clinicians, but they are problematic in terms of accurate calculation in meta‐analyses and their subsequent interpretation (Hutton 2009). For binary data presented in the 'Table 1', where possible we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes, we would have estimated MDs between groups. We prefer not to calculate effect size measures (SMDs). However, if scales of very considerable similarity were used, we would have presumed that there was a small difference in measurement, and we would have calculated effect size and transformed the effect back to the units of one or more of the specific instruments. If SMDs were used, this would only have been calculated for endpoint data.
Unit of analysis issues
1. Cluster trials and cross‐over trials
We did not anticipate that drug discontinuation studies would use cluster randomisation or cross‐over designs; and in the unlikely event that we did encounter such designs, we planned to use methods described in the Cochrane Handbook for Systemic Reviews of Interventions to avoid 'unit of analysis' issues in data synthesis (Higgins 2011b).
2. Studies with multiple treatment groups
Where a study involved more than two treatment arms, we presented the additional treatment arms in comparisons. Binary data were simply added and combined within a two‐by‐two table. If data were continuous, we would have combined data following the guidance in the Cochrane Handbook for Systemic Reviews of Interventions (Higgins 2011b). Where the additional treatment arms were not relevant, we did not use these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss to follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we would not reproduce these data or use them within analyses (except for the outcome 'Leaving the study early'). If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we would have marked such data with an asterisk (*) to indicate that such a result may well be prone to bias.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we would have presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis). Those leaving the study early were all to be assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes, we would have used the rate of those who stay in the study — in that particular arm of the trial — for those who did not. We would have undertaken a sensitivity analysis testing how prone the primary outcomes were to change when we compared data only from people who completed the study to that point to the intention‐to‐treat analysis using the above assumptions.
3. Continuous
3.1. Attrition
In the case where attrition for a continuous outcome was between 0% and 50%, and data only from people who had completed the study to that point were reported, we would have reproduced these.
3.2. Standard deviations (SDs)
If SDs were not reported, we tried to obtain the missing values from the authors. If not available, where there were missing measures of variance for continuous data, but an exact standard error (SE) and 95% CIs available for group means, and either P value or t value available for differences in mean, we would have calculated them according to the rules described in the Cochrane Handbook for Systemic Reviews of Interventions (Higgins 2011b):
when only the SE is reported, SDs are calculated by the formula: SD = SE*√(n).
The Cochrane Handbook for Systemic Reviews of Interventions presents detailed formulae for estimating SDs from P values, t or F values, 95% CIs, ranges or other statistics (Higgins 2011b). If these formulae did not apply, we would have calculated the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study's outcome and thus to lose information. We nevertheless would have examined the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3. Last observation carried forward (LOCF)
We anticipated that in some studies the method of last observation carried forward (LOCF) would be employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). Therefore, where LOCF data have been used in the trial, if less than 50% of the data have been assumed, we would have presented and used these data and indicated that they were the product of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise. Had such situations or participant groups arise, we would have fully discussed these.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise. Had such methodological outliers arisen, we would have discussed these in detail.
3. Statistical heterogeneity
3.1. Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2. Employing the I² statistic
We investigated heterogeneity between studies by considering the I² method alongside the Chi² P value. The I² provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I² depends on both the magnitude and direction of effects as well as the strength of evidence for heterogeneity (e.g. P value from Chi² test, or 95% CIs for I²). We interpreted I² estimates greater than or equal to around 50% accompanied by a statistically significant Chi² statistic as evidence of substantial levels of heterogeneity (Deeks 2011). When substantial levels of heterogeneity were found in the primary outcome, we would have explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in section 10.1 of the Cochrane Handbook for Systemic Reviews of Interventions (Sterne 2011).
1. Protocol versus full study
We tried to locate protocols of included randomised trials. If the protocol was available, we would compare outcomes in the protocol and in the published report. If the protocol was not available, we compared outcomes listed in the Methods section of the trial report with actually reported results.
2. Funnel plot
We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We would not use funnel plots for outcomes where there are 10 or fewer studies, or where all studies are of similar size. In other cases, where funnel plots are possible, we would seek statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model: it puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size.
We chose a fixed‐effect model for all analyses.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
Clinical state, stage or problem
We proposed to undertake this review and provide an overview of the effects of haloperidol discontinuation for people with schizophrenia in general. In addition, we would have reported data on subgroups of people in the same clinical state, stage and with similar problems.
2. Investigation of heterogeneity
If inconsistency was high, we would have reported this. First, we would have investigated whether data had been entered correctly. Second, if data were correct, we would have visually inspected the graph and we would have removed studies outside of the company of the rest to see if homogeneity was restored.
When unanticipated clinical or methodological heterogeneity were obvious, we would simply have stated hypotheses regarding these for future reviews or versions of this review. We did not anticipate undertaking analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. For the primary outcomes, we would have included these studies and if there was no substantive difference when the implied randomised studies were added to those with better description of randomisation, then we would have employed all data from these studies.
2. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (Dealing with missing data), we were to compare the findings of the primary outcomes when using our assumptions and when using data only from people who complete the study to that point. If there was a substantial difference, we would have reported results and discussed them but continued to employ our assumption.
Where assumptions had to be made regarding missing SDs data (Dealing with missing data), we were to compare the findings of the primary outcomes when using our assumptions and when using data only from people who completed the study to that point. We would have undertaken a sensitivity analysis to test how prone results were to change when completer‐only data only were compared to the imputed data using the above assumption. If there was a substantial difference, we would have reported results and discussed them but continued to employ our assumption.
3. Risk of bias
We were to analyse the effects of excluding trials that are judged to be at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available) allocation concealment, blinding and outcome reporting for the meta‐analysis of the primary outcome. If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, we would have included data from these trials in the analysis.
4. Imputed values
We were to undertake a sensitivity analysis to assess the effects of including data from trials where we used imputed values. If substantial differences were noted in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we would have not pooled data from the excluded trials with the other trials contributing to the outcome, but would have presented them separately.
5. Fixed‐effect and random‐effects model
We synthesised data using a fixed‐effect model. However, we synthesised data for the primary outcomes using a random‐effects model to evaluate whether this altered the significance of the results.
Results
Description of studies
For substantive descriptions of the studies please see Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The initial search of the Cochrane Schizophrenia specialised register yielded 41 citations; further searches in 2017 and 2019 found 10 more records. Searching the reference list of a similar Cochrane Review found a further three records (Leucht 2012). We screened 54 records and obtained 29 full‐text articles for further inspection. We excluded 11 studies after full‐text assessment for eligibility (see below and Characteristics of excluded studies). Two studies from searching Cochrane Schizophrenia's register met the inclusion criteria of this review (Gilbertson 1997; Ruskin 1991); and we included three studies from our other searching (Eklund 1990; Nishikawa 1984; Ota 1973). For a summary of trial selection, please see Figure 1.
1.
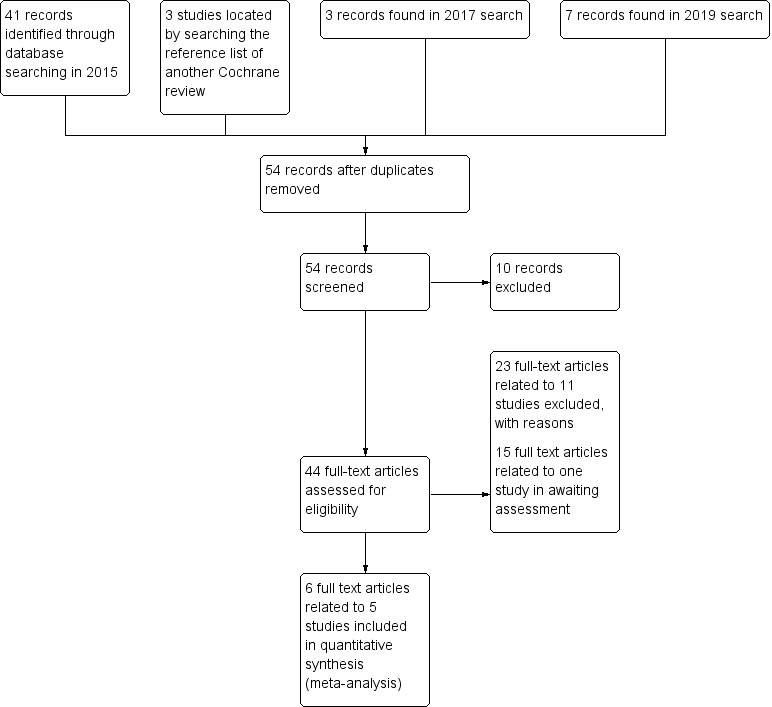
Study flow diagram (for searching up to January 2019)
Included studies
This review includes five studies (Eklund 1990; Gilbertson 1997; Nishikawa 1984; Ota 1973; Ruskin 1991). They are individually described in the Characteristics of included studies table.
1. Length of studies
Of the five included studies, two were short term (< 3 months (Gilbertson 1997; Ota 1973)); one was medium term (> 3 to 6 months (Ruskin 1991)); and two were long term (> 6 months (Nishikawa 1984; Eklund 1990)). The longest study lasted one year (Nishikawa 1984).
2. Participants
232 people with schizophrenia participated in the included studies. The Diagnostic and Statistical Manual of Mental Disorders, third edition (DSM‐III) diagnostic criteria for schizophrenia were used in two trials (Nishikawa 1984; Ruskin 1991). Gilbertson 1997 used the DSM‐III‐R criteria and Eklund 1990 the Research Diagnostic Criteria. In Ota 1973 chronic schizophrenia was diagnosed clinically without using specific diagnostic criteria. All participants in all included studies were stable on haloperidol at the time of randomisation.
3. Setting
Gilbertson 1997 and Ota 1973 were conducted in hospitals; and Nishikawa 1984 and Ruskin 1991 involved outpatients. Eklund 1990 included both inpatients and outpatients.
4. Study size
The number of participants ranged between 21 and 50. The largest study was Nishikawa 1984 (N = 50), and the smallest was Gilbertson 1997 (N = 21).
5. Interventions
5.1 Haloperidol discontinuation
In all five studies this was achieved by gradually replacing existing haloperidol treatment with placebo. Haloperidol was used in oral forms in four studies and in long‐acting intramuscular form in Eklund 1990.
5.2 Control
The comparison intervention for the control group was haloperidol continuation either by oral administration (Gilbertson 1997; Nishikawa 1984; Ota 1973, Ruskin 1991); or intramuscular administration (Eklund 1990).
6. Outcomes
We were able to use data on three outcomes: global state (improvement and relapse); satisfaction with treatment (leaving the studies early); and cognitive functioning.
Ruskin 1991 reported on relapse. Nishikawa 1984 reported on relapse, leaving the study early and (dis)satisfaction with treatment as indicated by a strong request to change the prescribed drug. Ota 1973 reported on global state improvement as measured by the Clinical Global Impression, and on relapse defined as deterioration of global state as measured by the Clinical Global Impression. Eklund 1990 reported usable data on relapse and on leaving the study early. Gilbertson 1997 reported measuring the following cognitive functions: recent memory (as measured by Wechsler Memory Scale‐Revised (WMS‐R); remote memory (as measured by Boston Remote Memory Battery; attention (as measured by Trail Making A & B); and Visual Continuous Performance Test (CPT). However, it provided usable data only for WMS‐R and Boston Remote Memory Battery.
6.1 Rating scales used by the included studies
a. Clinical Global Impression Scale (CGI) (Guy 1976)
This is a rating scale which measures severity of illness and clinical improvement based on a seven‐point scoring system (high = poor).
b. Boston Remote Memory Battery (Albert 1979)
This tests recognition of faces of famous people and events from different decades.
c. Trail Making A & B (Reitan 1959)
This is one of the most popular neuropsychological tests. It provides information on visual search, scanning, speed of processing, mental flexibility, and executive functions.
d. Visual Continuous Performance Test (CPT) (Nestor 1990)
This is a computer‐controlled, modified version of the continuous performance test in which specific attentional effects can be empirically distinguished from nonspecific factors by signal detection theory. It is used to assess sustained attention in schizophrenia.
e. Wechsler Memory Scale‐Revised (WMS‐R) (Wechsler 1987)
This is derived from the Wechsler Memory Scale (WMS). WMS consists of seven subtests: information, orientation, mental control, logical memory, digit span, visual reproduction, and associate learning. The total score is the sum of the subtest scores. The memory quotient (MQ) is derived by adding an age correction and then referring the total to the table of MQs. The WMS‐R comprises a series of brief subtests, some taken from the WMS, each measuring a different facet of memory, which are summarized into five composite scores and finally two major scores using weights prescribed by Wechsler.
6.2 Missing outcomes
No study reported for the outcomes of death, service outcomes, mental state, general functioning, behaviour, adverse effects, quality of life and economic outcomes.
Excluded studies
We excluded 11 studies. Two trials had to be excluded because participants were not randomly assigned (Allen 1997; Lee 2014). Nine studies were excluded because they did not involve the intervention of haloperidol discontinuation (Barak 2005; Eli 2003; Fang 2012; Fleischhacker 1996; Glazer 1990; Himei 2005; NCT00044655 2002; Nordic 1986; Velligan 1999).
Awaiting assessment
One study is still awaiting assessment (Gaebel 2002). This trial involved a randomised comparison between risperidone and haloperidol for one year followed by a second year of randomised discontinuation of both risperidone and haloperidol. We have written to the corresponding author asking for information limited to the two haloperidol groups (continuation and discontinuation).
Ongoing studies
We know of no ongoing studies.
Risk of bias in included studies
For a graphical representation of the risk of bias in the single included study see Figure 2. We were unable to assess many potential types of biases in the included studies because of lack of necessary information as described for each included study in the Characteristics of included studies table.
2.
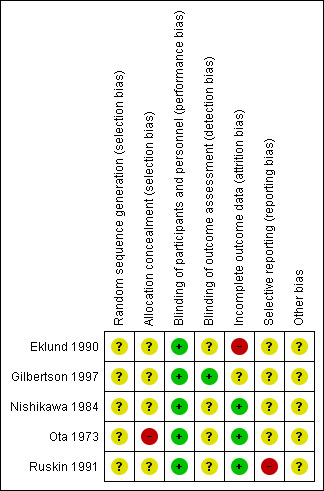
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
With the exception of Ota 1973 all other included studies were described as "randomised". We assumed Ota 1973 to be randomised because it was described as "double‐blind". However, all included studies did not describe the method used to generate the randomisation sequence. Allocation concealment was also not reported for any of the included studies. Accordingly, all included studies were assigned an unclear risk of selection bias.
Blinding
All included studies were reported as "double‐blind" and were assigned a low risk of performance bias. Three of the five included studies described the blinding procedure, which involved using placebo injections (Eklund 1990); unmarked blind capsules (Gilbertson 1997); or placebo tablets and placebo liquid (Ota 1973).
Blinding of outcome assessment was clearly described in only one of the included studies (Gilbertson 1997), so it was assigned a low risk of detection bias. The risk of detection bias in the other four included studies was unclear.
Incomplete outcome data
Attrition rates were low in two included studies (Nishikawa 1984; Ota 1973); and were judged to be associated with low attrition bias despite utilising completer‐only analysis in one of them (Ota 1973). Thirty per cent of the participants in Gilbertson 1997 were excluded from the final outcome analyses for different reasons. The potential attrition bias of this practice was labelled "unclear". Eklund 1990 used an Intent‐to‐treat analysis but the attrition rate was high (42%) and imbalanced as it was higher in the placebo group and the reasons differed. The attrition rate was also high in Ruskin 1991, and it was largely due to relapse. We judged the attrition bias to be high in the latter two studies.
Selective reporting
We were not able to locate the protocol for four of the included studies. Through comparing Methods and Results sections of the included studies, we found that a planned outcome (quality of life) was not reported in Ruskin 1991.
Other potential sources of bias
We did not detect any other potential sources of bias.
Effects of interventions
See: Table 1
1. Comparison 1: Haloperidol discontinuation versus haloperidol continuation
The five included studies collectively reported usable data on only three outcomes: global state (not improved and relapse); satisfaction with treatment (leaving the study early, and switching drugs); and cognitive functioning.
1.1 Global state: clinically important change ‒ not improved (CGI) ‒ short term
A single short‐term study of 49 participants reported data for number of participants showing no improvement in global state. There was a clear difference between the two treatment groups, favouring haloperidol continuation (RR 2.06, 95% CI 1.33 to 3.20, very low quality evidence; Analysis 1.1).
1.1. Analysis.
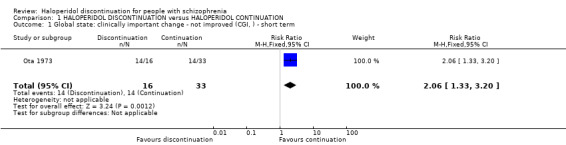
Comparison 1 HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION, Outcome 1 Global state: clinically important change ‐ not improved (CGI, ) ‐ short term.
1.2 Global state: relapse
Data for short‐, medium‐ and long‐term relapse were reported. Overall, pooled data showed haloperidol discontinuation was associated with higher relapse rates (up to 1 year: 4 RCTs, n = 165; RR 1.80, 95% CI 1.18 to 2.74, very low quality evidence). This effect was still found when Ota 1973, a trial with very serious risk of allocation bias due to 'implied randomisation', was removed from this analysis; (Analysis 1.2).
1.2. Analysis.
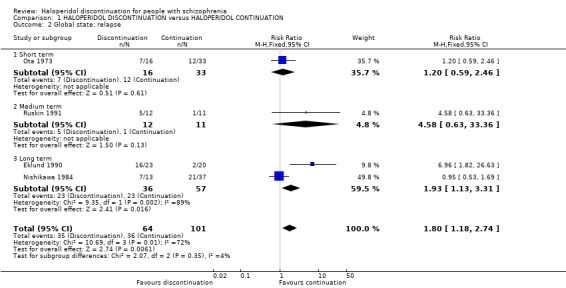
Comparison 1 HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION, Outcome 2 Global state: relapse.
1.2.1 Short term
We found no clear differences between the treatment groups for numbers of people relapsing by up to three months (1 RCT, n = 49; RR 1.20, 95% CI 0.59 to 2.46).
1.2.2 Medium term
We found no clear differences between the treatment groups for numbers of people relapsing between three and six months (1 RCT, n = 23; RR 4.58, 95% CI 0.63 to 33.36).
1.2.3 Long term
We did find an effect favouring haloperidol continuation for relapse beyond six months (2 RCTs, n = 93; RR 1.93, 95% CI 1.13 to 3.31).
1.3 Satisfaction with treatment: various measures ‒ long‐term
Trials reported data for leaving the study early and switching drugs, which we considered as proxy measures of satisfaction with treatment Analysis 1.3.
1.3. Analysis.
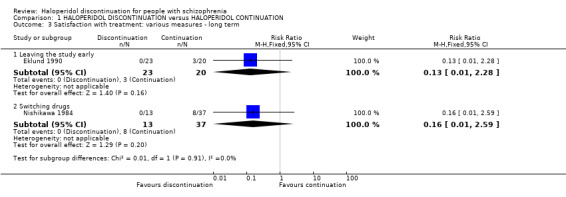
Comparison 1 HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION, Outcome 3 Satisfaction with treatment: various measures ‐ long term.
1.3.1 Leaving the study early
One study (n = 43) reported number of participants leaving the study early and found no clear difference between the treatment groups (RR 0.13, 95% CI 0.01 to 2.28, very low quality evidence).
1.3.2 Switching drugs
Another study (N = 50) reported numbers of people who switched treatment drugs during the trial. Again, we found no clear difference between haloperidol discontinuation or haloperidol continuation (RR 0.16, 95% CI 0.01 to 2.59).
1.4. Cognitive functioning: change in memory ‒ short term
Specific aspects of cognitive functioning were reported in one very small study (n = 21); Analysis 1.4.
1.4. Analysis.
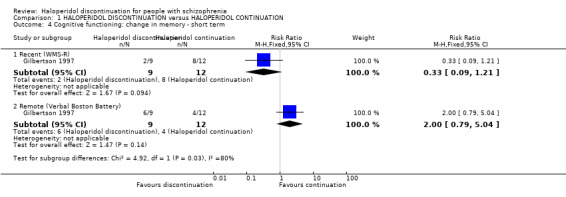
Comparison 1 HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION, Outcome 4 Cognitive functioning: change in memory ‐ short term.
1.4.1 Recent memory (WMS‐R)
We found no clear difference between haloperidol discontinuation group and haloperidol continuation group (RR 0.33, 95% CI 0.09 to 1.21).
1.4.2 Remote memory (Verbal Boston Battery)
We found no clear difference between haloperidol discontinuation group and haloperidol continuation group for verbal/remote memory (RR 2.00, 95% CI 0.79 to 5.04).
Discussion
Summary of main results
This review includes five studies with a total of 232 people with schizophrenia who were stable on treatment with haloperidol. Before seeing the data we chose key outcomes which we felt would best summarise the overall evidence.
1. Global state: clinically important change ‒ not improved (CGI); relapse
Discontinuation was clearly associated with increased risk of not being improved but the very low quality evidence came from one small (n = 49) short trial. Four trials (total n = 165) also reported very low quality evidence on relapse. Relapse was also clearly offset by use of continuation of haloperidol. The consistency of the two global state outcomes is important albeit with the proviso that evidence is so poor — and should be better (please see Implications for research).
2. Satisfaction with treatment: leaving the study early
Both longer‐term studies contributing to this outcome were small; and both the combined totals and individual proportions are equivocal (Eklund 1990; Nishikawa 1984). The event rates were all low.
That Nishikawa 1984 reported on leaving the study early for the reason of changing from the haloperidol continuation group to another drug (an outcome really only available to one group) may be the best indicator of rate of adverse effects in these trials. Around 22% (95% CI 11% to 37%) of people who were allocated to continue on haloperidol did not and were swapped to another medication. That such a high proportion of people did stay on haloperidol for a year may indicate that this group of people were content with their medication, had some degree of inertia, felt compelled within the confines of the trial, or that researchers felt people to be well on haloperidol or persuaded people to stay on the drug within the trial for longer than they might have otherwise.
3. Mental state, functioning, behaviour, quality of life
We were particularly surprised that there were no data on key outcomes of mental state and functioning. Considering how common drug discontinuation is, this important area of research is grossly under‐investigated.
Overall completeness and applicability of evidence
1. Completeness
The number of included studies is small considering that haloperidol is one of the earliest antipsychotic medications to be used in the treatment of schizophrenia. All included studies were relatively small (largest < 60 people). The power of these combined studies for any outcome of interest is limited, no outcomes are for longer than one year, and adverse effect ratings are absent. The data are pioneering, but woefully incomplete.
2. Applicability
These studies were conducted in different countries and different settings (outpatient or inpatient) over a period of about two decades. What findings we have are probably reasonably applicable.
Quality of the evidence
Four out of the five studies were randomised and all were double‐blind, but the methods of sequence generation and allocation concealment were not reported. In one of the studies, randomisation was implied. Therefore it is unclear whether the studies were adequately randomised and whether treatment allocation was really concealed. There is also no indication of whether blinding worked. Another shortcoming is the high attrition rates in some studies, the use of completers‐only analysis and the poor reporting of most outcomes. Without original study protocols being available, it is also not clear if there were cases of selective reporting. In summary, the overall methodology and reporting of the included studies in this review is unclear and this factor, combined with the small sample size, limits the quality of the evidence provided.
Potential biases in the review process
Because of lack of standard reporting for 'discontinuation' in the trials, searching for terms such as 'stopping', 'withdrawal' or 'discontinuation' cannot identify all the potential trials. Only two included studies were identified by the first search. We identified more relevant studies searching the references of other relevant Cochrane Reviews. These studies have not reported standard terminology for 'discontinuation' rather they reported randomisation of patients, who were already taking haloperidol, to haloperidol versus placebo (meaning discontinuing haloperidol). So we did not limit the search to 'discontinuation' terms in the update search and we were able to identify the studies missed in the first search. We informed the Cochrane Schizophrenia Group's register to re‐index and correct the indexing for the 'discontinuation' studies.
There is a possibility of publication bias, and of unpublished studies that we are not aware of. The small number of included studies precluded producing a funnel plot.
Agreements and disagreements with other studies or reviews
The present results are consistent with the results of a number of narrative reviews reporting that people with schizophrenia who were withdrawn from antipsychotic drugs relapsed significantly more frequently than participants who continued them (e.g. Baldessarini 1985, Davis 1975, Gilbert 1995). A Cochrane Review of people with schizophrenia stable on chlorpromazine demonstrated that cessation of chlorpromazine was associated with a significant increase in the risk of relapse (Almerie 2007). Relapse rates were also shown to be reduced by continuing antipsychotic treatment in a Cochrane Review restricted to second‐generation antipsychotics (Leucht 2009), and in another Cochrane Review involving all antipsychotic drugs (Leucht 2012).
A Cochrane Review of the efficacy of haloperidol in schizophrenia found results that were comparable to the present results. More people allocated haloperidol improved than those given placebo. Relapse data also favoured haloperidol. Data on leaving the study early marginally favoured haloperidol. However, adverse effect confirmed that haloperidol was a potent cause of movement disorders, suggesting that haloperidol should be a less favoured antipsychotic drug (Adams 2013).
Authors' conclusions
Implications for practice.
1. People with schizophrenia
1. People with schizophrenia What limited data there are suggest that discontinuing haloperidol may be associated with a higher risk of relapse ‐ in the long term. The available studies did not report usable data on the adverse effects of haloperidol treatment. For the outcome of patient satisfaction, there was no clear difference between proportions of those staying on treatment and those who discontinued. People with schizophrenia (and their carers) have often to balance the potential harm of relapse with the problems associated with staying on medication. Trials in this review are not good sources of evidence of the ongoing problems of taking this medication (evidence for this is better found elsewhere ‐ Adams 2013). Longer‐term use of an alternative medication may be preferable to taking haloperidol because of the known risk of adverse effects but all medications carry risks of some unwanted effects (Adams 2013). Judicious use of lower doses of medication offset but do not negate this risk (Donnelly 2013). The risks of relapse can be considerable but these too can be offset (but, again, not negated) by imaginative use of medication and intervention packages/contracts if long‐term use of medication is unacceptable.
2. Clinicians
This review suggests that haloperidol discontinuation could result in higher risk of relapse but the adverse effects of taking haloperidol were not reported in the studies included in this review. Given no alternative, haloperidol is a useful and potent antipsychotic drug which is likely to offset the damage of relapse. Should there be alternatives, other antipsychotics may well have less adverse effects and, certainly, lowest possible dose is preferable. For some people with a history of illness conducive to imaginative, collaborative, contractual arrangements regarding use of medication and services it may be possible to avoid long term use of drugs such as haloperidol — albeit with the increased risk of relapse.
3. Policy makers
After so many decades of wide use we should have better evidence of the benefits/risks of discontinuing haloperidol. It is hard to recommend discontinuation of medication when evidence of the effects of its withdrawal are so limited.
Implications for research.
1. General
This review contains a small number of studies into the benefit and harm of a widely used antipsychotic drug. The sample size was generally small and the longest included study in this review was for one year. The available studies did not report on outcomes that are important to clinicians and to patients, particularly side effects and social outcomes. However, the results of this review are consistent with similar Cochrane Reviews on continuing versus discontinuing antipsychotic treatment of schizophrenia. For instance, relapse rates were higher on discontinuing chlorpromazine medication for people with schizophrenia who were stable on this drug (Almerie 2007). A review of maintenance treatment of schizophrenia clearly demonstrated the superiority of antipsychotic drugs compared to placebo in preventing relapse (Leucht 2012).
2. Specific
2.1 Reviews
Several of the excluded studies which were not relevant for this review could be added to existing Cochrane Reviews or pose a question that may warrant new reviews (Table 3).
2. Excluded schizophrenia [or possible schizophrenia] trials which may be of relevance to other reviews.
| Study | Particular focus of study | Intervention | Cochrane review | |
| #1 | #2 | |||
| Barak 2005 | Haloperidol | Olanzapine | Duggan 2005 | |
| Fleischhacker 1996; Velligan 1999 | Quetiapine | Srisurapanont 2004; Suttajit 2013 | ||
| Fang 2012 | Acute agitation | Haloperidol IM and then oral haloperidol | Risperidone oral solution | Ostinelli 2017; Ostinelli 2018 |
| Eli 2003; NCT00191555 2005 | Haloperidol continuation | Switching to olanzapine | Leucht 2012 | |
| Himei 2005 | Switching to risperidone | Leucht 2012 | ||
| NCT00044655 2002 | Fluphenazine decanoate or haloperidol decanoate continuation | Switching to long‐acting injectable risperidone microspheres | Leucht 2012 | |
| Nordic 1986 | Tardive dyskinesia | Haloperidol | Haloperidol + biperiden | Momcilovic 2014; Bergman 2018 |
| Chlorprothixene | Dold 2015; Tardy 2014 | |||
| Perphenazine | Dold 2015 | |||
| Haloperidol + biperiden | Chlorprothixene | Bergman 2018 | ||
| Perphenazine | Bergman 2018 | |||
| Chlorprothixene | Perphenazine | ‐ | ||
| Glazer 1990 | Haloperidol | Molindone | ‐ | |
2.2 Trials
This review exposes the poor quality of the available relevant studies. Better designed and conducted trials may be warranted as haloperidol is still widely used. Trials should be for longer than one year and should examine outcomes reflecting quality of life, adverse effects and social participation in addition to relapse. We realise that design of such a study is a painstaking procedure necessitating much time and effort. However, we have given this some thought and a draft outline of a design is provided (Table 4).
3. Proposed design of future trial, assessing effects of haloperidol discontinuation for people with schizophrenia.
| Methods | Allocation: randomised ‒ clearly described generation of sequence and concealment of allocation Blinding: double ‐ described and tested Duration: 3 years |
| Participants | People with schizophrenia stable on haloperidol for at least 1 month N = 500 Age: any Sex: both |
| Interventions | 1. Haloperidol continuation 2. Haloperidol discontinuation (placebo after gradual withdrawal of haloperidol) |
| Outcomes | Relapse (primary outcome) Death ‒ suicide and natural causes Global state General functioning Behaviour Adverse effects ‒ general and specific Satisfaction with treatment (including subjective well‐being and family burden) Quality of life Economic outcomes Cognitive functioning |
What's new
| Date | Event | Description |
|---|---|---|
| 24 January 2019 | Amended | Search was updated and 7 new references were added to Gaebel 2002. |
Acknowledgements
We are grateful to the Association for Evidence‐based Medicine, Damascus for the training courses on conducting systematic reviews and for ongoing support. The Cochrane Schizophrenia Group Editorial Base in Nottingham produces and maintains standard text for use in the Methods section of their reviews. We have used this text as the basis of what appears here and adapted it as required.
The search term was developed by the Trials Search Co‐ordinator of the Cochrane Schizophrenia Group, Farhad, and the contact author of this protocol.
We would like to thank Farooq Naeem and Shuo Xiang for peer reviewing the protocol and Rachael Jones for peer review of the review stage.
Appendices
Appendix 1. Previous searches
Search in Protocol
Electronic searches
1. Cochrane Schizophrenia Group’s Trials Register
The Trials Search Co‐ordinator of the Cochrane Schizophrenia Group will search the Group's Specialised Register (http://onlinelibrary.wiley.com/o/cochrane/clabout/articles/SCHIZ/frame.html) using the following search terms:
(haloperi* or R‐1625 or haldol* or alased* or aloperidi* or bioperido* or buterid* or ceree* or dozic* or duraperido* or fortuna* or serena* or serenel* or seviu* or sigaperid* or sylad* or zafri*) in Title or Abstract of REFERENCE or (haloperi* or R‐1625 or haldol* or alased* or aloperidi* or bioperido* or buterid* or ceree* or dozic* or duraperido* or fortuna* or serena* or serenel* or seviu* or sigaperid* or sylad* or zafri*) in Intervention of STUDY
The Cochrane Schizophrenia Group'ss Specialised Register is compiled by systematic searches of major resources (including AMED, BIOSIS, 95% CINAHL, EMBASE, MEDLINE, PsycINFO, PubMed, and registries of Clinical Trials) and their monthly updates, hand‐searches, grey literature, and conference proceedings.
Data and analyses
Comparison 1. HALOPERIDOL DISCONTINUATION versus HALOPERIDOL CONTINUATION.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: clinically important change ‐ not improved (CGI, ) ‐ short term | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.33, 3.20] |
| 2 Global state: relapse | 4 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.18, 2.74] |
| 2.1 Short term | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.59, 2.46] |
| 2.2 Medium term | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.58 [0.63, 33.36] |
| 2.3 Long term | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.13, 3.31] |
| 3 Satisfaction with treatment: various measures ‐ long term | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Leaving the study early | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.28] |
| 3.2 Switching drugs | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 2.59] |
| 4 Cognitive functioning: change in memory ‐ short term | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Recent (WMS‐R) | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.09, 1.21] |
| 4.2 Remote (Verbal Boston Battery) | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.79, 5.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Eklund 1990.
| Methods | Allocation: randomly assigned. Blindness: double‐blind. Setting: inpatients and outpatients. Duration: 48 weeks. |
|
| Participants | Diagnosis: schizophrenia (Research Diagnostic Criteria). N = 43. Sex: not stated. Age: mean 52 years (range 25 to 65). History: "stable and requiring antipsychotic medication to prevent relapse"; "all participants were stable and given haloperidol decanoate injections 60 mg four weekly for 15 weeks before randomisation". |
|
| Interventions | 1. Haloperidol discontinuation: replacement of haloperidol decanoate injections with placebo injections 4 weekly. N = 23. 2. Haloperidol continuation: continuation of haloperidol decanoate injections 60 mg/4 weeks. N = 20. |
|
| Outcomes | Global state: relapse. Satisfaction with treatment: leaving the study early. Unable to use Mental state: Comprehensive Psychopathological Rating Scale (data not reported for both groups). Adverse effects: scales for evaluation of extrapyramidal side‐effects and tardive dyskinesia (data not reported for both groups). |
|
| Notes | The authors did not report on conflict of interest. Funding was from the Swedish Medical Research Council and Janssen Pharma. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double using placebo injections. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No further details regarding blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis was used but the attrition rate was high: 42% left the study early. The attrition rate was higher in the placebo group and the reasons differed. |
| Selective reporting (reporting bias) | Unclear risk | All planned outcomes are reported. |
| Other bias | Unclear risk | Partially funded by Janssen Pharma. |
Gilbertson 1997.
| Methods | Allocation: randomly assigned. Blindness: double‐blind using unmarked blind capsules. Setting: hospital‐based. Duration: 3 weeks. |
|
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (DSM‐III‐R). N = 30 (21 available for analyses). Sex: men. History: stable on haloperidol, "participants were judged to be clinically stable with a mean Bunney‐Hamburg Psychosis rating of 4.3 ± 1.6". |
|
| Interventions | 1. Haloperidol discontinuation: haloperidol discontinued and replaced with placebo. N = 9. 2. Haloperidol continuation: mean daily dose of 10.5 ± 4.2 mg, range 4 mg to 20 mg. N = 12. |
|
| Outcomes | Cognitive functioning: recent memory (Wechsler Memory Scale‐Revised ‐‐ WMS‐R), remote memory (Boston Remote Memory Battery). Unable to use Leaving the study early: 9 participants were "unavailable for analysis". Not clear why. Mental state: Bunney‐Hamburg Psychosis Scale (Bunney 1963) (lack of data). Cognitive functioning: Trail Making A & B, and Visual Continuous Performance Test for attention (lack of data). |
|
| Notes | The authors did not report on conflict of interest. They were funded by the National Institute of Mental Health, the Department of Veterans Affairs Research and Development Service, and the Bataan and Corregidor Medical Research Fund. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind using unmarked blind capsules that remained equivalent in appearance and number throughout the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Assessment of the clinical status of subjects was conducted blindly on a daily basis throughout the duration of the study. Neuropsychological testing was administered to each subject and scored by a trained research assistant who was blind with regard to the clinical and medication status of the patient". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 30 participants were initially recruited and randomised. 9 participants (30%) were excluded from the outcome analysis for different reasons. |
| Selective reporting (reporting bias) | Unclear risk | All planned outcomes are reported. |
| Other bias | Unclear risk | No indication of other bias. |
Nishikawa 1984.
| Methods | Allocation: randomly assigned.
Blinding: double; drug appearance was made identical.
Setting: outpatient. Duration: 1 year. |
|
| Participants | Diagnosis: schizophrenia (DSM‐III). N = 87. Sex: 53 men, 34 women. Age: mean 41 years. History: residual or in remission. |
|
| Interventions | 1. Haloperidol discontinuation: replaced with placebo. N = 13. 2. Haloperidol continuation: fixed doses 1, 3 or 6 mg/day. N = 37. 3. Propericiazine: fixed dose 10, 30 or 60 mg/day. N = 37. All 3 groups also given biperidine and nitrazepam. Haloperidol discontinuation was the only randomised intervention. |
|
| Outcomes | Global state: relapse. Satisfaction with treatment: "strong request to change the prescribed drug were indicators of dissatisfaction with treatment". |
|
| Notes | For the purpose of this review, we have considered only the haloperidol continuation group and the placebo group. We combined all haloperidol doses in a single group. No information available about funding or conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double, drug appearance was made identical with respect to powder, colour, taste and volume by adding a gastric acid. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double, drug appearance was made identical with respect to powder, colour, taste and volume by adding a gastric acid. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rates in the haloperidol and the placebo groups. |
| Selective reporting (reporting bias) | Unclear risk | All planned outcomes are reported. |
| Other bias | Unclear risk | No indication of other bias. |
Ota 1973.
| Methods | Allocation: implied randomisation. Blinding: double. Setting: inpatient. Duration: 90 days. | |
| Participants | Diagnosis: chronic schizophrenia maintained on haloperidol (mean dose 9.3 mg/day). N = 49. Sex: 24 men, 20 women. Age: mean 42.5 years. | |
| Interventions | 1. Haloperidol discontinuation: haloperidol tablets replaced with placebo tablets and liquid. N = 16. (12 analysed) 2. Haloperidol continuation: haloperidol tablets, mean dose 8.8 mg/day, and placebo liquid. N = 17. (17 analysed) 3. Haloperidol continuation: haloperidol liquid, mean dose: 10.4 mg/day, and placebo tablets. N = 16. (15 analysed) We combined the two haloperidol continuation groups in a single group. N = 33 (32 analysed) |
|
| Outcomes | Global state: improvement, relapse. Unable to use Mental state: Brief Psychiatric Rating Scale (no SD). Global state: Clinical Global Impression of Severity (no SD). Behaviour: Nurses Observation Scale for Inpatient Evalutation (no SD). |
|
| Notes | No information available about funding or conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is implied ‒ assumed due to double‐blinding. |
| Allocation concealment (selection bias) | High risk | Implied randomisation ‒ no further details how allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double, all participants received both tablets and liquid, active or placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double, all participants received both tablets and liquid, active or placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completer‐only analysis unlikely to be affected by low attrition rate (10%). |
| Selective reporting (reporting bias) | Unclear risk | All planned outcomes are reported. |
| Other bias | Unclear risk | No indication of other bias. |
Ruskin 1991.
| Methods | Allocation: randomly assigned.
Blinding: double, no further details.
Setting: outpatient. Duration: 6 months. |
|
| Participants | Diagnosis: schizophrenia (DSM‐III). N = 23. Age: mean 60 years. Sex: men. History: "The antipsychotic medication of all participants was switched to haloperidol for one month or until they were considered stable, then they were randomised to haloperidol discontinuation or to haloperidol continuation". |
|
| Interventions | 1. Haloperidol discontinuation: haloperidol dose reduced by 50% for 14 days, then changed to placebo. N = 12 (10 analysed). 2. Haloperidol continuation: at the same dose used at randomisation. N = 11 (8 analysed). |
|
| Outcomes | Global state: relapse. Unable to use Mental state: BPRS (data not reported for both groups) Quality of life: data not reported for both groups. Leaving the study early: no details. |
|
| Notes | No information available about funding or conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind: "participants and investigators were blind to treatment”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double blind: "participants and investigators were blind to treatment”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comparable low attrition rates in the two groups: 2/12 = 17% in the placebo group and 3/11 = 27% in the haloperidol group. |
| Selective reporting (reporting bias) | High risk | A planned outcome (quality of life) was not reported. |
| Other bias | Unclear risk | No indication of other bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 1997 | Allocation: not randomised. |
| Barak 2005 | Allocation: unclear. Participants: people with schizophrenia. Interventions: olanzapine versus haloperidol, not haloperidol discontinuation. |
| Eli 2003 | Allocation: randomly assigned. Participants: people with schizophrenia. Interventions: switching from haloperidol to olanzapine, not haloperidol discontinuation. |
| Fang 2012 | Allocation: randomly assigned. Participants: people with schizophrenia, acute agitation. Interventions: risperidone oral solution versus intramuscular haloperidol shifting to oral, not haloperidol, discontinuation. |
| Fleischhacker 1996 | Allocation: randomly assigned. Participants: people with schizophrenia. Interventions: haloperidol versus quetiapine, not haloperidol discontinuation. |
| Glazer 1990 | Allocation: randomly assigned. Participants: people with tardive dyskinesia, not schizophrenia. Intervention: haloperidol versus molindone; not haloperidol discontinuation. |
| Himei 2005 | Allocation: unclear. Participants: people with schizophrenia. Interventions: switching from haloperidol versus risperidone, not haloperidol discontinuation. |
| Lee 2014 | Allocation: not randomised. |
| NCT00044655 2002 | Allocation: randomly assigned. Participants: people with schizophrenia. Interventions: switching from long‐acting injectable fluphenazine or haloperidol decanoate to long‐acting injectable risperidone microspheres; not haloperidol discontinuation. |
| Nordic 1986 | Allocation: unclear. Participants: people with tardive dyskinesia not schizophrenia. Intervention: chlorprothixene versus perphenazine verus haloperidol versus haloperidol + biperiden; not haloperidol discontinuation. |
| Velligan 1999 | Allocation: randomly assigned. Participants: people with schizophrenia. Interventions: haloperidol versus quetiapine, not haloperidol discontinuation. |
Characteristics of studies awaiting assessment [ordered by study ID]
Gaebel 2002.
| Methods | Allocation: randomly assigned. Blindness: open. Duration: 8 weeks of acute treatment followed by a year of maintenance treatment, then people were randomised for a second year of continuing or discontinuing antipsychotic drug treatment. |
| Participants | Diagnosis: schizophrenia. N = 44. Age = 18 to 55 years. Sex: both. History: first episode. |
| Interventions | Year 1 1. Risperidone. N = 23. 2. Haloperidol: low‐dose. N = 21. Year 2 1. Risperidone: continuation. 2. Risperidone: discontinuation. 3. Haloperidol: continuation. 4. Haloperidol: discontinuation |
| Outcomes | Global state: Relapse, Clinical Global Impressions. Cognitive functioning. Functioning: Global Assessment of Functioning. |
| Notes |
Differences between protocol and review
1. Outcomes
We have changed the wording of 'Global state improvement' to standard Cochrane Schizophrenia Group wording of 'Clinically important change'.
Data were not presented for several of our prestated outcomes of importance. We did have data for satisfaction with treatment (measured by leaving the study early) — we have added this outcome to the 'Summary of findings' table.
2. Methods template
We have updated sections of the methods to include latest updates in Cochrane Schizophrenia's template. This includes changes such as updating references and some changes to the wording of paragraphs — not changes in the methodology of the review.
Contributions of authors
Adib Essali: analysing data, writing manuscript, responding to editors and referees and approval of the final version
Khaled Turkmani: extracting data, assessment risk of bias, analyzing data and writing manuscript
Shaimaa Aboudamaah: extracting data, assessment risk of bias
Alaa Aboudamaah: extracting data
Mohammad Reyad Diaa Aldeen: extracting data
Mohamad Essam Marwa: extracting data, assessment risk of bias and writing manuscript
Nawar Almounayer: extracting data
All authors contributed to writing the protocol
Sources of support
Internal sources
-
Manaaki Centre, Waikato District Health Board, Thames, New Zealand.
Employs review author Adib Essali.
-
Faculty of Medicine, Damascus University, Damascus, Syrian Arab Republic.
Review authors Khaled Turkmani, Shaimaa Aboudamaah, Mohammad Reyad Diaa Aldeen, Mohamad Essam Marwa and Nawar Almounayer are students at this university.
-
Damascus Health Unit, Damascus University, Damascus, Syrian Arab Republic.
Employs review author Alaa Aboudamaah.
External sources
No sources of support supplied
Declarations of interest
Adib Essali: none known
Khaled Turkmani: none known
Shaimaa Aboudamaah: none known
Alaa Aboudamaah: none known
Mohammad Reyad Diaa Aldeen: none known
Mohamad Essam Marwa: none known
Nawar Almounayer: none known
New
References
References to studies included in this review
Eklund 1990 {published data only}
- Eklund K. Low dose of haloperidol decanoate is effective against relapses in schizophrenia patients. A double‐blind placebo controlled study. Proceedings of the 17th Collegium Internationale Neuro‐Psychopharmacologicum Congress; 1990 Sep 10‐14; Kyoto, Japan. 1990:287. [CSzG: Ref12924]
- Eklund K, Forsman A. Minimal effective dose and relapse ‐ double‐blind trial: haloperidol decanoate vs. placebo. Clinical Neuropharmacology 1991;14(Suppl 2):S7‐15. [CSzG: Ref747] [PubMed] [Google Scholar]
Gilbertson 1997 {published data only}
Nishikawa 1984 {published data only}
- Nishikawa T, Tsuda A, Tanaka M, Hoaki Y, Koga I, Uchida Y. Prophylactic effect of neuroleptics in symptom‐free schizophrenics ‐ a comparative dose‐response study of haloperidol and propericiazine. Psychopharmacology 1984;82(3):153‐6. [CSzG: Ref2168] [DOI] [PubMed] [Google Scholar]
Ota 1973 {published data only}
- Ota KY, Kurland AA. A double‐blind comparison of haloperidol oral concentrate, haloperidol solutabs and placebo in the treatment of chronic schizophrenia. Journal of Clinical Pharmacology and New Drugs 1973;13(2):99‐110. [CSzG: Ref2318] [DOI] [PubMed] [Google Scholar]
Ruskin 1991 {published data only}
- Ruskin PE, Nyman G. Discontinuation of neuroleptic medication in older, outpatient schizophrenics. A placebo‐controlled, double‐blind trial. Journal of Nervous and Mental Disease 1991;179(4):212‐4. [CSzG: Ref2471; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allen 1997 {published data only}
- Allen DN, Gilbertson MW, Barry E, Kammen DP, Gurklis JA. Haloperidol improves memory in schizophrenia. Proceedings of the 150th Annual Meeting of the American Psychiatric Association; 1997 May 17‐22; San Diego, California, USA 1997.
Barak 2005 {published data only}
Eli 2003 {published data only}
- Eli Lilly Company. Efficacy study of switching stabilized schizophrenic patients from conventional to atypical antipsychotic treatment. Eli Lilly and Company Clinical Trial Registry 2003.
- NCT00191555. Double‐blind long‐term study comparing the efficacy and safety of olanzapine versus haloperidol in patients with schizophrenia previously stabilized with conventional antipsychotic treatment. clinicaltrials.gov/ct2/show/NCT00191555 (accessed 10 April 2019).
Fang 2012 {published data only}
- Fang M, Chen H, Li LH, Wu R, Li Y, Liu L, et al. Comparison of risperidone oral solution and intramuscular haloperidol with the latter shifting to oral therapy for the treatment of acute agitation in patients with schizophrenia. International Clinical Psychopharmacology 2012; Vol. 27, issue 2:107‐13. [DOI] [PubMed]
Fleischhacker 1996 {published data only}
- Copolov DL, Link CG, Kowalcyk B. A multicentre, double blind, randomized comparison of quetiapine (ICI 204,636, 'seroquel') and haloperidol in schizophrenia. Psychological Medicine 2000; Vol. 30, issue 1:95‐105. [MEDLINE: ] [DOI] [PubMed]
- Fleischhacker WW, Linz CG, Hurst BC. ICI 204636 ('seroquel') ‐ a putative new atypical antipsychotic: results from phase III trials. Schizophrenia Research 1996; Vol. 18, issue 2‐3:132.
- Hellewell JS, Hurst BC, Link CG. Switching from a conventional antipsychotic (haloperidol) to an atypical antipsychotic ('seroquel'‐ICI 204,636). European Neuropsychopharmacology 1996; Vol. 6, issue Suppl 4:123.
- Link C, Farrow L. "Seroquel"(tm) (ICI 204, 636) EPS and prolactin ‐ comparison with haloperidol. Proceedings of the 10th World Congress of Psychiatry; 1996 Aug 23‐28; Madrid, Spain 1996.
- Link C, Kowalcyzk B, Farrow L. "Seroquel"(tm) (ICI 204,636) EPS and prolactin: comparison with haloperidol. Proceedings of the 8th Congress of the Association of European Psychiatrists; 1996 Jul 7‐12; London, UK 1996.
- Zeneca P. Study 0014: a multicentre, double‐blind, randomised comparison of seroquel and haloperidol in the treatment of subjects with acute exacerbation of subchronic or chronic schizophrenia (5077IL/0014). Seroquel Trial Program: Zeneca Limited 1998.
Glazer 1990 {published data only}
- Glazer WM, Hafez H. A comparison of masking effects of haloperidol versus molindone in tardive dyskinesia. Schizophrenia Research 1990; Vol. 3, issue 5‐6:315‐20. [MEDLINE: ] [DOI] [PubMed]
- Glazer WM, Hafez HM, Benarroche CL. Molindone and haloperidol in tardive dyskinesia. Journal of Clinical Psychiatry 1985; Vol. 46, issue 8 Pt. 2:4‐7. [PubMed]
Himei 2005 {published data only}
Lee 2014 {published data only}
- Lee B, Vogel S, Sisk S, Daniel A, Yao J. The Effects of Dopamine Antagonism on Reward Learning in Schizophrenia. Archives of Clinical Neuropsychology 2014;29(6):580. [Google Scholar]
NCT00044655 2002 {published data only}
- Covell NH, McEvoy JP, Schooler NR, Stroup TS, Jackson CT, Rojas IA, et al. Effectiveness of switching from long‐acting injectable fluphenazine or haloperidol decanoate to long‐acting injectable risperidone microspheres: An open‐label, randomized controlled trial. Journal of Clinical Psychiatry 2012; Vol. 73, issue 5:669‐75. [DOI] [PMC free article] [PubMed]
- NCT00044655. Effectiveness of switching antipsychotic medications. clinicaltrials.gov/ct2/show/NCT00044655 (accessed 10 April 2019).
Nordic 1986 {published data only}
- Gerlach J. Tardive dyskinesia: pathophysiological mechanisms and clinical trials. L'Encephale 1988; Vol. 14:227‐32. [PubMed]
- Nordic Dyskinesia Study Group. Effect of different neuroleptics in tardive dyskinesia and parkinsonism. A video‐controlled multicenter study with chlorprothixene, perphenazine, haloperidol and haloperidol + biperiden. Psychopharmacology 1986; Vol. 90, issue 4:423‐9. [MEDLINE: ] [PubMed]
- Povlsen UJ, Noring U, Meidahl B, Korsgaard S, Waehrens J, Gerlach J. The effects of neuroleptics on tardive dyskinesias. A video‐controlled, randomized study of chlorprothixene, perphenazine, haloperidol and haloperidol + biperiden [Neuroleptikas virkning pa tardive dyskinesier. En videokontrolleret, randomiseret undersogelse med klorprotixen, perfenazin, haloperidol og haloperidol + biperiden]. Ugeskrift for Laeger 1987; Vol. 149, issue 25:1682‐5. [MEDLINE: ] [PubMed]
Velligan 1999 {published data only}
- Velligan DI, Prihoda T, Maples N, Ritch JL, Miller AL. Neurocognitive advantages of quetiapine. Proceedings of the 154th Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; New Orleans, Louisiana, USA. 2001.
- Velligan DI, Pultz J, Csernansky JG, Hoff AL, Mahurin R, Miller AL, et al. Changes in cognitive function with quetiapine fumarate versus haloperidol. Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; 1999 May 15‐20; Washington DC, USA. 1999.
- Velligan DI, Pultz J, Newcomer JW. Changes in cognitive function with quetiapine versus haloperidol. Proceedings of the 51st Institute on Psychiatric Services; 1999 Oct 25‐Nov 2; New Orleans, Louisiana, USA. 1999.
References to studies awaiting assessment
Gaebel 2002 {published data only}
- Gaebel W, Hans‐juergen M. Treatment strategies in first episode schizophrenia. Proceedings of the 12th World Congress of Psychiatry; 2002 Aug 24‐29; Yokohama, Japan. 2002.
- Gaebel W, Moeller HJ. Measures to prevent relapse in long‐term treatment: results from the German Research Network on Schizophrenia. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25; Toronto, Canada. 2006.
- Gaebel W, Moller HJ, Buchkremer G, Ohmann C, Riesbeck M, Wolwer WVWM, et al. Pharmacological long‐term treatment strategies in first episode schizophrenia‐‐study design and preliminary results of an ongoing RCT within the German Research Network on Schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. Germany, 2004; Vol. 254, issue 2:129‐40. [MEDLINE: ] [DOI] [PubMed]
- Gaebel W, Riesbeck M, Wolwer W, Klimke A, Eickhoff M, Wilmsdorff M, et al. Relapse prevention in first‐episode schizophrenia ‐ maintenance vs intermittent drug treatment with prodrome‐based early intervention: Results of a randomized controlled trial within the german research network on schizophrenia. Journal of Clinical Psychiatry 2011;72(2):205‐18. [DOI] [PubMed] [Google Scholar]
- Gaebel W, Riesbeck M, Wolwer W, Klimke A, Eickhoff M, Wilmsdorff M, et al. Rates and predictors of remission in first‐episode schizophrenia within 1 year of antipsychotic maintenance treatment. Results of a randomized controlled trial within the German Research Network on Schizophrenia. Schizophrenia Research 2014;152(2‐3):478‐86. [DOI] [PubMed] [Google Scholar]
- Gaebel W, Riesbeck M, Wilmsdorff M, Zielasek J. Pharmacological long‐term treatment strategies in first episode schizophrenia: preliminary results of an ongoing randomised clinical trial within the German research network on schizophrenia. Schizophrenia Bulletin 2005; Vol. 31:483. [DOI] [PubMed]
- Jager M, Riedel M, Bottlender R, Wilmsdorff M, Wolwer W, Gaebel W, et al. Pharmacological acute treatment in first episode schizophrenic disorders [Medikamentose Akutbehandlung schizophrener Ersterkrankungen]. Nervenheilkunde 2006;25(1‐2):32‐6. [Google Scholar]
- Moller H‐J, Riedel M, Jager M, Wickelmaier F, Maier W, Kuhn K‐U, et al. Short‐term treatment with risperidone or haloperidol in first‐episode schizophrenia: 8‐week results of a randomized controlled trial within the German Research Network on Schizophrenia. International Journal of Neuropsychopharmacology 2008;11(7):985‐97. [DOI] [PubMed] [Google Scholar]
- NCT00157378. Optimization of acute treatment in first episode schizophrenic patients by new pharmacological treatments. www.ClinicalTrials.gov/ct/show/ 2005.
- NCT00159120. Maintenance treatment vs stepwise drug discontinuation after one year of maintenance treatment in first‐episode schizophrenia. clinicaltrials.gov/ct2/show/NCT00159120 (accessed 12 September 2005).
- NCT00159133. Prodrome‐based early intervention with antipsychotics vs benzodiazepine in patients with first‐episode schizophrenia after one year maintenance treatment under further maintenance treatment vs stepwise discontinued drugs. clinicaltrials.gov/ct2/show/NCT00159133 (accessed 12 September 2005).
- Riedel M, Mayr A, Seemller F, Maier W, Klingberg S, Heuser I, et al. Depressive symptoms and their association with acute treatment outcome in first‐episode schizophrenia patients: Comparing treatment with risperidone and haloperidol. World Journal of Biological Psychiatry 2012;13(1):30‐8. [DOI] [PubMed] [Google Scholar]
- Schuhmacher A, Mossner R, Quednow BB, Kuhn KU, Wagner M, Cvetanovska G, et al. Influence of 5‐HT3 receptor subunit genes HTR3A, HTR3B, HTR3C, HTR3D and HTR3E on treatment response to antipsychotics in schizophrenia. Pharmacogenetics and Genomics 2009;19(11):843‐51. [DOI] [PubMed] [Google Scholar]
- Seemuller F, Schennach R, Mayr A, Musil R, Jager M, Maier W, et al. Akathisia and suicidal ideation in first‐episode schizophrenia. Journal of Clinical Psychopharmacology 2012;32(5):694‐8. [DOI] [PubMed] [Google Scholar]
- Wölwer W, Brinkmeyer J, Riesbeck M, Freimüller L, Klimke A, Wagner M, et al. Neuropsychological impairments predict the clinical course in schizophrenia. European Archives of Psychiatry and Clinical Neuroscience 2008; Vol. 258, issue Suppl 5:28‐34. [DOI] [PubMed]
Additional references
Adams 2013
- Adams CE, Bergman H, Irving CB, Lawrie S. Haloperidol versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD003082] [DOI] [PMC free article] [PubMed] [Google Scholar]
Albert 1979
- Albert MS, Butters N, Levin J. Temporal gradients in theretrograde amnesia of patients with alcoholic Korsakoff'sdisease. Archives of Neurology 1979;36(4):211‐6. [DOI] [PubMed] [Google Scholar]
Almerie 2007
- Almerie MQ, Alkhateeb H, Essali A, Matar HE, Rezk E. Cessation of medication for people with schizophrenia already stable on chlorpromazine. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD006329] [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baldessarini 1985
- Baldessarini RJ. Chemotherapy in psychiatry: principles and practice. Cambridge, MA: Harvard University Press, 1985. [Google Scholar]
Berger 2003
- Berger M. Mental illness. Psychische Erkrankungen. Klinik und Therapie. 2nd Edition. Urban & Fischer, 2003. [Google Scholar]
Bergman 2018
- Bergman H, Soares‐Weiser K. Anticholinergic medication for antipsychotic‐induced tardive dyskinesia. Cochrane Database of Systematic Reviews 2018, Issue 1. [DOI: 10.1002/14651858.CD000204.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhattacharjee 2016
- Bhattacharjee J, El‐Sayeh HG. Aripiprazole versus haloperidol for people with schizophrenia and schizophrenia‐like psychoses. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD012073; CD012073] [DOI] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d'efficacite therapeutique, 3: comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacite]. Therapie 1999;54(4):405‐11. [PubMed] [Google Scholar]
Bunney 1963
- Bunney WE, Hamburg DA. Methods for reliable longitudinal observation of behavior. Archives of General Psychiatry 1963;9:280‐94. [DOI] [PubMed] [Google Scholar]
Carpenter 1994
- Carpenter WT, Buchanan RW. Schizophrenia. New England Journal of Medicine 1994;330(10):681‐90. [DOI] [PubMed] [Google Scholar]
Davis 1975
- Davis JM. Overview: maintenance therapy in psychiatry: I. Schizophrenia. American Journal of Psychiatry 1975;132(12):1237‐45. [DOI] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. The 8th International Cochrane Colloquium. Cape Town: The Cochrane Collaboration, 25‐28 Oct 2000.
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dold 2015
- Dold M, Samara MT, Li C, Tardy M, Leucht S. Haloperidol versus first‐generation antipsychotics for the treatment of schizophrenia and other psychotic disorders. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD009831.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donnelly 2013
- Donnelly L, Rathbone J, Adams CE. Haloperidol dose for the acute phase of schizophrenia. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD001951.pub2; CD001951] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duggan 2005
- Duggan L, Fenton M, Rathbone J, Dardennes R, El‐Dosoky A, Indran S. Olanzapine for schizophrenia. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001359.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Essali 1993
- Essali MA, Maddocks PD. Seventeen years in the life of a depot neuroleptic clinic ‐ an audit study of schizophrenia and other psychosis. British Journal of Medical Economics 1993;6:3‐11. [Google Scholar]
Essali 2014
- Essali A, Turkmani K, Aboudamaah S, AbouDamaah A, Dia'a Aldeen MR, Marwa ME, et al. Haloperidol discontinuation for schizophrenia. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD011408; CD011408] [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. [DOI] [PubMed] [Google Scholar]
Gilbert 1995
- Gilbert P, Harris MJ, McAdams LA, Bowers MB, Charney DS, Glazer WM. Neuroleptic withdrawal in schizophrenic patients. A review of the literature. Archives of General Psychiatry 1995;52:173‐88. [DOI] [PubMed] [Google Scholar]
Hanafi 2017
- Hanafi I, Arafat S, Al Zayed L, Sukkar M, Albeirakdar A, Krayem D, et al. Haloperidol (route of administration) for people with schizophrenia. Cochrane Database of Systematic Reviews 2017, Issue 10. [DOI: 10.1002/14651858.CD012833; CD012833] [DOI] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Green S. Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Huf 2016
- Huf G, Alexander J, Gandhi P, Allen MH. Haloperidol plus promethazine for psychosis‐induced aggression. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD005146.pub3; CD005146] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2009
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. [DOI] [PubMed] [Google Scholar]
Irving 2006
- Irving CB, Adams CE, Lawrie S. Haloperidol versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003082.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986. [Google Scholar]
Khushu 2016
- Khushu A, Powney MJ. Haloperidol for long‐term aggression in psychosis. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2016, issue 11. [DOI: 10.1002/14651858.CD009830.pub2; CD009830] [DOI] [PMC free article] [PubMed]
Kudo 1999
- Kudo S, Ishizaki T. Pharmacokinetics of haloperidol: An update. Clinical Pharmacokinetics 1999;37:435‐56. [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [DOI] [PubMed] [Google Scholar]
Leucht 2005b
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [DOI] [PubMed] [Google Scholar]
Leucht 2007
- Leucht S, Engel RR, Bauml J, Davis JM. Is the superior efficacy of new generation antipsychotics an artifact of LOCF?. Schizophrenia Bulletin 2007;33(1):183‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leucht 2008
- Leucht C, Kitzmantel M, Kane J, Leucht S, Chua WL. Haloperidol versus chlorpromazine for schizophrenia. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004278.pub2; CD004278] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leucht 2009
- Leucht S, Arbter D, Engel RR, Kissling W, Davis JM. How effective are second‐generation antipsychotic drugs? A meta‐analysis of placebo‐controlled trials. Molecular Psychiatry 2009;14:429‐47. [DOI] [PubMed] [Google Scholar]
Leucht 2012
- Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Davis JM. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD008016] [DOI] [PubMed] [Google Scholar]
Lohse 2009
- Lohse MJ, Müller‐Oerlinghausen B. Psychopharmaka. In: Schwabe U, Pfaffrath D editor(s). Arzneimittelverordnungsreport. Heidelberg: Springer, 2009:767‐810. [Google Scholar]
López‐Munoz 2009
- López‐Munoz F, Alamo C. The consolidation of neuroleptic therapy: Janssen, the discovery of haloperidol and its introduction into clinical practice. Brain Research Bulletin 2009;79:130‐41. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
Marvaha 2004
- Marvaha S, Johnson S. Schizophrenia and employment ? A review. Social Psychiatry and Psychiatric Epidemiology 2004;39:337‐49. [DOI] [PubMed] [Google Scholar]
McGrath 2008
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiology 2008;30:67‐76. [DOI] [PubMed] [Google Scholar]
Momcilovic 2014
- Momcilovic S, Dickenson R, Adams CE. Anticholinergics for neuroleptic‐induced parkinsonism and tardive dyskinesia. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD011262] [DOI] [Google Scholar]
Moncrieff 2015
- Moncrieff J. Antipsychotic Maintenance Treatment: Time to Rethink?. PLoS Med 12(8): 2015;12(8):e1001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nestor 1990
- Nestor PG, Faux SF, McCarley RW, Shenton ME, Sands SF. Measurement of visual sustained attention in schizophreniausing signal detection analysis and a newly developedcomputerized CPT task. Schizophrenia Research 1990;3:329‐32. [DOI] [PubMed] [Google Scholar]
Ostinelli 2017
- Ostinelli EG, Brooke‐Powney MJ, Li X, Adams CE. Haloperidol for psychosis‐induced aggression or agitation (rapid tranquillisation). Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD009377.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostinelli 2018
- Ostinelli EG, Hussein M, Ahmed U, Rehman FU, Miramontes K, Adams CE. Risperidone for psychosis‐induced aggression or agitation (rapid tranquillisation). Cochrane Database of Systematic Reviews 2018, Issue 4. [DOI: 10.1002/14651858.CD009412.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Quraishi 1999
- Quraishi SN, David A, Brasil MA, Alheira FV. Depot haloperidol decanoate for schizophrenia. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD001361; CD001361] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ray 2017
- Ray S, Ray A, Gopi A, Hunter R. Haloperidol versus risperidone for schizophrenia. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD012728; CD012728] [DOI] [Google Scholar]
Reitan 1959
- Reitan RM, Tarshes EL. Differential effects of lateralized brain lesions on the Trail Making Test. Journal of Nervous and Mental Disease 1959;129:257‐62. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from cochrane.handbook.org.
Shepherd 1989
- Shepherd M, Watt D, Falloon I, Smeeton N. The natural history of schizophrenia: a five‐year follow‐up study of outcome and prediction in a representative sample of schizophrenics. Psychological Medicine. Monograph supplement 1989;15:1‐46. [DOI] [PubMed] [Google Scholar]
Shokraneh 2017
- Shokraneh F, Adams CE. Study‐based registers of randomized controlled trials: Starting a systematic review with data extraction or meta‐analysis. BioImpacts 2017;7(4):209‐17. [DOI: 10.15171/bi.2017.25] [DOI] [PMC free article] [PubMed] [Google Scholar]
Srisurapanont 2004
- Srisurapanont M, Maneeton B, Maneeton N, Lankappa S, Gandhi R. Quetiapine for schizophrenia. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD000967.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Suttajit 2013
- Suttajit S, Srisurapanont M, Xia J, Suttajit S, Maneeton B, Maneeton N. Quetiapine versus typical antipsychotic medications for schizophrenia. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD007815.pub2] [DOI] [PubMed] [Google Scholar]
Tardy 2014
- Tardy M, Huhn M, Engel RR, Leucht S. Perphenazine versus low‐potency first‐generation antipsychotic drugs for schizophrenia. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD009369.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsuang 1978
- Tsuang MT. Suicide in schizophrenics, manics, depressives, and surgical controls: a comparison with general population suicide mortality. Archives of General Psychiatry 1978;35(2):153‐5. [DOI] [PubMed] [Google Scholar]
Wechsler 1987
- Wechsler D. WMS‐R: Wechsler Memory Scale–Revised: Manual. Harcourt Brace Jovanovich, San Antonio: The Psychological Corporation, 1987. [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]
Xiberas 2001
- Xiberas X, Martinot JL, Mallet L, Artiges E, Loc'H C, Maziere B, et al. Extrastriatal and striatal D2 dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. British Journal of Psychiatry 2001;179:503‐8. [DOI] [PubMed] [Google Scholar]


