Abstract
Background
Inconsistent data have been reported for the effectiveness of intramuscular botulinum toxin type A (BTXA) in patients with limb spasticity after stroke. This meta-analysis of available randomized controlled trials (RCTs) aimed to determine the efficacy and safety of BTXA in adult patients with upper and lower limb spasticity after stroke.
Methods
An electronic search was performed to select eligible RCTs in PubMed, Embase, and the Cochrane library through December 2018. Summary standard mean differences (SMDs) and relative risk (RR) values with corresponding 95% confidence intervals (CIs) were employed to assess effectiveness and safety outcomes, respectively.
Results
Twenty-seven RCTs involving a total of 2,793 patients met the inclusion criteria, including 16 and 9 trials assessing upper and lower limb spasticity cases, respectively. For upper limb spasticity, BTXA therapy significantly improved the levels of muscle tone (SMD=-0.76; 95% CI -0.97 to -0.55; P<0.001), physician global assessment (SMD=0.51; 95% CI 0.35-0.67; P<0.001), and disability assessment scale (SMD=-0.30; 95% CI -0.40 to -0.20; P<0.001), with no significant effects on active upper limb function (SMD=0.49; 95% CI -0.08 to 1.07; P=0.093) and adverse events (RR=1.18; 95% CI 0.72-1.93; P=0.509). For lower limb spasticity, BTXA therapy was associated with higher Fugl-Meyer score (SMD=5.09; 95%CI 2.16-8.01; P=0.001), but had no significant effects on muscle tone (SMD=-0.12; 95% CI -0.83 to 0.59; P=0.736), gait speed (SMD=0.06; 95% CI -0.02 to 0.15; P=0.116), and adverse events (RR=1.01; 95% CI 0.71-1.45; P=0.949).
Conclusions
BTXA improves muscle tone, physician global assessment, and disability assessment scale in upper limb spasticity and increases the Fugl-Meyer score in lower limb spasticity.
1. Introduction
Stroke is characterized by sudden development of signs of focal and global cerebral function disturbance lasting more than 24 hours or leading to death. Currently, stroke has high morbidity, mortality, and disability and constitutes one of the top three causes of death in China [1, 2]. Further, stroke causes various degrees of disability in approximately 90% of patients, and nearly 1/4 individuals that experience a stroke develop recurrent stroke within a few weeks or months [3, 4]. Spasticity is an important cause of dysfunction and is observed in 19% of patients within 3 months and 38% within 12 months [5, 6]. Spasticity could affect rehabilitation and cause muscle and joint atrophy, which results in shortening of muscle fibers and ligaments, and hinder improvement in daily living activities [7]. However, few effective treatments are available for stroke and sequelae.
Botulinum toxin type A (BTXA) is a potent neurotoxin from the bacterium Clostridium botulinum, which blocks acetylcholine release at neuromuscular junctions. Numerous studies reported local injection of BTXA improves muscle tone, motion, and pain [8–13]. However, the therapeutic effects are often transient and vary by BTXA dose, for which no guidelines offer a unified standard [14]. However, injections of BTXA are widely used in patients with obvious spasticity and after 3 months in stroke patients, which might affect their rehabilitation process [15, 16]. Further, the treatment effectiveness of BTXA in upper or lower limb spasticity according to different follow-up durations and other patient characteristics remains limited and inconclusive.
Recently, numerous randomized controlled trials (RCTs) have investigated the efficacy and safety of BTXA for upper or lower limb spasticity after stroke and reported inconsistent findings. Clarifying the optimal treatment effects is particularly important in patients with limb spasticity. Therefore, we performed a large-scale analysis of available RCTs to determine the efficacy and safety of BTXA in patients with upper or lower limb spasticity. Furthermore, whether treatment effectiveness differs according to study or patient characteristics was assessed using metaregression and subgroup analyses.
2. Material and Methods
2.1. Data Sources, Search Strategy, and Selection Criteria
This review was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement issued in 2009 [17]. RCTs that investigated the efficacy and safety of BTXA for upper or lower limb spasticity after stroke were included in this study. The core search terms “botulinum toxin, type A” and “spasticity” were used to query the PubMed, Embase, and Cochrane library databases through December 2018. The detailed search strategies in PubMed are shown in Supplementary 1. A manual search of reference lists of relevant reviews and studies was also carried out to identify additional potential studies for inclusion.
The study selection process was independently undertaken by 2 investigators (Li-Chun Sun and Ying Chen), and any disagreement was resolved by group discussion until a consensus was reached. A study was included if the following inclusion criteria were met: (1) patients with upper or lower spasticity after stroke included; (2) BTXA as intervention; (3) control group administered a placebo; (4) outcomes as muscle tone, active upper limb function, physician global assessments, disability assessment scale, and adverse events for upper spasticity, and muscle tone, Fugl-Meyer score, gait speed, and adverse events for lower limb spasticity; (5) study design as RCT.
2.2. Data Collection and Quality Assessment
Data collection and quality assessment were performed by 2 authors (Rong Chen and Chuan Fu), and inconsistencies were examined and adjudicated by group discussion referring to the original studies. The information collected included first author's surname, publication year, country, sample size, mean patient age, percentage of males, time since event, spasticity sites, dose of BTXA, injection technique, duration of follow-up, and reported outcomes. Study quality was evaluated by the JADAD scale, based on randomization, concealment of treatment allocation, blinding, completeness of follow-up, and the use of intention-to-treat analysis [18]. The scoring system ranged from 0 (low quality) to 5 (high quality) in study quality assessment.
2.3. Statistical Analysis
Efficacy results of individual trials were assigned as continuous data, and safety results as dichotomous frequency data. Individual standard mean differences (SMDs) and relative risk (RR) values with 95% confidence intervals (CIs) were calculated from means, standard deviations, sample sizes, or event numbers. Summary effect estimates were calculated using the random-effects model since the actual underlying effect varies across included trials [19, 20]. Heterogeneity among the included trials was assessed by the I-square and Q tests; P values of the Q test < 0.10 were checked for statistical significance [21, 22]. Sensitivity analysis of the investigated outcomes was performed to evaluate the impact of each single trial on the overall findings [23]. The source of heterogeneity in estimates of efficacy results was identified by univariate metaregression [24]. Subgroup analyses of efficacy results were performed according to publication year, mean age, percentage male, time since event, follow-up duration, and study quality. Publication bias for the investigated outcomes was assessed by the Egger [25] and Begg tests [26]. P values in pooled analysis are two-sided, and a value below 0.05 was considered statistically significant. All statistical analyses were performed with the Stata software (version 10.0; Stata Corporation, College Station, TX, USA).
3. Results
3.1. Literature Search
The electronic search of electronic databases produced 498 articles, and 447 studies were excluded for irrelevance. The remaining 51 were selected for further evaluation, and 27 RCTs involving 2,793 spasticity patients were selected in the final analysis [27–53]. The manual search of reference lists from relevant reviews and these 27 studies yielded no additional eligible studies. The literature search and study selection are presented in Figure 1, and the baseline characteristics of included studies are shown in Table 1.
Figure 1.
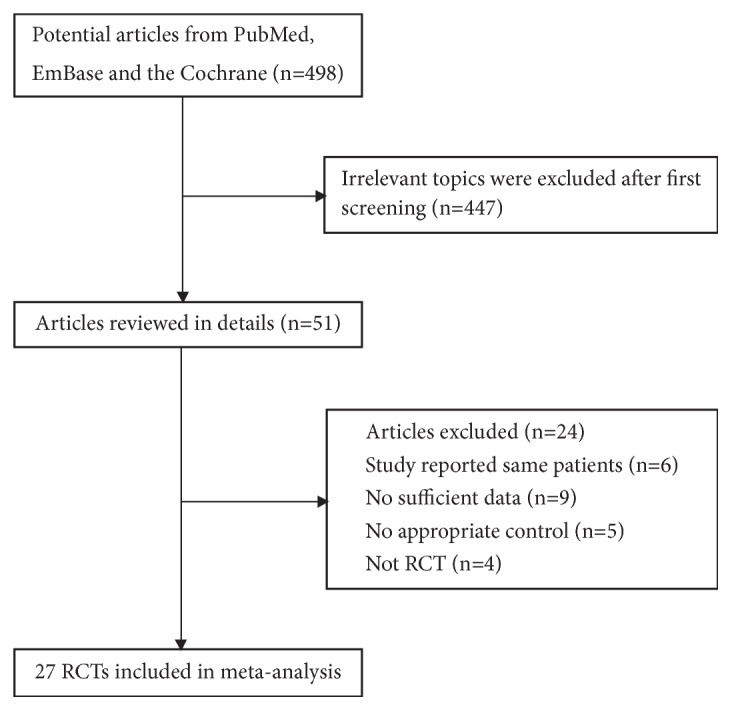
The literature search and study selection process.
Table 1.
Baseline characteristics of studies included in the current systematic review and meta-analysis.
| Study | Country | Sample size | Mean age | Percentage male (%) | Time since event | Spasticity sites | Dose of BTXA | Injection technique | Duration of follow-up | Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|
| Simpson 1996 [27] | US | 37 | 59.0 | 43.2 | 37.0 months | Upper | Botox 75, 150, 300 units | EMG | 16.0 weeks | 2 |
|
| ||||||||||
| Hesse 1998 [28] | Germany | 24 | 52.3 | 79.2 | 7.5 months | Upper | Dysport 1000 units | EMG | 12.0 weeks | 3 |
|
| ||||||||||
| Smith 2000 [29] | UK | 25 | 51.9 | 40.0 | >12.0 months | Upper | Dysport 500, 1000, 1500 units | NA | 12.0 weeks | 4 |
|
| ||||||||||
| Bakheit 2000 [30] | UK | 83 | 62.5 | 62.7 | >3.0 months | Upper | Dysport 500, 1000, 1500 units | Anatomical landmarks | 16.0 weeks | 4 |
|
| ||||||||||
| Bhakta 2000 [31] | UK | 40 | 57.0 | 57.5 | 37.2 months | Upper | Dysport 1000 units | Anatomical landmarks | 12.0 weeks | 5 |
|
| ||||||||||
| Brashear 2002 [32] | US | 126 | 61.5 | 50.0 | 56.4 months | Upper | Botox 200-240 units | NA | 12.0 weeks | 4 |
|
| ||||||||||
| Childers 2004 [33] | US | 91 | 60.0 | 67.0 | 25.8 months | Upper | Botox 90, 180, 360 units | EMG | 9.0 weeks | 5 |
|
| ||||||||||
| Yelnik 2007 [34] | France | 20 | 54.1 | 75.0 | 17.0 months | Upper | Dysport 500 units | ES | 4.0 weeks | 3 |
|
| ||||||||||
| Simpson 2009 [35] | US | 39 | 51.9 | 53.8 | > 24 hours | Upper | Botox 500 units | ES | 6.0 weeks | 4 |
|
| ||||||||||
| Meythaler 2009 [36] | US | 21 | 53.3 | 71.4 | > 6.0 months | Upper | Botox 300-400 units | EMG | 12.0 weeks | 4 |
|
| ||||||||||
| Mccrory 2009 [37] | Australia | 96 | 59.6 | 60.4 | 70.8 months | Upper | Dysport 750-1000 units | EMG or ES | 20.0 weeks | 5 |
|
| ||||||||||
| Kanovsky 2009 [38] | Germany | 148 | 55.6 | 64.2 | 55.0 months | Upper | Botox 400 units | EMG | 12.0 weeks | 5 |
|
| ||||||||||
| Kaji 2010 [39] | Japan | 109 | 63.2 | 67.9 | 82.9 months | Upper | Botox 120-150, 200-240 units | EMG nerve stimulator | 12.0 weeks | 5 |
|
| ||||||||||
| Rosales 2012 [40] | Hong Kong, Malaysia, the Philippines, Singapore, and Thailand |
163 | 55.1 | 66.9 | 1.7 months | Upper | Botox 500 units | NA | 24.0 weeks | 5 |
|
| ||||||||||
| Wolf 2012 [41] | US | 25 | 49.3 | 60.0 | 3.0-24.0 months | Upper | Dysport 300 units | NA | 12.0 weeks | 3 |
|
| ||||||||||
| Gracies 2015 [42] | Europe and the US | 238 | 52.8 | 64.3 | 61.2 months | Upper | Dysport 500, 1000 units | ES | 12.0 weeks | 5 |
|
| ||||||||||
| Elovic 2016 [43] | US | 317 | 56.0 | 46.4 | 28.0 months | Upper | Xeomin 400 units | EMG and/or ES ultrasound |
4.0 weeks | 5 |
|
| ||||||||||
| Prazeres 2018 [44] | Brazil | 40 | 52.3 | 60.0 | 33.1 months | Upper | Dysport NA | NA | 24.0 weeks | 4 |
|
| ||||||||||
| Burbaud 1996 [45] | France | 23 | 52.5 | 69.6 | 23.5 months | Lower | Dysport 1000 units | EMG | 12.0 weeks | 3 |
|
| ||||||||||
| Pittock 2003 [46] | Ireland | 234 | 55.5 | NA | > 3.0 months | Lower | Dysport 500, 1000, 1500 units | NA | 12.0 weeks | 4 |
|
| ||||||||||
| Kaji 2010 [47] | Japan | 120 | 62.5 | 80.0 | > 6.0 months | Lower | Botox 300 units | EMG | 12.0 weeks | 4 |
|
| ||||||||||
| Dunne 2012 [48] | Australia | 83 | 58.4 | 76.5 | 40.8 months | Lower | Botox 200, 300 units | EMG or ES | 12.0 weeks | 5 |
|
| ||||||||||
| Fietzek 2014 [49] | Germany | 52 | 54.3 | 57.7 | < 3.0 months | Lower | Botox 230 units | NA | 12.0 weeks | 4 |
|
| ||||||||||
| Tao 2015 [50] | China | 23 | 56.5 | 65.2 | < 1.5 months | Lower | Botox 200 units | EMG | 8.0 weeks | 3 |
|
| ||||||||||
| Ding 2015 [51] | China | 68 | 63.5 | 48.5 | 15.9 months | Lower | Lanzhou Institute of Biological Products 100 units/ampule |
Electrical nerve stimulator | 24.0 weeks | 3 |
|
| ||||||||||
| Ding 2017 [52] | China | 80 | 61.9 | 51.3 | 4.2 months | Lower | Lanzhou Institute of Biological Products 350 units |
Electrical nerve stimulator | 12.0 weeks | 3 |
|
| ||||||||||
| Wein [53] | North America, Europe, Russia, the United Kingdom, 66 and Asia 5 | 468 | 56.5 | 64.7 | 64.3 months | Lower | Botox ≤ 400 units | EMG and/or ES ultrasound | 6.0 weeks | 5 |
∗EMG: electromyography; ES: electrical stimulation; NA: not available; UK: United Kingdom; US: United States.
3.2. Study Characteristics
Of the 27 included trials, 18 were conducted in patients with upper limb spasticity [27–44], and the remaining 9 studies in individuals with lower limb spasticity [45–53]. Studies were published from 1996 to 2018, with 20-468 patients included in each trial. Twenty of the included trials were conducted in Western countries, 6 in Eastern countries, and the remaining 1 in multiple countries [53]. The mean patient age ranged from 49.3 to 63.5 years, with a percentage of males ranging from 40.0 to 80.0. Study quality was evaluated using the JADAD scale; 10 trials had a score of 5, 8 had 4 points, 8 had 3 points, and the remaining 1 had 2 points.
3.3. Upper Limb Spasticity
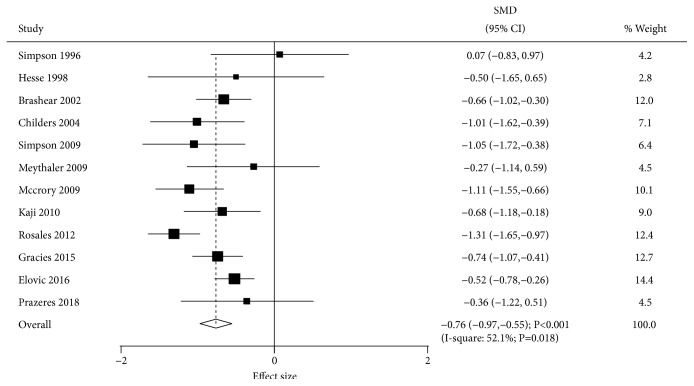
Data for the effect of BTXA on muscle tone were available in 12 trials. The summary SMD indicated that BTXA therapy was associated with lower muscle tone compared with the placebo group (SMD=-0.76; 95% CI -0.97 to -0.55; P<0.001; Figure 2). There was significant heterogeneity among these trials (I-square, 52.1%; P=0.018). Sensitivity analysis indicated the results were not altered by sequentially excluding individual trials. Univariable metaregression analyses indicated percentage of males (P=0.011) and time since event (P=0.006) altered BTXA's effects on muscle tone. Subgroup analysis indicated significant differences between BTXA and placebo for muscle tone in most subsets, while no significant differences between BTXA and placebo groups were observed for muscle tone for a duration of follow-up > 12 weeks and in low quality studies (Table 2).
Figure 2.
Effect of BTXA on muscle tone in upper limb spasticity.
Table 2.
Subgroup analysis for upper limb spasticity.
| Outcomes | Factors | Groups | SMD and 95% CI | P value | Heterogeneity (%) | P value for heterogeneity | P value for meta-regression |
|---|---|---|---|---|---|---|---|
| Muscle tone | Publication year | Before 2010 | -0.76 (-1.04 to -0.48) | <0.001 | 31.0 | 0.192 | 0.909 |
| 2010 or after | -0.77 (-1.10 to -0.43) | <0.001 | 71.9 | 0.007 | |||
| Mean age (year) | ≥ 55.0 | -0.80 (-1.10 to -0.50) | <0.001 | 69.7 | 0.003 | 0.528 | |
| < 55.0 | -0.70 (-0.96 to -0.44) | <0.001 | 0.0 | 0.598 | |||
| Percentage male (%) | ≥60.0 | -0.87 (-1.12 to -0.62) | <0.001 | 42.4 | 0.096 | 0.011 | |
| <60.0 | -0.59 (-0.86 to -0.32) | <0.001 | 30.0 | 0.232 | |||
| Time since event (months) | ≥24.0 | -0.69 (-0.87 to -0.50) | <0.001 | 26.1 | 0.220 | 0.006 | |
| <24.0 | -0.93 (-1.42 to -0.44) | <0.001 | 50.5 | 0.109 | |||
| Follow-up duration (weeks) | 4 | -0.98 (-1.28 to -0.68) | <0.001 | 65.8 | 0.005 | 0.231 | |
| 6 | -0.83 (-1.17 to -0.49) | <0.001 | 20.0 | 0.287 | |||
| 8 | -0.87 (-1.15 to -0.59) | <0.001 | 0.0 | 0.710 | |||
| 12 | -0.62 (-0.83 to -0.42) | <0.001 | 0.0 | 0.538 | |||
| > 12 | -0.55 (-1.30 to 0.20) | 0.152 | 69.0 | 0.040 | |||
| Study quality | High | -0.81 (-1.02 to -0.60) | <0.001 | 53.4 | 0.023 | 0.079 | |
| Low | -0.15 (-0.86 to 0.56) | 0.685 | 0.0 | 0.444 | |||
|
| |||||||
| Active upper limb function | Publication year | Before 2010 | 0.63 (-0.16 to 1.42) | 0.116 | 64.2 | 0.095 | 0.049 |
| 2010 or after | 0.22 (-0.27 to 0.71) | 0.379 | - | - | |||
| Mean age (year) | ≥ 55.0 | 0.49 (-0.08 to 1.07) | 0.093 | 70.0 | 0.036 | - | |
| < 55.0 | - | - | - | - | |||
| Percentage male (%) | ≥60.0 | 0.22 (-0.27 to 0.71) | 0.379 | - | - | 0.049 | |
| <60.0 | 0.63 (-0.16 to 1.42) | 0.116 | 64.2 | 0.095 | |||
| Time since event (months) | ≥24.0 | 0.49 (-0.08 to 1.07) | 0.093 | 70.0 | 0.036 | - | |
| <24.0 | - | - | - | - | |||
| Follow-up duration (weeks) | 4 | 0.55 (0.28 to 0.83) | <0.001 | 0.0 | 0.685 | 0.015 | |
| 6 | 1.11 (0.46 to 1.77) | 0.001 | 72.5 | 0.026 | |||
| 12 | 0.19 (-0.24 to 0.63) | 0.375 | 0.0 | 0.833 | |||
| Study quality | High | 0.60 (-0.11 to 1.30) | 0.096 | 81.1 | 0.022 | 0.239 | |
| Low | 0.11 (-0.79 to 1.01) | 0.811 | - | - | |||
|
| |||||||
| Physician global assessments | Publication year | Before 2010 | 0.72 (0.42 to 1.01) | <0.001 | 0.0 | 0.396 | 0.098 |
| 2010 or after | 0.42 (0.24 to 0.61) | <0.001 | 0.0 | 0.962 | |||
| Mean age (year) | ≥ 55.0 | 0.54 (0.34 to 0.74) | <0.001 | 10.1 | 0.349 | 0.638 | |
| < 55.0 | 0.44 (0.12 to 0.76) | 0.008 | - | - | |||
| Percentage male (%) | ≥60.0 | 0.56 (0.20 to 0.92) | 0.002 | 44.5 | 0.165 | 0.900 | |
| <60.0 | 0.50 (0.29 to 0.71) | <0.001 | 0.0 | 0.591 | |||
| Time since event (months) | ≥24.0 | 0.51 (0.35-0.67) | <0.001 | 0.0 | 0.457 | - | |
| <24.0 | - | - | - | - | |||
| Follow-up duration (weeks) | 4 | 0.86 (0.45 to 1.26) | <0.001 | 75.4 | 0.007 | 0.002 | |
| 6 | 1.16 (0.62 to 1.71) | <0.001 | 71.0 | 0.016 | |||
| 12 | 0.49 (0.28 to 0.71) | <0.001 | 0.0 | 0.750 | |||
| Study quality | High | 0.50 (0.34 to 0.66) | <0.001 | 2.4 | 0.393 | 0.449 | |
| Low | 0.87 (-0.08 to 1.82) | 0.073 | - | - | |||
|
| |||||||
| Disability assessment scale | Publication year | Before 2010 | -0.32 (-0.44 to -0.20) | <0.001 | 0.0 | 0.732 | 0.561 |
| 2010 or after | -0.26 (-0.43 to -0.09) | 0.003 | 0.0 | 0.335 | |||
| Mean age (year) | ≥ 55.0 | -0.33 (-0.44 to -0.21) | <0.001 | 0.0 | 0.737 | 0.377 | |
| < 55.0 | -0.22 (-0.42 to -0.02) | 0.030 | 0.0 | 0.480 | |||
| Percentage male (%) | ≥60.0 | -0.26 (-0.43 to -0.09) | 0.003 | 0.0 | 0.335 | 0.561 | |
| <60.0 | -0.32 (-0.44 to -0.20) | <0.001 | 0.0 | 0.732 | |||
| Time since event (months) | ≥24.0 | -0.30 (-0.40 to -0.20) | <0.001 | 0.0 | 0.641 | 0.649 | |
| <24.0 | -0.46 (-1.15 to 0.23) | 0.191 | - | - | |||
| Follow-up duration (weeks) | 4 | -0.33 (-0.63 to -0.03) | 0.030 | 59.2 | 0.118 | 0.119 | |
| 6 | -0.42 (-0.49 to -0.34) | <0.001 | 0.0 | 0.461 | |||
| 12 | -0.30 (-0.40 to -0.20) | <0.001 | 0.0 | 0.641 | |||
| Study quality | High | -0.30 (-0.40 to -0.20) | <0.001 | 0.0 | 0.756 | - | |
| Low | - | - | - | - | |||
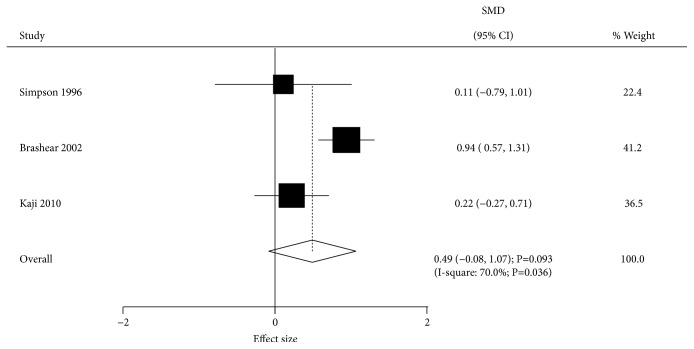
The effect of BTXA on active upper limb function was assessed by 3 trials. There was no significant difference between BTXA and placebo groups for active upper limb function (SMD=0.49; 95% CI -0.08 to 1.07; P=0.093; Figure 3). Significant heterogeneity was recorded across the included trials (I-square, 70.0%; P=0.036). Sensitivity analysis indicated that the lack of significant difference was not changed after excluding any particular trial. Univariable metaregression analysis indicated publication year (P=0.049), percentage male (P=0.049), and follow-up duration (P=0.015) could bias the effect of BTXA on active upper limb function. Subgroup analysis indicated BTXA therapy was associated with high active upper limb function with a follow-up of 4 or 6 weeks (Table 2).
Figure 3.
Effect of BTXA on active upper limb function in upper limb spasticity.
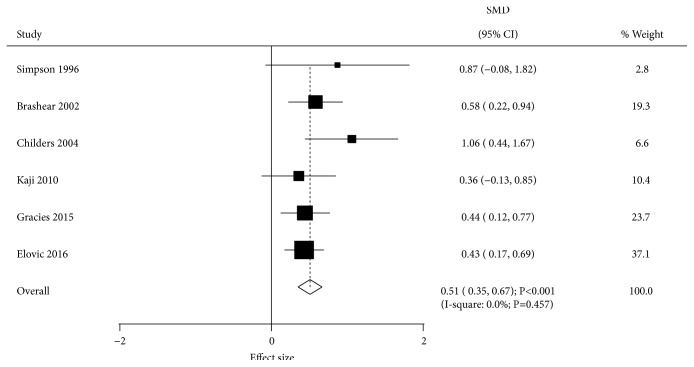
Data reporting the effect of BTXA on physician global assessment were available in 6 trials. BTXA therapy significantly increased physician global assessment compared with the placebo group (SMD-0.51; 95% CI 0.35 to 0.67; P<0.001; Figure 4). There was no evidence of heterogeneity among the included trials (I-square, 0.0%; P=0.457). Metaregression analysis indicated follow-up duration could affect the treatment effect of BTXA (P=0.002). Subgroup analysis indicated no significant difference between BTXA and placebo for physician global assessment when pooling low quality studies (Table 2).
Figure 4.
Effect of BTXA on physician global assessments in upper limb spasticity.
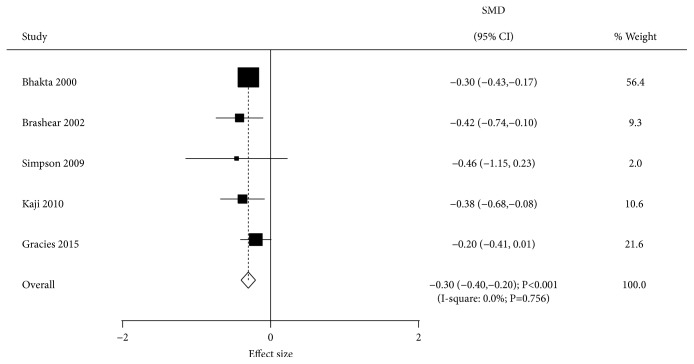
The effect of BTXA on disability assessment scale was evaluated in 5 trials. BTXA therapy significantly reduced this scale compared with placebo administration (SMD=-0.30; 95% CI -0.40 to -0.20; P<0.001; Figure 5). No evidence of heterogeneity across the included trials was detected (I-square, 0.0%; P=0.756). Univariable metaregression analysis indicated no factors affecting the treatment effect of BTXA. Subgroup analysis indicated significant effects of BTXA in most subsets, with no significant difference in disability assessment scale for time since event < 24.0 months (Table 2).
Figure 5.
Effect of BTXA on disability assessment scale in upper limb spasticity.
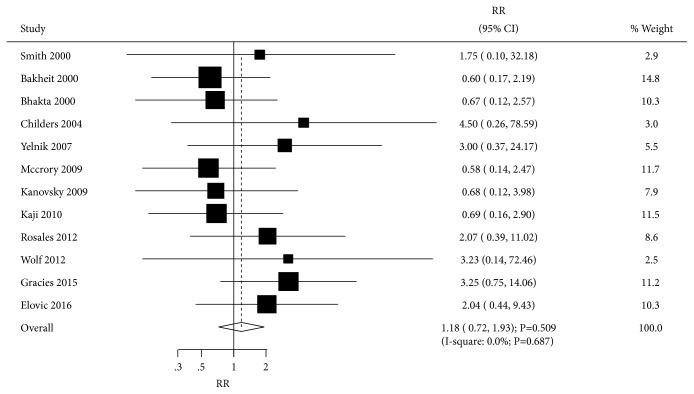
Data reporting the effect of BTXA on adverse events were available in 12 trials. BTXA therapy had no significant effect on the risk of adverse events compared with the placebo group (RR=1.18; 95% CI 0.72 to 1.93; P=0.509; Figure 6). There was no evidence of heterogeneity among the included trials (I-square, 0.0%; P=0.687), and the summary result was robust.
Figure 6.
Effect of BTXA on adverse events in upper limb spasticity.
3.4. Lower Limb Spasticity
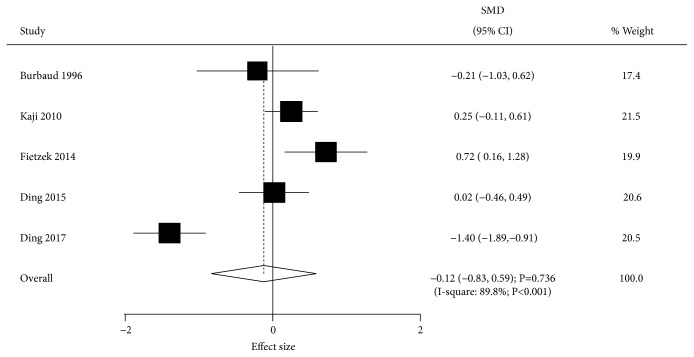
Data describing the effect of BTXA on muscle tone were available in 5 trials. BTXA had no significant effect on muscle tone compared with the placebo group (SMD=-0.12; 95% CI -0.83 to 0.59; P=0.736; Figure 7). Substantial heterogeneity across the included trials was observed (I-square, 89.8%; P<0.001). In sensitivity analysis, the conclusion was not altered after trials were sequentially excluded from the pooled results. Mean age (P<0.001), percentage of males (P=0.037), follow-up duration (P=0.049), and study quality (P<0.001) affected the effect of BTXA on muscle tone as assessed by univariable metaregression analysis. Subgroup analysis indicated BTXA could increase muscle tone after 4 weeks of follow-up (Table 3).
Figure 7.
Effect of BTXA on muscle tone in lower limb spasticity.
Table 3.
Subgroup analysis for lower limb spasticity.
| Outcomes | Factors | Groups | SMD and 95% CI | P value | Heterogeneity (%) | P value for heterogeneity | P value for meta-regression |
|---|---|---|---|---|---|---|---|
| Muscle tone | Publication year | Before 2010 | -0.21 (-1.03 to 0.62) | 0.618 | - | - | 0.762 |
| 2010 or after | -0.10 (-0.94 to 0.73) | 0.808 | 92.3 | <0.001 | |||
| Mean age (year) | ≥ 55.0 | -0.69 (-2.08 to 0.70) | 0.332 | 94.0 | <0.001 | <0.001 | |
| < 55.0 | 0.31 (-0.12 to 0.74) | 0.156 | 45.8 | 0.158 | |||
| Percentage male (%) | ≥60.0 | 0.18 (-0.16 to 0.51) | 0.298 | 0.3 | 0.317 | 0.037 | |
| <60.0 | -0.22 (-1.43 to 0.98) | 0.714 | 94.1 | <0.001 | |||
| Time since event (months) | ≥24.0 | -0.21 (-1.03 to 0.62) | 0.618 | - | - | 0.762 | |
| <24.0 | -0.10 (-0.94 to 0.73) | 0.808 | 92.3 | <0.001 | |||
| Follow-up duration (weeks) | 4 | 0.62 (0.01 to 1.22) | 0.045 | 85.1 | <0.001 | 0.049 | |
| 8 | -0.15 (-0.59 to 0.29) | 0.504 | - | - | |||
| 12 | 0.02 (-0.77 to 0.80) | 0.968 | 91.6 | <0.001 | |||
| > 12 | 0.02 (-0.46 to 0.50) | 0.934 | - | - | |||
| Study quality | High | 0.43 (-0.02 to 0.88) | 0.058 | 47.8 | 0.166 | <0.001 | |
| Low | -0.54 (-1.53 to 0.44) | 0.278 | 88.7 | <0.001 | |||
|
| |||||||
| Fugl-Meyer score | Publication year | Before 2010 | 1.20 (-1.88 to 4.28) | 0.445 | - | - | <0.001 |
| 2010 or after | 6.26 (3.39 to 9.12) | <0.001 | 94.8 | <0.001 | |||
| Mean age (year) | ≥ 55.0 | 6.26 (3.39 to 9.12) | <0.001 | 94.8 | <0.001 | <0.001 | |
| < 55.0 | 1.20 (-1.88 to 4.28) | 0.445 | - | - | |||
| Percentage male (%) | ≥60.0 | 0.57 (-1.44 to 2.57) | 0.580 | 0.0 | 0.595 | <0.001 | |
| <60.0 | 8.46 (7.90 to 9.02) | <0.001 | 27.8 | 0.239 | |||
| Time since event (months) | ≥24.0 | 1.20 (-1.88 to 4.28) | 0.445 | - | - | <0.001 | |
| <24.0 | 6.26 (3.39 to 9.12) | <0.001 | 94.8 | <0.001 | |||
| Follow-up duration (weeks) | 4 | 0.96 (-0.27 to 2.18) | 0.125 | 70.2 | 0.018 | <0.001 | |
| 8 | -0.56 (-0.89 to -0.23) | 0.001 | 0.0 | 0.622 | |||
| 12 | 6.66 (4.82 to 8.50) | <0.001 | 91.0 | <0.001 | |||
| > 12 | 9.04 (7.89 to 10.19) | <0.001 | - | - | |||
| Study quality | High | - | - | - | - | - | |
| Low | 5.09 (2.16 to 8.01) | 0.001 | 94.9 | <0.001 | |||
|
| |||||||
| Gait speed | Publication year | Before 2010 | -0.00 (-0.04 to 0.03) | 0.806 | 0.0 | 0.645 | 0.003 |
| 2010 or after | 0.13 (-0.02 to 0.28) | 0.089 | 85.2 | 0.001 | |||
| Mean age (year) | ≥ 55.0 | 0.13 (-0.03 to 0.29) | 0.116 | 89.9 | <0.001 | 0.129 | |
| < 55.0 | -0.01 (-0.06 to 0.04) | 0.712 | 0.0 | 0.712 | |||
| Percentage male (%) | ≥60.0 | 0.06 (-0.07 to 0.20) | 0.343 | 85.8 | 0.001 | 0.016 | |
| <60.0 | 0.17 (0.06 to 0.28) | 0.002 | - | - | |||
| Time since event (months) | ≥24.0 | -0.02 (-0.09 to 0.06) | 0.601 | - | - | 0.211 | |
| <24.0 | 0.09 (-0.01 to 0.19) | 0.087 | 85.5 | <0.001 | |||
| Follow-up duration (weeks) | 4 | 0.01 (-0.02 to 0.04) | 0.533 | 0.0 | 0.442 | 0.454 | |
| 8 | 0.07 (-0.03 to 0.18) | 0.158 | 79.7 | 0.002 | |||
| 12 | 0.02 (-0.04 to 0.09) | 0.434 | 66.9 | 0.029 | |||
| Study quality | High | 0.00 (-0.04 to 0.04) | 1.000 | 0.0 | 1.000 | 0.014 | |
| Low | 0.12 (-0.04 to 0.29) | 0.139 | 87.7 | <0.001 | |||
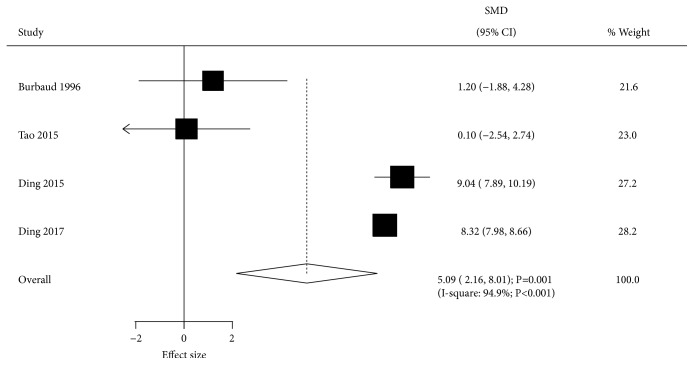
Data describing the effect of BTXA on Fugl-Meyer score were available in 4 trials. The summary result indicated that BTXA significantly increased Fugl-Meyer score compared with the placebo group (SMD=5.09; 95% CI 2.16 to 8.01; P=0.001; Figure 8). Substantial heterogeneity among the included trials was observed (I-square, 94.9%; P<0.001), and the results were stable after excluding individual trials. Univariable metaregression analysis suggested that publication year (P<0.001), mean age (P<0.001), percentage of males (P<0.001), time since event (P<0.001), and follow-up duration (P<0.001) could bias the effect of BTXA on Fugl-Meyer score. Subgroup analysis indicated no significant differences between BTXA and placebo in Fugl-Meyer score when pooling trials published before 2010, as well as those with mean patient age <55.0 years, percentage of males ≥60.0%, time since event ≥24.0 months, and more than 4 weeks of follow-up (Table 3).
Figure 8.
Effect of BTXA on Fugl-Meyer score in lower limb spasticity.
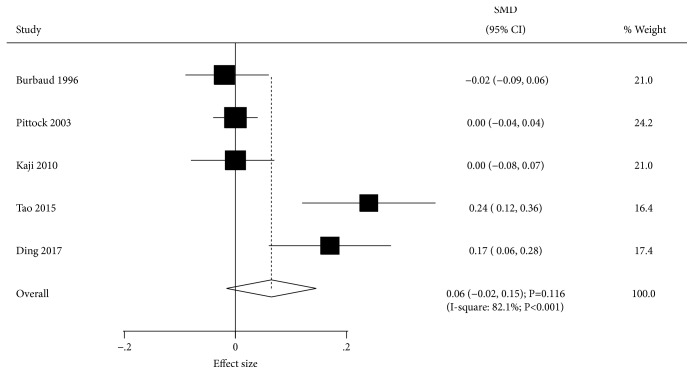
Data describing the effect of BTXA on gait speed were available in 5 trials. There was no significant difference between BTXA and placebo for gait speed (SMD=0.06; 95% CI -0.02 to 0.15; P=0.116; Figure 9). Although significant heterogeneity among the included trials was detected (I-square, 82.1%; P<0.001), the conclusion was not altered by the exclusion of any specific study. Univariable metaregression analysis indicated that publication year (P=0.003), percentage of males (P=0.016), and study quality (P=0.014) could bias the effect of BTXA on gait speed. BTXA therapy significantly increased gait speed with percentage of males < 60.0% (Table 3).
Figure 9.
Effect of BTXA on gait speed in lower limb spasticity.
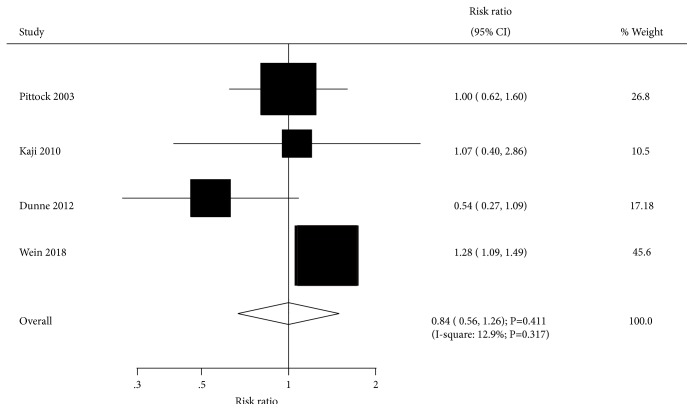
The effect of BTXA on adverse events was assessed by 4 trials. Overall, BTXA therapy had no significant effect on the risk of adverse events (RR=1.01; 95% CI 0.71-1.45; P=0.949; Figure 10). There was potential significant heterogeneity across the included trials (I-square, 53.6%; P=0.091), and sensitivity analysis indicated the conclusion was not affected by sequential exclusion of individual trials.
Figure 10.
Effect of BTXA on adverse events in lower limb spasticity.
3.5. Publication Bias
Publication bias was assessed for the investigated outcomes, and results are shown in Table 4. There were no significant publication biases for muscle tone (Egger's P value=0.591; Begg's P value=0.409), active upper limb function (Egger's P value=0.467; Begg's P value=1.000), physician global assessment (Egger's P value=0.136; Begg's P value=0.133), disability assessment scale (Egger's P value=0.370; Begg's P value=0.462), and adverse events (Egger's P value=0.081; Begg's P value=0.064) in patients with upper limb spasticity. Furthermore, no evidence of publication bias was found for muscle tone (Egger's P value=0.838; Begg's P value=0.806), Fugl-Meyer score (Egger's P value=0.226; Begg's P value=0.308), and adverse events (Egger's P value=0.209; Begg's P value=0.734) in patients with lower limb spasticity. Although the Egger's test suggested no publication bias for gait speed (P=0.136), potential publication bias was detected in the Begg's test in patients with lower limb spasticity (P=0.043). The conclusion regarding gait speed was not altered after adjustment by the trim and fill method [54].
Table 4.
Publication bias assessment for investigated outcomes.
| Limb spasticity | Outcomes | P value for Egger | P value for Begg |
|---|---|---|---|
| Upper | Muscle tone | 0.591 | 0.409 |
| Active upper limb function | 0.467 | 1.000 | |
| Physician global assessments | 0.136 | 0.133 | |
| Disability assessment scale | 0.370 | 0.462 | |
| Adverse events | 0.081 | 0.064 | |
|
| |||
| Lower | Muscle tone | 0.838 | 0.806 |
| Fugl-Meyer score | 0.226 | 0.308 | |
| Gait speed | 0.136 | 0.043 | |
| Adverse events | 0.209 | 0.734 | |
4. Discussion
BTXA is widely used for limb spasticity, but its effectiveness varies according to spasticity site and treatment dose. The aim of this study was to determine the efficacy and safety of BTXA therapy for upper and lower limb spasticity after stroke. In this comprehensive quantitative meta-analysis, 27 RCTs involving 2,793 spasticity patients with a broad range of characteristics could ensure the applicability of summary findings. Pooled results showed that BTXA therapy could significantly improve muscle tone, physician global assessment, and disability assessment scale in upper limb spasticity compared with the placebo group. In addition, BTXA versus placebo showed significant improvement in Fugl-Meyer score in lower limb spasticity. Finally, the treatment effects of BTXA might be affected by several predefined factors, including publication year, mean age, percentage of males, time since event, follow-up duration, and study quality.
A previous meta-analysis has investigated the efficacy and safety of BTXA for upper limb spasticity after stroke and traumatic brain injury [55]. The authors concluded BTXA therapy has beneficial effects with improved muscle tone, reduced disability assessment scale, and increased patients' global assessment score. In addition, they pointed out that BTXA therapy is well-tolerated in patients with upper limb spasticity after stroke. Another meta-analysis was performed in patients with lower limb spasticity and concluded BTXA therapy produces persistent benefits for muscle tone and Fugl-Meyer score compared with placebo administration [56]. The limitations of previous meta-analyses are that they just provide summary effect estimates according to the follow-up duration and in-depth stratified analyses are not illustrated. Therefore, the current meta-analysis was performed to determine the efficacy and safety of BTXA in patients with upper and lower limb spasticity and evaluate whether the treatment effectiveness of BTXA differs according to publication year, mean age, percentage male, time since event, follow-up duration, and study quality in upper and lower limb spasticity, respectively.
Summary results indicated BTXA was superior to placebo for upper limb spasticity in terms of muscle tone, physician global assessment, and disability assessment scale. Indeed, most included trials reported significant differences between BTXA and placebo in muscle tone, physician global assessment, and disability assessment scale, while several others found no statistically significant differences [27, 28, 36, 44]. A possible explanation might be the small sample sizes of these trials, which showed large standard deviations and broad 95% CIs. In addition, muscle tone assessment is highly subjective, with varying indexes among the included trials, which might produce potential bias. Furthermore, although summary results for active upper limb function were not statistically significant, an obvious trend of increase was detected, which requires further large-scale trials for verification. Finally, the risk of adverse events between BTXA and placebo showed no statistically significant difference, and all the included trials reported similar conclusions. This could be explained by an incidence of adverse events lower than expected as well as broad 95% CI.
As shown above, BTXA could improve Fugl-Meyer score, but had no significant effect on muscle tone, gait speed, and adverse events in patients with lower limb spasticity after stroke. First, only 2 of the included trials reported significant effects of BTXA on muscle tone, with discrepant results. Fietzek et al. indicated patients administered BTXA show high muscle tone compared with the placebo group [49]. This might be because muscle tone deterioration was observed in patients who initially had placebo, which is associated with reduced susceptibility to BTXA therapy. Secondly, pooled results for Fugl-Meyer score showed a significant increase in patients administered BTXA since the study by Ding et al. reported a large effect difference between the BTXA and placebo groups [51, 52]. This might be explained by large sample sizes in these two studies and sufficient power to detect statistical significance. Furthermore, these two patients received electrical nerve stimulation, which could affect the Fugl-Meyer score. Thirdly, gait speed results between BTXA and placebo had no statistically significant difference, while two trials performed in China reported inconsistent findings. Further large-scale trials are required for verification. Finally, pooled results for adverse events showed no statistically significant difference, likely because of low incidence of events and small sample sizes of the included trials.
Subgroup analysis for upper or lower limb spasticity could be affected by predefined factors. Firstly, publication year affected active upper limb function in upper limb spasticity, as well as Fugl-Meyer score and gait speed in lower limb spasticity. This might be because studies performed at different periods could affect the level of background rehabilitation therapy. Secondly, mean patient age significantly biased results for muscle tone and Fugl-Meyer score in lower limb spasticity. The recovery ability in different age groups varies, which might affect the effects of BTXA. Thirdly, the percentage of males mostly affected efficacy results, likely because mean age varied between men and women among the included trials. Fourthly, time since event in patients administered BTXA could bias results for muscle tone in upper limb spasticity and Fugl-Meyer score in lower limb spasticity. This indicates that time of BTXA administration could affect treatment effects. Fifthly, the duration of follow-up reflected the persistent effects of BTXA and affected most results. Treatment effects were tapered after prolonged follow-up, especially after 12 weeks. Finally, study quality affected the balance of patients' characteristics, which plays an important role in the treatment effects of BTXA.
The limitations of this study should be mentioned. Firstly, the effects of BTXA on limb spasticity based on dose were not assessed due diverse units and types. Secondly, effect estimates had large ranges and/or different units; therefore, weighted mean differences could not be calculated. Thirdly, stratified analyses based on several important factors were not conducted due to smaller number of included trials for each specific outcome, such as country, injection techniques, and rating scales. Fourthly, this study was not registered online, which might be a potential shortcoming. Finally, inherent limitations of meta-analyses include publication bias and use of pooled results.
5. Conclusion
In conclusion, pooled results indicated BTXA is superior to placebo in upper or lower limb spasticity after stroke. However, the treatment effects taper after 12 weeks. Furthermore, publication year, mean patient age, percentage of males, time since event, and study quality could significantly affect efficacy results in patients administered BTXA. Future large-scale RCTs should be performed to verify the current findings and assess treatment effects according to patients' characteristics.
Acknowledgments
This work was funded by 2017 Hainan Natural Science Foundation Project: clinical study on the efficacy of different doses of botulinum toxin A in the treatment of upper limb paralysis after stroke (Item No. 817325); 2016 Hainan Medical College Research and Development Fund (Item No. HY2016-19).
Data Availability
The data set supporting the results of this article is included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
Search strategies in PubMed.
Data set supporting the results of this article.
References
- 1.Go A. S., Mozaffarian D., Roger V. L., et al. Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. The top ten causes of death. WorldHealthOrganization, 2017, http://www.who.int/mediacentre/factsheets/fs310/en/index3.html.
- 3.Kim B. R., Lee J., Sohn M. K., et al. Risk factors and functional impact of medical complications in stroke. Annals of Rehabilitation Medicine. 2017;41(5):753–760. doi: 10.5535/arm.2017.41.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleindorfer D., Panagos P., Pancioli A., et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36(4):720–723. doi: 10.1161/01.STR.0000158917.59233.b7. [DOI] [PubMed] [Google Scholar]
- 5.Sommerfeld D. K., Eek E. U., Svensson A., Holmqvist L. W., Von Arbin M. H. Spasticity after stroke: its occurrence and association with motor impairments and activity limitations. Stroke. 2004;35(1):134–139. doi: 10.1161/01.STR.0000105386.05173.5E. [DOI] [PubMed] [Google Scholar]
- 6.Watkins C. L., Leathley M. J., Gregson J. M., Moore A. P., Smith T. L., Sharma A. K. Prevalence of spasticity post stroke. Clinical Rehabilitation. 2002;16(5):515–522. doi: 10.1191/0269215502cr512oa. [DOI] [PubMed] [Google Scholar]
- 7.Kimura A., Abo M., Kawate N., et al. Efficacy and safety of botulinum toxin type a in treating upper limb spasticity in post-stroke patients: a multicenter, double-blind, placebo-controlled trial followed by an open-label trial. The Japanese Journal of Rehabilitation Medicine. 2010;47(10):714–727. doi: 10.2490/jjrmc.47.714. [DOI] [Google Scholar]
- 8.Das T. K., Park D. M. Botulinum toxin in treating spasticity. British Journal of Clinical Practice. 1989;43:401–403. [PubMed] [Google Scholar]
- 9.Hesse S., Friedrich H. Botulinum toxin therapy for upper limb flexor spasticity: preliminary results. Journal of Rehabilitation Science. 1992;5:98–101. [Google Scholar]
- 10.Skeil D. A., Barnes M. P. The local treatment of spasticity. Clinical Rehabilitation. 1994;8(3):240–246. doi: 10.1177/026921559400800309. [DOI] [Google Scholar]
- 11.Dunne J. W., Heye N., Dunne S. L. Treatment of chronic limb spasticity with botulinum toxin A. Journal of Neurology, Neurosurgery & Psychiatry. 1995;58(2):232–235. doi: 10.1136/jnnp.58.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhakta B. B., Cozens J. A., Bamford J. M., Chamberlain M. A. Use of botulinum toxin in stroke patients with severe upper limb spasticity. Journal of Neurology, Neurosurgery & Psychiatry. 1996;61(1):30–35. doi: 10.1136/jnnp.61.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierson S. H., Katz D. I., Tarsy D. Botulinum toxin A in the treatment of spasticity: Functional implications and patient selection. Archives of Physical Medicine and Rehabilitation. 1996;77(7):717–721. doi: 10.1016/S0003-9993(96)90015-5. [DOI] [PubMed] [Google Scholar]
- 14.Santamato A., Panza F., Ranieri M., et al. Efficacy and safety of higher doses of botulinum toxin type A NT 201 free from complexing proteins in the upper and lower limb spasticity after stroke. Journal of Neural Transmission. 2013;120(3):469–476. doi: 10.1007/s00702-012-0892-x. [DOI] [PubMed] [Google Scholar]
- 15.McIntyre A., Lee T., Janzen S., Mays R., Mehta S., Teasell R. Systematic review of the effectiveness of pharmacological interventions in the treatment of spasticity of the hemiparetic lower extremity more than six months post stroke. Topics in Stroke Rehabilitation. 2012;19(6):479–490. doi: 10.1310/tsr1906-479. [DOI] [PubMed] [Google Scholar]
- 16.Speelman J. D. Treatment strategies in movement disorders. Journal of Inherited Metabolic Disease. 2005;28(3):441–444. doi: 10.1007/s10545-005-8049-9. [DOI] [PubMed] [Google Scholar]
- 17.Panic N., Leoncini E., De Belvis G., Ricciardi W., Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE. 2013;8(12, article e83138) doi: 10.1371/journal.pone.0083138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jadad A. R., Moore R. A., Carroll D., et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R., Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Ades A. E., Lu G., Higgins J. P. T. The interpretation of random-effects metaanalysis in decision models. Medical Decision Making. 2005;25(6):646–654. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 21.Deeks J. J., Higgins J. P. T., Altman D. G. Analyzing data and undertaking meta-analyses. In: Higgins J., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1. Oxford, UK: The Cochrane Collaboration; 2008. (chapter 9). [Google Scholar]
- 22.Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tobias A. Assessing the influence of a single study in meta-analysis. Stata Technical Bulletin. 1999;47:15–17. [Google Scholar]
- 24.Thompson S. G., Higgins J. P. T. How should meta-regression analyses be undertaken and interpreted? Statistics in Medicine. 2002;21(11):1559–1574. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 25.Egger M., Smith G. D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begg C. B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 27.Simpson D. M., Alexander D. N., O'Brien C. F., et al. Botulinum toxin type A in the treatment of upper extremity spasticity: a randomized, double-blind, placebo-controlled trial. Neurology. 1996;46(5):1306–1310. doi: 10.1212/WNL.46.5.1306. [DOI] [PubMed] [Google Scholar]
- 28.Hesse S., Reiter F., Konrad M., Jahnke M. T. Botulinum toxin type A and short-term electrical stimulation in the treatment of upper limb flexor spasticity after stroke: a randomized, double-blind, placebo-controlled trial. Clinical Rehabilitation. 1998;12(5):381–388. doi: 10.1191/026921598668275996. [DOI] [PubMed] [Google Scholar]
- 29.Smith S. J., Ellis E., White S., Moore A. P. A double-blind placebo-controlled study of botulinum toxin in upper limb spasticity after stroke or head injury. Clinical Rehabilitation. 2000;14(1):5–13. doi: 10.1191/026921500666642221. [DOI] [PubMed] [Google Scholar]
- 30.Bakheit A. M. O., Thilmann A. F., Ward A. B., et al. A randomized, double-blind, placebo-controlled, dose-ranging study to compare the efficacy and safety of three doses of botulinum toxin type A (Dysport) with placebo in upper limb spasticity after stroke. Stroke. 2000;31(10):2402–2406. doi: 10.1161/01.STR.31.10.2402. [DOI] [PubMed] [Google Scholar]
- 31.Bhakta B. B., Cozens J. A., Chamberlain M. A., Bamford J. M. Impact of botulinum toxin type A on disability and carer burden due to arm spasticity after stroke: a randomised double blind placebo controlled trial. Journal of Neurology, Neurosurgery & Psychiatry. 2000;69(2):217–221. doi: 10.1136/jnnp.69.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brashear A., Gordon M. F., Elovic E., et al. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. The New England Journal of Medicine. 2002;347(6):395–400. doi: 10.1056/NEJMoa011892. [DOI] [PubMed] [Google Scholar]
- 33.Childers M. K., Brashear A., Jozefczyk P., et al. Dose-dependent response to intramuscular botulinum toxin type a for upper-limb spasticity in patients after a stroke. Archives of Physical Medicine and Rehabilitation. 2004;85(7):1063–1069. doi: 10.1016/j.apmr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Yelnik A. P., Colle F. M., Bonan I. V., Vicaut E. Treatment of shoulder pain in spastic hemiplegia by reducing spasticity of the subscapular muscle: a randomised, double blind, placebo controlled study of botulinum toxin A. Journal of Neurology, Neurosurgery & Psychiatry. 2007;78(8):845–848. doi: 10.1136/jnnp.2006.103341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson D. M., Gracies J. M., Yablon S. A., Barbano R., Brashear A., Team T. B. S. Botulinum neurotoxin versus tizanidine in upper limb spasticity: a placebo-controlled study. Journal of Neurology, Neurosurgery and Psychiatry. 2009;80(4):380–385. doi: 10.1136/jnnp.2008.159657. [DOI] [PubMed] [Google Scholar]
- 36.Meythaler J. M., Vogtle L., Brunner R. C. A preliminary assessment of the benefits of the addition of botulinum toxin a to a conventional therapy program on the function of people with longstanding stroke. Archives of Physical Medicine and Rehabilitation. 2009;90(9):1453–1461. doi: 10.1016/j.apmr.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 37.McCrory P., Turner-Stokes L., Baguley I. J., et al. Botulinum toxin a for treatment of upper limb spasticity following stroke: a multi-centre randomized placebo-controlled study of the effects on quality of life and other person-centred outcomes. Journal of Rehabilitation Medicine. 2009;41(7):536–544. doi: 10.2340/16501977-0366. [DOI] [PubMed] [Google Scholar]
- 38.Kaňovský P., Slawek J., Denes Z., et al. Efficacy and safety of botulinum neurotoxin NT 201 in poststroke upper limb spasticity. Clinical Neuropharmacology. 2009;32(5):259–265. doi: 10.1097/WNF.0b013e3181b13308. [DOI] [PubMed] [Google Scholar]
- 39.Kaji R., Osako Y., Suyama K., et al. Botulinum toxin type A in post stroke upper limb spasticity. Current Medical Research and Opinion. 2010;26(8):1983–1992. doi: 10.1185/03007995.2010.497103. [DOI] [PubMed] [Google Scholar]
- 40.Rosales R. L., Kong K. H., Goh K. J., et al. Botulinum toxin injection for hypertonicity of the upper extremity within 12 weeks after stroke: a randomized controlled trial. Neurorehabilitation and Neural Repair. 2012;26(7):812–821. doi: 10.1177/1545968311430824. [DOI] [PubMed] [Google Scholar]
- 41.Wolf S. L., Milton S. B., Reiss A., Easley K. A., Shenvi N. V., Clark P. C. Further assessment to determine the additive effect of botulinum toxin type a on an upper extremity exercise program to enhance function among individuals with chronic stroke but extensor capability. Archives of Physical Medicine and Rehabilitation. 2012;93(4):578–587. doi: 10.1016/j.apmr.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 42.Gracies J.-M., Brashear A., Jech R., et al. Safety and efficacy of abobotulinumtoxinA for hemiparesis in adults with upper limb spasticity after stroke or traumatic brain injury: a double-blind randomised controlled trial. The Lancet Neurology. 2015;14(10):992–1001. doi: 10.1016/S1474-4422(15)00216-1. [DOI] [PubMed] [Google Scholar]
- 43.Elovic E. P., Munin M. C., Kaňovský P., Hanschmann A., Hiersemenzel R., Marciniak C. Randomized, placebo-controlled trial of incobotulinumtoxina for upper-limb post-stroke spasticity. Muscle & Nerve. 2016;53(3):415–421. doi: 10.1002/mus.24776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prazeres A., Lira M., Aguiar P., et al. Efficacy of physical therapy associated with botulinum toxin type A on functional performance in post-stroke spasticity: a randomized, double-blinded, placebo-controlled trial. Neurology International. 2018;10(2, article 7385) doi: 10.4081/ni.2018.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burbaud P., Wiart L., Dubos J. L., et al. A randomised, double blind, placebo controlled trial of botulinum toxin in the treatment of spastic foot in hemiparetic patients. Journal of Neurology, Neurosurgery & Psychiatry. 1996;61(3):265–269. doi: 10.1136/jnnp.61.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pittock S. J., Moore A. P., Hardiman O., et al. A double-blind randomised placebo-controlled evaluation of three doses of botulinum toxin type A (Dysport) in the treatment of spastic equinovarus deformity after stroke. Cerebrovascular Disease. 2003;15(4):289–300. doi: 10.1159/000069495. [DOI] [PubMed] [Google Scholar]
- 47.Kaji R., Osako Y., Suyama K., Maeda T., Uechi Y., Iwasaki M. Botulinum toxin type A in post-stroke lower limb spasticity: a multicenter, double-blind, placebo-controlled trial. Journal of Neurology. 2010;257(8):1330–1337. doi: 10.1007/s00415-010-5526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunne J. W., Gracies J.-M., Hayes M., Zeman B., Singer B. J. A prospective, multicentre, randomized, double-blind, placebo-controlled trial of onabotulinumtoxinA to treat plantarflexor/invertor overactivity after stroke. Clinical Rehabilitation. 2012;26(9):787–797. doi: 10.1177/0269215511432016. [DOI] [PubMed] [Google Scholar]
- 49.Fietzek U. M., Kossmehl P., Schelosky L., Ebersbach G., Wissel J. Early botulinum toxin treatment for spastic pes equinovarus-a randomized double-blind placebo-controlled study. European Journal of Neurology. 2014;21(8):1089–1095. doi: 10.1111/ene.12381. [DOI] [PubMed] [Google Scholar]
- 50.Tao W., Yan D., Li J.-H., Shi Z.-H. Gait improvement by low-dose botulinum toxin a injection treatment of the lower limbs in subacute stroke patients. Journal of Physical Therapy Science. 2015;27(3):759–762. doi: 10.1589/jpts.27.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding X. D., Zhang G. B., Chen H. X., et al. Color Doppler ultrasound-guided botulinum toxin type A injection combined with an ankle foot brace for treating lower limb spasticity after a stroke. European Review for Medical and Pharmacological Sciences. 2015;19(3):406–411. [PubMed] [Google Scholar]
- 52.Ding X., Huang L. I., Wang Q., Liu Y., Zhong J., Chen H. Clinical study of botulinum toxin a injection combined with spasmodic muscle therapeutic instrument on lower limb spasticity in patients with stroke. Experimental and Therapeutic Medicine. 2017;13(6):3319–3326. doi: 10.3892/etm.2017.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wein T., Esquenazi A., Jost W. H., et al. Onabotulinumtoxin A for the treatment of poststroke distal lower limb spasticity: a randomized trial. PM&R: The Journal of Injury, Function, and Rehabilitation. 2018;10(7):693–703. doi: 10.1016/j.pmrj.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Duvall S., Richard T. A nonparametric 'trim and fill' method for assessing publication bias in meta-analysis. Journal of the American Statistical Association. 2000;95:89–98. [Google Scholar]
- 55.Dong Y., Wu T., Hu X., Wang T. Efficacy and safety of botulinum toxin type A for upper limb spasticity after stroke or traumatic brain injury: a systematic review with meta-analysis and trial sequential analysis. European Journal of Physical and Rehabilitation Medicine. 2017;53(2):256–267. doi: 10.23736/S1973-9087.16.04329-X. [DOI] [PubMed] [Google Scholar]
- 56.Wu T., Li J. H., Song H. X., Dong Y. Effectiveness of botulinum toxin for lower limbs spasticity after stroke: a systematic review and meta-analysis. Topics in Stroke Rehabilitation. 2016;23(3):217–223. doi: 10.1080/10749357.2016.1139294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategies in PubMed.
Data set supporting the results of this article.
Data Availability Statement
The data set supporting the results of this article is included within the article.