Abstract
Esophageal cancer is a very deadly disease, killing more than 15,000 people in the United States annually. Almost 400,000 new cases happen in the worldwide every year. More than 50% esophageal cancer patients are diagnosed at an advanced stage when they need an esophageal stent to open the blocked esophagus for feeding and drinking. Esophageal stents have evolved in stages over the years. Current clinically used stents commonly include stainless steel or nitinol self-expandable metallic stent (SEMS) and self-expandable plastic stent (SEPS). There are many choices of different types of stents and sizes, with fierce competition among manufacturers. However, current stent technology, whether uncovered, partially covered, fully covered SEMS or SEPS, has their own advantages to solve the dysphagia, stricture, and fistula problems, but they also cause some clinical complications. The ideal stent remains elusive. New 3D printing technique may bring new promising potential to manufacturing personalized esophageal stents. Drug-eluting stents could be the new avenue to do more than just pry open a stricture or cover a defect in the esophageal lumen, a possibility of proving local anticancer therapy simultaneously. Additionally, the lack of esophageal cancer animal models also hinders the progress of stent development. This paper reviews these topics for a comprehensive understanding of this field. In a conclusion, the ultimate goal of the future esophageal stent would have multifunction to treat the underlying conditions and restore esophageal function to near normal.
1. Introduction
Esophageal cancer is a deadly disease, ranking sixth among all cancers in mortality [1]. About 17000 new esophageal cancer cases are diagnosed yearly and about 16000 deaths a year occur due to esophageal cancer in the United States (Cancer facts and figures. American Cancer Society). An estimated 456,000 new cases were diagnosed worldwide in 2012 [2], and there were over 500,000 new cases in 2018. More than 50% of esophageal cancer patients are diagnosed at an advanced stage when the esophagus has often been occluded by the tumor [3–5]. To open the occluded esophagus, a self-expanding esophageal stent is often used for drinking and feeding, which has become the primary palliative therapy of dysphagia [6]. Beyond just palliative therapy, researchers are exploring new therapeutic applications for an esophageal stent by wrapping a drug-eluting polymer film on the device [7, 8]. This wrapping polymer film provides the release of an anticancer drug to inhibit tumor growth. This kind of attempt in vascular stents has been proven to enhance treatments of blood vessel diseases [9, 10] and showed that it is feasible to treat both cancer-related stenosis as well as malignant gastrointestinal cancer [11]. This paper mainly focuses on reviewing the applications of self-expanding esophageal stents, biodegradable stent, and drug-eluting esophageal stents in malignant esophageal cancers, while the sizes, shapes, and manufactures of clinically used esophageal stents are not discussed as these aspects have been comprehensively reviewed in [6].
2. Self-Expandable Stents
2.1. Self-Expanding Metallic Stents (SEMS)
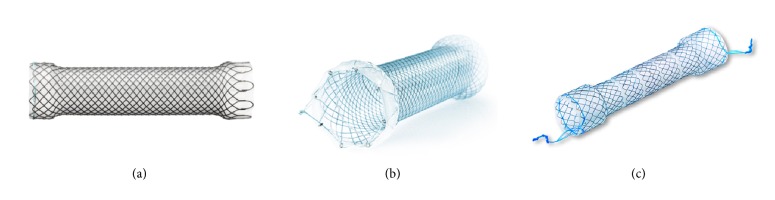
A variety of self-expanding esophagus stents have been widely used in the treatment of esophagus cancer [12, 13]. The esophagus stents include self-expanding plastic stents (SEPS) and self-expanding metal stents (SEMS). SEMS is the most widely used stents clinically for malignant esophageal cancer. Currently, SEMS are available in three main types: covered, partially covered, and uncovered (Figure 1). Although these types of SEMS have been widely used clinically, they cause complications differently. Multiple studies have reported that the conventional uncovered SEMS showed complications, such as bleeding, fistulae, recurrence of tissue growth, embedment, etc. [14]. The major complication is tissue regrowth to the mesh so that the stent is embedded in the tumor tissue, leading to new stricture or occlusion. Tumor ingrowth or overgrowth is one of the most delayed complications. In bare SEMS, about 17−36% showed tumor ingrowth [15]. Mayoral et al. analyzed the literature data of 81 patients receiving SEMS for esophageal cancer, and they found tumor overgrowth in 60% of the patients. Among these patients, 53% of the cases had a malignant growth while 47% had a nonmalignant growth over the stent [16]. To overcome the complications of tumor and granulation tissue ingrowth, a variety of covering polymer materials have been developed to fully cover the stent. However, after covering, new complications happened. The covered SEMS become prone to migration [17], leading secondary surgery to take out the stent from stomach. The most common complication of SEMS was migration (36.3%), followed by pain and obstruction [18]. To prevent the stent migration, the middle stem part of SEMS is covered but the proximal and distal ends of SEMS are exposed to allow embedding into the esophageal wall, thus helping to prevent the stent migration. An interesting method, through-the-scope (TTS) clips [19] or over-the-scope (OTS) [20], was developed to anchor the stent. Another interesting and promising method to anchor a stent to the esophageal wall is endoscopic suturing with an endoscopic suturing device [21]. To further review major advantages and drawbacks of SEMS, some representative SEMS of the most commonly used SEMS in the United States are listed in the table (Table 1). In general, these SEMS have common complications such as tumor tissue ingrowth and stent migration. The differences in migration rates among SEMS were not statistically significant [22].
Figure 1.
Photographs (from the company official websites) show Softcup esophagus SEMS stent (MICRO-TECH, Germany) (a), PermalumeTM Silicone coating SEMS (WallFlexTM from Boston Scientifi, USA) (b), and a segmented SEMS (Choostent; M.I. Tech, Pyeongtaek, Korea) (c).
Table 1.
Overview of advantages and drawbacks of some SEMS commonly used in clinics.
| Product | Manufacturer | Advantages | Drawbacks | Techniques to prevent migration |
|
| ||||
| Alimaxx-ES EndoMAXX |
Merit Medical | (i) Laser cut designed to meet specific anatomical requirements. (ii) Polyurethane cover helps decrease tissue ingrowth. (iii) Silicone lining provides a smooth inner lumen. (iv) Purse-string design of the proximal suture knot allows repositioning and removal immediately post-placement. |
(i) One case report that the patient presented with vomiting and dysphagia to solids [63]. | (i) Anti-migration struts design reduces stent migration. |
| Choo stent | M.I. Tech | (i) Fully covered with polyurethane to prevent tumor ingrowth. (ii) Cylindrical zigzag fashion of 12 or 15 bends to maximize its longitudinal flexibility. (iii) Easily removed under mild sedation. |
(i) The distal release mechanism may cause inconvenience in placement [64]. | (i) Distal flared extremity. |
| Niti-S Double-layered stent | Taewoong Medical | (i) Prevent tumor ingrowth and migration. | (i) May cause overgrowth at the proximal end of the stent [65]. | (i) Stent-inside-a-stent. |
| SX-ELLA-HV | Ella-CS | (i) The ends are non-traumatic. (ii) Covered film prevents tumor in-growth and occlusion. (iii) Unique delivery system. |
(i) The frequency of hemorrhage and fistula formation was considerable. (ii) It has similar migration rate to other fully covered stents [66]. |
(i) Collar anti-migration system. |
| Evolution | Cook Medical | (i) Silicone encases the exterior and interior surfaces of the stent to prevent tumor ingrowth. (ii) A dual purse string “lasso loop” on the proximal and distal ends of the stent facilitates repositioning. (iii) Unique delivery system. |
(i) Potential risk of aspiration [67]. | (i) Proximal and distal uncovered flares. |
| Ultraflex/Wallflex | Boston Scientific |
(i) A purse string facilitates stent repositioning or removal. | (i) Potential risk of bleeding [67]. | (i) Progressive step flared ends reduces migration. |
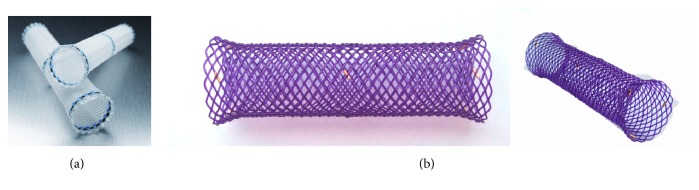
2.2. Self-Expanding Plastic Stents (SEPS)
Although SEMS has been used clinically more than two decades, the rigid metal and large-sized ends occasionally cause patients' chest pain, bleeding, and fistulas or perforations [24, 25], even large tracheal-bronchial fistula [26]. Thus, the self-expanding plastic stents (SEPS) provides the advantages in mitigating these complications [27]. Further, SEPS can be easily removed and seems not to be inferior to metallic stent [28]. They also provide significant improvement of dysphagia and quality of life, and to decrease the number of dilatation sessions in patients with benign strictures [29]. The currently available SEPS is the Polyflex stent (Boston Scientific, Natick, MA, USA), which is composed of a plastic wire and has a fully covered design with silicone and a proximal flare (Figure 2(a)) This stent material has been suggested to reduce reactive tissue hyperplasia [30]. However, studies reported that SEPS have significantly higher rate of migration than SEMS. Holm and coworkers reported stent migration was more frequent in proximal (30/44 stents, 68.1%) and distal (19/27 stents, 70.4%) compared with mid esophageal (3/10 stents, 30%). Migration was more frequent in stents placed for benign strictures (18/22 stents, 81.8%), anastomotic strictures (18/24 stents, 75%), and fistulae/leak (13/22 stents, 59.1%) compared with radiation-induced strictures (4/14 stents, 28.6%) [31]. Although SEPS causes high rate of migration, it appears to be safer than metallic stent as the plastic material may not cause significant tissue trauma [32]. Therefore, these SEPS are often used in benign strictures, as well as for treating esophageal leaks, fistulae, and perforations. In a recent review paper, Gwang Ha Kim reviewed that SEPS were used in 130 patients with a benign esophageal stricture, and results showed that after a median follow-up period of 13 months, only 52% of the patients were dysphagia-free, the early migration rate was 24%, the endoscopic reintervention rate was 21%, and major clinical complications occurred in 9% of patients [30, 32].
Figure 2.
Photographs (from the company official websites) show the self-expanding plastic Polyflex stent (Boston Scientifi, USA) (a) and SX-ELLA degradable esophageal stent (ELLA-CS, s.r.o. Czech Republic) (b).
In malignant management, SEPS became an alternative to SEMS since 2000s. Szegedi et al. compared the efficacy of SEPS and SEMS in palliation of advanced esophageal carcinoma [33]. They reported that the use of SEPS in palliation of esophageal cancer seems to be safer and more effective than SEMS in improving the quality of life of those patients. However, Conio and colleagues found that there was a significantly higher complication rate (hemorrhage, tumor overgrowth, and migration) with SEPS (Polyflex) compared to partially covered SEMS (Ultraflex) in 101 patients with unresectable carcinoma [34]. Twenty (44%) patients with a Polyflex SEPS stent and 18 (33%) with an Ultraflex SEMS stent had recurrent dysphagia because of tumor overgrowth, stent migration, hyperplastic granulomatous reaction, or food bolus impaction. Their results showed that more complications, especially late stent migration, were observed in the SEPS group.
2.3. Biodegradable Stents
To decrease the complications of tumor tissue or hyperplastic tissue growth and stent migration, and also to avoid the need for stent removal, new biodegradable stents (BD stent) have recently been developed. Two types of biodegradable polymer stents are available currently. One is the ELLA-BD stent (ELLA-CS, Hradec Kralove, Czech Republic), which is composed of polydioxanone, a surgical suture material (Figure 2(b)) [35], and the other one is the poly-L-lactic acid (PLLA)-BD stent (Marui Textile Machinery, Osaka, Japan), which consists of knitted PLLA monofilaments [23]. The advantage of biodegradable stent is that they do not have to be removed after they are implanted [36]. The gastric acid can hydrolysis the materials, which reduces the morbidity. Saito and colleagues developed Ultraflex-type stent by knitting poly-l-lactic acid (PLLA) monofilaments. They placed the biodegradable PLLA stent into 13 patients with different esophagus disorders including esophagus cancer. They found that no symptoms of restenosis were observed within the follow-up period of 7 months to 2 years. Further treatment with balloon dilatation or replacement of the biodegradable stent was not required [37]. However, spontaneous migration of the stents occurred between 10 to 21 d after placement in 10 of the 13 cases. The same research group continued to place the biodegradable PLLA stent into 2 cases with benign esophageal strictures. Their results showed that there were no symptoms of restenosis for 6 months. The PLLA esophageal stent provided a new possibility for the management of benign esophageal strictures after endoscopic submucosal dissection (ESD) [38]. van Boeckel PG compared the efficacy and safety of SEPS with the placement of biodegradable stents for the treatment of refractory benign esophageal strictures (RBES). Their results showed promising potential of the biodegradable stent for the treatment of RBES. They found that reinterventions were less using biodegradable stent than SEPS placement [39]. If using SEPS, twelve patients (67%) had recurrent dysphagia, but if using a biodegradable stent, only six patients (33%) have dysphagia. Obviously, biodegradable stents offered an advantage. However, biodegradable stents have also their drawbacks. Repici et al. conducted a prospective study in 21 patients using SX-Ella stents for RBES [40]. After 7 weeks, they reported that stent migration occurred in 9.5%. 45% of patients did not experienced dysphagia at the end of the follow-up period. However, 55% still suffered symptom recurrence of tissue ingrowth, and three patients experienced severe pain after placement. From these results, it can be seen that although biodegradable stents may provide a valuable alternative to SEPS and SEMS, and also may eliminate the need for repeat esophageal dilations, biodegradable stents still presented some complications of migration and tissue regrowth. Also, biodegradation may lead to the collapse of stents after placement due to the collapsed degradation of the stent, quickly losing the mechanical strength. For example, Nogales Rincon and colleagues placed a SX-ELLA esophageal degradable BD stent (ELLA-CS, Hradec Králové, Czech Republic) into a 74-year-old man with a previous history of surgery for pharyngolaryngeal neoplasia and reconstruction. They found that the stent degraded and collapsed after 9 weeks. The collapsed mesh did not allow the passage of a standard Pentax endoscope [41]. To overcome these issues of poor corrosion resistance and a quick loss of mechanical support of BD stents, Yuan et al. mixed biodegradable poly(ε-caprolactone) (PCL) and poly(trimethylene carbonate) (PTMC) as the coated membrane to coat magnesium alloy stents for a fully biodegradable esophageal stent. Their results showed that the new biodegradable stent had an ability to delay the degradation time and maintain mechanical performance in the long term [42, 43].
From these studies, it can be seen that the degradation properties of a BD stent determine its mechanical integrity. Studies showed that both ELLA-BD stent and PLLA-BD stents, the two currently available BD stents, can be degraded by hydrolysis, which is accelerated at low ambient pH. The stents began to degrade after 4 to 5 weeks and dissolved during a period of 2 to 3 months [23, 35]. The degradation rate of a BD stent is dependent not only on the properties of the polymer, but also the size and structure of the stent, and also influenced by surrounding environment, such as temperature, pH and type of body tissue/fluid. With time, these factors gradually affect the mechanical integrity of stent. Radial expansion force of stent would be also affected. The initial value of radial force of the current BD stent can be maintained in physiological saline solution (pH 7, 37°C) for 6-8 weeks. After seven weeks, the radial force was dramatically decreased and the stent was completely degraded after 2–4 months [44]. The polymer stent degraded faster with a lower pH. Therefore, in the future, when a new degradable stent will be designed, the polymer properties need to be optimized to achieve prolonged stent integrity. From these studies, due to the biodegradable features of the degradable stent, longer term studies are necessary to investigate the relationship between the expected disappearance of the stent and the patency of the management of esophagus disorders. The current evidence is insufficient to determine the relative efficacy or safety of esophageal biodegradable stents [45].
Thus, these esophageal stents had their own advantages and limitations. A fully covered SEMS decreases the recurrence of dysphagia associated with a bare SEMS, but it has a higher migration rate than a bare SEMS. Partially covered SEMS has a firm anchoring effect, preventing stent migration and the recurrence of dysphagia, but hyperplastic tissue reaction may easily happen. Likewise, SEPS reduces reactive hyperplasia, but the high rate of stent migration limited its application. A biodegradable stent brings an advantage of not requiring stent removal in comparison with SEMS and SEPS. However, it still has a somewhat high rate of hyperplastic reaction, and the risk of collapse, which does not satisfy expectations. Therefore, the question of which type of stent should be recommended for the effective treatment of complex and refractory benign strictures, also malignant tumor remains unclear [30].
2.4. 3D-Printed Stents
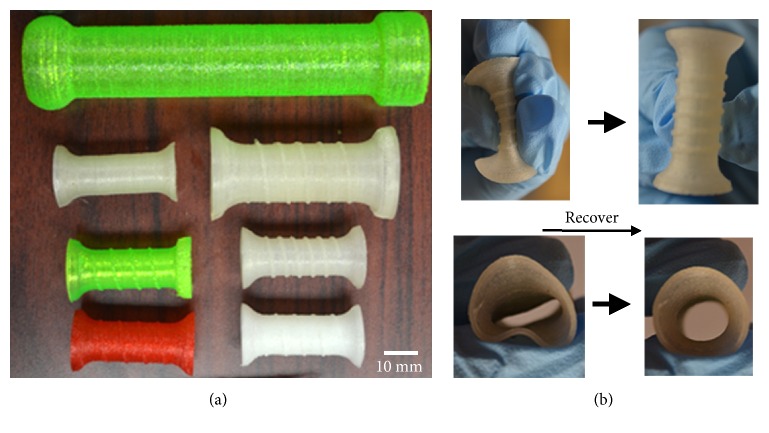
More recently, additive manufacturing technologies have been used to fabricate stents, for example, airway stent [46]. Existing airway stents have many shortcomings including the development of obstructing granulation tissue in the weeks and months following placement, mucous build up within the stent, and cough. Furthermore, existing airway stents are expensive and, if improperly sized for a given airway, may be easily dislodged (stent migration). 3D-printing technology may offer the best chance of personalizing stents and addressing many of the current limitations in stent design. Similarly, 3D printing provides a new potential to produce esophageal stents. The 3D printing technology provides an advantage over the current traditional technologies for the preparation of polymer stents, such as braided, knitted, laser-cut [47], and segmented [48]. In our study, we used a 3D printing technology to produce a flexible polymer esophageal stent (Figure 3) [49]. We found that our 3D printing technique can print an esophageal stent with different size and shape. This is the first study using 3D printing technique to produce a polymer esophageal stent. Although the function of the 3D-printed flexible polymer stent has not been proved in vivo, the in vitro study showed that the 3D-printed esophageal stent has promising potential to treat malignant esophageal diseases. It can self-expand, and 3D printing technique can design and print different sizes and shapes of the stent easily. Further studies need to be done to further show the function of a 3D-printed esophageal stent.
Figure 3.
Photographs show the different types of 3D-printed stents with different structures and material ratios (a). The stent was compressed and then recovered to the original shape (b). Reedited and reprinted with the permission from [23].
2.5. Drug-Eluting Stents
For those patients with inoperable esophageal malignancies, using a SEMS to mechanically open the blocked esophagus for drinking and feeding has become the primary palliative therapy of dysphagia [6]. Recently, drug-eluting self-expanding metallic stents (DE-SEMS) have been attempted with some efficacy for the management of occlusion of the esophagus beyond just palliative procedure [7, 8]. DE-SEMS, a drug-loaded polymer membrane coated SEMS, provides both the mechanical support to expand the blocked esophagus, and the released anticancer drug to inhibit tumor growth, which has been proven to prolong patient life-span[11].
Cannular stents have been widely used in across a variety of vascular and nonvascular organs for unblocking the occlusion of body conduits. Drug-eluting stents (DESs) are popular for vascular applications, bile conduit [50], but the development of DES for nonvascular organ, for instance, esophagus, has been slow, and no drug-eluting stent is clinically available for treating esophageal cancer [51]. However, research on DESs for esophagus is following a similar track. Sanjay Garg et al. developed a bilayer polymer film loading an anticancer drug (docetaxel), which can be covered on a SEMS [52]. They used biocompatible, biostable polyurethane PurSil AL 20 (PUS) to prepare the docetaxel loaded films as a SEMS covering material for the localized delivery of DTX to the esophagus. They investigated the effect of thickness on release behavior and the permeation through esophageal tissues, thus establishing the critical factors responsible for controlling the delivery of DTX. They found that the bilayer films structure exhibited sustained release (>30 days) and minimal DTX permeation through esophageal tissues in vitro. They also found that the release rate lied with the esophageal tissues, suggesting that DTX delivery may be sustained for longer periods in vivo compared to the in vitro. This kind of localized sustained delivery system in combination with the stent appeared to be a promising strategy to treat malignant esophagus cancer. Fan et al. used rabbit esophageal cancer models to evaluate the efficiency and safety of paclitaxel-eluting SEMS [53]. Paclitaxel is currently being used to treat several types of cancer including esophageal carcinoma [54], through inhibiting tumor growth by binding to β -tubulin and stabilizing polymerized microtubules [50, 55]. Thus, paclitaxel-eluting SEMS (PEMS) may have antitumor effects against or prevent tumor overgrowth of malignant esophageal strictures through the local release to the esophagus tissue. They found that in the 22 rabbits, the average tumor volume was significantly decreased from 7.00±4.30 cm3 in the SEMS group to 0.94±1.51 cm3 in the drug-eluting stents group (p<0.05), and that the tumor area in the drug-eluting stent group was also smaller than that in the SEMS groups (p<0.05). This brought a promising clinical trial potential.
Besides loading the paclitaxel into a covering polymer film, other anticancer drugs, for instance, 5-fluorouracil were also loaded in the covering film of SEMS [56, 57]. The new development of this type of drug-eluting stent was a series of new films featuring multilayered structures for improved mechanical properties and unidirectional, controlled drug release [56, 58]. These studies aimed at solving the problem of drug leaking to the stomach through the cannula of stent and esophagus, because a drug-eluting film covering on a SEMS may lack the unidirectional drug release control to target the mucosa tissue of the esophagus [53]. Drugs from the thin film were released to the stomach through the mesh into the canal of the stent, which compromises the drug delivery efficacy of the drug-eluting SEMS and significantly increases side effects. To overcome this kind of complications, Tian et al. developed multilayered films based on a series of poly(caprolactone) (PCL) and PEG polymers, which contained antitumor 5-fluorouracil [58]. The covering film contained a backing layer, which blocks the release of anticancer drug to the stomach, and a surface multiple drug layers, which loaded different drug concentrations in different layers to realize unidirectional, controlled drug release. Their results showed that drug release was dependent on the drug loading and environmental pH, and that ex vivo permeation behaviors showed the drug released from the multilayered film in a unidirectional and controlled manner. This kind of the multilayered films provided an attractive mode to produce polymer covering stents for localized treatment of stenosis or occlusion of esophageal cancer. Similarly, Guo's research group recently developed a paclitaxel or 5-fluorouracil loaded bilayered polymer films covering on a nitinol SEMS to treat unresectable cancer in a porcine model [59, 60]. The bilayered polymer film is consisted of a layer of 50% PTX or 5-FU and a layer of drug-free backing. Their results showed that majority of the loaded drugs permeated into esophagus from the outside of the film, and the backing layer blocked the release of the drug to the cannula. The drug concentrations were highest in the esophagus compared with in the heart, liver, spleen, lung, kidney and blood (81500.0 ± 9475.2 ng/g versus 3.9 ± 0.3 ng/mL of PTX in the plasma at 13 days). This new stent provided a dual function as both a stent and a local drug delivery device for esophageal cancer.
A local, more targeted release of an anticancer drug from a bilayered or multilayered drug-releasing films demonstrated enhanced efficacy of anticancer drugs, prevented or reduced the side effects associated with systemic administration of an anticancer drug, such as paclitaxel, 5-fluorouracil, and gemcitabine [7, 11, 60–62]. Therefore, this type of drug-eluting stent has potential for improving inhibition of esophageal tumor growth.
3. Animal Models for Esophageal Stents
Currently, two-dimensional (2D) cell culture, 3D cell spheroids models, animal xenograft/orthotopic models are widely used in cancer studies to evaluate the efficacy and functionalities of a new drug delivery system or a new treatment [68–71]. Usually these models are feasible in interrogating the antitumor effect of drug delivery systems in many types of cancers [72, 73]. However, in esophageal cancers with strictures or benign esophageal diseases, these models encounter challenges for the studies of esophageal stents. This is because the esophageal stents commonly used in esophageal diseases include bared SEMS, SEPS, BD, covered stents, and drug-eluting stents. The length and diameter of most FDA-approved stents currently marketed in the United States are 8-10 cm and 16-20 mm [74], which is not applicable in a 2D cell culture, 3D cell spheroid model, or xenograft animal models due to the lack of sufficient dimensions and 3D esophageal biological structure [75–77]. For orthotopic esophagus models, stenting is still a challenge. For example, the esophagi of small animals, mice or rats, are too small to allow for stenting, compared to the deployment procedures designed for humans [78–80]. Rabbit models belong to moderate-to-large-sized animal models, but there are few reports of rabbit tumor models established for human-sized esophageal stents [81]. Large-sized animal models, such as the dog or pig, could simulate the human body environment for esophageal stent deployment, but most of studied dog or pig models used healthy esophagi to examine the safety of stenting and tissue responses. They were not established for esophageal cancer conditions [82, 83]. Therefore, there are very few appropriate animal esophageal cancer models available for the study of esophageal cancers and esophageal stents. There is an unmet need for the establishment of esophageal cancer animal models.
Although there are few orthotopic esophageal cancer models, in the past decades there are many reports about the use of animals for the test of esophageal stents in vivo. Shaikh et al. used a nude mice xenograft tumor model to test docetaxel (DTX)-loaded polyurethane formulations for stent application [84]. To test the esophageal tissue responses to nitinol stents loaded with 50% 5-FU or PTX, Guo et al. used twenty-three healthy Bama minipigs to test 4 groups for stent implantation: PTX stent, 5-FU stent, blank film–covered stent, and bare stent. They found that severe tissue responses including inflammation, ulceration, and granulation occurred at the bare ends of the stent not in the middle part of the stent. This animal model demonstrated that the drug concentrations in the esophagus that had contact with the 5-FU stent or PTX stent were very high, which did not cause obvious tissue damage [59, 60]. Chan Sup Shim et al. used the same model, minipig esophageal model, to test the clinical feasibility of a newly developed fully covered, self-expanding, through-the-scope (TTS) esophageal stent [85]. Besides these models, other animal models have been used to test drug-eluting stents, for instance, a dog esophagus stricture model was used to test the tissue response to a rapamycin-eluting nanofiber membrane-covered metal stent and a paclitaxel-eluting stent [86, 87], and a rabbit model was also used to test the efficacy of an IN-1233-eluting covered stent in preventing tissue hyperplasia [88]. A rabbit model was successfully used to test the efficiency of long-term local drug delivery of 5-fluorouracil-containing self-expandable nitinol stent [57].
These healthy animal models have been used to test the tissue safety of stents. However, a tumor model is required for evaluating the antitumor effect of self-expandable stent or drug-eluting stents that have been or are being developed. Very recently, Huang et al. successfully established a rabbit esophageal tumor model using endoscopic and surgical implantation of VX2 tumor fragments [81, 89]. Both the endoscopic and the surgical method had a relatively high success rate of tumor implantation [93.7% (30/32) versus 97.1% (33/34)] and tumor growth [86.7% (26/30) versus 81.8% (27/33)]. They continued to further evaluate the feasibility of the animal models for stenting [81]. The self-expandable metal stents were randomly deployed in rabbits with severe esophageal stricture to investigate the safety using the rabbit malignant model. The results indicated that the rabbits that received stent placement survived longer than those without stent implantation (the mean survival time: 53.9 days versus 40.3 days, p= 0.016).
To comprehensively review these reported models, a table is listed here to show the existing middle-to-large animal esophagus models for diverse purposes. All kinds of stents including SEMS, SEPS, BD, covered stent or new developed stents were listed in Table 2.
Table 2.
Current animal esophagus models for stenting.
| Animal model | Inserted stent | Purposes | Results | Refs |
|
| ||||
| Healthy rabbit | SEMS with 125I loaded | To evaluate radiotolerance | Caused epithelial hyperplasia and stricture | [90] |
| Canine stricture | New covered SEMS | To test the antimigration | Half of stents migrated | [91] |
| Mongrel dogs | New nitinol stent | Anti-postcaustic stricture | Better than unstented group | [92] |
| Bama mini-pig | Nitinol stents loaded 5-FU or Paclitaxel (PTX) | To investigate tissue response; Drug release | Severe tissue response at the ends; highest drug concentrations in esophagus | [59, 60] |
| New Zealand rabbits | magnetocaloric nitinol stent with PTX | Drug eluting Release | biocompatible and safe | [93] |
| Healthy beagle dogs | Covered SEMS | Evaluate safety | No significant radiation toxicity | [94] |
| Benign dog cardia stricture | paclitaxel or rapamycin-eluting stent | Observe inflammatory reaction | Drug-eluting stent had better outcomes | [95] |
| A stricture model of rabbit | Three “piece” of SEMS with PLGA treads | Safety of the stent | The degradable part of the stent degraded; stent migrated | [96, 97] |
| Mini pig | Full covered SEMS | to evaluate the clinical feasibility | Easy deployment; | [82, 98] |
| Refractory benign strictures in dogs | SEMS, SEPS, BD | To evaluate the complications | 50% dogs had complications | [99] |
| Pig stricture model | ELLA-CS); PLA/PCL BD stent |
To treat stricture | Did not prevent high-grade stricture formation. | [100, 101] |
| Rabbit model. | IN-1233–eluting covered stents | To investigate the efficacy | decreased tissue hyperplasia |
[88] |
| Dog model | PCDL BD stent | To treat stenosis | The stent recovered its initial shape in vivo | [102] |
| Malignant rabbit models | SEMS, drug-eluting stent | To image cancer tissue, and treat | Successful in establishing a malignant esophagostenosis model in rabbits |
[7, 81, 103] |
From Table 1 we can see that at present, only the rabbit malignant model is a middle animal esophagus tumor model for stent deployment, while most of other animal models used healthy esophagi or benign stricture esophagi. This is because it is challenging to establish a large animal esophageal cancer model. The challenge is not only because of the complex surgery on a large animal, but also the potential difficulty of inoculating tumor cells or tissue in the orthotopic esophagus for tumor formation. In the future, new animal models or alternative animal modelling technologies still need to be developed and established for esophageal cancer stenting.
4. Conclusion
Esophageal cancer remains a leading cause of cancer-related deaths worldwide. Palliation therapy for dysphagia using esophageal stents including SEMS, SEPS, and biodegradable stents is the current major treatment of choice for those patients with inoperable esophageal malignancies. Although these stents have been used in clinics for years, it is highly needed to develop novel stents that can overcome some complications associated with current stents. In addition to improving the functionality of the drug-loaded stent with markedly reduced adverse effects, new ideal stents will allow to be tailored to individual needs at much lower cost. Additionally, there is an unmet need to develop a large animal esophageal cancer model in vivo and establish a functional esophageal cancer model in vitro to test stents and study esophageal cancers.
Acknowledgments
This review was supported by the National Cancer Institute of the National Institutes of Health under Award no. R03CA201960 and R03CA235244.
Disclosure
The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
The author declares that they have no conflicts of interest.
References
- 1.Lin E. W., Karakasheva T. A., Hicks P. D., Bass A. J., Rustgi A. K. The tumor microenvironment in esophageal cancer. Oncogene. 2016;35(41):5337–5349. doi: 10.1038/onc.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richman D. M., Tirumani S. H., Hornick J. L., et al. Beyond gastric adenocarcinoma: Multimodality assessment of common and uncommon gastric neoplasms. Abdominal Radiology. 2017;42(1):124–140. doi: 10.1007/s00261-016-0901-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park C. G., Kim M. H., Park M., et al. Polymeric nanofiber coated esophageal stent for sustained delivery of an anticancer drug. Macromolecular Research. 2011;19(11):1210–1216. doi: 10.1007/s13233-011-1112-5. [DOI] [Google Scholar]
- 4.Muller J. M., Erasmi H., Stelzner M., Zieren U., Pichlmaier H. Surgical therapy of oesophageal carcinoma. British Journal of Surgery. 1990;77(8):845–857. doi: 10.1002/bjs.1800770804. [DOI] [PubMed] [Google Scholar]
- 5.Enzinger P. C., Mayer R. J. Esophageal Cancer. The New England Journal of Medicine. 2003;349(23):2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 6.Hindy P., Hong J., Lam-Tsai Y., Gress F. A comprehensive review of esophageal stents. Gastroenterol Hepatol (N Y) 2012;8(8):526–534. [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y., Ma L., Huang J., Shuang J., Chen J., Fan Z. The effect of paclitaxel-eluting covered metal stents versus covered metal stents in a rabbit esophageal squamous carcinoma model. PLoS ONE. 2017;12(3) doi: 10.1371/journal.pone.0173262.e0173262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suk K. T., Kim J. W., Kim H. S. Human application of a metallic stent covered with a paclitaxel-incorporated membrane for malignant biliary obstruction: multicenter pilot study. Gastrointestinal Endoscopy. 2007;66(4):798–803. doi: 10.1016/j.gie.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 9.Perin E. C. Choosing a drug-eluting stent: a comparison between CYPHER and TAXUS. Reviews in Cardiovascular Medicine. 2005;6(supplement 1):S13–S21. [PubMed] [Google Scholar]
- 10.Zhu Y., Zhang H., Zhang Y., et al. Endovascular metal devices for the treatment of cerebrovascular diseases. Advanced Materials. 2018;31(8) doi: 10.1002/adma.201805452.1805452 [DOI] [PubMed] [Google Scholar]
- 11.Lee J. W., Yang S.-G., Na K. Gemcitabine-releasing polymeric films for covered self-expandable metallic stent in treatment of gastrointestinal cancer. International Journal of Pharmaceutics. 2012;427(2):276–283. doi: 10.1016/j.ijpharm.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Didden P., Spaander M. C., Bruno M. J., Kuipers E. J. Esophageal stents in malignant and benign disorders. Current Fungal Infection Reports. 2013;15(4):p. 319. doi: 10.1007/s11894-013-0319-3. [DOI] [PubMed] [Google Scholar]
- 13.Mangiavillano B., Pagano N., Arena M., et al. Role of stenting in gastrointestinal benign and malignant diseases. World Journal of Gastrointestinal Endoscopy. 2015;7(5):460–480. doi: 10.4253/wjge.v7.i5.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain P. Self-expanding metallic esophageal stents: A long way to go before a particular stent can be recommended. World Journal of Gastroenterology. 2011;17(48):5327–5328. doi: 10.3748/wjg.v17.i48.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabharwal T., Morales J. P., Salter R., Adam A. Esophageal cancer: Self-expanding metallic stents. Abdominal Imaging. 2005;30(4):456–464. doi: 10.1007/s00261-004-0277-1. [DOI] [PubMed] [Google Scholar]
- 16.Martinez J. C., Puc M. M., Quiros R. M. Esophageal stenting in the setting of malignancy. ISRN Gastroenterology. 2011;2011:9. doi: 10.5402/2011/719575.719575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang D. H., Hwang E., Meisenbach L. M., Kim M. P., Chan E. Y., Khaitan P. G. Clinical outcomes following self-expanding metal stent placement for esophageal salvage. The Journal of Thoracic and Cardiovascular Surgery. 2017;154(3):1145–1150. doi: 10.1016/j.jtcvs.2017.03.051. [DOI] [PubMed] [Google Scholar]
- 18.So H., Ahn J. Y., Han S., et al. Efficacy and safety of fully covered self-expanding metal stents for malignant esophageal obstruction. Digestive Diseases and Sciences. 2018;63(1):234–241. doi: 10.1007/s10620-017-4839-9. [DOI] [PubMed] [Google Scholar]
- 19.Vanbiervliet G., Filippi J., Karimdjee B. S., et al. The role of clips in preventing migration of fully covered metallic esophageal stents: A pilot comparative study. Surgical Endoscopy. 2012;26(1):53–59. doi: 10.1007/s00464-011-1827-6. [DOI] [PubMed] [Google Scholar]
- 20.González-Haba M., Ferguson M. K., Gelrud A. Spontaneous esophageal perforation (Boerhaave syndrome) successfully treated with an over-the-scope clip and fully covered metal stent. Gastrointestinal Endoscopy. 2016;83(3):p. 650. doi: 10.1016/j.gie.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 21.Yang J., Siddiqui A. A., Kowalski T. E., et al. Esophageal stent fixation with endoscopic suturing device improves clinical outcomes and reduces complications in patients with locally advanced esophageal cancer prior to neoadjuvant therapy: a large multicenter experience. Surgical Endoscopy. 2017;31(3):1414–1419. doi: 10.1007/s00464-016-5131-3. [DOI] [PubMed] [Google Scholar]
- 22.Jang S., Parsi M., Collins J., Vargo J. Predictors of esophageal self-expandable metal stent migration: An academic center study. Gastrointestinal Intervention. 2016;5(1):72–79. doi: 10.18528/gii150018. [DOI] [Google Scholar]
- 23.Tanaka T., Takahashi M., Nitta N., et al. Newly developed biodegradable stents for benign gastrointestinal tract stenoses: a preliminary clinical trial. Digestion. 2007;74(3-4):199–205. doi: 10.1159/000100504. [DOI] [PubMed] [Google Scholar]
- 24.Sze D. Y., Dake M. D. Delayed complications after esophageal stent placement for treatment of malignant esophageal obstructions and esophagorespiratory fistulas. Journal of Vascular and Interventional Radiology. 2001;12(4):465–474. doi: 10.1016/S1051-0443(07)61886-7. [DOI] [PubMed] [Google Scholar]
- 25.Farrugia M., Morgan R. A., Latham J. A., Glynos M., Mason R. C., Adam A. Perforation of the esophagus secondary to insertion of covered wallstent endoprostheses. CardioVascular and Interventional Radiology. 1997;20(6):470–472. doi: 10.1007/s002709900196. [DOI] [PubMed] [Google Scholar]
- 26.Fiorelli A., Esposito G., Pedicelli I., Reginelli A., Esposito P., Santini M. Large tracheobronchial fistula due to esophageal stent migration: Let it be! Asian Cardiovascular and Thoracic Annals. 2015;23(9):1106–1109. doi: 10.1177/0218492315587816. [DOI] [PubMed] [Google Scholar]
- 27.A. Siddiqui A., Loren D., Dudnick R., Kowalski T. Expandable polyester silicon-covered stent for malignant esophageal structures before neoadjuvant chemoradiation: A pilot study. Digestive Diseases and Sciences. 2007;52(3):823–829. doi: 10.1007/s10620-006-9513-6. [DOI] [PubMed] [Google Scholar]
- 28.Verschuur E. M. L., Repici A., Kuipers E. J., Steyerberg E. W., Siersema P. D. New design esophageal stents for the palliation of dysphagia from esophageal or gastric cardia cancer: A randomized trial. American Journal of Gastroenterology. 2008;103(2):304–312. doi: 10.1111/j.1572-0241.2007.01542.x. [DOI] [PubMed] [Google Scholar]
- 29.Martin R. C. G., Woodall C., Duvall R., Scoggins C. R. The use of self-expanding silicone stents in esophagectomy strictures: less cost and more efficiency. The Annals of Thoracic Surgery. 2008;86(2):436–440. doi: 10.1016/j.athoracsur.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 30.Ham Y. H., Kim G. H. Plastic and biodegradable stents for complex and refractory benign esophageal strictures. Clinical Endoscopy. 2014;47(4):295–300. doi: 10.5946/ce.2014.47.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holm A. N., de la Mora Levy J. G., Gostout C. J., Topazian M. D., Baron T. H. Self-expanding plastic stents in treatment of benign esophageal conditions. Gastrointestinal Endoscopy. 2008;67(1):20–25. doi: 10.1016/j.gie.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 32.Repici A., Hassan C., Sharma P., Conio M., Siersema P. Systematic review: the role of self-expanding plastic stents for benign oesophageal strictures. Alimentary Pharmacology & Therapeutics. 2010;31(12):1268–1275. doi: 10.1111/j.1365-2036.2010.04301.x. [DOI] [PubMed] [Google Scholar]
- 33.Szegedi L., Gál I., Kósa I., Kiss G. G. Palliative treatment of esophageal carcinoma with self-expanding plastic stents: A report on 69 cases. European Journal of Gastroenterology & Hepatology. 2006;18(11):1197–1201. doi: 10.1097/01.meg.0000236886.67085.2e. [DOI] [PubMed] [Google Scholar]
- 34.Conio M., Repici A., Battaglia G., et al. A randomized prospective comparison of self-expandable plastic stents and partially covered self-expandable metal stents in the palliation of malignant esophageal dysphagia. American Journal of Gastroenterology. 2007;102(12):2667–2677. doi: 10.1111/j.1572-0241.2007.01565.x. [DOI] [PubMed] [Google Scholar]
- 35.Stivaros S. M., Williams L. R., Senger C., Wilbraham L., Laasch H.-U. Woven polydioxanone biodegradable stents: A new treatment option for benign and malignant oesophageal strictures. European Radiology. 2010;20(5):1069–1072. doi: 10.1007/s00330-009-1662-5. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Y., Yang K., Cheng R., et al. The current status of biodegradable stent to treat benign luminal disease. Materials Today. 2017;20(9):516–529. doi: 10.1016/j.mattod.2017.05.002. [DOI] [Google Scholar]
- 37.Saito Y., Tanaka T., Andoh A., et al. Usefulness of biodegradable stents constructed of poly-l-lactic acid monofilaments in patients with benign esophageal stenosis. World Journal of Gastroenterology. 2007;13(29):3977–3980. doi: 10.3748/wjg.v13.i29.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito Y., Tanaka T., Andoh A., et al. Novel biodegradable stents for benign esophageal strictures following endoscopic submucosal dissection. Digestive Diseases and Sciences. 2008;53(2):330–333. doi: 10.1007/s10620-007-9873-6. [DOI] [PubMed] [Google Scholar]
- 39.van Boeckel P. G. A., Vleggaar F. P., Siersema P. D. A comparison of temporary self- expanding plastic and biodegradable stents for refractory benign esophageal strictures. Clinical Gastroenterology and Hepatology. 2011;9(8):653–659. doi: 10.1016/j.cgh.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Repici A., Vleggaar F. P., Hassan C., et al. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: the BEST (Biodegradable Esophageal Stent) study. Gastrointestinal Endoscopy. 2010;72(5):927–934. doi: 10.1016/j.gie.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 41.Nogales Rincon O., Huerta Madrigal A., Merino Rodriguez B., Gonzalez Asanza C., Cos Arregui E., Menchen Fernandez-Pacheco P. Esophageal obstruction due to a collapsed biodegradable esophageal stent. Endoscopy. 2011;43(2):E189–E190. doi: 10.1055/s-0030-1256324. [DOI] [PubMed] [Google Scholar]
- 42.Yuan T., Yu J., Cao J., et al. Fabrication of a delaying biodegradable magnesium alloy-based esophageal stent via coating elastic polymer. Materials. 2016;9(5):p. 384. doi: 10.3390/ma9050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan T., Zheng R., Yu J., et al. Fabrication and evaluation of polymer-based esophageal stents for benign esophagus stricture insertion. RSC Advances. 2016;6(20):16891–16898. doi: 10.1039/C5RA23763G. [DOI] [Google Scholar]
- 44.Yang K., Ling C., Yuan T., Zhu Y., Cheng Y., Cui W. Polymeric biodegradable stent insertion in the esophagus. Polymer. 2016;8(5):p. 158. doi: 10.3390/polym8050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imaz-Iglesia I., García-Pérez S., Nachtnebel A., et al. Biodegradable stents for the treatment of refractory or recurrent benign esophageal stenosis. Expert Review of Medical Devices. 2016;13(6):583–599. doi: 10.1080/17434440.2016.1184967. [DOI] [PubMed] [Google Scholar]
- 46.Xu J., Ong H. X., Traini D., Byrom M., Williamson J., Young P. M. The utility of 3D-printed airway stents to improve treatment strategies for central airway obstructions. Drug Development and Industrial Pharmacy. 2018;45(1):1–10. doi: 10.1080/03639045.2018.1522325. [DOI] [PubMed] [Google Scholar]
- 47.Peck R., Wattam J. Fracture of Memotherm metallic stents in the biliary tract. CardioVascular and Interventional Radiology. 2000;23(1):55–56. doi: 10.1007/s002709910008. [DOI] [PubMed] [Google Scholar]
- 48.Laasch H.-U., Marriott A., Wilbraham L., Tunnah S., England R. E., Martin D. F. Effectiveness of open versus antireflux stents for palliation of distal esophageal carcinoma and prevention of symptomatic gastroesophageal reflux. Radiology. 2002;225(2):359–365. doi: 10.1148/radiol.2252011763. [DOI] [PubMed] [Google Scholar]
- 49.Lin M., Firoozi N., Tsai C., Wallace M. B., Kang Y. 3D-printed flexible polymer stents for potential applications in inoperable esophageal malignancies. Acta Biomaterialia. 2019;83:119–129. doi: 10.1016/j.actbio.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 50.Lee D. K., Kim H. S., Kim K.-S., et al. The effect on porcine bile duct of a metallic stent covered with a paclitaxel-incorporated membrane. Gastrointestinal Endoscopy. 2005;61(2):296–301. doi: 10.1016/S0016-5107(04)02570-2. [DOI] [PubMed] [Google Scholar]
- 51.Tokar J. L., Banerjee S., Barth B. A., et al. Drug-eluting/biodegradable stents. Gastrointestinal Endoscopy. 2011;74(5):954–958. doi: 10.1016/j.gie.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 52.Shaikh M., Choudhury N. R., Knott R., Garg S. Engineering stent based delivery system for esophageal cancer using docetaxel. Molecular Pharmaceutics. 2015;12(7):2305–2317. doi: 10.1021/mp500851u. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y., Gao Y., Chen J., et al. Effect of a paclitaxel-eluting metallic stent on rabbit esophagus. Experimental and Therapeutic Medicine. 2016;12(5):2928–2936. doi: 10.3892/etm.2016.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.López De Cicco R., Watson J. C., Bassi D. E., Litwin S., Klein-Szanto A. J. Simultaneous expression of furin and vascular endothelial growth factor in human oral tongue squamous cell carcinoma progression. Clinical Cancer Research. 2004;10(13):4480–4488. doi: 10.1158/1078-0432.CCR-03-0670. [DOI] [PubMed] [Google Scholar]
- 55.Rowinsky E. K., Donehower R. C. Paclitaxel (taxol) The New England Journal of Medicine. 1995;332(15):1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 56.Guo Q., Guo S., Wang Z. A type of esophageal stent coating composed of one 5-fluorouracil-containing EVA layer and one drug-free protective layer: In vitro release, permeation and mechanical properties. Journal of Controlled Release. 2007;118(3):318–324. doi: 10.1016/j.jconrel.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 57.Guo S.-R., Wang Z.-M., Zhang Y.-Q., et al. In vivo evaluation of 5-fluorouracil-containing self-expandable nitinol stent in rabbits: Efficiency in long-term local drug delivery. Journal of Pharmaceutical Sciences. 2010;99(7):3009–3018. doi: 10.1002/jps.22066. [DOI] [PubMed] [Google Scholar]
- 58.Lei L., Liu X., Guo S., Tang M., Cheng L., Tian L. 5-Fluorouracil-loaded multilayered films for drug controlled releasing stent application: Drug release, microstructure, and ex vivo permeation behaviors. Journal of Controlled Release. 2010;146(1):45–53. doi: 10.1016/j.jconrel.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z., Liu J., Wu K., et al. Nitinol stents loaded with a high dose of antitumor 5-fluorouracil or paclitaxel: Esophageal tissue responses in a porcine model. Gastrointestinal Endoscopy. 2015;82(1):153.e151–160.e151. doi: 10.1016/j.gie.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 60.Liu J., Wang Z., Wu K., et al. Paclitaxel or 5-fluorouracil/esophageal stent combinations as a novel approach for the treatment of esophageal cancer. Biomaterials. 2015;53:592–599. doi: 10.1016/j.biomaterials.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 61.Rowinsky E. K., Eisenhauer E. A., Chaudhry V., Arbuck S. G., Donehower R. C. Clinical toxicities encountered with paclitaxel (Taxol) Seminars in Oncology. 1993;20(4) supplement 3:1–15. [PubMed] [Google Scholar]
- 62.Marupudi N. I., Han J. E., Li K. W., Renard V. M., Tyler B. M., Brem H. Paclitaxel: A review of adverse toxicities and novel delivery strategies. Expert Opinion on Drug Safety. 2007;6(5):609–621. doi: 10.1517/14740338.6.5.609. [DOI] [PubMed] [Google Scholar]
- 63.Gebrail R., Absah I. Successful use of esophageal stent placement to treat a postoperative esophageal stricture in a toddler. ACG Case Reports Journal. 2014;2(1):61–63. doi: 10.14309/crj.2014.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bona D., Laface L., Bonavina L., et al. Covered nitinol stents for the treatment of esophageal strictures and leaks. World Journal of Gastroenterology. 2010;16(18):2260–2264. doi: 10.3748/wjg.v16.i18.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verschuur E. M. L., Homs M. Y. V., Steyerberg E. W., et al. A new esophageal stent design (Niti-S stent) for the prevention of migration: A prospective study in 42 patients. Gastrointestinal Endoscopy. 2006;63(1):134–140. doi: 10.1016/j.gie.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 66.Uitdehaag M. J., Siersema P. D., Spaander M. C. W., et al. A new fully covered stent with antimigration properties for the palliation of malignant dysphagia: a prospective cohort study. Gastrointestinal Endoscopy. 2010;71(3):600–605. doi: 10.1016/j.gie.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 67.Doosti-Irani A., Mansournia M. A., Rahimi-Foroushani A., Haddad P., Holakouie-Naieni K. Complications of stent placement in patients with esophageal cancer: A systematic review and network meta-analysis. PLoS ONE. 2017;12(10) doi: 10.1371/journal.pone.0184784.e0184784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim S.-A., Lee E. K., Kuh H.-J. Co-culture of 3D tumor spheroids with fibroblasts as a model for epithelial-mesenchymal transition in vitro. Experimental Cell Research. 2015;335(2):187–196. doi: 10.1016/j.yexcr.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 69.Rajcevic U., Knol J. C., Piersma S., et al. Colorectal cancer derived organotypic spheroids maintain essential tissue characteristics but adapt their metabolism in culture. Proteome Science. 2014;12(1):p. 39. doi: 10.1186/1477-5956-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Myungjin Lee J., Mhawech-Fauceglia P., Lee N., et al. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Laboratory Investigation. 2013;93(5):528–542. doi: 10.1038/labinvest.2013.41. [DOI] [PubMed] [Google Scholar]
- 71.Larmour L. I., Jobling T. W., Gargett C. E. A review of current animal models for the study of cervical dysplasia and cervical carcinoma. International Journal of Gynecological Cancer. 2015;25(8):1345–1352. doi: 10.1097/IGC.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 72.Alley M. C., Scudiero D. A., Monks A., et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Research. 1988;48(3):589–601. [PubMed] [Google Scholar]
- 73.Monks A., Scudiero D., Skehan P., et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. Journal of the National Cancer Institute. 1991;83(11):757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 74.Sharma P., Kozarek R., Inadomi J. M. Role of esophageal stents in benign and malignant diseases. American Journal of Gastroenterology. 2010;105(2):258–273. doi: 10.1038/ajg.2009.684. [DOI] [PubMed] [Google Scholar]
- 75.Padrón J. M., Peters G. J. Cytotoxicity of sphingoid marine compound analogs in mono- and multilayered solid tumor cell cultures. Investigational New Drugs. 2006;24(3):195–202. doi: 10.1007/s10637-005-3691-5. [DOI] [PubMed] [Google Scholar]
- 76.Weaver V. M., Lelièvre S., Lakins J. N., et al. β4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2(3):205–216. doi: 10.1016/S1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fischbach C., Chen R., Matsumoto T., et al. Engineering tumors with 3D scaffolds. Nature Methods. 2007;4(10):855–860. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 78.Kim E.-Y., Park Y. S., Shin J. H., et al. The effectiveness of erythromycin in reducing stent-related tissue hyperplasia: An experimental study with a rat esophageal model. Acta Radiologica. 2012;53(8):868–873. doi: 10.1258/ar.2012.120351. [DOI] [PubMed] [Google Scholar]
- 79.Kim E.-Y., Shin J. H., Jung Y. Y., Shin D.-H., Song H.-Y. A rat esophageal model to investigate stent-induced tissue hyperplasia. Journal of Vascular and Interventional Radiology. 2010;21(8):1287–1291. doi: 10.1016/j.jvir.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 80.Kapisiz A., Karabulut R., Sonmez K., et al. Effect of stent placement, balloon or cutting balloon dilatation on stricture formation after caustic esophageal burn in rats. European Journal of Pediatric Surgery. 2011;21(4):258–262. doi: 10.1055/s-0031-1275700. [DOI] [PubMed] [Google Scholar]
- 81.Huang J., Shuang J., Xiong G., et al. Establishing a rabbit model of malignant esophagostenosis using the endoscopic implantation technique for studies on stent innovation. Journal of Translational Medicine. 2014;12(1):p. 40. doi: 10.1186/1479-5876-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Endo M., Kaminou T., Ohuchi Y., et al. Development of a new hanging-type esophageal stent for preventing migration: A preliminary study in an animal model of esophagotracheal fistula. CardioVascular and Interventional Radiology. 2012;35(5):1188–1194. doi: 10.1007/s00270-011-0240-9. [DOI] [PubMed] [Google Scholar]
- 83.Baron T. H., Burgart L. J., Pochron N. L. An internally covered (lined) self-expanding metal esophageal stent: tissue response in a porcine model. Gastrointestinal Endoscopy. 2006;64(2):263–267. doi: 10.1016/j.gie.2006.03.936. [DOI] [PubMed] [Google Scholar]
- 84.Shaikh M., Zhang H., Wang H., et al. In vitro and in vivo assessment of docetaxel formulation developed for esophageal stents. AAPS PharmSciTech. 2017;18(1):130–137. doi: 10.1208/s12249-016-0501-7. [DOI] [PubMed] [Google Scholar]
- 85.Cheon Y. K., Lee T. Y., Sung I. K., Shim C. S. Clinical feasibility of a new through-the-scope fully covered esophageal self-expandable metallic stent: An in vivo animal study. Digestive Endoscopy. 2014;26(1):32–36. doi: 10.1111/den.12056. [DOI] [PubMed] [Google Scholar]
- 86.Zhu Y. Q., Cui W. G., Cheng Y. S., et al. Biodegradable rapamycin-eluting nano-fiber membrane-covered metal stent placement to reduce fibroblast proliferation in experimental stricture in a canine model. Endoscopy. 2013;45(6):458–468. doi: 10.1055/s-0032-1326399. [DOI] [PubMed] [Google Scholar]
- 87.Jeon S. R., Eun S. H., Shim C. S., et al. Effect of drug-eluting metal stents in benign esophageal stricture: An in vivo animal study. Endoscopy. 2009;41(5):449–456. doi: 10.1055/s-0029-1214607. [DOI] [PubMed] [Google Scholar]
- 88.Kim E.-Y., Song H.-Y., Kim J. H., et al. IN-1233-eluting covered metallic stent to prevent hyperplasia: Experimental study in a rabbit esophageal model. Radiology. 2013;267(2):396–404. doi: 10.1148/radiol.12120361. [DOI] [PubMed] [Google Scholar]
- 89.Huang J., Zhang Y., Zhong H., et al. Comparison of endoscopic submuscosal implantation vs. surgical intramuscular implantation of VX2 fragments for establishing a rabbit esophageal tumor model for mimicking human esophageal squamous carcinoma. PLoS ONE. 2014;9(1) doi: 10.1371/journal.pone.0085326.e85326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo J.-H., Teng G.-J., Zhu G.-Y., He S.-C., Deng G., He J. Self-expandable stent loaded with 125I seeds: Feasibility and safety in a rabbit model. European Journal of Radiology. 2007;61(2):356–361. doi: 10.1016/j.ejrad.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 91.Ji J.-S., Lee B.-I., Kim H.-K., et al. Antimigration property of a newly designed covered metal stent for esophageal stricture: An in vivo animal study. Gastrointestinal Endoscopy. 2011;74(1):148–153. doi: 10.1016/j.gie.2011.03.1252. [DOI] [PubMed] [Google Scholar]
- 92.Zhou J., Jiang Y., Wang R., et al. Prevention of stricture development after corrosive esophageal burn with a modified esophageal stent in dogs. The Journal of Thoracic and Cardiovascular Surgery. 2008;136(5):1336–1342.e7. doi: 10.1016/j.jtcvs.2008.02.086. [DOI] [PubMed] [Google Scholar]
- 93.Jin Z., Wu K., Hou J., Yu K., Shen Y., Guo S. A PTX/nitinol stent combination with temperature-responsive phase-change 1-hexadecanol for magnetocaloric drug delivery: Magnetocaloric drug release and esophagus tissue penetration. Biomaterials. 2018;153:49–58. doi: 10.1016/j.biomaterials.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 94.Won J. H., Lee J. D., Wang H. J., et al. Self-expandable covered metallic esophageal stent impregnated with beta-emitting radionuclide: an experimental study in canine esophagus. International Journal of Radiation Oncology Biology Physics. 2002;53(4):1005–1013. doi: 10.1016/S0360-3016(02)02837-7. [DOI] [PubMed] [Google Scholar]
- 95.Wang L., Yue-Qi Z., Ying-Sheng C., Wen-Guo C., Ni-Wei C. Temporary placement of a paclitaxel or rapamycin-eluting stent is effective to reduce stenting induced inflammatory reaction and scaring in benign cardia stricture models. The Turkish Journal of Gastroenterology. 2015;25(1) supplement 1:69–74. doi: 10.5152/tjg.2014.6611. [DOI] [PubMed] [Google Scholar]
- 96.Liu J., Shang L., Liu J., Qin C. A novel biodegradable esophageal stent: Results from mechanical and animal experiments. American Journal of Translational Research. 2016;8(2):1108–1114. [PMC free article] [PubMed] [Google Scholar]
- 97.Liu J., Shang L., Liu J.-Y., Qin C.-Y. Newly designed 'pieced' stent in a rabbit model of benign esophageal stricture. World Journal of Gastroenterology. 2015;21(28):8629–8635. doi: 10.3748/wjg.v21.i28.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cannistrà M., Ruggiero M., Grande R., et al. The impact of BMI on early colorectal neoplastic lesions and the role of endoscopic diagnosis:. An Italian observational study. International Journal of Surgery. 2016;33:S71–S75. doi: 10.1016/j.ijsu.2016.05.049. [DOI] [PubMed] [Google Scholar]
- 99.Lam N., Weisse C., Berent A., et al. Esophageal stenting for treatment of refractory benign esophageal strictures in dogs. Journal of Veterinary Internal Medicine. 2013;27(5):1064–1070. doi: 10.1111/jvim.12132. [DOI] [PubMed] [Google Scholar]
- 100.Pauli E. M., Schomisch S. J., Furlan J. P., et al. Biodegradable esophageal stent placement does not prevent high-grade stricture formation after circumferential mucosal resection in a porcine model. Surgical Endoscopy. 2012;26(12):3500–3508. doi: 10.1007/s00464-012-2373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nonaka K., Miyazawa M., Aikawa M., et al. Experimental trial for perforation caused by esophageal endoscopic submucosal dissection using a biodegradable polymer stent in an animal model. Digestive Endoscopy. 2012;24(4):p. 286. doi: 10.1111/j.1443-1661.2011.01204.x. [DOI] [PubMed] [Google Scholar]
- 102.Yu X., Wang L., Huang M., et al. A shape memory stent of poly(ε-caprolactone-co-dl-lactide) copolymer for potential treatment of esophageal stenosis. Journal of Materials Science: Materials in Medicine. 2012;23(2):581–589. doi: 10.1007/s10856-011-4475-4. [DOI] [PubMed] [Google Scholar]
- 103.Ma L., Huang X., Wang X., et al. Study on the diagnosis of rabbit VX2 esophageal cancer and stent-therapy efficacy based on multiphoton microscopy. Scanning. 2015;37(2):152–157. doi: 10.1002/sca.21192. [DOI] [PubMed] [Google Scholar]