Abstract
Background
Extensive floodwater damage following hurricane Harvey raised concerns of excess mold infections in immunocompromised patients. This study sought to evaluate the impact of hurricane Harvey on the incidence of culture-positive invasive mold infections (cIMIs) in patients treated at MD Anderson Cancer Center (MDACC; Houston, TX).
Methods
All mold-positive culture results in the Microbiology Laboratory at MDACC in a 12-month period before and after hurricane Harvey were reviewed. cIMI cases were defined according to European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group criteria. Rates and causative agents of cIMIs pre- and post-Harvey were compared. In addition, we evaluated institution-wide trends in the use of systemically administered mold-active antifungal agents by segmented regression analysis.
Results
Inpatient cIMI rates per 1000 patient-days were comparable in the pre- and post-Harvey observation period (0.17 vs 0.21, P = .36). During both surveillance periods, the vast majority of cIMI cases were due to Aspergillus spp., Fusarium spp., or Mucorales. No emergence of unusual mold infections was seen, and the relative frequencies of mold genera recovered from cultures at the MDACC Microbiology Laboratory remained largely unaltered. The overall use of posaconazole was significantly higher in the post-Harvey period and the use of both voriconazole and liposomal amphotericin B began to increase significantly immediately after Harvey.
Conclusions
Our monocentric study employing stringent culture-based definitions of mold infections found no excess cases of IMIs in MDACC’s immunosuppressed patient population in the aftermath of a major flooding event. Increased use of some mold-active antifungals in the aftermath of hurricane Harvey was observed institutionally.
Keywords: hurricane Harvey, invasive mold infections
In August 2017, hurricane Harvey generated a 4-day downpour dumping >270 trillion gallons of rain onto Texas [1]. In the Houston metropolitan area, where one-third of the land mass was submerged [2], approximately 100 000 homes were destroyed or damaged by the hurricane and flooding. Extensive water damage and the area’s hot and humid climate created ideal conditions for excessive mold growth, potentially leading to massive unquantifiable mold exposure [3]. Mold-related diseases after geo-meteorological disasters range from allergic disease after spore inhalation to invasive soft tissue and pulmonary infections, with the potential for subsequent dissemination, mainly in immunocompromised hosts [3].
Houston is home to the Texas Medical Center, the world’s largest medical complex, including the University of Texas MD Anderson Cancer Center (MDACC), which provides care to one of the largest immunocompromised patient populations in the world. Despite recommendations from the Centers for Disease Control and Prevention (CDC) to avoid mold exposure during renovation [4], a substantial number of immunosuppressed patients were exposed to mold and water-damaged areas or were actively engaged in home repair after hurricane Harvey [5]. The majority of exposed patients wore no or incomplete personal protective equipment [5]. We therefore sought to assess the impact of hurricane Harvey on the rates and causative agents of culture-positive invasive mold infections (cIMIs) in patients treated at MDACC.
METHODS
cIMI Surveillance
We reviewed the records of MDACC’s infection control surveillance database. All mold-positive culture results in the Microbiology Laboratory at MDACC during a 12-month period before and after hurricane Harvey were evaluated. Among culture-positive results, cIMI cases were defined according to European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) criteria [6]. Patients were categorized by their underlying malignancies into solid tumor, leukemia, lymphoma/myeloma, or hematopoietic stem cell transplantation (HCT) recipient cohorts. Pre- and post-Harvey cIMI incidence rates were compared using the 2-tailed mid P value exact test. A P value of <.05 was considered significant.
Determination of Areas Affected by Hurricane Harvey
The ZIP codes of cIMI patients were compared with the Federal Emergency Management Agency (FEMA) map of counties that have been designated as public ± individual assistance areas after hurricane Harvey. Counties designated public assistance areas at a minimum were considered affected counties.
Antifungal Prophylaxis and Therapy
No institution-wide changes in indications for antifungal prophylaxis were made in the period immediately preceding or following hurricane Harvey. Generally, patients at high risk for invasive mold infections (eg, patients with hematological malignancies and anticipated prolonged neutropenia or HCT recipients with graft-vs-host disease requiring high doses of corticosteroids) received mold-active azole prophylaxis, most commonly posaconazole. Front-line treatment of suspected invasive mold infection generally consisted of liposomal amphotericin B for patients receiving mold-active prophylaxis, whereas treatment practices varied for patients not on prophylaxis.
To evaluate potential changes in antifungal use in the aftermath of hurricane Harvey, segmented regression analysis [7] was performed, with September 1, 2017, as the dividing point. Days of therapy per 1000 patient-days were retrieved from pharmacy administration records for systemic formulations of the 3 widely used mold-active azoles and liposomal amphotericin B. Data were assessed on a monthly basis for the 12-month periods before and after hurricane Harvey. Ordinary least squares (OLS) regression lines were calculated for both periods, allowing for the assessment of changes in both regression slope (the rate at which drug use was changing over time) and regression intercept (the predicted use as of September 1, 2017). The changes in regression β coefficient (slope) and intercept were calculated using a linear combination of parameter estimates. All calculations were performed using Stata v14.1 (StataCorp LP, College Station, TX).
Ethics Statement
As the study was solely based on cumulative routine surveillance and administrative pharmacy data, it was exempt from institutional review board approval.
RESULTS
One hundred eighty-eight and 195 cultures positive for mold pathogens were seen in the MDACC Microbiology Laboratory in the 12-month surveillance periods pre- and post-hurricane Harvey, respectively. The amount of fungal cultures ordered institution-wide and the rates of mold-positive cultures were essentially identical in both surveillance periods (n = 8698, 2.16% pre-Harvey; n = 8696, 2.24% post-Harvey). With the exception of Lomentospora/Scedosporium (6% vs 0%), no major differences were seen in the relative frequencies of mold genera recovered from fungal cultures (Table 1).
Table 1.
Molds Recovered From Cultures at the MDACC Microbiology Laboratory and Pathogens Associated With cIMI Cases in the 12-Month Periods Before and After Hurricane Harvey
| Pre-Harvey | Post-Harvey | |||||||
|---|---|---|---|---|---|---|---|---|
| Positive Cultures | cIMI Cases | Positive Cultures | cIMI Cases | |||||
| No. | % | No. | % | No. | % | No. | % | |
| Aspergillus | 65 | 35 | 21 | 62 | 81 | 42 | 29 | 67 |
| Aspergillus + Fusarium | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| Fusarium | 21 | 11 | 7 | 21 | 19 | 10 | 9 | 21 |
| Rhizopus | 3 | 2 | 3 | 9 | 3 | 2 | 3 | 7 |
| Rhizomucor | 1 | <1 | 1 | 3 | 0 | 0 | 0 | 0 |
| Syncephalastrum | 1 | <1 | 1 | 3 | 1 | <1 | 1 | 2 |
| Conidiobolus | 0 | 0 | 0 | 0 | 1 | <1 | 1 | 2 |
| Lomentospora | 11 | 6 | 1 | 3 | 0 | 0 | 0 | 0 |
| Coccidioides | 1 | <1 | 0 | 0 | 1 | <1 | 0 | 0 |
| Dermatophytes | 2 | 1 | 0 | 0 | 4 | 2 | 0 | 0 |
| Penicillium | 24 | 13 | 0 | 0 | 26 | 13 | 0 | 0 |
| Other molds | 37a | 20 | 0 | 0 | 36b | 18 | 0 | 0 |
| Unidentified (eg, sterile hyphae) | 22 | 12 | 0 | 0 | 21 | 11 | 0 | 0 |
| Total | 188 | 100 | 34 | 100 | 195 | 100 | 43 | 100 |
Abbreviations: cIMI, culture-positive invasive mold infection; MDACC, MD Anderson Cancer Center.
aOne Bipolaris, 1 Chaetomium, 1 Chrysosporium, 2 Cladosporium, 7 Curvularia, 2 Setosphaeria, 2 Fonsecaea, 2 Geotrichum, 1 Hormographiella, 7 Malbranchea, 1 Nigrospora, 4 Paecilomyces, 1 Phialemonium, 1 Scopulariopsis, 1 Stemphylium, 3 Trichoderma.
bTwo Bipolaris, 1 Chrysosporium, 1 Cladosporium, 7 Curvularia, 1 Epicoccum, 1 Exserohilum, 3 Geotrichum, 1 Geotrichum + Malbranchea, 4 Malbranchea, 2 Nigrospora, 7 Paecilomyces, 1 Phaeoacremonium, 4 Scopulariopsis, 1 Verticillium.
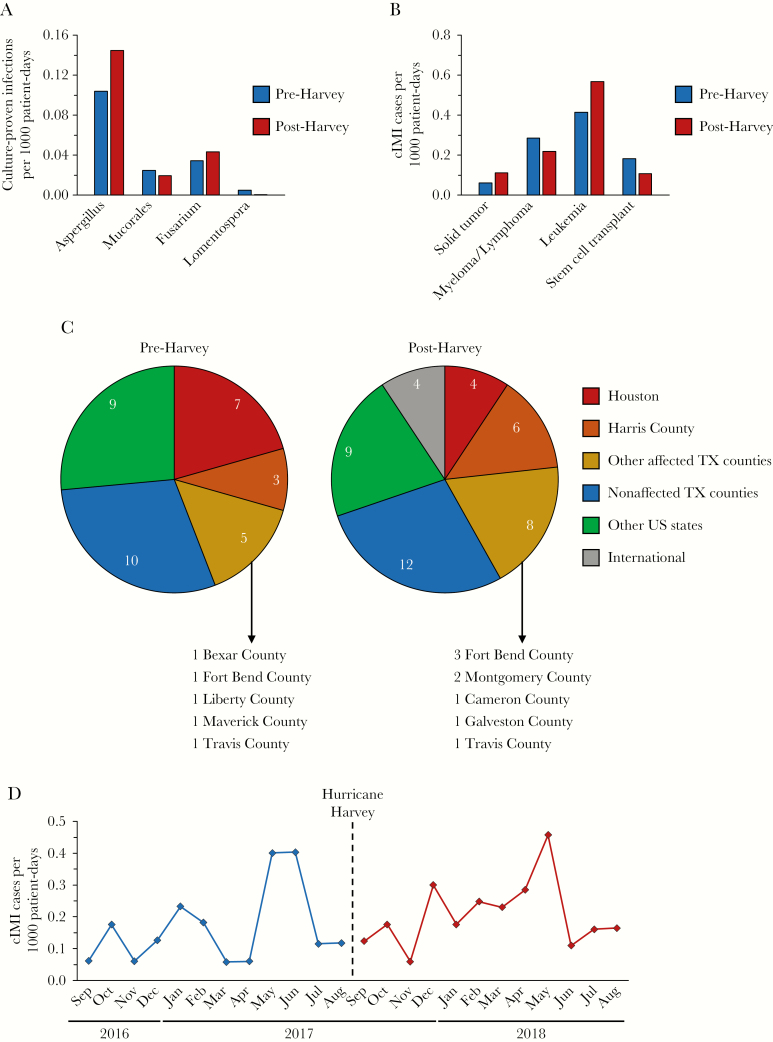
Thirty-four and 43 cIMI cases were identified pre- (202 365 patient-days) and post-Harvey (207 373 patient-days), respectively, resulting in cIMI rates of 0.17 and 0.21 per 1000 patient-days (P = .36). Comparing the causative agents, the vast majority of cIMI cases in both periods were due to either Aspergillus spp. (62% vs 67%), Fusarium spp., or Mucorales/Entomophthorales (Table 1), with no differences in the relative frequencies of these molds. The 35% post-Harvey increase in culture-proven invasive aspergillosis was not statistically significant (P = .30) (Figure 1A). Only 1 invasive infection due to Lomentospora prolificans was seen (pre-Harvey). There were no inpatient cIMI cases due to dimorphic fungi in either surveillance period (Table 1).
Figure 1.
Comparison of pre- and post-Harvey culture-positive invasive mold infection (cIMI) rates, causative agents, and places of residence of cIMI patients at MD Anderson Cancer Center (MDACC). A, Rates of cIMI cases per 1000 patient-days pre- and post-Harvey by causative agent. B, Pre- and post-Harvey cIMI rates at MDACC depending on the patients’ underlying disease. C, Place of residence of patients with cIMIs. D, Monthly cIMI rates per 1,000 patient-days at MDACC in a 12-month period before and after hurricane Harvey. Data presented in (A–C) were analyzed using the 2-tailed mid P value exact test. All P values were >.05 (not shown).
When stratified by underlying malignancy (Figure 1B), the highest cIMI rates were found in leukemia patients, with a nonsignificant increase from 0.42 to 0.57 per 1000 patient-days (P = .35), followed by lymphoma patients (0.29 pre- vs 0.22 post-Harvey, P = .67). The cIMI rate in solid tumor patients, who do not routinely receive antifungal prophylaxis, nearly doubled post-Harvey (0.06 vs 0.11), although this finding was not statistically significant (P = .21).
Given the vast catchment area of MDACC’s patient population, we superimposed the Federal Emergency Management Agency (FEMA) public assistance map with the ZIP codes of cIMI patients. Comparing the places of residence of pre- and post-Harvey cIMI patients, no shift or excess cIMI cases post-Harvey in patients from the Houston metropolitan area was found (Figure 1C). Taken together, patients from affected areas (Houston, Harris County, and other affected counties in Texas) accounted for 44% of the pre- and 42% of the post-Harvey cIMI cases, respectively. Importantly, 55% of the post-Harvey patients with solid tumors and cIMIs were from unaffected areas, as were 62% of such patients with leukemia, supporting the nonsignificance of the increased post-Harvey cIMI rates in these populations.
Finally, we compared monthly cIMI rates and antifungal drug use pre- and post-Harvey. No significant incline in cIMI cases was seen in temporal proximity to the hurricane (Figure 1D). In the 12-month period preceding hurricane Harvey, the use of both liposomal amphotericin B and voriconazole was declining (negative slope), but it subsequently increased in the post-Harvey period (Supplementary Figure 1, Supplementary Table 1). Although no significant increase in the slope for posaconazole was observed, the overall use (indicated by the intercept) was significantly greater after hurricane Harvey. However, this trend appeared to start in July 2017, thus preceding the hurricane. No changes in either the slope or intercept of isavuconazonium sulfate were found.
Discussion
Changes in climatic conditions affect the prevalence or virulence of pathogens and host susceptibility [8–10]. There is increasing evidence that natural disasters can cause large-scale alterations and disruptions of fungal habitats [3, 11–13], and the impact of flooding and water-damaged housing on the incidence of IMI in immunocompromised patients has been debated [3]. As hurricane Harvey caused unprecedented devastation and flooding in the Houston metropolitan area, an epidemiologic assistance investigation (Epi-Aid) was requested by Texas state officials to document trends in post-Harvey IMI and support guidance on cleanup activities. Despite concerns of extensive mold exposure in water-damaged homes or during repair activities [5], our study found no evidence of significantly increased cIMI rates or the emergence of unusual molds in MDACC’s immunocompromised patients in the first 12 months after hurricane Harvey. The overall cIMI rates of 0.17 and 0.21 per 1000 patient-days were within the institution’s usual prevalence range (data not shown). Although the case numbers were small and the observation period was rather short, we found no significant seasonal variation of cIMIs, consistent with prior data documenting the lack of a seasonally varying incidence of aspergillosis in our allogeneic HCT recipients [9].
Our observations match reports after hurricanes Katrina and Rita in New Orleans in that no increased IMI incidence was observed in immunocompromised patient populations [3, 12]. It was hypothesized that alternate housing and time-limited exposure scenarios contributed to the low incidence of IMI cases post-Katrina [12]. By contrast, post-Harvey CDC data suggest that a large proportion of surveilled immunocompromised patients stayed at their homes in the affected area during and after the hurricane [5].
Although data are scarce, mold-active prophylaxis has been well documented to prevent invasive disease in high-risk patients following water damage in the hospital setting [14]. Analysis of antifungal use at an institutional level indicated that a general decline in the use of liposomal amphotericin B and voriconazole reversed and turned into significantly increased use following hurricane Harvey. Additionally, an increase in use of posaconazole was seen in the post-Harvey period, but this appeared to have begun 2 months before hurricane Harvey’s landfall. Importantly, drug use data were obtained from administrative pharmacy databases, and, accordingly, it is impossible to know if the observed trends are related to hurricane Harvey or are simply coincidental. Nevertheless, these data suggest that clinicians may have had a lower threshold for the initiation of mold-active antifungals as treatment or prophylaxis in patients at risk in the aftermath of hurricane Harvey.
There are several limitations of our observational data. This study employed culture-based case definitions according to EORTC/MSG criteria, which have a high specificity but limited sensitivity. Though no suspicious alterations in relative frequencies of molds recovered from MDACC patients were seen (Table 1), future research is needed to determine whether there have been excess IMI cases not fitting the traditional diagnostic criteria (eg, biomarker-positive but culture-negative IMIs) or cases of severe pneumonia of unknown pathogen. Additionally, mildly symptomatic or asymptomatic patients were, in all likelihood, not captured by the infection control nosocomial surveillance. The very low rate of autopsies in our institution also raises the possibility of misclassification errors [15]. Although cIMI rates were unaffected post-Harvey, we provide no data regarding the time to cIMI diagnosis or the temporal relationship between exposure and infection. In addition, we did not use patient-level validated questionnaires to determine the intensity of exposure or perform detailed analyses of geo-climatic factors such as environmental spore counts, temperature, and precipitation. Instead, we used a general flood prediction map to categorize whether a patient was in a flooded zone as a broad indicator for possible mold exposure. Similarly, no information on mold-active antifungal prophylaxis of the different patient populations or individual cIMI patients was collected. Moreover, this study is based on data from a single center, and thus our findings may not be applicable to other centers in the Houston area and other types of immunocompromised patient populations (eg, solid organ recipients).
Although our study found no increased post-Harvey cIMI rates at MDACC and no infections due to unusual molds, long-term surveillance programs using a less strict IMI definition would be important to identify clusters of community- or even hospital-acquired IMIs among survivors of hurricane Harvey. For example, although not encountered in our 12-month post-Harvey surveillance period, the dimorphic mold Coccidioides immitis is endemic in Texas [16], proliferates in the environment in wet years, and becomes widely dispersed in dry years [17], emphasizing the importance of long-term surveillance. Finally, studies elucidating the impact of hurricane Harvey on the incidence of noninfectious mold-associated adverse health effects such as asthma are warranted.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
D.P.K. acknowledges the Texas 4000 Distinguished Professorship for Cancer Research. We thank Jing Yiang for statistical support and Mrs. Salli Saxton for administrative support.
Author contributions.D.P.K. conceived and designed the study and contributed to the manuscript. E.C.S., S.W., L.G., and S.L.A. collected data, performed analyses, and contributed to the manuscript. I.I.R. and R.F.C. gave feedback and read the manuscript. All authors approved the final version of the manuscript.
Financial support. This study was supported in part by the National Institutes of Health/National Cancer Institute under award number P30CA016672.
Potential conflicts of interest. D.P.K. reports research support from Astellas Pharma and honoraria for lectures from Merck & Co, Gilead, and United Medical. He has served as a consultant for Astellas Pharma, Cidara, Amplyx, Astellas, and Mayne and has served on the advisory board of Merck & Co. S.L.A. has served on advisory boards for and received research support from Merck & Co. I.I.R. is an inventor of nitroglycerin-based and minocycline-based catheter lock solution technologies with activity against Candida in biofilm, licensed by Novel Anti-Infective Technologies, LLC, and Citius Pharmaceuticals, in which he is a shareholder. He is also a consultant with Pfizer. R.F.C. has received research support from Merck, Chimerix, Shire, Oxford Immunotec, Gilead, Ansun pharmaceuticals, and Pulmotec. He has received honoraria from Merck, Chimerix, Ablynx, ADMA Biologics, Pulmotec, Astellas, Shire, Oxford Immunotec, Shionogie, Janssen, Ansun pharmaceuticals, Achaogen, and Xenex. All remaining authors report no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Harvey’s devastating impact by the numbers. CNN; September 1, 2017. https://www.cnn.com/2017/08/27/us/harvey-impact-by-the-numbers-trnd/index.html. Accessed 4 December 2018. [Google Scholar]
- 2. Cusick D. FEMA approves buyout funds for Houston homes flooded by Harvey. Scientific American; June 7, 2018. https://www.scientificamerican.com/article/fema-approves-buyout-funds-for-houston-homes-flooded-by-harvey/. Accessed 4 December 2018. [Google Scholar]
- 3. Benedict K, Park BJ. Invasive fungal infections after natural disasters. Emerg Infect Dis 2014; 20:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention; Homeowner’s and renter’s guide to mold cleanup after disasters. www.cdc.gov/mold/pdfs/homeowners_and_renters_guide.pdf. Accessed 4 December 2018. [Google Scholar]
- 5. Nancy C, Toda M, Beer K, et al. Hurricane-associated mold exposures among patients at risk of invasive mold infections - Houston, TX, 2017 [poster 127]. Poster presented at: InternationalConference on Emerging Infectious Diseases (ICEID); August 26–29, 2018; Atlanta, GA. [Google Scholar]
- 6. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002; 27:299–309. [DOI] [PubMed] [Google Scholar]
- 8. Garcia-Solache MA, Casadevall A. Global warming will bring new fungal diseases for mammals. MBio 2010; 1:e00061–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Panackal AA, Li H, Kontoyiannis DP, et al. Geoclimatic influences on invasive aspergillosis after hematopoietic stem cell transplantation. Clin Infect Dis 2010; 50:1588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tamerius JD, Comrie AC. Coccidioidomycosis incidence in Arizona predicted by seasonal precipitation. PLoS One 2011; 6:e21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flynn NM, Hoeprich PD, Kawachi MM, et al. An unusual outbreak of windborne coccidioidomycosis. N Engl J Med 1979; 301:358–61. [DOI] [PubMed] [Google Scholar]
- 12. Barbeau DN, Grimsley LF, Whitte LE, et al. Mold exposure and health effects following hurricanes Katrina and Rita. Annu Rev Public Health 2010; 31:165–78. [DOI] [PubMed] [Google Scholar]
- 13. Neblett Fanfair R, Benedict K, Bos J, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med 2012; 367:2214–25. [DOI] [PubMed] [Google Scholar]
- 14. Garner D, Machin K. Investigation and management of an outbreak of mucormycosis in a paediatric oncology unit. J Hosp Infect 2008; 70:53–9. [DOI] [PubMed] [Google Scholar]
- 15. Lewis RE, Cahyame-Zuniga L, Leventakos K, et al. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: a 20-year autopsy study. Mycoses 2013; 56:638–45. [DOI] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention. Coccidioidomycosis - United States, 1991–1992. MMWR Morb Mortal Wkly Rep 1993; 42:21–4. [PubMed] [Google Scholar]
- 17. Comrie AC. Climate factors influencing coccidioidomycosis seasonality and outbreaks. Environ Health Perspect 2005; 113:688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.