Abstract
Background
Detection of mycobacterial lipoarabinomannan antigen in urine has emerged as a potential point-of-care test for diagnosis of tuberculosis. This study aimed to evaluate the accuracy of the lateral flow urine lipoarabinomannan (LF-LAM) assay for diagnosis of active tuberculosis among Thai adults with advanced human immunodeficiency virus (HIV) infection.
Methods
HIV-infected adult patients with CD4 cell counts ≤200/μL and symptoms suggestive of active tuberculosis were prospectively recruited from both inpatient and outpatient settings at Siriraj Hospital and Chonburi Hospital in Thailand during the study period from December 2015 to March 2017. Freshly collected urine samples were applied to the Alere Determine TB LAM Ag test strip using a grade 1 cutoff, according to the manufacturer’s grading system. The diagnostic accuracy of the LF-LAM test was assessed against a microbiological reference standard (definite tuberculosis) or a composite reference standard (definite and probable tuberculosis).
Results
Of the 280 patients who were included, 72 (25.7%) had definite and 65 (23.2%) had probable tuberculosis. Among patients with definite tuberculosis, the LF-LAM test yielded a sensitivity of 75.0% and a specificity of 76.0%. It had the highest sensitivity (90.5%) in HIV-infected patients with CD4 cell counts <50/μL. It yielded a lower sensitivity (61.3%) but a higher specificity (86.0%) when compared with the composite reference standard. Among the 20 patients (14%) with false-positive results, strong band intensity was observed mostly in Mycobacterium avium complex infections. An incremental sensitivity of 11% was observed with use of acid-fast bacilli sputum smear or LF-LAM testing, compared with LF-LAM testing alone.
Conclusions
The LF-LAM test performed well in the diagnosis of active tuberculosis in selected patients with more advanced tuberculosis and coexisting HIV disease.
Keywords: active tuberculosis, diagnosis, HIV-infected adults, lateral flow urine lipoarabinomannan assay, LF-LAM, tuberculosis
Tuberculosis is among the leading causes of disease and death worldwide. In 2017, an estimated 920 000 (9%) of the 10 million new cases of tuberculosis occurred in persons with human immunodeficiency virus (HIV) infection, and an estimated 300 000 persons died of HIV-associated tuberculosis [1]. Confirming a diagnosis of tuberculosis in HIV-infected patients remains a challenge because clinical features can vary according to the patient’s immune status [2, 3]. Furthermore, compared with HIV-uninfected individuals, those with HIV infection have higher rates of negative sputum smear results and/or dissemination involving extrapulmonary sites, making it more difficult to obtain representative samples for microscopy and culture [4, 5].
Mycobacterial culture is the current reference standard for diagnosis of tuberculosis; however, culture is time consuming, and a delay in diagnosis can adversely affect patient outcome [6, 7]. Sputum smear microscopy is simple and inexpensive, but it has poor sensitivity, even when fluorescence stains are used [8]. Nucleic acid amplification testing provides sensitive and specific tuberculosis diagnosis, but enabling its use in limited-resource settings with minimally trained personnel remains a challenge. Similar to the limitations of culture for detecting HIV-associated tuberculosis, molecular assays are limited by the challenges associated with obtaining appropriate specimens from the site of disease, particularly in extrapulmonary tuberculosis. Even though a simpler rapid molecular test (Xpert MTB/RIF assay; Cepheid) is now available, its high cost makes it unaffordable in many limited-resource countries [8].
The lateral flow urine lipoarabinomannan (LF-LAM) assay is a commercially available point-of-care test for active tuberculosis (Alere Determine TB LAM Ag; Alere). The test detects lipoarabinomannan, a 19-kDa lipopolysaccharide major cell-wall component of Mycobacterium tuberculosis [9], which is released into the urine of patients with active tuberculosis disease. The sensitivities and specificities of the LF-LAM test reported from previous studies have varied according to differences in the following factors: study population, CD4 cell count, patient setting (inpatient vs outpatient), reference standard, number and type of specimens used, the positive cutoff point for band intensity, and the type of urine specimen used [10–17]. Most earlier studies were conducted in Africa. To date, few studies [18, 19] have reported the use of urine LF-LAM testing in Southeast Asian population with HIV-tuberculosis coinfection. The accuracy of diagnostic assays should be assessed in different geographic settings and among different populations.
Accordingly, the aim of the current multicenter study was to prospectively evaluate the accuracy of the LF-LAM test for diagnosis of active tuberculosis among Thai adults with advanced HIV infection. The secondary objective was to investigate the period of test positivity after initiation of antituberculosis treatment.
METHODS
Study Sites and Participants
This multicenter prospective cohort study included HIV-infected adult Thai patients with a CD4 cell count ≤200/μL and symptoms suggestive of active tuberculosis who were treated at Siriraj Hospital (Thailand’s largest university-based national tertiary referral center, located in Bangkok) or Chonburi Hospital (a provincial tertiary referral center located in Chonburi Province) during the December 2015 to March 2017 study period. Eligible patients who were treated as outpatients were recruited from the infectious disease clinic at Siriraj Hospital and the tuberculosis or HIV clinic at Chonburi Hospital, were aged ≥18 years, were HIV infected with a CD4 cell count ≤200/μL, and, were suspected of having active tuberculosis based on the presence of fever lasting >2 weeks along with ≥1 of the following: chronic cough, lymphadenopathy, pleural effusion, and weight loss. Patients were excluded if they had been treated for >2 days with ≥2 antituberculosis drugs within 3 months before enrollment.
Included patients were enrolled consecutively, and all who met the study criteria were included. Those unable to provide specimens for mycobacterial culture or urine for LF-LAM testing were excluded. The protocol for this study was approved by the institutional review boards of both Siriraj Hospital and Chonburi Hospital (COA nos. 659/2557 and COA nos. 24/58/o/h2, respectively).
Data Collection
After providing written informed consent, patients underwent a standardized interview, physical examination, chest radiography, and additional routine diagnostic investigations. All patients were required to provide ≥2 samples from sites where tuberculosis involvement was suspected, with ≥1 sputum specimen preferred. Other specimens, such as blood, pleural fluid, cerebrospinal fluid, lymph node, and/or bone marrow, were also obtained if the patient had clinical features suggestive of active infection at the site(s) in question. We did not exclude patients who were unable to provide a sputum specimen. Although a respiratory sample for culture was preferred, it was not required as long as the patient could provide ≥2 samples for culture from the suspected site(s) of infection. Patients with only 1 mycobacterial culture from any site could be included if the result showed positive culture for tuberculosis. All samples collected were based on clinical indications, and the selection of the sampling site was determined by nonstudy physicians.
Specimens of all types underwent both smear microscopy using light-emitting-diode fluorescence microscopy and mycobacterial culture using both liquid media with Mycobacteria Growth Indicator Tube (MGIT) using BACTEC 960 automated system (Becton Dickinson, Sparks, MD, USA) and solid media. Other investigations, including polymerase chain reaction assay for direct detection of M. tuberculosis from clinical specimens, were performed at the discretion of attending physicians. The clinical management of all patients was performed by attending physicians and consulting infectious disease physicians who were not involved in the study.
All specimens were processed by accredited laboratories (Department of Microbiology, Faculty of Medicine Siriraj Hospital, Mahidol University, and Department of Clinical Microbiology, Chonburi Hospital) using liquid culture according to standardized protocols. All patients were required to provide a spot urine sample collected in a sterile container. LF-LAM tests (manufacturer lot nos. 131120, 141123, 141124, 151124, and 151125) were performed on unprocessed urine samples within 3 days after collection. If not tested immediately, urine samples were stored at 4°C until use, according to the manufacturer’s recommendation. After the urine was applied to the test strip, the strip was visually inspected by 2 trained study staff members at each study site. The LF-LAM test result was graded according to the manufacturer’s new reference card, with band intensities graded on a scale of 1 to 4 (from lightest to darkest). We defined LF-LAM test positivity using the grade 1 cutoff. Staff members who interpreted the LF-LAM test were blinded to patient clinical data and tuberculosis diagnostic status. Any disagreement between interpreters was decided by the decision of a third interpreter. Moreover, the reference standard results were interpreted without knowledge of the results of LF-LAM testing. These results were not provided to the clinicians caring for enrolled participants, nor were they used for clinical care decision making.
Definite tuberculosis was defined as positive culture or polymerase chain reaction for M. tuberculosis complex from any clinical specimen. Patients were defined as having probable tuberculosis if they started antituberculosis treatment with subsequent documented clinical improvement at 2-month follow-up, without bacteriological confirmation, or if they died within 2 months after enrollment, with tuberculosis recorded as the cause of death on the death certificate or medical record. Patients categorized into the group without tuberculosis were those for whom an alternative diagnosis was available and those with a positive culture for nontuberculous mycobacteria (NTM) who were not receiving antituberculosis treatment. Follow-up data relating to subsequent clinical improvement at the 2-month time point and patient outcome were retrieved from electronic medical records. The treatment decisions that were made were based on the professional judgment of treating physicians who were not involved in this study.
Sample Size Calculation and Statistical Analysis
Based on the results of previous studies, we estimated that the LF-LAM test would yield 63% sensitivity and 96.6% specificity [20]. Using a 15% allowable error for sensitivity, a 3% allowable error for specificity, and a 40% prevalence of HIV-tuberculosis coinfection, the minimum sample size was calculated at 280 patients.
Descriptive statistics were used to characterize the study population, and descriptive statistical data are presented as frequency, frequency and percentage, or median and Interquartile range. The results of the 2 LF-LAM test readers were compared, and interobserver agreement was calculated using the κ statistic. Performance measures, including sensitivity, specificity, predictive values, and accuracy of the LF-LAM test or fluorescence smear microscopy, were calculated as percentages with 95% confidence intervals (CIs). The analysis for diagnostic accuracy of the LF-LAM test by CD4 cell count was also calculated as percentage with 95% CI. In our analysis, we evaluated the accuracy of the LF-LAM test or fluorescence smear microscopy compared combined group of probable tuberculosis and nontuberculosis against the microbiological reference standard (definite tuberculosis) or compared nontuberculosis with composite reference standard (combination of definite and probable tuberculosis). All statistical tests were 2 sided, with P values <.05 indicating statistical significance. Statistical calculations were performed using SPSS Statistics software, version 20 (SPSS).
RESULTS
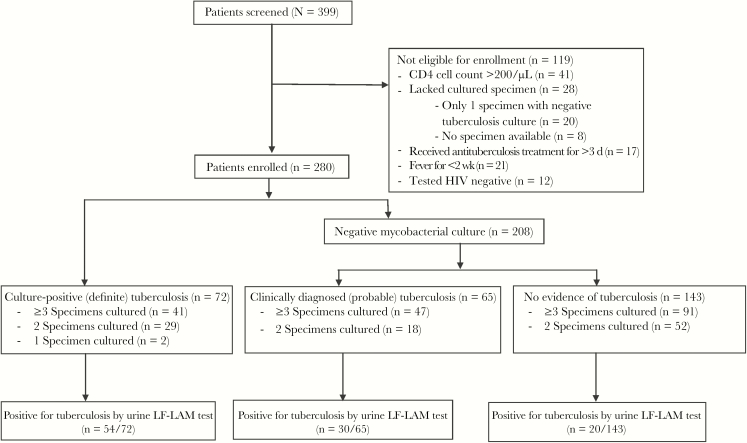
Of the 399 patients who were screened, 280 were enrolled (Figure 1); 157 were recruited from Chonburi Hospital and 123 from Siriraj Hospital. Approximately 80% of patients were hospitalized. Included patients were mostly young adults (median age, 39 years), and 62.5% were male. The median CD4 cell count was 33/μL (interquartile range, 12–68/μL). Other common symptoms in addition to fever included weight loss (in 87.5% of patients), chronic cough (62.1%), and lymphadenopathy (12.5%). Among 280 patients, only 15 patients could not provide sputum specimens but could provide other types of specimens.
Figure 1.
Flow diagram of patient enrollment and results of the lateral flow urine lipoarabinomannan (LF-LAM) assay. Abbreviation: HIV, human immunodeficiency virus.
Microbiologically confirmed tuberculosis was found in 72 patients, for a prevalence of definite tuberculosis of 25.7%. Of those patients, 34 had pulmonary tuberculosis only, 30 had disseminated tuberculosis, and 8 had isolated extrapulmonary tuberculosis. Among those with disseminated disease, 26 patients had concomitant pulmonary involvement, and 4 had disseminated disease due to isolation of tuberculosis from multiple body sites other than the lungs, including from blood, lymph node, pleural fluid, cerebrospinal fluid, and/or liver tissue. Among patients with isolated extrapulmonary tuberculosis, 3 had tuberculosis lymphadenitis, 2 had tuberculosis colitis, 2 had tuberculosis meningitis, and 1 had tuberculosis pleuritis. There were 65 patients with probable tuberculosis, for a prevalence of 23.2%. The combined prevalence rate of definite and probable tuberculosis was 48.9%. The baseline demographic and clinical characteristics of all patients are shown in Table 1. The overall mortality rate was 15.8%, and this rate was higher among patients with probable tuberculosis and those without tuberculosis than among those with definite tuberculosis. Among patients who died, we found no difference between the definite tuberculosis group and other groups regarding age, CD4 cell count, presence of multiple opportunistic infections, or number of opportunistic infections (data not shown).
Table 1.
Baseline Demographic and Clinical Characteristics of 280 Patients
| Baseline Characteristic | Patients, No. (%)a | P Valueb | |||
|---|---|---|---|---|---|
| All (n = 280) | Definite Tuberculosis (n = 72) |
Probable Tuberculosis (n = 65) |
No Tuberculosis (n = 143) |
||
| Age, median (IQR), y | 39 (32–48) | 38 (32–48) | 40 (35.5–47.5) | 39 (32–48) | .83 |
| Male sex | 175 (62.5) | 42 (58.3) | 48 (73.8) | 85 (59.4) | .99 |
| Enrolled at Siriraj Hospital | 123 (43.9) | 41 (56.9) | 20 (30.8) | 62 (43.4) | .08 |
| Inpatient setting | 229 (81.8) | 61 (84.7) | 52 (80.0) | 116 (81.1) | .64 |
| ART at enrollment | 29 (10.4) | 3 (4.2) | 8 (12.3) | 18 (12.6) | .09 |
| CD4 cell count, median (IQR), cells/μL | 33 (12–68) | 41 (12–86) | 33 (13–72.5) | 32 (12–61) | .15 |
| CD4 cell count | |||||
| <50/μL | 183 (65.4) | 42 (58.3) | 41 (63.1) | 100 (69.9) | .12 |
| 50–100/μL | 57 (20.4) | 15 (20.8) | 15(23.1) | 27 (18.9) | .87 |
| >100/μL to 200/μL | 40 (14.3) | 15 (20.8) | 9 (13.8) | 16 (11.2) | .09 |
| Presenting symptoms | |||||
| Weight loss | 245 (87.5) | 65 (90.3) | 55 (84.6) | 125 (87.4) | .69 |
| Chronic cough | 174 (62.1) | 47 (65.3) | 35 (53.8) | 92 (64.3) | >.99 |
| Lymphadenopathy | 35 (12.5) | 14 (19.4) | 5 (7.7) | 16 (11.2) | .15 |
| Specimens, no. | |||||
| Total | 778 | 197 | 187 | 394 | … |
| Sputum | 367 | 88 | 79 | 200 | … |
| Blood | 240 | 54 | 60 | 126 | … |
| Extrapulmonary | 171 | 55 | 48 | 68 | … |
| Deathc | 41 (15.8) | 5 (7.2) | 11 (18.6) | 25 (18.9) | .03 |
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range.
aData represent no. (%) of patients unless otherwise indicated.
b P values for comparison between definite and no-tuberculosis groups, with differences considered significant at P < .05.
cTwenty patients with no outcome data were excluded from the data on deaths, including 3 from the definite tuberculosis, 6 from the probable tuberculosis, and 11 from the no-tuberculosis group.
The diagnostic accuracy of LF-LAM testing and/or smear microscopy for culture-confirmed tuberculosis is shown in Table 2. When compared with the microbiological reference standard, the LF-LAM test yielded a sensitivity of 75.0% (95% CI, 63.9%–83.6%) and a specificity of 76.0% (69.7%–81.3%). In patients with a CD4 cell count <50/μL, the test showed a higher sensitivity (90.5%; 95% CI, 77.95–96.2%) and a higher negative predictive value (96.4%; 91.0%–98.6%). Compared with the LF-LAM test, fluorescence smear microscopy had a lower sensitivity (61.1%; 95% CI, 49.6%–71.5%) but a higher specificity (98.1%; 95.2%–99.2%). With use of either LF-LAM testing or smear microscopy, the sensitivity increased to 86.1% (95% CI, 76.3%–92.3%) compared with LF-LAM testing alone, with no observed reduction in specificity. When compared with the composite reference standard, the test yielded a lower sensitivity (61.3%; 95% CI, 53.0%–69.1%) but a higher specificity (86.0%; 79.4%–90.8%) (Table 3). Interobserver agreement was perfect between the 2 observers at both centers (κ = 1.0) for both presence or absence of test band and interpretation of band intensity. No invalid LF-LAM tests were observed in this study.
Table 2.
Diagnostic Accuracy of Lateral Flow Urine Lipoarabinomannan Assay and/or Smear Microscopy for Culture-Confirmed Tuberculosis
| Testing Method | Sensitivity | Specificity | PPV, % (95% CI) | NPV, % (95% CI) | ||
|---|---|---|---|---|---|---|
| No./Total | % (95% CI) | No./Total | % (95% CI) | |||
| LF-LAM assay | ||||||
| All patients | 54/72 | 75.0 (63.9–83.6) | 158/208 | 76.0 (69.7–81.3) | 51.9 (42.4–61.3) | 89.8 (84.4–93.4) |
| CD4 cell count | ||||||
| <50/μL | 38/42 | 90.5 (77.9–96.2) | 106/141 | 75.2 (67.4–81.6) | 52.1 (40.8–63.1) | 96.4 (91.0–98.6) |
| 50–100/μL | 9/15 | 60.0 (35.7–80.2) | 34/42 | 81.0 (66.7–90.0) | 52.9 (31.0–73.8) | 85.0 (70.9–92.9) |
| >100/μL to 200/μL | 7/15 | 46.7 (24.8–69.9) | 18/25 | 72.0 (52.4–85.7) | 50.0 (26.8–73.2) | 69.2 (50.0–83.5) |
| Smear microscopy | ||||||
| All patients | 44/72 | 61.1 (49.6–71.5) | 204/208 | 98.1 (95.2–99.2) | 91.7 (80.4–96.7) | 87.9 (83.1–91.5) |
| CD4 cell count | ||||||
| <50/μL | 30/42 | 71.4 (56.4–82.8) | 137/141 | 97.2 (92.9–98.9) | 88.2 (73.4–95.3) | 91.9 (86.5–95.3) |
| 50–100/μL | 5/15 | 33.3 (15.2–58.3) | 42/42 | 100 (91.6–100) | 100 (56.6–100) | 80.8 (68.1–89.2) |
| >100/μL to 200/μL | 9/15 | 60.0 (35.7–80.2) | 25/25 | 100 (86.7–100) | 100 (70.1–100) | 80.6 (63.7–90.8) |
| LF-LAM assay or smear | ||||||
| All patients | 62/72 | 86.1 (76.3–92.3) | 158/208 | 76.0 (69.7–81.3) | 55.4 (46.1–64.2) | 94.0 (89.4–96.7) |
| CD4 cell count | ||||||
| <50/μL | 40/42 | 95.2 (84.2–98.7) | 106/141 | 75.2 (67.4–81.6) | 53.3 (42.2–64.2) | 98.1 (93.5–99.5) |
| 50–100/μL | 10/15 | 66.7 (41.7–84.8) | 34/42 | 81.0 (66.7–90.0) | 55.6 (33.7–75.4) | 87.2 (73.3–94.4) |
| >100/μL to 200/μL | 12/15 | 80.0 (54.8–93.0) | 18/25 | 72.0 (52.4–85.7) | 63.2 (41.0–80.9) | 85.7 (65.4–95.0) |
Abbreviations: CI, confidence interval; LF-LAM, lateral flow urine lipoarabinomannan; NPV, negative predictive value; PPV, positive predictive value.
Table 3.
Diagnostic Accuracy of the Lateral Flow Urine Lipoarabinomannan Assay Compared With the Composite Reference Standard (Definite Plus Probable Tuberculosis)
| Patient Group | Sensitivity | Specificity | PPV, % (95% CI) | NPV, % (95% CI) | ||
|---|---|---|---|---|---|---|
| No./Total | % (95% CI) | No./Total | % (95% CI) | |||
| All patients | 84/137 | 61.3 (53.0–69.1) | 123/143 | 86.0 (79.4–90.8) | 80.8 (72.2–87.2) | 69.9 (62.7–76.2) |
| CD4 cell count | ||||||
| <50/μL | 57/83 | 68.7 (58.1–77.6) | 84/100 | 84.0 (75.6–89.9) | 78.1 (67.3–86.0) | 76.4 (67.6–83.3) |
| 50–100/μL | 15/30 | 50.0 (33.2–66.8) | 25/27 | 92.6 (76.6–97.9) | 88.2 (65.7–96.7) | 62.5 (47.0–75.8) |
| >100/μL to 200/μL | 12/24 | 50.0 (31.4–68.6) | 14/16 | 87.5 (64.0–96.5) | 85.7 (60.1–96.0) | 53.8 (35.5–71.2) |
Abbreviations: CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
Among the 20 patients without tuberculosis who had a positive LF-LAM test result, 5 had multiple infections, 7 had disseminated Mycobacterium avium complex, 6 had cryptococcal infections, 6 had pneumocystis pneumonia, and 1 each had Mycobacterium kansasii pulmonary infection, histoplasmosis, lymphoma, and unknown diagnosis with symptoms resolved after initiation of antiretroviral therapy, respectively (Table 4). Among the 30 patients with probable tuberculosis who had a positive LF-LAM test result, 18 had faint band intensities of grade 1, and 6 patients had strong band intensities of grade 4. Opportunistic infections in 30 patients with probable tuberculosis with positive LF-LAM test are listed in Table 5. Among the 54 patients who had positive tuberculosis culture and a positive LF-LAM test result, 15 patients, 7 patients, and 2 patients provided a follow-up urine specimen at 1, 2, and 4 weeks, respectively, after antituberculosis treatment. All of those urine specimens had a positive tuberculosis result with LF-LAM testing.
Table 4.
Opportunistic Infections in 20 Patients Without Tuberculosis With False-Positive Lateral Flow Urine Lipoarabinomannan Assay Results
| Patient | Band Intensity | MACa | Cryptococcosisa | PCPa | Other Infection |
|---|---|---|---|---|---|
| 1 | 4+ | Yes | No | No | … |
| 2 | 4+ | Yes | No | No | … |
| 3 | 4+ | Yes | No | No | … |
| 4 | 4+ | Yes | No | No | … |
| 5 | 4+ | Yes | No | Yes | … |
| 6 | 2+ | Yes | No | No | … |
| 7 | 1+ | Yes | No | No | … |
| 8 | 4+ | No | Yes | No | … |
| 9 | 2+ | No | Yes | Yes | Bacterial pneumonia |
| 10 | 1+ | No | Yes | Yes | … |
| 11 | 1+ | No | Yes | No | Bacterial pneumonia |
| 12 | 1+ | No | Yes | No | … |
| 13 | 1+ | No | Yesb | No | … |
| 14 | 1+ | No | No | Yes | … |
| 15 | 1+ | No | No | Yes | … |
| 16 | 1+ | No | No | Yes | Bacterial pneumonia |
| 17 | 1+ | No | No | No | Mycobacterium kansasii |
| 18 | 1+ | No | No | No | Histoplasmosis |
| 19 | 1+ | No | No | No | Lymphoma |
| 20 | 1+ | No | No | No | No proved OIc |
The LF-LAM test result was graded according to the manufacturer’s new reference card, with band intensities graded on a scale of 1 to 4 (from lightest to darkest).
Abbreviations: MAC, Mycobacterium avium complex; OI, opportunistic infection; PCP, Pneumocystis pneumonia.
aMAC infection was diagnosed based on positive culture blood, bone marrow, sputum, stool, or lymph node cultures; cryptococcosis, based on positive cerebrospinal fluid or blood culture; and PCP, based on clinical factors and marked improvement after trimethoprim-sulfamethoxazole therapy.
bCryptococcal antigenemia.
cSymptoms spontaneously resolved after antiretroviral therapy.
Table 5.
Opportunistic Infections in 30 Patients With Probable Tuberculosis and Positive Lateral Flow Urine Lipoarabinomannan Assay Results
| Patient | Band Intensity | MACa | Cryptococcosisa | PCPa | Others |
|---|---|---|---|---|---|
| 1 | 4+ | Yes | Yes | No | … |
| 2 | 4+ | No | Yes | No | … |
| 3 | 4+ | No | No | Yes | … |
| 4 | 4+ | No | No | No | Bacterial pneumonia |
| 5 | 4+ | No | No | No | No proved OI |
| 6 | 4+ | No | No | No | No proved OI |
| 7 | 3+ | No | No | No | No proved OI |
| 8 | 3+ | No | No | No | No proved OI |
| 9 | 2+ | No | No | Yes | … |
| 10 | 2+ | No | No | No | No proved OI |
| 11 | 2+ | No | No | No | No proved OI |
| 12 | 2+ | No | No | No | No proved OI |
| 13 | 1+ | Yes | No | No | … |
| 14 | 1+ | Yes | No | No | … |
| 15 | 1+ | No | Yes | No | … |
| 16 | 1+ | No | Yes | No | … |
| 17 | 1+ | No | Yesb | No | Salmonella bacteremia |
| 18 | 1+ | No | No | Yes | … |
| 19–30 | 1+ | No | No | No | No proved OI |
The LF-LAM test result was graded according to the manufacturer’s new reference card, with band intensities graded on a scale of 1 to 4 (from lightest to darkest).
Abbreviations: MAC, Mycobacterium avium complex; OI, opportunistic infection; PCP, Pneumocystis pneumonia.
aMAC infection was diagnosed based on positive culture blood, bone marrow, sputum, stool, or lymph node cultures; cryptococcosis, based on positive cerebrospinal fluid or blood culture; and PCP, based on clinical factors and marked improvement after trimethoprim-sulfamethoxazole therapy.
bCryptococcal antigenemia.
Discussion
In this multicenter study, the LF-LAM test was able to detect 75% of tuberculosis cases in Thai adult patients with advanced HIV disease. The sensitivity of the test varied from approximately 47% to 91%, depending on the reference standard and CD4 level. The highest LF-LAM test sensitivity (90.5%) was observed in HIV-infected patients with CD4 cell counts <50/μL. The specificity of the test ranged from 72% to 92.6%, depending on the reference standard and the CD4 level. The overall mortality rate was higher among patients with probable tuberculosis and those without tuberculosis than among those with definite tuberculosis. We have no good explanation for why the mortality rate was lower in patients with definite tuberculosis, because no factors were found to differ significantly between groups. However, the higher mortality rate among patients with probable tuberculosis may be partly due to how we defined probable tuberculosis, because we also included those who died within 2 months of enrollment and had tuberculosis recorded as the cause of death.
The reported performance of the LF-LAM test varied greatly among other published studies, depending on factors that include screening versus diagnosis of tuberculosis, outpatient versus inpatient care setting, CD4 level, and the type of reference standard that was used. A systematic review conducted by Shah et al [17] found a pooled sensitivity rate of 50% (95% CI, 24–82) for the diagnosis of tuberculosis with the LF-LAM test in HIV-infected patients with CD4 cell counts <200/μL (using the original grade 2 cutoff, which corresponds to the current grade 1 cutoff). The pooled sensitivity was higher (63%) when the CD4 cell count was <50/μL.
Our study found a higher sensitivity (75%) than many previous studies [10–15, 17, 21, 22], and this difference may have several explanations. First, it is likely that we included patients with high bacillary loads, because all included patients had suspected tuberculosis with more advanced symptoms. More specifically, for inclusion eligibility we required the presence of fever for >2 weeks plus ≥1 clinical presentation of tuberculosis. Moreover, approximately 40% of patients with definite tuberculosis had disseminated disease, which was shown in a prior study to effectuate higher levels of lipoarabinomannan antigen in the urine than localized disease [23]. Second, we recruited only patients with advanced HIV disease and CD4 cell counts ≤200/μL, and two-thirds of patients had a CD4 cell count <50/μL. In a previous study, the sensitivity was found to be higher in sick patients with a lower CD4 cell count [17]. Third, we used fresh urine samples, which we postulated would yield a greater sensitivity than frozen samples. However, to our knowledge, no studies have compared the stability of LF-LAM test results between fresh and frozen urine samples.
The overall 76% specificity of the LF-LAM test observed in this study was lower than the specificities reported from prior studies (90%; 95% CI, 72%–98%) [17] (most of which were conducted in Africa), and it did not exceed the 98% specificity proposed by an international expert committee for point-of-care tuberculosis tests [24]. Lower LF-LAM test specificities (range, 81%–88%) were reported from studies conducted in Tanzania, Zimbabwe, and Myanmar [19, 25]. In Thailand, a study by Suwanpimolkul et al [18] found an overall test specificity of 85%. The lower specificity in that study could be in part due to the use of the older grade 1 threshold (On a scale of 1–5).
The positivity threshold of the LF-LAM test does not explain the observed low specificity in our study because we used a modified reference card, and interobserver agreement at both participating centers was 100%. The low specificity may be due to cross-reactivity of the test with NTM infections that could also contain lipoarabinomannan molecules [26]. We found a false-positive rate of 14%, with strong band intensities (grade 4+) observed mostly in M. avium complex, whereas most other conditions had low band intensities (grade 1+). A prior study by Boehme et al [27] that evaluated the performance of the older LAM enzyme-linked immunosorbent assay observed cross-reactivity of the test with multiple species of mycobacteria. However, Boehme et al reported a small number of NTM cases, and they used healthy volunteers as negative controls to evaluate specificity; thus, a very high specificity (99%) was reported [27].
The cross-reactivity of LF-LAM testing with NTM disease was reported from prior study by Nel et al [28], which found an unexpectedly high false-positive rate in 19 of 21 patients with disseminated NTM disease. In the present study, the LF-LAM test had slightly higher sensitivity but lower specificity and positive predictive value than smear microscopy. An approximate 11% incremental benefit in sensitivity was observed when either acid-fast bacilli sputum smear or LF-LAM testing was used, compared with LF-LAM testing alone. The sensitivity increased from 75.0% to 86.1%, and the negative predictive value from 89.8% to 94% without compromising specificity or positive predictive value —all of which is consistent with the findings of previous studies [10, 29]. Thus, a negative result of LF-LAM testing or smear, especially in patients with a very low CD4 cell count, is useful for ruling out tuberculosis disease.
The LF-LAM test is generally not recommended for testing in patients who have already received antituberculosis treatment because it can show a false-negative result. However, our data suggests that the test result can remain positive for up to 4 weeks after tuberculosis treatment. This property of the LF-LAM test enables its use in patients already receiving antituberculosis treatment who are unable to provide appropriate specimens for culture testing.
The LF-LAM test has many attractive features. First, it is a point-of-care test that can be used in remote, unsophisticated, low-resource settings, with no need for electricity or a laboratory. In addition and importantly, the novel urine-based Fujifilm SILVAMP TB LAM assay (Fuji LAM; Fujifilm and the Foundation for Innovative New Diagnostics) has recently been announced. The Fuji LAM assay was reported to have a higher sensitivity than Alere LAM assay for diagnosis of active tuberculosis in hospitalized HIV-infected patients [30]. With this ability, tuberculosis diagnosis and a treatment decision may be made during a single clinical encounter, which can greatly increase the chance of improved patient outcomes. Second, the collection of urine is simple, and it is associated with minimal biohazard risk because no infectious aerosols are generated. Third, a complementary strategy that includes both LF-LAM testing and smear microscopy may be beneficial, because the LF-LAM test can identify the sickest patients, whereas smear microscopy can identify only the most infectious. Furthermore, inclusion of the LF-LAM test into routine investigation will help reduce mortality rates [25], presumably by increasing the proportion of patients who receive early tuberculosis treatment. Further studies are needed to assess the impact of the LF-LAM test on mortality rates in Asian populations.
To our knowledge, this is the first prospective study to evaluate the accuracy of the LF-LAM test using fresh urine samples for diagnosis of HIV-associated tuberculosis in Thailand. The strengths of this study include the assessment of LF-LAM test performance in an accredited laboratory setting where extensive diagnostic tests can be performed. Second, we enrolled both hospitalized and ambulatory patients. Third, we included multiple types of samples, reflecting a real-life clinical setting in which different patients have different sites of tuberculosis infection. These could help to reduce diagnostic misclassification. Fourth and last, testing was performed using fresh urine specimens that were collected in sterile containers and then tested or stored at 4°C to replicate point-of-care testing. This collection method was used to avoid cross-reactivity due to microbial replication from a contaminated nonsterile urine collection container left at room temperature [28].
The current study also had some mentionable limitations. First, the obtained specimens were not standardized. More specifically, specimens from pulmonary tuberculosis only, disseminated tuberculosis with pulmonary involvement, and extrapulmonary tuberculosis were collected and included, which could have compromised specificity. Second, we excluded 20 patients who provided only a single specimen that yielded a negative culture result. It is possible either that these patients had paucibacillary disease or that they would have been found to be positive for tuberculosis if they had >1 specimen been provided. Unfortunately, LF-LAM testing was not performed in these 20 patients. Third, not all patients with positive LF-LAM results provided a follow-up urine specimen for testing. Fourth and last, the longest duration between treatment and that urine collection was 4 weeks, so we were not able to determine how long LF-LAM results remain positive after tuberculosis treatment.
In conclusion, the LF-LAM test demonstrated good performance for diagnosing active tuberculosis among patients with advanced HIV-infection. An incremental sensitivity of 11% was observed with use of acid-fast bacilli sputum smear or LF-LAM testing, compared with LF-LAM testing alone. This study found a lower specificity for LF-LAM testing than those reported from studies conducted in African settings.
Acknowledgments
We gratefully acknowledge the patients who agreed to participate in this study. We also thank the personnel from the Department of Microbiology, Faculty of Medicine Siriraj Hospital, Mahidol University, and from the Department of Clinical Microbiology, Chonburi Hospital, for their contributions to this study.
Financial support. This work was supported by the Research Development Fund, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (grant R015832014). The test strips used in this study were donated by R.X. Company, Nakhon Pathom, Thailand.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors declare no personal or professional conflicts of interest, and no financial support from the companies that produce and/or distribute the drugs, devices, or materials described in this report. Neither the company that donated the test strips nor the study sponsor had any role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study, and had final responsibility for the decision to submit for publication. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2018. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 2. Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev 2011; 24:351–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chamie G, Luetkemeyer A, Walusimbi-Nanteza M, et al. Significant variation in presentation of pulmonary tuberculosis across a high resolution of CD4 strata. Int J Tuberc Lung Dis 2010; 14:1295–302. [PMC free article] [PubMed] [Google Scholar]
- 4. Peter JG, van Zyl-Smit RN, Denkinger CM, Pai M. Diagnosis of TB: state of the art. Eur Respir Monograph 2012; 58:124–43. [Google Scholar]
- 5. Lawn SD, Wood R. Tuberculosis in antiretroviral treatment services in resource-limited settings: addressing the challenges of screening and diagnosis. J Infect Dis 2011; 204(suppl 4):S1159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walzl G, McNerney R, du Plessis N, et al. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis 2018; 18:e199–210. [DOI] [PubMed] [Google Scholar]
- 7. Zumla A, Raviglione M, Hafner R, von Reyn CF. Tuberculosis. N Engl J Med 2013; 368:745–55. [DOI] [PubMed] [Google Scholar]
- 8. Steingart KR, Schiller I, Horne DJ, et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014; 1:1–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brennan PJ. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinb) 2003; 83:91–7. [DOI] [PubMed] [Google Scholar]
- 10. Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis 2012; 12:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dorman SE. New diagnostic tests for tuberculosis: bench, bedside, and beyond. Clin Infect Dis 2010; 50(suppl 3:S173–7. [DOI] [PubMed] [Google Scholar]
- 12. Peter JG, Theron G, van Zyl-Smit R, et al. Diagnostic accuracy of a urine lipoarabinomannan strip-test for TB detection in HIV-infected hospitalised patients. Eur Respir J 2012; 40:1211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peter JG, Theron G, Dheda K. Can point-of-care urine LAM strip testing for tuberculosis add value to clinical decision making in hospitalised HIV-infected persons? PLoS One 2013; 8:e54875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakiyingi L, Moodley VM, Manabe YC, et al. Diagnostic accuracy of a rapid urine lipoarabinomannan test for tuberculosis in HIV-infected adults. J Acquir Immune Defic Syndr 2014; 66:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bjerrum S, Kenu E, Lartey M, et al. Diagnostic accuracy of the rapid urine lipoarabinomannan test for pulmonary tuberculosis among HIV-infected adults in Ghana-findings from the DETECT HIV-TB study. BMC Infect Dis 2015; 15:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization. The use of lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis and screening of active tuberculosis in people living with HIV: policy guidance. 2015. Available at: http://apps.who.int/iris/bitstream/handle/10665/193633/9789241509633_eng.pdf?sequence=1. Accessed 2015. [Google Scholar]
- 17. Shah M, Hanrahan C, Wang ZY, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database Syst Rev 2016; 5:1–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suwanpimolkul G, Kawkitinarong K, Manosuthi W, et al. Utility of urine lipoarabinomannan (LAM) in diagnosing tuberculosis and predicting mortality with and without HIV: prospective TB cohort from the Thailand Big City TB Research Network. Int J Infect Dis 2017; 59:96–102. [DOI] [PubMed] [Google Scholar]
- 19. Thit SS, Aung NM, Htet ZW, et al. The clinical utility of the urine-based lateral flow lipoarabinomannan assay in HIV-infected adults in Myanmar: an observational study. BMC Med 2017; 15:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dorman S, Manabe YC, Nicol MP, et al. Accuracy of Determine TB-LAM lateral flow test for diagnosis of TB in HIV + adults: interim results from a multicenter study. Program and abstracts of the 19th Conference on Retroviruses and Opportunistic Infections, Mar 5–8, 2012, Seattle, Washington: 2012. Abstract 149aLB. [Google Scholar]
- 21. Lawn SD. Point-of-care detection of lipoarabinomannan (LAM) in urine for diagnosis of HIV-associated tuberculosis: a state of the art review. BMC Infect Dis 2012; 12:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drain PK, Losina E, Coleman SM, et al. Diagnostic accuracy of a point-of-care urine test for tuberculosis screening among newly-diagnosed HIV-infected adults: a prospective, clinic-based study. BMC Infect Dis 2014; 14:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah M, Martinson NA, Chaisson RE, et al. Quantitative analysis of a urine-based assay for detection of lipoarabinomannan in patients with tuberculosis. J Clin Microbiol 2010; 48:2972–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization. High priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting—28–29 April 2014. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 25. Peter JG, Zijenah LS, Chanda D, et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016; 387:1187–97. [DOI] [PubMed] [Google Scholar]
- 26. Qvist T, Johansen IS, Pressler T, et al. Urine lipoarabinomannan point-of-care testing in patients affected by pulmonary nontuberculous mycobacteria—experiences from the Danish Cystic Fibrosis cohort study. BMC Infect Dis 2014; 14:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boehme C, Molokova E, Minja F, et al. Detection of mycobacterial lipoarabinomannan with an antigen-capture ELISA in unprocessed urine of Tanzanian patients with suspected tuberculosis. Trans R Soc Trop Med Hyg 2005; 99:893–900. [DOI] [PubMed] [Google Scholar]
- 28. Nel JS, Lippincott CK, Berhanu R, et al. Does disseminated nontuberculous mycobacterial disease cause false-positive determine TB-LAM lateral flow assay results? a retrospective review. Clin Infect Dis 2017; 65:1226–8. [DOI] [PubMed] [Google Scholar]
- 29. Zijenah LS, Kadzirange G, Bandason T, et al. Comparative performance characteristics of the urine lipoarabinomannan strip test and sputum smear microscopy in hospitalized HIV-infected patients with suspected tuberculosis in Harare, Zimbabwe. BMC Infect Dis 2016; 16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Broger T, Sossen B, du Toit E, et al. Novel high sensitivity tuberculosis point-of-care test for people living with HIV (September 21, 2018). Access date: February 26, 2019. Available at: https://ssrn.com/abstract=3254479. [Google Scholar]