Abstract
Genetic predisposition is necessary for polycystic kidney disease (PKD) initiation, although there are other, incompletely identified downstream processes that are required for cyst growth. Their characterization may provide a unique opportunity for clinical interventions. One of the poorly studied phenomena in PKD is high ATP content in cysts. Unfortunately, neither origins of uncontrolled ATP release, nor consequences of abnormal purinergic signaling in relation to epithelial transport are well explored in the polycystic kidney. We tested the distribution of pannexin-1 (Panx1) and P2X7, two proteins potentially involved in ATP release, in the kidneys of the Pkd1RC/RC mice, a model of autosomal dominant PKD (ADPKD). Abundances of both proteins were abnormally increased in the cyst lining cells compared to non-dilated collecting ducts. To establish if pannexin-1 contributes to ATP release in the collecting ducts (CD), we measured luminal accumulation of ATP in M1 cell renal CD monolayers, and found that treatment with probenecid, a Panx1 blocker, prevents ATP release. Single channel patch clamp analysis of polarized M1 cells revealed that apical stimulation of P2X receptors with αβ-MeATP acutely reduces ENaC activity. We conclude that in ADPKD progression, an abnormal hyperexpression of both PANX1 and P2RX7 occurs in the cyst lining epithelial cells. High abundance of both proteins is not typical for non-dilated CDs but, when happens in cysts, pannexin1/P2X7 cooperation elevates ATP release into the luminal space. High ATP level is a pathogenic factor facilitating cystogenesis by reducing ENaC-mediated reabsorption from the lumen.
Keywords: ADPKD, polycystic kidney, ATP release, pannexin, P2X, ENaC
Introduction
Polycystic kidney diseases are hereditary nephropathies characterized by a spontaneous growth of fluid-filled cysts along the nephron. Autosomal dominant PKD (ADPKD) is caused by mutation in the PKD1 or PKD2 genes encoding polycystins 1 and 2 whereas the autosomal recessive form (ARPKD) is due to mutations in PKHD1 encoding fibrocystin, a protein capable of interacting with the polycystin-½ complex [1, 2]. ADPKD cysts start as tubular or ductal dilatation in a minority (~1%) of nephrons [3]. During cyst growth, numerous cellular abnormalities have been observed in cyst-lining cells, including alterations in apical–basal polarity, extracellular matrix rearrangements, cell proliferation, cAMP elevation, Warburg effect and abnormal fluid transport [4].
ATP is a molecule providing energy for a plethora of enzymatic reactions in the cell. It is also an important humoral factor responsible for para- and autocrine signaling. In the kidney, ATP plays a major role in orchestrating renal autoregulation, hemodynamics and epithelial transport. For example, in normal collecting ducts (CD) ATP is responsible for ion transport regulation and limits activity of ENaC during high salt consumption via activation of P2Y2 receptors [5]. P2X and P2Y receptors co-exist in renal vasculature, glomeruli, nephron segments and the collecting duct system and regulate cell contractility, Ca2+ release from ER, ion channel activity and other functions [6].
There are data obtained from human biopsies and recently confirmed in PCK rats, a model of ARPKD, that PKD cysts accumulate an abnormally high level of ATP [7–9]. The involvement of auto- and paracrine ATP effects in the development of PKD was reviewed by Rangan [10] and Ilatovskaya and colleagues [11]. Both papers suggest that general ATP release mechanisms identified earlier in the kidney contributes to cyst growth: destruction (via apoptosis and necrosis) of cystic and normal renal cells, exocytosis in vesicles, and release via connexin/pannexin channels located in the membrane of epithelial cells, or other sources like infiltrated immune cells or nerve terminals. ATP excess in the lumen can serve as a pivotal pathogenic factor of cyst expansion driving impaired sodium reabsorption, increased chloride secretion and cell proliferation [12–15]. However, the origins and effectors of ATP release in PKD remain poorly understood.
Pannexin-1 was characterized as a non-junctional membrane channel existing in two conformations: with low (~50 pS) and high conductance (~500 pS) [16]. In the high conductive state pannexin-1 pores are capable of releasing large (up to 1 kDa) molecules including ATP [17] and interleukin-1β [18]. PANX1 appears as a hexameric assembly of pannexin-1 subunits that form a transmembrane channel called pannexon. There is accumulating evidence summarized in the excellent review [16] that pannexin-1 matches all requirements to serve as an ATP release channel (biophysical properties, co-localization and expression in ATP releasing cells, response to physiological stimuli). PANX1-dependent ATP release has been firmly shown in CNS, pancreatic β-cells, blood and immune cells [16, 19, 20]. PANX1 is expressed in various excitable and non-excitable cells including the kidney, brain, various epithelial and endothelial cells, erythrocytes, and lymphocytes, whereas the expression of PANX2 and PANX3 is restricted to the brain and skin/bone, respectively [16]. According to data obtained in Panx1 knockout mice, in the kidney Panx1 is expressed in principal and intercalated cells of the distal nephron and descending thin limb, and pannexin-1 is necessary for ATP release into the urine [21, 22].
There is strong evidence that pannexin-1 interacts with P2X7 receptors that change its conformation and form a channel highly permeable for ATP [16, 23–25]. Pannexin-1 can be immunoprecipitated with P2X7 protein by antibodies directed to either Panx1 or P2X [26–28]. Contribution of the pannexin-1/P2X7 interaction to ATP release has recently been demonstrated in cardioprotection [29], vascular inflammation in lungs [30], bone [25], and neuromuscular transmission [31]. A central role of the autocrine signaling loop involving ATP-induced P2X7 activation and further amplified by pannexin-1 dependent ATP release has been shown in motility of dendritic cells [32]. Unfortunately, the cooperation of pannexin-1 and P2X receptors in renal physiology is not well characterized.
Our study investigates cystic expression of pannexin-1 and P2X7 in Pkd1RC/RC mice, a rodent model of ADPKD [33], and their involvement in ATP release which serves as a pathogenic factor reducing sodium reabsorption in cystogenesis.
Methods
Animals and cell cultures.
Pkd1RC/RC mouse homozygous breeders were provided by the Mayo Clinic Translational Polycystic Kidney Disease Center and housed in the Henry Ford Health System animal facility (IACUC protocol 1580). Breeding and housing of the animals was performed in a standard 12/12hr light/dark cycle with water and food (Envigo Teklad 8640) provided ad libitum. Produced litters were weaned at 6 weeks and grown till 6 months of age. Only males were used in the experiments. Mice were anesthetized with isoflurane and the kidneys were cleared of blood by retrograde perfusion via abdominal aorta [34]. M1 cell culture was purchased from ATCC (#CRL-2038) and maintained under standard culture conditions recommended by the vendor: DMEM:F-12 1:1, 5% FBS (ATCC #30–2006; 30–2020), 1% PenStrep and 1% Insulin-Transferrin-Selenium Mix (Gibco #15140–122; 41400–045) in a 5% CO2/37°C incubator.
Immunohistochemistry.
After antigen retrieval (S2369) samples were blocked with Dako system (X0590, X0909) and stained with primary antibody Anti-PANX1, 1:20 (HPA16930, Sigma-Aldrich) or Anti-P2X7 Receptor, 1:50 (APR-004, Alomone Labs). For secondary staining we used biotinylated Anti-Rabbit Antibody 1:200 (BA-1000, Vector Laboratories, Burlingame, CA) and Streptavidin-HRP (Dako K0609). Visualization was conducted with DAB+ Substrate (Dako K3468). Images were captured at 40x magnification with Nikon microscope equipped with a histology camera and Leica Aperio slide scanner. For signal intensity quantification Fuji software package (NIH, Bethesda) was employed. Mean intensity was measured in apical regions of proximal tubules (taken as background), non-dilated collecting ducts and cyst lining cells.
Growing cells on permeable supports and ATP release assay.
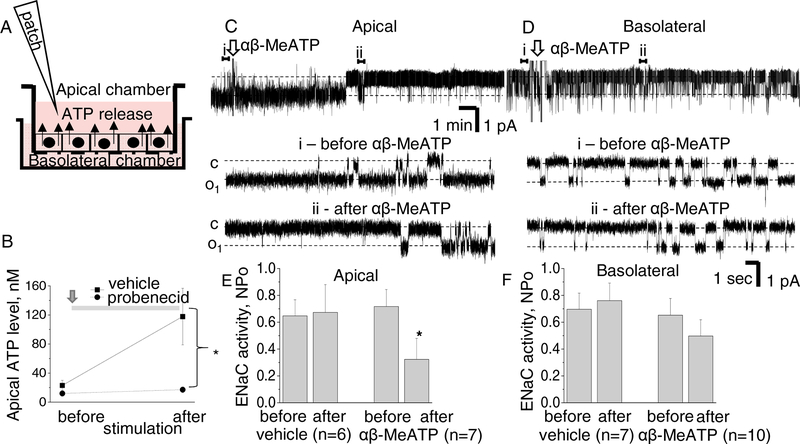
M1 cells were seeded onto Transwell Corning 3801 snapwells and cultured for several days until an epithelial monolayer formed [35]. Figure 3A demonstrates that similarly to the Ussing system, snapwells have apical and basolateral chambers separated by a cellular monolayer grown on the permeable 0.22 μm pore membrane. Epithelial Volt Ohm Meter (Warner Instruments) was used to verify formation of a high-resistive monolayer, and experiments were performed when resistance reached ~1 kΩ cm2. ATP production was stimulated with a 60 min long slow rocking. Samples of the media were collected from the apical chamber before and after flow stimulation and ATP level was measured with bioluminescent luciferin/luciferase assay kit (Invitrogen #A22066). Probenecid (Santa Cruz, CAS 57-66-9) was applied to study its effect on ATP release.
Figure 3. ATP release by M1 cells and the effect of P2X stimulation on ENaC.
A - scheme of Transwell Corning system demonstrates that M1 cells form polarized tight epithelial monolayer which separates apical and basolateral chambers. B - accumulation of ATP in the apical chamber during 1 hr mechanical stimulation with rocking is inhibited by treatment with 50 μM probenecid. C,D - 100 μM αβ-MeATP was applied into apical (C) or basolateral (D) chambers. Upper traces are recordings of ENaC activity, arrows denote application of the drug. Representative sections before (i) and after (ii) are expanded below. “c” and “o1” denote closed and opened states of the channel, respectively; scale bars for entire and expanded traces are shown; holding potential is −40 mV; openings are downward. E,F - Summary graphs demonstrate that apical application of αβ-MeATP acutely decreases activity of ENaC whereas basolateral or control application of vehicle have no effect.
Patch-clamp experiments.
Single-channel currents were acquired and subsequently analyzed with an Axopatch 200B amplifier (Axon Instruments) interfaced via a Digidata 1550B to a PC running the pClamp 10.6 suite of software (Axon Instruments). Bath solution contained (in mM): 150 NaCl, 1 CaCl2, 2 MgCl2, 10 HEPES (pH 7.4). Pipette solutions for were (in mM): 140 LiCl, 2 MgCl2 and 10 HEPES (pH 7.4). Snapwells with grown M1 cells were detached from the holder and transferred to the inverted microscope Nikon Ti-S. When mounted into the bath, snapwell has isolated apical and basolateral chambers that allows applying drugs specifically from one side. Patch-clamp micropipettes approached apical membrane and conventional cell-attached voltage clamp was performed to record ENaC activity at −40 mV holding potential as used earlier [35]. P2X agonist αβ-MeATP (α,β-Methyleneadenosine 5ʹ-triphosphate) (Tocris #3209) was applied in 100 μM concentration.
Statistics.
All data presented as mean±SEM. Significance of difference in independent groups was verified with non-parametric Mann-Whitney U test, in paired experiments – with Wilcoxon signed-rank test. *p<0.05; *** p<0.001
Results
Augmented Pannexin-1 expression in ADPKD cysts.
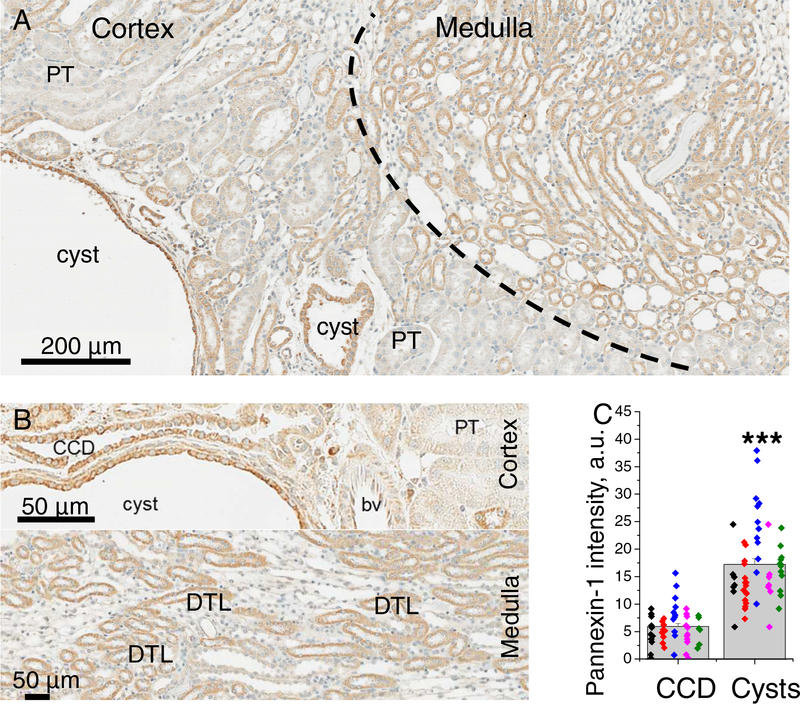
Pannexin-1 was shown to mediate urinary ATP excretion in healthy c57 mice [21]; here we first studied the distribution of this protein in Pkd1RC/RC mouse kidneys. Immunohistochemical staining on Figure 1 demonstrates pannexin-1 signal in the nephron, similarly to Hanner and colleagues [21]. In the cortex, the protein presence was detected in proximal tubules but significantly more intense levels were typical for cortical collecting ducts. Labeling was highly abundant at the apical membrane of collecting ducts and less in the cytoplasm; also all CCD cell were Panx1-positive indicating expression in both principal and intercalated cells (Figure 1B). In the medulla, a large portion of tubules (descending thin limbs, according to [21]) exhibit strong signal. Surprisingly, cyst lining cells demonstrated an increased abundance of pannexin-1, which was ~2.8 fold higher than in collecting ducts (Figure 1C). Therefore, development of ADPKD cysts could be associated with enhanced expression of Panx1 in cystic epithelium.
Figure 1. Pannexin-1 distribution in Pkd1RC/RC mouse kidneys.
A - staining at 10x magnification shows Panx1 expression in cortex and medulla. B - in the cortex, Panx1 signal is prominent in cysts and non-dilated collecting ducts; in the medulla - in DTL. C -quantification reveals significantly higher labelling in cysts vs collecting ducts. Each color represents one animal; CCD - collecting duct, PT -proximal tubule, DTL - descending thin limb, bv - blood vessel
High abundance of P2X7 receptors in ADPKD cysts.
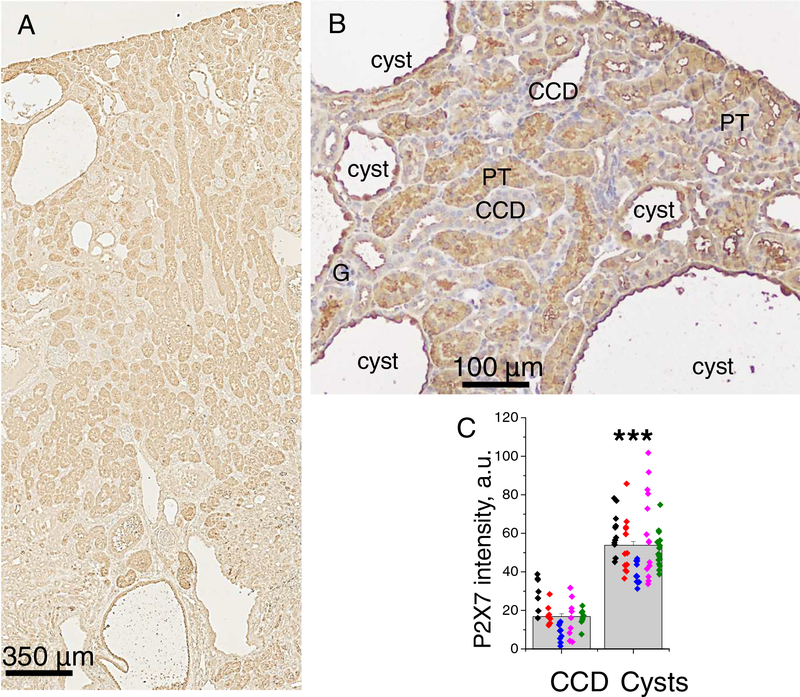
Normal collecting ducts express both P2Y and P2X receptors. However, we recently demonstrated in PCK rats that the development of cysts is accompanied by loss of P2Y and transition to prevalent P2X signaling [9]. Also, P2X7 receptor was characterized as a factor facilitating activation of pannexin-1 channels and ATP release. Here, we studied the expression of the P2X7 receptor in ADPKD mouse kidneys. Figure 2A shows the distribution of this protein in renal sections of a 6 months old mouse. A moderate staining level was detected in collecting ducts, and prominent labeling was found in large portion of proximal tubules. Most importantly, P2X7 abundance was dramatically increased in dilated CCDs, especially in the apical membrane (Figure 2B) and reached a ~3.2 fold higher level in mature cysts compared to non-dilated collecting ducts (Figure 2C).
Figure 2. P2X7 distribution in Pkd1RC/RC mouse kidneys.
A - P2X7 staining in cortex and medulla. B - P2X7 abundance elevates during transition of CCDs to cysts; non-dilated CCD have low signal, developing cysts exhibit increased level especially in principal cells, mature cysts have strong P2X7 labeling in apical membranes of the cyst lining-cells. C - summary graph quantifies an enhanced expression of P2X in cysts vs collecting ducts. Each color represents one animal.
Probenecid precludes ATP release by collecting duct cells.
High expression of pannexin-1 and P2X7 in cysts is a unique phenomenon which is important because these proteins can contribute to ATP release. We studied apical ATP release stimulated in M1 cells by shear-stress (1 hour rocking) with or without application of probenecid, a pannexin-1 inhibitor [36]. The cells form a polarized highly differentiated monolayer suitable for studies of epithelial physiology [37, 38]. Mechanical stimulation leads to accumulation of ATP in the apical chamber from 23±7 to 118±29 nM whereas treatment with 50 μM probenecid blunts this effect (1 and 10 μM probenecid provided no effect, data not shown). We conclude that collecting ducts are capable of releasing ATP via a Panx1-dependent mechanism.
P2X stimulation decreases activity of ENaC.
ATP is a powerful downregulator of ENaC activity and P2Y signaling is recognized as a key mediator of this effect [39–41]. The contribution of P2X receptors is less studied; data from amphibian cells indicate that the effect depends on polarity of application: ENaC inhibition was observed in non-polarized oocytes whereas basolateral application of MeS-ATP induced ENaC activation in A6 cell monolayer [42, 43]. Here we tested the effect of αβ-MeATP, a P2X agonist used in our experiments earlier [9] on polarized mammalian M1 cells. Cell attached analysis of ENaC activity was performed from the apical membranes whereas 100μM αβ-MeATP (or vehicle) was applied into apical or basolateral chambers. Figures 3C,D demonstrate 20 min recordings of ENaC activity (upper panels) and expanded representative sections before and after αβ-MeATP applications. As seen on the traces, apical (3C) but not basolateral (3D) application of the drug decreases ENaC activity. Summary graphs also demonstrates the lack of effect of vehicle application on ENaC activity (3E,F). These experiments support the hypothesis that ATP accumulation in the cyst space can reduce the activity of ENaC in cyst-lining cells.
Discussion
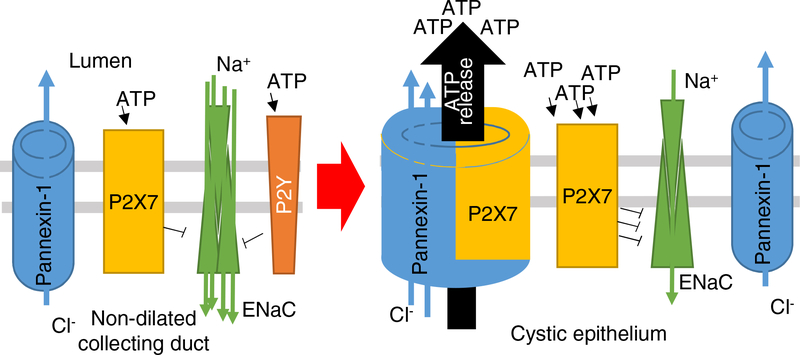
ADPKD is the most prevalent inherited progressive kidney disease affecting 1:1000 live births in the USA [44]. Paracrine factors are important players in cellular physiology and pathophysiology of PKD, and extracellular milieu of kidney cysts has a potential to serve as paracrine factors promoting cystogenesis. For example, cystic fluid collected from DBA/2FG-pcy/pcy mice is capable of stimulating cyst growth when applied to MDCK 3D culture [45]. The origins of the secretagogues and mitogens in cysts were not fully determined, however, later a number of publications suggested that abnormal cystic ATP accumulation and purinergic signaling may contribute to the development of both ARPKD and ADPKD. Our data collected in Pkd1RC/RC mice reveal high expression of pannexin-1 and P2X7 membrane proteins capable of ATP release. There is evidence coming from other areas of research that these two proteins interact, facilitating ATP release; moreover, the significance of Panx1 for ATP urinary secretion was demonstrated earlier [19, 21]. Tanner and colleagues used two-photon in vivo microscopy to study renal transport of sulfonefluorescin, an organic anion dye secreted by proximal tubules, and found that probenecid reduces accumulation of sulfonefluorescin in normal proximal tubules and Han:SPRD proximal cysts [46]. We assume that in the distal cysts luminal permeability to organic anions such as ATP or sulforhodamine B is mediated by pannexin-1, and can be reduced by probenecid, similar to our experiments shown in Figure 3. Therefore, we report that activation of pannexin-1 channels by P2X7 receptors can be highly important in the development of PKD and serves as a pivotal pathogenic mechanism of cystogenesis (Figure 4). Recently we demonstrated the remodeling of the P2 receptor profile in ARPKD cysts towards prevalence of P2X over P2Y signaling [9]; together these observations delineate this phenomenon as common for both forms of PKD. Interestingly, impaired P2Y signaling was suggested earlier as a factor unleashing vasopressin-dependent cAMP production in ADPKD [47].
Figure 4. Schematic illustration of the mechanism.
In normal collecting ducts ATP acts via P2Y and P2X receptors. However, in the developing cysts P2X receptors become more prevalent. Also, cystic epithelium enhances expression of pannexin-1 which is able to form a channel with P2X7 capable of facilitating ATP release into the cyst. High ATP levels promote cystogenesis by different mechanisms, particularly decreasing activity of ENaC-dependent reabsorption in cysts cells
As a powerful regulator of epithelial water-electrolyte transport, ATP can decrease reabsorption in the collecting duct system, where ~70% of ADPKD cysts develop. Particularly, as a paracrine agent, ATP limits activity of the epithelial sodium channel and increases chloride secretion in M1 cells [48]. Veizis et al. have shown that amiloride sensitive Na+ absorption is decreased in CD cells from the non-orthologous bpk mouse model of ARPKD [49] and later we demonstrated that impaired ENaC activity exacerbates development of PCK rat cysts [12, 13]. Involvement of ENaC in the development of ADPKD remains poorly understood. This study provides a rationale for targeting of pannexin-1 dependent ATP release in ADPKD, and suggests potential novel ways to correct abnormal renal management of sodium observed in hypertensive patients with ADPKD [50].
Supplementary Material
Highlights:
ADPKD cysts epithelia exhibit a hyperexpression of pannexin-1 and P2X7 proteins
Pannexin-1 in renal epithelial cells mediates ATP release into the lumen
P2X7 activation shown earlier to facilitate Panx1 conductance, reduces ENaC activity
We suggest a new mechanism of pathogenic ATP accumulation in cystogenesis
Acknowledgement
We are grateful to Dr. Peter C. Harris (Mayo Clinic Translational Polycystic Kidney Disease Center) for providing animals and critical reading of the manuscript. We also thank D’Anna Potter for technical assistance and HFHS Histology Core for service. Funding: P30 DK090868 via P&F Grant, R00 HL116603 (TSP) and P30 DK090728 (PCH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Torres VE, Harris PC, Mechanisms of Disease: autosomal dominant and recessive polycystic kidney diseases, Nat. Clin. Pract. Nephrol, 2 (2006) 40–55. [DOI] [PubMed] [Google Scholar]
- [2].Bergmann C, Senderek J, Kupper F, Schneider F, Dornia C, Windelen E, Eggermann T, Rudnik-Schoneborn S, Kirfel J, Furu L, Onuchic LF, Rossetti S, Harris PC, Somlo S, Guay-Woodford L, Germino GG, Moser M, Buttner R, Zerres K, PKHD1 mutations in autosomal recessive polycystic kidney disease (ARPKD), Hum. Mutat, 23 (2004) 453–463. [DOI] [PubMed] [Google Scholar]
- [3].Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE, Polycystic kidney disease, Nature Reviews Disease Primers, 4 (2018) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Antignac C, Calvet JP, Germino GG, Grantham JJ, Guay-Woodford LM, Harris PC, Hildebrandt F, Peters DJM, Somlo S, Torres VE, Walz G, Zhou J, Yu ASL, The Future of Polycystic Kidney Disease Research—As Seen By the 12 Kaplan Awardees, Journal of the American Society of Nephrology, 26 (2015) 2081–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pochynyuk O, Rieg T, Bugaj V, Schroth J, Fridman A, Boss GR, Insel PA, Stockand JD, Vallon V, Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone, FASEB J, 24 (2010) 2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Solini A, Usuelli V, Fiorina P, The Dark Side of Extracellular ATP in Kidney Diseases, Journal of the American Society of Nephrology, 26 (2015) 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schwiebert EM, Wallace DP, Braunstein GM, King SR, Peti-Peterdi J, Hanaoka K, Guggino WB, Guay-Woodford LM, Bell PD, Sullivan LP, Grantham JJ, Taylor AL, Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys, American Journal of Physiology - Renal Physiology, 282 (2002) F763–F775. [DOI] [PubMed] [Google Scholar]
- [8].Wilson PD, Hovater JS, Casey CC, Fortenberry JA, Schwiebert EM, ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys, J Am Soc. Nephrol, 10 (1999) 218–229. [DOI] [PubMed] [Google Scholar]
- [9].Palygin O, Ilatovskaya DV, Levchenko V, Klemens CA, Dissanayake L, Williams AM, Pavlov TS, Staruschenko A, Characterization of purinergic receptor expression in ARPKD cystic epithelia, Purinergic Signalling, 14 (2018) 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rangan G, Role of extracellular ATP and P2 receptor signaling in regulating renal cyst growth and interstitial inflammation in polycystic kidney disease, Front. Physiol, 4 (2013) 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ilatovskaya DV, Palygin O, Staruschenko A, Functional and therapeutic importance of purinergic signaling in polycystic kidney disease, American Journal of Physiology - Renal Physiology, 311 (2016) F1135–F1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pavlov TS, Levchenko V, Ilatovskaya DV, Palygin O, Staruschenko A, Impaired epithelial Na+ channels activity contributes to cystogenesis and development of autosomal recessive polycystic kidney disease in PCK rats, Pediatric research, 77 (2015) 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ilatovskaya DV, Levchenko V, Pavlov TS, Isaeva E, Klemens CA, Johnson J, Liu P, Kriegel AJ, Staruschenko A, Salt-deficient diet exacerbates cystogenesis in ARPKD via epithelial sodium channel (ENaC), EBioMedicine, 40 (2019) 663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hooper KM, Unwin RJ, Sutters M, The isolated C-terminus of polycystin-1 promotes increased ATP-stimulated chloride secretion in a collecting duct cell line, Clin Sci (Lond), 104 (2003) 217–221. [DOI] [PubMed] [Google Scholar]
- [15].Rajagopal M, Wallace DP, Chloride secretion by renal collecting ducts, Curr Opin Nephrol Hypertens, 24 (2015) 444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dahl G, ATP release through pannexon channels, Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 370 (2015) 20140191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bao L, Locovei S, Dahl G, Pannexin membrane channels are mechanosensitive conduits for ATP, FEBS Letters, 572 (2004) 65–68. [DOI] [PubMed] [Google Scholar]
- [18].Pelegrin P, Surprenant A, Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor, The EMBO Journal, 25 (2006) 5071–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dahl G, The Pannexin1 membrane channel: distinct conformations and functions, FEBS Letters, 592 (2018) 3201–3209. [DOI] [PubMed] [Google Scholar]
- [20].Taruno A, ATP Release Channels, International Journal of Molecular Sciences, 19 (2018) 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hanner F, Lam L, Nguyen MTX, Yu A, Peti-Peterdi J, Intrarenal localization of the plasma membrane ATP channel pannexin1, American journal of physiology. Renal physiology, 303 (2012) F1454–F1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Abed AB, Kavvadas P, Chadjichristos CE, Functional roles of connexins and pannexins in the kidney, Cellular and Molecular Life Sciences, 72 (2015) 2869–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Locovei S, Scemes E, Qiu F, Spray DC, Dahl G, Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex, FEBS Letters, 581 (2007) 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tozzi M, Larsen AT, Lange SC, Giannuzzo A, Andersen MN, Novak I, The P2X7 receptor and pannexin-1 are involved in glucose-induced autocrine regulation in β-cells, Scientific Reports, 8 (2018) 8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cheung WY, Fritton JC, Morgan SA, Seref-Ferlengez Z, Basta-Pljakic J, Thi MM, Suadicani SO, Spray DC, Majeska RJ, Schaffler MB, Pannexin-1 and P2X7-Receptor Are Required for Apoptotic Osteocytes in Fatigued Bone to Trigger RANKL Production in Neighboring Bystander Osteocytes, Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research, 31 (2016) 890–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G, The Pannexin 1 Channel Activates the Inflammasome in Neurons and Astrocytes, Journal of Biological Chemistry, 284 (2009) 18143–18151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hung S-C, Choi CH, Said-Sadier N, Johnson L, Atanasova KR, Sellami H, Yilmaz Ö, Ojcius DM, P2X4 Assembles with P2X7 and Pannexin-1 in Gingival Epithelial Cells and Modulates ATP-induced Reactive Oxygen Species Production and Inflammasome Activation, PLOS ONE, 8 (2013) e70210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li S, Tomić M, Stojilkovic SS, Characterization of novel Pannexin 1 isoforms from rat pituitary cells and their association with ATP-gated P2X channels, General and Comparative Endocrinology, 174 (2011) 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vessey DA, Li L, Kelley M, Pannexin-I/P2X 7 Purinergic Receptor Channels Mediate the Release of Cardioprotectants Induced by Ischemic Pre- and Postconditioning, Journal of Cardiovascular Pharmacology and Therapeutics, 15 (2010) 190–195. [DOI] [PubMed] [Google Scholar]
- [30].Sharma AK, Charles EJ, Zhao Y, Narahari AK, Baderdinni PK, Good ME, Lorenz UM, Kron IL, Bayliss DA, Ravichandran KS, Isakson BE, Laubach VE, Pannexin-1 channels on endothelial cells mediate vascular inflammation during lung ischemia-reperfusion injury, American Journal of Physiology-Lung Cellular and Molecular Physiology, 315 (2018) L301–L312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Miteva AS, Gaydukov AE, Shestopalov VI, Balezina OP, Mechanism of P2X7 receptor-dependent enhancement of neuromuscular transmission in pannexin 1 knockout mice, Purinergic Signalling, 14 (2018) 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sáez PJ, Vargas P, Shoji KF, Harcha PA, Lennon-Duménil A-M, Sáez JC, ATP promotes the fast migration of dendritic cells through the activity of pannexin 1 channels and P2X7 receptors, Science Signaling, 10 (2017) eaah7107. [DOI] [PubMed] [Google Scholar]
- [33].Hopp K, Ward CJ, Hommerding CJ, Nasr SH, Tuan H-F, Gainullin VG, Rossetti S, Torres VE, Harris PC, Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity, The Journal of Clinical Investigation, 122 (2012) 4257–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pavlov TS, Ilatovskaya DV, Palygin O, Levchenko V, Pochynyuk O, Staruschenko A, Implementing Patch Clamp and Live Fluorescence Microscopy to Monitor Functional Properties of Freshly Isolated PKD Epithelium, (2015) e53035. [DOI] [PMC free article] [PubMed]
- [35].Karpushev AV, Levchenko V, Ilatovskaya DV, Pavlov TS, Staruschenko A, Novel role of Rac1/WAVE signaling mechanism in regulation of the epithelial Na+ channel, Hypertension, 57 (2011) 996–1002. [DOI] [PubMed] [Google Scholar]
- [36].Silverman W, Locovei S, Dahl G, Probenecid, a gout remedy, inhibits pannexin 1 channels, American journal of physiology. Cell physiology, 295 (2008) C761–C767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pavlov TS, Levchenko V, Staruschenko A, Role of Rho GDP Dissociation Inhibitor α in Control of Epithelial Sodium Channel (ENaC)-mediated Sodium Reabsorption, The Journal of Biological Chemistry, 289 (2014) 28651–28659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pavlov TS, Levchenko V, Karpushev AV, Vandewalle A, Staruschenko A, Peroxisome proliferator-activated receptor gamma antagonists decrease Na+ transport via the epithelial Na+ channel, Mol. Pharmacol, 76 (2009) 1333–1340. [DOI] [PubMed] [Google Scholar]
- [39].Toney GM, Vallon V, Stockand JD, Intrinsic control of sodium excretion in the distal nephron by inhibitory purinergic regulation of the epithelial Na(+) channel, Current opinion in nephrology and hypertension, 21 (2012) 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mironova E, Suliman F, Stockand JD, Renal Sodium Excretion Consequent to Pharmacogenetic Activation of Gq-DREADD in Principal Cells, American Journal of Physiology-Renal Physiology, (2019) doi: 10.1152/ajprenal.00612.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang Y, Li L, Kohan DE, Ecelbarger CM, Kishore BK, Attenuation of lithium-induced natriuresis and kaliuresis in P2Y₂ receptor knockout mice, American journal of physiology. Renal physiology, 305 (2013) F407–F416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Thai TL, Yu L, Eaton DC, Duke BJ, Al-Khalili O, Lam HYC, Ma H, Bao H-F, Basolateral P2X₄ channels stimulate ENaC activity in Xenopus cortical collecting duct A6 cells, American journal of physiology. Renal physiology, 307 (2014) F806–F813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wildman SS, Marks J, Churchill LJ, Peppiatt CM, Chraibi A, Shirley DG, Horisberger J-D, King BF, Unwin RJ, Regulatory Interdependence of Cloned Epithelial Na+ Channels and P2X Receptors, Journal of the American Society of Nephrology, 16 (2005) 2586–2597. [DOI] [PubMed] [Google Scholar]
- [44].Torres VE, Harris PC, Pirson Y, Autosomal dominant polycystic kidney disease, Lancet, 369 (2007) 1287–1301. [DOI] [PubMed] [Google Scholar]
- [45].Yamaguchi T, Nagao S, Takahashi H, Ye M, Grantham JJ, Cyst fluid from a murine model of polycystic kidney disease stimulates fluid secretion, cyclic adenosine monophosphate accumulation, and cell proliferation by Madin-Darby canine kidney cells in vitro, American Journal of Kidney Diseases, 25 (1995) 471–477. [DOI] [PubMed] [Google Scholar]
- [46].Tanner GA, Sandoval RM, Dunn KW, Two-photon in vivo microscopy of sulfonefluorescein secretion in normal and cystic rat kidneys, American Journal of Physiology-Renal Physiology, 286 (2004) F152–F160. [DOI] [PubMed] [Google Scholar]
- [47].Chebib FT, Sussman CR, Wang X, Harris PC, Torres VE, Vasopressin and interactive calcium, cyclic AMP and purinergic signaling in Polycystic Kidney Disease, Nature reviews. Nephrology, 11 (2015) 451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cuffe JE, Bielfeld-Ackermann A, Thomas J, Leipziger J, Korbmacher C, ATP stimulates Cl− secretion and reduces amiloride-sensitive Na+ absorption in M-1 mouse cortical collecting duct cells, The Journal of Physiology, 524 (2000) 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Veizis EI, Carlin CR, Cotton CU, Decreased amiloride-sensitive Na+ absorption in collecting duct principal cells isolated from BPK ARPKD mice, Am J Physiol Renal Physiol, 286 (2004) F244–F254. [DOI] [PubMed] [Google Scholar]
- [50].Torres VE, Wilson DM, Burnett JC, Johnson CM, Offord KP, Effect of Inhibition of Converting Enzyme on Renal Hemodynamics and Sodium Management in Polycystic Kidney Disease, Mayo Clinic Proceedings, 66 (1991) 1010–1017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.