Abstract
Research into the function of microglia has dramatically accelerated during the last few years, largely due to recent genetic findings implicating microglia in virtually every neurodegenerative disorder. In Alzheimer’s disease, the majority of risk loci discovered through genome-wide association-studies were found in or near genes expressed most highly in microglia leading to the hypothesis that microglia play a much larger role in disease progression than previously thought. From this body of work produced in the last several years, we find that almost every function of microglia has been proposed to influence the progression of Alzheimer’s disease (AD) from altered phagocytosis and synaptic pruning to cytokine secretion and changes in trophic support. By studying key Alzheimer’s risk-genes such as TREM2, CD33, ABCA7, and MS4A6A, we will be able to distinguish true disease-modulatory pathways from the full range of microglial related functions. To successfully carry out these experiments, more advanced microglial models are needed. Microglia are quite sensitive to their local environment, suggesting the need to more fully recapitulate an in vivo environment to study this highly plastic cell type. Likely only by combining the above approaches, will the field fully elucidate the molecular pathways that regulate microglia and influence neurodegeneration, in turn uncovering potential new targets for future therapeutic development.
Keywords: Neurodegeneration, Microglia, Alzheimer’s disease, neuroinflammation, genome-wide association studies
Alzheimer’s Disease and the Amyloid Cascade Hypothesis
Alzheimer’s Disease (AD) is the most common form of dementia and the sixth leading cause of death in the Unites States1. Unlike most other causes of death, the incidence of AD continues to rise, and cases are expected to double within the next 30 years as our population ages. Basic and translational science coupled with medical advances, have greatly increased human lifespan, but with this comes increased risk of developing age-related diseases, such as AD. Thus, it is critically important to focus our research efforts on increasing the healthy years of life in older individuals.
Alzheimer’s disease was first identified in 1908 by a German Neurologist, Alois Alzheimer who described patients who exhibited disorientation, confusion, and progressive memory loss. His pathological examinations further revealed brain atrophy and the accumulation of key pathologies including intraneuronal neurofibrillary tangles, extracellular plaques, and morphological changes in microglia, the primary immune cells of the brain2. One hundred years past this original characterization, the diagnosis of AD remains largely similar, though somewhat more precise. Clinicians look for insidious onset of amnesic presentation, difficulties finding words, impaired facial recognition, and deficits in problem solving3. Researchers today are still searching for validated biomarkers through neuroimaging, cerebrospinal fluid (CSF), blood, or urine tests as well as genetic risk profiling, but none have yet proved to be reliably conclusive in large-scale clinical trials.
While the majority of AD occurs ‘sporadically’ in aged individuals, much can be learned from the rarer familial forms of AD (fAD). fAD accounts for around 2% of all AD cases, and often occurs earlier in life with onset in the 30s or 40s. Familial Alzheimer’s disease occurs due to inherited genetic mutations within the genes presenilin-1, presenilin-2, or amyloid precursor protein (APP). Each of these mutations effects the production and processing of beta-amyloid (Aβ), which is the primary component of the extracellular plaques that were first described by Alois Alzheimer. The identification and subsequent understanding of the functional effects of these mutations, led to the proposal by Hardy and Higgins in 1992 of the ‘amyloid cascade hypothesis’ of AD4. This hypothesis posits that Aβ accumulation is the initial cause of AD that in turn induces a series of downstream pathological cascades including neurofibrillary tangle formation, inflammatory responses, as well as synaptic and neuronal loss. In strong support of this hypothesis, imaging studies have now clearly shown that Aβ begins to accumulate some 10–15 years prior to diagnosis. As a response to this hypothesis and the evidence that Aβ pathology is one of the first recognizable signs of AD, many drugs have been developed to clear Aβ from the brain in an attempt to relieve the symptoms of AD and potentially halt disease progression. To date, many therapies targeting Aβ synthesis or clearance have been tested in clinical trials (bapineuzumab, solanezumab, tarenflurbil, phenserine, gammagard etc.) but unfortunately none have yet proved to be effective in reducing memory deficits or halting disease progression in late stage trials. Famously, one compound; PF-04494700 a drug licensed by Pfizer, actually caused AD patients to deteriorate faster than their placebo counterparts.
A likely issue with Aβ centered treatments may be that patients are treated too late in the disease process. Since Aβ has already been accumulating for ~10 years by the time patients are first diagnosed with AD or mild cognitive impairment (MCI), removal of Aβ from the brain is unlikely to resolve the additional downstream consequences of AD neuropathology. In others words, once neuroinflammation, tau pathology, and neurodegeneration begin, it may make little difference in disease progression to remove the initial insult of Aβ plaques. Instead, therapies that better target these downstream processes may be far more effective at later stages of disease. Yet, Aβ therapies could still be useful if treatments can be begun during the prodromal phases of the disease. Thus, research into earlier diagnosis and accurate biomarkers remains critical.
Microglia in AD pathogenesis
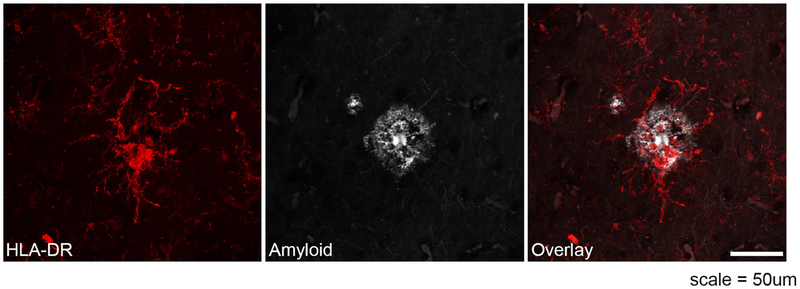
As mentioned previously, signs of microglial activation in AD, as assessed by broad morphological analysis, was first described by Alois Alzheimer in 19082. Since then, many groups have clearly demonstrated the close spatial-temporal relationship between Aβ plaques and activated microglia in both AD patients and mouse models (Figure 1). Several studies have also further visualized beta-amyloid itself within microglia cell bodies, suggesting an important role for microglia phagocytosis in the clearance of beta-amyloid5. Because microglia are preferentially activated in close proximity to Aβ plaques, many groups hypothesized that the plaques are responsible for activating microglia, further explaining the prominent hypothesis that beta-amyloid initiates the Alzheimer’s disease cascade. Yet, we now have evidence that microgliosis occurs prior to visible Aβ plaque deposition6. Furthermore, recent evidence suggests that microgla may even contribute to the seeding of plaques as pharmacological depletion of microglia leads to a significant reduction in plaque pathology in 5xfAD transgenic mice7. The next big questions are: what process leads to this microglial activation, and what are microglia doing to promote plaque formation or to inhibit plaque clearance?
Fig. 1.
Disease-associated microglia surrounding Aβ plaques. Immunofluorescent stain of human Alzheimer's patient tissue demonstrates microglia (stained with DAM marker HLA-DR, red) surrounding Aβ plaques (gray). HLA is upregulated in microglia around plaques. The scale represents 50 μm.
Because microglia are highly sensitive to changes in their environment, these cells have proven difficult to study. Thus far, murine models have served as the primary tool to study microglial genetics and function. While these model systems have led to important discoveries of microglial ontogeny and function, it has also become clear that there are important differences between murine microglia and human microglia which are particularly evident in aging and disease8,9. Thus, we must be careful not to simply conclude that findings in mouse models will necessarily translate to human microglia. In order to study human microglia, several labs have developed techniques to isolate human microglia from brain tissue removed during surgical resection of epileptic foci or brain tumors10–12. This approach provides one of the very few methods to study viable human brain-derived microglia, but remains logistically very challenging. Another innovative technique to overcome the difficulty of studying human microglia has been to isolate microglia or their nuclei from postmortem brain tissue. These techniques have allowed researchers to discover important human-specific changes that occur as microglia age13. Still, it is likely that the agonal state preceding death, co-morbid infectious or inflammatory conditions such as pneumonia, or post-mortem delay influence microglial gene expression and activation state which may may obscure and greatly complicate data interpretation. Given these complications, several groups including our own have developed protocols to differentiate human microglia from pluripotent stem cells14–20. Producing human microglia in vitro allows scientists to study these cells using better-controlled and more mechanistic approaches including the use of drug libraries and genetic manipulation such as CRISPR.
Although a fully defined microglia differentiation protocol is extremely useful for experiments that aim to study the mechanistic functions of human microglia, microglia in isolation may function quite differently than those in the brain environment. More comprehensive models of human microglia in a brain-like environment continue to be developed and include studies that involve engrafting human iPS-derived microglia into 3D neuronal cultures, brain organoids, or murine brains14,21,22 In order to recapitulate how human microglia react to realistically complex disease environments such as beta-amyloid plaques, neurofibrillary tangles, or traumatic brain injury, etc., a chimeric xenotransplantation system is likely to best mimic human disease and thus help narrow the focus of pre-clinical targets to ones which most accurately reflect what occurs in patients.
Genome-wide Association Studies
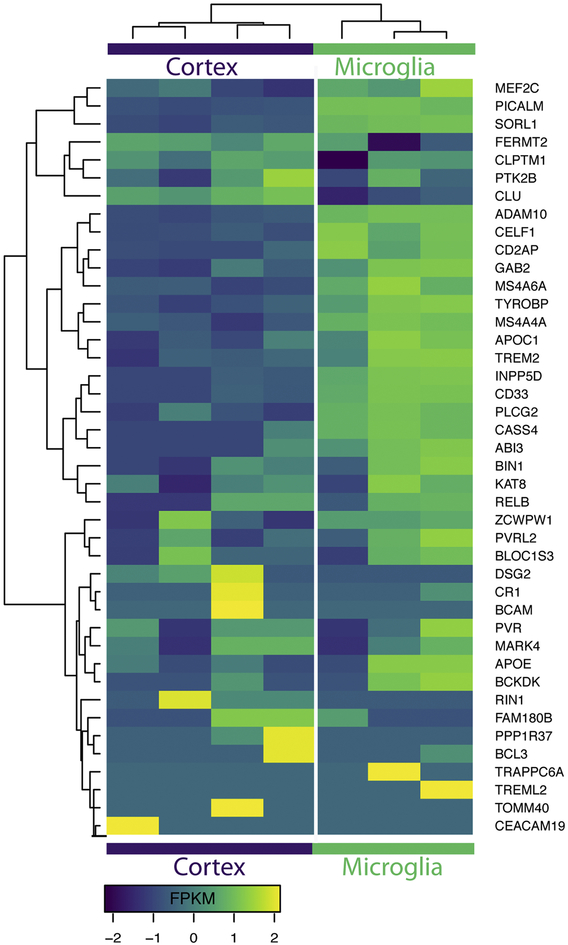
Some clues as to how microglia may be effecting the progression of Alzheimer’s disease can be found by studying which microglia-specific gene variants cause risk for or protection from AD. In recent years, the power of genomics has allowed geneticists to uncover many single nucleotide polymorphisms (SNPs) that are correlated with differential AD risk. These studies have confirmed the previously established importance of Apolipoprotein E (APOE), while also uncovering many new risk-SNPs. SNP variants may occurr within gene coding regions, or influence disease risk through known promoters, enhancers. Additionally SNPs may be sign posts which are inherited alongside mutations which are in map linkage disequilibrium. For this review, we will only discuss SNPs which are correlated with actual changes in gene expression or protein function. Surprisingly, around two thirds of these new AD-risk SNPs are exclusively or most highly expressed in microglia. This data has been corroborated by many groups including a recent study of over 300,000 individuals that reported 48 AD-risk SNPs (FDR < 10−5), 29 of which are most highly expressed by microglia (60.4%)23,24 (Figure 2). This data hints that changes in microglial function may influence differential risk for AD, suggesting that these brain-resident immune cells play a far greater role in disease development and progression than previously thought. While this review will not cover the role of every microglial specific risk gene, we provide a broad overview of all current AD-risk SNPs in Table 1.
Fig. 2.
Alzheimer’s risk genes are enriched in microglia over total cortex expression. Transcriptome data from Zhang et al. [24] was used to generate this heatmap of expression levels of each AD risk gene in the brain cortex (left) versus expression level in microglial cells (right). Data are displayed in frequency per kilobase million reads (FPKM).
Table 1.
Alzheimer’s disease risk-loci and their proposed functions
| SNP ID | Proposed gene affected | Function | Citation |
|---|---|---|---|
| rs3764650 rs3752246 |
ABCA7 | lipid transport | Allen et al. Neurology 2012 |
| rs616338 | ABI3 | actin polymerization | Sims et al. Nat Genet 2017 |
| rs2305421 | ADAM10 | cleaved TNFa and E-cadherin | Akhter et al. Neurobio Aging 2018 |
| rs4420638 | APOC1 | lipid metabolism | Lin et al. J Hu Genetics 2016 |
| rs5167 | APOC4 | lipid metabolism | Allan et al. Genomics 1995 |
| rs2075650 | APOE | lipid metabolism | Lin et al. J Hu Genetics 2016 |
| rs889555 | BCKDK | unknown immune function | NA |
| rs2965101 rs2927438 |
BCL3 | NF-kB immune regulation and survival | Poveda et al. Exp Mol Med 2017 |
| rs744373 rs7561528 |
BIN1 | endocytosis and phagocytosis | Prokic et al. J Mol Md 2014, Gold et al. J Exp Med 2004 |
| rs597668 | BLOC1S3 | endosome and lysosome trafficking | Seshadri et al. JAMA 2010 |
| rs7274581 rs6024870 |
CASS4 | cell adhesion and axonal transport | Beck et al. Oncoscience 2014 |
| rs9349407 rs9296559 |
CD2AP | cytoskeletal remodeling | Guimas et al. Cell Mol Life Sci 2018 |
| rs3865444 rs3826656 |
CD33 | phagocytosis | Griciuc et al Neuron 2013 |
| rs2965109 | CEACAM16 | antigen cell adhesion | Kammerer et. Al J Biol Chem 2012 |
| rs714948 | CEACAM19 | antigen cell adhesion | Kleita et al. Int J Oncol 2013 |
| rs10838725 | CELF1 | transcription regulation | Dasgupta and Ladd Wiley Interdiscp Rev RNA 2013 |
| rs35577563 | CLPTM1 | telomere regulation | Carkic et al. J Oral Sci 2016 |
| rs11136000 | CLU | complement, apoptosis, lipid transport | Karch and Goate Biol Psy 2015 |
| rs679515 rs3818361 |
CR1 | complement | Rogers et al. Neurobiol Aging 2006 |
| rs8093731 | DSG2 | lysosomal function | Karch and Goate Biol Psy 2015 |
| rs11767557 rs11771145 |
EPHA1 | immune response, cell adhesion and motility | Misra et al. Indian J Med Res 2018, Aasheim et al. Blood 2005 |
| rs10415983 | EXOC3L2 | exocytosis | Dayeh et al. PLoS Genet 2014 |
| rs12287076 | FAM180B | unknown | NA |
| rs17125944 | FERMT2 | actin polymerization | Yasuda-Yamahara et al. Matrix Biol 2018 |
| rs1385600 | GAB2 | cell growth and apoptosis | Bagyinsky et al. Clin Interv Aging 2014 |
| rs5848 | GRN | lysosomal function | Paushter et al. Acta Neuropathol 2018 |
| rs9271192 | HLA-DRB5-DBR1 | antigen presentation | Karch and Goate Biol Psy 2015 |
| rs35349669 | INPP5D | myeloid proliferation and survival | Efthymiou and Goate Mol Neurodegener 2017 |
| rs7196161 | KAT8 | cell survival | Patillon et al. PLoS One 2012 |
| rs8100183 | MARK4 | inflammasome | Li et al. Nat Commun 2017 |
| rs190982 | MEF2C | immune profliferation and antigen presentation | Sao et al. Phychiatry Clin Neurosci 2018 |
| rs558678 rs554311 |
MS4A2 | hematopoietic immune response | Keuk et al. Immuno Cell Bio 2015 |
| rs610932 rs11824773 |
MS4A4A | signal transduction phagocytosis | Greer et al. Cell 2016 and unpublished data from our lab |
| rs10897011 rs7926729 |
MS4A4E | unknown immune function | Hollingworth et al. Nat Genetics 2011 |
| rs610932 rs983392 |
MS4A6A | phagocytosis | unpublished data from our lab |
| rs17643262 | NKPD1 | lipid synthesis | Amin et al. Biol Psych 2017 |
| rs2718058 | NME8 | cytoskeletal and axonal transport | Liu et al. Oncotarget 2016 |
| rs3851179 rs541458 |
PICALM | endocytosis | Zhao et al. Nat Neurosci 2016 |
| rs72824905 | PLCG2 | calcium signaling | Conway et al. Molecular Neurodegener 2018 |
| rs145999145 | PLD3 | APP processing | Satoh et al. Alzheimers Res Ther |
| rs3848140 | PPP1R37 | phosphotase activity | Han er al. PLoS One 2017 |
| rs2058716 | PRKD3 | inflammatory signaling | Baker et al. PLoS One 2018 |
| rs28834970 | PTK2B | inflammation | Beck et al. Oncoscience 2014 |
| rs2301275 | PVR | immune activation | Stamm et al. Oncogene 2018 |
| rs10402271 rs1871047 |
PVRL2 | chlosterol metabolism | Lin et al. J Hu Genetics 2016 |
| rs2376866 rs117612135 |
RELB | immune migration | Dohler et al. Front Immunol 2017 |
| rs10498633 | SLC24H4-RIN3 | cardiocascular function | Giri et al. Clin Interv Aging 2016 |
| rs12285364 | SORL1 | lipoprotein receptor | Holstege et al. Eur J Hum Genet 2017 |
| rs760136 rs10524523 |
TOMM40 | chlosterol metabolism | Lin et al. J Hu Genetics 2016 |
| rs28367893 | TRAPPC6A | protein transport | Chang et al. Oncotarget 2015 |
| rs75932628 | TREM2 | phagocytosis, migration, activation | Gratuze et al. Mol Neurodegen 2018 |
| rs9381040 | TREML2 | immune activation, phagocytosis | Zheng et al. Neurobiol Aging 2017 |
| rs1476679 | ZCWPW1 | histone modification | Gao et al. Oncotarget 2016 |
Of the GWAS-risk genes, SNPs within Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) are associated with the highest risk of developing AD, increasing disease risk by 2–4 fold. As the name suggests, TREM2 is exclusively expressed on cells within the myeloid lineage. Thus, in the brain, TREM2 expression is dominated by microglia. Additionally, recent comparisons of human peripheral blood monocytes and both iPSC-derived and brain-derived microglia further suggest that TREM2 expression is greatly enriched in microglia versus other monocyte lineages10. Several of the AD-associated SNPs occur within the Trem2 coding region, including R47H, R62H, and H157Y. R47H TREM2 mutations, in particular have been ardently studied, uncovering relationships between carriers of this variant and increased CSF biomarkers such as tau, p-tau181, and soluble Trem2 (sTrem2), each of which have been associated with worse disease progression11–13. Research on the function of R47H and other TREM2 variants thus far suggests that AD-risk is incurred through a partial loss of function28, however, the localization of the R47H and R62H mutations within the ligand binding domain of TREM2 suggest perhaps a more nuanced alteration in specific microglial responses.
Thus far, the majority of studies examining TREM2 in relation to AD have utilized TREM2 deletion that in general appears to reduce microglial activation in response to varying stimuli. For example, murine AD models with TREM2−/− exhibit decreased microglial activation resulting in less microglial migration to beta-amyloid plaques and delayed plaque clearance29. In addition, plaques in TREM2−/− mice are less compacted, leading to increased plaque-associated neuritic dystrophy30,31. These data collectively suggest that microglial activation is necessary for clearance of plaques, and that suppression of these activation programs may accelerate plaque accumulation. Interestingly, total microglial numbers are also decreased in TREM2−/− mice potentially due to their inability to initiate activation-related proliferation and/or impaired microglial survival. Indeed, Trem2 expression normally decreases with some forms of microglial activation such as LPS treatment, but is conversely elevated in microglia adjacent to beta-amyloid plaques. Furthermore, microglia that lack trem2 do not seem to activate normally in response to injury18,19. In addition, as a transmembrane protein, recent studies have demonstrated that TREM2 can be proteolytically cleaved, resulting in sTREM2 which may serve as a promising biomarker for AD and may also provide additional immunomodulatory functions34–36.
In addition to specific mutations in Trem2, other microglial AD-risk genes, membrane spanning 4-domains subfamily A members 4A and 6A (MS4A4A, MS4A6A) have recently been associated with altered sTrem2 levels in patient CSF. The MS4A family is itself linked to altered AD risk; in autopsied AD brains and blood samples from AD-patients with MS4A risk SNPs, expression of both MS4A4A and MS4A6A is increased. Importantly, these elevated expression levels also parallel increasing Braak tangle and plaque scores37–39. Interestingly, an AD-risk SNP (rs6591561) associated with increased expression of both MS4A genes, is also correlated with reduced levels of sTrem2. Conversely, rs1582763, a SNP associated with decreased MS4A4A and MS4A6A is linked to increased sTrem2 and protection from Alzheimer’s disease27. However, MS4A proteins likely also influence disease risk independently of their effect on sTrem2. For example, unpublished data from our lab suggests these proteins play a role in regulating phagocytosis. Furthermore, other members of the MS4A family such as CD20 (MS4A1) have previously been implicated in immune regulation independent of Trem2 signaling.
CD33 or Siglec-3, is another myeloid cell specific receptor that has been significantly associated with Alzheimer’s disease40,41. Sialic acid binding triggers Immunoreceptor Tyrosine-based Inhibitory Motif (ITIM) signaling through Siglec proteins such as CD33, which has previously been shown to induce SYK-mediated signaling cascades that lead to changes in phagocytosis that are similar to those triggered by TREM2/DAP12 signaling42. CD33 expression is also increased in human AD brains and correlates with increased plaque burden as well as swifter disease progression40. Within BV2 immortalized microglia and murine CD33 knockout models of AD reduced expression of CD33 is associated with impaired clearance of beta-amyloid43. Extrapolation from these data may seem confusing given that they suggest increased expression of CD33 in microglia would be predicted to increase beta-amyloid phagocytosis while also leading to increased plaque burden. However, this combination can be resolved if we again consider the microglial seeding hypothesis whereby increased phagocytosis of beta-amyloid would lead to higher levels of plaque seeding leading to increased plaque load.
In addition, an elegant recent study of monocyte-derived microglia-like6 (MDMi) cells recently demonstrated that the CD33 AD risk SNP rs3865444 is associated with increased expression and membrane localization of full-length CD33 and decreased expression of a shorter splicing variant that lacks the immunoglobulin V-set domain, which together lead to reduced phagocytic activity44. In parallel, it was discovered that a protective SNP (rs12459419) leads to increased splicing of exon 2 leading to a shorter length protein.45 While our understanding of CD33 biology continues to improve, additional research is still needed to determine whether the main role of CD33 in AD is through modulation of Aβ phagocytosis or whether additional immune regulatory aspects of altered CD33 signaling play a more important role in disease pathogenesis.
Of additional interest, ATP-binding cassette transporter A7 (ABCA7) is a membrane transporter expressed highly by neurons, microglia, oligodendrocytes, and endothelial cells, but still seems to have the largest effect on disease risk through microglia24. In AD, SNPs in ABCA7 seem to be associated with a gain of function that may enhance phagocytosis of apoptotic cells and beta-amyloid38,46–49. On a broader scale, human post-mortem tissue analysis has shown that SNPs in ABCA7, which increase ABCA7 expression, correlate with increased hippocampal atrophy. Inversely, when ABCA7 was deleted from the J20 amyloid model of AD, a decrease in plaque deposition was observed. These data again suggest that changes in microglial phagocytosis of beta-amyloid may underlie the effects of microglial risk genes on disease. On the other hand, our studies to date have been guided by the existing knowledge in the field and the somewhat biased expectation that any studies of AD-associated microglial function should by definition examine beta-amyloid phagocytosis. Yet, a growing number of studies suggest that phagocytosis of other CNS-derived substrates such as synapses or myelin could be at least as important to disease progression and we and others are finding that microglial genes can differentially effect phagocytosis of differing substrates. Likewise, many other less studied functions of microglia, could also be critically involved in this disease. Thus, it seems a more comprehensive, unbiased analysis of the effects of AD risk genes on human microglial function and gene expression are desperately needed to improve our understanding of these cells and their role in AD.
Now that it has become clear that microglia are crucial in AD pathogenesis, the field needs to better understand how these cells influence disease risk and whether the normal function of microglia in disease is generally protective or pathogenic. Though many of these risk genes eventually effect production or clearance of Aβ plaques, it is not known whether this is the mechanism that confers altered disease risk or whether this is merely a byproduct of a more important pathway or our somewhat biased experimental designs. By understanding the broader role of microglia and the immune system in AD we will be able to gain insight into the elusive causes of late onset Alzheimer’s disease in order to better target disease-modifying therapies that can prove to be effective in clinical trials.
Microglia in Homeostasis and Disease
In homeostatic conditions, microglia are responsible for promoting neuronal health through secretion of trophic factors and synaptic remodeling as well as clearing pathogens, protein aggregates, myelin, and dead cell debris. These immune cells tile to form a grid through the brain, ensuring that no section goes unsurveiled. Homeostatic microglia are highly ramified and each of their processes is appreciably motile, constantly probing their environment for potential pathogens50. When a threat arises, microglia quickly become activated in order to address the insult. Activated microglia can secrete pro-inflammatory cytokines, clear pathogenic materials through phagocytosis and lysosomal degradation, and may also induce astrogliosis and astrocyte-associated changes to the blood brain barrier. After the pathogen has been cleared, microglia will typically return to a homeostatic state.
In some cases, however, microglia activation fails to resolve. In these circumstances, the constitutively active microglia often become detrimental to brain health. They may aberrantly over-prune synapses, kill neurons through phagoptosis, or induce unnecessary astrogliosis through pro-inflammatory cytokine secretion. Through prolonged, unnecessary microglial activation, severe neurodegeneration may occur. For example, aberrant inflammation in traumatic brain re-injury results in an inability for lesions to heal51. Chronic microglial activation has also been strongly implicated in many neurodegenerative diseases, playing a role in multiple sclerosis, amyotrophic lateral sclerosis, Huntington’s disease and Alzheimer’s disease25,38,52.
As mentioned previously, problems can also arise if, conversely, microglia are unable to become appropriately activated in response to an insult, such as in Trem2 knockout models. When microglia are constitutively homeostatic, they may not be able to properly remove pathogens, debris, or dead cells. In this case, these hazardous materials may build up creating further imbalances in brain homeostasis. Because microglia are responsible for supporting brain health and homeostasis through many avenues, microglia may influence the onset of AD in various ways, some of which are explored below.
Migration, phagocytosis, and lysosomal degradation
Many of the Alzheimer’s risk genes highly expressed in microglia effect microglial phagocytosis of beta-amyloid. Given the widespread interest in and adoption of the amyloid cascade hypothesis4, it follows that the majority of research on microglia in AD has often begun with examinations of this question. However, amyloid targeted therapeutics have thus far failed to improve or delay cognition in late stage clinical trials, leading some to speculate that beta-amyloid deposition could be a sign post of other more detrimental issues rather than a pathogen directly. If therapies can be developed that can reset and enhance microglial-mediated clearance of beta-amyloid, many would predict that this might stop or delay disease progression. Yet, as with other amyloid targeting therapies such an approach would likely only be useful if initiated during very early prodromal phases of the disease.
Phagocytosis of beta-amyloid is a complex system which includes migration towards the beta-amyloid plaques, endocytosis of beta-amyloid and lysosomal degradation into its constituent amino acids. The build up of beta-amyloid plaques observed in AD brains may be occurring from deficits in any or all of these components. These dysfunctions may be beta-amyloid specific or may also effect a broader range of phagocytosis of other substrates including apoptotic cells, myelin, or debris.
The ability of a microglia to migrate is crucial to its immune surveillance activity. In order to clear something from the brain, microglia must first follow chemotactic cues towards the debris or pathogens. This process is complex to study given that there are many chemokines, but often the mechanisms can be extrapolated from macrophage biology. When neurons die, for example, ADP and nucleotides released from the dying cell form a chemoattractive gradient sensed by the puranergic receptor P2RY12 on microglia53–55. When P2YR12 is chemically blocked, microglia are unable to activate in response to ADP/ATP and additionally do not migrate along their concentration gradient. In vivo, blockade of P2YR12 would likely inhibit microglial activation in response to dead neurons leading to a build up of apoptotic debris in the brain56. This is similar to what occurs with trem2 responses to beta-amyloid in which knockout of trem2 inhibits microglial migration toward amyloid plaques leading to increased beta-amyloid accumulation in AD mouse models. Correspondingly, it has been suggested that trem2 and its’ co-receptor dap12 may act as an actual phagocytic receptor for beta-amyloid. However, a large number of receptors on microglia have been posited to bind beta-amyloid and thus additional research is needed to tease out which receptors are necessary for directed migration and which are more important for beta-amyloid internalization.
If a microglia cell is able to properly migrate towards its target, the cell will still need to express the receptors and machinery to complete phagocytosis of this substrate. We still do not fully understand all the components involved in microglial phagocytosis, but much has been learned from assuming homology with other myeloid cells. In terms of neural phagocytosis, one of the major signals for a microglia cell to engulf its target is exposed phosphatidylserine. This phospholipid becomes exposed on the cell surface during the early stages of apoptosis and in response to oxidative stress, ATP depletion, or increased calcium ion levels all of which are signs of cellular stress and increase with age57–59. Interestingly tau-laden neurons have also been shown to aberrantly expose phosphatidylserine60,61. Microglial recruitment to these neurons may be a partial mechanism for how tau causes neurotoxicity. Indeed, PET imaging in mice has shown tau accumulation to precede microglial activation which strongly correlated with a reduction in brain volume62. Other groups however cite microglia as the mediators of tau spreading though phagocytosis remains important in either case60.
Protein aggregates, on the other hand, often must become opsonized before they can be recognized by a microglia cell. The most well-studied opsonins are IgG antibodies and the complement system both of which have been associated with AD63–65. Though, for beta-amyloid proteins, it has also been suggested that opsonization is not necessary. Many toll-like receptors, g-protein coupled receptors, and several AD-risk genes (trem2, abca7) have been proposed to serve as beta-amyloid receptors. For some of these receptors, it is likely that beta-amyloid does indeed bind, but rather than triggering phagocytosis of Aβ, this ligand may trigger downstream pro-inflammatory signaling cascades. It is difficult to distinguish receptors necessary for activation from those necessary for engulfment since removal of the former may still inhibit beta-amyloid phagocytosis by causing the cells to remain in a homeostatic state. This may be the case with AD-risk genes such as TREM2 and ABCA7. However, cell culture based studies have begun to provide initial evidence that beta-amyloid can indeed be recognized by TREM2, albeit only when bound to Apolipoprotein E66.
After a microglia cell has successfully sensed, migrated to, and engulfed a particle. It must still degrade the particle. For most substrates that have been engulfed, the phagocytic vesicle containing the cargo will merge with early and late endosomes to load digestive enzymes and acidify the pH before finally merging with a lysosome to form a phagolysosome67. Within the phagolysosome, particles are broken up by hydrolytic enzymes suitable for the low pH of the lysosome and can then be released from the cell. The specific proteins involved in this pathway differ depending on the cell type and the substrate being engulfed. Currently, the downstream signaling pathways involving specific processing of apoptotic cells68 or beta-amyloid69,70 have not been found to be linked to disease progression directly. However, more research into microglia-specific responses to phagocytic substrates in homeostatic or activated states will be required to better understand how these immune cells are able to respond to pathogenic stimuli in both early and later stages of disease progression. General knowledge from other immune cell types demonstrates that when phagolysosome formation or function is disrupted, this results in a build up of debris within enlarged phagolysosomes and can even result in cell death through necrosis71.
Several groups have showed that activating microglia not only boosts the migration to and engulfment of Aβ, but microglia treated with pro-inflammatory cytokines or LPS can actually degrade Aβ more efficiently. This is in part because activation induces acidification of the lysosomes which encourages faster and more complete degradation of proteins and cellular debris. If a microglia is unable to properly activate in response to neuroinflammatory stimuli, lysosomal efficiency would not increase, resulting in further reduction of the ability of microglia to process pathogenic debris. It is possible that a cascade like this may be the multifactorial trigger promoting disease progression, however, there is also significant data suggesting that many other important microglia functions are also altered in Alzheimer’s disease as discussed below.
Cytokine secretion, astrogliosis, and blood-brain barrier breakdown
Cytokines and chemokines are important mediators of neuroinflammation. Somewhat contradictory to the story surrounding Trem2 which concludes that hindering microglial activation increases AD risk, pro-inflammatory cytokines such as CCL2 and TNFα are increased in human AD brains. In addition, homeostatic cytokines such as CX3CL1 are dramatically decreased. CX3CL1 is secreted from neurons and acts as a homeostatic signal for the microglia receptor CX3CR1. In studies of AD models deficient for CX3CR1, AD brains displayed decreased beta-amyloid plaque deposition and substantially less neurodegeneration72–74. Not surprisingly, CX3CR1−/− mice showed increased levels of CCL2 and TNFα further confirming their activated state as a result of the absence of homeostatic signaling. Yet in stark contrast to this, deletion of CX3CR1 in tau transgenic models leads to increased neurofibrillary tangle pathology and behavioral deficits75. Thus, the effects of microglia activation can be diametrically opposite between the two hallmark AD pathologies. A similar relationship has also been described following treatment of AD mice with LPS, which leads to increased microglial activation and reduced beta-amyloid plaques, but enhanced tangle pathology76. Effects from pro-inflammatory cytokines can of course be pleiotropic as cytokines may have autocrine and paracrine effects signaling both back to microglia as well as to astrocytes furthering the spread of neuroinflammation, perhaps providing a partial explanation for these findings. Alternatively, perhaps the key role of microglia in AD is as an intermediary that transduces the proinflammatory-inducing effects of beta-amyloid plaques into increased neuritic dystrophy and tau pathology. In support of this are recent findings regarding the influence of TREM2 deletion and mutations on plaque barrier formation28.
In terms of pro-inflammatory cytokines, CCL2 levels are increased in patients with Alzheimer’s disease and may potentially provide a reasonable biomarker for disease progression77. The mechanism of CCL2 in disease progression is still unclear though there is evidence that CCL2 expression alters phagocytosis of beta-amyloid plaques and effects disease progression through this axis78. Others propose that CCL2 is mainly effective through recruitment of peripheral mononuclear phagocytes though it remains unclear and controversial whether these cells actually migrate into the brain during human disease79. TNFα, is similarly increased in Alzheimer’s patient brains as well as model systems and seems to also increase phagocytosis of beta-amyloid80. Though the effect of TNFα may be broader in that it is secreted by neurons as well and has independent effects on neuronal survival and proliferation81.
Many important microglial-derived pro-inflammatory cytokines such as CCL2, TNFα, Il 1β, IL-6 and others also influence astrocyte activation or astrogliosis82,83. Even in injury models, removal of microglial cytokines inhibits astrogliosis from occurring further proving that microglia are often responsible for induction of astrocyte reactivity84,85. Like microgliosis, astrogliosis is particularly prevalent near plaques suggesting they play a role either in barrier formation to protect neurons and/or in the chemoattractive recruitment of microglia to the plaque environment86,87. Conversely there is also evidence that astrogliosis is detrimental in that increased astrocyte derived IL-1β, iNOS, and ROS secretion acts as a positive feedback mechanism to increase neuroinflammation and may even harm the blood brain barrier85,87 which would allow for further recruitment of peripheral phagocytes into the brain via CCL2/CCR2 signaling.
Damage Associated Microglia
Since the direct pathways through which microglia influence Alzheimer’s disease remain unclear, several groups have begun to study microglial biology using broader unbiased approaches. For example, Keren-Shaul et al. used single-cell RNA-sequencing to uncover a specific population of microglia whose temporal appearance mirrored the progression of plaque pathology in the 5x-fAD mouse model30. These Damage Associated Microglia (DAM) are formed via a two-step process the second of which appears to be TREM2 dependent since in TREM2−/− mice, microglia remain in the intermediate activation phase throughout disease progression. Therefore, DAM have been hypothesized to be beneficial in the context of AD knowing that Trem2 loss of function mutations are known to exacerbate disease severity and age of onset.
Interestingly, Krasemann et al. have discovered a similar set of genes which they have denoted the microglia neurodegenerative phenotype or MGnD31. Here, the authors have described a more generalized phenotypic change associated with several neurodegenerative diseases and demonstrate that this activation state is influenced by APOE. Using mouse models of amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and Alzheimer’s disease, the authors highlight genes that are induced or repressed commonly across disease type. This list includes many of the same genes discovered in Keren-Shaul et al. including increased apoe, hla, clec7a, and cd11c expression as well as decreased p2ry12, cx3cr1 and tmem119 expression. Although the gene sets discovered in each paper are not identical, it seems likely that each group has independently discovered a similar set of cells. Indeed DAM microglia have been shown to be similarly occurring in ALS as well. Interestingly, MGnDs and the corresponding loss of more homeostatic microglia have been proposed to be detrimental in contrast to the subsequent conclusions of Keren-Shaul et al. Whether the MgnD and DAM phenotype is equivalent and more importantly whether they are detrimental or beneficial will likely depend on the nature of the disease process and timing. For example, one might predict that DAM phenotypes are protective against beta-amyloid given the effects of TREM2 deletion on DAMs and plaque load whereas DAM cells might conversely by detrimental in the context of tau pathology or synaptic pruning. Continued validation of these unbiased approaches and extension of these studies to include examination of human microglia are critically needed and will hopefully help narrow down the true roles of microglia in neurodegenerative disease.
Microglia as a therapeutic target
Since microglia effect so many crucial pathways in the brain, therapies which effect this cell type may have unexpected off-target effects. Fortunately some of the most important microglial functions, such as synaptic pruning, occur predominantly early in life and thus it may not be detrimental to dampen these processes in Alzheimer’s patients. Another concern is that microglia share many transcriptional and functional pathways with peripheral monocytes and macrophages. For this reason, small molecule therapies may produce unwanted side effects on these peripheral targets. Currently, in AD, is not yet clear whether immune activation or suppression will be therapeutic as examples in this review have been presented in support of both possibilities. In either case, broad activation or suppression of myeloid cells would likely be detrimental for patients. Sustaining myeloid activation globally may cause chronic inflammation similar to macrophage activation syndrome88,89. On the other hand, general suppression of immune activation in aged patients who already experience an increased risk of infection and immune impairment would leave patients increasingly vulnerable to infectious disease. For these reasons, the most successful microglial therapies will need to be precisely targeted towards microglia but not other monocytes and thus need to capitalize on our growing understanding of the genetic and functional differences between these closely related cells.
If cell-specificity can be sufficiently achieved, it is possible that broad activation or suppression of microglia may be effective although the timing of these approaches will likely be critical. Recent data from mouse studies in which microglia are ablated using a CSF-1 blockade demonstrated no cognitive detriments from complete removal of microglia in otherwise normal WT mice89. While behavioral studies in mice are much less nuanced than human cognition, this research suggests that therapeutic microglia suppression, perhaps via more subtle means such as reduced proliferation90, may be therapeutically tractable. Although ideally a specific pathway of microglia activity such as migration, phagocytosis, or cytokine signaling pathways could be isolated and specifically modulated, the effect of microglia on AD pathogenesis does not seem to be that simple. Indeed, this review has provided evidence for disruption in all three of those pathways in AD and likely further study of microglia enriched risk genes will uncover additional microglia functions that influence disease progression.
Perspectives
This review presents a broad overview of the current data positing that the immune system, primarily microglia, plays a much larger role in disease development and progression than previously understood. With the rapid growth of research focusing on microglia in AD, many different functional pathways have been proposed to alter disease risk. Of these, most pathways can be broadly altered by changing microglial activation state. In order to separate these individual pathways from the pleiotropic effects of broad microglia activation, more research towards understanding the spectrum of human microglial activation states will be required. We have learned a great deal from studying peripheral macrophages, but given the key transcriptome and functional differences between peripheral macrophages and microglia, we must assume that microglial activation is likewise quite different. Furthermore, even murine microglia in vivo have been shown to significantly differ from human microglia and these differences are enhanced in aging, making it particularly difficult to study age-related human disease in traditional murine models. While mouse models are extremely useful for studying microglia in their natural environment, they are inherently biased based on what we currently understand to cause Alzheimer’s disease and thus will always produce data related to those original assumptions. In order to create a more accurate model of microglia in Alzheimer’s disease using patient derived iPS-microglia, one potential promising approach will be to utilze brain organoid models or generate chimeric mouse models to study the complex interactions between human microglia, neurons, astrocytes, and AD neuropathology.
Highlights.
Microglia are associated with the progression of Alzheimer’s disease.
Key microglia functions in AD: cytokine secretion, phagocytosis, trophic support.
Human in vitro models allow for controlled studies of molecular microglia function.
Understanding human microglial function in AD may elucidate new, targeted therapies.
Acknowledgements
The authors would like to thank Morgan Coburn for contributing the images for Figure 1. This work was supported by NIH grants AG048099, AG056303, and AG016573, CIRM RT3–07893 (M.B.J.) and NINDS T32 NS082174 (A.M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alzheimers Association update. Alzheimers Dement 11, 104–105 (2015). [PubMed] [Google Scholar]
- 2.Alzheimer A, Stelzmann RA, Schnitzlein HN & Murtagh FR An English translation of Alzheimer’s 1907 paper, ‘Uber eine eigenartige Erkankung der Hirnrinde’. Clin Anat 8, 429–431 (1995). [DOI] [PubMed] [Google Scholar]
- 3.McKhann GM et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 7, 263–269 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy JA & Higgins GA Alzheimer’s disease: the amyloid cascade hypothesis. Science 256, 184–185 (1992). [DOI] [PubMed] [Google Scholar]
- 5.Wisniewski HM, Moretz RC & Lossinsky AS Evidence for induction of localized amyloid deposits and neuritic plaques by an infectious agent. Annals of Neurology 10, 517–522 (1981). [DOI] [PubMed] [Google Scholar]
- 6.Boza-Serrano A, Yang Y, Paulus A & Deierborg T Innate immune alterations are elicited in microglial cells before plaque deposition in the Alzheimer’s disease mouse model 5xFAD. Sci Rep 8, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sosna J et al. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol Neurodegener 13, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman BA et al. Diverse Brain Myeloid Expression Profiles Reveal Distinct Microglial Activation States and Aspects of Alzheimer’s Disease Not Evident in Mouse Models. Cell Rep 22, 832–847 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Ueda Y, Gullipalli D & Song W-C Modeling complement-driven diseases in transgenic mice: Values and limitations. Immunobiology 221, 1080–1090 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Durafourt BA et al. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia 60, 717–727 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Bennett ML et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. U.S.A 113, E1738–1746 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosselin D et al. An environment-dependent transcriptional network specifies human microglia identity. Science 356, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olah M et al. A transcriptomic atlas of aged human microglia. Nat Commun 9, 539 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abud EM et al. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron 94, 278–293.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muffat J et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med 22, 1358–1367 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haenseler W et al. A Highly Efficient Human Pluripotent Stem Cell Microglia Model Displays a Neuronal-Co-culture-Specific Expression Profile and Inflammatory Response. Stem Cell Reports 8, 1727–1742 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takata K et al. Induced-Pluripotent-Stem-Cell-Derived Primitive Macrophages Provide a Platform for Modeling Tissue-Resident Macrophage Differentiation and Function. Immunity 47, 183–198.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Pandya H et al. Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nat. Neurosci 20, 753–759 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douvaras P et al. Directed Differentiation of Human Pluripotent Stem Cells to Microglia. Stem Cell Reports 8, 1516–1524 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McQuade A et al. Development and validation of a simplified method to generate human microglia from pluripotent stem cells. Molecular Neurodegeneration 13, 67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ormel PR et al. Microglia innately develop within cerebral organoids. Nature Communications 9, 4167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci 21, 941–951 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marioni RE et al. GWAS on family history of Alzheimer’s disease. Transl Psychiatry 8, 99 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y et al. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J. Neurosci 34, 11929–11947 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lill CM et al. The role of TREM2 R47H as a risk factor for Alzheimer’s disease, frontotemporal lobar degeneration, amyotrophic lateral sclerosis, and Parkinson’s disease. Alzheimers Dement 11, 1407–1416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jay TR, von Saucken VE & Landreth GE TREM2 in Neurodegenerative Diseases. Mol Neurodegener 12, 56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deming Y et al. The MS4A gene cluster is a key regulator of soluble TREM2 and Alzheimer disease risk. bioRxiv (2018). doi: 10.1101/352179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kober DL et al. Neurodegenerative disease mutations in TREM2 reveal a functional surface and distinct loss-of-function mechanisms. eLife Sciences 5, e20391 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleinberger G et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med 6, 243ra86 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Condello C, Yuan P, Schain A & Grutzendler J Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques. Nat Commun 6, 6176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y et al. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J. Exp. Med 213, 667–675 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keren-Shaul H et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 0, (2017). [DOI] [PubMed] [Google Scholar]
- 33.Krasemann S et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 47, 566–581.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suárez-Calvet M et al. Early changes in CSF sTREM2 in dominantly inherited Alzheimer’s disease occur after amyloid deposition and neuronal injury. Sci Transl Med 8, 369ra178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suárez-Calvet M et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol Med 8, 466–476 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong L et al. Soluble TREM2 induces inflammatory responses and enhances microglial survival. J. Exp. Med 214, 597–607 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proitsi P et al. Alzheimer’s disease susceptibility variants in the MS4A6A gene are associated with altered levels of MS4A6A expression in blood. Neurobiology of Aging 35, 279–290 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Allen M et al. Novel late-onset Alzheimer disease loci variants associate with brain gene expression. Neurology 79, 221–228 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karch CM et al. Expression of Novel Alzheimer’s Disease Risk Genes in Control and Alzheimer’s Disease Brains. PLOS ONE 7, e50976 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang T et al. CD33 in Alzheimer’s disease. Mol. Neurobiol 49, 529–535 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Hollingworth P et al. Common variants in ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet 43, 429–435 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linnartz B & Neumann H Microglial activatory (immunoreceptor tyrosine-based activation motif)- and inhibitory (immunoreceptor tyrosine-based inhibition motif)-signaling receptors for recognition of the neuronal glycocalyx. Glia 61, 37–46 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Griciuc A et al. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 78, 631–643 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan KJ et al. A human microglia-like cellular model for assessing the effects of neurodegenerative disease gene variants. Sci Transl Med 9, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malik M et al. CD33 Alzheimer’s Risk-Altering Polymorphism, CD33 Expression, and Exon 2 Splicing. J Neurosci 33, 13320–13325 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jehle AW et al. ATP-binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages. J Cell Biol 174, 547–556 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka N, Abe-Dohmae S, Iwamoto N & Yokoyama S Roles of ATP-Binding Cassette Transporter A7 in Cholesterol Homeostasis and Host Defense System. JAT 18, 274–281 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Kim WS et al. Deletion of Abca7 Increases Cerebral Amyloid-β Accumulation in the J20 Mouse Model of Alzheimer’s Disease. J. Neurosci 33, 4387–4394 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramirez LM et al. Common variants in ABCA7 and MS4A6A are associated with cortical and hippocampal atrophy. Neurobiology of Aging 39, 82–89 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Davalos D et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci 8, 752–758 (2005). [DOI] [PubMed] [Google Scholar]
- 51.Donat CK, Scott G, Gentleman SM & Sastre M Microglial Activation in Traumatic Brain Injury. Front Aging Neurosci 9, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mammana S et al. The Role of Macrophages in Neuroinflammatory and Neurodegenerative Pathways of Alzheimer’s Disease, Amyotrophic Lateral Sclerosis, and Multiple Sclerosis: Pathogenetic Cellular Effectors and Potential Therapeutic Targets. International Journal of Molecular Sciences 19, 831 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Simone R et al. TGF-β and LPS modulate ADP-induced migration of microglial cells through P2Y1 and P2Y12 receptor expression. J. Neurochem 115, 450–459 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Corriden R & Insel PA New insights regarding the regulation of chemotaxis by nucleotides, adenosine, and their receptors. Purinergic Signal 8, 587–598 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore CS et al. P2Y12 expression and function in alternatively activated human microglia. Neurol Neuroimmunol Neuroinflamm 2, e80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eyo UB et al. P2Y12R-Dependent Translocation Mechanisms Gate the Changing Microglial Landscape. Cell Reports 23, 959–966 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown GC & Neher JJ Microglial phagocytosis of live neurons. Nature Reviews Neuroscience 15, 209–216 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Suzuki J et al. Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J. Biol. Chem 288, 13305–13316 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tyurina YY et al. Nitrosative stress inhibits the aminophospholipid translocase resulting in phosphatidylserine externalization and macrophage engulfment: implications for the resolution of inflammation. J. Biol. Chem 282, 8498–8509 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Asai H et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci 18, 1584–1593 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brelstaff J, Tolkovsky AM, Ghetti B, Goedert M & Spillantini MG Living Neurons with Tau Filaments Aberrantly Expose Phosphatidylserine and Are Phagocytosed by Microglia. Cell Rep 24, 1939–1948.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishikawa A et al. In Vivo Visualization of Tau Accumulation, Microglial Activation, and Brain Atrophy in a Mouse Model of Tauopathy rTg4510. Journal of Alzheimer’s Disease 61, 1037–1052 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Marsh SE et al. The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial function. Proc. Natl. Acad. Sci. U.S.A 113, E1316–1325 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu H et al. Complement component C3 and complement receptor type 3 contribute to the phagocytosis and clearance of fibrillar Aβ by microglia. Glia 60, 993–1003 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong S et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yeh FL, Wang Y, Tom I, Gonzalez LC & Sheng M TREM2 Binds to Apolipoproteins, Including APOE and CLU/APOJ, and Thereby Facilitates Uptake of Amyloid-Beta by Microglia. Neuron 91, 328–340 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Desjardins M, Huber LA, Parton RG & Griffiths G Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J. Cell Biol 124, 677–688 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou Z & Yu X Phagosome maturation during the removal of apoptotic cells: receptors lead the way. Trends Cell Biol 18, 474–485 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tam JH, Seah C & Pasternak SH The Amyloid Precursor Protein is rapidly transported from the Golgi apparatus to the lysosome and where it is processed into beta-amyloid. Mol Brain 7, 54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tam JHK, Cobb MR, Seah C & Pasternak SH Tyrosine Binding Protein Sites Regulate the Intracellular Trafficking and Processing of Amyloid Precursor Protein through a Novel Lysosome-Directed Pathway. PLoS One 11, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Turk B & Turk V Lysosomes as “Suicide Bags” in Cell Death: Myth or Reality? J Biol Chem 284, 21783–21787 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen P, Zhao W, Guo Y, Xu J & Yin M CX3CL1/CX3CR1 in Alzheimer’s Disease: A Target for Neuroprotection. Biomed Res Int 2016, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee S et al. CX3CR1 Deficiency Alters Microglial Activation and Reduces Beta-Amyloid Deposition in Two Alzheimer’s Disease Mouse Models. The American Journal of Pathology 177, 2549–2562 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Z, Condello C, Schain A, Harb R & Grutzendler J CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar Aβ phagocytosis. J Neurosci 30, 17091–17101 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhaskar K et al. Regulation of tau pathology by the microglial fractalkine receptor. Neuron 68, 19–31 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zuroff L, Daley D, Black KL & Koronyo-Hamaoui M Clearance of cerebral Aβ in Alzheimer’s disease: reassessing the role of microglia and monocytes. Cell Mol Life Sci 74, 2167–2201 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Westin K et al. CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer’s disease. PLoS ONE 7, e30525 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kiyota T et al. CCL2 accelerates microglia-mediated Abeta oligomer formation and progression of neurocognitive dysfunction. PLoS ONE 4, e6197 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guedes JR, Lao T, Cardoso AL & El Khoury J Roles of Microglial and Monocyte Chemokines and Their Receptors in Regulating Alzheimer’s Disease-Associated Amyloid-β and Tau Pathologies. Front. Neurol 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma J, Jiang T, Tan L & Yu J-T TYROBP in Alzheimer’s Disease. Mol Neurobiol 51, 820–826 (2015). [DOI] [PubMed] [Google Scholar]
- 81.Bhaskar K et al. Microglial Derived Tumor Necrosis Factor-α Drives Alzheimer’s Disease-Related Neuronal Cell Cycle Events. Neurobiol Dis 62, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Benveniste EN & Benos DJ TNF-alpha- and IFN-gamma-mediated signal transduction pathways: effects on glial cell gene expression and function. FASEB J 9, 1577–1584 (1995). [DOI] [PubMed] [Google Scholar]
- 83.Hanisch U-K Microglia as a source and target of cytokines. Glia 40, 140–155 (2002). [DOI] [PubMed] [Google Scholar]
- 84.Selmaj KW, Farooq M, Norton WT, Raine CS & Brosnan CF Proliferation of astrocytes in vitro in response to cytokines. A primary role for tumor necrosis factor. J. Immunol 144, 129–135 (1990). [PubMed] [Google Scholar]
- 85.Balasingam V, Tejada-Berges T, Wright E, Bouckova R & Yong VW Reactive astrogliosis in the neonatal mouse brain and its modulation by cytokines. J. Neurosci 14, 846–856 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kamphuis W et al. Glial fibrillary acidic protein isoform expression in plaque related astrogliosis in Alzheimer’s disease. Neurobiology of Aging 35, 492–510 (2014). [DOI] [PubMed] [Google Scholar]
- 87.Osborn LM, Kamphuis W, Wadman WJ & Hol EM Astrogliosis: An integral player in the pathogenesis of Alzheimer’s disease. Progress in Neurobiology 144, 121–141 (2016). [DOI] [PubMed] [Google Scholar]
- 88.Grom AA, Horne A & De Benedetti F Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol 12, 259–268 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lull ME & Block ML Microglial activation and chronic neurodegeneration. Neurotherapeutics 7, 354–365 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Olmos-Alonso A et al. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Brain 139, 891–907 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]