Abstract
Objective:
To compare sagittal walking gait biomechanics between participants with knee osteoarthritis (KOA) who increased quadriceps strength following a lower-extremity strengthening intervention (responders) and those who did not increase strength following the same strengthening protocol (non-responders) both at baseline and following the lower extremity strengthening protocol.
Design:
Fifty-three participants with radiographic KOA (47% female, 62.3±7.1 years, BMI = 28.5±3.9 kg/m2) were enrolled in 10 sessions of lower extremity strengthening over a 28-day period. Maximum isometric quadriceps strength and walking gait biomechanics were collected on the involved limb at baseline and 4-weeks following the strengthening intervention. Responders were classified as individuals who increased quadriceps strength greater than the upper limit of the 95% confidence interval for the minimal detectable change in quadriceps strength (29 Nm) determined in a previous study. 2 × 2 functional analyses of variance were used to evaluate the effects of group (responders and non-responders) and time (baseline and 4-weeks) on time-normalized waveforms for knee flexion angle, vertical ground reaction force (vGRF), and internal knee extension moment.
Results:
A significant group x time interaction for knee flexion angle demonstrated greater knee flexion angle in the first half of stance at baseline and greater knee extension in the second half of stance at 4-weeks in responders compared to non-responders. There was no significant group x time interaction for vGRF or internal knee extension moment.
Conclusions:
Quadriceps strengthening may be used to stimulate small changes in knee flexion angle in individuals with KOA.
Keywords: knee flexion angle, vertical ground reaction force, internal knee extension moment, waveform analysis, functional data analysis
Introduction
Knee osteoarthritis (KOA) affects multiple joint tissues causing pain and disability during activities of daily living [1]. Aberrant walking gait biomechanics are common in those with KOA [2–4] and have been suggested to contribute to KOA progression [5, 6]. Individuals with symptomatic KOA demonstrate reduced peak internal knee extension moments (KEM) compared to individuals without KOA [2, 7] as well as asymptomatic individuals with radiographic KOA [7]. Those with KOA demonstrate less knee flexion excursion during stance, or knee range of motion in the sagittal plane throughout stance [3, 4, 8], and lower vertical ground reaction force (vGRF) [3, 8] compared to individuals without KOA. Less KEM and knee excursion during stance are characteristics associated with a “stiffened-knee” gait, which likely alter tibiofemoral contact characteristics and might lead to deleterious changes in KOA progression [5, 9–11].
Individuals with KOA often experience quadriceps dysfunction, which is associated with characteristics of “stiffened-knee” gait strategies [12]. Quadriceps muscle action is important for generating an adequate KEM to attenuate ground reaction force (GRF) applied to the lower extremity during early stance [13]. The quadriceps also assist with forward propulsion during the second half of stance, which is important for locomotion and performance of activities of daily living [13, 14]. Cross-sectional studies indicate greater quadriceps strength associates with greater peak knee flexion angle (KFA) in individuals with KOA [15, 16], suggesting individuals with greater quadriceps strength can better control increased knee flexion during the early part of the stance phase of walking. While cross-sectional studies have demonstrated the association between lesser quadriceps strength and lesser KFA and KEM [16–18], there is little evidence evaluating the effects of increasing quadriceps strength on walking gait biomechanics in individuals with KOA. Understanding the influence of quadriceps strengthening on gait biomechanics is critical for developing the most appropriate therapeutic methods for altering aberrant gait biomechanics in those with KOA.
The primary purpose of this study was to compare KFA, vGRF, and KEM throughout stance between participants with KOA who did (responders) and did not (non-responders) increase quadriceps strength via a lower-extremity strengthening intervention; between-group comparisons were made before and after the lower extremity strengthening protocol. We hypothesized no differences in walking biomechanics would exist between the responders and non-responders prior to the strengthening protocol (baseline). We also hypothesized responders would demonstrate greater vGRF throughout stance, greater knee flexion excursion due to greater KFA in the first half of stance, and greater KEM throughout stance compared to non-responders following the lower extremity strengthening intervention (4-week follow-up). A gait strategy encompassing a greater vGRF, KFA, and KEM would be hypothesized to promote both optimal energy attenuation and propulsion needed for maintaining joint tissue health and physical function in individuals with KOA.
Method
Data collected in the current study were part of a larger randomized control trial (RCT, NCT02634814). The primary purpose of the RCT was to maximize voluntary activation of the quadriceps in individuals with KOA with 10 sessions over a 4-week period of progressive, lower extremity strengthening directed by a licensed physical therapist [19]. The RCT was designed to resemble the standard of care at our clinic for patients with KOA as well as previously published therapeutic exercise regimes for KOA [19–21]. All main outcomes were assessed in the same order at a baseline session prior to the intervention, and at a 4-week follow-up session (mean ± standard deviation; 28±4 days). Quadriceps maximum voluntary isometric strength (MVIC) was collected in the involved limb, defined as the more symptomatic limb in the case of bilateral KOA [19], and followed by collection of walking gait biomechanics. Quadriceps MVIC was evaluated first, as it was the primary outcome of the larger RCT. The Institutional Review Board at the XXX approved all methods, and all participants provided written consent prior to participation.
Participants
We included participants with radiographically defined KOA (Kellgren-Lawrence [K-L] grade 2–4) between the ages of 40 and 75 years with a normalized Western Ontario and McMaster Osteoarthritis Index (WOMAC) function ≥ 31 out of 100, indicating symptomatic KOA. Potential participants with a BMI > 35 kg/m2 were excluded from this study. Participants were required to demonstrate a quadriceps central activation ratio (CAR) in the involved limb ≤ 92% [22, 23]. CAR was collected as previously reported, using an exogenous train of stimuli to activate muscle not recruited voluntarily [23]. CAR was calculated as the maximal voluntary torque normalized to the torque produced by the voluntary contraction and superimposed electrical stimulus together [24]. Thirty-nine participants had bilateral KOA. However, a chi-square test of independence revealed no significant difference in the percentage of bilateral cases between responders and non-responders (X2 (2) = 2.530, p = 0.112); therefore, we included bilateral and unilateral KOA participants in both groups for the current study.
Lower-Extremity Strengthening Intervention
All participants were enrolled in 10 sessions of supervised, progressive lower extremity strengthening directed by a licensed physical therapist over a 28-day period. The 45-minute strengthening sessions consisted of 15-minutes of warming up on a cycle ergometer and stretching, 20-minutes of daily adjustable progressive resistance exercise for knee extension, knee flexion, and hip abduction exercises, and 10-minutes of balance progressions. As part of the larger clinical trial, participants were block-randomized in blocks of 6 into one of 3 treatment groups upon enrollment. One group (n = 17) used transcutaneous electrical nerve stimulation (TENS) in combination with the lower extremity strengthening exercises to determine if TENS affected outcomes related to neuromuscular activation and muscle strength. A second group received placebo TENS (n = 13), and the third group (n = 23) received only the lower extremity strengthening exercises. A chi-square test of independence concluded there were no statistically significant differences between group assignments for responders and non-responders (X2 (2) = 2.589, p = 0.274). Therefore, we combined our treatment groups for the current analyses.
Quadriceps Strength
Maximum isometric quadriceps strength was collected using an isokinetic dynamometer (HUMAC Norm; CSMi, Stoughton, MA). Participants were seated with their hips and knees flexed to 85º and 70º respectively and arms folded across their chest [25]. Participants’ pelvis and torso were secured with a seat belt attached to the chair, and the padded lever arm of the dynamometer was secured to the involved leg approximately 3cm proximal to the lateral malleolus and adjusted to align the knee joint axis of rotation with the dynamometer axis of rotation. The torque signal was output to an analog to digital converter (16-bit, NI USB-6221; National Instruments Corp., Austin, TC), sampled at 2000 Hz and displayed in real-time on a 56cm computer screen using a custom built software program (LabVIEW; National Instruments Corp., Austin, TX).
Participants performed 3 submaximal contractions at increasing intensities followed by a series of practice maximal effort trials during which participants were instructed to straighten their leg as fast and with as much force as possible, with 60 seconds of rest between each trial [25]. Participants performed practice trials until the maximum torque value was within 10% of the previous trial (3–5 practice trials were performed for each participant) [23]. The average of the 3 greatest practice trials was used as a torque threshold (MVIC) and displayed on a computer screen with an additional target line set to 120% torque threshold [23]. The torque signal was provided in real time, and participants completed 2 maximal effort trials with instructions to straighten their leg as hard and as fast as they could to attempt to reach the 120% target line in order to ensure they reached the MVIC threshold calculated from the practice trials [23]. The 2 test trials only counted if they exceeded 100% of the previously calculated torque threshold, to ensure fatigue did not affect results. These procedures were conducted in a method that ensured maximal effort on each trial, based on results from a previous study measuring quadriceps activation [23].
A second custom-built LabVIEW program was used to analyze quadriceps MVIC. Torque data were corrected for baseline passive torque resulting from the weight of the participant’s limb attached to the dynamometer lever arm, and were filtered using a 4th order, zero phase shift, low pass Butterworth filter with a cutoff frequency of 150 Hz [25]. Quadriceps MVIC was defined as the data point corresponding to the peak torque achieved in the trial, and normalized to body mass (Nm/kg) [25]. As conducted in a previous reliability study, demonstrating strong intra (intraclass correlation coefficient [ICC] = 0.98) and inter (ICC = 0.97) tester reliability of MVIC measurement, we averaged 2 maximal effort trials in order to determine MVIC [23]. We classified responders as participants who increased quadriceps strength greater than the upper limit of the 95% confidence interval (CI) for the minimal detectable change (MDC) in quadriceps strength as determined in individuals with KOA in a previous study [26]. In the previous study, the MDC at a 90% CI was reported, and we calculated an MDC using a 95% CI using this data, as 95% CIs are a more conservative statistical approach. If a participant increased their quadriceps MVIC by a MDC of 29 Nm [26], he or she was considered a responder. The MDC was not normalized to body mass, as the purpose of the MDC approach was to determine if an increase in strength could be detected based on the sensitivity of a measurement, which does not vary in respect to between-participant variability in mass.
Walking Gait Biomechanics
Synchronized 3D motion of the lower extremity and GRF were recorded with high-speed video (Vicon Motion Systems) and a force plate (40 × 60 cm, FP406010, Bertec Corporation, Columbus, Ohio, United States). For gait analysis, participants wore comfortable walking shoes (same shoes were worn at baseline and 4-weeks), shorts, and a t-shirt. Participants were outfitted with 22 retro reflective markers (anterior superior iliac spines (ASIS), greater trochanters, medial and lateral femoral epicondyles, medial and lateral malleoli, first metatarsal head, fifth metatarsal head, and posterior calcanei) as well as rigid clusters of 3–4 additional markers secured over the sacrum, lateral thighs, and lateral shanks for a total of 35 retro reflective markers. The knee- and ankle-joint centers were defined as the midpoints of the medial and lateral epicondyle marker and malleoli markers, respectively [27]. The hip-joint center was estimated as a percentage of the distance from the ASIS markers using the Bell method [28]. Participants walked at a self-selected speed across a 6m walkway, which included 2 force plates embedded in the floor and a set of timing gates. Between 5–10 practice trials were performed for each participant to calculate average self-selected walking speed and the starting position for each participant, which was maintained for all walking trials to ensure the involved foot contacted the middle of the force plate. After the practice walking trials, five walking trials were collected and considered acceptable for analysis if the involved limb struck the middle of the force plate 2) participants did not aim for the force plates, 3) gait speed was within ±5% of self-selected walking speed, and 4) gait kinematics were not visibly altered during the trial [29–31].
GRFs were sampled at 1200 Hz and low pass filtered at 75 Hz [29–31] in order to capture impulsive loading rates linked to joint tissue breakdown [32, 33]. Marker trajectories were sampled at 120 Hz, post-processed with Vicon Nexus v1.8.5 motion capture software (Vicon Motion Systems), low pass filtered at 10 Hz with a fourth-order Butterworth filter, and then synchronized to the force data by upsampling the marker positions to 1200 Hz via linear interpolation [29–31]. For each of the five acceptable walking trials, the stance phase for the involved limb was defined as the interval between heel strike (vGRF > 20 N) and toe off (vGRF < 20 N). KFA was calculated referenced to the thigh segment coordinate system using Euler angles such that flexion represented a positive value [27, 29]. KEM was calculated using the synchronized joint kinematic and GRF data, and a standard inverse dynamics approach. vGRF was normalized to body weight for each subject, and KEM was normalized to the product of body weight and Works, Natick, MA, USA).
Statistical Analysis
Prior to the primary analysis, demographic variables assessed at baseline only (age, weight, BMI, KL-grade, sex, and number of strengthening sessions) were compared between responders and non-responders using independent Student’s t-tests for continuous variables and chi-square tests of independence for categorical variables (Table 1). We conducted repeated measures analyses of variances (ANOVA) to determine if there were differences in quadriceps strength, WOMAC function subscale, WOMAC pain subscale, tolerability of walking, and walking speed between responders and non-responders at baseline and 4-weeks (Table 2, α=0.05; SPSS, Version 19.0, IBM Corp., Somers, NY, USA). We checked that all data used in the primary analysis met assumptions necessary for a two-way ANOVA: Our dependent variables were continuous, our independent variables consisted of two categorical groups, our observations were independent, there were no significant outliers, dependent variables were normally distributed, and there was homogeneity of variance between groups for our independent variables. For our primary analysis, 2 × 2 functional ANOVAs [34] were used to evaluate the interaction between group (responders and non-responders) and time (baseline and 4-weeks) on the time-normalized waveforms for each biomechanical variable; this approach detects significant differences in biomechanical variables throughout the entirety of stance, rather than just at discrete peaks [34, 35]. If there was no significant interaction effect, main effects for time and group were evaluated. Estimates of pairwise comparison functions were plotted for responders and non-responders at baseline and 4-weeks, as well as 95% CIs to identify group differences, which were considered different if 95% CIs did not overlap zero [34]. The functional ANOVAs were performed using the functional data analysis (FDA) package in R statistical computing software (version 3.4.3).
Table 1.
Descriptive Statistics at Baseline
| Combined Groups (N = 53) | Non-Responders (n = 38) | Responders (n = 15) | |
|---|---|---|---|
| Age (years) | 62.40 (7.08) | 62.89 (7.53) | 61.13 (5.84) |
| Weight (kg) | 85.58 (15.45) | 83.06 (13.66) | 91.97 (18.23) |
| Body Mass Index (kg/m2) | 28.48 (4.14) | 28.14 (4.26) | 29.33 (3.82) |
| Number of Bilateral KOA | Bilateral=39 | Bilateral=30 | Bilateral=9 |
| cases | Unilateral=13 | Unilateral=7 | Unilateral=6 |
| KL grade | KL 2 = 17 | KL 2 = 12 | KL 2 =5 |
| KL 3 = 29 | KL 3 = 20 | KL 3 = 9 | |
| KL 4 = 7 | KL 4 = 6 | KL 4 = 1 | |
| Sex | 27 females | 21 females | 6 females |
| 26 males | 17 males | 9 males | |
| Number of strengthening sessions completed | 9.64 (0.74) | 9.63 (0.78) | 9.67 (0.62) |
WOMAC = Western Ontario and McMaster Osteoarthritis Index, KL = Kellgren Lawrence Independent student’s t-tests and chi-square tests of independence confirmed no statistically significant difference (at the p ≤ 0.05 level) between groups for all variables
Table 2.
Demographics Assessed at Both Baseline and 4-weeks
| WOMAC function (0=no disability, 100=maximum disability) | Baseline | 50.47 (14.21) | 49.92 (13.91) | 51.86 (15.35) |
| 4-weeks | 30.63 (16.22)A | 28.48 (15.87)A | 36.08 (16.36)A | |
| WOMAC pain (0 = no pain, 100 = maximum pain) | Baseline | 43.49 (16.71) | 43.29 (16.82) | 44.00(17.03) |
| 4-weeks | 27.92 (17.80)A | 24.60(16.25)A | 36.33(19.31)A | |
| Strength (Nm) | Baseline | 111.14 (47.46) | 108.15 (50.30) | 118.74 (39.91) |
| 4-weeks | 134.07 (58.7)AB | 118.74(39.91)AB | 181.42(49.59)AB | |
| Strength (Nm/kg) | Baseline | 1.29 (0.49) | 1.27 (0.52) | 1.33 (0.45) |
| 4-weeks | 1.56 (0.62)AB | 1.38 (0.56)AB | 2.01 (0.54)AB | |
| VAS – Tolerability of Walking 20m (0=not tolerable, 10=very tolerable) | Baseline | 7.39 (2.47) | 7.50 (2.39) | 7.10 (2.74) |
| 4-weeks | 8.78 (1.63)A | 8.66 (1.81)A | 9.08 (1.05)A | |
| Walking Speed (m/s) | Baseline | 1.12 (0.18) | 1.14 (0.19) | 1.07 (0.14) |
| 4-weeks | 1.16 (0.154)A | 1.18 (0.16)A | 1.13 (0.15)A |
WOMAC = Western Ontario and McMaster Osteoarthritis Index, BW = Body VAS = Visual Analog Scale
Data at 4-weeks is statistically significantly different (p ≤ 0.05) at 4-weeks compared to baseline
Data is statistically significantly different (p ≤ 0.05) between responders and non-responders at 4-weeks
Results
All data assessed at baseline only is presented in Table 1. All data assessed at both baseline and 4-weeks is presented in Table 2. Both strength (F1,51 = 51.000, p < 0.001) and strength normalized to body weight (F1,51 = 56.549, p < 0.001) increased over time in responders, but not in non-responders (Table 2). Pain (F1,51 = 231.460, p ≤0.001), function (F1,51 = 57.248, p ≤0.001) and tolerability of walking (F1,51 = 19.798, p ≤ 0.001) improved in both groups from baseline to the 4-weeks but were not different between groups (Table 2). Walking speed increased in both groups from baseline to 4-weeks (F (1,51) = 11.391, p = 0.001, Table 2). All other demographics were not different between responders and non-responders over time (p ≤ 0.05, Tables 1 and 2). All statistical assumptions stated in the statistical analysis were met. Ensemble curves with surrounding 95% CIs of each variable are presented in Supplementary Figure 1.
Knee Flexion Angle
We found a significant group × time interaction for KFA during 45 – 78% of stance, indicating responders demonstrated greater KFA in the first half of stance compared to non-responders at baseline and less KFA (greater knee extension) in the second half of stance at 4-weeks compared to non-responders. Figure 1A represents the between-group difference in the alterations made from baseline to 4-weeks. At baseline, responders demonstrated significantly greater KFA (2.82°) during 12 – 60% of stance compared to non-responders (Figures 2A and 3A). At 4-weeks, responders demonstrated greater knee extension (1.67°) during 61–85% of stance phase compared to non-responders (Figures 2B and 3B).
Figure 1.
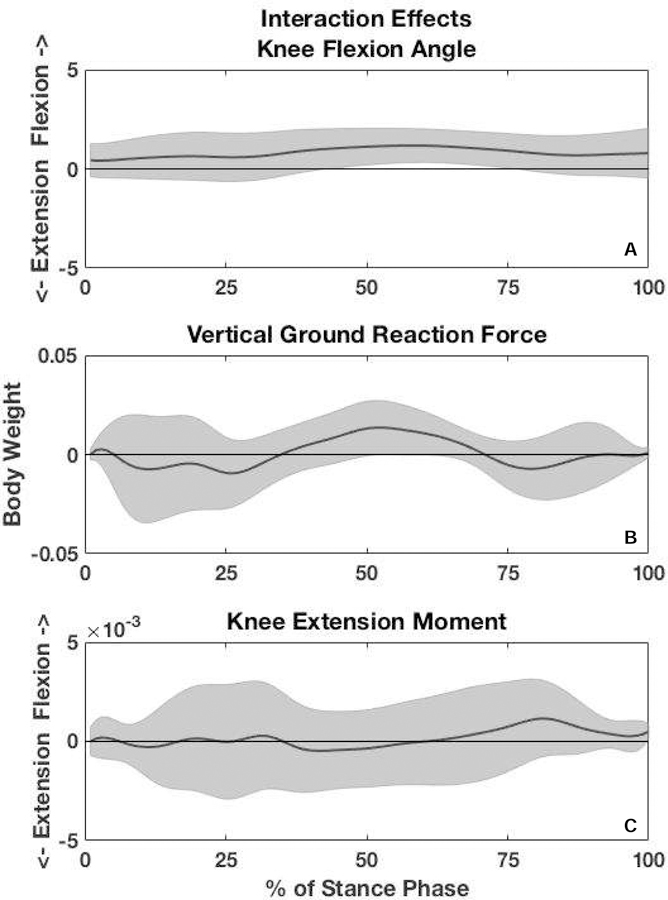
Interaction effects between response groups (strength responders and strength non-responders) and time (baseline and 4-weeks). The y-axis represents the differences between groups in the changes made from baseline to 4-weeks. The shaded gray area encompasses the upper and lower 95% confidence intervals. There was a significant interaction in knee flexion angle in mid-stance. Vertical ground reaction force approached a significant interaction, but there was no interaction for internal knee extension moment.
Figure 2.
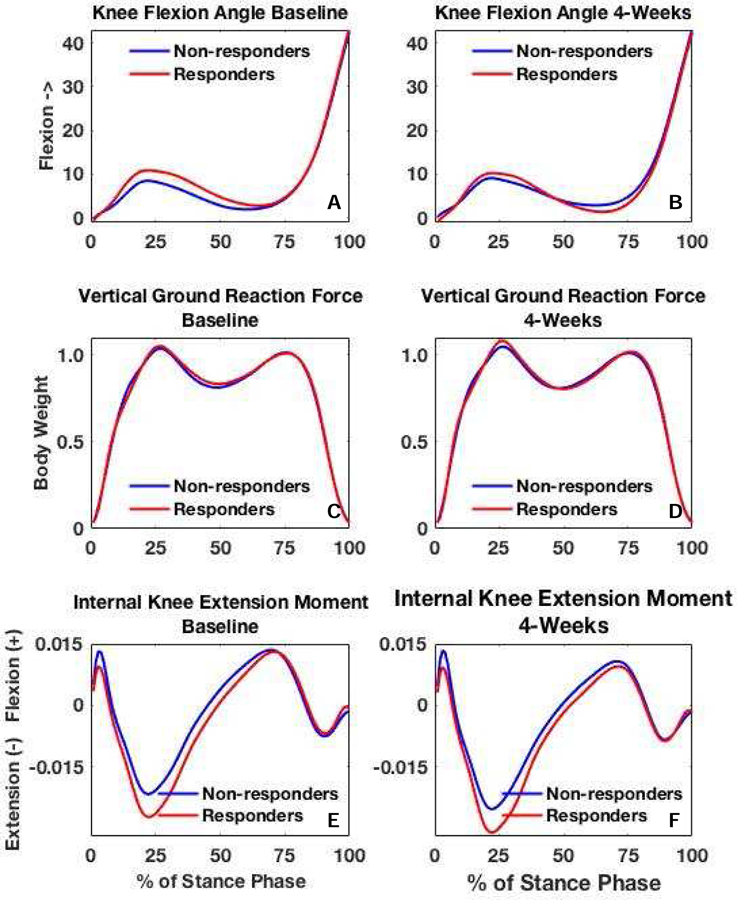
Ensemble averages are presented for knee flexion angle, vertical ground reaction force, and internal knee extension moment between responders and non-responders at baseline and 4-weeks.
Figure 3.
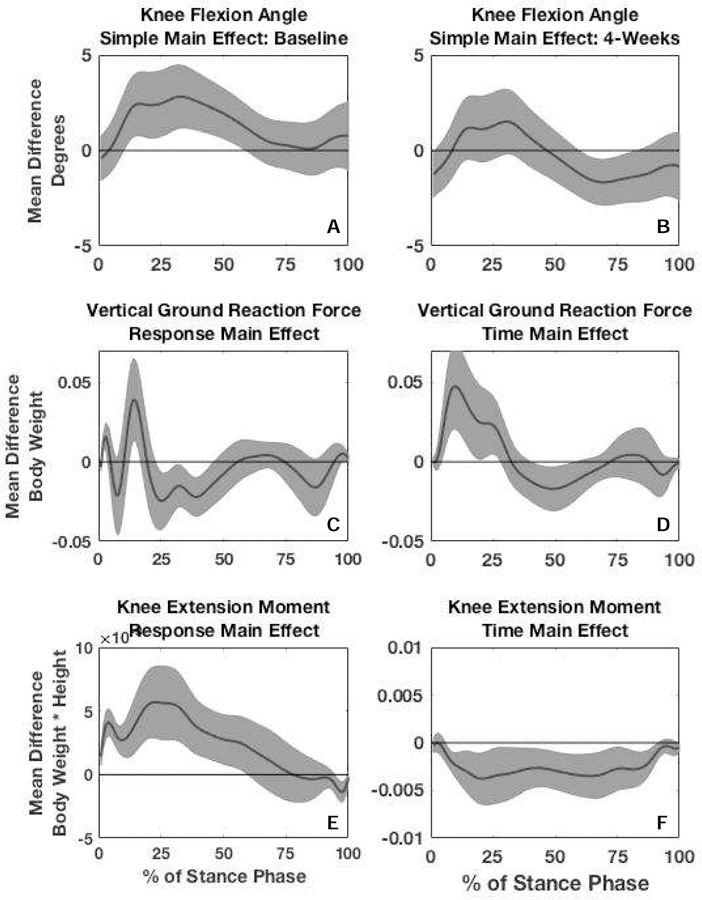
Simple main effects for knee flexion angle (KFA) were calculated by plotting the mean difference in KFA (solid black line) between responders and non-responders at baseline (3A) and 4-weeks (3B) and surrounding 95% confidence intervals (gray shading around solid black line). Main effect for response for vertical ground reaction force (vGRF) (3C) and internal knee extension moment (KEM) (3E) were calculated by plotting the mean difference in vGRF and KEM between responders and non-responders collapsed across time. Main effect for time for vGRF (3D) and KEM (3F) was calculated by plotting the mean difference in vGRF and KEM between 4-weeks and baseline collapsed across response group. Significant differences were identified as any part of stance phase where the 95% confidence intervals of the difference did not overlap zero.
vGRF
There was no significant group x time interaction for vGRF (Figure 1B); however, there were significant main effects for both time and group for vGRF. When data were collapsed across groups vGRF at 4-weeks was significantly higher (5% BW) during 6–30% of stance (the first peak) and significantly lower (2% BW) during 42–60% of stance compared to baseline (Figures 2C–D and 3D). When time points were collapsed, responders demonstrated lower vGRF during 2–4% and 14–17% of stance (4% BW) and significantly higher vGRF (2% BW) during 24–48% of stance compared to non-responders (Figures 2C–D and 3C).
Internal Knee Extension Moment
There was no significant group x time interaction for KEM (Figure 1C); however, there were significant main effects for time and group. All participants demonstrated significantly greater KEM (0.004 Nm/BW*height) during 7 – 93% of stance (Figures 2E–F and 3F) at 4-weeks compared to baseline. Responders demonstrated significantly greater KEM (0.006 Nm/BW*height) during 1 – 60% of stance compared to non-responders (Figures 2E–F and 3E).
Discussion
Contrary to our hypothesis, differences existed between responders and non-responders at baseline. Responders demonstrated greater KFA during the first half of stance compared to non-responders at baseline. Responders at 4-weeks did not increase KFA in the first half of stance following strengthening, rather they increased knee extension angle during the second half of stance. Although responders did demonstrate greater knee flexion excursion throughout stance at 4-weeks compared to non-responders, it was due to an increase in knee extension in the second half of stance rather than an increase in KFA in the first half of stance. All participants demonstrated greater vGRF during the first third of stance, lower vGRF in the middle of stance, and greater KEM throughout the majority of stance following the intervention. Although the current data did not generally support our hypotheses, the data do suggest that individuals with KOA who increase quadriceps strength may reduce their “stiffened-knee” gait strategy by increasing knee extension in the second half of stance, which may have important implications on disease progression.
The “stiffened-knee” gait strategy, characterized by decreased knee flexion excursion and a lower KEM throughout stance, may be adopted in individuals with KOA, potentially due to an impaired capacity of the quadriceps to eccentrically control knee flexion and stabilize the joint during the weight acceptance phase of gait [16]. A decrease in knee excursion throughout stance consequently decreases the tibiofemoral contact area during loading, which may cause a deleterious increase in pressure on the new contact area of the tibiofemoral cartilage [36]. Adult articular cartilage is mechanosensitive, meaning overloading and underloading of the tissue may result in negative effects on joint tissue health [5]. Increasing contact force and pressure may cause overloading at a certain location of the cartilage and consequently cause underloading in other locations of the cartilage [11, 37]. Adopting a “stiffened-knee” gait strategy may be a response to knee pain in individuals with KOA, as opposed to a protective mechanism [38]. In individuals with KOA, less KEM is associated with worse self-reported pain [10]. In the current study, responders and non-responders both demonstrated less pain at 4-weeks; suggesting physical therapy, regardless of strength changes, contributed to pain reduction. Responders demonstrated greater KFA in the first half of stance at baseline and greater knee extension in the second half of stance at 4-weeks. While the mean difference in KFA between responders and non-responders is small (2.82°of greater KFA at baseline and 1.67°o f greater knee extension at 4-weeks for responders), a small magnitude during a single step may have larger cumulative effects over the course of a single day [39]. Individuals with symptomatic KOA average approximately 6476 steps per day [40], thereby small differences in knee loading on knee tissues. Future research should focus on the influence of KFA and its effect on cartilage stress and strain during loading of the joint in individuals with KOA.
Those with KOA demonstrate lower vGRF in the first peak of stance [8], greater vGRF at mid-stance [41], and less vGRF in the second peak of stance [41] compared to individuals without KOA. Similar to previous reports, all participants in our cohort demonstrated greater vGRF during the first peak of stance and lower vGRF mid stance at 4-weeks compared to baseline. Individuals with KOA absorb and generate less energy and power at the hip, knee, and ankle compared to asymptomatic individuals during walking gait [42], potentially to reduce reaction forces at the knee. Increased quadriceps strength results in greater knee stability and energy absorption during walking [43], which may have allowed responders to have more control and generate a lower vGRF immediately following heel strike while still reaching a higher vGRF at the first peak during stance. Greater quadriceps strength may allow an individual to resist a greater external knee flexion moment during stance; however, it is unclear if greater loading at the knee joint is beneficial or harmful to joint tissues in individuals with KOA [9, 10]. Future longitudinal studies should continue to determine the effects of knee joint loading on measures of joint tissue.
Individuals with severe KOA demonstrate less KEM during gait compared to asymptomatic or healthy individuals [2, 7]. All individuals in our cohort demonstrated greater KEM throughout stance phase at 4-weeks compared to baseline. Across time, responders demonstrate greater KEM throughout the first half of stance. An increase in vGRF and KFA in the first half of stance will generate a greater external knee flexion moment [44], which requires the knee extensor to generate an equivalent KEM to prevent the knee from going into excessive flexion [44]. Strengthening in individuals with KOA likely increases the ability of the quadriceps muscle to resist a greater external knee flexion moment, resulting in less knee stiffness during walking gait [2, 7]. In our entire cohort, KEM increased throughout stance phase and vGRF was higher at the beginning of stance phase and lower during mid-stance from baseline to 4-weeks for all participants, which may be a beneficial change. Increases in knee flexion excursion and KEM, regardless of maximum isometric strength response, may be a result of individuals altering lower extremity neuromuscular control during gait, regardless of a quadriceps strength response. It is possible changes in gait biomechanics in non-responders were caused by improvements in submaximal neuromuscular control or proprioception during gait. Overall, participants in the current study exhibited less of a stiffened gait strategy following the strengthening intervention.
Gait retraining is another method used to favorably alter walking gait biomechanics in individuals with KOA. [45] However in the current study, responders altered KFA over the course of 4-weeks by strengthening their quadriceps muscles in their involved limb. Gait retraining studies seek to make similar gait modifications (often cuing a decrease external knee adduction moment), and while immediate retention is successful, future work is needed to optimize long-term retention and transfer outside of the laboratory [46–48]. The combination of gait retraining with strength training may result in more optimal adjustments to walking gait compared to strength training alone in individuals with KOA. Future studies should determine the effects of quadriceps strengthening in addition to gait retraining to determine if long-term alterations in gait can be retained.
Twenty-eight percent of our cohort improved their quadriceps strength by at least 29 Nm between baseline and 4-weeks. Although only 28% of individuals increased their quadriceps strength by the MDC of 29 Nm, almost 78% of individuals in our cohort increased their quadriceps by some amount, with the average change for the entire cohort being 22.9 Nm. There were no differences in walking speed, self-reported disability, or self-reported tolerability of walking between the responders and non-responders. Future studies should determine which participants are more likely to benefit from a lower-extremity strengthening protocol. Future studies may also determine if increasing the number of strength training sessions provides additional benefit in strength gains and changes in biomechanics.
This study is the first of our knowledge to compare gait biomechanics in participants with KOA who did and did not increase quadriceps strength following a strengthening intervention. However, there are some limitations to our study. We specifically recruited participants with symptomatic KOA, and inclusion criteria included participants with a WOMAC function score of at least 31 out of 100 [49], indicating self-reported disability. Inclusion criteria also required that participants have a CAR ≤ 92% [22, 23], indicating a neuromuscular activation deficit in the quadriceps muscle. The individuals in the current study had symptomatic KOA as well as quadriceps dysfunction, which may limit the generalizability of our results to the participants who were included in the RCT. Our participants may have been more likely to improve their quadriceps strength because they demonstrated quadriceps dysfunction at baseline. We also do not know if individuals who were responders were able to maintain changes in gait after the study ended or if the small changes made in gait are meaningful in decreasing progression of the disease. While the percentage of patients with bilateral KOA was not different between groups, it is possible biomechanical responses may be different in those with unilateral and bilateral KOA. Future work with larger sample sizes should evaluate differences in the response to strength training in those with unilateral compared to bilateral KOA. Our sample size was not large enough to analyze the effect of TENS between responders and non-responders, and this may be a limitation in our statistical analysis. Also, the number of responders (15) was less than the number of non-responders (38). While changes in KFA are small (2.67°), and statistical significance does not always translate to clinical significance, given the cumulative nature of walking, these small changes may have an impact over time. Future studies should determine retention of altered walking biomechanics due to quadriceps strengthening as well as the long-term effect(s) of those biomechanical changes on knee articular cartilage health.
In the current study, individuals who responded to the strengthening protocol demonstrated favorable modifications in the stance phase of gait. Responders at 4-weeks demonstrated greater knee flexion excursion throughout stance via greater knee extension in the second half of stance phase compared to baseline. vGRF was lower during the initial loading phase following heel strike, but peak vGRF during the first half of stance phase was higher in responders compared to non-responders at 4-weeks. KEM was greater throughout stance phase in responders compared to non-responders at both baseline and 4-weeks. The current study provides evidence that quadriceps strengthening may be a clinically useful intervention to elicit favorable changes in gait for individuals with KOA.
Supplementary Material
Acknowledgments
Role of the funding source: Research reported in this manuscript was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (1R21AR067560-01). The funding source had no role in the study design, collection, analysis and interpretation of data, writing of the manuscript, and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: There are no reported competing interests.
References
- 1.Heidari B Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Caspian J Intern Med 2011; 2: 205–212. [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufman KR, Hughes C, Morrey BF, Morrey M, An KN. Gait characteristics of patients with knee osteoarthritis. J Biomech 2001; 34: 907–915. [DOI] [PubMed] [Google Scholar]
- 3.Mundermann A, Dyrby CO, Andriacchi TP. Secondary gait changes in patients with medial compartment knee osteoarthritis: increased load at the ankle, knee, and hip during walking. Arthritis Rheum 2005; 52: 2835–2844. [DOI] [PubMed] [Google Scholar]
- 4.Winters JD, Rudolph KS. Quadriceps rate of force development affects gait and function in people with knee osteoarthritis. European Journal of Applied Physiology 2014; 114: 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andriacchi TP, Favre J. The nature of in vivo mechanical signals that influence cartilage health and progression to knee osteoarthritis. Curr Rheumatol Rep 2014; 16: 463. [DOI] [PubMed] [Google Scholar]
- 6.Debbi EM, Wolf A, Goryachev Y, Rozen N, Haim A. Alterations in sagittal plane knee kinetics in knee osteoarthritis using a biomechanical therapy device. Ann Biomed Eng 2015; 43: 1089–1097. [DOI] [PubMed] [Google Scholar]
- 7.Astephen JL, Deluzio KJ, Caldwell GE, Dunbar MJ. Biomechanical changes at the hip, knee, and ankle joints during gait are associated with knee osteoarthritis severity. J Orthop Res 2008; 26: 332–341. [DOI] [PubMed] [Google Scholar]
- 8.Childs JD, Sparto PJ, Fitzgerald GK, Bizzini M, Irrgang JJ. Alterations in lower extremity movement and muscle activation patterns in individuals with knee osteoarthritis. Clin Biomech (Bristol, Avon) 2004; 19: 44–49. [DOI] [PubMed] [Google Scholar]
- 9.Chehab EF, Favre J, Erhart-Hledik JC, Andriacchi TP. Baseline knee adduction and flexion moments during walking are both associated with 5 year cartilage changes in patients with medial knee osteoarthritis. Osteoarthritis and Cartilage 2014; 22: 1833–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erhart-Hledik JC, Favre J, Andriacchi TP. New insight in the relationship between regional patterns of knee cartilage thickness, osteoarthritis disease severity, and gait mechanics. Journal of Biomechanics 2015; 48: 3868–3875. [DOI] [PubMed] [Google Scholar]
- 11.Saxby DJ, Bryant AL, Modenese L, Gerus P, Killen BA, Konrath J, et al. Tibiofemoral Contact Forces in the Anterior Cruciate Ligament-Reconstructed Knee. Med Sci Sports Exerc 2016; 48: 2195–2206. [DOI] [PubMed] [Google Scholar]
- 12.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res 1991; 9: 113–119. [DOI] [PubMed] [Google Scholar]
- 13.Shelburne KB, Torry MR, Pandy MG. Contributions of muscles, ligaments, and the ground-reaction force to tibiofemoral joint loading during normal gait. J Orthop Res 2006; 24: 1983–1990. [DOI] [PubMed] [Google Scholar]
- 14.Pandy MG, Andriacchi TP. Muscle and joint function in human locomotion. Annu Rev Biomed Eng 2010; 12: 401–433. [DOI] [PubMed] [Google Scholar]
- 15.Farrokhi S, O’Connell M, Fitzgerald GK. Altered gait biomechanics and increased knee-specific impairments in patients with coexisting tibiofemoral and patellofemoral osteoarthritis. Gait & Posture 2015; 41: 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray AM, Thomas AC, Armstrong CW, Pietrosimone BG, Tevald MA. The associations between quadriceps muscle strength, power, and knee joint mechanics in knee osteoarthritis: A cross-sectional study. Clinical Biomechanics 2015; 30: 1140–1145. [DOI] [PubMed] [Google Scholar]
- 17.Bennell KL, Hinman RS, Metcalf BR. Association of sensorimotor function with knee joint kinematics during locomotion in knee osteoarthritis. Am J Phys Med Rehabil 2004; 83: 455–463; quiz 464–456, 491. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt LC, Rudolph KS. Influences on knee movement strategies during walking in persons with medial knee osteoarthritis. Arthritis Rheum 2007; 57: 1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pietrosimone BG, Saliba SA, Hart JM, Hertel J, Kerrigan DC, Ingersoll CD. Effects of transcutaneous electrical nerve stimulation and therapeutic exercise on quadriceps activation in people with tibiofemoral osteoarthritis. J Orthop Sports Phys Ther 2011; 41: 4–12. [DOI] [PubMed] [Google Scholar]
- 20.Deyle GD, Allison SC, Matekel RL, Ryder MG, Stang JM, Gohdes DD, et al. Physical Therapy Treatment Effectiveness for Osteoarthritis of the Knee: A Randomized Comparison of Supervised Clinical Exercise and Manual Therapy Procedures Versus a Home Exercise Program. Physical Therapy 2005; 85: 1301–1317. [PubMed] [Google Scholar]
- 21.Deyle GD, Henderson NE, Matekel RL, Ryder MG, Garber MB, Allison SC. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee: A randomized, controlled trial. Annals of Internal Medicine 2000; 132: 173–181. [DOI] [PubMed] [Google Scholar]
- 22.Pietrosimone BG, Hertel J, Ingersoll CD, Hart JM, Saliba SA. Voluntary quadriceps activation deficits in patients with tibiofemoral osteoarthritis: a meta-analysis. Pm r 2011; 3: 153–162; quiz 162. [DOI] [PubMed] [Google Scholar]
- 23.Luc BA, Harkey MH, Arguelles GD, Blackburn JT, Ryan ED, Pietrosimone B. Measuring voluntary quadriceps activation: Effect of visual feedback and stimulus delivery. J Electromyogr Kinesiol 2016; 26: 73–81. [DOI] [PubMed] [Google Scholar]
- 24.Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve 1996; 19: 861–869. [DOI] [PubMed] [Google Scholar]
- 25.Davis HC, Troy Blackburn J, Ryan ED, Luc-Harkey BA, Harkey MS, Padua DA, et al. Quadriceps rate of torque development and disability in individuals with anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 2017; 46: 52–56. [DOI] [PubMed] [Google Scholar]
- 26.Kean CO, Birmingham TB, Garland SJ, Bryant DM, Giffin JR. Minimal detectable change in quadriceps strength and voluntary muscle activation in patients with knee osteoarthritis. Arch Phys Med Rehabil 2010; 91: 1447–1451. [DOI] [PubMed] [Google Scholar]
- 27.Blackburn JT, Norcross MF, Cannon LN, Zinder SM. Hamstrings stiffness and landing biomechanics linked to anterior cruciate ligament loading. J Athl Train 2013; 48: 764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell AL, Brand RA, Pedersen DR. Prediction of hip joint centre location from external landmarks. Human Movement Science 1989; 8: 3–16. [Google Scholar]
- 29.Luc-Harkey BA, Harkey MS, Stanley LE, Blackburn JT, Padua DA, Pietrosimone B. Sagittal plane kinematics predict kinetics during walking gait in individuals with anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 2016; 39: 9–13. [DOI] [PubMed] [Google Scholar]
- 30.Pietrosimone B, Blackburn JT, Harkey MS, Luc BA, Hackney AC, Padua DA, et al. Greater Mechanical Loading During Walking Is Associated With Less Collagen Turnover in Individuals With Anterior Cruciate Ligament Reconstruction. Am J Sports Med 2016; 44: 425–432. [DOI] [PubMed] [Google Scholar]
- 31.Pietrosimone B, Loeser RF, Blackburn JT, Padua DA, Harkey MS, Stanley LE, et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics six-months following anterior cruciate ligament reconstruction. J Orthop Res 2017. [DOI] [PMC free article] [PubMed]
- 32.Blackburn JT, Pietrosimone BG, Harkey MS, Luc BA, Pamukoff DN. Comparison of three methods for identifying the heelstrike transient during walking gait. Med Eng Phys 2016; 38: 581–585. [DOI] [PubMed] [Google Scholar]
- 33.Radin EL, Martin RB, Burr DB, Caterson B, Boyd RD, Goodwin C. Effects of mechanical loading on the tissues of the rabbit knee. J Orthop Res 1984; 2: 221–234. [DOI] [PubMed] [Google Scholar]
- 34.Park J, Seeley MK, Francom D, Reese CS, Hopkins JT. Functional vs. Traditional Analysis in Biomechanical Gait Data: An Alternative Statistical Approach. J Hum Kinet 2017; 60: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H, Son S, Seeley MK, Hopkins JT. Functional Fatigue Alters Lower-extremity Neuromechanics during a Forward-side Jump. Int J Sports Med 2015; 36: 1192–1200. [DOI] [PubMed] [Google Scholar]
- 36.Favre J, Erhart-Hledik JC, Chehab EF, Andriacchi TP. Baseline ambulatory knee kinematics are associated with changes in cartilage thickness in osteoarthritic patients over 5 years. J Biomech 2016; 49: 1859–1864. [DOI] [PubMed] [Google Scholar]
- 37.Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng 2000; 2: 691–713. [DOI] [PubMed] [Google Scholar]
- 38.Seeley MK, Park J, King D, Hopkins JT. A novel experimental knee-pain model affects perceived pain and movement biomechanics. J Athl Train 2013; 48: 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maly MR, Robbins SM, Stratford PW, Birmingham TB, Callaghan JP. Cumulative knee adductor load distinguishes between healthy and osteoarthritic knees-A proof of principle study. Gait & Posture 2013; 37: 397–401. [DOI] [PubMed] [Google Scholar]
- 40.White DK, Tudor-Locke C, Zhang Y, Fielding R, LaValley M, Felson DT, et al. Daily walking and the risk of incident functional limitation in knee osteoarthritis: an observational study. Arthritis Care Res (Hoboken) 2014; 66: 1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen CP, Chen MJ, Pei YC, Lew HL, Wong PY, Tang SF. Sagittal plane loading response during gait in different age groups and in people with knee osteoarthritis. Am J Phys Med Rehabil 2003; 82: 307–312. [DOI] [PubMed] [Google Scholar]
- 42.Resende RA, Fonseca ST, Silva PL, Magalhaes CM, Kirkwood RN. Power at hip, knee and ankle joints are compromised in women with mild and moderate knee osteoarthritis. Clin Biomech (Bristol, Avon) 2012; 27: 1038–1044. [DOI] [PubMed] [Google Scholar]
- 43.Palmieri-Smith RM, Thomas AC. A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev 2009; 37: 147–153. [DOI] [PubMed] [Google Scholar]
- 44.Creaby MW, Hunt MA, Hinman RS, Bennell KL. Sagittal plane joint loading is related to knee flexion in osteoarthritic gait. Clinical Biomechanics 2013; 28: 916–920. [DOI] [PubMed] [Google Scholar]
- 45.Barrios JA, Crossley KM, Davis IS. Gait retraining to reduce the knee adduction moment through real-time visual feedback of dynamic knee alignment. J Biomech 2010; 43: 2208–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richards R, van der Esch M, van den Noort JC, Harlaar J. The learning process of gait retraining using real-time feedback in patients with medial knee osteoarthritis. Gait Posture 2018; 62: 1–6. [DOI] [PubMed] [Google Scholar]
- 47.Richards RE, van den Noort JC, van der Esch M, Booij MJ, Harlaar J. Effect of real-time biofeedback on peak knee adduction moment in patients with medial knee osteoarthritis: Is direct feedback effective? Clin Biomech (Bristol, Avon) 2017. [DOI] [PubMed]
- 48.van den Noort JC, Steenbrink F, Roeles S, Harlaar J. Real-time visual feedback for gait retraining: toward application in knee osteoarthritis. Med Biol Eng Comput 2015; 53: 275–286. [DOI] [PubMed] [Google Scholar]
- 49.Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: the patient acceptable symptom state. Annals of the Rheumatic Diseases 2005; 64: 34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


