Abstract
Problem:
Chlamydia infections in women can ascend to the upper genital tract, and repeated infections are common, placing women at risk for sequelae. The protective role of anti-chlamydia antibodies to surface exposed antigens in ascending and incident infection is unclear.
Method of Study:
A whole-bacterial ELISA was used to quantify chlamydia-specific IgG and IgA in serum and cervical secretions of 151 high-risk women followed longitudinally. Correlations were determined between antibody and cervical burden and causal mediation analysis investigated the effect of antibody on ascension. We examined the relationship of antibody to incident infection using the marginal Cox model.
Results:
Serum and cervical anti-chlamydia IgG and cervical IgA levels correlated inversely with cervical burden. While lower burden was associated with reduced ascension, causal mediation analysis revealed that the indirect effects of antibody mediated through reductions in bacterial burden were insufficient to prevent ascension. Analysis of women uninfected at enrollment revealed that serum and cervical anti-chlamydia IgG were associated with increased risk of incident infection; hazard ratio increased 3.6-fold (95% CI, 1.3 – 10.3), and 22.6-fold (95% CI, 3.1 – 165.2) with each unit of serum and cervical IgG, respectively.
Conclusions:
Although anti-chlamydia IgG and IgA correlated with reduced cervical chlamydia burden, they failed to prevent ascension and increased levels of anti-chlamydia IgG were associated with increased risk for incident infection.
Keywords: chlamydia, women, antibody, genital tract, endometrium, incident infection, causal mediation
INTRODUCTION
Chlamydia trachomatis is highly prevalent due to its asymptomatic nature, and its ability to evade immunity results in chronic and repeated infections that can lead to serious reproductive sequelae in women. Chlamydia elementary bodies (EBs) rapidly invade host epithelial cells through multiple mechanisms1–4, where they transform to reticulate bodies and replicate within a protective vacuole (inclusion) before re-differentiating to infectious EBs that escape to infect adjacent cells and hosts exposed through sexual contact. CD4 T cell mediated immunity is essential for host defense against chlamydia and plays a key role in eradication of infection and resistance to reinfection5–8. In contrast, a protective role for antibody in chlamydial immunity is unresolved.
Anti-chlamydia antibodies against surface exposed proteins could neutralize infectious extracellular EBs prior to host cell invasion, and thereby prevent infection or reduce infectious burden. Antibody-mediated chlamydia neutralization has been demonstrated in vitro9–11, but animal studies suggesting a protective role for this mechanism are limited12,13. Other studies support a role for antibody in limiting infection by enhancement of chlamydia opsonophagocytosis and degradation14,15, but this process appears dependent on T-cell interferon-γ (IFN-γ) production15.
In two longitudinal analyses of highly exposed women, chlamydia-specific CD4 T cell IFN-γ responses, and not serum anti-chlamydia IgG titers, were associated with a reduced risk of reinfection16,17. Same serovar repeated infections are frequent18,19, and high titers of anti-chlamydia IgG antibodies are associated with enhanced disease20–22. These findings suggest high IgG titers are a marker of repeated and/or prolonged chlamydia exposure, and do not protect from reinfection.
We previously investigated the role for anti-chlamydia EB IgG in limiting chlamydial infection16. We observed both that serum titers of anti-EB IgG were associated with reduced cervical burden, and that women with lower cervical burden were at reduced risk for endometrial infection23. Paradoxically, anti-EB IgG titers failed to correlate with absence of endometrial infection16. These data suggest anti-EB IgG antibodies are ineffective at limiting ascension and its associated risk for disease. However, these studies did not investigate mucosal IgG, or IgA, which may exhibit more potent, neutralizating effects24. Brunham et al. reported that anti-chlamydia IgA, but not IgG, was more frequently detected in genital secretions of women who did not develop salpingitis, compared to those who did, suggesting a role for local IgA in limiting ascension25. Levels of HIV specific mucosal IgA were shown to correlate with resistance to HIV infection in Kenyan sex workers26, and IgA was shown to act through both extracellular neutralization and intracellular viral inhibition after transcytosis27,28.
Despite these protective effects, some antibodies can also mediate enhanced uptake of pathogens. Antibody mediated enhancement of infection has been well descried for viruses29,30. In addition, IgG specific to the major outer membrane protein (MOMP) of chlamydia has been shown to enhance chlamydial uptake and transcytosis through polarized genital tract epithelial cells, and increase murine genital tract infection11. To explore the potential that direct effects of antibody might counteract limitation of ascension mediated through lowering cervical burden, we performed a causal mediation analysis. We determined that the probability of endometrial infection was significantly reduced by both serum anti-EB IgG and cervical anti-EB IgA mediated through effects on burden. However, the burden-independent effects of antibody on ascension negated these protective effects, leading to an overall effect that was neither protective or detrimental. We also determined that both serum and cervical anti-EB IgG correlated with significantly increased, rather than decreased risk, of repeat infection. These novel results indicate that natural infection induces high levels of antibody to surface exposed chlamydial EB proteins in women that can effectively lower cervical burden, but are insufficient to prevent endometrial infection or reinfection and serve as biomarkers of enhanced susceptibility.
METHODS
Patient Population
The Institutional Review Boards for Human Subject Research at the University of Pittsburgh and the University of North Carolina approved the study and all participants provided written informed consent prior to inclusion. Samples were collected from participants recruited into a previously described T cell Response Against Chlamydia (TRAC) cohort16, which enrolled 246 asymptomatic women (age 15–30 years) at high risk for sexually transmitted infection from three urban sites in Pittsburgh, Pennsylvania from 2011–2015. Eligibility criteria included clinical evidence of mucopurulent cervicitis, diagnosis of chlamydia prior to treatment, or reported sexual contact with a man recently diagnosed with chlamydia urethritis or nongonococcal urethritis. Women diagnosed with pelvic inflammatory disease or who were pregnant were excluded.
Demographic and clinical data were obtained using a standardized questionnaire and examination. Cervical secretions were adsorbed onto an ophthalmic sponge (Beaver-Visitec, Waltham, MA) placed within the endocervix (30 seconds) of each patient recruited into the TRAC cohort. An endometrial biopsy was then obtained to determine extent of infection. Cervical and endometrial swab samples were tested for C. trachomatis, N. gonorrhoeae, and M. genitalium using nucleic acid amplification tests (NAAT), (Aptima Combo 2; Gen-Probe, and APTIMA MG; Gen-Probe) as described previously16. Cervical chlamydia burden was estimated via quantitative PCR using genomic DNA extracted from reserved cervical swab eluates as template23. Women with positive cervical and endometrial Chlamydia NAATs were designated Endo+, while those with a positive cervical NAAT only were defined as Endo-. Serum was collected for antibody assays. All women were treated with ceftriaxone and azithromycin at enrollment regardless of infection status. Participants returned for follow-up visits at 1, 4, 8, and 12 months after enrollment, microbiologic, medical, sexual history and exposure, and contraception data were gathered at each follow-up visit, and all participants that tested positive for chlamydia were treated with azithromycin.
Antibodies in Serum and Cervical Secretions
Cervical secretions were eluted from sponges as previously described31. IgG and IgA assays were performed on cervical secretions and serum samples collected from the first 151 participants enrolled into the TRAC cohort. Concentrations of anti-EB IgG and IgA were measured using a whole-EB ELISA that detects chlamydial outer membrane and periplasmic proteins, but not intracellular proteins31. Concentrations of total IgG and IgA in serum and secretions were measured using a previously described ELISA which readily detects secretory IgA in mucosal secretions32. Concentrations of anti-EB IgG or IgA antibody in each sample were determined from standard curves that consisted of pooled human serum from CT-infected subjects, then divided by the concentration of total IgG or IgA to obtain the specific activity. Non-specific antibody values were quantitated using 2 IgG and 9 IgA myeloma antibodies as well as anti-HIV-1 gp120 and gp41 human IgG1 mAbs derived from transformed human B cells or hybridomas as previously described31. The cut-off for detection of a specific anti-EB antibody response was defined by a value that was > the mean + 3 standard deviations (SD) of the non-specific antibody values, which implies a value greater than 99% of the non-specific antibody values.
Statistical Analysis
Anti-EB antibody and cervical chlamydia burden values were log transformed and tested for normal distribution using the Shapiro–Wilk normality tests. Pearson correlation was used to measure the strength of association of the four antibodies to each other, and to cervical burden. A comparison of cervical chlamydial burden in Endo+ versus Endo- women was conducted by unpaired t test.
Antibody and ascension by causal mediation analysis.
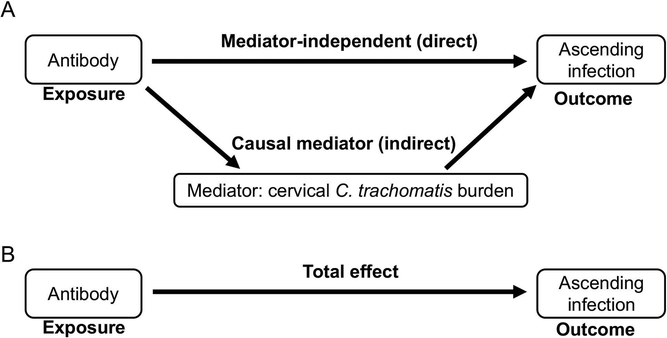
We considered ascending infection (Endo+ vs. Endo-) as a binary outcome, and we defined chlamydia cervical burden as a mediator, and antibody as an exposure. These definitions were based on prior data from this cohort that revealed women with lower cervical burden had higher titers of serum anti-chlamydia IgG, implying antibody drives a reduction in burden16. This led us to use a well-established causal mediation algorithm published by Imai and Tingley33 to investigate the association of antibody with endometrial infection. Using causal mediation, the relationship between antibody and ascension is decomposed into a direct link and an indirect link. The causal mediation effect represents the indirect effect of the exposure (antibody) on the outcome (ascension) through the mediating variable (bacterial burden). Whereas, the direct effect is the effect of the exposure (antibody) on the outcome (ascension) that is independent of the mediator (burden). The total effect is the sum of the indirect and direct effects (Figure 1). The Imai and Tingley algorithm models the exposure as binary and two levels of exposure can be accommodated. For this analysis, we compared the median antibody level of participants with EB-specific responses versus the median antibody level of participants with responses below the cut-off for EB antibody specificity to determine if detection of an EB-specific antibody response was associated with reduced endometrial infection. Since we previously determined that oral contraceptive pill (OCP) use and N. gonorrhoeae co-infection were significant risk factors for ascending infection16, we treated these as confounders.
Figure 1.
(A) Diagram of causal mediation analysis of the relation of antibody (Exposure), cervical C. trachomatis burden (Mediator) and ascending infection (Outcome). Sensitivity analysis allows for assessing unobserved confounder bias. (B) The sum of mediator-dependent and –independent effects equals the total effect.
Mediation processes are framed in terms of intermediate variables between an independent variable and a dependent variable, where t is the independent variable (antibody), Y is the dependent variable (ascension), and M is the mediator variable (cervical chlamydial burden) that is supposed to transmit the causal effect of t to Y33. The total effect of t on Y is referred to as the total effect (TE), and that effect is then partitioned into a combination of a direct effect (DE) of t on Y, and an indirect effect (IE) of t on Y that is transmitted through M.
We defined the causal mediation effect of antibody as follows:
Yi denotes the outcome (ascension) for subject i; t is the exposure (antibody) status, t=0 denotes absence of EB-specific response, t=1 denotes presence of EB-specific response; E denotes expected value. This determines what change would occur to the outcome, if one changes the mediator (cervical chlamydia burden) value that would be realized under the presence of an EB-specific response, M i (1), to the value that would be observed under the absence of an EB-specific response, M i (0), while holding the exposure status constant. If antibody has no effect on cervical chlamydia burden, M(1)=M(0), then the causal mediation effect is zero.
Depending on level of t, we have:
In causal mediation analysis, we are interested in the average causal mediation effect between exposed (median of participants with an EB-specific response) and control (median of participants without an EB-specific response) populations:
The direct effect represents the effect of the exposure on outcome while holding the level of the mediator constant. We defined the direct effect as follows:
For t=0,1. For example, ζi (1) represents the direct effect of the presence of an EB-specific response on subject i’s ascension outcome while holding the chlamydia burden level constant. Similarly, averaging over the relevant population, we can define the average direct effects.
To investigate whether the effect of anti-EB antibody on burden is different in women with EB-specific antibody responses and those without, we used statistical software R mediation package (Version 4.4.6) for biostatistical interactions. A key assumption of mediation is that no unmeasured factors confound the relation between mediator and outcome. To investigate for unobserved confounder bias in our mediation analysis, we performed a sensitivity analysis that quantified the degree to which unmeasured confounders must be present to reverse the direction of the mediation effect. If the inference of a causal mediation effect is sensitive, a slight violation of the assumption (small ρ value) may lead to a substantially different conclusion (reverse direction of effect). The null hypothesis is that unexplained variances in mediator and outcomes are independent, and therefore the sensitivity parameter, correlation (ρ), between these two variances should be zero. Results of this sensitivity analysis shows how much the casual mediation effect changes as a function of ρ. We used Imai and Tingley algorithms and statistical software R package mediation for both mediation and sensitivity analyses33–35.
Antibody and incident infection.
Since active infection drives antibody production we stratified our analysis into separate evaluations of uninfected and infected women. We used the non-parametric Mann–Whitney U test to determine if antibody levels differed among women who developed incident infection during a year of follow-up versus those who did not. Women who tested positive for chlamydia infection at any follow-up visit were treated with azithromycin. These women were assumed to be cured of their infection and at risk for repeat infection. We therefore proceeded to determine the relative hazard ratios (HR) for antibody effects on incident infection using the Wei–Lin–Weissfeld method, a marginal Cox model that accounts for multiple chlamydial infections per person and adjusts for within-subject correlations36. Adjustments were made for previously identified risk factors associated with incident infection, including age, current or incident gonorrhea, chlamydia infection at enrollment; and sex with new, uncircumcised, or infected partners16. To formally test situations in which the antibody effect differs according to infection status (Uninfected, Endo-, and Endo+), we added an interaction term of antibody × infection status at enrollment to the model. This enabled a single test of antibody effects in the entire group rather than performing separate tests in the three groups; these analyses were performed in SAS 9.4 (SAS Institute Inc.). All analyses used a P value of < 0.05 as significant.
RESULTS
Baseline Characteristics of Participants
Data from the first 151 women recruited into TRAC, whose anti-EB IgG, and anti-EB IgA had been quantified in serum and cervical secretions via ELISA, were analyzed in this study. Among them, 56 (37%) were negative for chlamydia infection at cervix and endometrium, 56 (37%) had cervical infection only (Endo-), and 39 (26%) had cervical and endometrial infection (Endo+). Consistent with our previous report for 225 women of this cohort, women in this study were young (median age 21 years; range, 18–35 years), single (89%), and African American (66%) (Supplementary Table 1). The majority (57%) reported a previous diagnosis of chlamydial infection, and (25%) reported having had ≥2 prior infections. About 68% of participants reported a past history of at least one bacterial STI such as N. gonorrhoeae (23%) and/or T. vaginalis (27%). 117 women (77%) completed at least 3 follow-up visits, and 87 (58%) completed 4. There were no sociodemographic differences between women who attended all visits and those who did not (data not shown).
Relationship between Anti-chlamydia EB Antibodies and Ascension
We determined anti-EB IgG and IgA antibody levels in serum and cervical secretions to explore both systemic and mucosal responses to multiple outer membrane components of the infectious developmental form of chlamydia31. We first determined the strength of relationships among the anti-EB antibodies measured at enrollment. Although all antibody relationships were significant and positively correlated, only the correlation between serum and cervical anti-EB IgG levels were strong (R=0.57, P = 1.82E-13). The positive correlations were weak to moderate for serum and cervical anti-EB IgA levels (R=0.26, P = 0.002), serum anti-EB IgG and IgA levels (R=0.26, P = 0.0013), and cervical anti-EB IgG and IgA levels (R=0.36, P = 1.18E-5).
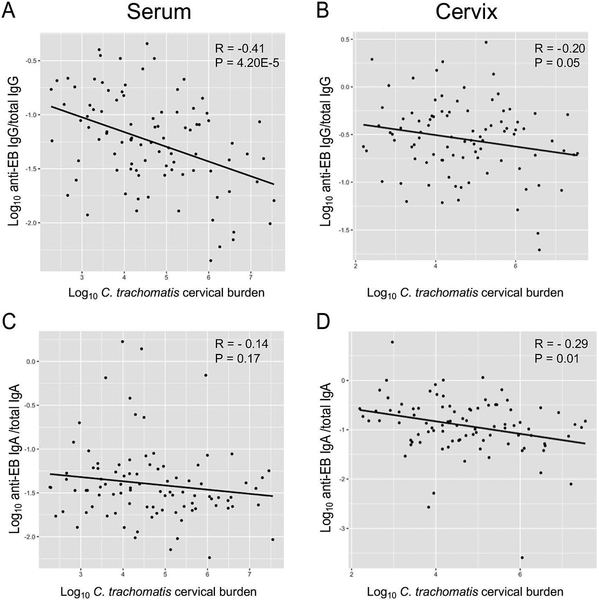
We next determined the relationship of the various antibody subtypes to cervical chlamydia load. Inverse correlations were significant for both serum and cervical anti-EB IgG and cervical chlamydia burden, with the strength of the inverse correlation being stronger for serum anti-EB IgG compared to cervical anti-EB IgG (R = - 0.41, P = 4.20E-5 and R = - 0.20, P = 0.05 respectively). A weak inverse correlation of borderline significance was observed between anti-EB IgA in cervical secretions and cervical chlamydia load (R = - 0.29, P = 0.01), and a non-significant weak inverse correlation was observed between serum anti-EB IgA levels and chlamydia burden (R = - 0.14, P = 0.17) (Figure 2).
Figure 2.
Correlations of cervical chlamydia burden with anti-chlamydia EB antibodies among all participants. Correlation plot for chlamydia cervical burden versus serum anti-EB IgG (A), cervical anti-EB IgG (B), serum anti-EB IgA (C), and cervical anti-EB IgA (D). X-axis: Log10 chlamydia DNA copies/swab. Y-axis: Log10 ratio of the concentration of anti-EB antibody/total antibody as determined by whole-EB ELISA.
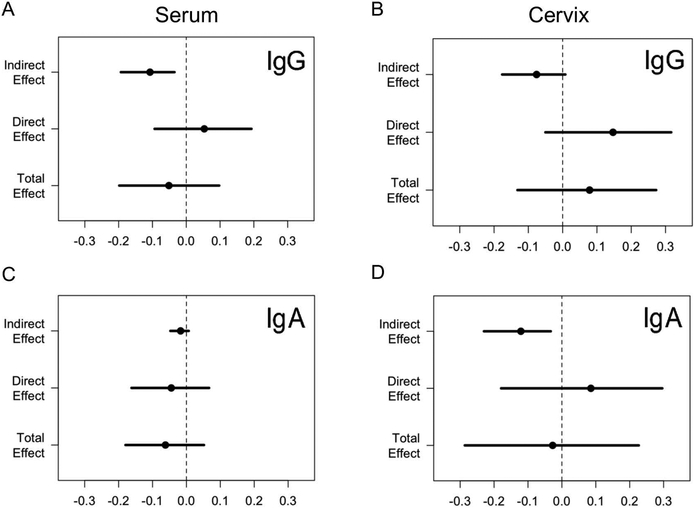
We next investigated the association between chlamydia load and ascension. Cervical burden was significantly lower in 56 women with cervical infection only, compared to 39 women with endometrial infection (P = 3.51E-08, t test) (Supplementary Figure 1), consistent with prior findings within the cohort23. Finally, we performed a causal mediation analysis to test whether antibody impacted ascension (Figure 1). Since inverse correlations were determined between each antibody subtype and cervical chlamydia burden, we delineated the effects of antibody subtypes on ascension that are mediated through their effects on burden (indirect), as well as effects that are independent of their effects on pathogen load (direct) (Figure 1A). The combination of burden/mediator-dependent, indirect effects and burden-independent, direct effects comprises the total effect (Figure 1B). The results are listed in Table 1 and graphically displayed in Figure 3. The antibody effect mediated through reduced burden was a decreased probability of ascension. This was significant for anti-EB serum IgG and cervical IgA, borderline for cervical IgG, and non-significant for serum IgA (Table 1). The direct effects independent of burden negated the protective role of antibodies, leading to a non-significant relationship between serum and mucosal anti-EB IgG and serum and mucosal anti-EB IgA on the probability of endometrial infection.
Table 1.
Effects of antibody on probability of chlamydia ascension to the endometrium determined by causal mediator analysis.
| Antibody Subtype | Average indirect effect or causal mediator effect through burden |
Average direct effect independent of burden |
Average total effect | |||
|---|---|---|---|---|---|---|
| (95% CI) | P Value | (95% CI) | P Value | (95% CI) | P Value | |
| Serum IgG | −0.11# | 0.055 | −0.055 | |||
| (−0.19, −0.04) | 0.001 | (−0.1, 0.2) | 0.445 | (−0.2, 0.1) | 0.496 | |
| Serum IgA | −0.02 | −0.04 | −0.06 | |||
| (−0.05, 0.01) | 0.180 | (−0.16, 0.07) | 0.452 | (−0.18, 0.05) | 0.296 | |
| Cervix IgG | −0.07 | 0.15 | 0.08 | |||
| (−0.17, 0.01) | 0.082 | (−0.05, 0.33) | 0.128 | (−0.13, 0.27) | 0.449 | |
| Cervix IgA | −0.12 | 0.09 | −0.03 | |||
| (−0.22, −0.03) | 0.002 | (−0.17, 0.31) | 0.459 | (−0.29, 0.23) | 0.845 | |
Each value represents the percent decreased (-values) or increased (+values) probability of ascension. For example, there is an 11% decreased risk of ascension for women with a specific serum anti-EB IgG response.
Figure 3.
Graphical summary of causal mediation analysis results for effects of serum anti-EB IgG (A), cervical anti-EB IgG (B), serum anti-EB IgA (C) and cervical anti-EB IgA (D) on ascension. X-axis represents the effect value; each dot represents the point estimate, and each line represents the confidence interval. The dotted vertical line indicates absence of an effect of antibody on ascension.
A sensitivity analysis determined that the observed mediated effect was unlikely to be caused by an unmeasured confounder for all antibody subtypes assessed (Supplementary Figure 2). These analyses strengthen the validity of the results of causal mediation. To exclude the possibility that the effects of antibody on burden differed between women with EB-specific antibody responses and those without, we used R mediation analysis to detect biostatistical interactions of antibody and pathogen load and determined that no interaction existed for any antibody subtype (P=0.93, 0.99, 0.51, and 0.80 for serum IgG and IgA, cervix IgG and IgA respectively).
Relationship of Anti-chlamydia EB Antibodies with Incident Infection
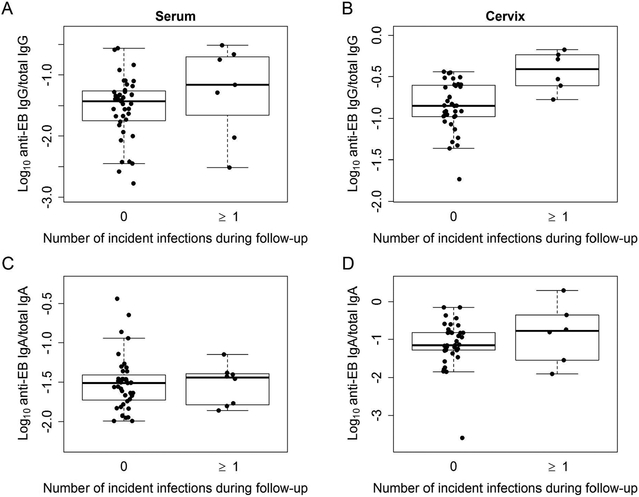
We next examined the relationship between anti-EB antibodies and incident infection. Recognizing that active infection drives induction of antibody, we stratified our analysis of incident infection into two separate evaluations of uninfected and infected women to determine if baseline antibody levels altered incident infection risk. Anti-EB serum and cervical IgG were higher in uninfected women who experienced incident infection(s), compared to those that did not (Figure 4A-B and Table 2). Although this relationship was not observed with anti-EB serum and cervical IgA levels, higher levels of mucosal anti-EB IgA clearly failed to prevent infection (Figure 4C-D and Table 2). After determining that baseline anti-EB IgG levels varied between uninfected women who became infected versus those who did not, we formally tested the association of anti-EB IgG levels with increased risk of incident chlamydia infection and adjusted for 6 potential confounders: age, gonorrhea, chlamydia infection at enrollment, sex with new, uncircumcised, or infected partners (Table 2) 16. Each unit of anti-EB serum IgG significantly increased the HR of incident infection 3.63-fold (95% CI, 1.28 – 10.34). Cervical anti-EB IgG was even more pronounced at 22.57-fold (95% CI, 3.08 – 165.16).
Figure 4.
Relationship of anti-EB antibodies in uninfected women at enrollment to incident infection during follow-up. Box and whisker plots for serum anti-EB IgG (A), cervical anti-EB IgG (B), serum anti-EB IgA (C), and cervical anti-EB IgA (D). Each dot represents one woman. The middle line of each box indicates the median value of antibody data. The upper and lower limit of each box represents the 75% (Q3) and 25% (Q1), respectively. The box spans the interquartile range (IQR=Q3-Q1).Upper whisker = min(max(antibody values), Q3 + 1.5 * IQR) and lower whisker = max(min(antibody values), Q1 – 1.5 * IQR).
Table 2.
Univariable analysis of baseline antibody levels ✕ chlamydia infection status indicates increased risk for incident infection in women who were uninfected at enrollment.
| Interaction term | Description | Unadjusted | Adjusted* | ||
|---|---|---|---|---|---|
| Estimated HR# (95% CI) |
P value** | Estimated HR (95% CI) |
P value** | ||
| Serum anti-EB IgG × chlamydia infection status | Uninfected | 3.31 (1.20 , 9.15) | ref*** | 3.63 (1.28 , 10.34) | ref |
| Endo- | 1.04 (0.64 , 1.69) | 0.044 | 0.90 (0.51 , 1.56) | 0.020 | |
| Endo+ | 0.85 (0.46 , 1.59) | 0.026 | 0.98 (0.51 , 1.9) | 0.038 | |
| Serum anti-EB IgA × chlamydia infection status | Uninfected | 0.88 (0.4 , 1.93) | ref | 0.89 (0.46 , 1.74) | ref |
| Endo- | 1.23 (0.89 , 1.69) | 0.441 | 1.02 (0.72 , 1.45) | 0.735 | |
| Endo+ | 0.77 (0.36 , 1.66) | 0.821 | 0.90 (0.42 , 1.95) | 0.983 | |
| Cervical anti-EB IgG × chlamydia infection status | Uninfected | 19.54 (3.17 , 120.54) | ref | 22.57 (3.08 , 165.16) | ref |
| Endo- | 0.85 (0.59 , 1.22) | 0.001 | 1.05 (0.69 , 1.6) | 0.003 | |
| Endo+ | 1.13 (0.57 , 2.21) | 0.004 | 1.27 (0.61 , 2.65) | 0.007 | |
| Cervical anti-EB IgA × chlamydia infection status | Uninfected | 1.16 (0.50 , 2.68) | ref | 1.13 (0.45 , 2.85) | ref |
| Endo- | 1.07 (0.70 , 1.65) | 0.877 | 1.22 (0.78 , 1.9) | 0.890 | |
| Endo+ | 0.97 (0.58 , 1.63) | 0.733 | 0.98 (0.56 , 1.7) | 0.790 | |
HR: hazard ratio. Each value represents the fold-change in incident infection risk for each unit increase in log10 anti-EB antibody/total antibody
Adjusted for age, gonorrhea, and sex with new, uncircumcised, or infected partners.
P value indicates the statistical comparison of the effect of antibody on incident infection between the various groups, with uninfected women as the reference group.
ref: Reference level
In contrast, IgG antibody levels at baseline did not predict an altered risk of incident infection in Endo- or Endo+ infected women, with statistical differences being observed between the HR for uninfected women and those who were Endo- and Endo+ (P =0.044 and 0.026 for serum anti-EB IgG; and P = 0.001 and 0.004 for cervical anti-EB IgG respectively). Baseline levels of IgA antibodies in infected women were not associated with altered risk of incident infection (Table 2 and Supplementary Figure 3).
DISCUSSION
Chlamydial infection reaching the upper genital tract of women increases risk for tubal factor infertility and chronic pelvic pain. A chlamydia vaccine that induces complete immunity to infection would be optimal, but a vaccine that prevents ascension could eliminate these morbidities. Thus, defining immune responses that limit chlamydial ascension is important for vaccine development. Prior analysis of a subset of infected women in the TRAC cohort revealed that Endo- women had a lower mean cervical chlamydia burden when compared to Endo+ women, and that higher titers of serum anti-EB IgG correlated inversely with cervical burden23. However, we did not detect any relationship between anti-EB IgG levels and ascension16. In this study, we confirmed higher cervical chlamydia burdens were present in Endo+ women, that serum and mucosal levels of anti-EB IgG were strongly correlated with each other, and that both were inversely correlated with pathogen load. Since cervical IgG is a composite of IgG transported from the serum into cervical secretions37,38 and IgG produced by local plasma cells, it is logical that anti-EB IgG levels in serum and cervical secretions were highly correlated and that both were associated with reduced burden. The correlation between serum and cervical anti-EB IgA levels was weakly positive, and although both correlated inversely with burden, the correlation was weak and not significant for serum IgA; this can be explained by the fact that mucosal IgA is locally produced by IgA-producing plasma cells.
The inverse relationships of antibody with burden and burden with ascension suggests that higher levels of antibody should be associated with reduced risk of ascension. However, causal mediation analysis did not detect a significant relationship between any antibody subtype and ascension when the total effect was determined. Examination of the indirect effects of antibody on ascension, mediated through alterations in cervical burden, revealed that both serum anti-EB IgG and cervical anti-EB IgA were associated with a significantly decreased probability of ascension. This contrasts with burden-independent effects, which, while not significant, abrogated the protective effects of antibody mediated through burden. These findings suggest the possibility for antibody-mediated enhanced uptake of chlamydia that has been well-described for non-neutralizing antibodies in viral infections29,30. Evidence that antibody might promote chlamydial infection directly via transcytosis has been obtained in cell culture and in vivo. Anti-IgG antibody against chlamydial MOMP enhanced neonatal receptor FcR (FcRn)-dependent uptake and translocation of chlamydia in polarized epididymal epithelia grown on transwells, and female mice challenged with chlamydia opsonized with anti-MOMP IgG displayed delayed infection clearance and exacerbated oviduct disease11. Prolonged infection is likely to result in higher titers of serum and mucosal anti-EB antibodies, elicited by continued antigen exposure. An ineffectual T cell response would result in prolonged infection. Thus, detection of high levels of anti-EB antibodies may reflect poor development of protective T cell immunity and serve as a marker of increased risk or susceptibility. Baseline levels of systemic and mucosal anti-EB IgG in women uninfected at enrollment correlated with a significantly enhanced risk of incident infection which is further evidence that elevated antibodies of this isotype serve as a marker of inferior anti-chlamydial immunity. These data are in stark contrast to results from this same cohort that revealed significant increases in frequencies of peripheral blood CD4 T cell IFN-γ responses to chlamydial proteins were associated with protection from incident infection23.
Although we did not examine IgG subtypes associated with Th1 versus Th2 responses, given the considerable data related to the protective effects of Th1 responses and the detrimental effects of Th2 responses on chlamydia infection5–8,39–41, it is plausible that the subset of women with the highest anti-EB IgG responses are Th2-skewed. This would have resulted in an inadequate IFN-γ response with high amounts of anti-chlamydial antibody at the site of infectious challenge. Naglak et al. showed that IFN-γ was necessary for antibody-mediated opsonophagocytosis and enhancement of chlamydia killing via neutrophils15. Reduced neutrophil opsonophagocytosis and killing of infectious EBs would promote enhanced susceptibility to infection. Determination of the subclass of anti-chlamydia serum and mucosal antibodies could aid in determining the polarization of the immune response and the relative contribution of serum-transported versus locally-produced antibodies. The absence of any correlation between levels of anti-EB antibodies present at enrollment and incident infection rate in infected women is likely due to the strong influence of active infection on antibody production. This would mask any Th2 versus Th1 bias, preventing detection of Th2-prone women with an increased risk of incident infection.
Strengths of our study include rigorous biostatistical methods, including mediation analysis that determined the mechanism of effects of anti-EB antibodies on ascending infection by dissecting them into mediator-dependent and -independent effects; appropriate adjustments for known behavioral and clinical characteristics that influence infection; and a sensitivity analysis that revealed a low likelihood of unmeasured confounders influencing the observed causal mediator effect. An important limitation is the lack of information regarding specificity of serum- and secretion-derived anti-chlamydial antibodies. EB antibody ELISA detects antigens exposed on the surface of EBs, such as MOMP, but not cytoplasmic or inclusion membrane chlamydia antigens31. The latter may have a protective role; e.g., IgG specific for IncA, a chlamydia inclusion membrane protein can be transported intracellularly by FcRn, resulting in recruitment of autophagic proteins and reduced infection11. Other inclusion membrane proteins that are cytoplasmic facing can be bound by intracellular IgG, with resulting reductions in chlamydial progeny42. Although our analyses indicated that the total effect of serum or mucosal anti-EB antibodies were insufficient to protect from ascension or incident infection, it is possible that antibody to specific chlamydia antigens could have a protective effect. Although labor intensive, chlamydial protein and peptide libraries43–46, in combination with isotype analysis could be used to correlate chlamydia protein-specific antibody responses with endometrial ascension and rates of incident infection in highly exposed individuals. Detection of chlamydial antigens associated with protection against ascension or reinfection would identify vaccine candidates.
Our failure to detect correlations of antibody levels to surface exposed chlamydial proteins with protection from ascension or from incident infection in women naturally infected with chlamydia does not indicate vaccines that incorporate these antigens will be ineffective. Data from murine models indicate significant, although not complete, protection can be induced by vaccination with intact EBs or chlamydia MOMP, when administered with Th1-inducing adjuvants47–49, and that antibodies contribute to the protection induced by vaccination48. Data from murine models also demonstrate a protective role for polymeric immunoglobulin receptor (pIgR) mediated delivery of secretory IgA, against extracellular antigens11. Rather, our data reinforce the importance of eliciting robust and appropriate functional T cell responses, generating a milieu where antibody to surface exposed antigens can mediate protection. Furthermore, we conclude that high levels of anti-EB IgG in serum or secretions from uninfected women serve as a biomarker for an ineffective T cell response, identifying women at highest risk for reproductive sequelae after chlamydial infection.
Supplementary Material
Acknowledgments.
We thank the women who agreed to participate in this study; Ingrid Macio, Melinda Petrina, Carol Priest, Abi Jett, and Lorna Rabe, for their efforts in the clinic and the microbiology laboratory; Pam Kozlowski for her ELISA expertise and advice; and the staff at the Allegheny County Health Department STD Clinic, for their efforts. This work was supported by the National Institute of Allergy and Infectious Diseases (grants R01 AI119164, U19 AI084024 and U19 AI113170).
Footnotes
Potential conflicts of interest. All authors: No reported conflicts. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REFERENCES
- 1.Fadel S, Eley A. Chlamydia trachomatis OmcB protein is a surface-exposed glycosaminoglycan-dependent adhesin. J. Med Microbiol. 2007;56(Pt 1):15–22. [DOI] [PubMed] [Google Scholar]
- 2.Wehrl W, Brinkmann V, Jungblut PR, Meyer TF, Szczepek AJ. From the inside out--processing of the Chlamydial autotransporter PmpD and its role in bacterial adhesion and activation of human host cells. Mol Microbiol. 2004;51(2):319–334. [DOI] [PubMed] [Google Scholar]
- 3.Davis CH, Raulston JE, Wyrick PB. Protein disulfide isomerase, a component of the estrogen receptor complex, is associated with Chlamydia trachomatis serovar E attached to human endometrial epithelial cells. Infect Immun. 2002;70(7):3413–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subbarayal P, Karunakaran K, Winkler AC, et al. EphrinA2 receptor (EphA2) is an invasion and intracellular signaling receptor for Chlamydia trachomatis. PLoS Pathog. 2015;11(4):e1004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramsey KH, Rank RG. Resolution of chlamydial genital infection with antigen-specific T- lymphocyte lines. Infect Immun. 1991;59(3):925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect Immun. 2000;68(12):6979–6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rank RG, Whittum-Hudson JA. Protective immunity to chlamydial genital infection: evidence from animal studies. J Infect Dis. 2010;201 Suppl 2:S168–177. [DOI] [PubMed] [Google Scholar]
- 8.Gondek DC, Olive AJ, Stary G, Starnbach MN. CD4+ T cells are necessary and sufficient to confer protection against Chlamydia trachomatis Infection in the murine upper genital tract. J Immunol. 2012;189(5):2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crane DD, Carlson JH, Fischer ER, et al. Chlamydia trachomatis polymorphic membrane protein D is a species-common pan-neutralizing antigen. Proc Natl Acad Sci USA. 2006;103(6):1894–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan J, Stephens RS. Antigen conformation dependence of Chlamydia trachomatis infectivity neutralization. J Infect Dis. 1997;176(3):713–721. [DOI] [PubMed] [Google Scholar]
- 11.Armitage CW, O’Meara CP, Harvie MC, Timms P, Blumberg RS, Beagley KW. Divergent outcomes following transcytosis of IgG targeting intracellular and extracellular chlamydial antigens. Immunol Cell Biol. 2014;92(5):417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rank RG, Batteiger BE. Protective role of serum antibody in immunity to chlamydial genital infection. Infect Immun. 1989;57(1):299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen AW, Follmann F, Erneholm K, Rosenkrands I, Andersen P. Protection against Chlamydia trachomatis infection and upper genital tract pathological changes by vaccine-promoted neutralizing antibodies directed to the VD4 of the Major Outer Membrane Protein. J Infect Dis. 2015;212(6):978–989. [DOI] [PubMed] [Google Scholar]
- 14.Igietseme JU, Eko FO, He Q, Black CM. Antibody regulation of Tcell immunity: implications for vaccine strategies against intracellular pathogens. Expert Rev Vaccines. 2004;3(1):23–34. [DOI] [PubMed] [Google Scholar]
- 15.Naglak EK, Morrison SG, Morrison RP. IFNgamma is required for optimal antibody-mediated immunity against genital Chlamydia infection. Infect Immun. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell AN, Zheng X, O’Connell CM, et al. Analysis of factors driving incident and ascending infection and the role of serum antibody in Chlamydia trachomatis genital tract infection. J Infect Dis. 2016;213(4):523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen CR, Koochesfahani KM, Meier AS, et al. Immunoepidemiologic profile of Chlamydia trachomatis infection: importance of heat-shock protein 60 and interferon- gamma. J Infect Dis. 2005;192(4):591–599. [DOI] [PubMed] [Google Scholar]
- 18.Blythe MJ, Katz BP, Batteiger BE, Ganser JA, Jones RB. Recurrent genitourinary chlamydial infections in sexually active female adolescents. J Pediatr. 1992;121(3):487–493. [DOI] [PubMed] [Google Scholar]
- 19.Batteiger BE, Tu W, Ofner S, et al. Repeated Chlamydia trachomatis genital infections in adolescent women. J Infect Dis. 2010;201(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Hakim EA, Gordon UD, Akande VA. The relationship between serum Chlamydia antibody levels and severity of disease in infertile women with tubal damage. Arch Gynecol Obstet. 2010;281(4):727–733. [DOI] [PubMed] [Google Scholar]
- 21.Ness RB, Soper DE, Richter HE, et al. Chlamydia antibodies, chlamydia heat shock protein, and adverse sequelae after pelvic inflammatory disease: The PID evaluation and clinical health (PEACH) study. Sex Transmit Dis. 2008;35(2):129–135. [DOI] [PubMed] [Google Scholar]
- 22.LaVerda D, Albanese LN, Ruther PE, et al. Seroreactivity to Chlamydia trachomatis Hsp10 correlates with severity of human genital tract disease. Infect Immun. 2000;68(1):303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell AN, Zheng X, O’Connell CM, et al. Identification of Chlamydia trachomatis antigens recognized by T Cells from highly exposed women who limit or resist genital tract infection. J Infect Dis. 2016;214(12):1884–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norrby-Teglund A, Ihendyane N, Kansal R, et al. Relative neutralizing activity in polyspecific IgM, IgA, and IgG preparations against group A streptococcal superantigens. Clin Infect Dis. 2000;31(5):1175–1182. [DOI] [PubMed] [Google Scholar]
- 25.Brunham RC, Peeling R, Maclean I, McDowell J, Persson K, Osser S. Postabortal Chlamydia trachomatis salpingitis: correlating risk with antigen-specific serological responses and with neutralization. J Infect Dis. 1987;155(4):749–755. [DOI] [PubMed] [Google Scholar]
- 26.Kaul R, Plummer F, Clerici M, Bomsel M, Lopalco L, Broliden K. Mucosal IgA in exposed, uninfected subjects: evidence for a role in protection against HIV infection. AIDS. 2001;15(3):431–432. [DOI] [PubMed] [Google Scholar]
- 27.Devito C, Broliden K, Kaul R, et al. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J Immunol. 2000;165(9):5170–5176. [DOI] [PubMed] [Google Scholar]
- 28.Devito C, Hinkula J, Kaul R, et al. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS. 2000;14(13):1917–1920. [DOI] [PubMed] [Google Scholar]
- 29.Khandia R, Munjal A, Dhama K, et al. Modulation of Dengue/Zika Virus pathogenicity by antibody-dependent enhancement and strategies to protect against enhancement in Zika Virus infection. Front Immunol. 2018;9:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ong EZ, Zhang SL, Tan HC, Gan ES, Chan KR, Ooi EE. Dengue virus compartmentalization during antibody-enhanced infection. Sci Rep. 2017;7:40923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albritton HL, Kozlowski PA, Lillis RA, et al. A novel whole-bacterial enzyme linked-immunosorbant assay to quantify Chlamydia trachomatis specific antibodies reveals distinct differences between systemic and genital compartments. PLoS One. 2017;12(8):e0183101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozlowski PA, Lynch RM, Patterson RR, Cu-Uvin S, Flanigan TP, Neutra MR. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J Acquir Immune Defic Syndr. 2000;24(4):297–309. [DOI] [PubMed] [Google Scholar]
- 33.Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods. 2010;15(4):309–334. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Nelson S, Albert JM. Estimation of causal mediation effects for a dichotomous outcome in multiple-mediator models using the mediation formula. Stat Med. 2013;32(24):4211–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei LJ, Lin DY, Weissfeld L. Regression-Analysis of Multivariate Incomplete Failure Time Data by Modeling Marginal Distributions. J Am Stat Assoc. 1989;84(408):1065–1073. [Google Scholar]
- 37.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–725. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida M, Kobayashi K, Kuo TT, et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest. 2006;116(8):2142–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holland MJ, Bailey RL, Conway DJ, et al. T helper type-1 (Th1)/Th2 profiles of peripheral blood mononuclear cells (PBMC); responses to antigens of Chlamydia trachomatis in subjects with severe trachomatous scarring. Clin Exper Immunol. 1996;105(3):429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gondek DC, Roan NR, Starnbach MN. T cell responses in the absence of IFN-gamma exacerbate uterine infection with Chlamydia trachomatis. J Immunol. 2009;183(2):1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawkins RA, Rank RG, Kelly KA. A Chlamydia trachomatis-specific Th2 clone does not provide protection against a genital infection and displays reduced trafficking to the infected genital mucosa. Infect Immun. 2002;70(9):5132–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rzomp KA, Moorhead AR, Scidmore MA. The GTPase Rab4 interacts with Chlamydia trachomatis inclusion membrane protein CT229. Infect Immun. 2006;74(9):5362–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Budrys NM, Gong S, Rodgers AK, et al. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obstet Gynecol. 2012;119(5):1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hufnagel K, Lueong S, Willhauck-Fleckenstein M, et al. Immunoprofiling of Chlamydia trachomatis using whole-proteome microarrays generated by on-chip in situ expression. Sci Rep. 2018;8(1):7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sachse K, Rahman KS, Schnee C, et al. A novel synthetic peptide microarray assay detects Chlamydia species-specific antibodies in animal and human sera. Sci Rep. 2018;8(1):4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curtis KA, Hanson DL, Price KA, Owen SM. Performance characteristics of an antibody-based multiplex kit for determining recent HIV-1 infection. PLoS One. 2017;12(5):e0176593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rank RG, Batteiger BE, Soderberg LSF. Immunization against chlamydial genital infection in guinea pigs with UV-inactivated and viable chlamydiae administered by different routes. Infect Immun. 1990;58:2599–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farris CM, Morrison SG, Morrison RP. CD4+ T cells and antibody are required for optimal major outer membrane protein vaccine-induced immunity to Chlamydia muridarum genital infection. Infect Immun. 2010;78(10):4374–4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pal S, Peterson EM, Rappuoli R, Ratti G, De La Maza LM. Immunization with the Chlamydia trachomatis major outer membrane protein, using adjuvants developed for human vaccines, can induce partial protection in a mouse model against a genital challenge. Vaccine. 2006;24(6):766–775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.