Abstract
Objective:
To describe changes in visual acuity (VA) and macular morphology at 5 years in eyes with non-fibrotic scars (NFS) identified at 1 year in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT).
Design:
Prospective cohort study within a randomized clinical trial.
Participants:
Participants in CATT.
Methods:
Participants assigned to ranibizumab or bevacizumab and to 1 of 3 dosing regimens were released from the clinical trial protocol after 2 years and recalled at 5 years. NFS was identified on color images at year 1 as flat, small, well-circumscribed areas of pigmentation with varying degrees of central hypopigmentation without exposure of underlying choroidal vessels, at the site of baseline choroidal neovascularization (CNV). Follow-up images were assessed for changes in and around the NFS.
Main outcome measures:
Changes in pigmentation, VA, development of fibrotic scar (FS), non-geographic (NGA) and geographic atrophy (GA), retinal fluid on optical coherent tomography, and fluorescein leakage.
Results:
Among 474 eyes with images at 1, 2 and 5 years, 39 (8.2%) had NFS at 1 year with a mean VA of 80 letters (≈ 20/25). Among these eyes, FS developed in 5% at 2 and 28% at 5 years. NGA was observed in 34%, 47% and 65% of eyes at 1, 2 and 5 years, respectively. GA developed in 5% of eyes at 2 and 21% at 5 years. Among eyes with NFS, FS or no scar at 1 year, mean VA at 5 years was 73 letters (≈ 20/32), 48 (≈ 20/100) and 62 (≈ 20/63), respectively. At 5 years, NFS eyes had less GA, less intraretinal fluid, more subretinal fluid, and less sub-RPE fluid (all p<0.01). Among NFS eyes, mean thickness of the retina, subretinal tissue complex and total retina did not change across years 1 to 5 (p>0.50). The proportion of eyes with fluid on OCT also did not change (p=0.36). Subretinal hyperreflective material disappeared by 5 years in 40% of eyes with NFS.
Conclusion:
These results indicate that, on average, eyes with NFS after anti-VEGF treatment have good visual acuity not only at 1 and 2 years, but also through 5 years
Treatment of neovascular age-related macular degeneration (nAMD) with intravitreal anti-vascular endothelial growth factor (anti-VEGF) is well established and is associated with improvement in visual acuity during the first 2 years for most patients.1–5 However, with continued follow-up, visual acuity often declines and the presence and area of scarring and macular atrophy increases.6–11
The morphological appearance at the site of nAMD after anti-VEGF treatment has been described in reports from several clinical trials of anti-VEGF treatment.12–17 Quantitative measurements and qualitative descriptions, mainly of macular atrophy and fibrotic scar, derived from multiple imaging modalities have provided a greater understanding of the pathological processes and their progression over time. However, non-fibrotic scar (NFS) may be a unique sequela of anti-VEGF therapy that has not been fully investigated. NFS is identified on color images as a flat, small, well-circumscribed area of pigmentation with varying degrees of central hypopigmentation and with corresponding features on fluorescein angiograms.18,19 In the Comparison of Age-related Macular Degeneration Treatments Trials (CATT), NFS developed in 21% of treated eyes by 2 years, with 13% in the first year.18 In this paper we describe the morphological changes that occur at 2 years and 5 years in and around NFS that had developed by 1 year after initiation of anti-VEGF therapy for nAMD in the study eyes of participants in CATT and the CATT Follow-up Study.
Methods:
Details of the methods used in CATT and CATT Follow-up Study have been described previously.1,8,14,20,21 Key features relevant to this report are summarized below.
Enrollment of Subjects
Participants in CATT (N=1185) enrolled in the clinical trial through 43 clinical centers in the United States between February 2008 and December 2009. Study eyes had untreated active (leakage on FA and fluid on OCT) choroidal neovascularization (CNV) associated with AMD. Either CNV or fluid was required to be present at the foveal center. Patients were excluded if scar involved the foveal center but eyes with non-foveal scars with an area of <50% of the total CNV lesion area were allowed. Subjects were randomly assigned to treatment with intravitreal injections of ranibizumab or bevacizumab and to 1 of 3 dosing regimens for the initial 2 years of the study: monthly injections, monthly evaluation with injection only when signs of active neovascularization were present (pro re nata [PRN]), or monthly injections for 1 year followed by PRN injections for 1 year. Institutional review boards associated with each center approved the study and written informed consent was obtained from all subjects. The study was compliant with Health Insurance Portability and Accountability Act regulations. The CATT was registered with ClinicalTrials.gov (NCT00593450). All research adhered to the tenets of the Declaration of Helsinki.
Follow-up of Subjects
Color fundus photographs (CFP), fluorescein angiograms (FA), optical coherence tomograms (OCT), and visual acuity measurements were obtained at baseline, 1, 2 and 5 years. At 2 years, participants were released from the clinical trial protocol and were managed according to best medical judgment. CATT participants alive at the end of the clinical trial (N=1117) were targeted to participate in the CATT Follow-up Study at approximately 5 years after initiation of the anti-VEGF therapy. A total of 203 of these patients died between year 2 and the close of the examinations for the CATT Follow-up Study, leaving 914 available for the year 5 visit.
Assessment of Images
Digital images of CFP and FA, and OCT scans were graded applying the same methods in all years.20Briefly, dual-Reader grading was performed on each image set for various morphological features by trained and certified CATT Readers masked to demographic and clinical details. CFP and FA images were evaluated at the Scheie Ophthalmology Reading Center in the University of Pennsylvania while OCTs were evaluated at the Duke Reading Center in North Carolina.21 Grading was performed on CFP and FA images to identify fluorescein leakage, CNV type (occult, minimally classic, and predominantly classic), hemorrhage, serous pigment epithelial detachment, blocked fluorescence, presence of fibrotic scar (FS), non-fibrotic scar (NFS), non-geographic atrophy (NGA), geographic atrophy (GA) and retinal pigment epithelial tear. Morphological features from both eyes at and away from the foveal center were identified. Discrepancies on grading results were adjudicated between the readers and the director of the reading center and unresolved discrepancies were reviewed by the principal investigator to complete a final consensus grading form. OCT scan features that were graded included the location of fluid (intraretinal, subretinal and sub-RPE); thickness at the foveal center of the retina, subretinal fluid, and subretinal tissue complex; presence of subretinal hyper-reflective material (SHRM); retinal pigment epithelial elevation; epi-retinal membrane; and vitreo-macular attachment. Dual Reader grading of OCT discrepancies were arbitrated by a third, independent Senior Reader.
Assessment of the non-fibrotic scar (NFS)
An ophthalmologist evaluated 1-year CFP and FA images to identify NFS among CATT subjects for whom all three follow up visit (1, 2 and 5 years) images were available. NFS were typically flat, small, well circumscribed areas of pigmentation with varying degrees of central hypopigmentation on CFP images (Figure 1). The peripheral pigmentary changes in these scars often followed the outline of previously active CNV lesion. The hypopigmented areas were flat and choroidal vessels were not visible. Hyperfluorescence of the depigmented area appeared early on FA and persisted or increased in intensity in the late phase. Hypofluorescence on FA surrounding the hyperfluorescence corresponded to the pigmented borders observed on CFP.18
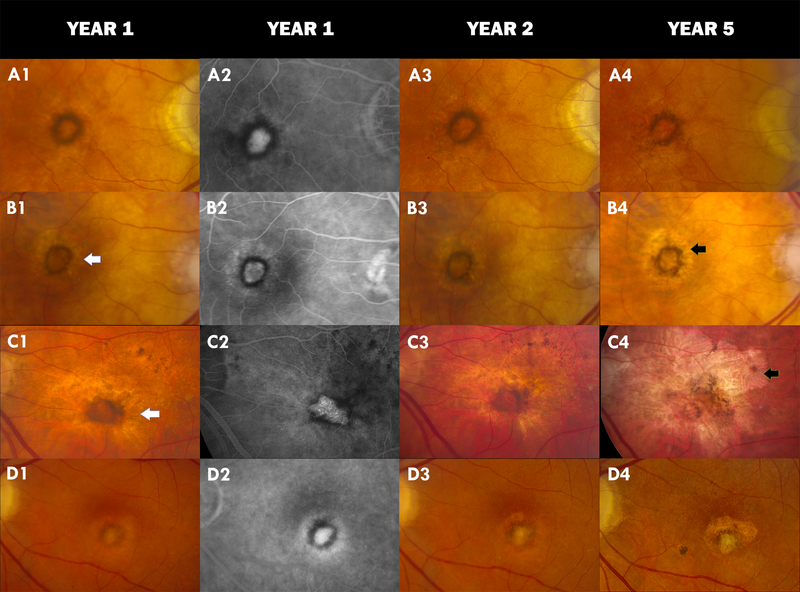
Figure 1:
Color fundus photograph (CFP) and Fluorescein Angiogram (FA) images of non-fibrotic scar (NFS) in four different CATT subjects: A1 shows a typical NFS at year 1 with hyperpigmentation encircling an area of hypopigmentation on CFP. A2 is the corresponding FA showing an inner hyperfluorescence surrounded by circular hypofluorescence. No changes are seen at year 2 (A3) but part of the pigmentation at 7 o’clock has disappeared in the year 5 CFP (A4). B1 and B2 show a halo of non-geographic atrophy (NGA) surrounding the NFS in year 1. This persists in year 2 (B3) and intensifies into geographic atrophy (GA) at year 5 (B4); C1–C3 show NGA surrounding the NFS at years 1 and 2 with development of a large GA at year 5 (C4) and the disappearance of more than half of the circular hyperpigmentation of the NFS; D1 and D2 show a NFS which develops fibrosis within the hyperpigmented ring at year 2 (D3) that is more pronounced at year 5 (D4). Some amount of fibrosis is also observed superiorly overlying an area of geographic atrophy. White arrow=NGA. Black arrow=GA.
NFS were distinguished from fibrotic scars (FS) which were identified on color stereo images as white or yellow mounds of fibrous-appearing tissue that were well defined in shape and appeared solid and on FA had either hyperfluorescence due to tissue staining or blocked fluorescence of the underlying choroid. They were also differentiated from non-geographic atrophy (NGA) - defined as area(s) of pigment disturbances including hypo-pigmentation and hyper-pigmentation that typically corresponded to hyper-fluorescence and hypo-fluorescence on FA in areas contiguous or previously occupied by CNV. The hypo-pigmented areas of NGA do not meet the criteria for GA that include sharp borders and visible choroidal vessels. NFS could occur alone or along with other morphological features. All identified NFS at 1 year by the ophthalmologist were subjected to further independent evaluation by a retina specialist and eyes with indeterminate NFS were excluded (2.5%). Year 2 and 5 images were evaluated for the presence of NGA, GA, or FS involving or contiguous to the year-1 NFS. Active CNV and pigmentation changes in and around NFS was also documented.
Statistical Analysis
We performed descriptive analyses for VA change over time, incidence of FS, NGA and GA over time, presence of active CNV, and hyperpigmentation changes over time, with mean (SD) for continuous measures, and proportion for categorical measures. We used the analysis of variance for comparison of continuous measures and Fisher exact test for comparison of proportions among scar groups. We used generalized linear models for repeated measures for evaluating change of morphological characteristics over time (years 1, 2, and 5) for eyes in each scar group. All statistical analyses were performed in SAS v9.4 (SAS Institute Inc., Cary, NC), and p<0.05 was considered to be statistically significant.
Results:
Among the 474 subjects who had photographic images at all three follow up visits (1, 2 and 5 years), 39 study eyes developed NFS by 1 year. Among 263 eyes without any scar (nonfibrotic or fibrotic) at 2 years and with complete data available at the 5-year follow-up exam, 5 (2%) developed NFS while 47 (18%) developed FS by 5 years.
Morphological changes observed in the 39 eyes that had developed NFS at 1 year through 5 years are given in Table 1. Only a small percentage of eyes (5%) developed FS at year 2 but at 5 years 28% had developed FS. Similarly, GA developed in 5% and 21% of eyes at 2 and 5 years, respectively. NGA was already present in 34% of the eyes at 1 year and increased to 47% and 65% in years 2 and 5 respectively. Fluorescein leakage, observed in 50% of eyes with NFS in 1 year, decreased to 29% and 20% in years 2 and 5, respectively (p=0.02)
Table 1.
Morphological characteristics at 1, 2, and 5 years in 39 eyes with non-fibrotic scar at 1 year
| Year | ||||
|---|---|---|---|---|
| Characteristics on color fundus photographs and fluorescein angiogram | 1 n (%) |
2 n (%) |
5 n (%) |
P |
| Non-fibrotic scar, yes (%) | 39 (100) | 39 (100) | 39 (100) | -- |
| Fibrotic scar, yes (%) | 0 (0) | 2 (5) | 11 (28) | 0.004 |
| Geographic atrophy, yes (%) | 0 (0) | 2 (5) | 8 (21) | 0.02 |
| Non-geographic atrophy, yes (%) | 11 (34) | 18 (47) | 24 (65) | 0.04 |
| Leakage on angiography, yes (%) | 18 (50) | 11 (29) | 7 (20) | 0.02 |
| OCT Characteristics | ||||
| Any fluid, yes (%) | 24 (65) | 28 (72) | 30 (77) | 0.36 |
| Intraretinal fluid, yes (%) | 13 (35) | 14 (36) | 16 (41) | 0.78 |
| Subretinal fluid, yes (%) | 15 (42) | 20 (51) | 20 (51) | 0.42 |
| Sub-Retinal Pigment Epithelium fluid, yes (%) | 8 (28) | 10 (29) | 9 (23) | 0.54 |
| Retinal thickness, um; mean (SD) | 165 (39) | 168 (52) | 163 (56) | 0.67 |
| Subretinal fluid thickness, um; mean (SD) | 5.7 (17) | 8.9 (21) | 7.0 (26) | 0.61 |
| Subretinal tissue complex thickness, um; mean (SD) | 91.1 (68) | 94.7 (68) | 97.8 (78) | 0.77 |
| Total retinal thickness, um; mean (SD) | 261 (78) | 272 (88) | 271 (94) | 0.56 |
| Subretinal hyper-reflective material, yes (%) | 16 (44) | 14 (37) | 19 (49) | 0.39 |
The comparison of the development of accompanying morphological features among the 3 groups (eyes that developed NFS at 1 year, fibrotic scars at 1 year and no scar at 1 year) at each follow up visit is given in Tables 2, 3 and 4. As shown in Table 2, the number of eyes with GA increased in all three groups from 1 year to 5 years but in eyes that had NFS at 1 year the development of GA was the least across all time points (p=0.002). The number of eyes with NGA also increased over time in all the groups but were similar among groups at all time points. The number of eyes with leakage of fluorescein dye decreased over time in all the three groups but were similar among groups at all time points. The mean (SD) of baseline total CNV lesion area in mm2 was 2.3 (2.6), 8.2 (8.8) and 6.3 (6.1), for the NFS group, FS group and no scar group, respectively (p<0.0001). Mean lesion size increased over time for the 3 types of lesions (p<0.0001) and the mean area for NFS remained the smallest.
Table 2:
Morphological characteristics in color fundus photographs at 1, 2, and 5 years in eyes with no scar, non-fibrotic scar, fibrotic scar and no scar at Year 1
| Characteristics on color fundus photographs and fluorescein angiogram | Scar Group | Year | P* | ||
|---|---|---|---|---|---|
| 1 | 2 | 5 | |||
| n (%) | n (%) | n (%) | |||
| Geographic atrophy, yes (%) | No scar (N=367) | 83 (23) | 95 (26) | 166 (45) | <0.0001 |
| Nonfibrotic scar (N=39) | 0 (0) | 2 (5) | 8 (21) | 0.02 | |
| Fibrotic scar (N=68) | 12 (18) | 16 (24) | 37 (54) | <0.0001 | |
| P§ | 0.0004 | 0.008 | 0.002 | ||
| Non-geographic atrophy, yes (%) | No scar (N=367) | 137 (42) | 167 (46) | 235 (72) | <0.0001 |
| Nonfibrotic scar (N=39) | 11 (34) | 18 (47) | 24 (65) | 0.04 | |
| Fibrotic scar (N=68) | 30 (49) | 33 (50) | 43 (73) | 0.009 | |
| P§ | 0.36 | 0.79 | 0.61 | ||
| Leakage on angiography, yes (%) | No scar (N=367) | 162 (45) | 120 (33) | 79 (25) | <0.0001 |
| Nonfibrotic scar (N=39) | 18 (50) | 11 (29) | 7 (20) | 0.02 | |
| Fibrotic scar (N=68) | 28 (43) | 15 (23) | 13 (22) | 0.006 | |
| P§ | 0.81 | 0.24 | 0.84 | ||
| CNV Lesion Size (mm2):mean (SD) | Nonfibrotic scar (N=39) | 2.6 (3.2) | 3.4 (4.3) | 5.8 (7.2) | 0.04 |
| Fibrotic scar (N=68) | 10.5 (9.4) | 10.4 (8.8) | 17.1 (12.0) | <0.0001 | |
| No scar (N=367) | 6.9 (6.7) | 7.7 (7.3) | 12.5 (10.8) | <0.0001 | |
| P§ | <0.0001 | <0.0001 | <0.0001 | ||
For the comparison of difference among three scar groups.
For the comparison of difference among three time points within a given scar group.
CNV = Choroidal neovascularization
Table 3:
Fluid in OCT at 1, 2, and 5 years in eyes with non-fibrotic scar, fibrotic scar and no scar at Year 1
| OCT Characteristics | Scar Group | Year | P* | ||
|---|---|---|---|---|---|
| 1 | 2 | 5 | |||
| n (%) | n (%) | n (%) | |||
| Any fluid, yes (%) | No scar (N=367) | 254 (70) | 278 (76) | 304 (83) | <0.0001 |
| Nonfibrotic scar (N=39) | 24 (65) | 28 (72) | 30 (77) | 0.36 | |
| Fibrotic scar (N=68) | 45 (67) | 55 (81) | 58 (85) | 0.03 | |
| P§ | 0.78 | 0.57 | 0.54 | ||
| Intraretinal fluid, yes (%) | No scar (N=367) | 160 (45) | 171 (48) | 212 (58) | <0.0001 |
| Nonfibrotic scar (N=39) | 13 (35) | 14 (36) | 16 (41) | 0.78 | |
| Fibrotic scar (N=68) | 41 (62) | 50 (76) | 55 (81) | 0.02 | |
| P§ | 0.01 | <0.0001 | <0.0001 | ||
| Subretinal fluid, yes (%) | No scar (N=367) | 117 (33) | 144 (40) | 144 (40) | 0.008 |
| Nonfibrotic scar (N=39) | 15 (42) | 20 (51) | 20 (51) | 0.42 | |
| Fibrotic scar (N=68) | 14 (22) | 16 (25) | 21 (32) | 0.33 | |
| P§ | 0.10 | 0.02 | 0.16 | ||
| Sub-RPE fluid, yes (%) | No scar (N=367) | 128 (40) | 156 (45) | 151 (42) | 0.12 |
| Nonfibrotic scar (N=39) | 8 (28 | 10 (29) | 9 (23) | 0.54 | |
| Fibrotic scar (N=68) | 10 (19) | 14 (24) | 17 (26) | 0.51 | |
| P§ | 0.005 | 0.003 | 0.007 | ||
For the comparison of difference among three scar groups.
For the comparison of difference among three time points within a given scar group.
RPE = Retinal pigment epithelium
Table 4:
Thickness of OCT characteristics at 1, 2, and 5 years in eyes with non-fibrotic scar, fibrotic scar and no scar at Year 1
| OCT Feature Thickness, um | Scar Group | Year | P* | ||
|---|---|---|---|---|---|
| 1 | 2 | 5 | |||
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| Retinal thickness | No scar (N=367) | 156 (53) | 156 (56) | 144 (85) | 0.007 |
| Nonfibrotic scar (N=39) | 165 (39) | 168 (52) | 163 (56) | 0.67 | |
| Fibrotic scar (N=68) | 161 (84) | 159 (91) | 162 (156) | 0.98 | |
| P§ | 0.63 | 0.51 | 0.25 | ||
| Subretinal fluid thickness | No scar (N=367) | 7.4 (26) | 9.4 (39) | 5.9 (22) | 0.32 |
| Nonfibrotic scar (N=39) | 5.7 (17) | 8.9 (21) | 7.0 (26) | 0.61 | |
| Fibrotic scar (N=68) | 8.3 (32) | 3.9 (19) | 1.3 (11) | 0.13 | |
| P§ | 0.90 | 0.50 | 0.24 | ||
| Subretinal tissue complex thickness | No scar (N=367) | 127 (117) | 127 (111) | 107 (101) | 0.0005 |
| Nonfibrotic scar (N=39) | 91 (68) | 95 (68) | 98 (78) | 0.77 | |
| Fibrotic scar (N=68) | 178 (102) | 157 (97) | 98 (81) | <0.0001 | |
| P§ | 0.0002 | 0.01 | 0.75 | ||
| Total retinal thickness, | No scar (N=367) | 290 (141) | 293 (138) | 258 (138) | <0.0001 |
| Nonfibrotic scar (N=39) | 261 (78) | 272 (88) | 271 (94) | 0.56 | |
| Fibrotic scar (N=68) | 347 (144) | 320 (144) | 278 (191) | 0.02 | |
| P§ | 0.003 | 0.18 | 0.62 | ||
For the comparison of difference among three scar groups.
For the comparison of difference among three time points within a given scar group.
OCT = Optical Coherence Tomography
Table 3 shows the fluid on OCT over time among the three groups. While the number of eyes with any fluid increased over time in all of the groups it was not significant in the NFS group (p=0.36). In the NFS group the individual types of fluid (intraretinal, subretinal and sub-RPE) also did not show significant variations at 1, 2 or 5 years and the relative frequency of these three types of fluid remained ordered throughout the follow up period. Intraretinal fluid was present in more eyes in the no scar and FS groups when compared to the NFS group at all follow up time points. However more eyes in the NFS group had sub-retinal fluid than the other two groups at 2 years (p=0.02). Sub-RPE fluid was seen in more eyes at all follow up time points in the group with no scar when compared to the FS and NFS groups. OCT features such as retinal thickness, subretinal fluid thickness, subretinal complex thickness and total retinal thickness were also not significantly different from baseline at each follow up time points (p>0.05) in the NFS group (Table 4). However the mean retinal thickness, subretinal complex thickness and total retinal thickness grew thinner in the no scar group.
The mean visual acuity over time in eyes that developed NFS at 1 year, fibrotic scars at 1 year and no scar at 1 year is given in Table 5. Eyes with NFS at 1 year had a VA of 66, 80, 77 and 73 letters at baseline, years 1, 2 and 5, respectively (p<0.0001). In contrast, eyes that developed FS at 1 year had a mean VA of 54, 61, 63 and 48 at baseline, 1, 2 and 5 years, respectively (p<0.0001). In eyes that did not develop either NFS or FS the mean VA was 65, 73, 72 and 62 at baseline, 1,2 and 5 years respectively (p<0.0001). Only the NFS group showed a mean VA at 5 years that was better than the VA at baseline, improving from 66 letters (≈ 20/50) to 73 letters (≈ 20/32).
Table 5.
Visual acuity over time in eyes that developed non-fibrotic scar, fibrotic scar and no scar at Year 1
| Status at Year 1 | Visual Acuity | Year | P* | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 5 | |||
| Nonfibrotic scar (N=39) | ≥20/20; n (%) | 0 (0) | 19 (49) | 12 (31) | 7 (18) | |
| 20/25–20/40; n (%) | 18 (46) | 19 (49) | 20 (51) | 21 (54) | ||
| ≤ 20/50; n (%) | 21 (54) | 1 (3) | 7 (18) | 11 (28) | ||
| Mean (SD); letters | 66 (9) | 80 (9) | 77 (8) | 73 (11) | <0.0001 | |
| Fibrotic scar (N=68) | ≥20/20; n (%) | 0 (0) | 7 (10) | 9 (13) | 4 (6) | |
| 20/25–20/40; n (%) | 12 (18) | 25 (37) | 22 (32) | 17 (25) | ||
| ≤ 20/50; n (%) | 56 (82) | 36 (53) | 37 (54) | 47 (69) | ||
| Mean (SD); letters | 54 (15) | 61 (21) | 63 (18) | 48 (27) | <0.0001 | |
| No scar (N=367) | ≥20/20; n (%) | 0 (0) | 73 (20) | 74 (20) | 43 (12) | |
| 20/25–20/40; n (%) | 181 (49) | 207 (56) | 201 (55) | 162 (44) | ||
| ≤ 20/50; n (%) | 186 (51) | 87 (24) | 92 (25) | 161 (44) | ||
| Mean (SD); letters | 65 (12) | 73 (14) | 72 (15) | 62 (23) | <0.0001 | |
| P** | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| P*** | 0.66 | 0.002 | 0.04 | 0.006 | ||
P* = For comparing mean VA in each group over time
P** = For comparing mean VA among three groups
P***= For comparing mean VA between nonfibrotic scar group and no scar group.
Table 6 shows the percentage of eyes that had subretinal hyperreflective material (SHRM) over time in the 3 groups. While approximately 90% of eyes had SHRM at baseline in the NFS and FS groups, only 71% had baseline SHRM in the no scar group. At 5 years SHRM was present in 49% of eyes in the NFS group compared with 63% in the no scar group and 93% in FS group (p<0.0001). At 1 year, 24 eyes with NFS (62%) had 360 degrees of peripheral pigmentation, 9 (23%) had almost complete pigmentation (>270 degrees) and 6 (15%) had at least a semicircular pigmentation. The peripheral pigmentation deceased in 8 (21%) eyes at year 2 and in 21 (54%) eyes at year 5 while it increased in 6 eyes (15%) at year 5.
Table 6.
Subretinal Hyper Reflective Material (SHRM) over time in eyes that developed non-fibrotic scar, fibrotic scar and no scar at Year 1
| At Year 1 | Subretinal hyperreflective material (SHRM) | P* | |||
|---|---|---|---|---|---|
| Baseline | Year 1 | Year 2 | Year 5 | ||
| n (%) | n (%) | n (%) | n (%) | ||
| Non-Fibrotic scar (N=39) | 34 (90) | 16 (44) | 14 (37) | 19 (49) | <0.0001 |
| Fibrotic scar (N=68) | 60 (88) | 60 (90) | 56 (82) | 63 (93) | 0.26 |
| No scar (N=367) | 257 (71) | 146 (40) | 137 (38) | 228 (63) | <0.0001 |
| P§ | 0.001 | <0.0001 | <0.0001 | <0.0001 | |
For the comparison of difference among three scar groups.
For the comparison of difference among three time points within a given scar group.
Discussion
Eyes with NFS that develops during the initial year of anti-VEGF treatment evolved over the next 4 years, with changes taking place both in and around the scar. NGA increased from being present in one-third of the eyes at 1 year to being present in nearly half of eyes at 5 years. GA, which was not present in any eyes with NFS at 1 year, developed in 21% of eyes by 5 years. This increased incidence of GA is relatively modest when compared to the more substantial percentage of eyes with FS at 1 year that later developed GA.12 The appearance of new FS in color images was observed at year 5 in one-fourth of the eyes with NFS with most occurring between years 2 and 5. This later appearance may have been due to the absence of strict oversight of trial conditions during this period, which may have resulted in under-treatment, or may have simply been due to the natural history of the disease.
We reported previously that approximately 20% of eyes in CATT developed NFS by 2 years.18 The development of NFS is relatively uncommon after the initial two years of therapy, with an incidence of less than 2% between years 2 and 5. In contrast, the incidence of fibrotic scar was 18% during this period. The results of the 5 year follow up of CATT subjects suggests that almost all of the NFS form within two years of starting anti-VEGF therapy for nAMD and their occurrence was scarce after this period even though the strict oversight of trial conditions was absent.
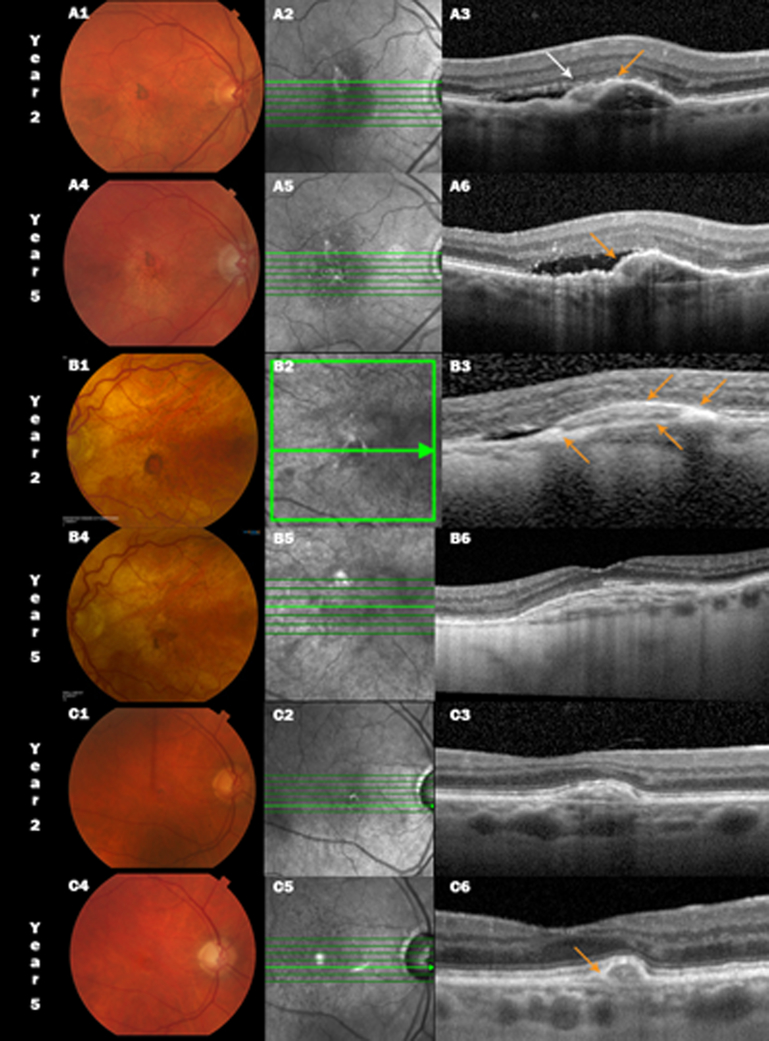
Although some risk factors such as classic CNV and SHRM at baseline are common to both NFS and FS, they differ in their presentation on color images, visual outcomes, types of retinal fluid seen on OCT and in changes to SHRM during follow up. NFS, described more comprehensively in an earlier article, have no raised yellowish mounds of fibrotic scar tissue on stereo color images.18 The mean SHRM thickness at baseline in eyes that subsequently develop NFS by 2 years has been reported to be 148μ, which is greater than the SHRM thickness in eyes that do not develop any scar (119 μ), but less that the SHRM thickness in eyes that develop a FS (168μ).19 At 5 years, the frequency of SHRM was reduced by 40% in eyes with NFS when compared to their presence at baseline. In contrast, the no scar group had only a reduction of 9% even though the baseline eyes had the lowest frequency of SHRM among the 3 groups and the FS group had the highest frequency of SHRM and did not show any appreciable reduction in frequency during follow up. It appears that the SHRM in some eyes with NFS begins compacting and disappearing similar to the example shown in Figure 2 where the SHRM appears to become thin on follow up OCT scans taken at year 2 and 5. The layers of scar tissue seen in the same example remain confined under the elevated PED.
Figure 2:
Color fundus photograph (CFP, left), infrared scanning laser ophthalmoscopic (IR, center) and spectral domain optical coherence tomographic (SDOCT, right) images of non-fibrotic scar (NFS) in three CATT subjects at year 2 and 5. A small pigment encircled NFS is seen at 2 years (A1, B1 and C1) diminishes at year 5 (A4, B4 and C4). Pigmented areas appear bright on the IR images (A2–C2). SDOCT A3 and A6 show compact subretinal hyperreflective material (SHRM, white arrow) over the fibrovascular pigment epithelial detachment (PED) with adjacent subretinal fluid (SRF), however, there is a hyperreflective layer extending from the retinal pigment epithelium (RPE) across part of the inner border of the SHRM (orange arrow). By year 5, the SHRM is now encased in the RPE layer contiguous with the inner border of the PED, subretinal fluid persists with focal sites of greater penetration of OCT signal into the choroid. B1, B2 and B3 show a NFS at 2 years in another study eye along with a similar appearance as in the previous eye (A3) but with an even more prominent region of heaped up hyperreflectivity corresponding to the sites of the dark pigment ring and a second “RPE layer” extending across the SHRM. However the SHRM has either resolved or cannot be distinguished from the layered reflectance in the fibrovascular PED (B6) and the SRF has resolved. In the third example C1–C3, the year 2 compact SHRM (C3) contracts further at year 5 with a more pronounced hyperreflective RPE layer over the lesion (C6).
Unlike the FS group and the no scar group, the frequency of any fluid on OCT remained constant at all follow up time points in the NFS group. The frequency of individual types of fluid on OCT (intraretinal, subretinal and sub-RPE) also did not fluctuate significantly in the NFS group. However the NFS group had less intraretinal fluid than the other groups at both baseline and follow up. Conversely, although not reaching a significant difference except at year 2, subretinal fluid was present in a larger proportion of eyes in the NFS group compared to the other groups. The no scar group had a higher frequency of sub-RPE fluid compared to the other groups and this may be related to the large number of occult lesions in this group which anatomically lie below the RPE layer and do not enter the sub-RPE layer. The various mean retinal measurements also remained constant in NFS group throughout the follow up period in contrast to the other groups.
Despite the continued evolution of NFS over 5 years, VA remained remarkably good at a mean of 73 letters (≈ 20/32) and was better than the 5-year mean VA in eyes that had FS at 1 year (48 letters, ≈ 20/100) and better than eyes that did not have a scar at 1 year (62 letters, ≈ 20/63). Many factors could contribute to the good vision observed in the NFS group: smaller sized CNV lesions; the reduced frequency and size of GA16,17; the reduced frequency and thinning of SHRM22; the lower frequency of intraretinal fluid that is known to cause more retinal destruction and reduction of vision23; the higher frequency of eyes with subretinal fluid that is known to be associated with better visual acuity and lesser number of GA.24
The pigment ring of the NFS is not well understood. It is most likely due to multi-layering of migrating RPE cells best seen on OCT (Fig 2B3) at both edges of the PED. The splitting is most apparent on the left side. RPE “encasing” the CNV is apparent in each of these cases (Figure 2) and may explain the process that limited the enlargement of these lesions. At the edges, the RPE is not yet stretched thin, so it still appears dark, but in the center of the lesion it appears lighter because the RPE cells have “stretched”, diluting their pigment. The hypopigmentation could also be the result of replicating RPE cells that fill in for dead RPE cells, halving the pigment with each replication. Histopathological studies of eyes with NFS may help us better understand this phenotypic presentation.
Limitations of this study include the relatively small number of eyes with NFS at 1 year that were followed through 5 years and the lack of availability of spectral domain OCT at baseline and 1 year. Despite these limitations, it is apparent that the development of a nonfibrotic scar is a far more preferable than the development of a fibrotic scar. Non-fibrotic scars tend to maintain better VA over time and develop far fewer destructive pathological changes than a fibrotic scar. Better understanding of nonfibrotic scars and how and why they differ from fibrotic scars may lead to the identification of future therapeutic targets that reduce fibrosis and scar formation.
Supplementary Material
Acknowledgments
Financial Support: This study was supported by cooperative agreements U10 EY017823, U10 EY017825, U10 EY017826, U10 EY017828 and R21EY023689 from the National Eye Institute, National Institutes of Health, and Department of Health and Human Services. ClinicalTrials.gov number, NCT00593450. The funding organization had no role in the design or conduct of this research.
Financial Disclosure(s): The author(s) have made the following disclosure(s): Dr. Ying is a consultant to Chengdu Kanghog Biotech co. Ltd, Ziemer Ophthalmic Systems AG and Synergy Research. Dr. Kim is a consultant to Allergan and Synergy Research. Dr Jaffe has a consultancy relationship with Heidelberg Engineering, Alcon/Novartis, Genentech/Roche, and Neurotech. Dr. Ferris is a consultant for Foundation for Fighting Blindness, Janssen Research & Development, LLC, Viewpoint Therapeutics, Inc, EMMES, Acucela, Apellis, SemaThera, PTC Therapeutics, Bausch and Lomb - Patent/Royalties -AMD Supplements and is on the Data and Safety Monitoring Committee of Allergan, Genentech/Roche and Norvo Nordisk and is also on the executive committee of DRCR network. Dr. Maguire serves on a data and safety monitoring committee for Genentech/Roche. Dr Toth received Alcon royalties through Duke University. Other members of the writing committee have no financial relationships to declare. Dr Jaffe is a consultant for Heidelberg Engineering and Novartis.
Footnotes
Meeting Presentation: Association for Research in Vision and Ophthalmology Annual Meeting, May 2018 at the Hawaii Convention Center, 1801 Kalakaua Ave, Honolulu, HI 96815
Conflict of Interest: No conflicting relationship exists for any other author than described above.
References:
- 1.CATT Research Group., et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IVAN Study Investigators, Chakravarthy U, Harding SP, Rogers CA, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012;119:1399–411. [DOI] [PubMed] [Google Scholar]
- 3.Brown DM, Kaiser PK, Michels M, et al. ; ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432–44. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld PJ, Brown DM, Heier JS, et al. ; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–31. [DOI] [PubMed] [Google Scholar]
- 5.Berg K, Pedersen TR, Sandvik L, Bragadóttir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology 2015;122:146–52. [DOI] [PubMed] [Google Scholar]
- 6.Garweg JG, Zirpel JJ, Gerhardt C, Pfister IB. The fate of eyes with wet AMD beyond four years of anti-VEGF therapy. Graefes Arch Clin Exp Ophthalmol 2018;256:823–831. [DOI] [PubMed] [Google Scholar]
- 7.Munk MR, Ceklic L, Ebneter A, et al. Macular atrophy in patients with long-term anti-VEGF treatment for neovascular age-related macular degeneration. Acta Ophthalmol 2016;94:757–64. [DOI] [PubMed] [Google Scholar]
- 8.Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group., Five-Year Outcomes with Anti-Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016;123:1751–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillies MC, Campain A, Barthelmes D, et al. ; Fight Retinal Blindness Study Group. Long-Term Outcomes of Treatment of Neovascular Age-Related Macular Degeneration: Data from an Observational Study. Ophthalmology 2015;122:1837–45. [DOI] [PubMed] [Google Scholar]
- 10.Peden MC, Suñer IJ, Hammer ME, Grizzard WS. Long-term outcomes in eyes receiving fixed-interval dosing of anti-vascular endothelial growth factor agents for wet age-related macular degeneration. Ophthalmology 2015;122:803–8. [DOI] [PubMed] [Google Scholar]
- 11.Rofagha S, Bhisitkul RB, Boyer DS, et al. ; SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology 2013;120:2292–9. [DOI] [PubMed] [Google Scholar]
- 12.Daniel E, Pan W, Ying GS, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials. Development and Course of Scars in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2018;125:1037–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Channa R, Sophie R, Bagheri S, et al. Regression of choroidal neovascularization results in macular atrophy in anti-vascular endothelial growth factor-treated eyes. Am J Ophthalmol 2015;159:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffe GJ, Martin DF, Toth CA, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2013;120:1860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grunwald JE, Pistilli M, Daniel E, et al. ; Comparison of Age-Related Macular Degeneration Treatments Trials Research Group. Incidence and Growth of Geographic Atrophy during 5 Years of Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2017;124:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grunwald JE, Pistilli M, Ying GS, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Growth of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2015;122:809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grunwald JE, Daniel E, Huang J, et al. ; CATT Research Group. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2014;121:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniel E, Toth CA, Grunwald JE; Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2014;121:656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casalino G, Stevenson MR, Bandello F, Chakravarthy U. Tomographic Biomarkers Predicting Progression to Fibrosis in Treated Neovascular Age-Related Macular Degeneration: A Multimodal Imaging Study. Ophthalmology Retina 2017;5:451–61. [DOI] [PubMed] [Google Scholar]
- 20.Grunwald JE, Daniel E, Ying GS, et al. ; CATT Research Group. Photographic assessment of baseline fundus morphologic features in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2012;119:1634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folgar FA, Jaffe GJ, Ying GS, et al. ; Comparison of Age-Related Macular Degeneration Treatments Trials Research Group. Comparison of optical coherence tomography assessments in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2014;121:1956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willoughby AS, Ying GS, Toth CA, et al. ; Comparison of Age-Related Macular Degeneration Treatments Trials Research Group. Subretinal Hyperreflective Material in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2015;122:1846–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah N, Maguire MG, Martin DF, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Angiographic Cystoid Macular Edema and Outcomes in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016;123:858–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma S, Toth CA, Daniel E, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Macular Morphology and Visual Acuity in the Second Year of the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016;123:865–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.