Abstract
Glutamate dysregulation is known to contribute to many psychiatric disorders including schizophrenia. Aberrant cortico-striatal activity and therefore glutamate levels might be relevant to this disease characterized by reduced prepulse inhibition (PPI), however, the molecular and behavioral mechanism of the pathophysiology of schizophrenia remains unclear. The focus of this study was to contribute to the current understanding of the glutamate and neurogranin (Ng) pathway, in relation to the cortico-striatal pathology of schizophrenia using a mouse model. A variant of the Ng gene has been detected in people with schizophrenia, implicating maladaptation of cortical glutamate signaling and sensorimotor gating. To test Ng-mediated PPI regulation in the mouse model, we utilized Ng null mice, viral-mediated Ng expression, and genetics approaches. Our results demonstrate that lack of Ng in mice decreases PPI. Ng over-expression in the prefrontal cortex (PFC) increases PPI, while Ng expression in either the nucleus accumbens (NAc) or hippocampus induces no change in PPI. Using optogenetics and chemogenetics, we identified that cortico-striatal activation is involved in PPI regulation. Finally, pharmacological regulation of Ng using glutamate receptor inhibitors demonstrated altered PPI between genotypes. In this study, we have investigated the impact of Ng expression on sensorimotor gating. This study contributes to a better understanding of the glutamatergic theory of schizophrenia, opening novel therapeutic avenues that may lead to glutamatergic treatments to ameliorate the symptoms of schizophrenia.
Keywords: Schizophrenia, Neurogranin, Prepulse Inhibition, Nucleus Accumbens, Prefrontal Cortex
INTRODUCTION
Schizophrenia affects approximately 51 million people worldwide and presents a severe cost to the individual as well as society (Fleishman, 2003). Schizophrenia is characterized by 3 symptom categories of positive, negative, and cognitive. Currently the most efficacious treatment available is aimed to reduce only the presence of positive symptoms; this treatment of modulating dopamine signaling in the brain may relieve positive symptoms but the majority of a patient’s life thereafter is spent in a state of economic dependency due, in part, to the continuation of the negative and cognitive symptoms associated with the disease (Howes et al., 2015). Better treatments are therefore necessary to further increase the quality of life in these patients. Research has highlighted a role for glutamate in mediating the symptomatology of schizophrenia, notably the negative and cognitive aspects of the disease (Javitt, 2010), although its mechanism is not yet known.
Glutamate signaling via the N-methyl-D-aspartate receptor (NMDAR) has been shown to be associated with schizophrenia pathophysiology based on the ability of pharmacological NMDAR antagonists, such as phencyclidine or ketamine, to induce schizophrenia-like symptoms in otherwise healthy subjects (Krystal et al., 1994; Malhotra et al., 1997). Therefore, research has led to the glutamate hypothesis of schizophrenia, which holds that hypo-NMDAR function contributes to the symptomatology of schizophrenia, particularly the negative and cognitive aberrations present in the patient population (Gonzalez-Burgos and Lewis, 2012; Kantrowitz and Javitt, 2010; Snyder and Gao, 2013). In addition, genome-wide association studies and copy number variation studies have elucidated the neurogranin gene (Ng) as a possible mechanism of schizophrenia pathology. A genetic variant of Ng has been identified in schizophrenia patients and is possibly linked to negative symptoms and cognitive aberrations (Broadbelt et al., 2006; Ohi et al., 2012; Pohlack et al., 2011; Ruano et al., 2008; Smith et al., 2011; Su et al., 2015; Walton et al., 2013). This is in large, partly, due to the primary role of Ng within neurons modulating intracellular calmodulin (CaM) availability to facilitate NMDAR calcium-mediated signaling (Hoffman et al., 2014). The functional consequences of this regulation include modulation of synaptic plasticity, especially at dendrites (Petersen et al., 2015), which ultimately delineates activation of glutamate-mediated long-term potentiation or long-term depression (Zhong et al., 2009; Zhong and Gerges, 2012). Reduced function or expression of Ng may therefore be a critical component of NMDAR hypo-function associated with schizophrenia and its symptomatology.
Prepulse inhibition (PPI) is a diminution of the startle reflex - a protective body response to an unexpected and intense stimulation. The startle response is sensitive to sensory, cognitive, and pharmacological manipulations and thus is useful in an extensive variety of research studies (Mena et al., 2016; Ziermans et al., 2012). The use of PPI in animal studies has made it possible to identify the underlying neuronal brain circuitries (Geyer et al., 2002). Thus, this method is a very applicable paradigm for schizophrenia research as PPI occurs in all mammals and primates, including humans.
In this study, we have investigated the regulation of Ng to understand its contributions to PPI using transgenic mice, brain-specific Ng expression, pharmacology, and optogenetic approaches. Specifically, our study demonstrates how functional regulation of Ng in the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), and 47 hippocampus control PPI in mice.
MATERIALS AND METHODS
Animals
C57BL/6J mice, Pval-Cre mice, Ng null mice were purchased from Jackson Laboratories (Bar Harbor, ME). Ng null mice were generated by in-house breeding at LSUHSC. The Ng null mice (C57BL/6J) were backcrossed with wild-type 129S1 mice to generate mixed background (C57BL/6J x129S1) mice. We then bred C57BL/6 ×129S1 Ng +/− mice together to generate the Ng +/+ and Ng −/− mice that we used. Since significant genetic association of Ng is observed in male patients with schizophrenia (Ruano et al., 2008), only male mice aged 8–16 weeks were used. Mice were housed in standard Plexiglas cages under a 12h light/ dark cycle (lights on at 6:00 AM) at a constant temperature (24±0.5°C) and humidity (60±2%) with food and water available ad libitum. The animal care and handling procedures were in accordance with LSUHSC institutional and National Institutes of Health (NIH) guidelines.
Adeno-Associated Virus (AAV)-mediated Ng Expression
AAV-Ng constructs and stereotaxic injections of AAV viruses were conducted as described previously (Reker et al., 2018). Mice were anesthetized with isoflurane gas and placed in a digital stereotaxic alignment system (Model 1900; David Kopf Instruments). Two holes for bilateral injection were drilled into the skull above the target brain region. The injector (33 Ga; Plastics One) was connected to a Hamilton syringe (10 μl) and virus infusion was controlled by a syringe pump. To infuse the virus, 1 μl of AAV virus (AAV-oNg, AAV-dnNg or AAV-GFP) was bilaterally injected into either the NAc (AP: 1.34 mm; ML: ±1.3 mm; DV: 3.5 mm), hippocampus (AP: −1.70 mm; ML: ± 0.8 mm; DV: 2.2 mm) or medial prefrontal cortex (mPFC) (AP: 1.94 mm; ML: ± 0.3.mm; DV: 2.0 mm) at a rate of 0.1 μl/min. Injectors remained in place for an additional 5 min after each infusion.
Startle and PPI Paradigm
All mice were individually housed one-week before startle and PPI experiments. Mice were allowed to acclimate for 1h in the PPI experimental room on test day. Following this acclimation period, mice were subjected to the PPI experimental protocol as previously described (Oliveros et al., 2010). Each animal was individually placed within a Plexiglas cylinder (12.7 cm long×1.5 cm in diameter) resting on a Plexiglas frame (12.7 cm×20.3 cm) housed within the startle response chamber (38.1 cm×40.6 cm×58.4 cm, San Diego Instruments, San Diego, CA, USA). Startle amplitudes were detected and measured by a piezoelectric accelerometer mounted directly below the midline of the animal on the underside of the Plexiglas frame. Mice were allowed to acclimate to the startle response chamber for 5 min prior to the onset of a PPI session.
Background noise intensity was 65 dB, while startle pulse intensity was 120 dB (Swerdlow et al., 2001). PPI sessions consisted of 58 trials. Each trial was followed by an inter-trial interval (ITI) with a duration averaging 15 s. The PPI session consisted of the following eight distinct trial types, pseudo-randomly presented five different times during the session: no stimulus, startle pulse alone, prepulse alone with prepulse intensities of 4, 8, or 16 dB (PP4, PP8, PP16) above background (65dB), and prepulse (PP4, PP8, PP16) with startle pulse trials (120 dB). All pre-pulses and startle pulses had a duration of 20 ms and 40 ms. For pre-pulse with startle pulse trials, the interval between onset of the prepulse and onset of the startle pulse was 100 ms. Startle responses were collected for each individual trial throughout the PPI session for each individual mouse subject. To calculate PPI, the startle responses for each prepulse with startle pulse trial yielded a %PPI value, and these were averaged into one %PPI value for each mouse. PPI was calculated as a percentage of the pulse alone startle amplitude using the following formula: PPI= [1–(startle magnitude after prepulse-startle pulse pair/startle magnitude after startle pulse alone)] ×100. To test the pharmacological inhibition of either NMDAR or mGluR5 in both genotypes, Saline, CGP37849 (0.5 mg/kg or 1.0mg/kg), or MPEP (20mg/kg) were administrated by intraperitoneal injection 30 min prior to PPI experiments (n = 9 ~ 10 per group).
Optogenetics and DREADD in PPI
Stereotaxic surgeries for optogenetics and designer receptor exclusively activated by designer drugs (DREADD) virus infusion were conducted the same way as the AAV virus described above. For excitatory optogenetics stimulation, AAV-CaMKIIs-ChR2 vector was expressed followed by the optic fiber implantation to the target brain regions 3 weeks later. After 5 days of recovery, baseline PPI was measured. The effect of excitatory neuronal activation was measured by optogenetic stimulation using blue laser for 30 min (10Hz) (Lobo et al., 2010) immediately followed by the PPI session (n = 7 per group). The effect of GABAergic activation was measure by DREADD stimulation. AAV-Syn-DIO-hM3Dq vector was infused to the target brain of Cre-Parvalbumin mice. After a 3-week incubation period, baseline PPI was measured with saline injection prior to PPI sessions. To measure the effect of parvalbumin-specific stimulation in the same animals, CNO (1mg/kg, i.p., 30 min) was injected and PPI was measured (n = 7 per group). All surgical sites were confirmed by histology, and around 15% of mice were excluded for incorrect optic-fiber placement or lack of viral expression.
Open-field Activity
Spontaneous locomotor activity was measured in open-field chambers (41 × 41 cm). After stereotaxic surgery, mice were handled and weighed daily for 1 week prior to activity testing. On the test day, mice were weighed and placed immediately in the activity chambers. Horizontal distance traveled (cm) was recorded for 1 h using a video-tracking system (Opto4-Varimex Columbus Instruments, OH). For assessment of activity in the center of the field, the chamber floor was divided post hoc into a central zone (21 × 21 cm; center equidistant from all four walls of the chamber) and a peripheral zone (the remaining area of the floor). Distance traveled and time spent in each area were calculated from the locomotor activity data. The speed to movement ratio was categorized by resting, ambulatory, and stereotypic behaviors (n = 8 per group).
Marble-burying Test
To assess anxiety and compulsive behaviors related to brain specific-Ng expression, we used the marble burying test. Each mouse was placed in a cage containing 20 marbles, evenly spaced (4 cm apart) on top of bedding at a depth of 5 cm. After 30 minutes, the number of un-buried marbles was recorded for each animal (n = 7 per group).
Elevated Plus Maze
The elevated plus maze test has been validated for measurement of anxiety-like behavior in rodents. The plus-shape maze was elevated 60 cm off the floor having two open arms (37 × 8 cm) and two closed arms (37 × 8 × 14 cm) extending from a common central platform (8 × 8 cm). Each mouse was placed on the central platform and allowed to explore for 5 min. The dwelling time and entry number of each arm were analyzed using the video-tracking system (EthoVision XT, Noldus, VA). Following each session, the maze was cleaned with 70% ethanol to remove odors (n = 8 per group).
Statistical Analysis
All data were expressed as mean ± standard error of the mean (SEM). Statistics were performed by either two-tailed Student’s t test (Prism, GraphPad Software, La Jolla, CA) or two-way ANOVA followed by Tukey post-hoc test (SigmaStat, SYSTAT software, Point Richmond, CA). PPI analyses were stratified by genotype and prepulse intensity regardless of the results of interaction tests, as genotype-specific and prepulse intensity-specific comparisons were of interest. The criterion for statistical significance was p < 0.05.
RESULTS
Ng expression regulates PPI in mice.
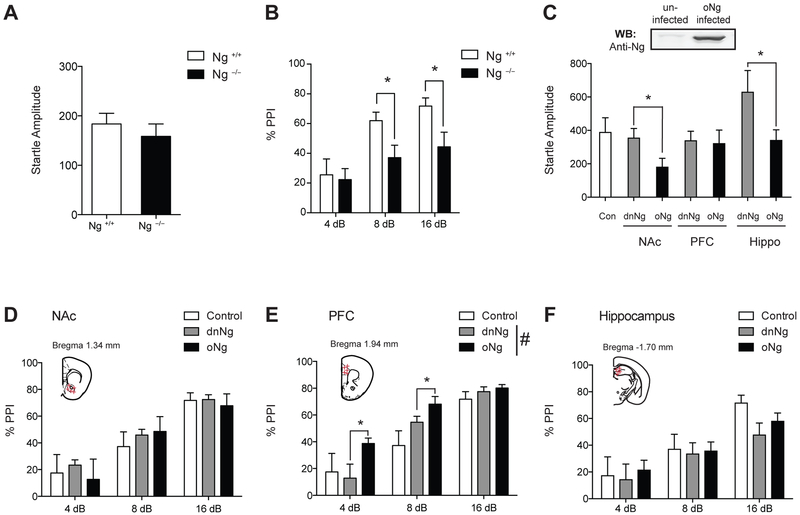
To test the hypothesis that Ng expression is critical to regulating certain domains of behaviors associated with schizophrenia, we administered PPI sessions to Ng null (Ng −/−) mice. We first measured an acoustic startle reflex response between Ng +/+ mice and Ng −/− mice and found no differences between genotypes (Fig.1A). Then, we tested whether weaker prepulse inhibits the reaction of a subsequent acoustic startle reflex in both Ng +/+ mice and Ng −/− mice, to measure abnormality in sensorimotor gating. Two-way ANOVA identified an overall significant main effect of genotype (F(1,61)=7.62, p <0.05), dB (F(2,61)=6.68, p <0.05 ) but there was no interaction between genotypes and the different decibel (dB) levels (F(2,61)=1.41, p =0.253). Tukey post-hoc test for individual comparisons identified that Ng −/− mice showed a significantly decreased PPI at the 8dB and 16 dB (Fig.1B). This evidence shows that lack of Ng expression in the brain results in an abnormal PPI, thus demonstrating a deficit in sensorimotor gating as a proxy for schizophrenia associated behaviors of Ng −/− mice.
Figure 1.
Ng expression in the brain regulates PPI. A, There are no differences in startle response between Ng+/+ mice and Ng −/− mice. B, Ng −/− mice show a significantly decreased PPI compared to Ng+/+ mice. (n = 8 ~ 14; C57BL/6J x129S1 cross). C, Western blot analysis of the expression of oNg in the HEK293T cells (infected) and control (uninfected) cell. Startle changes in response to brain-specific Ng expression in C57BL/6J mice. D, Ng expression in the NAc does not induce changes in PPI. E, Overexpression of Ng (oNg) in the mPFC significantly increases PPI compared to that of dominant-negative Ng (dnNg) expression. F, Ng expression in the hippocampus does not induce changes in PPI. Control indicates baseline startle and PPI of C57BL/6J mice without stereotaxic surgery. Statistical significance was measured by Two-way ANOVA followed by Tukey’s test. (n = 8 per group). Data presented as mean ± SEM.
Then, to examine the effects of Ng-mediated PPI in relation to the brain region, we examined the behavioral response of either Ng overexpression (oNg) or Ng dominant negative (dnNg) expression in the prefrontal cortex, nucleus accumbens, and hippocampus of C57BL/6J mice (Fig.1C). Using stereotaxic surgery, AAV-oNg or AAV-dnNg vectors were administered into the target brain regions of C57BL/6J mice bilaterally. Both startle and PPI changes were measured after 3-weeks post-surgery compared to C57BL/6J mice without surgery. oNg expression in the NAc significantly decreased startle response (t(18)=2.23, p <0.05) compared to that of dnNg expression, while dnNg expression in the hippocampus significantly increased startle response (t(18)=2.20, p <0.05) (Fig.1C). However, mPFC-specific Ng expressions did not induce any changes in startle response. To validate these acoustic startle response change by Ng expression, we measured anxiety and compulsive behaviors, but there were no significant differences (Supplemental Fig.1). Although Ng expression in both the NAc and the hippocampus induce startle changes (Fig.1C), there was no significant change in PPI (Fig.1D and 1F). Interestingly, oNg expression in the mPFC significantly increased PPI in mice (Fig.1E) compared to that of dnNg expression. Two-way ANOVA identified a significant main effect of Ng overexpression (F(1,44)=8.37, p <0.05) and dB (F(2,44)=4,48, p<0.001) without interactions between them (F(2,44)=1.90, p=0.10). These findings demonstrate that Ng overexpression in the mPFC increase sensorimotor gating response, while Ng expression in the NAc or hippocampus may regulate acoustic startle response in mice.
Ng expression in the mPFC regulates PPI.
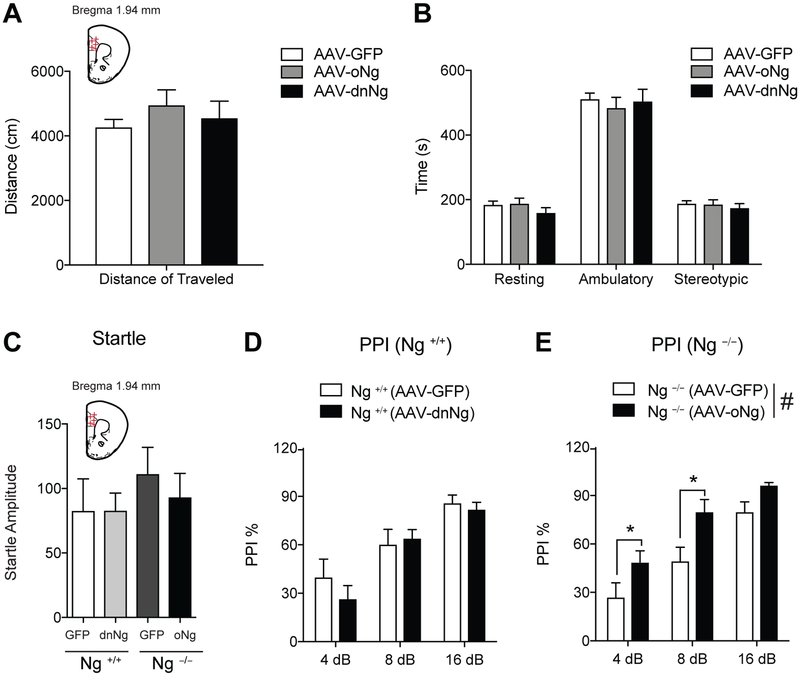
Since Ng over-expression in the mPFC increased PPI, we examined whether oNg or dnNg expression in the mPFC induces any changes in locomotor activity or anxiety using an open field test. Mice were infused with AAV-oNg, AAV-dnNg, or AAV-GFP into the mPFC region. Both oNg and dnNg expression in the mPFC of C57BL/6J mice did not induce any motor function changes including distance of travel nor anxiety compared to AAV-GFP control mice (Fig. 2A and B).
Figure 2.
Ng expression in the mPFC regulates PPI response. A and B, Neither AAV-mediated oNg nor dnNg expression in the C57BL/6J mice induces any locomotor changes compared to those of AAV-GFP expression. C, Both AAV-dnNg expression in the mPFC of Ng +/+ mice and AAV-oNg expression in the mPFC of Ng −/− does not induce acoustic startle changes compared to AAV-GFP control mice. D, dnNg expression in the mPFC of Ng +/+ mice does not induce PPI change. E, oNg expression in the mPFC of Ng −/− mice demonstrate significantly increased PPI response compared to that of AAV-GFP expression in the mPFC of Ng −/− mice. Statistical significance was measured by one-way ANOVA followed by Tukey’s test. (n = 8 per group, C57BL/6J x129S1 cross). Data presented as mean ± SEM.
To elucidate the role of Ng in the mPFC on PPI, we examined if this behavior could be induced in a reversed manner using AAV-dnNg expression in the mPFC compared to that of AAV-GFP expression. First, we measured acoustic startle response changes by AAV-dnNg or AAV-oNg expression in the mPFC of Ng transgenics mice compared the that of AAV-GFP expression and found no significant changes in these mice (Fig. 2C). One-way ANOVA indicates that the functional Ng inhibition using dominant negative expression in the mPFC did not decrease PPI in the Ng +/+ mice (Fig.2D). In contrast, we tested whether overexpression of Ng in the mPFC of Ng −/− mice can restore PPI levels compared to that of AAV-GFP expression. One-way ANOVA identified a significant main effect of Ng over expression in Ng −/− mice (F(1,53)=14.65, p <0.001) and dB (F(2,53)=23.76, p<0.001), but there was no significant interaction between expression and dB levels. Tukey post hoc test for individual comparisons identified that Ng −/− mice showed significantly increased PPI at the 4 dB and 8 dB (Fig.2E). Overall, our findings demonstrate that Ng expression in the mPFC is critical to regulate PPI while functional Ng inhibition by dnNg expression is not involved in the behavioral regulation of PPI. Taken together with the results in WT animals, this suggests that circuitry, rather than structure specific, function mediates PPI.
Optogenetics and DREADD regulation of cortico-striatal circuitry and PPI.
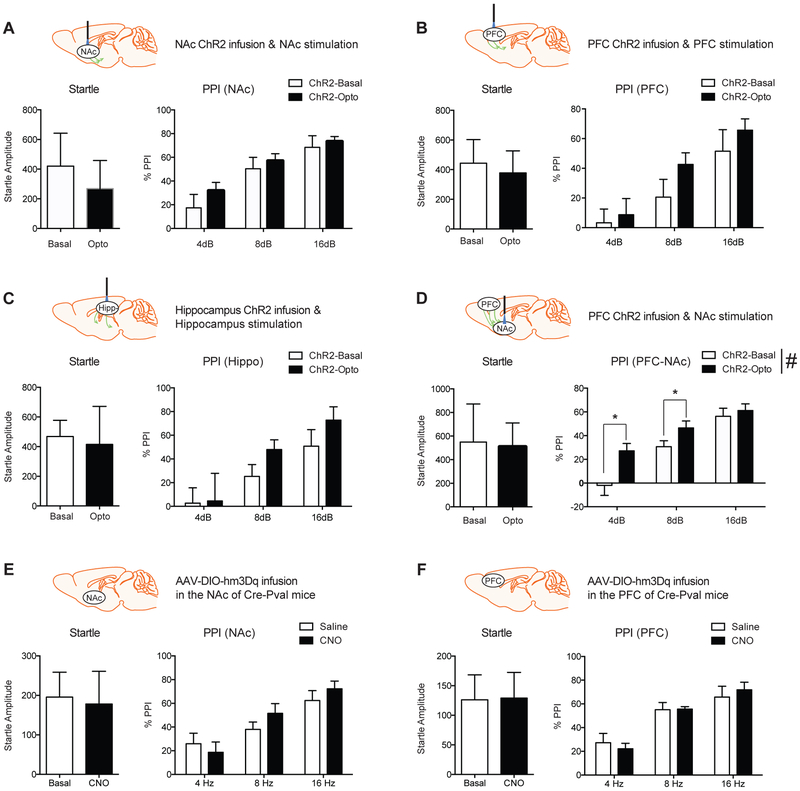
Since Ng is known to be expressed in the excitatory neurons including the cortico-striatal circuitry (Singec et al., 2004), we examined whether PPI can be controlled by excitatory neuron-specific activation in the mPFC (Bubser and Koch, 1994), NAc (Wan and Swerdlow, 1996), or Hippocampus (Bast and Feldon, 2003). To understand the brain circuitry related to PPI, we applied CaMKII promoters driven optogenetics approaches. Optogenetics stimulation in mPFC, NAc, or Hippocampus did not induce significant changes in the startle and PPI (Fig.3A–C). However, the activation of cortico-striatal circuitry (Ahmari et al., 2013), which is established by ChR2 viral infusion into the mPFC and light activation at the NAc, increased PPI compared to basal expression. Two-way ANOVA indicated a significant main effect of optogenetic stimulation (F(1,50)=10.25, p<0.05) and dB levels (F(2,50)=26.29, p <0.001) without significant interactions between them (F(2,50)=1.82, p =0.174) (Fig.3D). Tukey post hoc test for individual comparisons identified that optogenetic stimulation significantly increased PPI at 4 and 8 dB.
Figure 3.
Optogenetics and DREADD activation in the cortico-striatal circuitry and PPI. A, B, and C, Excitatory neuron-specific optogenetic stimulation does not induce any changes in startle response. (n = 7). D, Cortico-striatal optogenetic stimulation increases PPI significantly. Statistical significance was measured by Two-way ANOVA followed by Tukey’s test. (n = 7 per group). E and F, Pv-FSI neuron-specific DREADD activation in either the NAc or mPFC by CNO (1mg/kg, i.p) treatment does not induce PPI changes compared to saline control. Data presented as mean ± SEM.
Since GABAergic regulation mediated by Parvalbumin expressing fast-spiking interneurons (Pv-FSI) is known to regulate excitatory neuronal activity for behaviors associated with schizophrenia (Gonzalez-Burgos and Lewis, 2012; Jinno and Kosaka, 2004), we examined whether activation of Pv-FSI in the mPFC or NAc alters PPI responding. Using Cre-dependent DREADD (AAV-DIO-hm3Dq) expression in the Cre-Pval mice, the effect of Pval activation during the PPI session was measured in response to saline and CNO injection. There were no significant differences in both startle and PPI by GABAergic interneuron activation (Fig.3E and F). These findings indicate that excitatory neuronal regulation in the cortico-striatal circuitry is critical in regulating PPI.
Pharmacological modulation of glutamate receptors and PPI.
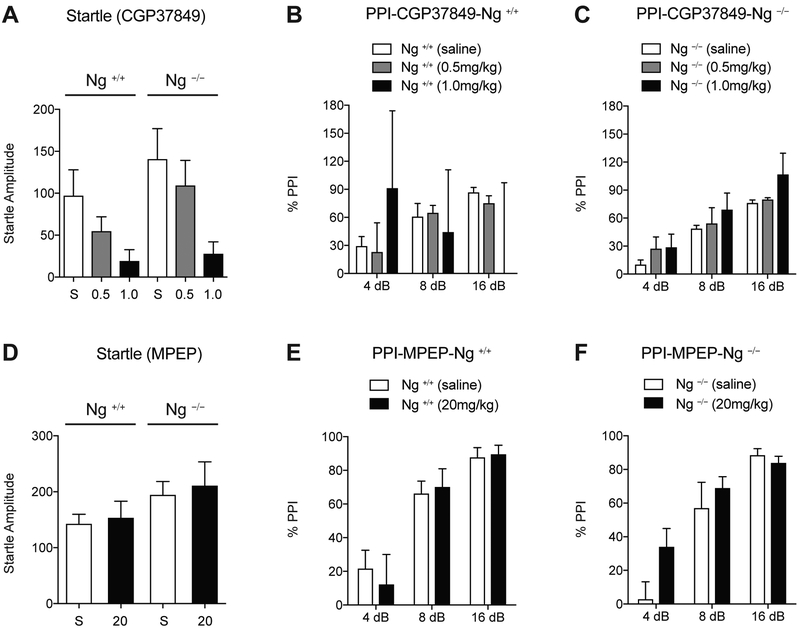
To further explore the connection between Ng and PPI, pharmacological experiments were utilized (Geyer et al., 2001). Since Ng regulates ligand-mediated NMDAR response and subsequently the activation of the PKC by mGluR5 receptor (Reker et al., 2018), we tested the effect of both NMDAR antagonist (CGP37849, i.p.) and mGluR5 antagonist (MPEP, i.p.) in Ng +/+ and Ng −/− mice during PPI. Two-way ANOVA identified that CGP37849 treatment significantly decreases the startle response (F(2,31)=4.79, p <0.05). However, there were no significant differences in genotype (F(1,31)=1,63, p = 0.21) and interaction between CGP37849 treatment and genotypes (F(2,31)=0.23, p=0.80) (Fig.4A). Regarding PPI levels, saline treatment showed significantly decreased PPI in Ng −/− mice (Fig.4B and 4C). Two-way ANOVA indicates differences in genotype (F(1,53)=4.48, p < 0.05) and dB change (F(2,53)=29.39, p < 0.001). 0.5mg/kg of CGP37849 treatment did not induce significant changes compared to saline control in both genotypes. However, two-way ANOVA identified that 1mg/kg of CGP37849 treatment induces high variability in the dB change of Ng +/+ mice (F(2,74)=0.25, p = 0.98) along with a loss of PPI at all prepulse levels (Fig.4B), but for Ng −/− mice , the dB change was significant for PPI (F(2,71)=13.86, p < 0.001) (Fig.4C). These finding indicate that NMDAR inhibition was only effective in PPI of Ng +/+ mice although CGP37849 treatment decreased startle response in both genotypes. This may be because Ng −/− mice already have hypo-NMDAR function or a higher tolerance to the effect of NMDAR inhibition.
Figure 4.
NMDA glutamate receptor antagonist only disrupts PPI in Ng +/+ mice. A, NMDA antagonist treatment (CGP37849, 1mg/kg, i.p., 30 min prior to testing, n = 10) decreases startle response in both genotypes. B, 1mg/kg of CGP37849 treatment disrupts dB dependent PPI in Ng +/+ mice although 0.5 mg/kg of CGP37849 treatment still demonstrates statistical significance through 4 dB, 8 dB, and 16 dB of prepulse. C, Ng −/− mice still demonstrate statistical significance against dB levels by CGP37849 treatments. D, mGluR5 inhibitor treatment (MPEP, 20mg/kg, i.p., 30 min prior to testing) does not induce startle response change in both genotypes. E and F, Mice with Ng +/+ or Ng −/− genotype do not demonstrate any differences in PPI after MPEP treatment (n = 9 per group, C57BL/6J x129S1 cross). Data presented as mean ± SEM.
Another glutamate receptor, mGluR5, is known to increase Ng phosphorylation via Gq-dependent PKC activation (Reker et al., 2018). We therefore examined the role of functional inhibition of Ng using mGluR5 antagonist (MPEP) compared to that of saline treatment (Henry et al., 2002). Two-way ANOVA indicated no significant main effect of startle response against genotype (F(1,41)=3.92, p=0.06), MPEP treatment (F(1,41)=0.23, p=0.63), and interactions between them (F(1,41)=0.01, p =0.92) (Fig.4D). Two-way ANOVA indicated significant effect of dB in Ng +/+ mice (F(2,56)=18.25, p<0.01), without effect of MPEP treatment (F(1,56)=0.26, p =0.61) and interactions between them (F(1,56)=0.25, p=0.78) (Fig.4E). Similarly, two-way ANOVA indicated significant effect of dB in Ng −/− mice (F(2,56)=23.91, p<0.01), without effect of MPEP treatment (F(1,56)=2.49, p =0.12) and interactions between them (F(1,56)=1.60, p =0.21) (Fig.4E).These findings demonstrate that mGlrR5 inhibition or functional inhibition of Ng did not induce PPI changes, suggesting a significant role of Ng expression in PPI modulation rather than its functional regulation.
DISCUSSION
PPI deficits are a common symptom of schizophrenia. Using this method in animal models, we have provided support to the glutamatergic theory of this mental health disorder. Specifically, we identified that Ng expression in the mPFC regulates PPI. Lack of Ng decreases PPI in Ng−/− mice, however, Ng overexpression in the mPFC of both C57BL/6J mice and Ng−/− mice increase PPI. Further, through the use of optogenetic activation of cortico-striatal glutamate, we have identified the wider circuit that contributes to the regulation of PPI.
The role of Ng in schizophrenia stems from its association with the glutamatergic system and its role in the regulation of NMDAR-mediated Ca2+-CaM pathway. Previous studies have provided evidence toward specific human chromosomal segments that indicate a connection between Ng and the development of schizophrenia (Pohlack et al., 2011). More specifically, varying single-nucleotide polymorphism (SNP) within the specific chromosomal segment were found to be consistent among people with schizophrenia within a given population of males (Rose et al., 2012; Thong et al., 2013). Further evidence highlighting the connection between Ng and schizophrenia is that Ng modulates both NMDAR and mGluR5 mediated signaling through the Ca2+-CaM complex formation and mGluR5-dependent phosphorylation of Ng. Therefore, Ng may be a suitable model to explain NMDAR hypo-function in schizophrenia and our genetic mouse model offers great advantage investigating the role of specific brain regions and/or circuitry leading to behavioral dysregulation.
PPI deficits in rodents are not animal models of schizophrenia per se, but they are an ideal study model to understand the sensorimotor gating deficits observed in patients with schizophrenia (Geyer et al., 2001). In 1992, Swerdlow et al. suggested that the loss of neural communication between limbic and basal ganglia structures is a possible neural basis of PPI (Swerdlow et al., 1992a; Swerdlow et al., 1992b). Although the acoustic startle reflex is controlled by the caudal pontine reticular nucleus (PnC), it can be utilized to measure the motor response to sensorimotor gating. Modulation of PPI is regulated by the NAc and its adjacent cortical sub-regions such as the mPFC and hippocampus (Kohl et al., 2013).
Rodent neonatal ventral hippocampal lesions (NVHL) is a convincing model to mimic the behavioral and biological features of schizophrenia in humans. As the hippo-campus is anatomically and functionally interconnected with the mPFC and NAc, the NVHL model demonstrated a reduction of PPI and disruption of prefrontal dopamine-glutamate interactions during the early development stage (O’Donnell et al., 2002; Tseng et al., 2007). In this study, we found that both Ng expression in the mPFC and corticostriatal connectivity toward NAc is critical in PPI regulation. Connections between the striatal and the prefrontal regions can demonstrate a gradient of hypo to hyper-connectivity and this facet has been related to schizophrenia (Fornito et al., 2012). Evidence presented in this paper supports the existence of an excitatory mPFC to NAc path-way that, when activated, can rescue an aberrant, decreased PPI response and the startle response to above the non-stimulated baseline. In conjunction with established neural pathways modulated in schizophrenia, the mPFC-NAc excitatory circuit adds a novel piece to the etiopathophysiology of this disease.
More scientific research on schizophrenia utilizing NMDAR antagonist models offer a promising avenue to better understand the circuitry involved in this disorder. NMDAR antagonist and PPI models may have particular relevance to the treatment of cognitive rather than positive symptoms of schizophrenia (Geyer, 2006). This is important given the paucity of new treatments for schizophrenia over recent years, especially with regards to negative symptoms and cognitive deficits, which predict functional disability in people with schizophrenia (Moghaddam and Javitt, 2012). For example, Phencyclidine (PCP) and other non-competitive NMDAR antagonists, have been shown to disrupt PPI in mice and humans, mimicking the PPI deficits seen in schizophrenia (Geyer et al., 2001). Even the pharmacological effect of PCP on PPI was more potent than any other behavioral changes. Thus, functional suppression of NMDAR strongly supports the theory of hypo-glutamatergic tone as a mechanism of schizophrenia. As mentioned previously, mGluR5 has also been shown to regulate PPI in mice (Barnes et al., 2015; Henry et al., 2002). In fact, blocking the expression of mGluR5 in conjugation with an NMDAR antagonist results in further decreases in PPI compared to NMDAR antagonist alone. Consistently, positive allosteric modulation of mGluR5 reduces neuronal firing in the mPFC mediated by the pharmacological inhibition of NMDAR(Lecourtier et al., 2007). However, the mGluR5 receptor antagonist, MPEP has not been shown to decrease PPI. The evidence from this paper further supports that there is a connection between the glutamate system and PPI regulation, by showing that viral over-expression of Ng in the mPFC increases PPI levels significantly over baseline.
Since NMDAR signaling in the GABAergic interneuron affects cortical excitation, we examined whether neural activation of either fast-spiking interneuron or pyramidal neurons in the mPFC regulates PPI. We found that optogenetic activation of corticostriatal excitatory neuron increases PPI although activation of parvalbumin specific inter-neuron did not induce PPI change. Ng was found to be highly expressed in pyramidal neurons by colocalizing CaMKII alpha subunit (Guadano-Ferraz et al., 2005). This finding supports the idea that NMDAR hypofunction diminishes the inhibitory control of cortical output, which implicates a pathophysiology of schizophrenia (Homayoun and Moghaddam, 2007).
Acoustic startle reflex is an unconscious defensive response to sudden sound or stimuli regulated by the brainstem. Interestingly, patients with anxiety disorders (Ludewig et al., 2002), autism (Csomor et al., 2008), or post-traumatic stress disorders (PTSD) (Orr et al., 2002)also demonstrated an increased startle amplitude compared to that of healthy controls. This higher startle amplitude also influences PPI calculation by decreasing PPI values, and so, we made sure to measure the startle response as well. In our rodent experiments, we observed significantly different startle baselines depending on mouse strain, drug treatment, and brain-specific Ng expression. For example, C57BL/6 mice showed higher startle response compared to Ng transgenic mice with a C57BL/6 × 129/SV mixed background mice. In addition, we also detected that over expression of Ng in the NAc significantly decreases the startle response while dominant negative expression of Ng in the hippocampus increases startle response (Fig. 1C), indicating a role for Ng in these structures in anxiety or compulsive behaviors. As these are common comorbidities in schizophrenia (Buckley et al., 2009), we examined marble burying in our Ng mice to evaluate the role of this signaling system, outlined above, in mediating anxiety. Ng −/− mice did not exhibit altered anxiety as per the marble burying test compared to Ng +/+ mice (Supplemental Fig.1A). However, C57BL/6J mice with over-expression of Ng in the NAc displaced and buried significantly more marbles from their initial locations, which may induce compulsive responding in these mice (Supplemental Fig.1B). Interestingly, human clinical studies demonstrate a slower decline in startle responses in individuals with obsessive compulsive disorder (Hoenig et al., 2005). To further investigate how dnNg in mice hippocampus increases startle response, anxiety in mice was measured by a plus maze experiment. There was no difference in arm entry number in these mice (Supplemental Fig.1C), and mice with dnNg expression in the hippocampus significantly decreased the dwelling time in the open arms compared the AAV-GFP expression control (Supplemental Fig.1D). These findings suggest that decreased Ng activity by mutation of phosphorylation site in the hippocampus increases anxious behaviors in mice, which may explain the increased startle response observed in Fig.1C.
Overall, our findings yield a novel mechanism to explain glutamate theory of schizophrenia as measured using PPI. Using optogenetics and DREADD approaches, we demonstrated a possible role of cortico-striatal connectivity in PPI. Moreover, manipulation of glutamate pharmacology using the Ng systems model presented here offers a means by which novel treatments, or refinements of existing treatments, may be developed to treat schizophrenia.
Supplementary Material
Supplemental Figure 1. Compulsivity and anxiety measurements using marble burying and open field test in response to brain-specific Ng expression. A, There are no differences in marble burying between Ng +/+ mice and Ng −/− mice. B, oNg in the NAc significantly increases marble burying (t12=4,18, p <0.001, n=7) measured by unpaired t-test. C, Ng expression in the hippocampus does not induce statistical differences in number of entries during the plus maze anxiety test. D, One-way ANOVA identified that mice with hippocampus-specific dnNg expression demonstrate significantly decreased dwelling time in open arms (F(2, 23)=5,43, p < 0.01, n=8).
Genetic deletion of neurogranin in mice decreases prepulse inhibition.
Neurogranin expression in the medial prefrontal cortex regulates prepulse inhibition.
Cortico-striatal connectivity is critical in the regulation of prepulse inhibition.
NMDAR antagonist and prepulse inhibition models are particularly relevant to the treatment of cognitive symptoms of schizophrenia.
ACKNOWLEDGEMENTS
We would like to thank Dr. Alfredo Oliveros and Dr. David Hinton for their comments and critiques of this manuscript.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest. This work was supported by COBRE (1P20GM121307-01A1) from NIGMS, NARSAD Young Investigator Award (20882) from the Brain & Behavior Research Foundation, BRF Seed Funding Program from Biomedical Research Foundation to HWN.
ABBREVIATIONS
- Ng
Neurogranin
- PPI
prepulse inhibition
- PFC
prefrontal cortex
- NAc
nucleus accumbens
- AAV
adeno-associated virus
- oNg
overexpression neurogranin
- dnNg
dominant negative neurogranin
- NMDAR
N-methyl-D-aspartate receptor
- DREADD
designer receptor exclusively activated by designer drugs
- Pv-FSI
parvalbumin expressing fast-spiking interneurons
- CNO
clozapine-n-oxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, Gordon JA, Hen R, 2013. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science 340, 1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Pinto-Duarte A, Kappe A, Zembrzycki A, Metzler A, Mukamel EA, Lucero J, Wang X, Sejnowski TJ, Markou A, Behrens MM, 2015. Disruption of mGluR5 in parvalbumin-positive interneurons induces core features of neurodevelopmental disorders. Mol Psychiatry 20, 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast T, Feldon J, 2003. Hippocampal modulation of sensorimotor processes. Prog Neurobiol 70, 319–345. [DOI] [PubMed] [Google Scholar]
- Broadbelt K, Ramprasaud A, Jones LB, 2006. Evidence of altered neurogranin immunoreactivity in areas 9 and 32 of schizophrenic prefrontal cortex. Schizophrenia Res 87, 6–14. [DOI] [PubMed] [Google Scholar]
- Bubser M, Koch M, 1994. Prepulse inhibition of the acoustic startle response of rats is reduced by 6-hydroxydopamine lesions of the medial prefrontal cortex. Psychopharmacology 113, 487–492. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS, Castle DJ, 2009. Psychiatric comorbidities and schizophrenia. Schizophr Bull 35, 383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csomor PA, Yee BK, Vollenweider FX, Feldon J, Nicolet T, Quednow BB, 2008. On the influence of baseline startle reactivity on the indexation of prepulse inhibition. Behav Neurosci 122, 885–900. [DOI] [PubMed] [Google Scholar]
- Fleishman M, 2003. Economic grand rounds: psychopharmacosocioeconomics and the global burden of disease. Psychiatr Serv 54, 142–144. [DOI] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Pantelis C, Bullmore ET, 2012. Schizophrenia, neuroimaging and connectomics. Neuroimage 62, 2296–2314. [DOI] [PubMed] [Google Scholar]
- Geyer MA, 2006. Are cross-species measures of sensorimotor gating useful for the discovery of procognitive cotreatments for schizophrenia? Dialogues Clin Neurosci 8, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR, 2001. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology 156, 117–154. [DOI] [PubMed] [Google Scholar]
- Geyer MA, McIlwain KL, Paylor R, 2002. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry 7, 1039–1053. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA, 2012. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull 38, 950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadano-Ferraz A, Vinuela A, Oeding G, Bernal J, Rausell E, 2005. RC3/neurogranin is expressed in pyramidal neurons of motor and somatosensory cortex in normal and denervated monkeys. J Comp Neurol 493, 554–570. [DOI] [PubMed] [Google Scholar]
- Henry SA, Lehmann-Masten V, Gasparini F, Geyer MA, Markou A, 2002. The mGluR5 antagonist MPEP, but not the mGluR2/3 agonist LY314582, augments PCP effects on prepulse inhibition and locomotor activity. Neuropharmacology 43, 1199–1209. [DOI] [PubMed] [Google Scholar]
- Hoenig K, Hochrein A, Quednow BB, Maier W, Wagner M, 2005. Impaired prepulse inhibition of acoustic startle in obsessive-compulsive disorder. Biol Psychiatry 57, 1153–1158. [DOI] [PubMed] [Google Scholar]
- Hoffman L, Chandrasekar A, Wang X, Putkey JA, Waxham MN, 2014. Neurogranin alters the structure and calcium binding properties of calmodulin. J Biol Chem 289, 14644–14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B, 2007. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27, 11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O, McCutcheon R, Stone J, 2015. Glutamate and dopamine in schizophrenia: an update for the 21st century. Journal of psychopharmacology 29, 97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, 2010. Glutamatergic theories of schizophrenia. Isr J Psychiatry Relat Sci 47, 4–16. [PubMed] [Google Scholar]
- Jinno S, Kosaka T, 2004. Parvalbumin is expressed in glutamatergic and GABAergic corticostriatal pathway in mice. J Comp Neurol 477, 188–201. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC, 2010. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull 83, 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S, Heekeren K, Klosterkotter J, Kuhn J, 2013. Prepulse inhibition in psychiatric disorders--apart from schizophrenia. J Psychiatr Res 47, 445–452. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr., Charney DS, 1994. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51, 199–214. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Homayoun H, Tamagnan G, Moghaddam B, 2007. Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-Methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex. Biol Psychiatry 62, 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ, 2010. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330, 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig S, Ludewig K, Geyer MA, Hell D, Vollenweider FX, 2002. Prepulse inhibition deficits in patients with panic disorder. Depress Anxiety 15, 55–60. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Adler CM, Kennison SD, Elman I, Pickar D, Breier A, 1997. Clozapine blunts N-methyl-D-aspartate antagonist-induced psychosis: a study with ketamine. Biol Psychiatry 42, 664–668. [DOI] [PubMed] [Google Scholar]
- Mena A, Ruiz-Salas JC, Puentes A, Dorado I, Ruiz-Veguilla M, De la Casa LG, 2016. Reduced Prepulse Inhibition as a Biomarker of Schizophrenia. Front Behav Neurosci 10, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Javitt D, 2012. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 37, 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Lewis BL, Weinberger DR, Lipska BK, 2002. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb Cortex 12, 975–982. [DOI] [PubMed] [Google Scholar]
- Ohi K, Hashimoto R, Yasuda Y, Nemoto K, Ohnishi T, Fukumoto M, Yamamori H, Umeda-Yano S, Okada T, Iwase M, Kazui H, Takeda M, 2012. Impact of the genome wide supported NRGN gene on anterior cingulate morphology in schizophrenia. PLoS One 7, e29780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveros A, Heckman MG, Del Pilar Corena-McLeod M, Williams K, Boules M, Richelson E, 2010. Sensorimotor gating in NTS1 and NTS2 null mice: effects of d-amphetamine, dizocilpine, clozapine and NT69L. J. Exp. Biol 213, 4232–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Pitman RK, 2002. Psychophysiology of post-traumatic stress disorder. Psychiatric Clinics of North America 25, 271–293. [DOI] [PubMed] [Google Scholar]
- Pohlack ST, Nees F, Ruttorf M, Witt SH, Nieratschker V, Rietschel M, Flor H, 2011. Risk variant for schizophrenia in the neurogranin gene impacts on hippocampus activation during contextual fear conditioning. Mol Psychiatry 16, 1072–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reker AN, Oliveros A, Sullivan JM 3rd, Nahar L, Hinton DJ, Kim T, Bruner RC, Choi DS, Goeders NE, Nam HW, 2018. Neurogranin in the nucleus accumbens regulates NMDA receptor tolerance and motivation for ethanol seeking. Neuropharmacology 131, 58–67. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Morris DW, Fahey C, Robertson IH, Greene C, O’Doherty J, Newell FN, Garavan H, McGrath J, Bokde A, Tropea D, Gill M, Corvin AP, Donohoe G, 2012. The effect of the neurogranin schizophrenia risk variant rs12807809 on brain structure and function. Twin Res Hum Genet 15, 296–303. [DOI] [PubMed] [Google Scholar]
- Ruano D, Aulchenko YS, Macedo A, Soares MJ, Valente J, Azevedo MH, Hutz MH, Gama CS, Lobato MI, Belmonte-de-Abreu P, Goodman AB, Pato C, Heutink P, Palha JA, 2008. Association of the gene encoding neurogranin with schizophrenia in males. J Psychiatr Res 42, 125–133. [DOI] [PubMed] [Google Scholar]
- Singec I, Knoth R, Ditter M, Volk B, Frotscher M, 2004. Neurogranin is expressed by principal cells but not interneurons in the rodent and monkey neocortex and hippocampus. J Comp Neurol 479, 30–42. [DOI] [PubMed] [Google Scholar]
- Smith RL, Knight D, Williams H, Dwyer S, Richards A, Kirov G, O’Donovan MC, Owen MJ, 2011. Analysis of neurogranin (NRGN) in schizophrenia. Am J Med Genet B Neuropsychiatr Genet 156B, 532–535. [DOI] [PubMed] [Google Scholar]
- Snyder MA, Gao WJ, 2013. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front Cell Neurosci 7, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Long J, Pan R, Xie X, Mao X, Zhou Y, Chen Q, Wei B, 2015. Influence of NRGN rs12807809 polymorphism on symptom severity in individuals with schizophrenia in the Han population but not the Zhuang population of south China. Acta Neuropsychiatr, 1–7. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Caine SB, Braff DL, Geyer MA, 1992a. The neural substrates of sensorimotor gating of the startle reflex: a review of recent findings and their implications. J Psychopharmacol 6, 176–190. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Caine SB, Geyer MA, 1992b. Regionally selective effects of intracerebral dopamine infusion on sensorimotor gating of the startle reflex in rats. Psychopharmacology 108, 189–195. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL, 2001. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology 156, 194–215. [DOI] [PubMed] [Google Scholar]
- Thong JY, Qiu A, Sum MY, Kuswanto CN, Tuan TA, Donohoe G, Sitoh YY, Sim K, 2013. Effects of the neurogranin variant rs12807809 on thalamocortical morphology in schizophrenia. PLoS One 8, e85603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Lipska BK, O’Donnell P, 2007. Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia. Biol Psychiatry 62, 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton E, Geisler D, Hass J, Liu J, Turner J, Yendiki A, Smolka MN, Ho BC, Manoach DS, Gollub RL, Roessner V, Calhoun VD, Ehrlich S, 2013. The impact of genome-wide supported schizophrenia risk variants in the neurogranin gene on brain structure and function. PLoS One 8, e76815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan FJ, Swerdlow NR, 1996. Sensorimotor gating in rats is regulated by different dopamine-glutamate interactions in the nucleus accumbens core and shell subregions. Brain Res 722, 168–176. [DOI] [PubMed] [Google Scholar]
- Zhong L, Cherry T, Bies CE, Florence MA, Gerges NZ, 2009. Neurogranin enhances synaptic strength through its interaction with calmodulin. EMBO J. 28, 3027–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Gerges NZ, 2012. Neurogranin targets calmodulin and lowers the threshold for the induction of long-term potentiation. PLoS One 7, e41275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziermans TB, Schothorst PF, Sprong M, Magnee MJ, van Engeland H, Kemner C, 2012. Reduced prepulse inhibition as an early vulnerability marker of the psychosis prodrome in adolescence. Schizophrenia Res 134, 10–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Compulsivity and anxiety measurements using marble burying and open field test in response to brain-specific Ng expression. A, There are no differences in marble burying between Ng +/+ mice and Ng −/− mice. B, oNg in the NAc significantly increases marble burying (t12=4,18, p <0.001, n=7) measured by unpaired t-test. C, Ng expression in the hippocampus does not induce statistical differences in number of entries during the plus maze anxiety test. D, One-way ANOVA identified that mice with hippocampus-specific dnNg expression demonstrate significantly decreased dwelling time in open arms (F(2, 23)=5,43, p < 0.01, n=8).