Abstract
Individuals with disorders that include psychotic symptoms (i.e. psychotic disorders) experience broad cognitive impairments in the chronic state, indicating a dimension of abnormality associated with the experience of psychosis. These impairments negatively impact functional outcome, contributing to the disabling nature of schizophrenia, bipolar disorder, and psychotic depression. The robust and reliable nature of cognitive deficits has led researchers to explore the timing and profile of impairments, as this may elucidate different neurodevelopmental patterns in individuals who experience psychosis. Here, we review the literature on cognitive deficits across the life span of individuals with psychotic disorder and psychotic-like experiences, highlighting the dimensional nature of both psychosis and cognitive ability. We identify premorbid generalized cognitive impairment in schizophrenia that worsens throughout development, and stabilizes by the first-episode of psychosis, suggesting a neurodevelopmental course. Research in affective psychosis is less clear, with mixed evidence regarding premorbid deficits, but a fairly reliable generalized deficit at first-episode, which appears to worsen into the chronic state. In general, cognitive impairments are most severe in schizophrenia, intermediate in bipolar disorder, and the least severe in psychotic depression. In all groups, cognitive deficits are associated with functional outcome. Finally, while the generalized deficit is the clearest and most reliable signal, data suggests specific deficits in verbal memory across all groups, specific processing speed impairments in schizophrenia and executive functioning impairments in bipolar disorder. Cognitive deficits are a core feature of psychotic disorders that provide a window into understanding developmental course and risk for psychosis.
Introduction
Psychosis refers to the experience of delusions, hallucinations, and thought disorder. In the context of disorders that almost always or often include psychotic symptoms, such as schizophrenia and bipolar disorder, psychotic symptoms are significant enough to cause distress and/or functional impairment. However psychotic experiences occur on a spectrum. There is evidence that approximately 20% of individuals in the general population have subtle psychotic-like experiences (van Os et al. 2009), while approximately 2–5% meet criteria for schizotypal personality disorder (Chemerinski et al. 2013), a disorder characterized by magical thinking, ideas of reference, and perceptual aberrations that do not rise to the level of primary psychosis. In recognition of mental illness as continuous in nature, the NIMH put forth Research Domain Criteria to conceptualize and research aspects of psychopathology through a dimensional lens (Insel et al. 2010). Under the research domain criteria framework, questions arise regarding whether associated features of psychosis, such as cognitive dysfunction, also occur along a spectrum. Cognitive dysfunction represents an excellent opportunity to assess dimensional characteristics of mental illness, as cognitive impairment is present in individuals with putatively “distinct” mental disorders that share the feature of psychosis. While research domain criteria does not directly address the trajectory of phenotypic dimensions, a fact recently critiqued (Mittal and Wakschlag 2017), understanding the timing of cognitive impairments in psychosis contributes to a richer understanding of its etiology. Examining the time course of cognitive impairment in psychotic disorders also allows for assessment of differential trajectories, introducing an opportunity to test questions of dissociation. In the spirit of research domain criteria, and with an eye towards future neurodevelopmental investigations, we present a review of broad cognitive dysfunction across the psychosis spectrum, across the life course.
Although not a symptom of psychotic disorders, cognitive dysfunction is a core feature of illnesses that include psychotic symptoms. From the definition of schizophrenia as dementia praecox (Kraepelin 1919), cognitive deficits have been identified in individuals in the chronic states of illness. Despite the co-occurrence of cognitive deficits with psychosis, anti-psychotic medications do not improve cognitive impairment (Nielsen et al. 2015). In fact, anti-psychotics demonstrate minimal efficacy in improving daily functioning, and research suggests that functional impairment is most strongly associated with cognitive impairment, not severity of psychotic symptoms (Velligan et al. 1997). Therefore, cognitive deficits represent an important target for improving the lives of patients suffering from psychotic disorders.
The relevance of understanding cognitive impairment is further highlighted when considering its presence early in the disease course. While psychotic symptoms typically emerge between ages 18 and 25, cognitive deficits are observed much earlier on in the lifespan of those who go on to develop schizophrenia (Cornblatt et al. 1999). This suggests that cognitive deficits are a marker of abnormal neurodevelopment, especially within the context of genetic risk and early developmental insults (Rapoport et al. 2012). While schizophrenia is often considered a neurodevelopmental disorder, researchers continue to debate this model in bipolar disorder, which some evidence suggests is neurodegenerative in nature (Goodwin et al. 2008). Therefore, understanding the timing, specificity, and severity of cognitive deficits in psychotic disorders creates a window into brain function across the life span. In this review, we examine the data on cognitive functioning across the life course of individuals who experience psychosis, starting with available data on premorbid cognition, and then considering individuals at ultra-high risk for developing psychosis, first-episode psychosis, and finally the chronic state. We work to uncover the current landscape of research on cognitive dysfunction in psychotic disorders, and briefly discuss implications for functional outcome, treatment, and developmental course.
Cognitive Functioning in Schizophrenia
Premorbid cognitive function in schizophrenia
There is a general consensus that children and adolescents exhibit premorbid cognitive impairments prior to the onset of schizophrenia. As will be detailed in this section, this consensus follows decades of empirical research documenting both general (e.g., IQ) and specific (e.g., processing speed) impairments in individuals that later develop schizophrenia. Consistent with premorbid impairments, first-degree relatives also show cognitive impairments (Ivleva et al. 2012; Keri et al. 2001; McIntosh et al. 2005; Niendam et al. 2003), and the presence of psychosis in a first-degree relative increases the severity of childhood premorbid impairment in schizophrenia (Seidman et al. 2013). Despite the consistency of research regarding premorbid cognitive impairments in schizophrenia, there is less consensus regarding their exact nature (i.e., which cognitive domains show premorbid impairments specific to schizophrenia) and course.
General cognitive impairments
Children and adolescents who later develop schizophrenia show deficits in general cognitive abilities (Figure 1). Consistent IQ deficits are observed among individuals who subsequently develop schizophrenia (Aylward et al. 1984; Daban et al. 2006; Dickson et al. 2012; Khandaker et al. 2011; Mollon and Reichenberg 2017; Woodberry et al. 2008), with evidence that risk for schizophrenia increases by 3.7% for every one point decrease in IQ (Khandaker et al. 2011). Premorbid reduction in IQ has a medium effect size(Khandaker et al. 2011; Woodberry et al. 2008), which translates to a premorbid IQ deficit of around 8–10 points (Mollon and Reichenberg 2017; Seidman et al. 2013). This is approximately half of the deficit seen in first-episode and chronic schizophrenia patients (Reichenberg and Harvey 2007). In terms of the relationship between premorbid school achievement and schizophrenia, poor premorbid school performance is generally associated with increased risk for schizophrenia (Crow et al. 1995; Fuller et al. 2002; Kendler et al. 2016; MacCabe et al. 2008; Strauss et al. 2012; Watt and Lubensky 1976). However, one meta-analysis found that children who later develop schizophrenia do not show deficits in premorbid academic performance (Dickson et al. 2012), and other research has pointed to intact early school functioning but later difficulties in schooling that are associated with severity of symptoms (Helling et al. 2003) and poorer outcomes (Cannon et al. 1999; Ang and Tan 2004). Therefore, early premorbid cognitive function in schizophrenia appears to be associated with some functional impairment, as measured by academic performance, but this may only become apparent later in schooling with increased severity of symptoms.
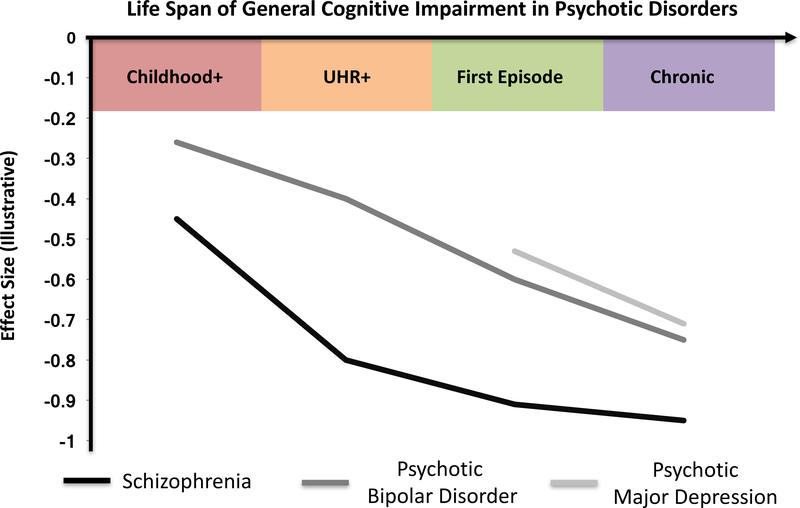
Figure 1:
Estimated general cognitive impairment across the life span in schizophrenia, psychotic bipolar disorder, and psychotic depression. Effect size estimates based on studies presented in this review (e.g. Mollon & Reichenberg, 2017; Seidman et al., 2016; Heinrichs & Zakzanis, 1998; Mesholam-Gately et al., 2009; Daban et al., 2006; Hill et al., 2013; Martinez-Aran et al., 2004; Schatzberg et al., 2000). No data is currently available for premorbid or ultra-high risk psychotic depression.
Childhood+ = children who develop disorder; UHR+ = ultra high risk individuals who develop disorder.
Specific cognitive impairments
In addition to generalized cognitive impairments in terms of IQ and academic achievement, specific premorbid impairments exist in a number of cognitive domains (Heinrichs and Zakzanis 1998; Daban et al. 2006; Mesholam-Gately et al. 2009; Trotta et al. 2015). These domains include attention (Cannon et al. 2006; Erlenmeyer-Kimling et al. 2000; Cornblatt et al. 1999; Kern et al. 2011; Welham et al. 2010), memory (MacCabe et al. 2013; Erlenmeyer-Kimling et al. 2000; McIntosh et al. 2005; Meier et al. 2014; Reichenberg and Harvey 2007), reasoning (Addington and Addington 2005; Niendam et al. 2003; Reichenberg et al. 2010; Reichenberg and Harvey 2007; Reichenberg et al. 2002; Tiihonen et al. 2005; Welham et al. 2010), and executive functioning (Cannon et al. 2006; Meier et al. 2014; Reichenberg and Harvey 2007).
There is also considerable support for premorbid impairments in processing speed (McIntosh et al. 2005; Kern et al. 2011; Bachman et al. 2010; Seidman et al. 2013; Meier et al. 2014; Niendam et al. 2003), consistent with research indicating a processing speed impairment across the course of schizophrenia, including in prodromal, first-episode, and chronic phases of the illness (Heinrichs and Zakzanis 1998; Mesholam-Gately et al. 2009; Seidman et al. 2013; Seidman et al. 2010). Meta-analysis of cognitive performance in almost 2000 chronic patients, for instance, demonstrates that digit symbol coding ability is significantly more impaired than episodic memory, executive functioning, and working memory ability, suggesting processing speed as one of the most sensitive areas of cognitive impairment in schizophrenia (Dickinson et al. 2007). One hypothesis for why tasks like digit symbol coding are so sensitive to impairment is their reliance on multiple aspects of cognition, particularly executive functioning, verbal fluency and memory (Knowles et al. 2015; R. S. Keefe and Harvey 2015). In the premorbid state of schizophrenia, processing speed impairments are therefore multifaceted, both relying on other areas of cognition that are impaired and potentially contributing to (or interacting with) deficit development of the host of cognitive impairments seen in later stages of the disorder, such as verbal abilities (Brebion et al. 2006).
Verbal abilities are consistently associated with premorbid impairments (Mollon and Reichenberg 2017; Parellada et al. 2017), particularly receptive language (Addington and Addington 2005; Reichenberg et al. 2002; Seidman et al. 2013; Cannon et al. 2002; Jones et al. 1994; Kremen et al. 2010; Meier et al. 2014; Niendam et al. 2003; Welham et al. 2010). For example, children later diagnosed with schizophrenia exhibit receptive language impairments in childhood, which predict psychotic symptoms at age eleven (Cannon et al. 2002). Among children with receptive language disorder diagnoses, 10% later developed schizophrenia (Howlin et al. 2000), consistent with an early deficit in receptive language as a risk-factor for schizophrenia.
Neurodevelopment model of cognitive impairments
Premorbid cognitive deficits support the neurodevelopmental model of schizophrenia, which posits that the earliest signs of the disorder are mild abnormalities in cognitive development (Mollon and Reichenberg 2017; Murray and Lewis 1987; Weinberger 1987). This model conceptualizes cognitive dysfunction in schizophrenia as aberrant neurodevelopment during the first two decades of life (Bora 2015; Dickson et al. 2012; Khandaker et al. 2011; MacCabe et al. 2013), with deficits in the acquisition of cognitive abilities compared to normative individuals (Bora and Pantelis 2015; Kremen et al. 1998; Reichenberg et al. 2010). The presence of cognitive deficits years prior to the onset of psychotic symptoms suggests that cognitive dysfunction is at the core of schizophrenia, with abnormal neurodevelopment manifesting through performative lag as early as preschool age (Seidman and Mirsky 2017). As outlined elegantly by Kahn & Keefe (2013), multiple lines of evidence converge on the notion that schizophrenia is a cognitive illness that is neurodevelopmental in nature: cognitive deficits exist prior to psychosis onset, cognitive ability is influenced by genetics, and cognition is an independent predictor of functional outcome(Kahn and Keefe 2013). Furthermore, despite the vast heterogeneity in symptom profiles of schizophrenic patients, an estimated 98% of schizophrenia patients have lower cognitive functioning than expected based on their mother’s education level (R. S. Keefe et al. 2005). Cognitive impairment early in illness is therefore a critical piece of evidence for abnormal neurodevelopment and should be considered a core feature and risk-factor for symptom manifestation later in life (Seidman and Mirsky 2017).
While the presence of premorbid cognitive impairment in schizophrenia is well-described, there are mixed findings regarding the nature of this aberrant neurodevelopment, including whether schizophrenia is associated with static premorbid deficits versus increasing cognitive deficits prior to the onset of the disorder. There is some evidence for static deficits (Cornblatt et al. 1999; Crow et al. 1995; Jones et al. 1994), including in attention (Cornblatt et al. 1999) and verbal abilities (Meier et al. 2014; Reichenberg et al. 2010). Yet the majority of research shows increasing cognitive deficits prior to the onset of schizophrenia, especially immediately prior to the onset of the disorder (Lewandowski et al. 2011b; Mesholam-Gately et al. 2009; Mollon and Reichenberg 2017; Parellada et al. 2017). One hypothesis helping to explain these discrepant findings is that verbal deficits may emerge early and remain static during childhood, contribute to an increasing impairments in non-verbal abilities in adolescence, with these non-verbal deficits further exacerbating verbal deficits during adolescence (Mollon and Reichenberg 2017). Schizophrenia is largely associated with premorbid deficits in the acquisition of cognitive abilities during neurodevelopment (Bora 2015), as opposed to the loss of previously acquired cognitive abilities, indicating these deficits increase in severity throughout development (Mollon and Reichenberg 2017).
Schizophrenia: Ultra-high Risk
Prior to first-episode psychosis, prediction of who will develop a primary psychotic disorder remains elusive; however, researchers have identified markers of increased risk. These individuals, often referred to as ultra-high risk, clinical high risk, or prodromal, are identified through dimensions of attenuated psychotic symptoms and/or familial risk. Approximately 13–23% of individuals meeting these ultra-high risk criteria convert to psychosis within two years (Seidman et al. 2016) (Lemos-Giraldez et al. 2009) (Bechdolf et al. 2010). Identification of ultra-high risk individuals in longitudinal studies allows for a particularly rich comparison of neurocognitive function in those who do and do not convert to psychosis, providing a window into the functioning of those at the highest risk.
Ultra-High Risk Compared to Healthy Controls
Based on a multitude of studies, there is reliable evidence that ultra-high risk individuals are globally cognitively impaired when compared to a normative sample (Hawkins et al. 2004) (R. S. Keefe et al. 2006) (Fusar-Poli et al. 2012) (Simon et al. 2007). At-risk participants demonstrate deficits in working memory, executive function, verbal fluency, attention, and memory (Hawkins et al. 2004) (R. S. Keefe et al. 2006); (Seidman et al. 2016). In a meta-analysis, general intelligence and all cognitive sub-domains, with the exception of processing speed, were found to be impaired in ultra-high risk participants (Fusar-Poli et al. 2012). This global deficit in ultra-high risk was replicated with a small but reliable effect size (Cohen’s d = 0.30) for cognitive impairment averaged across 19 neuropsychological tasks (Seidman et al. 2016). Further, individuals who endorse psychotic symptoms between ages 8–12 have a significantly lower predicted age based on their cognitive performance (Gur et al. 2014). This impairment is present at 8 years old, becomes even more pronounced after age 16, and is observed in all tested domains (executive function, memory, complex cognition, social cognition, and sensorimotor) with the biggest lag in complex cognition (which includes verbal reasoning, non-verbal reasoning, and spatial processing) and social cognition.
Ultra-High Risk Compared to First-episode Schizophrenia
Cognitive ability in individuals at-risk for schizophrenia is globally impaired compared to healthy controls, but the magnitude of impairment is less than can be seen in patients who have recently experienced their first-episode of psychosis (Keefe et al., 2006; Simon et al., 2010). Ultra-high risk participants have performed significantly better than first-episode subjects on all tasks (CPT, verbal recall, digit symbol, verbal fluency, and working memory) except for finger tapping on which they had similar performance (R. S. Keefe et al. 2006). This intermediate impairment between controls and first-episode patients was replicated across all neuropsychological tasks in a separate analysis of ultra-high risk subjects (Hou et al. 2016). Intermediate levels of cognitive impairment have also been observed in areas of visual working memory, verbal memory, executive functioning, visual spatial skills, and mental control (Goghari et al. 2014) (C. C. Liu et al. 2015). Impairments similar to first-episode patients have been reported for sustained attention (Francey et al. 2005) and relational memory (Greenland-White et al. 2017). Overall, ultra-high risk participants demonstrate global impairments that are intermediate in magnitude between healthy controls and first-episode. That said, there may be domains that are impaired to the same degree as first-episode psychosis, even during the prodromal state, particularly in the domains of memory and attention.
Cognitive Deficits Based on Clinical Versus Familial Risk
Definition of ultra-high risk is not uniform, and several studies have worked to parse out differences in cognitive ability based on the category of risk. For instance, verbal memory is significantly worse in individuals whose risk is defined based on clinical symptoms compared to those whose risk is defined based on family history (1st degree relative with psychotic disorder) (Seidman et al. 2010). Additionally, while their composite cognition scores were similar, their profiles of impairment differed; individuals with clinical risk showed deficits in executive functioning and verbal memory while individuals with familial risk had deficits in vocabulary and visuospatial skills. Children with a lower familial risk (only one affected 2nd-degree relative) have cognitive scores similar to healthy controls, but those with higher familial risk (at least one affected 1st degree relative or at least two affected 2nd-degree relatives) demonstrate lower full-scale IQ, scholastic achievement, verbal comprehension, working memory, verbal memory, and executive functioning (Dickson et al. 2014). When patients are stratified by the level of clinical risk, which may indicate different stages of the prodrome (Klosterkotter et al. 2001), those identified as “basic symptom at-risk” had better working memory and verbal memory than those at ultra-high risk and first-episode subjects (Simon et al. 2007). Basic symptom participants showed cognitive performance worse than healthy controls but similar to other psychiatric controls who endorsed no psychotic symptoms, suggesting that cognitive deficits are attenuated, but present at this early prodromal stage.
Ultra-High Risk+ Compared to Ultra-High Risk-
Longitudinal studies of ultra-high risk subjects allow for the comparison of cognitive ability between individuals who do and do not convert to a primary psychotic disorder, usually over the course of 1–3 years, providing clues about incremental risk and time course. Research suggests that ultra-high risk+ subjects (i.e., those who convert) demonstrate greater cognitive impairment than ultra-high risk- subjects (i.e. those who do not convert) (R. S. Keefe et al. 2006) (Seidman et al. 2010), with some evidence of ultra-high risk+ subjects having cognitive deficits similar to first-episode participants, and ultra-high risk- subjects looking similar to healthy controls (R. S. Keefe et al. 2006). Interestingly, the tests most impaired in the first-episode subjects, symbol coding and verbal memory, are the most impaired in the ultra-high risk+ group (Seidman et al. 2010), further suggesting that ultra-high risk subjects who will convert to psychosis demonstrate a cognitive profile similar to those with the illness. Meta-analysis has found that ultra-high risk+ individuals have significantly lower general intelligence, poorer verbal fluency, verbal and visual memory, and working memory compared to ultra-high risk- individuals (Fusar-Poli et al. 2012). Greater impairments in working memory, attention, and worse declarative and verbal memory abilities can also differentiate ultra-high risk+ from ultra-high risk- subjects (Lencz et al. 2006) (Seidman et al. 2016). Finally, conversion to psychosis is associated with poor verbal memory and sustained attention (Seidman et al. 2010) (R. S. Keefe et al. 2006). These longitudinal findings demonstrate that individuals at high risk who will ultimately develop a psychotic disorder are more cognitively impaired than individuals presenting with a similar level of risk, who do not develop a psychotic disorder within 1–3 years. Those who do convert look more similar to first-episode patients, suggesting that the intermediate deficit discussed earlier may partially reflect the average of two groups (converters and non-converters). Of further interest is the potential importance of poor verbal memory and attention, both of which predicted conversion to psychosis and both of which were more impaired in first-episode psychosis than ultra-high risk on average.
More Specific Impairments in Ultra-High Risk Schizophrenia
Broad domain-level deficits in ultra-high risk patients are clear, and growing research into this population is working to reveal more specific impairments. For instance, in the context of broad memory and IQ impairments, visual reproduction of a stimulus and story recall on a logical memory task have discriminated ultra-high risk+ patients from those who did not convert (Brewer et al. 2005) – an association not seen for other memory, attentional, or executive functioning tasks. Abnormalities in perceptual processing are also observed in clinical high risk patients, with deficits in visual form perception (Kimhy et al. 2007) and perceptual organization (S. Silverstein et al. 2006). Perceptual processing abnormalities may contribute to, or possibly interact with, abnormalities in attributional bias in the ultra-high risk state (An et al. 2010), which is in turn related to paranoid symptoms. Unpacking executive dysfunction into “cold” (e.g. working memory) and “hot” (e.g. decision making) functions, there are specific relationship between “hot” executive functioning (decision making ability) and psychotic symptom severity (MacKenzie et al. 2017), suggesting that certain aspects of these broad cognitive domains are more critical for understanding relationships with current and impending psychotic experiences. Finally, specific analysis of latent inhibition, a learning process dependent on both attentional and perceptual resources, identified a deficit in latent inhibition in ultra-high risk individuals, indicating impaired ability to learn from past experiences to adjust expectations for future experiences (Kraus et al. 2016). While not comprehensive, these findings, particularly those predicting conversion to psychosis and relationships with symptom severity, suggest the importance of unpacking deficits observed within neurocognitive domains.
Summary
Together, these data suggest that prodromal psychosis is characterized by global cognitive impairment, with some specific risk associated with poor memory and attention during this stage. Individuals with a strong familial risk, more pronounced attenuated psychosis, and those who will ultimately convert to a psychotic disorder, demonstrate the greatest impairment in cognition on average. Cognitive deficits are reliably present at this stage of illness, with some data suggesting that deficits are similar in magnitude and pattern to those seen in first-episode psychosis.
Schizophrenia: First-episode
Measuring cognitive ability in first-episode schizophrenia allows for the measurement of cognition prior to long-term treatment with anti-psychotics and prior to any potential degenerating process associated with the illness (e.g. (Kirkpatrick et al. 2008)). Although definitions of “first-episode” are variable, the phrase refers to the identification of individual who have recently transitioned to a primary psychotic disorder. The majority of first-episode subjects discussed in this review were tested following their index hospitalization, within 24 months of their first contact with psychiatric treatment for psychosis.
First-episode Compared to Chronic Schizophrenia
Comparison of first-episode schizophrenia with chronic schizophrenia and healthy controls has been the primary question of interest for many studies, allowing researchers to assess whether cognitive deficits emerge through a degenerating process following first-episode psychosis, or are already present when the psychosis emerges. Meta-analysis of over 2,000 first-episode patients across 47 studies has revealed medium-to-large impairments in first-episode schizophrenia across 10 cognitive domains, with all tested domains revealing significant impairment (Mesholam-Gately et al. 2009). General cognitive ability had an effect size of −.91, demonstrating almost one standard deviation of overall cognitive impairment in first-episode schizophrenia. Areas of greatest impairment included immediate verbal memory (Cohen’s d = −1.2) and processing speed (Cohen’s d = −0.96). Motor skills exhibited the least impairment, but still represented a significant deficit compared to controls (Cohen’s d = −0.64). The authors compared their results to those in chronic patients presented in Heinrichs & Zakzanis (1998). Chronic subjects were, on average, 9 years older, had greater illness chronicity, and had substantially longer duration of medication exposure; yet their impairments were similar to those observed in first-episode subjects (chronic patients: Cohen’s ds ranged from −.46 to −1.41). Verbal memory also represented the greatest deficit in chronic schizophrenia subjects (Cohen’s d=−1.41) suggesting a stable profile of impairment across the illness course. Deficits in global cognitive impairment between first-episode and chronic schizophrenia remain similar when reducing methodological heterogeneity (e.g. medication use) (McCleery et al. 2014), as these groups exhibit comparable deficits in processing speed, attention/vigilance, verbal learning, visual learning, and reasoning and problem solving performance. In both groups, processing speed was approximately 0.5 standard deviations below their mean performance across all domains, revealing a specific deficit in processing speed in both early and chronic stages of illness, on the backdrop of a generalized deficit.
Evidence is strong that first-episode schizophrenia patients demonstrate cognitive deficits similar to those observed in chronic stages, suggesting that duration of anti-psychotic medication use is not a potent contributor to cognitive impairment in schizophrenia. In fact, recent meta-analysis of medication-naïve first-episode patients found medium to large effect sizes in all cognitive domains compared to healthy controls, with the largest impairment in verbal memory, processing speed, and working memory (Fatouros-Bergman et al. 2014). These findings across 23 studies strongly support the presence of significant cognitive impairment in the early stages of psychosis that is independent of medication use.
Longitudinal Analysis of First-Episode Schizophrenia
Longitudinal studies of first-episode schizophrenia patients provide important insight into the trajectory of cognitive impairments into the chronic state. In general, these studies point to a stable cognitive profile in the years following a patient’s first break. For instance, over two years, only 10% of a large cohort of first episode patients demonstrated decline or improvement in cognition, indicating that 90% of patients had stable cognitive functioning (Sanchez-Torres et al. 2017). Over 10 years, in two independent studies, schizophrenia-spectrum patients exhibited stable cognitive performance on all tests (Bergh et al. 2016; Rund et al. 2016), even in the context of improved clinical symptoms. Duration of untreated psychosis was unrelated to cognitive performance (Rund et al. 2016). Global cognitive ability measured at first-episode therefore appears to reflect a patient’s cognitive capacity during the chronic course, arguing against neurotoxic effects of schizophrenia.
Additional Cognitive Abnormalities in First-episode Schizophrenia
Large cognitive batteries, such as the MATRICS Consensus Cognitive Battery, provide standardized measurement of broad cognitive domains, but do not address a number of more specific aspects of cognitive performance. We therefore mention several more nuanced findings of cognitive ability in first-episode psychosis to provide a more complete picture. For instance, analysis of verbal learning in antipsychotic-naïve first-episode patients revealed significant impairment in both short and long-term memory recall (Hill et al. 2004). Memory impairment was associated with reduced use of organizational strategies to facilitate retrieval of words, based on semantic similarities (e.g. animals), suggesting that first-episode patients’ robust verbal memory impairment may, in part, be due to minimal use of memory-facilitating strategies as used by healthy controls. Another area of cognition not yet discussed is Jumping to Conclusions, a data-gathering bias measured as the amount of information needed for an individual to make a decision (McKay et al. 2006). Jumping to conclusions studies typically ask participants to determine which jar colored balls are being pulled from, when revealed one-by-one, with jars of different color ratios (e.g. 80:20 vs. 60:40). When tested in first-episode schizophrenia, 49% of first-episode participants demonstrated the jumping to conclusions bias, compared to 26% of controls, which was a significant difference. Participant IQ and delusional severity were significant predictors of jumping to conclusions and both IQ and working memory ability were significantly lower in first-episode subjects who jumped to conclusions, compared to those who did not. These data suggest that cognitive impairment contributes to a cognitive bias, which is associated with more severe psychotic symptoms in early stages of psychosis. Finally, in a study on prospective memory, first-episode patients were significantly impaired on both cue identification (identifying when they observed a cue that signaled a to-be-performed action) and intentional retrieval (retrieval of the intended action from long-term memory following the specified cue) (D. Liu et al. 2017). Cue identification impairments remained after controlling for working memory and retrospective memory.
Summary
First-episode schizophrenia is characterized by large deficits in overall cognitive ability. Analysis of specific cognitive domains reveals a similar pattern of impairment as seen in chronic schizophrenia, with comparable magnitude. Verbal memory and processing speed were most robustly impaired in first-episode patients, consistent with findings in both ultra-high risk and chronic stages (discussed below). These deficits are observed even in the absence of neuroleptics and prior to chronicity of illness, and therefore are not a byproduct of anti-psychotics or a result of degeneration as the illness progresses. Longitudinal studies suggest a stable cognitive profile following one’s first break. Data therefore suggests that global cognitive deficits are a core feature of schizophrenia that are present in close to their final form at the first-episode of psychosis.
Chronic Schizophrenia
At this point, there is no mystery regarding whether cognitive impairment is present in chronic schizophrenia. A robust and reliable cognitive deficit has been observed in up to 80% of patients with schizophrenia (Reichenberg et al. 2009) and a large literature has emerged working to elucidate the specificity of these deficits as well as their correlates. Here, we broadly review that literature.
Generalized Deficit
As previously mentioned, in 1998, Heinrichs and Zakzanis produced a striking meta-analysis of cognitive deficits in schizophrenia (Heinrichs and Zakzanis 1998). The data, analyzed across 204 studies and 22 cognitive variables, revealed an undeniable generalized deficit in chronic schizophrenia. Patients were significantly impaired for all cognitive variables compared to controls, with moderate-to-large effect sizes. These deficits were not only large in magnitude, but appear relatively stable over time. For instance, no significant differences in cognitive ability have been found between patients aged 18–70, suggesting that schizophrenia is characterized by static impairments and that cognitive ability does not degenerate as part of the disease process (Goldberg et al. 1993). Individuals who experience an unstable remission or are continually psychotic during the first year have lower overall cognitive scores than those who achieved remission, but this difference remains 10 years later (Rund et al. 2016). In fact, illness remission in the first year was the only significant predictor of cognitive course, not duration of untreated psychosis, symptom scores, or medication use. It appears that at the start of the illness a general cognitive deficit is present, without strong evidence for a steep decline in the context of aging.
The generalized deficit is therefore a stable feature of schizophrenia and has been further characterized in multiple studies. Shared variance in cognitive ability across 17 cognitive domains is the best predictor of diagnostic group (schizophrenia or healthy control), explaining 63.6% of the diagnosis-related variance (Dickinson et al. 2008). This generalized deficit factor has been further replicated across patients well-characterized for schizophrenia, schizoaffective disorder, and bipolar disorder with psychotic features, revealing that a single-factor model is the best fit for characterizing cognitive ability in psychosis (Hochberger et al. 2016). A common factor explained 53% of the variance in cognitive ability in schizophrenia and 50% in schizoaffective disorder, suggesting that the broad profile of cognitive impairment manifests as dysfunction shared across domains.
Specific Deficits
Within the context of a robust generalized deficit, researchers have explored whether pockets of specificity can be observed. Processing speed and social cognition have been found to best distinguish patients from controls above and beyond the generalized deficit (Kern et al. 2011). Furthermore, processing speed, visual learning, and attention/vigilance best distinguish patients with and without competitive employment, suggesting that impairments in these areas contribute to functional disability (Kern et al. 2011). Processing speed, and even more specifically digit symbol coding, has been found to be particularly sensitive to impairments in schizophrenia, leading some to suggest that processing speed represents a specific deficit in schizophrenia that contributes to the generalized deficit (Dickinson et al. 2007). Digit symbol coding’s sensitivity may be due to its reliance on multiple cognitive demands, including sustained attention, memory, motor speed, and strategy formation (Glosser et al. 1977). An analysis of digit symbol in schizophrenia revealed that, although patients are impaired on the test overall, they demonstrate difficulty in benefiting from predictable information about the target, suggesting that a deficit in relational memory fails to aid cognitive processing speed (Bachman et al. 2010).
Of all the psychotic disorders and stages of illness, chronic schizophrenia has been most extensively studied for cognitive specificity, with consortium such as the Cognitive Neuroscience Test Reliability and Clinical Applications (CNTRACS) working to identify cognitive neuroscience-driven paradigms for investigating mechanism within broad cognitive domains (Gold et al. 2012). While a comprehensive description of findings is not within the breadth of this review, several findings stand out. Under the large umbrella of executive functioning is cognitive control, the ability to maintain goal representation in the service of performing a specific task. Chronic patients are impaired in cognitive control tasks (Barch et al. 2003) and more specifically demonstrate a reliance on reactive versus proactive cognitive control (Barch and Ceaser 2012), indicating a “late correction” for behavior as opposed to a preparedness and active maintenance of goals. Deficits in cognitive control have been related to disorganized symptoms of schizophrenia (Lesh et al. 2013). Working memory is also a multifaceted construct, including encoding, visuospatial and verbal buffer systems, representation, and maintenance (Baddeley 2000). Schizophrenia patients demonstrate deficits in all of these areas, making specificity challenging, however there is some suggestion that the encoding, representation, and maintenance of information are driving the observed working memory deficits (Barch and Sheffield 2014). In addition to very early perceptual deficits in chronic schizophrenia, such as visual integration (S. M. Silverstein et al. 2012), early task-dependent processes have recently been shown to be impaired in schizophrenia. More specifically, rapid instructed task learning (RITL), the ability to rapidly transfer task instruction into goal-directed behavior, is reduced in schizophrenia, suggesting impaired learning processes (Sheffield et al. 2018). Again, this overview is sparse compared to the impressive literature on cognitive impairment in chronic schizophrenia, but provides insight into the rich application of cognitive neuroscience research to understanding the generalized cognitive deficit across the life course of schizophrenia patients.
Impact of Medications on Cognition
Associations between medications use, primarily anti-psychotics, and cognition has been of great interest to researchers, due both to their influence on the dopamine system (which is critical for cognitive functioning (Backman et al. 2010)) and the hope that medications might improve cognitive ability in schizophrenia. In one of the most robust tests of this question, Keefe and colleagues tested whether medication influenced cognitive ability in 817 schizophrenia patients in randomized double-blind treatment trials for anti-psychotics (R. S. Keefe et al. 2007). Patients received one of four medications for 18 months and after two months there was a small (approximately d=0.20) but significant improvement in cognitive ability for all of the anti-psychotics tested. While cognitive improvement was stable after two years, all areas of cognition improved in those first two months, with no specificity for any cognitive domain. Small, but positive influence of anti-psychotic use on global cognition has also been observed in early psychosis patients taking either haloperidol or second-generation antipsychotics over six months (Davidson et al. 2009). While there is some suggestion that first-generation anti-psychotics have negligible if not adverse impact of cognitive performance (Elie et al. 2010) with second-generation anti-psychotics having a more positive impact (Kane et al. 1988), a recent meta-analysis found no global differences in cognition for first- versus second-generation anti-psychotics (Nielsen et al. 2015). One major limitation in interpreting these findings is the impact of practice effects. Recently, practice effects were estimated to have an effect size of 0.18 using data from 813 psychotic disorder patients across multiple clinical trials (R. S. E. Keefe et al. 2017). This is a notably similar effect size to the previously reported change in cognition following two months of anti-psychotic treatment (R. S. Keefe et al. 2007) but is smaller than the medium effect sizes for medication effects reported by Davidson and colleagues (2009). Inclusion of healthy controls at each time point would help address the concern of practice effects, however neither study showing a positive effect of medications included a healthy comparison group. Overall, cognitive benefits of anti-psychotics in schizophrenia are mixed and modest at best (Hill et al. 2010) suggesting the need for adjunctive or alternative pharmacological treatments for meaningful improvement.
Summary
Together, findings across the lifespan of schizophrenia widely suggest cognitive deficits that can be observed as early as childhood, are robust in ultra-high risk individuals who develop the disorder, and are stable and severe from the first-episode of psychosis throughout the remainder of the life course (Figure 1). While deficits in verbal memory and attention may be early markers of abnormal psychiatric development, there is less specificity at the chronic stage, with the strongest evidence pointing to particularly robust deficits in processing speed. Cognitive deficits are minimally impacted by anti-psychotics and, as will be discussed in more detail below, contribute to the functional disability that takes such a large toll on this patient population. Cognitive deficits in schizophrenia appear to be neurodevelopmental in nature, and revelations about their development are a necessary step towards early intervention.
Bipolar Disorder
Premorbid cognitive functioning in bipolar disorder
In comparison to premorbid cognitive function in schizophrenia, fewer studies have been conducted on premorbid cognitive deficits in bipolar disorder. Of these studies, even fewer have examined premorbid impairments associated with bipolar disorder with psychotic features (Daban et al. 2006). In terms of research examining bipolar disorder in general (i.e., including bipolar disorder with and without psychotic features), studies largely fail to find evidence of significant premorbid cognitive impairments (Lewandowski et al. 2011a; Martino et al. 2015; Mollon and Reichenberg 2017; Parellada et al. 2017). For example, several studies failed to find premorbid IQ deficits among children and adolescents who subsequently developed bipolar disorder (Cannon et al. 2002; Sorensen et al. 2012; Zammit et al. 2004). Furthermore, there is evidence that higher cognitive functioning is associated with the development of bipolar disorder (Tiihonen et al. 2005; MacCabe et al. 2013). A significant limitation of the existing research is the lack of studies comparing bipolar disorder with and without psychotic features on premorbid cognitive functioning (Parellada et al. 2017).
In contrast to premorbid cognitive function in bipolar disorder generally, a mild global cognitive impairment exists in individuals with bipolar disorder with psychotic features, including evidence that the presence of psychotic features may worsen IQ deficits (Daban et al. 2006). In early onset bipolar disorder with psychotic features, research demonstrates premorbid IQ deficits (Paya et al. 2013). Similarly, individuals at high-risk for schizophrenia who later develop bipolar disorder show global cognitive deficits (Olvet et al. 2010; Ratheesh et al. 2013), and medium-sized impairments in processing speed and executive functioning are observed in the premorbid phase (Ratheesh et al. 2013). Retrospective examination of premorbid cognitive functioning in individuals who later developed bipolar disorder with psychotic features identified intermediate impairment between schizophrenia and controls, with no significant differences between either group (Seidman et al. 2013). However, 22.9% of individuals in this study who developed bipolar disorder with psychotic features showed evidence of premorbid cognitive impairment. Yet several studies have failed to find premorbid IQ deficits in bipolar disorder with psychotic features (Guerra et al. 2002; Simonsen et al. 2011; Zanelli et al. 2010). Mixed evidence also comes from a study finding that both low and high scholastic performance are associated with risk for developing bipolar disorder with psychotic features (MacCabe et al. 2010). Taken together, research more consistently points to mild premorbid global cognitive impairments in bipolar disorder with psychotic features in comparison to premorbid cognitive function in bipolar disorder generally.
Comparison with Premorbid Cognitive Function in Schizophrenia
Research indicates that schizophrenia and bipolar disorder with psychotic features are distinguished by the degree of premorbid impairment, with schizophrenia showing more severe premorbid cognitive impairments than bipolar disorder (Figure 1) (Daban et al. 2006; Trotta et al. 2015). Even among the studies finding premorbid cognitive impairments in bipolar disorder with psychotic features, these impairments are generally less severe than found in premorbid schizophrenia (Daban et al. 2006; Seidman et al. 2013; Kendler et al. 2016). Although schizophrenia is associated with greater premorbid IQ deficits than bipolar disorder in several studies (Simonsen et al. 2011; Zanelli et al. 2010), others have failed to find this association (Olvet et al. 2010; Seidman et al. 2002). Thus, while bipolar disorder with psychotic feature is more consistently associated with premorbid impairments than in bipolar disorder generally, these impairments are generally less severe than premorbid cognitive deficits in schizophrenia.
Mixed Findings on Premorbid Cognitive Function in Bipolar Disorder
Potential reasons for these mixed results regarding the presence of premorbid cognitive impairments in bipolar disorder include both small sample sizes as well as differing findings depending on how the sample (i.e., conscript, birth cohort, or high-risk) was collected (Daban et al. 2006; Trotta et al. 2015). Furthermore, premorbid cognitive impairments in bipolar disorder may be restricted to individuals with earlier onset of the disorder (Parellada et al. 2017) and therefore more severe versions of the disorder (i.e., psychotic symptoms; earlier onset). One theory that potentially helps explain the limited evidence for premorbid cognitive deficits in bipolar disorder is termed the staging model, which theorizes that as symptoms of the illness arise, cognitive functioning deteriorates, and these cognitive deficits are compounded by the effects of number of episodes, life stress, and illness progression (Kapczinski et al. 2009). However, not all longitudinal studies of cognitive functioning in bipolar disorder support the notion of deteriorating cognitive functioning over the course of the disorder. Several studies have observed static cognitive deficit trajectories in the bipolar disorder (Samame et al. 2014; Bora and Ozerdem 2017; Martino et al. 2018), and several studies demonstrate cognitive improvements after the first manic episode (Torres et al. 2014; Torrent et al. 2018). It is also plausible that premorbid cognitive functioning impairments in bipolar disorder are associated with specific genetic variants (Arts et al. 2013; Bryzgalov et al. 2018; Flowers et al. 2016) that may be associated with neural development (Tabares-Seisdedos et al. 2008) and thus may not be present in all individuals premorbidly. Overall, the heterogeneity of findings regarding the presence of premorbid cognitive deficits in bipolar disorder during childhood and adolescence points to the necessity that future studies incorporate consistent methodology using large samples to better understand whether premorbid deficits exist in bipolar disorder.
First-episode bipolar disorder
Deficits occur across the spectrum of cognitive domains early after the onset of bipolar disorder with psychotic features, including in samples of first-episode bipolar disorder with psychotic features (FEBP+) and recent onset psychosis diagnosed with bipolar disorder (Barrett et al. 2009; Dickerson et al. 2011; Reichenberg et al. 2009; Zabala et al. 2010). These cognitive impairments have generally been localized to attention, processing speed, verbal learning/memory, and executive functioning (Albus et al. 1996; Daglas et al. 2016; Demmo et al. 2016; Elshahawi et al. 2011; Hellvin et al. 2012; Trisha et al. 2017). There is also cumulating evidence for impairments in verbal memory and fluency early in the course of bipolar disorder, including in samples of FEBP+ (Albus et al. 1996; Daglas et al. 2016; Demmo et al. 2016; Elshahawi et al. 2011; Hellvin et al. 2012; Trisha et al. 2017), majority (~70%) FEBP+ samples (Muralidharan et al. 2014; Torres et al. 2014), samples with recent onset psychosis diagnosed with bipolar disorder (Ayres et al. 2007; Zanelli et al. 2010), as well as samples of early onset bipolar disorder with psychotic features (McClellan et al. 2004). Impairments in executive functioning are also consistently observed, with impairments demonstrated in FEBP+ (Albus et al. 1996; Daglas et al. 2016; Demmo et al. 2016; Elshahawi et al. 2011; Hellvin et al. 2012; Trisha et al. 2017) and majority (~70%) FEBP+ samples (Muralidharan et al. 2014; Torres et al. 2014). Executive functioning impairments have been found most consistently in cognitive flexibility (Daglas et al. 2015; Trisha et al. 2017), with these impairments correctly classifying the bipolar diagnosis of 80% of patients with first-episode psychosis (Pena et al. 2011).
Bipolar Disorder With and Without Psychotic Features
While research consistently indicates that FEBP+ is associated with cognitive impairments, there is a lack of consensus regarding the relative severity of FEBP+ impairments compared to other forms of bipolar disorder. For example, some studies indicate that FEBP+ has less severe cognitive deficits in comparison to multiple-episode patients (Elshahawi et al. 2011; Hellvin et al. 2012), especially regarding attention and executive function (Elshahawi et al. 2011) and the presence of prior depressive episodes does not confer additional cognitive impairments (Muralidharan et al. 2014). There is also mixed evidence regarding whether FEBP+ exhibits significantly greater impairments compared to bipolar disorder without psychotic features (FEBP-), with some studies demonstrating similar severity of neuropsychological deficits (Demmo et al. 2016; Lewandowski et al. 2011a; Trisha et al. 2017), and others finding greater impairments in FEBP+ (Albus et al. 1996). Thus, FEBP+, if anything, appears to be intermediate in severity between FEBP- and chronic bipolar disorder, although additional research should attempt to resolve disparities in these findings (Bora and Pantelis 2015), including the effect of depressive symptoms on FEBP+.
Bipolar Disorder Compared to Schizophrenia
The majority of research indicates that first-episode schizophrenia shows greater cognitive impairments than FEBP+ (Figure 1) (Mojtabai et al. 2000; Dickerson et al. 2011; Reichenberg et al. 2009; Woodward 2016; Zanelli et al. 2010). First-episode schizophrenia is associated with greater impairments on most cognitive measures, including measures of verbal memory, executive functioning, and processing speed (Barrett et al. 2009; Demmo et al. 2016; Hill et al. 2009). Yet there is some evidence for similar severity in deficits between schizophrenia and FEBP+ (Albus et al. 1996; Zabala et al. 2010; Ayres et al. 2007; McClellan et al. 2004), especially in processing speed and attention (Albus et al. 1996; Dickerson et al. 2011). Taken together, the synthesis of this research indicates that any differences in cognitive impairments between schizophrenia and FEBP+ are quantitative rather than qualitative in nature (Hill et al. 2009; Mojtabai et al. 2000; Pena et al. 2011; Reichenberg et al. 2009; Zanelli et al. 2010).
Specific Deficits
Nuanced studies of cognitive dysfunction in first-episode bipolar disorder with psychotic features is notably limited, as many studies including affective psychosis combine patient groups to study the broader category of “first episode psychosis”. Impaired semantic verbal fluency has been observed in first episode psychotic bipolar patients in the context of intact cognitive functioning over a wide range of cognitive domains (e.g. processing speed, working memory, executive functioning) (Kravariti et al. 2009). On an antisaccade task, increased error rates were observed in a large group of first episode psychosis patients, but when separated by diagnostic group, bipolar disorder patients did not demonstrate significant impairment, as this finding was largely driven by the schizophrenia group (Harris et al. 2009). Finally, cognitive flexibility is an area of particular deficit in first episode psychotic bipolar disorder patients compared to bipolar patients without a history of psychosis (Trisha et al. 2018). There is much room for growth in the understanding of specific cognitive deficits in first episode psychotic bipolar disorder. One limitation to studying first episode subjects is the difficulty in knowing whether a truly bipolar illness will manifest, as opposed to schizoaffective disorder or an affectively-charged schizophrenia; however as larger longitudinal studies take shape, analysis of first episode bipolar disorder will help elucidate aspects of cognition more vulnerable to decline over the course of illness.
Summary
While the findings point to significant mild to moderate cognitive impairments across a broad range of cognitive domains in FEBP+, questions remain regarding the contributions of several clinical variables to these cognitive impairments. For example, stable impairments in cognitive functioning after first-episode treatment have been observed (Demmo et al. 2017; Hill et al. 2009), while other research shows improvements after first-episode remission (Torres et al. 2014), including in one test of processing speed (Daglas et al. 2016). These improvements are above-and-beyond practice effects observed in the comparative healthy control groups tested at similar time intervals, as reflected in significant group by time interactions. Several methodological issues may contribute to the heterogeneity of findings, including a lack of consistency regarding what is considered first-episode with psychotic features (Demmo et al. 2016). As previously mentioned, these samples varied in inclusion criteria for first-episode, from a first manic episode with psychotic features (Demmo et al. 2016; Elshahawi et al. 2011; Trisha et al. 2017), to first hospital admission for psychotic symptoms (Barrett et al. 2009; Pena et al. 2011; Zanelli et al. 2010). Furthermore, samples varied on several characteristics, including active symptomology (versus remission), duration of illness, and the number of untreated depressive episodes.
Chronic bipolar disorder
In comparison to premorbid and first-episode cognitive functioning, by far the greatest amount of research has been conducted on chronic bipolar disorder. Similar to FEBP+, chronic bipolar disorder with psychotic features (CBP+) is associated with impairments across the gamut of cognitive domains (Bora 2017; Bora et al. 2010b; Vohringer et al. 2013), especially in attention, executive function, and memory (Glahn et al. 2007; Jenkins et al. 2017; Ozdel et al. 2007). In comparison to FEBP+, more studies have examined domains of impairment specifically associated with psychotic features in chronic bipolar disorder, with some finding that verbal learning/memory and aspects of executive functioning may be specific trait markers of CBP+ (Bora et al. 2009; Martinez-Aran et al. 2008), with these domains typically showing the largest effect sizes (Bora et al. 2010b). Impairments in verbal learning/memory are typically seen in CBP+ (Bora 2017; Bora et al. 2009, 2010a, 2010b), with verbal memory impairment relating to global functioning, longer duration of illness, and a higher number of manic episodes (Martinez-Aran et al. 2004). However, verbal memory deficits may be a marker for bipolar disorder more generally, as opposed to specifically related to CBP+ (Aminoff et al. 2013; Bora et al. 2007).
There is also significant evidence for impairments in executive functioning in CBP+ (Bora et al. 2007; Ozdel et al. 2007; Selva et al. 2007), and these impairments may be specific to those with psychotic features (Bora et al. 2007). Two aspects of executive functioning implicated in CBP+ are cognitive flexibility (Bora et al. 2007; Savitz et al. 2009) and working memory (Bora 2017; Bora et al. 2010b; Glahn et al. 2007; Jimenez-Lopez et al. 2017; Kim et al. 2015), although sometimes these impairments show modest (e.g.,Cohen’s d=.29) effect sizes (Bora 2017; Jimenez-Lopez et al. 2017). Working memory impairments in CBP+ may also be localized to more demanding working memory measures (Frydecka et al. 2014). One study demonstrated that working memory impairments are specifically related to the presence of hallucinations (Jenkins et al. 2017).
Bipolar Disorder With and Without Psychotic Features
There is some empirical support for greater cognitive impairments in CBP+ in comparison to chronic bipolar disorder without psychotic features (CBP-). While a few studies that have found that CBP+ and CBP- show similar cognitive profiles (Ancin et al. 2013; Savitz et al. 2009), the majority of studies demonstrate more severe cognitive impairments in CBP+ as compared to CBP- (Bora 2017; Frydecka et al. 2014; Levy and Weiss 2010; Simonsen et al. 2011). Importantly, more severe cognitive impairments in CBP+ exist in the domains of verbal memory/learning and executive functioning (Levy and Weiss 2010), supporting the notion that mechanisms underlying CBP+ may be partially independent of those for bipolar disorder more generally (Glahn et al. 2007). Thus, the presence of a history of psychotic symptoms in bipolar disorder may represent either a more severe subtype of bipolar disorder (Simonsen et al. 2011), or a separate nosological identity, as CBP+ is associated with a younger onset of illness (Bora et al. 2010b) and poorer outcome (Bora et al. 2010a; Torres et al. 2007).
Bipolar Disorder Compared to Schizophrenia
Schizophrenia shows greater cognitive impairments compared to CBP+ (Figure 1). Despite some evidence of similar performance across neurocognitive measures (Ivleva et al. 2012; Jimenez-Lopez et al. 2017), studies generally find that schizophrenia is associated with greater cognitive impairments than CBP+ (Hill et al. 2013; Sperry et al. 2015; Kim et al. 2015; Sheffield et al. 2012; Vohringer et al. 2013). For example, in comparison to 57.7% of psychotic bipolar patients, 84% of schizophrenia patients were cognitively impaired (Reichenberg et al. 2010). Yet as with FEBP+, even among studies finding more severe impairments in schizophrenia compared to CBP+, most studies find that the groups differ in magnitude, but not pattern, but impairment (Barch and Sheffield 2014; Van Rheenen et al. 2016; Jimenez-Lopez et al. 2017; Sheffield et al. 2012).
Specific Deficits
Investigation of specific deficits in psychotic bipolar disorder pales in comparison to the breadth of studies in chronic schizophrenia, but databases such as the Bipolar and Schizophrenia Spectrum on Intermediate Phenotypes (B-SNIP) are helping to fill this gap (Keshavan et al. 2011). For instance, in a task of context processing, psychotic bipolar patients demonstrated reduced target sensitivity and increase false alarms, largely due to impaired response inhibition (Reilly et al. 2017), which was specific to bipolar patients compared to schizophrenia or schizoaffective disorder. In an executive function task of set-shifting, psychotic bipolar disorder patients had increased rates of regressing to a previously established response (i.e., regressive errors) (Hill et al. 2015). Importantly, while increased perseverative errors were also observed in psychotic bipolar disorder, only regressive errors remained a significant deficit after controlling for general cognitive ability, suggesting a specific deficit within the larger impairment of executive functioning. Within the realm of working memory, psychotic bipolar participants demonstrate impairment for high-demand, but not low-demand measures, suggesting intact working memory functioning that breaks down as demand increases (Frydecka et al. 2014). Finally, in a study of dichotic listening, in which individuals had to focus attention on auditory stimuli in one ear or the other to guide behavior, psychotic bipolar patients were significantly impaired and demonstrated performance similar to patients with schizophrenia (Bozikas et al. 2014). Interestingly, this impairment was present at the start and end of their hospitalization, indicating the presence of this cognitive deficit in the context of symptom improvement.
Summary
Research generally points to cognitive impairments in CBP+ being trait-like features present even during periods of symptom remission (Bora et al. 2010b; Glahn et al. 2007). There is cumulating research that cognitive deficits in chronic bipolar disorder may be stable over time, including from elderly chronic bipolar disorder populations, which failed to find evidence substantiating accelerated cognitive decline (Schouws et al. 2012). However, more empirical studies need to be conducted specifically examining the longitudinal course of cognitive deficits in CBP+, as well as the contribution of several factors to cognitive impairment, including the contribution of chronic antipsychotic medication usage (Donaldson et al. 2003). Overall, it appears that bipolar disorder with psychotic features is intermediate on the spectrum of cognitive impairment between bipolar disorder without psychotic features and schizophrenia, with evidence of increasing cognitive impairments, including in verbal abilities and executive functioning, as the disorder progresses from early to chronic stages (Figure 1).
Major Depressive Disorder, with Psychotic Features
In the United States, approximately 14% of individuals diagnosed with Major Depressive Disorder report psychotic experiences (Johnson et al. 1991). Depression with psychotic features signifies individuals who endorse delusions or hallucinations during, but not independent of, a depressive episode. Although psychotic features are a diagnostic specifier in the DSM-5, there is debate about whether depression with psychotic features represents a distinct psychiatric disorder (Keller et al. 2007). Those with psychotic features have been found to experience more frequent and severe psychomotor abnormalities (Glassman and Roose 1981), longer episodes of depression (Coryell et al. 1987), and greater likelihood of depression recurrence (Aronson et al. 1988) compared to those with non-psychotic depression. An additional feature of psychotic depression that distinguishes it from non-psychotic depression is the profile of cognitive impairment.
First-episode Psychotic Depression
To our knowledge there is no research on individuals at high risk for psychotic depression or premorbid cognition functioning in psychotic depression. However, even at their first-episode of psychosis, psychotic depression patients have deficits in full-scale IQ and demonstrate impairments in verbal learning, category fluency, and Trails B even after adjusting for IQ (Zanelli et al. 2010). Across 16 tasks, effect sizes range from −0.2 to −0.9 compared with healthy controls, but psychotic depression’s cognitive profile is comparable to individuals from other psychotic disorder groups, indicating a level of severity similar to first-episode schizophrenia (Zanelli et al. 2010). Hill and colleagues also observed a broad impairment in psychotic depression, in a small sample of antipsychotic naïve first-episode psychotic depression patients (Hill et al. 2004). Similar to schizophrenia, psychotic depression patients were impaired in all areas of cognition including visual memory, verbal memory, and spatial abilities.
Chronic Psychotic Depression
In more chronic stages of psychotic depression, a global deficit emerges. For instance, psychotic depression participants have been shown to be significantly impaired on tests of attention, working memory, executive functioning, verbal memory recall (both immediate and delayed), and delayed logical memory recall and recognition, although they demonstrate intact simple attention (Gomez et al. 2006). Effect sizes between −0.82 and −1.86 have also been observed on Trails B, WAIS Vocabulary, WAIS Block Design, Stroop, Paragraph Recall Test, and immediate visual memory when compared to healthy controls (Schatzberg et al. 2000). Although both studies had relatively small sample sizes (<30 in each group), these data support the notion that psychotic depression participants have a fairly generalized cognitive impairment in relation to non-psychiatric controls.
MDD With and Without Psychotic Features
Research in the field of psychotic depression has not only addressed cognitive ability compared with healthy subjects, but has also explored cognitive ability in psychotic depression compared with non-psychotic depression. This question works to isolate the contribution of psychosis to the cognitive profile, as individuals with non-psychotic depression also experience global cognitive impairment (Goodall et al. 2018). One meta-analysis of five studies found that psychotic depression patients were impaired in all areas of cognition when compared to non-psychotic depression (effect sizes ranging from −0.37 to −0.73) (Fleming et al. 2004). Areas of cognition that revealed the strongest impairment in psychotic depression were verbal memory, executive functioning, and processing speed. A more recent meta-analysis that included 12 studies observed a significant global cognitive impairment in psychotic depression compared with non-psychotic depression, as calculated by a summary measure of effect sizes across all domains (Zaninotto et al. 2015). Within the context of a global impairment, significant specific deficits in verbal learning, visual learning, and processing speed were observed. Overall, these meta-analyses reveal that the presence of psychosis in depression contributes to greater cognitive impairment than the experience of depression without psychotic features.
Further analysis of psychotic depression compared with non-psychotic depression has revealed the impact of medication use and cortisol on cognitive impairment. For instance, the Zaninotto meta-analysis found a greater global impairment in drug-free psychotic depression than was observed in the whole psychotic depression sample, suggesting that untreated psychotic depression is associated with greater cognitive impairment than psychotic depression that is treated with medications (“medications” varied across studies and included anti-depressants, anti-psychotics, mood stabilizers, and anxiolytics). This finding may also be influenced by anti-depressant use in the medicated patients, as anti-depressants have a modest, positive effect on a wide range of cognitive domains (e.g. processing speed, attention, memory) (Prado et al. 2018). Gomez and colleagues (2006) measured cortisol levels in psychotic depression, non-psychotic depression, and healthy participants and analyzed whether cortisol levels were associated with cognitive functioning (Gomez et al. 2006). This investigation was based on previous research revealing associations between higher cortisol and lower cognitive ability in depression (O’Brien et al. 1996) as well as hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis in psychotic depression (Belanoff et al. 2001) – a biological system that regulates the production of cortisol. Psychotic depression participants performed significantly worse than non-psychotic depression participants on measures of working memory, executive functioning, processing speed, and verbal memory. Cortisol levels were found to be elevated in psychotic depression and correlated negatively with verbal memory and processing speed across all subjects. This finding was recently replicated and extended to implicate genetic variation in psychotic depression as a mechanism for the relationship between cortisol and cognitive performance (Keller et al. 2017).
Summary
Although more work is needed, current evidence in psychotic depression reveals a broad profile of cognitive impairment when compared to both healthy subjects as well as patients with non-psychotic depression (Figure 2). There is some suggestion from meta-analyses that processing speed, verbal memory, and executive functioning are most impaired in the context of psychotic depression. The majority of studies match psychotic depression and non-psychotic depression participants on endogenous depressive symptoms, reducing the likelihood that the more severe cognitive impairment observed in psychotic depression is due to more severely depressed mood. In fact, in DSM-5, severity of depression and presence of psychotic features have been separated, reflecting that psychotic symptoms are not simply a manifestation of exceptionally depressed mood. Instead, psychotic features in the context of depressed mood are associated with a broad cognitive impairment that is present at the first-episode of psychosis, is potentially less severe with appropriate treatment, and may be related to elevated cortisol.
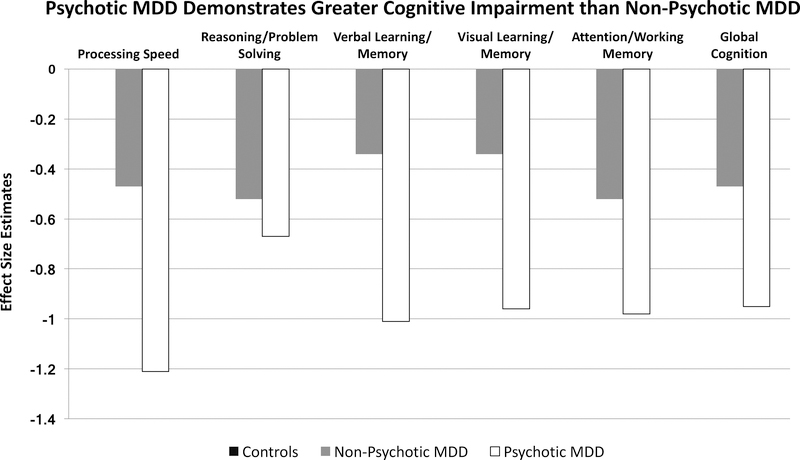
Figure 2:
Illustration of cognitive deficits in MDD patients with and without psychotic symptoms. While non-psychotic MDD patients demonstrate deficits in cognition, those with psychotic symptoms exhibit even greater deficits in all areas of cognition. This pattern suggest that the presence of psychosis contributes to greater cognitive impairment. Estimates based on data from Bora et al., 2013; Rock et al., 2014; Zaninotto et al., 2015
Psychotic-Like Experiences
A small literature exists on cognitive deficits in individuals who endorse psychotic-like experiences. These individuals exemplify how conceptualization of symptoms as dimensional can yield richer understanding of brain-behavior relationships. Psychotic-like experiences are often self-reported experiences of perceptual abnormality and/or reality distortion in the general population that, by definition, cause minimal functional difficulties (van Os et al. 2009). Due to the robust findings of cognitive impairment in primary psychotic disorders, researchers have started investigating cognitive functioning in those who endorse psychotic-like experiences.
In one of the first studies to directly ask whether cognition is intact in individuals who endorse psychotic-like experiences, Barnett and colleagues (2012) utilized a population-based sample in Great Britain of 2918 participants (Barnett et al. 2012). Of those, 22% reported psychotic-like experiences at age 53, and those who reported psychotic-like experiences had poorer childhood cognitive test scores at ages 8 and 15. Cognitive deficits were also associated with greater general health problems as reported on a self-report questionnaire at age 53, however this association was not significant after controlling for psychotic-like experiences, suggesting that childhood cognitive deficits are a specific risk factor for the experience of psychosis in adulthood. Presence of sub-clinical psychosis has been associated with processing speed deficits as measured by the digit symbol task (Rossler et al. 2015). In data from the Human Connectome Project, 21.6% of individuals endorsed at least one psychotic-like experience, and psychotic-like experiences were significantly negatively associated with general cognitive ability, replicating previous findings (Sheffield et al. 2016). Further, individuals who endorse symptoms of schizotypy in the general population have below average cognitive performance (Dinn et al. 2002) (Park et al. 1995).
Research in psychotic-like experiences, although in a relatively early stage, suggests that cognitive impairment can be observed even in individuals from the general population who experience subtle, non-impairing psychotic experiences. These data support the research domain criteria conceptualization of psychosis-risk as being related to dimensional impairments in global cognitive functioning.
Functional Implications for Cognitive Impairment in Psychotic Disorders
A primary interest for understanding cognitive deficits across the psychosis spectrum is understanding their role in functional impairment. Functional limitations are a critical facet of diagnosing a mental health disorder, and psychotic disorders are among the most disabling (Rossler et al. 2005), causing significant burden to families and society at large (Ohaeri 2003). Understanding illness-related factors that contribute to functional disability is of great interest, as it carries the hope of specific interventions to support patients in achieving greater independence. Somewhat surprisingly, severity of positive psychotic symptoms is minimally associated with functional outcome (e.g (Tabares-Seisdedos et al. 2008)), suggesting that delusions and hallucinations are not the primary barrier to work and social functioning. In fact, of those who were admitted to a hospital for first-episode psychosis, 65% of those who achieved syndromal recovery (i.e. resolution of symptoms) failed to achieve functional recovery (Tohen et al. 2000). Instead of pointing to symptom resolution as a marker of functioning, research consistently demonstrates a critical role of cognitive deficits in functional capacity.
Cognition and Function Status in Schizophrenia
Relationships between functional outcome and cognitive ability can be seen even in individuals at high-risk of developing psychosis, suggesting they shape the trajectory of that individuals’ capabilities prior to disease onset. Poor verbal memory has been associated with poor concurrent social functioning in ultra-high risk individuals (Niendam et al. 2006) as well as poor functional outcome 3–5 years (Carrion et al. 2013) and 13 years (Lin et al. 2011) after assessment of memory. Processing speed, attention, and verbal fluency measures at baseline also predict functioning several years later (Lin et al. 2011) (Carrion et al. 2013), both in the realms of social functioning and role functioning. In first-episode psychosis, patients considered to have “good” premorbid adjustment show better overall cognitive functioning (Rabinowitz et al. 2002); however premorbid functioning is not a perfect predictor of psychotic disorder status, as 40% of ultra-high risk subjects identified as having poor functional outcome did not transition to having a primary psychotic disorder (Carrion et al. 2013).
In chronic schizophrenia, cross-sectional work has also revealed associations between cognitive capacity and current functioning. For instance, functional capacity as measured by a performance-based skills test is associated with relational memory, cognitive control, and processing speed abilities, while verbal memory is associated with self-reported functioning (Sheffield et al. 2014). Associations between cognitive performance and real-world functioning appear to be mediated by functional capacity. Patients with poor cognitive ability have poorer functional capacity, which predicts more impaired real-world functioning in three areas (interpersonal skills, work skills, and community activities) (Bowie et al. 2006). Therefore, cognitive deficits in schizophrenia appear to contribute to functional impairment through deficits in the ability to perform skills of daily living.
Longitudinal studies have further exposed cognitive capacity as a robust predictor of future functional outcome. A review of longitudinal studies from Green and colleagues (2004) described medium effect sizes (Cohen’s d=.50) for the relationship between cognitive constructs and functional outcome in schizophrenia (Green et al. 2004). In these studies, cognitive ability predicted the rate of improvement in work performance (Bell and Bryson 2001) (Bryson and Bell 2003), change in social and community outcomes (Dickerson et al. 1999) and the number of hours worked 12 and 24 months after cognitive testing (Gold et al. 2002). Baseline cognition predicted activities of daily living (ADLs) four years later, and change in cognition was related to change in ADLs. Further, attention, working memory, and verbal fluency predicted community outcome, as measured by work/school functions and independent living (Jaeger et al. 2003). The relatively stable cognitive deficits observed in schizophrenia are therefore predictive of functional outcomes in a range of areas, including work, social functioning and independent living across the course of illness.
Cognition and Functional Status in Affective Psychosis
Cognitive deficits also have functional implications in affective disorders, although few studies have investigated this association in affective psychosis specifically. In bipolar disorder generally, social functioning deficits are associated with deficits in planning and problem solving, suggesting a role of executive difficulties in problems with daily living (Laes and Sponheim 2006). Change in cognitive scores over the course of one year predicted functioning changes over that follow-up period, as did the overall deficit in processing speed (Tabares-Seisdedos et al. 2008). Interestingly, the majority of studies assessing cognition and functioning in bipolar disorder have implicated verbal memory as a particularly strong predictor of functional outcome (Sanchez-Moreno et al. 2009). For instance, across 12 studies, 50% of them revealed verbal memory ability as a significant predictor of psychosocial functioning, and in several of those studies, verbal memory was the only cognitive domain associated with functional outcome (Wingo et al. 2009). Additionally, low-functioning euthymic bipolar patients had significantly lower verbal memory and executive functioning compared to high-functioning bipolar patients (Martinez-Aran et al. 2007). In a study on depression, patients whose cognition worsened from baseline to 6-month follow-up represented those patients who remained significantly disabled at 6-months (Jaeger et al. 2006), although it is unclear how many of those patients suffered from psychotic symptoms.
Cognitive deficits across the psychosis spectrum have critical implications for patients’ ability to function in their daily life. In all psychotic disorders, cognitive deficits are associated with poor current functioning, but also predict functional outcome up to 13 years later. The longevity and predictability of these functional impairments may not be surprising, given the chronicity and stability of cognitive deficits across the course of illness. The presence of cognitive deficits prior to first-episode, and association between those early cognitive abilities and functioning, further highlights the importance of intervening on cognitive impairment early, to help these individuals achieve and maintain a higher lifetime level of functioning.
Discussion
In this review, we outline decades of research indicating the presence of cognitive deficits in psychotic disorders across the life course. Considering these findings as a whole, we document robust, reliable, and stable cognitive impairment in chronic psychosis, regardless of affective status, suggesting that psychotic disorders are incontrovertibly associated with cognitive dysfunction (Figure 1). Differences among diagnostic categories emerge when considering timing and magnitude, and possibly specificity, although the data is somewhat mixed. In schizophrenia, IQ deficits are observed during childhood, become more severe during adolescence, and reach a stable level of impairment (approximately 1 standard deviation below the norm for global cognition) by the first-episode of psychosis. This general impairment remains consistent during the chronic state, contributes to functional impairment, and is minimally influenced by psychiatric medications. In affective psychosis, there is some evidence of mild global impairment during childhood (particularly for those later developing psychotic bipolar disorder), but these premorbid impairments are less severe than in schizophrenia. By the first-episode of psychosis, bipolar and depression patients demonstrate a global deficit similar in pattern to schizophrenia, but less severe in magnitude (Figure 1). In schizophrenia, there is some evidence of specific impairments in verbal memory and processing speed, while affective psychosis may demonstrate greater impairment in the domains of verbal memory and executive functioning; however these deficits exist in the context of a generalized deficit across all psychotic disorders and even in those experiencing psychotic-like experiences.
Covariation Between Cognitive Deficits and Psychotic Symptom Severity
Surprisingly, despite the clear co-occurrence of psychotic symptoms and cognitive impairment, there is little evidence to suggest that severity of psychotic experiences is directly associated with severity of cognitive deficits (e.g. (Bell et al. 1994; Heydebrand et al. 2004)). Global IQ, as well as performance on tasks of cognitive flexibility, verbal fluency, and processing speed, do not demonstrate significant linear associations with positive symptoms, suggesting that those with more frequent and severe delusions and hallucinations are not those also experiencing the worst cognitive impairment (Bell et al. 1994; Berman et al. 1997) (Heydebrand et al. 2004). The lack of association between severity of cognitive and psychotic symptoms is elusive, and diminishes the frequent assumption that presence of psychotic symptoms impairs performance on cognitive tasks. Instead, data suggests that negative symptoms are more closely related to cognitive ability (Berman et al. 1997) (Heydebrand et al. 2004; Harvey et al. 2006), as well as functional outcome (Evans et al. 2004), possibly related to impaired reward processing (Barch and Dowd 2010); although these data are largely specific to schizophrenia. While it is not in the scope of this review to explore mechanism, the lack of relationship between psychotic symptoms and cognitive impairment is notable. The timing of when these symptoms arise may provide some insight into their relationship, as cognitive impairment in schizophrenia appears prior to onset of psychotic symptoms, suggesting that presence of positive symptoms does not directly cause cognitive impairment.
Non-affective Versus Affective Psychosis
When considering timing of cognitive and psychotic symptoms, there is a notable discrepancy between affective and non-affective disorders. Schizophrenia patients demonstrate a fairly reliable deficit in IQ during childhood, well before their first-episode of psychosis. Bipolar and depression patients, however, appear to develop cognitive deficits closer to their first-episode, showing much less robust premorbid impairments. These differences in cognitive development have contributed to two hypotheses: 1) that schizophrenia is a disorder of neurodevelopment, and 2) that bipolar disorder takes a neurodegenerative course (Lewandowski et al. 2011b). These hypotheses are further bolstered by the fact that first-episode bipolar patients demonstrate less impaired cognition than first-episode schizophrenia, but in the chronic state, these differences are not as strong. Differential timing of cognitive impairment provides a marker of different disease states, and begs the question of whether the mechanism that causes or contributes to cognitive impairment in both affective and non-affective psychosis is the same, just with different timing, or whether cognitive impairment is simply a shared phenotype with distinct etiologies. Working to elucidate this question are large-scale studies such as the Philadelphia Neurodevelopmental Cohort (Gur et al. 2014), which addresses early development of psychotic disorders, and the Bipolar and Schizophrenia Network on Intermediate Phenotype (B-SNIP) (Hill et al. 2013), which includes large cohorts of chronic patients with both affective and non-affective psychosis. Studies of this nature allow for the continued analysis of differential and shared phenotypes, including possible neurobiological mechanisms underlying cognitive impairment across the psychosis spectrum.
Improving Cognitive Function Across the Spectrum of Psychotic Disorders
Finally, given the clear presence of cognitive deficits across the psychosis spectrum, and its contribution to functional impairment, how can cognition be improved? As noted earlier, anti-psychotic medications have minimal efficacy in improving cognitive ability (Nielsen et al. 2015), leading many to consider non-pharmacological alternatives. Cognitive remediation is the leading avenue of research, in the hopes that systematic training in cognitive tasks might improve cognitive functioning. To date, the results are mixed. In chronic patients, meta-analysis reveals small to moderate improvement in global cognition, which appears stable over follow-up (Wykes et al. 2011). Cognitive remediation is most effective when adjunctive rehabilitation support is provided, particularly with supportive, as opposed to drill and practice, remediation protocols. In first-episode patients, meta-analysis demonstrates smaller, non-significant effect sizes for general cognition, possibly due to a small number of trials with fewer participants than is seen with chronic subjects (Revell et al. 2015). Cognitive remediation is getting more attention for use in bipolar disorder (Miskowiak et al. 2018), but current results on efficacy are mixed (Demant et al. 2015; Bonnin et al. 2016). In general, cognitive remediation represents an important avenue of continued research, and more data is needed in ultra-high risk subjects to assess efficacy of remediation on buffering the effects of first-episode psychosis. Combination of cognitive and functional remediation is also a promising direction (Torrent et al. 2013), providing a more comprehensive approach to cognitive and functional improvement. Lastly, there is also a growing body of research examining the effects of aerobic exercise on cognitive function in psychosis (Falkai et al. 2017) (Su et al. 2016; Firth et al. 2017). This research suggests that regular aerobic exercise improves global cognition in schizophrenia, possibly through the mechanism of increased brain volume, particularly within the hippocampus. The most effective duration, frequency, and type of exercise is still being investigated, as is the persistence of cognitive improvement following completion of the exercise intervention.
Summary
Cognitive impairment is a dimensional phenotype present across the psychosis spectrum. Although not linearly related in terms of severity, there is no doubt that the presence of or vulnerability to psychotic experiences confers risk for cognitive deficits. In terms of development, schizophrenia patients exhibit cognitive impairment during childhood that continues to decline, and then stabilize, during the chronic state. Bipolar patients, on the other hand, demonstrate minimal impairment early on in the life course, but show deficits by the first-episode, that appear to worsen as the disease progresses. Across psychotic disorders, a global deficit is present, with impairments apparent in nearly all areas of cognition. Assessment of domain specificity within psychotic disorders, on a backdrop of global impairment, reveals the largest deficit in processing speed in schizophrenia (e.g. Dickinson et al., 2007) and executive functioning in bipolar disorder (e.g. Bora et al., 2010b) as well relatively greater impairment in verbal memory across all groups (Mesholam-Gately et al., 2009; Daglas et al.,2016; Gomez et al., 2006). Research on associations between psychotic symptoms and cognitive impairment, differential developmental mechanisms across affective and non-affective psychosis, and treatment options for cognitive impairment are important steps for improving the lives of individuals with psychosis.
References
- Addington J, & Addington D (2005). Patterns of premorbid functioning in first episode psychosis: relationship to 2-year outcome. Acta Psychiatr Scand, 112(1), 40–46, doi: 10.1111/j.1600-0447.2005.00511.x. [DOI] [PubMed] [Google Scholar]
- Albus M, Hubmann W, Wahlheim C, Sobizack N, Franz U, & Mohr F (1996). Contrasts in neuropsychological test profile between patients with first-episode schizophrenia and first-episode affective disorders. Acta Psychiatr Scand, 94(2), 87–93. [DOI] [PubMed] [Google Scholar]
- Aminoff SR, Hellvin T, Lagerberg TV, Berg AO, Andreassen OA, & Melle I (2013). Neurocognitive features in subgroups of bipolar disorder. Bipolar Disord, 15(3), 272–283, doi: 10.1111/bdi.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An SK, Kang JI, Park JY, Kim KR, Lee SY, & Lee E (2010). Attribution bias in ultra-high risk for psychosis and first-episode schizophrenia. Schizophr Res, 118(1–3), 54–61, doi: 10.1016/j.schres.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Ancin I, Cabranes JA, Santos JL, Sanchez-Morla E, & Barabash A (2013). Executive deficits: a continuum schizophrenia-bipolar disorder or specific to schizophrenia? J Psychiatr Res, 47(11), 1564–1571, doi: 10.1016/j.jpsychires.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Ang YG, & Tan HY (2004). Academic deterioration prior to first episode schizophrenia in young Singaporean males. Psychiatry Res, 121(3), 303–307. [DOI] [PubMed] [Google Scholar]
- Aronson TA, Shukla S, Gujavarty K, Hoff A, DiBuono M, & Khan E (1988). Relapse in delusional depression: a retrospective study of the course of treatment. Compr Psychiatry, 29(1), 12–21. [DOI] [PubMed] [Google Scholar]
- Arts B, Simons CJ, & Os J (2013). Evidence for the impact of the CACNA1C risk allele rs1006737 on 2-year cognitive functioning in bipolar disorder. Psychiatr Genet, 23(1), 41–42, doi: 10.1097/YPG.0b013e328358641c. [DOI] [PubMed] [Google Scholar]
- Aylward E, Walker E, & Bettes B (1984). Intelligence in schizophrenia: meta-analysis of the research. Schizophr Bull, 10(3), 430–459. [DOI] [PubMed] [Google Scholar]
- Ayres AM, Busatto GF, Menezes PR, Schaufelberger MS, Coutinho L, Murray RM, et al. (2007). Cognitive deficits in first-episode psychosis: a population-based study in Sao Paulo, Brazil. Schizophr Res, 90(1–3), 338–343, doi: 10.1016/j.schres.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Bachman P, Reichenberg A, Rice P, Woolsey M, Chaves O, Martinez D, et al. (2010). Deconstructing processing speed deficits in schizophrenia: application of a parametric digit symbol coding test. Schizophr Res, 118(1–3), 6–11, doi: 10.1016/j.schres.2010.02.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman L, Lindenberger U, Li SC, & Nyberg L (2010). Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neurosci Biobehav Rev, 34(5), 670–677, doi: 10.1016/j.neubiorev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Baddeley A (2000). The episodic buffer: a new component of working memory? Trends Cogn Sci, 4(11), 417–423. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, MacDonald AW 3rd, Braver TS, & Cohen JD (2003). Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol, 112(1), 132–143. [PubMed] [Google Scholar]
- Barch DM, & Ceaser A (2012). Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci, 16(1), 27–34, doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, & Dowd EC (2010). Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull, 36(5), 919–934, doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, & Sheffield JM (2014). Cognitive impairments in psychotic disorders: common mechanisms and measurement. World Psychiatry, 13(3), 224–232, doi: 10.1002/wps.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JH, McDougall F, Xu MK, Croudace TJ, Richards M, & Jones PB (2012). Childhood cognitive function and adult psychopathology: associations with psychotic and non-psychotic symptoms in the general population. Br J Psychiatry, 201, 124–130, doi: 10.1192/bjp.bp.111.102053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SL, Mulholland CC, Cooper SJ, & Rushe TM (2009). Patterns of neurocognitive impairment in first-episode bipolar disorder and schizophrenia. Br J Psychiatry, 195(1), 67–72, doi: 10.1192/bjp.bp.108.054874. [DOI] [PubMed] [Google Scholar]
- Bechdolf A, Thompson A, Nelson B, Cotton S, Simmons MB, Amminger GP, et al. (2010). Experience of trauma and conversion to psychosis in an ultra-high-risk (prodromal) group. Acta Psychiatr Scand, 121(5), 377–384, doi: 10.1111/j.1600-0447.2010.01542.x. [DOI] [PubMed] [Google Scholar]
- Belanoff JK, Kalehzan M, Sund B, Fleming Ficek SK, & Schatzberg AF (2001). Cortisol activity and cognitive changes in psychotic major depression. Am J Psychiatry, 158(10), 1612–1616, doi: 10.1176/appi.ajp.158.10.1612. [DOI] [PubMed] [Google Scholar]
- Bell MD, & Bryson G (2001). Work rehabilitation in schizophrenia: does cognitive impairment limit improvement? Schizophr Bull, 27(2), 269–279. [DOI] [PubMed] [Google Scholar]
- Bell MD, Lysaker PH, Milstein RM, & Beam-Goulet JL (1994). Concurrent validity of the cognitive component of schizophrenia: relationship of PANSS scores to neuropsychological assessments. Psychiatry Res, 54(1), 51–58. [DOI] [PubMed] [Google Scholar]
- Bergh S, Hjorthoj C, Sorensen HJ, Fagerlund B, Austin S, Secher RG, et al. (2016). Predictors and longitudinal course of cognitive functioning in schizophrenia spectrum disorders, 10years after baseline: The OPUS study. Schizophr Res, 175(1–3), 57–63, doi: 10.1016/j.schres.2016.03.025. [DOI] [PubMed] [Google Scholar]
- Berman I, Viegner B, Merson A, Allan E, Pappas D, & Green AI (1997). Differential relationships between positive and negative symptoms and neuropsychological deficits in schizophrenia. Schizophr Res, 25(1), 1–10, doi: 10.1016/S0920-9964(96)00098-9. [DOI] [PubMed] [Google Scholar]
- Bonnin CM, Torrent C, Arango C, Amann BL, Sole B, Gonzalez-Pinto A, et al. (2016). Functional remediation in bipolar disorder: 1-year follow-up of neurocognitive and functional outcome. Br J Psychiatry, 208(1), 87–93, doi: 10.1192/bjp.bp.114.162123. [DOI] [PubMed] [Google Scholar]
- Bora E (2015). Neurodevelopmental origin of cognitive impairment in schizophrenia. Psychol Med, 45(1), 1–9, doi: 10.1017/s0033291714001263. [DOI] [PubMed] [Google Scholar]
- Bora E (2017). Neurocognitive features in clinical subgroups of bipolar disorder: A meta-analysis. J Affect Disord, 229, 125–134, doi: 10.1016/j.jad.2017.12.057. [DOI] [PubMed] [Google Scholar]
- Bora E, & Ozerdem A (2017). Meta-analysis of longitudinal studies of cognition in bipolar disorder: comparison with healthy controls and schizophrenia. Psychol Med, 47(16), 2753–2766, doi: 10.1017/S0033291717001490. [DOI] [PubMed] [Google Scholar]
- Bora E, & Pantelis C (2015). Meta-analysis of Cognitive Impairment in First-Episode Bipolar Disorder: Comparison With First-Episode Schizophrenia and Healthy Controls. Schizophr Bull, 41(5), 1095–1104, doi: 10.1093/schbul/sbu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Vahip S, Akdeniz F, Gonul AS, Eryavuz A, Ogut M, et al. (2007). The effect of previous psychotic mood episodes on cognitive impairment in euthymic bipolar patients. Bipolar Disord, 9(5), 468–477, doi: 10.1111/j.1399-5618.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, & Pantelis C (2009). Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord, 113(1–2), 1–20, doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, & Pantelis C (2010a). Cognitive impairment in affective psychoses: a meta-analysis. Schizophr Bull, 36(1), 112–125, doi: 10.1093/schbul/sbp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Yucel M, & Pantelis C (2010b). Neurocognitive markers of psychosis in bipolar disorder: a meta-analytic study. J Affect Disord, 127(1–3), 1–9, doi: 10.1016/j.jad.2010.02.117. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Reichenberg A, Patterson TL, Heaton RK, & Harvey PD (2006). Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry, 163(3), 418–425, doi: 10.1176/appi.ajp.163.3.418. [DOI] [PubMed] [Google Scholar]
- Bozikas VP, Kosmidis MH, Giannakou M, Kechayas P, Tsotsi S, Kiosseoglou G, et al. (2014). Controlled shifting of attention in schizophrenia and bipolar disorder through a dichotic listening paradigm. Compr Psychiatry, 55(5), 1212–1219, doi: 10.1016/j.comppsych.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Brebion G, David AS, Bressan RA, & Pilowsky LS (2006). Processing speed: a strong predictor of verbal memory performance in schizophrenia. J Clin Exp Neuropsychol, 28(3), 370–382, doi: 10.1080/13803390590935390. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Francey SM, Wood SJ, Jackson HJ, Pantelis C, Phillips LJ, et al. (2005). Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am J Psychiatry, 162(1), 71–78, doi: 10.1176/appi.ajp.162.1.71. [DOI] [PubMed] [Google Scholar]
- Bryson G, & Bell MD (2003). Initial and final work performance in schizophrenia: cognitive and symptom predictors. J Nerv Ment Dis, 191(2), 87–92, doi: 10.1097/01.NMD.0000050937.06332.3C. [DOI] [PubMed] [Google Scholar]
- Bryzgalov LO, Korbolina EE, Brusentsov II, Leberfarb EY, Bondar NP, & Merkulova TI (2018). Novel functional variants at the GWAS-implicated loci might confer risk to major depressive disorder, bipolar affective disorder and schizophrenia. BMC Neurosci, 19(Suppl 1), 22, doi: 10.1186/s12868-018-0414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, et al. (2002). Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry, 59(5), 449–456. [DOI] [PubMed] [Google Scholar]
- Cannon M, Jones P, Huttunen MO, Tanskanen A, Huttunen T, Rabe-Hesketh S, et al. (1999). School performance in Finnish children and later development of schizophrenia: a population-based longitudinal study. Arch Gen Psychiatry, 56(5), 457–463. [DOI] [PubMed] [Google Scholar]
- Cannon M, Moffitt TE, Caspi A, Murray RM, Harrington H, & Poulton R (2006). Neuropsychological performance at the age of 13 years and adult schizophreniform disorder: prospective birth cohort study. Br J Psychiatry, 189, 463–464, doi: 10.1192/bjp.bp.105.020552. [DOI] [PubMed] [Google Scholar]
- Carrion RE, McLaughlin D, Goldberg TE, Auther AM, Olsen RH, Olvet DM, et al. (2013). Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA Psychiatry, 70(11), 1133–1142, doi: 10.1001/jamapsychiatry.2013.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemerinski E, Triebwasser J, Roussos P, & Siever LJ (2013). Schizotypal personality disorder. J Pers Disord, 27(5), 652–679, doi: 10.1521/pedi_2012_26_053. [DOI] [PubMed] [Google Scholar]
- Cornblatt B, Obuchowski M, Roberts S, Pollack S, & Erlenmeyer-Kimling L (1999). Cognitive and behavioral precursors of schizophrenia. Dev Psychopathol, 11(3), 487–508. [DOI] [PubMed] [Google Scholar]
- Coryell W, Endicott J, & Keller M (1987). The importance of psychotic features to major depression: course and outcome during a 2-year follow-up. Acta Psychiatr Scand, 75(1), 78–85. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Done DJ, & Sacker A (1995). Childhood precursors of psychosis as clues to its evolutionary origins. Eur Arch Psychiatry Clin Neurosci, 245(2), 61–69. [DOI] [PubMed] [Google Scholar]
- Daban C, Martinez-Aran A, Torrent C, Tabares-Seisdedos R, Balanza-Martinez V, Salazar-Fraile J, et al. (2006). Specificity of cognitive deficits in bipolar disorder versus schizophrenia. A systematic review. Psychother Psychosom, 75(2), 72–84, doi: 10.1159/000090891. [DOI] [PubMed] [Google Scholar]
- Daglas R, Allott K, Yucel M, Pantelis C, Macneil CA, Berk M, et al. (2016). The trajectory of cognitive functioning following first episode mania: A 12-month follow-up study. Aust N Z J Psychiatry, 50(12), 1186–1197, doi: 10.1177/0004867415622272. [DOI] [PubMed] [Google Scholar]
- Daglas R, Yucel M, Cotton S, Allott K, Hetrick S, & Berk M (2015). Cognitive impairment in first-episode mania: a systematic review of the evidence in the acute and remission phases of the illness. Int J Bipolar Disord, 3, 9, doi: 10.1186/s40345-015-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M, Galderisi S, Weiser M, Werbeloff N, Fleischhacker WW, Keefe RS, et al. (2009). Cognitive effects of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: a randomized, open-label clinical trial (EUFEST). Am J Psychiatry, 166(6), 675–682, doi: 10.1176/appi.ajp.2008.08060806. [DOI] [PubMed] [Google Scholar]
- Demant KM, Vinberg M, Kessing LV, & Miskowiak KW (2015). Effects of Short-Term Cognitive Remediation on Cognitive Dysfunction in Partially or Fully Remitted Individuals with Bipolar Disorder: Results of a Randomised Controlled Trial. PLoS One, 10(6), e0127955, doi: 10.1371/journal.pone.0127955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmo C, Lagerberg TV, Aminoff SR, Hellvin T, Kvitland LR, Simonsen C, et al. (2016). History of psychosis and previous episodes as potential explanatory factors for neurocognitive impairment in first-treatment bipolar I disorder. Bipolar Disord, 18(2), 136–147, doi: 10.1111/bdi.12377. [DOI] [PubMed] [Google Scholar]
- Demmo C, Lagerberg TV, Aminoff SR, Hellvin T, Kvitland LR, Simonsen C, et al. (2017). Course of neurocognitive function in first treatment bipolar I disorder: One-year follow-up study. Psychiatry Res, 249, 286–292, doi: 10.1016/j.psychres.2016.12.048. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Boronow JJ, Ringel N, & Parente F (1999). Social functioning and neurocognitive deficits in outpatients with schizophrenia: a 2-year follow-up. Schizophr Res, 37(1), 13–20. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Vaughan C, Origoni A, Khushalani S, Dickinson D, et al. (2011). Cognitive functioning in recent onset psychosis. J Nerv Ment Dis, 199(6), 367–371, doi: 10.1097/NMD.0b013e31821cd0ff. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ragland JD, Gold JM, & Gur RC (2008). General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry, 64(9), 823–827, doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, & Gold JM (2007). Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry, 64(5), 532–542, doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Dickson H, Cullen AE, Reichenberg A, Hodgins S, Campbell DD, Morris RG, et al. (2014). Cognitive impairment among children at-risk for schizophrenia. J Psychiatr Res, 50, 92–99, doi: 10.1016/j.jpsychires.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Dickson H, Laurens KR, Cullen AE, & Hodgins S (2012). Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol Med, 42(4), 743–755, doi: 10.1017/S0033291711001693. [DOI] [PubMed] [Google Scholar]
- Dinn WM, Harris CL, Aycicegi A, Greene P, & Andover MS (2002). Positive and negative schizotypy in a student sample: neurocognitive and clinical correlates. Schizophr Res, 56(1–2), 171–185. [DOI] [PubMed] [Google Scholar]
- Donaldson S, Goldstein LH, Landau S, Raymont V, & Frangou S (2003). The Maudsley Bipolar Disorder Project: the effect of medication, family history, and duration of illness on IQ and memory in bipolar I disorder. J Clin Psychiatry, 64(1), 86–93. [PubMed] [Google Scholar]
- Elie D, Poirier M, Chianetta J, Durand M, Gregoire C, & Grignon S (2010). Cognitive effects of antipsychotic dosage and polypharmacy: a study with the BACS in patients with schizophrenia and schizoaffective disorder. J Psychopharmacol, 24(7), 1037–1044, doi: 10.1177/0269881108100777. [DOI] [PubMed] [Google Scholar]
- Elshahawi HH, Essawi H, Rabie MA, Mansour M, Beshry ZA, & Mansour AN (2011). Cognitive functions among euthymic bipolar I patients after a single manic episode versus recurrent episodes. J Affect Disord, 130(1–2), 180–191, doi: 10.1016/j.jad.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Rock D, Roberts SA, Janal M, Kestenbaum C, Cornblatt B, et al. (2000). Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: the New York High-Risk Project. Am J Psychiatry, 157(9), 1416–1422, doi: 10.1176/appi.ajp.157.9.1416. [DOI] [PubMed] [Google Scholar]
- Evans JD, Bond GR, Meyer PS, Kim HW, Lysaker PH, Gibson PJ, et al. (2004). Cognitive and clinical predictors of success in vocational rehabilitation in schizophrenia. Schizophr Res, 70(2–3), 331–342, doi: 10.1016/j.schres.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Falkai P, Malchow B, & Schmitt A (2017). Aerobic exercise and its effects on cognition in schizophrenia. Curr Opin Psychiatry, 30(3), 171–175, doi: 10.1097/YCO.0000000000000326. [DOI] [PubMed] [Google Scholar]
- Fatouros-Bergman H, Cervenka S, Flyckt L, Edman G, & Farde L (2014). Meta-analysis of cognitive performance in drug-naive patients with schizophrenia. Schizophr Res, 158(1–3), 156–162, doi: 10.1016/j.schres.2014.06.034. [DOI] [PubMed] [Google Scholar]
- Firth J, Stubbs B, Rosenbaum S, Vancampfort D, Malchow B, Schuch F, et al. (2017). Aerobic Exercise Improves Cognitive Functioning in People With Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr Bull, 43(3), 546–556, doi: 10.1093/schbul/sbw115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SK, Blasey C, & Schatzberg AF (2004). Neuropsychological correlates of psychotic features in major depressive disorders: a review and meta-analysis. J Psychiatr Res, 38(1), 27–35. [DOI] [PubMed] [Google Scholar]
- Flowers SA, Ryan KA, Lai Z, McInnis MG, & Ellingrod VL (2016). Interaction between COMT rs5993883 and second generation antipsychotics is linked to decreases in verbal cognition and cognitive control in bipolar disorder. BMC Psychol, 4, 14, doi: 10.1186/s40359-016-0118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francey SM, Jackson HJ, Phillips LJ, Wood SJ, Yung AR, & McGorry PD (2005). Sustained attention in young people at high risk of psychosis does not predict transition to psychosis. Schizophr Res, 79(1), 127–136, doi: 10.1016/j.schres.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Frydecka D, Eissa AM, Hewedi DH, Ali M, Drapala J, Misiak B, et al. (2014). Impairments of working memory in schizophrenia and bipolar disorder: the effect of history of psychotic symptoms and different aspects of cognitive task demands. Front Behav Neurosci, 8, 416, doi: 10.3389/fnbeh.2014.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R, Nopoulos P, Arndt S, O’Leary D, Ho BC, & Andreasen NC (2002). Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry, 159(7), 1183–1189, doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Deste G, Smieskova R, Barlati S, Yung AR, Howes O, et al. (2012). Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry, 69(6), 562–571, doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, et al. (2007). The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry, 62(8), 910–916, doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Glassman AH, & Roose SP (1981). Delusional depression. A distinct clinical entity? Arch Gen Psychiatry, 38(4), 424–427. [DOI] [PubMed] [Google Scholar]
- Glosser G, Butters N, & Kaplan E (1977). Visuoperceptual processes in brain damaged patients on the digit symbol substitution test. Int J Neurosci, 7(2), 59–66. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Brett C, Tabraham P, Johns L, Valmaggia L, Broome M, et al. (2014). Spatial working memory ability in individuals at ultra high risk for psychosis. J Psychiatr Res, 50, 100–105, doi: 10.1016/j.jpsychires.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Barch DM, Carter CS, Dakin S, Luck SJ, MacDonald AW 3rd, et al. (2012). Clinical, functional, and intertask correlations of measures developed by the Cognitive Neuroscience Test Reliability and Clinical Applications for Schizophrenia Consortium. Schizophr Bull, 38(1), 144–152, doi: 10.1093/schbul/sbr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Goldberg RW, McNary SW, Dixon LB, & Lehman AF (2002). Cognitive correlates of job tenure among patients with severe mental illness. Am J Psychiatry, 159(8), 1395–1402, doi: 10.1176/appi.ajp.159.8.1395. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Hyde TM, Kleinman JE, & Weinberger DR (1993). Course of schizophrenia: neuropsychological evidence for a static encephalopathy. Schizophr Bull, 19(4), 797–804. [DOI] [PubMed] [Google Scholar]
- Gomez RG, Fleming SH, Keller J, Flores B, Kenna H, DeBattista C, et al. (2006). The neuropsychological profile of psychotic major depression and its relation to cortisol. Biol Psychiatry, 60(5), 472–478, doi: 10.1016/j.biopsych.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Goodall J, Fisher C, Hetrick S, Phillips L, Parrish EM, & Allott K (2018). Neurocognitive Functioning in Depressed Young People: A Systematic Review and Meta-Analysis. Neuropsychol Rev, 28(2), 216–231, doi: 10.1007/s11065-018-9373-9. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Martinez-Aran A, Glahn DC, & Vieta E (2008). Cognitive impairment in bipolar disorder: neurodevelopment or neurodegeneration? An ECNP expert meeting report. Eur Neuropsychopharmacol, 18(11), 787–793, doi: 10.1016/j.euroneuro.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, & Heaton RK (2004). Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res, 72(1), 41–51, doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Greenland-White SE, Ragland JD, Niendam TA, Ferrer E, & Carter CS (2017). Episodic memory functions in first episode psychosis and clinical high risk individuals. Schizophr Res, 188, 151–157, doi: 10.1016/j.schres.2017.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra A, Fearon P, Sham P, Jones P, Lewis S, Mata I, et al. (2002). The relationship between predisposing factors, premorbid function and symptom dimensions in psychosis: an integrated approach. Eur Psychiatry, 17(6), 311–320. [DOI] [PubMed] [Google Scholar]
- Gur RC, Calkins ME, Satterthwaite TD, Ruparel K, Bilker WB, Moore TM, et al. (2014). Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry, 71(4), 366–374, doi: 10.1001/jamapsychiatry.2013.4190. [DOI] [PubMed] [Google Scholar]
- Harris MS, Reilly JL, Thase ME, Keshavan MS, & Sweeney JA (2009). Response suppression deficits in treatment-naive first-episode patients with schizophrenia, psychotic bipolar disorder and psychotic major depression. Psychiatry Res, 170(2–3), 150–156, doi: 10.1016/j.psychres.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Koren D, Reichenberg A, & Bowie CR (2006). Negative symptoms and cognitive deficits: what is the nature of their relationship? Schizophr Bull, 32(2), 250–258, doi: 10.1093/schbul/sbj011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins KA, Addington J, Keefe RS, Christensen B, Perkins DO, Zipurksy R, et al. (2004). Neuropsychological status of subjects at high risk for a first episode of psychosis. Schizophr Res, 67(2–3), 115–122, doi: 10.1016/j.schres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, & Zakzanis KK (1998). Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology, 12(3), 426–445. [DOI] [PubMed] [Google Scholar]
- Helling I, Ohman A, & Hultman CM (2003). School achievements and schizophrenia: a case-control study. Acta Psychiatr Scand, 108(5), 381–386. [DOI] [PubMed] [Google Scholar]
- Hellvin T, Sundet K, Simonsen C, Aminoff SR, Lagerberg TV, Andreassen OA, et al. (2012). Neurocognitive functioning in patients recently diagnosed with bipolar disorder. Bipolar Disord, 14(3), 227–238, doi: 10.1111/j.1399-5618.2012.01004.x. [DOI] [PubMed] [Google Scholar]
- Heydebrand G, Weiser M, Rabinowitz J, Hoff AL, DeLisi LE, & Csernansky JG (2004). Correlates of cognitive deficits in first episode schizophrenia. Schizophr Res, 68(1), 1–9, doi: 10.1016/S0920-9964(03)00097-5. [DOI] [PubMed] [Google Scholar]
- Hill SK, Beers SR, Kmiec JA, Keshavan MS, & Sweeney JA (2004). Impairment of verbal memory and learning in antipsychotic-naive patients with first-episode schizophrenia. Schizophr Res, 68(2–3), 127–136, doi: 10.1016/S0920-9964(03)00125-7. [DOI] [PubMed] [Google Scholar]
- Hill SK, Bishop JR, Palumbo D, & Sweeney JA (2010). Effect of second-generation antipsychotics on cognition: current issues and future challenges. Expert Rev Neurother, 10(1), 43–57, doi: 10.1586/ern.09.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Harris MS, Rosen C, Marvin RW, Deleon O, et al. (2009). A comparison of neuropsychological dysfunction in first-episode psychosis patients with unipolar depression, bipolar disorder, and schizophrenia. Schizophr Res, 113(2–3), 167–175, doi: 10.1016/j.schres.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, et al. (2013). Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry, 170(11), 1275–1284, doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Ragozzino ME, Rubin LH, Bishop JR, Gur RC, et al. (2015). Regressing to Prior Response Preference After Set Switching Implicates Striatal Dysfunction Across Psychotic Disorders: Findings From the B-SNIP Study. Schizophr Bull, 41(4), 940–950, doi: 10.1093/schbul/sbu130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberger WC, Hill SK, Nelson CL, Reilly JL, Keefe RS, Pearlson GD, et al. (2016). Unitary construct of generalized cognitive ability underlying BACS performance across psychotic disorders and in their first-degree relatives. Schizophr Res, 170(1), 156–161, doi: 10.1016/j.schres.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou CL, Xiang YT, Wang ZL, Everall I, Tang Y, Yang C, et al. (2016). Cognitive functioning in individuals at ultra-high risk for psychosis, first-degree relatives of patients with psychosis and patients with first-episode schizophrenia. Schizophr Res, 174(1–3), 71–76, doi: 10.1016/j.schres.2016.04.034. [DOI] [PubMed] [Google Scholar]
- Howlin P, Mawhood L, & Rutter M (2000). Autism and developmental receptive language disorder--a follow-up comparison in early adult life. II: Social, behavioural, and psychiatric outcomes. J Child Psychol Psychiatry, 41(5), 561–578. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry, 167(7), 748–751, doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Ivleva EI, Morris DW, Osuji J, Moates AF, Carmody TJ, Thaker GK, et al. (2012). Cognitive endophenotypes of psychosis within dimension and diagnosis. Psychiatry Res, 196(1), 38–44, doi: 10.1016/j.psychres.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J, Berns S, Uzelac S, & Davis-Conway S (2006). Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res, 145(1), 39–48, doi: 10.1016/j.psychres.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Jaeger J, Czobor P, & Berns SM (2003). Basic neuropsychological dimensions in schizophrenia. Schizophr Res, 65(2–3), 105–116. [DOI] [PubMed] [Google Scholar]
- Jenkins LM, Bodapati AS, Sharma RP, & Rosen C (2017). Working memory predicts presence of auditory verbal hallucinations in schizophrenia and bipolar disorder with psychosis. J Clin Exp Neuropsychol, 1–11, doi: 10.1080/13803395.2017.1321106. [DOI] [PubMed] [Google Scholar]
- Jimenez-Lopez E, Aparicio AI, Sanchez-Morla EM, Rodriguez-Jimenez R, Vieta E, & Santos JL (2017). Neurocognition in patients with psychotic and non-psychotic bipolar I disorder. A comparative study with individuals with schizophrenia. J Affect Disord, 222, 169–176, doi: 10.1016/j.jad.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Johnson J, Horwath E, & Weissman MM (1991). The validity of major depression with psychotic features based on a community study. Arch Gen Psychiatry, 48(12), 1075–1081. [DOI] [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, & Marmot M (1994). Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet, 344(8934), 1398–1402. [DOI] [PubMed] [Google Scholar]
- Kahn RS, & Keefe RS (2013). Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry, 70(10), 1107–1112, doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- Kane J, Honigfeld G, Singer J, & Meltzer H (1988). Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry, 45(9), 789–796. [DOI] [PubMed] [Google Scholar]
- Kapczinski F, Dias VV, Kauer-Sant’Anna M, Frey BN, Grassi-Oliveira R, Colom F, et al. (2009). Clinical implications of a staging model for bipolar disorders. Expert Rev Neurother, 9(7), 957–966, doi: 10.1586/ern.09.31. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, et al. (2007). Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry, 64(6), 633–647, doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Eesley CE, & Poe MP (2005). Defining a cognitive function decrement in schizophrenia. Biol Psychiatry, 57(6), 688–691, doi: 10.1016/j.biopsych.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Keefe RS, & Harvey PD (2015). Understanding symbol coding in schizophrenia. Biol Psychiatry, 78(11), 744–746, doi: 10.1016/j.biopsych.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Perkins DO, Gu H, Zipursky RB, Christensen BK, & Lieberman JA (2006). A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophr Res, 88(1–3), 26–35, doi: 10.1016/j.schres.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Davis VG, Harvey PD, Atkins AS, Haig GM, Hagino O, et al. (2017). Placebo Response and Practice Effects in Schizophrenia Cognition Trials. JAMA Psychiatry, 74(8), 807–814, doi: 10.1001/jamapsychiatry.2017.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM Jr., et al. (2017). HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry, 22(4), 527–536, doi: 10.1038/mp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J, Schatzberg AF, & Maj M (2007). Current issues in the classification of psychotic major depression. Schizophr Bull, 33(4), 877–885, doi: 10.1093/schbul/sbm065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Mezuk B, Sundquist K, & Sundquist J (2016). A Swedish National Prospective and Co-relative Study of School Achievement at Age 16, and Risk for Schizophrenia, Other Nonaffective Psychosis, and Bipolar Illness. Schizophr Bull, 42(1), 77–86, doi: 10.1093/schbul/sbv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri S, Kelemen O, Benedek G, & Janka Z (2001). Different trait markers for schizophrenia and bipolar disorder: a neurocognitive approach. Psychol Med, 31(5), 915–922. [DOI] [PubMed] [Google Scholar]
- Kern RS, Gold JM, Dickinson D, Green MF, Nuechterlein KH, Baade LE, et al. (2011). The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res, 126(1–3), 124–131, doi: 10.1016/j.schres.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Morris DW, Sweeney JA, Pearlson G, Thaker G, Seidman LJ, et al. (2011). A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr Res, 133(1–3), 250–254, doi: 10.1016/j.schres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Barnett JH, White IR, & Jones PB (2011). A quantitative meta-analysis of population-based studies of premorbid intelligence and schizophrenia. Schizophr Res, 132(2–3), 220–227, doi: 10.1016/j.schres.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim JW, Koo TH, Yun HR, & Won SH (2015). Shared and distinct neurocognitive endophenotypes of schizophrenia and psychotic bipolar disorder. Clin Psychopharmacol Neurosci, 13(1), 94–102, doi: 10.9758/cpn.2015.13.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D, Corcoran C, Harkavy-Friedman JM, Ritzler B, Javitt DC, & Malaspina D (2007). Visual form perception: a comparison of individuals at high risk for psychosis, recent onset schizophrenia and chronic schizophrenia. Schizophr Res, 97(1–3), 25–34, doi: 10.1016/j.schres.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, & Bowie CR (2008). Is schizophrenia a syndrome of accelerated aging? Schizophr Bull, 34(6), 1024–1032, doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosterkotter J, Hellmich M, Steinmeyer EM, & Schultze-Lutter F (2001). Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry, 58(2), 158–164. [DOI] [PubMed] [Google Scholar]
- Knowles EE, Weiser M, David AS, Glahn DC, Davidson M, & Reichenberg A (2015). The puzzle of processing speed, memory, and executive function impairments in schizophrenia: fitting the pieces together. Biol Psychiatry, 78(11), 786–793, doi: 10.1016/j.biopsych.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E (1919). Dementia praecox and paraphrenia, trans. Barclay RM Kriefer RE, [Reprinted 1971]([WM]). [Google Scholar]
- Kraus M, Rapisarda A, Lam M, Thong JYJ, Lee J, Subramaniam M, et al. (2016). Disrupted latent inhibition in individuals at ultra high-risk for developing psychosis. Schizophr Res Cogn, 6, 1–8, doi: 10.1016/j.scog.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravariti E, Reichenberg A, Morgan K, Dazzan P, Morgan C, Zanelli JW, et al. (2009). Selective deficits in semantic verbal fluency in patients with a first affective episode with psychotic symptoms and a positive history of mania. Bipolar Disord, 11(3), 323–329, doi: 10.1111/j.1399-5618.2009.00673.x. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Buka SL, Seidman LJ, Goldstein JM, Koren D, & Tsuang MT (1998). IQ decline during childhood and adult psychotic symptoms in a community sample: a 19-year longitudinal study. Am J Psychiatry, 155(5), 672–677, doi: 10.1176/ajp.155.5.672. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Vinogradov S, Poole JH, Schaefer CA, Deicken RF, Factor-Litvak P, et al. (2010). Cognitive decline in schizophrenia from childhood to midlife: a 33-year longitudinal birth cohort study. Schizophr Res, 118(1–3), 1–5, doi: 10.1016/j.schres.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laes JR, & Sponheim SR (2006). Does cognition predict community function only in schizophrenia?: a study of schizophrenia patients, bipolar affective disorder patients, and community control subjects. Schizophr Res, 84(1), 121–131, doi: 10.1016/j.schres.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Lemos-Giraldez S, Vallina-Fernandez O, Fernandez-Iglesias P, Vallejo-Seco G, Fonseca-Pedrero E, Paino-Pineiro M, et al. (2009). Symptomatic and functional outcome in youth at ultra-high risk for psychosis: a longitudinal study. Schizophr Res, 115(2–3), 121–129, doi: 10.1016/j.schres.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, et al. (2006). Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry, 59(9), 863–871, doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Lesh TA, Westphal AJ, Niendam TA, Yoon JH, Minzenberg MJ, Ragland JD, et al. (2013). Proactive and reactive cognitive control and dorsolateral prefrontal cortex dysfunction in first episode schizophrenia. Neuroimage Clin, 2, 590–599, doi: 10.1016/j.nicl.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B, & Weiss RD (2010). Neurocognitive impairment and psychosis in bipolar I disorder during early remission from an acute episode of mood disturbance. J Clin Psychiatry, 71(2), 201–206, doi: 10.4088/JCP.08m04663yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski KE, Cohen BM, Keshavan MS, & Ongur D (2011a). Relationship of neurocognitive deficits to diagnosis and symptoms across affective and non-affective psychoses. Schizophr Res, 133(1–3), 212–217, doi: 10.1016/j.schres.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski KE, Cohen BM, & Ongur D (2011b). Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol Med, 41(2), 225–241, doi: 10.1017/s0033291710001042. [DOI] [PubMed] [Google Scholar]
- Lin A, Wood SJ, Nelson B, Brewer WJ, Spiliotacopoulos D, Bruxner A, et al. (2011). Neurocognitive predictors of functional outcome two to 13 years after identification as ultra-high risk for psychosis. Schizophr Res, 132(1), 1–7, doi: 10.1016/j.schres.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Liu CC, Hua MS, Hwang TJ, Chiu CY, Liu CM, Hsieh MH, et al. (2015). Neurocognitive functioning of subjects with putative pre-psychotic states and early psychosis. Schizophr Res, 164(1–3), 40–46, doi: 10.1016/j.schres.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Liu D, Ji C, Zhuo K, Song Z, Wang Y, Mei L, et al. (2017). Impaired cue identification and intention retrieval underlie prospective memory deficits in patients with first-episode schizophrenia. Aust N Z J Psychiatry, 51(3), 270–277, doi: 10.1177/0004867416640097. [DOI] [PubMed] [Google Scholar]
- MacCabe JH, Lambe MP, Cnattingius S, Sham PC, David AS, Reichenberg A, et al. (2010). Excellent school performance at age 16 and risk of adult bipolar disorder: national cohort study. Br J Psychiatry, 196(2), 109–115, doi: 10.1192/bjp.bp.108.060368. [DOI] [PubMed] [Google Scholar]
- MacCabe JH, Lambe MP, Cnattingius S, Torrang A, Bjork C, Sham PC, et al. (2008). Scholastic achievement at age 16 and risk of schizophrenia and other psychoses: a national cohort study. Psychol Med, 38(8), 1133–1140, doi: 10.1017/s0033291707002048. [DOI] [PubMed] [Google Scholar]
- MacCabe JH, Wicks S, Lofving S, David AS, Berndtsson A, Gustafsson JE, et al. (2013). Decline in cognitive performance between ages 13 and 18 years and the risk for psychosis in adulthood: a Swedish longitudinal cohort study in males. JAMA Psychiatry, 70(3), 261–270, doi: 10.1001/2013.jamapsychiatry.43. [DOI] [PubMed] [Google Scholar]
- MacKenzie LE, Patterson VC, Zwicker A, Drobinin V, Fisher HL, Abidi S, et al. (2017). Hot and cold executive functions in youth with psychotic symptoms. Psychol Med, 47(16), 2844–2853, doi: 10.1017/S0033291717001374. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Torrent C, Tabares-Seisdedos R, Salamero M, Daban C, Balanza-Martinez V, et al. (2008). Neurocognitive impairment in bipolar patients with and without history of psychosis. J Clin Psychiatry, 69(2), 233–239. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Vieta E, Colom F, Torrent C, Sanchez-Moreno J, Reinares M, et al. (2004). Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord, 6(3), 224–232, doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Vieta E, Torrent C, Sanchez-Moreno J, Goikolea JM, Salamero M, et al. (2007). Functional outcome in bipolar disorder: the role of clinical and cognitive factors. Bipolar Disord, 9(1–2), 103–113, doi: 10.1111/j.1399-5618.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- Martino DJ, Igoa A, Marengo E, Scapola M, & Strejilevich SA (2018). Longitudinal relationship between clinical course and neurocognitive impairments in bipolar disorder. J Affect Disord, 225, 250–255, doi: 10.1016/j.jad.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Martino DJ, Samame C, Ibanez A, & Strejilevich SA (2015). Neurocognitive functioning in the premorbid stage and in the first episode of bipolar disorder: a systematic review. Psychiatry Res, 226(1), 23–30, doi: 10.1016/j.psychres.2014.12.044. [DOI] [PubMed] [Google Scholar]
- McCleery A, Ventura J, Kern RS, Subotnik KL, Gretchen-Doorly D, Green MF, et al. (2014). Cognitive functioning in first-episode schizophrenia: MATRICS Consensus Cognitive Battery (MCCB) Profile of Impairment. Schizophr Res, 157(1–3), 33–39, doi: 10.1016/j.schres.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan J, Prezbindowski A, Breiger D, & McCurry C (2004). Neuropsychological functioning in early onset psychotic disorders. Schizophr Res, 68(1), 21–26, doi: 10.1016/s0920-9964(03)00058-6. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Harrison LK, Forrester K, Lawrie SM, & Johnstone EC (2005). Neuropsychological impairments in people with schizophrenia or bipolar disorder and their unaffected relatives. Br J Psychiatry, 186, 378–385, doi: 10.1192/bjp.186.5.378. [DOI] [PubMed] [Google Scholar]
- McKay R, Langdon R, & Coltheart M (2006). Need for closure, jumping to conclusions, and decisiveness in delusion-prone individuals. J Nerv Ment Dis, 194(6), 422–426, doi: 10.1097/01.nmd.0000221353.44132.25. [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Reichenberg A, Keefe RS, Fisher HL, Harrington H, et al. (2014). Neuropsychological decline in schizophrenia from the premorbid to the postonset period: evidence from a population-representative longitudinal study. Am J Psychiatry, 171(1), 91–101, doi: 10.1176/appi.ajp.2013.12111438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, & Seidman LJ (2009). Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology, 23(3), 315–336, doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Miskowiak KW, Burdick KE, Martinez-Aran A, Bonnin CM, Bowie CR, Carvalho AF, et al. (2018). Assessing and addressing cognitive impairment in bipolar disorder: the International Society for Bipolar Disorders Targeting Cognition Task Force recommendations for clinicians. Bipolar Disord, doi: 10.1111/bdi.12595. [DOI] [PubMed] [Google Scholar]
- Mittal VA, & Wakschlag LS (2017). Research domain criteria (RDoC) grows up: Strengthening neurodevelopment investigation within the RDoC framework. J Affect Disord, 216, 30–35, doi: 10.1016/j.jad.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojtabai R, Bromet EJ, Harvey PD, Carlson GA, Craig TJ, & Fennig S (2000). Neuropsychological differences between first-admission schizophrenia and psychotic affective disorders. Am J Psychiatry, 157(9), 1453–1460, doi: 10.1176/appi.ajp.157.9.1453. [DOI] [PubMed] [Google Scholar]
- Mollon J, & Reichenberg A (2017). Cognitive development prior to onset of psychosis. Psychol Med, 1–12, doi: 10.1017/s0033291717001970. [DOI] [PubMed] [Google Scholar]
- Muralidharan K, Torres IJ, Silveira LE, Kozicky JM, Bucker J, Fernando N, et al. (2014). Impact of depressive episodes on cognitive deficits in early bipolar disorder: data from the Systematic Treatment Optimization Programme for Early Mania (STOP-EM). Br J Psychiatry, 205(1), 36–43, doi: 10.1192/bjp.bp.113.135525. [DOI] [PubMed] [Google Scholar]
- Murray RM, & Lewis SW (1987). Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed), 295(6600), 681–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen RE, Levander S, Kjaersdam Telleus G, Jensen SO, Ostergaard Christensen T, & Leucht S (2015). Second-generation antipsychotic effect on cognition in patients with schizophrenia--a meta-analysis of randomized clinical trials. Acta Psychiatr Scand, 131(3), 185–196, doi: 10.1111/acps.12374. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Johnson JK, McKinley M, Loewy R, O’Brien M, et al. (2006). Neurocognitive performance and functional disability in the psychosis prodrome. Schizophr Res, 84(1), 100–111, doi: 10.1016/j.schres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Rosso IM, Sanchez LE, Hadley T, Nuechterlein KH, et al. (2003). A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. Am J Psychiatry, 160(11), 2060–2062, doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- O’Brien JT, Ames D, Schweitzer I, Colman P, Desmond P, & Tress B (1996). Clinical and magnetic resonance imaging correlates of hypothalamic-pituitary-adrenal axis function in depression and Alzheimer’s disease. Br J Psychiatry, 168(6), 679–687. [DOI] [PubMed] [Google Scholar]
- Ohaeri JU (2003). The burden of caregiving in families with a mental illness: a review of 2002. Current Opinion in Psychiatry, 16(4), 457–465. [Google Scholar]
- Olvet DM, Stearns WH, McLaughlin D, Auther AM, Correll CU, & Cornblatt BA (2010). Comparing clinical and neurocognitive features of the schizophrenia prodrome to the bipolar prodrome. Schizophr Res, 123(1), 59–63, doi: 10.1016/j.schres.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdel O, Karadag F, Atesci FC, Oguzhanoglu NK, & Cabuk T (2007). Cognitive functions in euthymic patients with bipolar disorder. Ann Saudi Med, 27(4), 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parellada M, Gomez-Vallejo S, Burdeus M, & Arango C (2017). Developmental Differences Between Schizophrenia and Bipolar Disorder. Schizophr Bull, 43(6), 1176–1189, doi: 10.1093/schbul/sbx126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Holzman PS, & Lenzenweger MF (1995). Individual differences in spatial working memory in relation to schizotypy. J Abnorm Psychol, 104(2), 355–363. [DOI] [PubMed] [Google Scholar]
- Paya B, Rodriguez-Sanchez JM, Otero S, Munoz P, Castro-Fornieles J, Parellada M, et al. (2013). Premorbid impairments in early-onset psychosis: differences between patients with schizophrenia and bipolar disorder. Schizophr Res, 146(1–3), 103–110, doi: 10.1016/j.schres.2013.01.029. [DOI] [PubMed] [Google Scholar]
- Pena J, Ojeda N, Segarra R, Eguiluz JI, Garcia J, & Gutierrez M (2011). Executive functioning correctly classified diagnoses in patients with first-episode psychosis: evidence from a 2-year longitudinal study. Schizophr Res, 126(1–3), 77–80, doi: 10.1016/j.schres.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Prado CE, Watt S, & Crowe SF (2018). A meta-analysis of the effects of antidepressants on cognitive functioning in depressed and non-depressed samples. Neuropsychol Rev, 28(1), 32–72, doi: 10.1007/s11065-018-9369-5. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J, De Smedt G, Harvey PD, & Davidson M (2002). Relationship between premorbid functioning and symptom severity as assessed at first episode of psychosis. Am J Psychiatry, 159(12), 2021–2026, doi: 10.1176/appi.ajp.159.12.2021. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, & Gogtay N (2012). Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry, 17(12), 1228–1238, doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratheesh A, Lin A, Nelson B, Wood SJ, Brewer W, Betts J, et al. (2013). Neurocognitive functioning in the prodrome of mania--an exploratory study. J Affect Disord, 147(1–3), 441–445, doi: 10.1016/j.jad.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM, et al. (2010). Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry, 167(2), 160–169, doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, & Harvey PD (2007). Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychol Bull, 133(5), 833–858, doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, et al. (2009). Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr Bull, 35(5), 1022–1029, doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Rabinowitz J, Caspi A, Schmeidler J, Mark M, et al. (2002). A population-based cohort study of premorbid intellectual, language, and behavioral functioning in patients with schizophrenia, schizoaffective disorder, and nonpsychotic bipolar disorder. Am J Psychiatry, 159(12), 2027–2035, doi: 10.1176/appi.ajp.159.12.2027. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Hill SK, Gold JM, Keefe RS, Clementz BA, Gershon E, et al. (2017). Impaired Context Processing is Attributable to Global Neuropsychological Impairment in Schizophrenia and Psychotic Bipolar Disorder. Schizophr Bull, 43(2), 397–406, doi: 10.1093/schbul/sbw081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell ER, Neill JC, Harte M, Khan Z, & Drake RJ (2015). A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophr Res, 168(1–2), 213–222, doi: 10.1016/j.schres.2015.08.017. [DOI] [PubMed] [Google Scholar]
- Rossler W, Ajdacic-Gross V, Muller M, Rodgers S, Kawohl W, Haker H, et al. (2015). Association between processing speed and subclinical psychotic symptoms in the general population: focusing on sex differences. Schizophr Res, 166(1–3), 316–321, doi: 10.1016/j.schres.2015.05.026. [DOI] [PubMed] [Google Scholar]
- Rossler W, Salize HJ, van Os J, & Riecher-Rossler A (2005). Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol, 15(4), 399–409, doi: 10.1016/j.euroneuro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Rund BR, Barder HE, Evensen J, Haahr U, ten Velden Hegelstad W, Joa I, et al. (2016). Neurocognition and Duration of Psychosis: A 10-year Follow-up of First-Episode Patients. Schizophr Bull, 42(1), 87–95, doi: 10.1093/schbul/sbv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samame C, Martino DJ, & Strejilevich SA (2014). Longitudinal course of cognitive deficits in bipolar disorder: a meta-analytic study. J Affect Disord, 164, 130–138, doi: 10.1016/j.jad.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Sanchez-Moreno J, Martinez-Aran A, Tabares-Seisdedos R, Torrent C, Vieta E, & Ayuso-Mateos JL (2009). Functioning and disability in bipolar disorder: an extensive review. Psychother Psychosom, 78(5), 285–297, doi: 10.1159/000228249. [DOI] [PubMed] [Google Scholar]
- Sanchez-Torres AM, Moreno-Izco L, Lorente-Omenaca R, Cabrera B, Lobo A, Gonzalez-Pinto AM, et al. (2017). Individual trajectories of cognitive performance in first episode psychosis: a 2-year follow-up study. Eur Arch Psychiatry Clin Neurosci, doi: 10.1007/s00406-017-0857-z. [DOI] [PubMed] [Google Scholar]
- Savitz J, van der Merwe L, Stein DJ, Solms M, & Ramesar R (2009). Neuropsychological status of bipolar I disorder: impact of psychosis. Br J Psychiatry, 194(3), 243–251, doi: 10.1192/bjp.bp.108.052001. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Posener JA, DeBattista C, Kalehzan BM, Rothschild AJ, & Shear PK (2000). Neuropsychological deficits in psychotic versus nonpsychotic major depression and no mental illness. Am J Psychiatry, 157(7), 1095–1100, doi: 10.1176/appi.ajp.157.7.1095. [DOI] [PubMed] [Google Scholar]
- Schouws SN, Stek ML, Comijs HC, Dols A, & Beekman AT (2012). Cognitive decline in elderly bipolar disorder patients: a follow-up study. Bipolar Disord, 14(7), 749–755, doi: 10.1111/bdi.12000. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Cherkerzian S, Goldstein JM, Agnew-Blais J, Tsuang MT, & Buka SL (2013). Neuropsychological performance and family history in children at age 7 who develop adult schizophrenia or bipolar psychosis in the New England Family Studies. Psychol Med, 43(1), 119–131, doi: 10.1017/s0033291712000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, et al. (2010). Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry, 67(6), 578–588, doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Kremen WS, Koren D, Faraone SV, Goldstein JM, & Tsuang MT (2002). A comparative profile analysis of neuropsychological functioning in patients with schizophrenia and bipolar psychoses. Schizophr Res, 53(1–2), 31–44. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, & Mirsky AF (2017). Evolving Notions of Schizophrenia as a Developmental Neurocognitive Disorder. J Int Neuropsychol Soc, 23(9–10), 881–892, doi: 10.1017/S1355617717001114. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA, et al. (2016). Association of Neurocognition With Transition to Psychosis: Baseline Functioning in the Second Phase of the North American Prodrome Longitudinal Study. JAMA Psychiatry, 73(12), 1239–1248, doi: 10.1001/jamapsychiatry.2016.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selva G, Salazar J, Balanza-Martinez V, Martinez-Aran A, Rubio C, Daban C, et al. (2007). Bipolar I patients with and without a history of psychotic symptoms: do they differ in their cognitive functioning? J Psychiatr Res, 41(3–4), 265–272, doi: 10.1016/j.jpsychires.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Sheffield JM, Gold JM, Strauss ME, Carter CS, MacDonald AW 3rd, Ragland JD, et al. (2014). Common and specific cognitive deficits in schizophrenia: relationships to function. Cogn Affect Behav Neurosci, 14(1), 161–174, doi: 10.3758/s13415-013-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Kandala S, Burgess GC, Harms MP, & Barch DM (2016). Cingulo-opercular network efficiency mediates the association between psychotic-like experiences and cognitive ability in the general population. Biol Psychiatry Cogn Neurosci Neuroimaging, 1(6), 498–506, doi: 10.1016/j.bpsc.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Ruge H, Kandala S, & Barch DM (2018). Rapid instruction-based task learning (RITL) in schizophrenia. J Abnorm Psychol, 127(5), 513–528, doi: 10.1037/abn0000354. [DOI] [PubMed] [Google Scholar]
- Sheffield JM, Williams LE, Cohen N, & Heckers S (2012). Relational memory in psychotic bipolar disorder. Bipolar Disord, 14(5), 537–546, doi: 10.1111/j.1399-5618.2012.01036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S, Uhlhaas PJ, Essex B, Halpin S, Schall U, & Carr V (2006). Perceptual organization in first episode schizophrenia and ultra-high-risk states. Schizophr Res, 83(1), 41–52, doi: 10.1016/j.schres.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Keane BP, Barch DM, Carter CS, Gold JM, Kovacs I, et al. (2012). Optimization and validation of a visual integration test for schizophrenia research. Schizophr Bull, 38(1), 125–134, doi: 10.1093/schbul/sbr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AE, Cattapan-Ludewig K, Zmilacher S, Arbach D, Gruber K, Dvorsky DN, et al. (2007). Cognitive functioning in the schizophrenia prodrome. Schizophr Bull, 33(3), 761–771, doi: 10.1093/schbul/sbm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C, Sundet K, Vaskinn A, Birkenaes AB, Engh JA, Faerden A, et al. (2011). Neurocognitive dysfunction in bipolar and schizophrenia spectrum disorders depends on history of psychosis rather than diagnostic group. Schizophr Bull, 37(1), 73–83, doi: 10.1093/schbul/sbp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen HJ, Saebye D, Urfer-Parnas A, Mortensen EL, & Parnas J (2012). Premorbid intelligence and educational level in bipolar and unipolar disorders: a Danish draft board study. J Affect Disord, 136(3), 1188–1191, doi: 10.1016/j.jad.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Sperry SH, O’Connor LK, Ongur D, Cohen BM, Keshavan MS, & Lewandowski KE (2015). Measuring Cognition in Bipolar Disorder with Psychosis Using the MATRICS Consensus Cognitive Battery. J Int Neuropsychol Soc, 21(6), 468–472, doi: 10.1017/s1355617715000442. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Allen DN, Miski P, Buchanan RW, Kirkpatrick B, & Carpenter WT Jr. (2012). Differential patterns of premorbid social and academic deterioration in deficit and nondeficit schizophrenia. Schizophr Res, 135(1–3), 134–138, doi: 10.1016/j.schres.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CY, Wang PW, Lin YJ, Tang TC, Liu MF, & Chen MD (2016). The effects of aerobic exercise on cognition in schizophrenia: A 3-month follow-up study. Psychiatry Res, 244, 394–402, doi: 10.1016/j.psychres.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Tabares-Seisdedos R, Balanza-Martinez V, Sanchez-Moreno J, Martinez-Aran A, Salazar-Fraile J, Selva-Vera G, et al. (2008). Neurocognitive and clinical predictors of functional outcome in patients with schizophrenia and bipolar I disorder at one-year follow-up. J Affect Disord, 109(3), 286–299, doi: 10.1016/j.jad.2007.12.234. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Haukka J, Henriksson M, Cannon M, Kieseppa T, Laaksonen I, et al. (2005). Premorbid intellectual functioning in bipolar disorder and schizophrenia: results from a cohort study of male conscripts. Am J Psychiatry, 162(10), 1904–1910, doi: 10.1176/appi.ajp.162.10.1904. [DOI] [PubMed] [Google Scholar]
- Tohen M, Strakowski SM, Zarate C Jr., Hennen J, Stoll AL, Suppes T, et al. (2000). The McLean-Harvard first-episode project: 6-month symptomatic and functional outcome in affective and nonaffective psychosis. Biol Psychiatry, 48(6), 467–476. [DOI] [PubMed] [Google Scholar]
- Torrent C, Bonnin Cdel M, Martinez-Aran A, Valle J, Amann BL, Gonzalez-Pinto A, et al. (2013). Efficacy of functional remediation in bipolar disorder: a multicenter randomized controlled study. Am J Psychiatry, 170(8), 852–859, doi: 10.1176/appi.ajp.2012.12070971. [DOI] [PubMed] [Google Scholar]
- Torrent C, Reinares M, Martinez-Aran A, Cabrera B, Amoretti S, Corripio I, et al. (2018). Affective versus non-affective first episode psychoses: A longitudinal study. J Affect Disord, 238, 297–304, doi: 10.1016/j.jad.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Torres IJ, Boudreau VG, & Yatham LN (2007). Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatr Scand Suppl(434), 17–26, doi: 10.1111/j.1600-0447.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- Torres IJ, Kozicky J, Popuri S, Bond DJ, Honer WG, Lam RW, et al. (2014). 12-month longitudinal cognitive functioning in patients recently diagnosed with bipolar disorder. Bipolar Disord, 16(2), 159–171, doi: 10.1111/bdi.12154. [DOI] [PubMed] [Google Scholar]
- Trisha C, Golnoush A, Jan-Marie K, Torres IJ, & Yatham LN (2017). Cognitive functioning in first episode bipolar I disorder patients with and without history of psychosis. J Affect Disord, 227, 109–116, doi: 10.1016/j.jad.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Trisha C, Golnoush A, Jan-Marie K, Torres IJ, & Yatham LN (2018). Cognitive functioning in first episode bipolar I disorder patients with and without history of psychosis. J Affect Disord, 227, 109–116, doi: 10.1016/j.jad.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Trotta A, Murray RM, & MacCabe JH (2015). Do premorbid and post-onset cognitive functioning differ between schizophrenia and bipolar disorder? A systematic review and meta-analysis. Psychol Med, 45(2), 381–394, doi: 10.1017/s0033291714001512. [DOI] [PubMed] [Google Scholar]
- van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, & Krabbendam L (2009). A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med, 39(2), 179–195, doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- Van Rheenen TE, Bryce S, Tan EJ, Neill E, Gurvich C, Louise S, et al. (2016). Does cognitive performance map to categorical diagnoses of schizophrenia, schizoaffective disorder and bipolar disorder? A discriminant functions analysis. J Affect Disord, 192, 109–115, doi: 10.1016/j.jad.2015.12.022. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Mahurin RK, Diamond PL, Hazleton BC, Eckert SL, & Miller AL (1997). The functional significance of symptomatology and cognitive function in schizophrenia. Schizophr Res, 25(1), 21–31, doi: 10.1016/S0920-9964(97)00010-8. [DOI] [PubMed] [Google Scholar]
- Vohringer PA, Barroilhet SA, Amerio A, Reale ML, Alvear K, Vergne D, et al. (2013). Cognitive impairment in bipolar disorder and schizophrenia: a systematic review. Front Psychiatry, 4, 87, doi: 10.3389/fpsyt.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt NF, & Lubensky AW (1976). Childhood roots of schizophrenia. J Consult Clin Psychol, 44(3), 363–375. [DOI] [PubMed] [Google Scholar]
- Weinberger DR (1987). Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry, 44(7), 660–669. [DOI] [PubMed] [Google Scholar]
- Welham J, Scott J, Williams GM, Najman JM, Bor W, O’Callaghan M, et al. (2010). The antecedents of non-affective psychosis in a birth-cohort, with a focus on measures related to cognitive ability, attentional dysfunction and speech problems. Acta Psychiatr Scand, 121(4), 273–279, doi: 10.1111/j.1600-0447.2009.01470.x. [DOI] [PubMed] [Google Scholar]
- Wingo AP, Harvey PD, & Baldessarini RJ (2009). Neurocognitive impairment in bipolar disorder patients: functional implications. Bipolar Disord, 11(2), 113–125, doi: 10.1111/j.1399-5618.2009.00665.x. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, Giuliano AJ, & Seidman LJ (2008). Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry, 165(5), 579–587, doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- Woodward ND (2016). The course of neuropsychological impairment and brain structure abnormalities in psychotic disorders. Neurosci Res, 102, 39–46, doi: 10.1016/j.neures.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, & Czobor P (2011). A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry, 168(5), 472–485, doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Zabala A, Rapado M, Arango C, Robles O, de la Serna E, Gonzalez C, et al. (2010). Neuropsychological functioning in early-onset first-episode psychosis: comparison of diagnostic subgroups. Eur Arch Psychiatry Clin Neurosci, 260(3), 225–233, doi: 10.1007/s00406-009-0046-9. [DOI] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, David AS, Dalman C, Hemmingsson T, Lundberg I, et al. (2004). A longitudinal study of premorbid IQ Score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Arch Gen Psychiatry, 61(4), 354–360, doi: 10.1001/archpsyc.61.4.354. [DOI] [PubMed] [Google Scholar]
- Zanelli J, Reichenberg A, Morgan K, Fearon P, Kravariti E, Dazzan P, et al. (2010). Specific and generalized neuropsychological deficits: a comparison of patients with various first-episode psychosis presentations. Am J Psychiatry, 167(1), 78–85, doi: 10.1176/appi.ajp.2009.09010118. [DOI] [PubMed] [Google Scholar]
- Zaninotto L, Guglielmo R, Calati R, Ioime L, Camardese G, Janiri L, et al. (2015). Cognitive markers of psychotic unipolar depression: a meta-analytic study. J Affect Disord, 174, 580–588, doi: 10.1016/j.jad.2014.11.027. [DOI] [PubMed] [Google Scholar]