Abstract
Background & Aims
Inflammation affects regeneration of the intestinal epithelia; long noncoding RNAs (lncRNAs) regulate cell functions such as proliferation, differentiation, and migration. We investigated the mechanisms by which the lncRNA H19, imprinted maternally expressed transcript (H19) regulates regeneration of intestinal epithelium using cell cultures and mouse models of inflammation.
Methods
We performed RNA-seq transcriptome analyses of intestinal tissues from mice with lipopolysaccharide (LPS)-induced sepsis to identify lncRNAs associated with inflammation; findings were confirmed by quantitative real-time polymerase chain reaction and in situ hybridization analyses of intestinal tissues from mice with sepsis or dextran sulfate sodium (DSS)-induced mucosal wound healing and patients with ulcerative colitis compared to healthy individuals (controls). We screened cytokines for their ability to induce expression of H19 in HT-29 cells and intestinal epithelial cells (IECs), and confirmed findings in crypt epithelial organoids derived from mouse small intestine. IECs were incubated with different signal transduction inhibitors and effects on H19 lncRNA levels were measured. We assessed intestinal epithelial proliferation or regeneration in H19ΔEx1/+ mice given LPS or DSS vs wild-type littermates (control mice). H19 was overexpressed in IECs using lentiviral vectors and cell proliferation was measured. We performed RNA antisense purification, RNA immunoprecipitation, and luciferase reporter assays to study functions of H19 in IECs.
Results
In RNA-sequencing transcriptome analysis of lncRNA expression in intestinal tissues from mice, we found that levels of H19 lncRNA changed significantly with LPS exposure. Levels of H19 lncRNA increased in intestinal tissues of patients with ulcerative colitis, mice with LPS-induced and polymicrobial sepsis, or mice with DSS-induced colitis, compared with controls. Increased H19 lncRNA localized to epithelial cells in the intestine, regardless of Lgr5 messenger RNA expression. Exposure of IECs to interleukin 22 (IL22) increased levels of H19 lncRNA with time and dose, which required STAT3 and protein kinase A activity. IL22 induced expression of H19 in mouse intestinal epithelial organoids within 6 hours. Exposure to IL22 increased growth of intestinal epithelial organoids derived from control mice, but not H19ΔEx1/+ mice. Overexpression of H19 in HT-29 cells increased their proliferation. Intestinal mucosa healed more slowly after withdrawal of DSS from H19ΔEx1/+ mice vs control mice. Crypt epithelial cells from H19ΔEx1/+ mice proliferated more slowly than those from control mice after exposure to LPS. H19 lncRNA bound to p53 and microRNAs that inhibit cell proliferation, including microRNA 34a and let-7; H19 lncRNA binding blocked their function, leading to increased expression of genes that promote regeneration of the epithelium.
Conclusions
The level of lncRNA H19 is increased in inflamed intestinal tissues from mice and patients. The inflammatory cytokine IL22 induces expression of H19 in IECs, which is required for intestinal epithelial proliferation and mucosal healing. H19 lncRNA appears to inhibit p53 protein and microRNA 34a and let-7 to promote proliferation of IECs and epithelial regeneration.
Keywords: gene regulation, mouse model, ulcerative colitis, tissue repair
Graphical Abstract
INTRODUCTION
Long noncoding RNAs (lncRNAs) represent a large and diverse class of non-protein coding transcripts longer than 200 nucleotides1. High-throughput sequencing analysis of the whole mammalian genome and transcriptome revealed a vast number of lncRNAs, and growing evidence shows that several lncRNAs have biological roles in regulating gene expression, controlling protein function, and organizing multiprotein complex assembly1. lncRNAs act as intracellular signals, decoys, guides, and scaffolds via DNA, RNA, and protein interactions. Several lncRNAs have also been proposed to participate in cell signaling, thereby impacting cellular functions and homeostasis in vivo2,3. Mounting studies support a role of lncRNAs in the pathogenesis of diseases1.
The intestinal epithelium is a single layer of columnar cells lining the luminal surface of the intestinal mucosa that is regenerated throughout adult life. It provides a critical barrier to harmful intraluminal entities, including foreign antigens and micro-organisms and their toxins. In the normal physiological state, renewal of the intestinal epithelium is governed by canonical Wnt signaling4. Intestinal epithelial injury often results from inflammatory bowel disease, which causes exacerbated inflammation in the intestinal mucosa. It also can occur in several critical conditions, such as sepsis, severe burn injury, and gastrointestinal radiation injury, during which bacteria or bacterial products are permitted to translocate across the intestinal epithelial barrier and into the bloodstream. Epithelial regeneration is a critical step for wound healing of the intestinal mucosa and plays an important role in sustaining intestinal epithelial integrity in response to inflammation and in critically ill patients. Previous studies suggest that interleukin (IL) 6 and IL22 promote intestinal epithelial regeneration in pathological conditions through distinctive receptor-mediated signal pathways5–7, suggesting a role for inflammatory cytokine-associated signaling. In addition to inflammatory cytokine-associated signaling pathways, studies have shown that intestinal epithelial cells (IECs) express a number of negative regulators to control regeneration of intestinal epithelium, for example, p53 protein8. Recently, microRNAs (miRNAs), such as the let-7 family members have been identified as negative regulators of IEC proliferation9. It remains unknown whether and how lncRNAs participate in regulation of intestinal epithelial regeneration and homeostasis.
In this report, we provided evidence that H19, an evolutionarily conserved and maternally expressed imprinted lncRNA10, is induced by inflammation in IECs. We defined the effects of inflammatory mediators on expression of H19 lncRNA in IECs, investigated the role of H19 in intestinal epithelial wound healing, and elucidated the underlying molecular mechanisms by which H19 lncRNA promotes re-establishment and sustains homeostasis of intestinal epithelium. Our study revealed that H19 lncRNA is an inflammatory lncRNA induced by IL22 that antagonizes negative regulators of intestinal epithelial proliferation and thus plays an important role in sustaining intestinal epithelial regeneration under inflammatory conditions.
MATERIAL AND METHODS
Detailed protocols are provided in the Supplementary Materials and Methods.
RESULTS
Inflammation results in the induction of intestinal H19 long noncoding RNA that is localized to Lgr5+ and Lgr5− epithelial cells in the intestinal mucosa
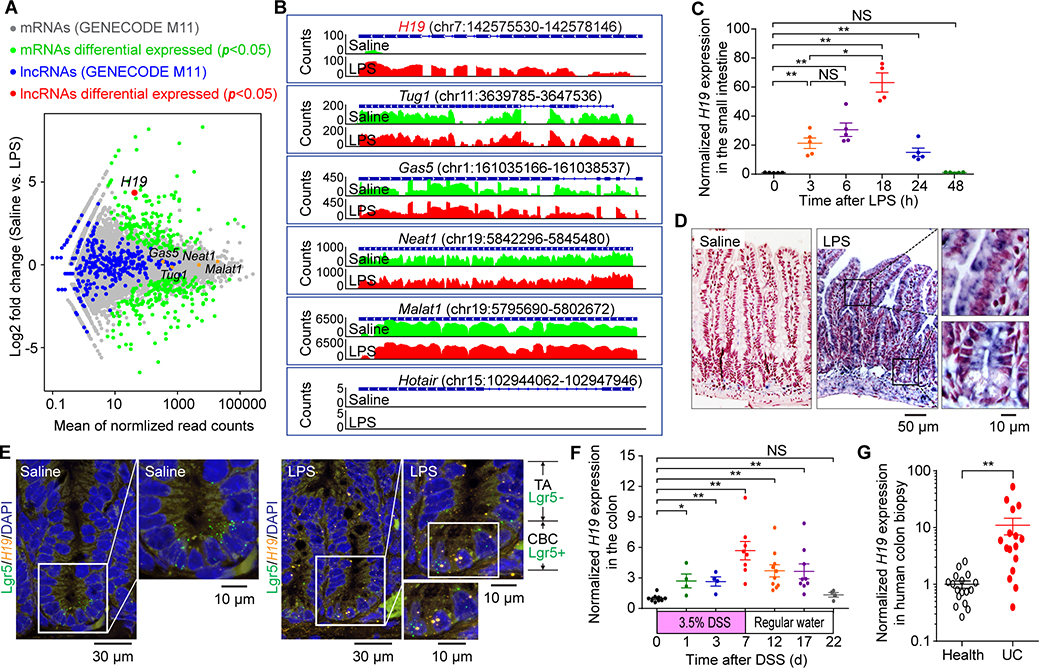
Although lncRNAs are thought to be a vast family of functional molecules associated with diverse biological processes in cells, their roles in sustaining tissue homeostasis in vivo remain largely unknown. To fill this knowledge gap, we profiled gene expression in the small intestine of mice with lipopolysaccharide (LPS)-induced sepsis using RNA sequencing (RNA-seq) transcriptome analysis. LPS challenge for 24 hours resulted in alterations in the expression of a large number of protein-coding genes associated with various biological processes (Figure 1A and Supplementary Figure 1A). However, from among 10,481 mouse lncRNAs (GENECODE, M11), only the levels of H19 gene transcripts showed significant change in the small intestine in response to LPS-induced sepsis (Figure 1A). Our further analysis revealed that H19 gene is normally transcriptionally silent in adult mouse small intestine, but is strongly activated by LPS treatment compared to other frequently studied lncRNAs (Figure 1B). Using quantitative real-time RT-PCR, we found that de novo expression of intestinal H19 occurred within 3 hours, peaked at 18 hours, and was gradually silenced by 48 hours after LPS treatment in mice (Figure 1C). We also observed that LPS challenge activated intestinal H19 expression in both male and female mice (Supplementary Figure 1B) and the effect was both dose-dependent and tissue-specific (Supplementary Figure 1C and D). Remarkably, in situ hybridization analysis revealed that LPS-evoked sepsis led to dramatically increased H19 expression in villus and crypt epithelial cells of the mouse small intestine (Figure 1D). The intestinal crypt epithelium contains Lgr5+ crypt base-columnar stem cells and Lgr5− transit-amplifying (TA) progenitor cells. Using a dual-color fluorescent in situ hybridization assay, we further found that LPS-induced H19 lncRNA is localized to Lgr5+ crypt base-columnar stem cells near the crypt bottom and Lgr5− epithelial cells within the TA zone in crypts (Figure 1E).
Figure 1. H19 is an early-response gene in inflammation of the intestinal epithelium.
(A) Bland-Altman plot showing comparative intestinal gene expression in LPS (2 mg/kg, intraperitoneally for 24 hours) and saline-treated mice. n=3 males for each group. (B) University of California, Santa Cruz Browser images illustrating normalized mouse small intestinal tissue RNA-Seq read densities across introns of indicated lncRNA genes at 24 hours after the indicated treatments. (C) quantitative real-time PCR (qRT-PCR) analysis of H19 expression in the small intestine of mice subjected to LPS treatment. (D) Representative micrographs showing cellular localization of LPS-induced H19 transcripts (blue) in the mouse small intestine by in situ hybridization using antisense RNA probes to H19 lncRNA. Slides were counterstained with Nuclear Fast Red (red). (E) Merged confocal fluorescent microscopy image for the localization of H19 transcripts and Lgr5 messenger RNA in the small intestinal crypts. Mouse small intestine was stained using RNAscope® Multiplex Fluorescent Assay with probes for H19 transcripts (orange) and Lgr5 mRNA (green) followed by counterstaining with 4′,6-diamidino-2-phenylindole (blue). (F, G) qRT-PCR analysis of H19 expression in colons of mice subjected to DSS-induced colitis (F) or colon biopsies from healthy subjects and patients with ulcerative colitis (UC) (G). Bars = mean ± SEM. *P< 0.05, **P< 0.01, NS = not significant.
Intestinal H19 expression was also triggered by TNF treatment and polymicrobial sepsis induced by cecal ligation and puncture in mice (Supplementary Figure 1E and F). Furthermore, using a dextran sulfate sodium (DSS)-induced colitis and recovery mouse model (Supplementary Figure 2), we observed highly H19 expression in the colon during acute colitis and recovery phase in mice (Figure 1F). We also found that H19 levels in the colonic mucosa were significantly higher in patients with ulcerative colitis compared to healthy individuals (Figure 1G). Together, these data indicate that H19 is unique among lncRNAs in its rapid response to acute inflammation in IECs.
Interleukin 22 induces H19 expression in intestinal epithelial cells in vitro
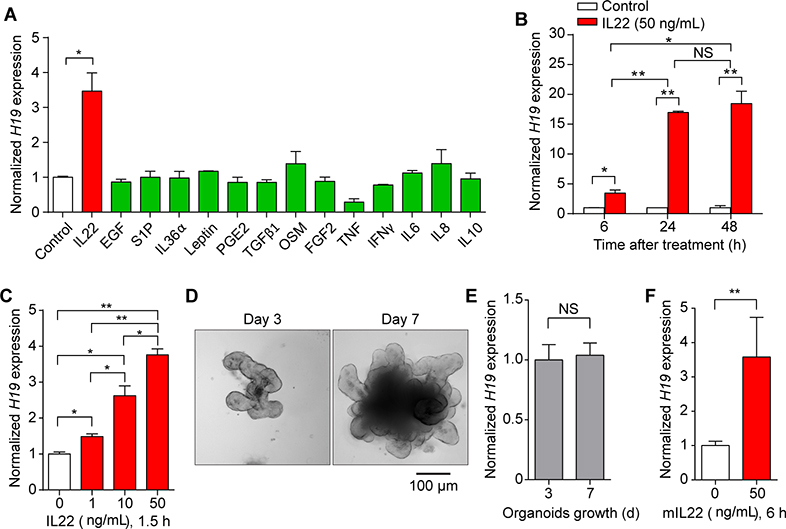
Given the induction of H19 lncRNA in IECs in vivo under acute inflammatory conditions, we searched for factors that might trigger H19 gene expression in HT-29 cells (a human IEC line). To this end, 14 inflammation-associated molecules were screened for their ability to induce H19 gene expression in IECs in vitro. Among these, only IL22 treatment induced H19 lncRNA in HT-29 cells (Figure 2A). We further focused on characterizing the effect of IL22 on induction of H19 expression in IECs.
Figure 2. IL22 is a potent inducer of H19 gene expression in mouse and human intestinal epithelial cells.
(A) qRT-PCR analysis showing that IL22 is a distinctive cytokine-inducing H19 expression in HT-29 cells. Six hours treatment with indicated molecules. (B, C) qRT-PCR analysis showing that IL22 induces H19 expression in HT-29 cells in a time- (B) and dose- (C) dependent manner. (D, E) Typical brightfield images (D) and qRT-PCR-measured H19 expression profile (E) of mouse primary small intestinal crypt epithelial organoids cultured in Matrigel droplets for the indicated times. (F) IL22 induced H19 expression in mouse primary IECs. Mouse small intestinal epithelial organoids formed in 7-day Matrigel culture were treated with or without IL22 (50 ng/mL) for 6 hours followed by measurement of H19 expression using qRT-PCR. n=4 in panels A–C, n=9 in panels E and F. Bars, mean ± SEM. *P< 0.05, **P< 0.01, NS = not significant.
Notably, we found that IL22 induced intestinal epithelial H19 expression in a time- and dose-dependent fashion (Figure 2B and C), similar to that observed in the small intestine of mice after LPS treatment (Figure 1C and Supplementary Figure 1C). Treatment of mouse intestine explant with IL22 also resulted in increased H19 expression (Supplementary Figure 3), suggesting that H19 expression in murine IECs is activated by IL22. To further test this notion, we isolated crypt epithelial organoids from the mouse small intestine and assessed H19 expression in the organoids after cultured them with or without IL22 treatment. We found that growth of mouse crypt epithelial organoids under the naïve condition (Figure 2D) was not associated with alterations in H19 lncRNA expression (Figure 2E), whereas stimulation with IL22 for 6 hours increased H19 lncRNA levels significantly in the cells (Figure 2F). Together, the data suggest that the inflammatory cytokine IL22 activates H19 gene expression in IECs.
H19 expression is activated by IL22 in intestinal epithelial cells through Protein Kinase A and STAT3-associated signal mechanisms
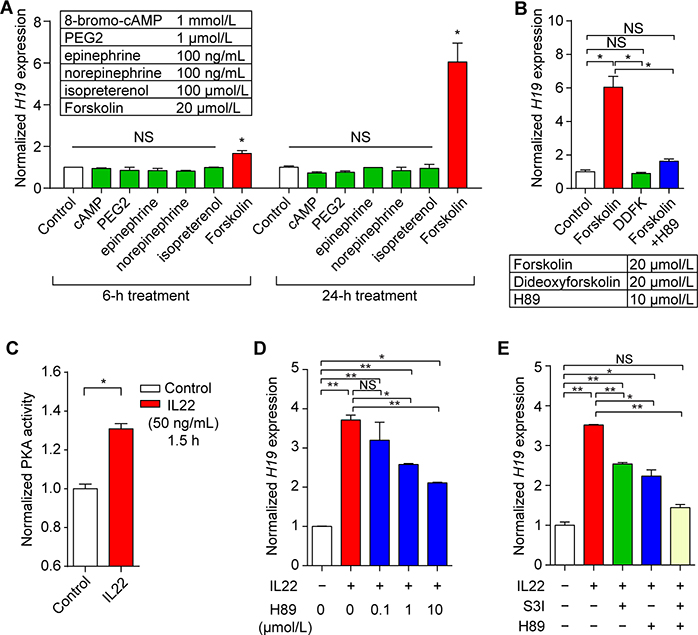
We next sought to delineate the intracellular signal pathways linking IL22 to H19 expression in IECs. Previously, Voutilainen et al. reported that a Protein Kinase A (PKA) activator, namely, forskolin, induces H19 expression in vitro11. Consistent with this, we found a late effect of forskolin treatment on H19 expression in HT-29 cells (Figure 3A and B); however, we did not observe up-regulation of H19 by G-protein-coupled receptor agonists that are known to activate PKA (Figure 3A). This suggests that activation of PKA independent of G-protein-coupled receptors may be involved in the induction of H19 expression in IECs.
Figure 3. PKA and STAT3 mediate the effect of IL22 on H19 expression in intestinal epithelial cells.
(A) Screening the effect of PKA activators on H19 expression in IECs using qRT-PCR. (B) Additional results of qRT-PCR assay show a PKA-dependent effect of forskolin on induction of H19 expression in IECs at 24-hour treatment. DDFK, dideoxyforskolin. (C) IL22 induced PKA activation in IECs. HT-29 cells were subjected to the indicated treatments followed by processing for analysis of PKA activity. (D) The PKA inhibitor H89 dose-dependently blocked the IL22 effect on H19 expression in HT-29 cells. Cells were pretreated with H89 at the indicated concentrations for 30 minutes before treatment with IL22 (50 ng/mL) for 1.5 hours followed by measurement of H19 expression by qRT-PCR assay. (E) qRT-PCR analysis showing an additive effect of inhibitors for STAT3 and PKA on IL22-induced H19 expression. Cells were pretreated with the indicated inhibitors (1 μmol/L for H89 and 100 μmol/L for S3I-201) for 30 minutes before treatment with IL22 (50 ng/mL) for 1.5 hours. S3I, S3I-201. n=4 each. Bars, mean ± SEM. *P< 0.05, **P< 0.01, NS = not significant.
To determine whether and how PKA mediates IL22-induced H19 expression in IECs, we executed the following set of experiments. First, we observed that IL22 significantly activates PKA in HT-29 cells (Figure 3C). Next, we found that H89 (a potent PKA inhibitor targeting the catalytic subunits) suppressed IL22-induced H19 expression in a dose-dependent manner (Figure 3D). Similarly, pretreatment with PKI (14–22), a synthetic peptide with highly specific inhibitory activity against the PKA catalytic subunit, but not pretreatment with Rp-cAMPS (a PKA inhibitor that blocks adenosine 3′,5′-cyclic monophosphate [cAMP]–binding sites), attenuated the effect of IL22 on H19 expression in HT-29 cells (Supplementary Figure 4). Together, these data strongly suggested that IL22 induces H19 expression in IECs through activation of PKA independent of cAMP.
Emerging evidence shows that STAT3-mediated signaling is a common pathway utilized by IL22 in IECs6,7. We further investigated a possible synergistic effect of STAT3 signaling and PKA on IL22–induced H19 expression. Treatment of IECs with S3I-201 (STAT3 inhibitor) and H89 blocked the effect of IL22 on H19 expression in an additive manner (Figure 3E), suggesting that IL22 induces H19 expression in IECs via both STAT3 signaling and PKA activation.
H19 plays an important role in sustaining the renewal of epithelial cells during inflammation and enhancing regeneration of the intestinal epithelium in colitis
IL22 is known to play an important role in promoting intestinal homeostatic events, such as epithelial stem cell proliferation and healing of intestinal inflammatory injury6,7,12. Because our data indicate that H19 gene is a downstream target of IL22 signaling, we hypothesized that de novo synthesized H19 lncRNA is involved in the maintenance of intestinal epithelium homeostasis under inflammatory conditions. Acute inflammation-induced alteration of intestinal epithelial homeostasis is characterized by impaired permeability of the intestinal epithelium, potentiated cytostatic state in crypt epithelial cells, and delayed epithelial cell turnover. However, we did not observe a notable correlation between an increase in H19 lncRNA and alteration of intestinal permeability in LPS-treated wild-type C57BL/6 mice (Supplementary Figure 5), indicating that inflammation-induced de novo synthesized H19 lncRNA does not affect the permeability of intestinal epithelium in vivo.
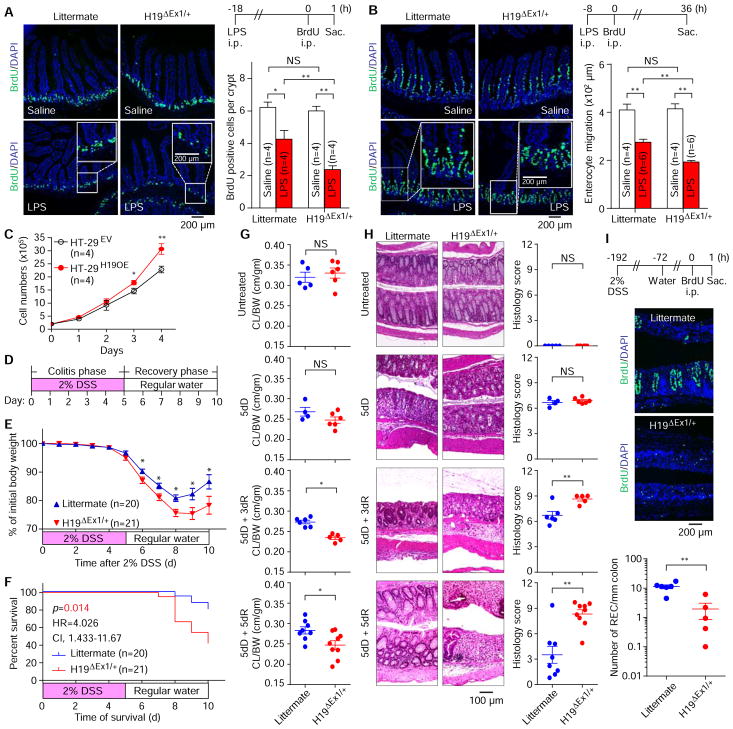
To further test our hypothesis, we examined whether de novo synthesized H19 lncRNA regulates crypt epithelial cell proliferation and enterocyte migration along the crypt-villus axis in the small intestine using H19ΔEx1/+ mice (mutants with maternal deletion of exon 1 in H19 gene). Comparing H19ΔEx1/+ mice with their wild-type littermate controls, we found that H19 lncRNA is not essential for gut development and maintenance of intestinal mucosa under normal physiological conditions (Supplementary Figure 6A). In addition, we verified that induction of intestinal H19 expression by LPS was abolished in H19ΔEx1/+ mice (Supplementary Figure 6B). Using bromodeoxyuridine (BrdU) pulse-chase assay, we revealed that features of intestinal epithelial regeneration and homeostasis, including crypt epithelial cell proliferation and enterocyte migration along the crypt-villus axis, were significantly impaired in both H19ΔEx1/+ lncRNA mice and their wild-type littermate after LPS challenge (Figure 4A and B), suggesting that acute inflammatory insults induce cytostasis or growth arrest in crypt epithelial cells of these mice. However, we noticed a more profound cytostatic and epithelial cell growth arrest response in LPS-treated H19ΔEx1/+ mice than that in their wild-type littermate controls (Figure 4A and B). To determine whether H19 levels impact IEC growth, we established HT-29H19OE cells that overexpress H19 via lentiviral vector-mediated gene transfer technology. HT-29H19OE cells grew significantly faster than HT-29EV cells transduced with recombinant lentivirus engineered with control vector DNA (Figure 4C). This suggests that ectopic expression of H19 increased epithelial cell proliferation. Together, these results indicate that H19 lncRNA synthesized de novo plays a role in abrogating the inflammation-induced cytostatic response, and sustaining intestinal epithelial regeneration.
Figure 4. Critical role of H19 lncRNA in proliferation and regeneration of intestinal epithelium.
(A, B) Bromodeoxyuridine (BrdU) pulse-chase analysis showing the effect of LPS treatment on impairment of crypt epithelial cell proliferation (A) and enterocyte migration along the crypt-villus axis (B) was potentiated in H19ΔEx1/+ mice. (C) in vitro proliferation assay showing that HT-29H19OE cells (ectopic expression of H19) exhibit increased proliferation. (D) Treatment scheme for a mouse DSS colitis recovery model. (E, F) Changes in body weights (E) and survival (F) in mice with DSS-induced colitis and recovery. (G, H) Analysis of colon length-to-body weight ratio (CL/BW) (G) and H & E staining of colon sections (H) of mice subjected to DSS-induced colitis and recovery for the indicated time periods. dD, days of DSS. dR, days of recovery. (I) Assessment of regenerated epithelial clusters (REC) in the colonic mucosa of mice subjected to 3-day recovery from DSS-induced colitis using BrdU (green) pulse-chase analysis. The schematic of the experimental protocol was illustrated on the top of the panel. Bars, mean ± SEM. *P< 0.05, **P< 0.01, NS = not significant.
We further studied the role of H19 lncRNA in the intestinal mucosal inflammatory injury and wound-healing response using H19ΔEx1/+ mice and their wild-type littermate controls with DSS-induced colitis. To this end, a DSS-water treatment scheme outlined using Figure 4D was followed. Monitoring body weight, survival, colon length, and histology revealed that H19 deficiency does not have a notable influence on the development of DSS-induced colitis in male or female mice (Figure 4E–H and Supplementary Figure 6C and D). By contrast, during the recovery phase of colitis, H19ΔEx1/+ mice of both sexes exhibited increased weight loss, a decline in survival, significant reduction in colon length, and a delay in restoration of colon epithelial integrity and resolution of colitis when compared to their wild-type littermate controls (Figure 4E–H and Supplementary Figure 6C–G). In addition, the BrdU labeling assays revealed that H19ΔEx1/+ mice display poor epithelial regeneration in the colonic mucosa during the recovery phase of DSS-colitis compared to their wild-type littermate controls (Figure 4I). Taken together, these results demonstrate that inflammation-induced H19 lncRNA is a critical molecule for intestinal epithelial wound healing and repair.
H19 lncRNA mediates the effect of Interleukin 22 on enhancing the growth of intestinal epithelial organoids
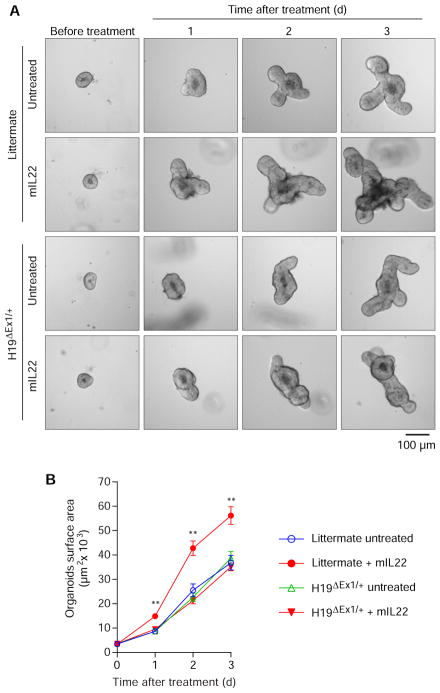
Recently, Lindemans et al. reported that IL22 potently activates intestinal epithelial organoid growth12. Because our data indicated that IL22 induces H19 expression in IECs and H19 lncRNA synthesized de novo plays a critical role in wound healing of gut epithelium, we hypothesized that H19 lncRNA mediates the stimulatory effect of IL22 on intestinal epithelial growth. We compared the IL22 growth response of intestinal epithelial organoids from H19ΔEx1/+ mice to their wild-type littermate controls. IL22 treatment significantly increased the size of intestinal epithelial organoids from wild-type littermate controls, and this was absent in the organoids from H19ΔEx1/+ mice (Figure 5A and B), suggesting that H19 lncRNA synthesized de novo relays the IL22 signal to promote IEC growth.
Figure 5. H19 lncRNA is required for enhancing the growth of intestinal epithelial organoids by IL22 in vitro.
(A, B) Representative bright-field images (A) and surface area expansion rates (B) of small intestinal epithelial organoids from H19ΔEx1/+ mice and their wild-type littermate controls after culture with or without IL22 (10 ng/mL) in Matrigel droplets for the indicated times. Photographs and measurements were taken at the indicated time points. n=15–20 each. Bars, mean ± SEM. **P< 0.01.
Inflammation-induced H19 lncRNA binds to cytostatic regulators and antagonizes their activities in intestinal epithelial cells in vivo and in vitro
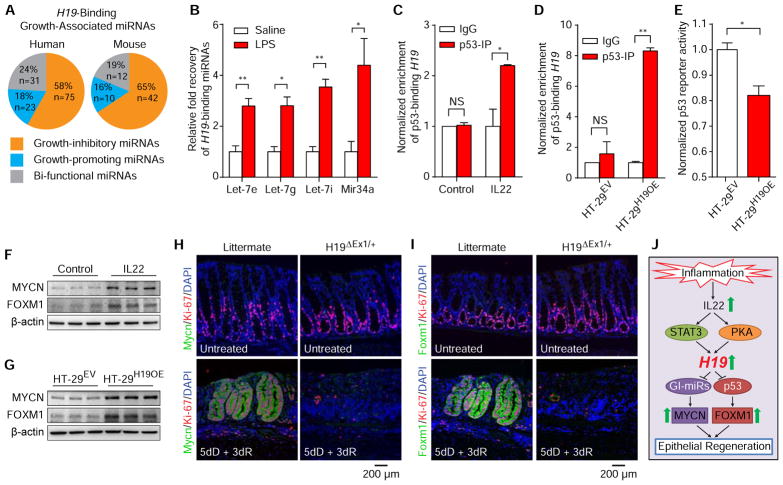
The biochemical functions of the H19 lncRNA remain enigmatic, but a recent study suggests that it binds to the microRNA let-7 to reduce its bioavailability13. Similarly, it has been proposed that lncRNAs interact with transcription factors to modulate their function1. This led us to hypothesize that H19 lncRNA interacts with negative regulators of IEC growth to abrogate their effect. We first used a bioinformatics approach to analyze the potential interactions between H19 lncRNA and known cell growth regulatory miRNAs. Remarkably, a group of cell growth inhibitory miRNAs (GI-miRNAs) was revealed to be the predominant cluster among cell growth-associated H19-binding miRNAs (Figure 6A and Supplementary Table 1). Using an RNA antisense purification assay-based approach, we further confirmed that 2 of predicted H19-binding GI-miRNAs, Mir34a and let-7, were indeed bound by intestinal H19 lncRNA (Figure 6B). The qRT-PCR demonstrated that although LPS treatment profoundly induced H19 expression (Figure 1C), it only slightly affected the expression of Mir34a and let-7 (Supplementary Figure 7) in the mouse intestine. Pearson correlation coefficient analysis revealed a strong correlation between H19 lncRNA levels and the quantity of H19-bound Mir34a and let-7 (Supplementary Table 2). Collectively, the data suggest that inflammation-induced H19 exhibits potential to function as a competitive inhibitor for GI-miRNAs in vivo.
Figure 6. H19 lncRNA binds cytostatic regulators to attenuate their activity and release repression of their gene targets in intestinal epithelial cells.
(A) in silico analysis of growth-associated H19-binding miRNAs. (B) RNA antisense purification (RAP) of H19 lncRNA from the small intestine of mice 18 hours after the indicated treatments followed by qRT-PCR of H19-bound Mir34a and let-7. n=3 each. (C, D) Immunoprecipitation of p53 from whole cell lysates of either HT-29 cells subjected to 24-hour of the indicated treatments (C) or indicated cell types (D) followed by qRT-PCR analysis of H19 transcripts. n=4 each. (E) Luciferase reporter assay showing lower levels of p53 activity in HT-29H19OE cells compared to HT-29EV cells. n=4 each. Bars, mean ± SEM. *P< 0.05, **P< 0.01, NS = not significant. (F, G) Immunoblot analysis of MYCN and FOXM1 expression in HT-29 cells after 24 hours of the indicated treatments (F) or in indicated cells types (G). (H, I) Expression analysis by immunofluorescent staining of Mycn (H) and Foxm1 (I) in mice after the indicated treatments. dD, days of DSS. dR, days of recovery. (J) Model for the role of de novo synthesized H19 lncRNA in linking IL22 signaling to the regeneration of intestinal epithelium.
Similarly, RNA immunoprecipitation assay of H19-p53 interaction with an antibody against p53 (a transcription factor that mediates growth inhibition in IECs) revealed that p53-binding complexes in IL22-treated HT-29 cell extracts contains significantly high level of H19, lncRNA while the amount of H19 lncRNA in the immune complexes recovered from vehicle-treated samples is negligible (Figure 6C). Notably, immunoblot analysis revealed that IL22 treatment did not affect p53 expression in these cells (Supplementary Figure 8). In addition, ectopic expression of H19 lncRNA increased recovery of the bound to p53 protein in HT-29 cells (Figure 6D), suggesting that p53 protein is enriched with IL22–induced H19 lncRNA. Using a luciferase reporter assay, we found that H19 lncRNA significantly attenuated p53 activity in IECs (Figure 6E), demonstrating that H19 lncRNA is an inhibitor of p53 function. Together, the data suggest that induction of H19 lncRNA during inflammation causes a decrease in p53 activity in IECs.
Additionally, we found that treatment of HT-29 cells with IL22 induced the expression of a group of positive regulators of epithelial cell proliferation, including MYCN and FOXM1 (Figure 6F), which are target genes repressed by the H19-binding GI-miRNAs and p53, respectively14–16. Ectopic expression of H19 in the cells (Figure 6G) also induced MYCN and FOXM1 expression. In vivo, we found that wild-type littermates, but not H19ΔEx1/+ mice exhibited an increase in Mycn (Figure 6H) and Foxm1 (Figure 6I) expression in the colonic mucosa during the recovery phase of DSS-induced colitis. Collectively, these results suggest that under inflammatory conditions, de novo synthesized H19 binds and attenuates the activity of the cytostatic regulators including GI-miRNAs and p53 protein, which in turn promote expression of growth-promoting genes Mycn and Foxm1 in IECs, thus contributing to regeneration of the intestinal mucosa.
DISCUSSION
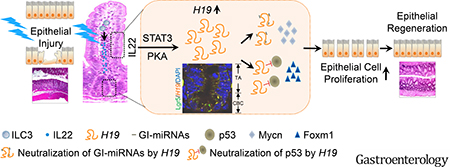
H19 lncRNA is an imprinted and maternally expressed transcript that is found in various tissues during embryogenesis but is silenced after birth17. Its expression can be reactivated in numerous disease conditions such as tumors and rheumatoid arthritis18,19. H19-deficient mice appear healthy, develop normally, and are fertile20, suggesting that H19 lncRNA is not essential for embryonic development and viability. However, little is known about the role of H19 lncRNA in inflammation and disease, particularly in the gut. In the present study, we found that H19 lncRNA is an early-response molecule to inflammation in IECs and its de novo expression is strongly activated by IL22 through STAT3 and a cAMP-independent PKA-mediated mechanism. Remarkably, we found that de novo synthesized H19 lncRNA is critical to antagonize the acute inflammation-induced cytostatic response in intestinal crypt epithelial cells and to promote regeneration of injured intestinal epithelium in the DSS-induced mouse colitis and recovery model. Mechanistically, H19 lncRNA exhibits a strong preference for binding and inhibiting p53 and multiple cell growth-inhibitory miRNAs, such as Mir34a and let-7, thereby increasing the expression of a subset of cell growth-promoting genes in the regenerative epithelium. Together, our findings support a model that de novo synthesized H19 lncRNA is an important intermediate signaling molecule linking IL22 signals to other regulatory networks that control repair of the intestinal epithelium under inflammatory conditions (Figure 6J).
While lncRNAs are present in various mammalian cells and tissues, understanding their roles in biological process and disease pathogenesis has been challenging. However, recent advances in the unbiased and systematic study of gene expression with RNA-seq technology have markedly increased our knowledge about the links between lncRNA expression and disease pathogenesis. For example, gene expression profiling with RNA-seq has revealed that numerous lncRNAs are expressed in either a tissue- or cell-specific manner1. It is becoming increasingly apparent that specific patterns of lncRNA expression coordinate cell state, differentiation, development, and disease. The lncRNA genes have been shown to be expressed in the gut tissue21. By performing RNA-seq combined with qRT-PCR analysis, we found that from among 10,481 mouse lncRNA transcripts in the mouse intestinal tissue, only H19 expression is increased by LPS-induced systemic inflammation. Intestinal H19 lncRNA levels were also dramatically up-regulated in the acute phase and the recovery phase of DSS-induced colitis in mice, and in inflamed colonic tissue from patients with inflammatory bowel disease. With in situ hybridization, we found that inflammation-induced H19 lncRNA is localized to IECs in the intestinal mucosa. Together, these observations strongly suggest that H19 lncRNA is an inflammatory molecule associated with wound healing in the gut.
Previous investigations described diverse cellular functions of H19 lncRNA. For example, H19 lncRNA is known to play a role in the regulation of the H19/Igf2 imprinted gene network22. Other studies have shown that H19 lncRNA is involved in tumorigenesis, though its role is controversial23,24. H19 lncRNA has been reported to regulate myogenic differentiation, although its pro- or anti-myogenic function is still debated25,26. Moreover, H19 lncRNA has been shown to be important in maintaining the capacity for hematopoietic stem cell repopulation27. Recent studies found that ectopic expression of H19 results in an increase in colonic epithelial cell monolayer permeability in vitro28, suggesting a link between H19 and IEC function. Therefore, it appears that H19 lncRNA functions in a context- and cell-type-dependent manner. To date, no prospective studies have evaluated the in vivo role of H19 lncRNA in the gut. In the present study, we found that H19ΔEx1/+ mice, which lack H19 expression, do not show apparent intestinal defects, suggesting H19 lncRNA is not essential for intestinal development and homeostasis in normal physiological states. In contrast, under the inflammatory conditions, H19ΔEx1/+ mice display impaired intestinal epithelial cell migration along the crypt-villus axis and arrested proliferation of crypt epithelial cells compared with their wild-type littermate controls. H19ΔEx1/+ mice showed delayed recovery and restoration of epithelial integrity after DSS-induced acute colonic injury, suggesting a functional role of inflammation-induced H19 lncRNA in regeneration and repair of the intestinal epithelium. Collectively, our experiments reveal for the first time that de novo expression of H19 plays an important role in intestinal epithelial wound healing in vivo.
In addition to determining the impact of H19 expression on intestinal epithelial regeneration, we also examined whether an increase in H19 lncRNA affects the intestinal epithelial barrier function in vivo. In LPS-treated mice, however, we found that induction of H19 lncRNA is not associated with alterations in intestinal epithelial permeability, suggesting that increase in H19 lncRNA levels may not significantly influence intestinal barrier function in vivo. Interestingly, Zou et al. recently noted that overexpression of H19 results in repressing expression of several tight-junction-associated molecules and disrupting intestinal epithelial barrier function in vitro28. This discrepancy may be due to differences in the in vitro and in vivo systems used in the 2 studies. It is possible that a lack of association between H19 expression level and intestinal barrier permeability in LPS-treated mice might not be recapitulated in other models. Further investigation is needed to fully understand the in vivo role of H19 lncRNA in regulation of intestinal barrier function in other animal models.
One major finding of the present study is that H19 expression in IECs is strongly activated by IL22, a type 3 innate lymphoid cell signature inflammatory cytokine. Previous studies have shown that IL22 promotes intestinal-stem-cell-mediated epithelial regeneration and wound healing through the IL22R/STAT3 signaling axis12. Interestingly, our results show that IL22 induces H19 expression in IECs not only through STAT3 but also via PKA. Importantly, we demonstrated that overexpression of H19 increases in IEC proliferation, whereas lack of H19 expression impairs intestinal epithelial regeneration. Furthermore, we demonstrated that H19 expression is required for IL22-induced proliferation and growth of intestinal epithelial organoids. Collectively, our studies suggest that H19 lncRNA is an essential downstream molecule of IL22-activated wound healing signaling pathways in IECs.
Although our data showed that, from among 14 inflammatory and mucosal healing-associated molecules, IL22 uniquely induced H19 expression, evidence suggests that additional H19 expression inducers exist. For instance, forskolin was reported to trigger H19 expression in fetal adrenal cells11. Recently, bile acid was shown to have a potent effect on induction of H19 expression in bile duct epithelial cells29. In a pilot study, we found that B27 and N2 (commercial serum-free cell growth supplements containing several different ingredients that support survival of neurons and various organoids in vitro30,31) induced H19 expression in IECs (Supplementary Figure 9). Taken together, it is likely that H19 may be up-regulated by factors in multiple injury-related settings, independent of IL22 in the gut.
Exactly how H19 lncRNA promotes intestinal epithelial regeneration is unknown. However, it has been proposed that lncRNAs regulate cell functions through competitive binding of miRNAs1. Furthermore, emerging evidence suggests that lncRNAs can influence transcription factor function through competitive binding and post-translational modifications, which in turn regulate cellular events32. In line with this idea, using in silico analysis, we found that miRNAs with cell growth inhibitory function (i.e. GI-miRNAs) were the predominant cluster of growth-related miRNAs binding to H19 lncRNA. With an H19 lncRNA antisense purification assay, we confirmed that 2 GI-miRNAs, Mir34a and let-7, were markedly bound by H19 lncRNA in vivo. Subsequently, in IECs in vivo and in vitro, we found an association between H19 levels and altered expression of a cell proliferation enhancer, Mycn, which is a known downstream target of Mir34a and let-7. We further showed that H19 lncRNA directly interacts with p53 and regulates its activity, and that H19 lncRNA levels are associated with changes in the expression of Foxm1, a p53 target gene. Taken together, we speculate that H19 lncRNA promotes intestinal epithelial regeneration through at least 2 mechanisms. First, it competitively binds GI-miRNAs to activate expression of positive regulators of cell growth. Second, it modulates activity of cell growth-associated transcription factors. Future studies are needed to confirm these hypotheses and determine the underlying mechanisms.
Regeneration of the intestinal epithelium is driven by the proliferative potential of intestinal stem cells and TA cells in intestinal crypts. Previously, we and other investigators found that sepsis induces cytostasis or growth arrest in intestinal crypt epithelial cells33, suggesting that severe acute inflammation decreases the proliferation of stem cells and TA cells in intestines. We confirmed this finding in the present study (Figure 4A). Despite this observation, however, there is currently a lack of understanding of how severe inflammation impairs the proliferation of stem cells and TA cells in the intestine. Interestingly, our current study revealed that Lgr5− TA progenitor cells and Lgr5+ stem cells in intestinal crypts express H19 during inflammation, and the inhibitory effect of severe inflammation on the proliferation of crypt epithelial cells is potentiated in H19ΔEx1/+mice compared to their wild-type littermate controls. It is apparent that inflammation-induced H19 lncRNA rescues the proliferative potential of stem cells and TA cells in the intestine. Given the fact that H19 lncRNA is an antagonist of p53 protein and GI-miRNAs that inhibit intestinal epithelial proliferation, we anticipate that severe inflammation induces growth arrest of IECs through activation of a set of intrinsic negative regulators of cell proliferation in intestinal stem and TA cells, such as p53 protein and GI-miRNA network. Although future studies should focus on testing this hypothesis, our current work suggests that inflammation-induced H19 lncRNA plays an important role in antagonizing inflammation-activated negative regulators of cell proliferation, which in turn sustains homeostasis of the intestinal epithelium during inflammation.
We also noticed that H19 lncRNA is reported to give rise to microRNA 675 (MIR675) that promotes cell growth and differentiation26. Indeed, we found that HT-29 cells with H19 overexpression showed the high levels of MIR675 (Supplementary Figure 10A and B), suggesting an association between H19 overexpression and MIR675 biogenesis in vitro. On the other hand, we revealed that neither IECs treated with IL22 in vitro nor intestine of wild-type mice with either LPS-induced sepsis or DSS-induced colitis displayed an increase in Mir675 levels (Supplementary Figure 10C, D, and E). In addition, treatment of intestine explants with IL22 showed no effect on Mir675 expression, though induction of H19 expression occurred (Supplementary Figures 3 and 10F). We failed to show a correlation between H19 lncRNA levels and Mir675 expression using in vivo and ex vivo models (Supplementary Figure 10G, H, and I). Thus, there is no evidence of a link between de novo synthesized H19 lncRNA and MIR675 in IECs under inflammatory conditions.
Finally, our data indicate that persistent de novo expression of H19 occurs in the colonic mucosa of patients with ulcerative colitis. We showed that inflammation-induced H19 lncRNA sustains the potential for IEC proliferation in vivo. Given the overwhelming observations that inflammation and IL22 contribute to colorectal carcinogenesis34,35, it will be of interest to examine whether H19 lncRNA impacts development of colitis-associated colorectal cancer through integration of inflammatory signals with cell proliferation networks.
In conclusion, we discovered a novel pathway in IECs in which the lncRNA H19 pathway links IL22 to cell growth regulation to maintain intestinal epithelial homeostasis under inflammatory conditions. We demonstrated that this pathway plays an important role in repressing the activity of p53 and GI-miRNAs as well as promoting epithelial regeneration. Under inflammatory conditions, de novo synthesized H19 lncRNA links IL22–activated STAT3 and a cAMP-independent PKA with expression of cell proliferation-associated genes (such as MYCN and FOXM1), via antagonism of p53 protein and GI-miRNAs, which subsequently induces proliferation and promotes repair of intestinal epithelium. Therefore, inflammation-induced H19 lncRNA relays the IL22 signal to promote an anti-cytostatic response and epithelial regeneration. Our work expands knowledge of H19 lncRNA function in inflammation and the regulatory potential of H19 lncRNA under pathophysiological conditions. This finding may reveal new therapeutic avenues to intervene in cases of inflammation-associated gut mucosal pathology, such as delayed mucosal healing and inflammatory bowel disease–associated colon cancers.
Supplementary Material
Acknowledgments
Funding: This study was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK064240 (X.D.T.), National Institute of General Medical Sciences grants R01GM117628 (X.D.T.) and R01GM122406 (X.D.T.), US Department of Veterans Affairs Merit Award I01BX001690 (X.D.T.), Dorothy M. and Edward E. Burwell Professorship (X.D.T.), and Ministry of Health of the People’s Republic of China Special Project Grant No. 201002020 (J.Q.). The sponsors had no role in study design or collection, analysis, or interpretation of the data.
The authors thank members of the Jun Sun laboratory for advice on preparing mouse intestinal epithelial organoids.
Non-standard abbreviations
- BrdU
bromodeoxyuridine
- CBC
Crypt-base-columnar
- DSS
dextran sulfate sodium
- GI-mRNA
cell growth inhibitory-miRNA
- IL22
interleukin-22
- lncRNA
long noncoding RNA
- RAP
RNA Antisense Purification
- REC
regenerated epithelial cluster
- RIP
RNA Immunoprecipitation
- TA
transit-amplifying
- UC
ulcerative colitis
Footnotes
Author contributions: Study concept and design: H.G. and X.D.T. were involved in the overall design of experiments and interpretation of results. H.G., H.F.B., and F.L. performed in vivo experiments. H.G., H.F.B., F.L., P.W., and X.W. performed in vitro experiments. H.G. and L.W. designed and prepared constructs. H.G. and L.W. performed RNA-seq studies. L.W. and A.P. analyzed RNA-seq data. H.G. performed in situ hybridization, immunofluorescent staining, and experiments for RNA antisense purification and RNA immunoprecipitation assays. H.F.B. performed protein kinase A assays. F.L. performed experiments involving human specimens. P.M.C. and X.D.T. performed pathology and histological evaluation of tissue samples. K.P. provided H19ΔEx1/+ mice and littermates. J.Y. and L.L. supervised the analysis of RNA-sequencing studies. J.Q. supervised the experiments involving human materials. J.S. provided samples to confirm H19 expression in mouse tissues. M.S.B. and K.P. contributed intellectually to experiments involving H19 knockout mice. I.G.D.P. provided intellectual input for the project. H.G. and L.W. prepared figures. H.G. and X.D.T. wrote the manuscript with input from all authors. X.D.T. conceived and orchestrated the project.
Disclosures: The authors disclose no conflicts.
Accession number for RNA-seq data in the Gene Expression Omnibus database: GSE97371
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geng H, Tan XD. Functional diversity of long non-coding RNAs in immune regulation. Genes Dis. 2016;3:72–81. doi: 10.1016/j.gendis.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotzin JJ, Spencer SP, McCright SJ, et al. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature. 2016;537:239–243. doi: 10.1038/nature19346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sallam T, Jones MC, Gilliland T, et al. Feedback modulation of cholesterol metabolism by the lipid-responsive non-coding RNA LeXis. Nature. 2016;534:124–8. doi: 10.1038/nature17674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van der Flier LG, Sabates-Bellver J, Oving I, et al. The Intestinal Wnt/TCF Signature. Gastroenterology. 2007;132:628–32. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 5.Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugimoto K, Ogawa A, Mizoguchi E, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–44. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickert G, Neufert C, Leppkes M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–72. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii S, Fujimori T, Kawamata H, et al. Development of colonic neoplasia in p53 deficient mice with experimental colitis induced by dextran sulphate sodium. Gut. 2004;53:710–6. doi: 10.1136/gut.2003.028779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madison BB, Liu Q, Zhong X, et al. LIN28B promotes growth and tumorigenesis of the intestinal epithelium via Let-7. Genes Dev. 2013;27:2233–45. doi: 10.1101/gad.224659.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juan V, Crain C, Wilson C. Evidence for evolutionarily conserved secondary structure in the H19 tumor suppressor RNA. Nucleic Acids Res. 2000;28:1221–7. doi: 10.1093/nar/28.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voutilainen R, Ilvesmaki V, Ariel I, et al. Parallel regulation of parentally imprinted H19 and insulin-like growth factor-II genes in cultured human fetal adrenal cells. Endocrinology. 1994;134:2051–6. doi: 10.1210/endo.134.5.7512497. [DOI] [PubMed] [Google Scholar]
- 12.Lindemans CA, Calafiore M, Mertelsmann AM, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–4. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kallen AN, Zhou XB, Xu J, et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol Cell. 2013;52:101–12. doi: 10.1016/j.molcel.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barsotti AM, Prives C. Pro-proliferative FoxM1 is a target of p53-mediated repression. Oncogene. 2009;28:4295–305. doi: 10.1038/onc.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buechner J, Tomte E, Haug BH, et al. Tumour-suppressor microRNAs let-7 and mir-101 target the proto-oncogene MYCN and inhibit cell proliferation in MYCN-amplified neuroblastoma. Br J Cancer. 2011;105:296–303. doi: 10.1038/bjc.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei JS, Song YK, Durinck S, et al. The MYCN oncogene is a direct target of miR-34a. Oncogene. 2008;27:5204–13. doi: 10.1038/onc.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartolomei MS, Zemel S, Tilghman SM. Parental imprinting of the mouse H19 gene. Nature. 1991;351:153–5. doi: 10.1038/351153a0. [DOI] [PubMed] [Google Scholar]
- 18.Kondo M, Suzuki H, Ueda R, et al. Frequent loss of imprinting of the H19 gene is often associated with its overexpression in human lung cancers. Oncogene. 1995;10:1193–8. [PubMed] [Google Scholar]
- 19.Stuhlmuller B, Kunisch E, Franz J, et al. Detection of oncofetal h19 RNA in rheumatoid arthritis synovial tissue. Am J Pathol. 2003;163:901–11. doi: 10.1016/S0002-9440(10)63450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava M, Frolova E, Rottinghaus B, et al. Imprint control element-mediated secondary methylation imprints at the Igf2/H19 locus. J Biol Chem. 2003;278:5977–83. doi: 10.1074/jbc.M208437200. [DOI] [PubMed] [Google Scholar]
- 21.Liang L, Ai L, Qian J, et al. Long noncoding RNA expression profiles in gut tissues constitute molecular signatures that reflect the types of microbes. Sci Rep. 2015;5:11763. doi: 10.1038/srep11763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaffer CR, Grinberg A, Pfeifer K. Regulatory mechanisms at the mouse Igf2/H19 locus. Mol Cell Biol. 2001;21:8189–96. doi: 10.1128/MCB.21.23.8189-8196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimizu T, Miroglio A, Ripoche MA, et al. The H19 locus acts in vivo as a tumor suppressor. Proc Natl Acad Sci U S A. 2008;105:12417–22. doi: 10.1073/pnas.0801540105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matouk IJ, DeGroot N, Mezan S, et al. The H19 non-coding RNA is essential for human tumor growth. PLoS One. 2007;2:e845. doi: 10.1371/journal.pone.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giovarelli M, Bucci G, Ramos A, et al. H19 long noncoding RNA controls the mRNA decay promoting function of KSRP. Proc Natl Acad Sci U S A. 2014;111:E5023–8. doi: 10.1073/pnas.1415098111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dey BK, Pfeifer K, Dutta A. The H19 long noncoding RNA gives rise to microRNAs miR-675-3p and miR-675-5p to promote skeletal muscle differentiation and regeneration. Genes Dev. 2014;28:491–501. doi: 10.1101/gad.234419.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venkatraman A, He XC, Thorvaldsen JL, et al. Maternal imprinting at the H19-Igf2 locus maintains adult haematopoietic stem cell quiescence. Nature. 2013;500:345–9. doi: 10.1038/nature12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou T, Jaladanki SK, Liu L, et al. H19 long noncoding RNA regulates intestinal epithelial barrier function via microRNA 675 by interacting with RNA-binding protein HuR. Mol Cell Biol. 2016;36:1332–41. doi: 10.1128/MCB.01030-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Liu R, Yang J, et al. The role of long noncoding RNA H19 in gender disparity of cholestatic liver injury in multidrug resistance 2 gene knockout mice. Hepatology. 2017;66:869–884. doi: 10.1002/hep.29145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Stevens B, Chang J, et al. NS21: re-defined and modified supplement B27 for neuronal cultures. J Neurosci Methods. 2008;171:239–47. doi: 10.1016/j.jneumeth.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brewer GJ, Torricelli JR, Evege EK, et al. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–76. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 32.Marchese FP, Raimondi I, Huarte M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017;18:206. doi: 10.1186/s13059-017-1348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Hao L, Bu HF, et al. Spherical nucleic acid targeting microRNA-99b enhances intestinal MFG-E8 gene expression and restores enterocyte migration in lipopolysaccharide-induced septic mice. Sci Rep. 2016;6:31687. doi: 10.1038/srep31687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–16. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 35.Kirchberger S, Royston DJ, Boulard O, et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med. 2013;210:917–31. doi: 10.1084/jem.20122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.