Abstract
Recently, a great attention has been given for applying a low-cost and effective adsorbents instead of expensive and dangerous chemical materials as a promising approach to treat wastewater. In this work, residue powder of brown macroalga Padina gymnospora (RPG), after extracting most of its active components by 70% methanol, was used as an adsorbent material for wastewater treatment. This work also reduces the costs of residue disposal. The adsorption ability of RPG is studied for removing Cd2+ and Cr3+from wastewater. We investigated metal adsorption isotherms and kinetics, the effect of initial metal concentration, contact time, adsorbent dosage, temperature, pH and the RPG reusability on metal ions removal. The results showed that the removal % generally increases with decreasing concentration of metal ions. RPG has higher metal removal percentages reaching 96.2% and 78.8% for Cd2+ and Cr3+, respectively, with a maxiumum adsorption capacity of 96.46 and 31.52 mg/g for Cd2+and Cr3+,respectively at pH 6.2, 50 mg, 25 °C and initial metal concentration of 100 mg/L. The metal ions removal % increased by increasing the dosage of adsorbent and it decreased after a certain limit. The metal removal % slightly changes with increasing temperature for Cd2+ and decreased at high-temperature for Cr3+. The adsorption increased with increasing pH value from 3 to 5, and decreases at pH value of 6.2 then it increased again at pH 8. The removal % and adsorption capacity at pH 8 reaches 99.58%, 99.65%, 99.85 mg/g and 39.86 mg/g for Cd2+ and Cr3+, respectively. The results also showed that RPG can be reused several times for metal ions removal. In addition, Tempkin isotherms and pseudo-second-order kinetic fit the adsorption of Cd2+ and Cr3+ well.
Keyword: Environmental science
1. Introduction
In the present, modern technology creates considerable amounts of hazardous heavy metals in the aquatic environment. These heavy metals include, for example, Cu2+, Cr3+, Cr6+, Cd2+, Ni2+, Zn2+, Pb2+, Hg2+ and As2+. For examples, the industries of tanneries, metal plating, and mining discharge several contaminated heavy metals like cadmium and chromium into their aqueous waste streams [1, 2]. Generally, heavy metals are very toxic, naturally non-degradable, accumulated in body organisms and uptake of a certain quantities of them cause several illnesses and disorders for living organisms [3]. In this regards, exposure to cadmium causes human cancer, renal damage, and kidney disorder [4, 5].
Chromium occurs in two forms, hexavalent and trivalent forms. Cr (III) in low doses is a necessary nutrient metal in the human diet [6]. Deficiency of Cr (III) in the human body causes cardiovascular disease, hyperglycemia, and other health effects. On the other hand, high doses of Cr (III) could negatively affect human life. Cr (III) induces skin rash and its high accumulations in cell cause DNA damage [7]. Cr (VI) is also carcinogenic for stomach and lung and also causes a headache, diarrhea, nausea, and vomiting [8, 9]. So, the elimination of these heavy metals from aqueous solutions and wastewater is the critical topic of many approaches [10].
Traditional methods, like chemical reduction and precipitation, for removing heavy metal such as cadmium and chromium from wastewater is an expansive processes due to reagents consumption and toxic sludge generation, which requires subsequent disposal [11]. Also, these techniques have low efficiency and inapplicability to a wide range of pollutants [12]. For this reason, increasing attention has been given to some alternative and economical techniques for the removal of cadmium and chromium, including membrane separation, adsorption, ion exchange and biological reduction [13, 14, 15, 16]. Adsorption has been found to be superior to other techniques for water treatment in terms of initial cost, flexibility and simplicity of design, ease of operation, insensitivity to toxic pollutants and does not produce harmful substances like most processes [17, 18]. Therefore, recently removal of heavy metal-contaminated aqueous solutions by using different adsorbents such as green sands [19], iron slag [9, 20], fly ash [21, 22] and waste iron [23], agricultural by-products like hazelnut shell, orange peel, rice husk, pecan shells, jackfruit, activated carbon [24, 25, 26] and potato peels charcoal [27] were applied. Interstingly, algal biomass from Spirogyra species [28], Chlorella Vulgaris [29], Ulva Lactuca [30, 31], Oedogonium and Nostoc specieses [32] showed efficient absorbing capacity.
In this work residue of residue of Padina gymnospora (RPG), after the extraction of its photochemicals, was used as an adsorbent material for wastewater treatment. RPG is selected for many reasons such as, its low-cost, natural and abundant adsorbent. Also, the cost of regeneration and reuse of RPG is inexpensive that may play an important economical role in making this a practical process. In addition, the reuse of RPG as a low-cost adsorbent has a vital role in minimizing the cost of residue disposal. The adsorption ability of RPG is studied for removing Cd2+ and Cr3+ from wastewater. In more details, metal adsorption isotherms and kinetics, the effect of initial metal concentration, contact time, adsorbent dosage, temperature, pH and the RPG reusability on metal ions removal were studied by batch experiments.
2. Experimental
2.1. Adsorbent preparation
Padina gymnospora (brown alga) was collected from the Egyptian red sea shores, Washed with tap water several times and then washed several times with distilled water to get rid of salt and granular materials from the surface. After drying the algal material at room temperature, was ground into fine powder by an electrical mill. P. gymnospora powder was soaked in 70% methanol for three days with continuous shaking at room temperature to extract its active component. After evaporation of the solvent on a rotatory evaporator, the residue powder of P. gymnospora was dried at room temperature and saved in airtight plastic bags to be used as a natural adsorbent in wastewater treatment.
2.2. Biosorbent characterization
The residue algal biomass is characterized before and after metal ions adsorption process using fourier-transform infrared spectroscopy (FTIR) (Tensor – 27. Bruker FTIR mode), in a wave number range of 400–4000 cm−1 under normal conditions, and Scanning electron microscopy (SEM)(a JEOL, JSM-52500 LV SEM, Japan).
2.3. Adsorption experiments
Cd2+ and Cr3+ metal ions were chosen for the adsorption process. The solutions of the tested metal ions were prepared by the desired concentrations by dissolving the analytical salts of CdCl2.H2O and CrCl3.H2O in distilled water and then mixed with RPG. The metal adsorption isotherms and kinetics, the effect of initial metal concentration, contact time, adsorbent dosage, temperature, pH and the RPG reusability on metal ions removal were studied by batch experiments.
All the experiments were conducted in the batch mode in various conditions in terms of metal ions initial concentrations of (10–100 mg/l), adsorbent dose (0.1–0.7 g/l), pH (3–8), contacted time (180 min), and temperature (25–80 °C).
To study the effect of the initial metal concentration the Adsorption assays were done at various metal ions concentrations (10, 25, 50 and 100 mg/L) by mixing 50 mg of RPG adsorbent with100 mL of the solutions at pH 6.2 and 25 °C with continuous shaking.
Biomass weight effect was done at 25 °C and pH 6.2 for 180 min where different amounts of adsorbent (10, 30, 50 and 70 mg) are added and shaken with 100 mL solution of 100 mg/L concentration. The influence of temperature on adsorption assay is tested at 25, 40, 60 and 80 °C and at pH 6.2, 50 mg of adsorbent and 100 mL of 100 mg/L solution for 180 min.
To study the effect of pH, a solution of different pH values (pH 3, 5, 6.2 and 8) were prepared and examined at a constant temperature of 25 °C and 50 mg of adsorbent was mixed with 100 mL of a solution of 100 mg metal/L for 180 min.
The reusability of RPG was examined by repeating the adsorption cycle four times with the same adsorbent using 50 mg of the catalyst at pH 6.2 and constant temperature (25 °C) for 180 min. After each run, the solid was collected by filtration and washed several times by distilled water to be used in the next run.
2.4. Calculations of metal ions removal percentages and adsorption capacity
After specific interval time, during the study of each parameter, the slurry samples were filtered to remove adsorbent and then concentration of the residual metal ions in aqueous solutions was analyzed by atomic absorption spectrometer (Perkin Elmer A Analyst 100, USA). The quantity of metal uptake at equilibrium in mg/g, Adsorption capacity (qe), can be calculated using Eq. (1):
| (1) |
Also, Adsorption capacity after time t (t), given by Eq. (2):
| (2) |
In addition, the metal ions removal percentages were calculated from Eq. (3):
| (3) |
Where, Co and Ce are the initial and equilibrium metal concentrations in the solution (mg/L), respectively. Also, Ct is the metal concentration after time t in the solution (mg/L), V is the solution volume (in mL) and m is the adsorbent mass (in mg) [33, 34].
2.5. Adsorption isotherm
The adsorption isotherm models explain mathematically the attitude of adsorbent species between solid and liquid phases. Langmuir, Freundlich and Tempkin isotherm models are used for the elucidation of adsorption of Cd2+ and Cr3+metals by RPG.
The Langmuir isotherm considers monolayer adsorption at active sites of the adsorbent with no interaction between molecules of adsorbed metals. The Langmuir isotherm is described by Eq. (4) [35]:
| (4) |
Where qe is the equilibrium adsorption capacity (mg/g), Qo is the maximum quantity of metal adsorbed on residue algal biomass (RPG) (mg/g), Ce is the concentration of the tested metal ions in solution at equilibrium (mg/L) and KL is the Langmuir constant (L/mg).
Freundlich isotherm model assumes multilayer adsorption on heterogeneous surfaces with the association between metal ion molecules. This model equation is written as the following(Eq. (5)) [36]:
| (5) |
Where KF is the adsorbent capacity and n is Freundlich adsorption intensity constant.
The third model is Tempkin isotherm, it characterized by decreasing adsorption heat linearly with coverage and distribution of binding energies regularly [37, 38]. Eq. (6) of Tempkin isotherm can be written as follows:
| (6) |
Where KT (L/mol) is Tempkin binding constant at equilibrium relating to the maximum binding energy; B (RT/b) is constant regarding the adsorption heat and R (8.314 J/mol K) is the gas constant and T (K) is the absolute temperature.
2.6. Adsorption kinetics
The kinetic models namely pseudo first order, pseudo-second order, intraparticle diffusion, and simple Elovich models were applied to analyze the behavior of metal ions adsorption and steps that control its rate.
2.6.1. Pseudo-first-order model
This kinetic model describes the adsorption of metal ion from an aqueous solution. The pseudo first order equation is represented by Eq. (7) [39, 40, 41]:
| ln (qe – qt) = lnqe – k t | (7) |
where qe is the equilibrium mass of adsorbed metal ions (mg/g), qt is the adsorption quantity at time t (mg/g), k (min−1) is the constant of first-order rate. In pseudo first order, the rate of filling of adsorption positions is dependent on the number of free active positions.
2.6.2. Pseudo-second order model
In this model, the adsorption rate was directly proportional to the square of the number of empty positions. The equation of this model can be described by Eq. (8) [42, 43, 44]:
| (8) |
where k1 is the second-order rate constant.
2.6.3. Intraparticle diffusion kinetic models
The intraparticle diffusion kinetic model assumes that the solubilized ions transport from aqueous solutions to the adsorbent materials then intraparticle diffusion assay takes place [45]. This model equation was given by Eq. (9):
| (9) |
Where, k2 is the constant of intraparticle diffusion rate and I is the intercept which is corresponding to the boundary layer thickness.
2.6.4. Elovich kinetic model
This kinetic model considers the adsorption of heterogeneous surfaces and it is a special type of second-order kinetics. The Elovich model Eq. (10) is represented as follow [46]:
| (10) |
Where α (mg/min) is the rate of initial adsorption at contact time t = 0 min and β (g/mg) is the extent of surface coverage and activating energy.
2.7. Statistical analysis
The adsorption data was a mean of three independent experiments. The regression coefficient (R2) values of Langmuir, Freundlich, Tempkin isotherm, pseudo-first-order, pseudo-second-order, Intraparticle diffusion, and Elovich kinetic models were determined by using statistical functions of Microsoft Excel, 2007 version.
3. Results and discussion
3.1. Biosorbent characterization
3.1.1. Fourier-transform infrared analysis (FTIR)
The different functional groups on the algal biomass surface that interact with heavy metal ions were indicated by FTIR analysis in two cases (before and after the adsorption process). FTIR spectra for free P. gymnospora residue and P. gymnospora residue loaded with Cd2+ and Cr3+ were analyzed (Fig. 1). From these data, a broad band of (OH) group was detected at 3450 cm−1 before adsorption process, but after adsorption broad band of (OH) group were detected at 3470 cm−1 and 3465 cm−1 with Cd2+ and Cr3+ metals ions, respectively. The interaction between adsorbed metals ions and the (OH) groups that present in the surface of the alga residue was responsible for the previous difference in the wave number value [47]. The (NH) of amide group was detected as a strong band at 3280 cm−1 before adsorption and at 3289 cm−1 and 3290 cm−1 after adsorption of Cd2+ and Cr3+, respectively. The peak at 3137 cm−1 before adsorption process was referred to the presence of C-H bond stretching of methyl and methoxy groups and they were detected at 3150 cm−1 and 3147 cm−1 after adsorption of Cd2+ and Cr3+, respectively [48]. The absorbance at 2683 cm−1 before adsorption was related to (SH) group and this band appears at 2697 cm−1 and 2692 cm−1 with Cd2+ and Cr3+, respectively. The C=C stretching vibration bond exhibited the peak at 1660 cm−1 before adsorption and at 1649 cm−1 and 1640 cm−1 after adsorption of Cd2+ and Cr3+, respectively and that probably was because of the aromatic bond [49]. The peak at 1404 cm−1 before adsorption showed the presence of (C-O) bond that shifts to 1426 cm−1 and 1413 cm−1 after adsorption of Cd2+ and Cr3+, respectively [30]. The absorbance at 1593 cm−1 before and after adsorption assay corresponded to the bending band of amid group [47].
Fig. 1.
FT-IR of RPG before and after adsorption processes by 50 mg of RPG at 25 °C and pH 6.2.
3.1.2. Scanning electron microscopy (SEM) characterization
The SEM images of RPG before and after metal adsorption at 2000 × magnification were illustrated (Fig. 2). By SEM examination, the morphology of the biosorbents surface before and after metal uptake is visualized. It was found that considerable changes to the morphology of the biosorbents surface were observed. Before Cd2+ and Cr3+ uptake, the cells are smooth and have certain dimensions and the surface was characterized by a number of micro and macro pores (Fig 2a and b). The destruction and swelling of the cells, their surface is developed into puckers and the pores were covered with the adsorbed metal particles after metal adsorption (Fig 2c and d). These resulted in changes perhaps because of the deposition of metal ions around the cell surface and associate with their functional groups. These changes may be produced during the exposure of the samples to heavy metal solutions; where it takes place the replacement of the metal ions for some of the cations that was initially found in the cell wall matrix and make stronger cross-linking. Because of the ion-exchange mechanism, the heavy metals fill the ready unoccupied binding sites [50].
Fig. 2.
SEM image of RPG before soaking in 70% methanol (a) and after soaking in 70% methanol (b) and after adsorption of (c) Cd2+ and (d) Cr3+.
3.2. Optimization of initial metal concentration and contact time
The effect of initial metal concentration and contact time on the removal % and the amounts of adsorbed metal ions using RPG as a green adsorbent was monitored (Figs. 3 and 4). It was deduced that at the initial stage, the adsorption rate was very high during the first 30 min then it decreased till it reached the equilibrium state (Fig. 3). The rapid adsorption rate at the initial level of the adsorption assay probably refered to a great number of unoccupied adsorption positions existing on the surface of the adsorbent. Afterward a certain time, the empty positions were covered by the adsorbed metal ions and this induced a repulsive force between the adsorbed molecules on the surface of adsorbent and in bulk phase and consequently the adsorption rate decreases [51] (Fig 3a and b). For Cd2+solution, the RPG catalyst represents 94, 97, 97 and 96.2% removal percentage after approximately 180 min with concentrations 10, 25, 50 and 100 ppm, respectively. In addition, the RPG catalyst removed up to 62.6, 88.7, 85.8 and 84.2% after approximately 180 min of treatment after mixing Cr3+solution at concentrations of 10, 25, 50 and 100 ppm, respectively. This activity may be attributed to the synergetic effect of the active ingredients of RPG. The two metal ions Cd2+and Cr3+ represented the same behavior where the metal removal % increases with decreasing initial metal concentration. Except for Cr3+ metal ions with initial concentration 10 ppm, which showed a lower removal % and this can be imputed to the very low driving force of the concentration gradient with the lower initial metal concentration. Thus it was unable to overcome the intraparticle diffusion strength to mass transfer between the solid and liquid phases [52].
Fig. 3.
Effect of metal ions concentration and contact time on the removal % of metal ion adsorbed by 50 mg of RPG at 25 °C and pH 6.2. a)Cd2+ b)Cr3+.
Fig. 4.
Effect of metal ions concentration and contact time on the amount of metal ion adsorbed by 50 mg of RPG at 25 °C and pH 6.2. a)Cd2+ b)Cr3+.
The amounts of adsorbed metals ions increased with the increase in the initial metal concentration (Fig. 3). This phenomenon may be because of the excess in the driving force of the concentration gradient with the higher initial metal concentration which has the capability to overcome the strength of mass transfer between the solid and liquid phases [52]. The maximum amounts of metals ions adsorbed were found to be 96.46 and 31.52 mg/g for Cd2+ and Cr3+respectively at 100 ppm concentration and temperature of 25 °C and pH value of 6.2.
3.3. RPG weight effect
The adsorbent quantity utilized in adsorption was essentially significant as indicated by the sorbent–sorbate equilibrium in the system and the treatment cost of adsorbent per unit of metal solution. The catalyst weight action on the removal % of Cd2+ and Cr3+ was studied (Fig. 5). The removal % of Cd2+ and Cr3+increase with an increase in catalyst weight from 10 to 50 mg, but then it decreased (Fig. 5). This behavior may be owed to the increase in the surface area of the catalyst with increasing the catalyst weight of catalyst [53]. On the other hand, by increasing the catalyst weight from 50 to 70 mg, the removal % decreased because of huge bio-sorbent quantities those causes cell agglomeration and then consequent decrease in intercellular distance. It also induced ‘screen effect’ among a dense layer of cells, causing the ‘protection’ of binding positions from metal ions [54]. The obtained data are in agreement with those of researchers who revealed that the sorbed metal removal % decreases at high concentrations of adsorbent [55,56].
Fig. 5.
Effect of catalyst weight on the removal % of Cd2+ and Cr3+ at 25 °C and pH 6.2.
3.4. Optimization of pH
The initial pH of the aqueous solution commands the metal adsorption capacity because it affected the adsorbent surface and ionization/dissociation of the sorbent molecules [57].
The effect of the initial pH of the aqueous solution on the removal % of Cd2+ and Cr3+utilizing RPG was studied (Fig. 6). The lower adsorption capacity for Cd2+ and Cr3+at acidic medium was attributed to the protonation assay of the adsorbent surface and the high portability of H3O+ ions which compete with the metal ions during the adsorption process [58]. With more and more increase in pH, the concentration of hydrogen ions as competitors was reduced and therefore these resulted in an increase in the adsorption of metal ions by the adsorbents [59]. By increasing the pH of the solution, the number of +ve charged free positions was reduced and the number of -ve charged available positions was increase. Thus, the -ve charged adsorbent surface increased + ve charged metal ions adsorption through electrostatic forces of attraction and ion exchange [60]. The metal removal % increased by pH increases up to pH 5. However, increasing pH to 6.2 led to a decrease in metal ions removal % not only due to the formation of soluble metal hydroxyl complexes (Cadmium ions in form of Cd(OH)2, and Chromium ions in the form of Cr(OH)2+) but also to the ionized nature of the cell wall surface of RPG powder under the studied pH. Generally, the decrease in adsorption of M(II) ions after pH 5 was perhaps as a result of the formation of M(OH)2 and soluble hydroxyl complexes such as MOH+, aqueous M(OH)∖2, and M(OH)3-. Moreover, the adsorbent perhaps deteriorated with accumulation of M(II) ions making binding to adsorption sites impossible [61]. The metal removal % increased again at pH 8 where, the removal % and adsorption capacity reaches 99.58%, 99.65%, 99.85 mg/g and 39.86 mg/g for Cd2+ and Cr3+, respectively. At pH = 8, an increase in the metal removal % occurred because of probably occurring various impacts resulted from other techniques like the dominant existence of hydrated species of heavy metals, alterations on the adsorbent surface, deposition of the appropriate salts [62] and the precipitation of metal ions as hydroxides. Cr(III) ions were removed due to the precipitation of Cr(OH)3 at pH above 7 [2], while Cd(II) due to the formation of Cd(OH)+ which was the dominating species of Cd(II) in the pH range 8–10 [63].
Fig. 6.
Effect of pH on the removal of Cd2+ and Cr3+ by 50 mg of RPG at 25 °C.
3.5. Optimization of temperature
It is essential to indicate that the biosorption assay is generally not performed at a high temperature as it will increase the operational cost [64]. So we studied the effect of temperature on the adsorption process in order to determine the optimum operating temperature. The metal adsorption was done at various temperatures 25, 40, 60, and 80 °C utilizing RPG as an adsorbent. The thermal effect is different for both cations (Fig. 7). These results refered to the endothermic behavior, which can be explained by the fact that the temperature may favor the agglomeration process for chromium till 60 °C, above which the temperature would have negative or no effect on adsorption for Cr3+and Cd2+ respectively.
Fig. 7.
Effect of temperature on the removal of Cd2+ and Cr3+ by 50 mg of RPG at pH 6.2.
According to the experimental outcomes, a decrease in the adsorption capacity occurs by an increase in the solution temperature over 60 °C (Fig. 7). This confirms that the technique of adsorption of chromium metal over 60 °C was an exothermic assay [65]. Because the reactions of adsorption are usually exothermic, the capacity of biosorption increased with the reduction of temperature. At high temperature, the removal % of Cr3+ions decreases and this probably because of destroying of active positions in the RPG. There are several other researchers that have also found the same results [66,67]. For Cd2+ metal, there was a slight effect of temperature on the removal % with increasing temperature from 25 to 80 °C, in agreement it was indicated that if the maximum limit of metal ions or adsorbate removal was attained by the adsorbent, there will be no effect for increasing the adsorbent dose [68].
3.6. Reusability of RPG
For the examination of the RPG reusability, the adsorption cycle was duplicated four times with the same adsorbent (Fig. 8). The operating conditions were adjusted at 50 mg adsorbent mass, 100 mg/L initial concentrations for the studied metal ions, 100 mL solution volume, 180 min reaction time and pH 6.2. After each run, the solid was collected after filtration and washed several times by distilled water to be used in the second run. The material showed high stability for the studied four runs and achieved high removal percentages for all the studied metal pollutants with a noticeable decrease in the efficiency from cycle 1 to cycle 4. The estimated removal percentages for Cd2+ ions were 96.2 %, 96.1%, 96.1% and 96 % for cycle 1, cycle 2, cycle 3 and cycle 4, respectively. The recorded removal percentages for chromium ions were decreased to 78.8%, 78.2%, 76.8% and 75.6% with repeating the removal cycles from cycle 1 to cycle 4 in order. This reduction can be attributed to the deposition of metal ions particles on the surface of RPG, which resulted in blockage of adsorbent pores and finally in decrease in adsorption capacity [69]. This results were also confirmed from SEM analysis (Fig. 2c and d). The results of the present work show that the generation of RPG can be re-used decreases from cycle 1 to cycle 4 is in agreement with previous studies [69, 70, 71, 72]. These results also show that RPG is an excellent reusable and easily regenerated adsorbent for the removal of both Cd2+ and Cr3+ ions from the aqueous environment.
Fig. 8.
Reusability of RPG for removal of Cd2+ and Cr3+ by 50 mg of RPG at 25 °C and pH 6.2.
3.7. Adsorption isotherm
To study the applicability of the Langmuir, Freundlich, and Tempkin isotherms for the Cd2+ and Cr3+, metal ions adsorption process at different initial metal ions concentrations, linear plots of Ce/qe against Ce, log qe against log Ce, and qe against Ln Ce were designed (Figs. 9, 10 and 11). In this regards, we indicated the straight line plots of Langmuir and Freundlich and Tempkin isotherms of the tested metals, respectively. The values of Qo, KL, KF, 1/n, KT, B, and R2 for the models were listed in Table 1. The values of R2 (Correlation coefficient) are taken into consideration as a measure of the applicability and goodness of fit of the experimental data to the isotherm models. The isotherms of Cd2+ and Cr3+metal ions adsorption onto adsorbent did not follow Freundlich or Langmuir isotherm (Table 1). The value of R2 is the greatest in case of Tempkin isotherm for Cd2+ and Cr3+ indicating that the adsorption assay approximately follows the Tempkin isotherm model. The observed correlation coefficients for Tempkin isotherms were 0.9984 and 0.8221 for Cd2+ and Cr3+ions adsorption, respectively at 25 °C.
Fig. 9.
Langmuir isotherms for the adsorption of Cd2+ and Cr3+ by 50 mg of RPG at 25 °C and pH 6.2.
Fig. 10.
Freundlich isotherms for the adsorption of Cd2+ and Cr3+ by 50 mg of RPG at 25 °C and pH 6.2.
Fig. 11.
Tempkin isotherms for the adsorption of Cd2+ and Cr3+ by 50 mg of RPG at 25 °C and pH 6.2.
Table 1.
Isotherm constants for Cd2+ and Cr3+ by 50 mg of RPG at 25 °C and pH 6.2.
| Metal ions |
Langmuir isotherm |
||
|---|---|---|---|
| Constants | Q o(mg/g) | KL (L/mg) | R2 |
| Cd2+ | -2083 | 0.025 | 0.025 |
| Cr3+ |
68.9 |
0.074 |
0.0506 |
| Freundlich isotherm | |||
| 1/n |
KF |
R2 |
|
| Cd2+ | 1.075 | 58.61 | 0.8852 |
| Cr3+ |
0.946 |
7.16 |
0.6464 |
| Temkin isotherm | |||
| B (J/mol) |
KT(L/mole) |
R2 |
|
| Cd2+ | 43.78 | 4.84 | 0.9984 |
| Cr3+ | 12.3 | 2.26 | 0.8221 |
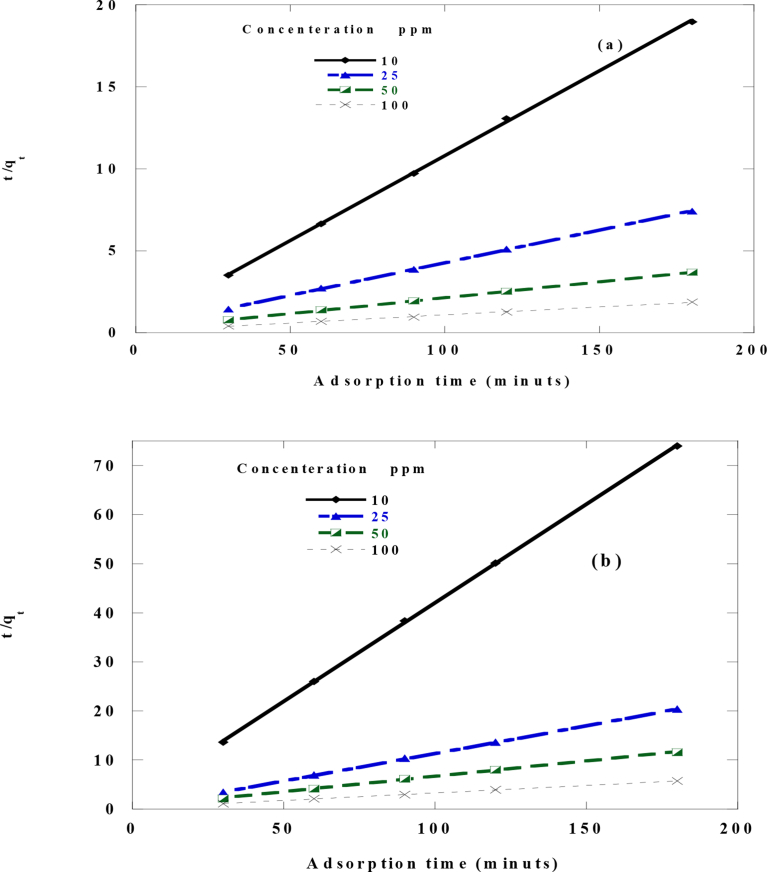
3.8. Adsorption kinetics
To study the good fitness of the first order, second order, intraparticle diffusion and Elovich kinetic models for metal ions adsorption onto RPG from metal ion solution linear plots of ln (qe – qt) against t, versus t, against and qt versus ln t must be plotted as shown in Figs. 12, 13, 14 and 15 respectively. The values of k, k1, k2, qe, I, α, β and R2 were calculated from the slope and intercept of the plots and are shown in Table 2.
Fig. 12.
Pseudo-first-order sorption kinetics of metal ions by 50 mg of RPG at 25 °C and pH 6.2. a)Cd2+ b)Cr3+.
Fig. 13.
Pseudo-second-order sorption kinetics of metal ions by 50 mg of RPG at 25 °C and pH 6.2. a)Cd2+ b)Cr3+.
Fig. 14.
Plots for evaluating intraparticle diffusion rate constant for sorption of metal ions by 50 mg of RPG at 25 °C and pH 6.2. a)Cd2+ b)Cr3+.
Fig. 15.
Plots for evaluating Elovich kinetic model for sorption of Metal ions by 50 mg of RPG at 25 °C and pH 6.2. a)Cd2+ b)Cr3+.
Table 2.
Parameters of the kinetic models for Cd2+ and Cr3+ by 50 mg of RPG at 25 °C and pH 6.2.
| First order kinetic model | ||||||||
|---|---|---|---|---|---|---|---|---|
| Metal ions |
Cd2+ |
Cr3+ |
||||||
| concentration (ppm) | 100 | 50 | 25 | 10 | 100 | 50 | 25 | 10 |
| K | 18.47 × 10−3 | 12.2 × 10−3 | 11.95 × 10−3 | 11.96 × 10−3 | 11.3 × 10−3 | 10.3 × 10−3 | 18.7 × 10−3 | 13.3 × 10−3 |
| Qe | 7.17 | 4.66 | 3.11 | 1.83 | 3.41 | 2.38 | 1.62 | 1.08 |
| R2 | 0.9821 | 0.9609 | 0.883 | 0.7587 | 0.855 | 0.7876 | 0.8047 | 0.8695 |
| qeexp | 96.46 | 48.8 | 24.26 | 9.484 | 31.52 | 15.53 | 8.83 | 2.43 |
| Second order kinetic model | ||||||||
| K1 | 0.93 × 10−3 | 1.7 × 10−3 | 5.5 × 10−3 | 23.9 × 10−3 | 4.1 × 10−3 | 9.6 × 10−3 | 84.6 × 10−3 | 86 × 10−3 |
| qe | 102.759 | 51.87 | 25.1 | 9.708 | 32.6 | 15.9 | 8.88 | 2.49 |
| R2 | 0.9986 | 0.9998 | 0.9996 | 0.9994 | 0.9998 | 0.9992 | 0.9999 | 0.9993 |
| qe exp | 96.46 | 48.8 | 24.26 | 9.484 | 31.52 | 15.53 | 8.83 | 2.43 |
| Intraparticle diffusion kinetic model | ||||||||
| K2 | 7.28 | 3.62 | 1.76 | 0.681 | 2.28 | 1.11 | 0.62 | 0.1 |
| I | 17.25 | 8.83 | 5.19 | 2.24 | 6.7 | 3.5 | 2.3 | 0.57 |
| R2 | 0.8359 | 0.8339 | 0.7772 | 0.7385 | 0.7793 | 0.7542 | 0.6724 | 0.743 |
| Elovich kinetic model | ||||||||
| β (g/mg) | 0.075 | 0.162 | 0.526 | 2.049 | 0.39 | 1.09 | 8.6 | 7.6 |
| α (mg/min) | 132 | 106 | 3744 | 753623 | 3352 | 97331 | 8.8 × 1029 | 89946 |
| R2 | 0.8958 | 0.9742 | 0.9737 | 0.9206 | 0.9742 | 0.9647 | 0.8199 | 0.9932 |
The linear fit of all kinetic models and the values of R2 for each graph (Table 2) indicated that the adsorption kinetics of the investigated metal ions onto RPG follow pseudo-second-order model. Also, the values of experimental qe (qe Exp.) values were in agreement with the calculated ones (qe), resulted from the linear plots of pseudo-second-order model shown in Fig. 13 and Table 2 at all studied initial metal ions concentrations showing that the adsorption of Cd2+ and Cr3+metals on RPG follows the pseudo-second-order kinetic model.
The pseudo-second-order adsorption mechanism predominated and the adsorption process seemed to be controlled by a chemical process involving electrons sharing or exchange between adsorbed Cd2+ and Cr3+ metal ions and the biosorbent RPG. The pseudo-second-order adsorption mechanism occurs in two steps the first one was the external diffusion step which involves Cd2+ and Cr3+ molecules movement from the bulk of the solution towards the external surface of RPG. This step was followed by the adsorption of the metal ions molecules on the surface of RPG adsorbent and chemically bonded to its surface. FTIR data show the presence of functional groups which had the ability to make chemical bonds with heavy metals. That also can be considered as another prove for adsorption mechanism. Morever, it was found that the pseudo-second-order rate constant (k) value was decreased with an increase in the initial concentration of the investigated metal ions. Similar kinetics was also observed in many literatures [53,73, 74, 75].
3.9. Comparison of adsorption capability of RPG with other adsorbents
A comparison between the adsorption capacities (qm) values of different adsorbents reported in the literature and that of RPG for adsorption of Cd2+ and Cr3+ ions (Table 3). It may be shown that qm values differ widely for different adsorbents [76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92]. Comparison of qm values also stated that RPG exhibited a reasonable capacity for adsorption of Cd2+ and Cr3+ ions from aqueous solutions.
Table 3.
Adsorption capacity of different adsorbents for Cd2+ and Cr3+ adsorption adsorbent.
| Cd2+ |
Cr3+ |
||||
|---|---|---|---|---|---|
| Adsorbent | Adsorption capacity (mg/g) | Reference | Adsorbent | Adsorption capacity (mg/g) | Reference |
| Orange peel–Fe2O3 | 71.43 | [76] | carrot residues | 45.09 | [77] |
| Prunus avium leaves | 45.45 | [91] | spirogyra spp | 30.21 | [79] |
| Kiwi cortex | 15.9 | [88] | modified lignin | 25 | [81] |
| Unmodified rice straw | 13.9 | [92] | Untreated pinussylvestris bark | 8.69 | [82] |
| Coffee grounds | 15.65 | [83] | Treated pinussylvestris bark | 9.77 | [82] |
| Activated carbon | 10.3 | [84] | Sphagnum moss peat | 29 | [85] |
| Agave bagasse | 14 | [89] | modified and non-modified carbon nanotubes | 18 | [7] |
| commercial activated carbon | 14.93 | [78] | Grain-less stalk of corn | 7.3 | [90] |
| RPG | 99.85 | This work | RPG | 39.86 | This work |
4. Conclusions
According to the experimental results, residue of Padina gymnospora (RPG), after extraction of most of its components by 70% methanol has been evaluated as a promising efficient adsorbent for the elimination of Cd2+ and Cr3+ from wastewater. Various parameters such as initial metal concentration, contact time, adsorbent dosage, temperature, pH and the RPG reusability were studied to demonstrate the potential use of RPG. This study shows that the RPG has higher metal uptake percentage reaching 96.46% and 84.2% for Cd2+ and Cr3+, respectively at pH 6.2, 50 mg of RPG, 25 °C and initial metal concentration of 100 mg/L. Different adsorption isotherms and kinetic models are investigated. The results indicate that metal ion adsorption on the RPG follows Tempkin isotherm (R2 = 0.9984 and 0.8221 for Cd2+ and Cr3+metals, respectively). Also, the pseudo-second-order kinetic model is fitted for both metal ions. This chemical mechanism of adsorption is confirmed by FTIR data that show the presence of functional groups which are able to make chemical bonds with heavy metals. The maximum amounts of metal ions adsorbed are found to be 96.46 and 31.52 mg/g for Cd2+ and Cr3+, respectively at 50 mg of RPG, 100 mg/L initial metal concentration, 25 °C and pH 6.2 which increase to 99.85 mg/g and 39.86 mg/g for Cd2+ and Cr3+, respectively at pH 8. The metal ions removal % increase by increasing the dosage of adsorbent and it decreases after a certain limit. The metal removal % slightly changes with increasing temperature for Cd2+ and decreases at high-temperature for Cr3+. The adsorption increases with increasing pH value from 3 to 5, and decreases at pH value of 6.2 then it increases again at pH 8. Finally, residue of Padina gymnospora can be used as a low-cost green adsorbent for adsorption of Cd2+ and Cr3+ from waste water.
Declarations
Author contribution statement
Hussein S Mohamed, N.K. Soliman, Doaa A Abdelrheem, Arwa A Ramadan, Ahmed H Elghandour, Sayed A Ahmed: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thanks Dr. Hamada AbdElgawad, Antwerp University, Belgium, for his support.
References
- 1.Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscipl. Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Gang, Chang Qing, Zhang Mingyue, Han Xiaoting. Effect of pH on the removal of Cr(III) and Cr(VI) from aqueous solution by modified polyethyleneimine. React. Funct. Polym. 2013;73:1439–1446. [Google Scholar]
- 3.Wuana R.A., Okieimen F.E. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Isrn Ecol. 2011;2011 [Google Scholar]
- 4.Sihabudeen M.M., Ali A.A., Hussain A.Z. Removal of heavy metals from ground water using Eucalyptus carbon as adsorbent. Int. J. Chem. Tech. Res. 2016;9:254–257. http://www.sphinxsai.com/2016/ch_vol9_no3/1/(254-257)V9N3CT.pdf [Google Scholar]
- 5.Karri V., Kumar V., Ramos D., Oliveira E., Schuhmacher M. Comparative in vitro toxicity evaluation of heavy metals (Lead, Cadmium, Arsenic, and Methylmercury) on HT-22 hippocampal cell line. Biol. Trace Elem. Res. 2017:1–14. doi: 10.1007/s12011-017-1177-x. [DOI] [PubMed] [Google Scholar]
- 6.Fay M., Abadin H., Wilbur S. Agency for Toxic Substances and Disease Registry (US); 2012. Toxicological Profile for Chromium, Atlanta (GA)https://www.atsdr.cdc.gov/toxprofiles/tp7.pdf [PubMed] [Google Scholar]
- 7.Atieh M.A., Bakather O.Y., Tawabini B.S., Bukhari A.A., Khaled M., Alharthi M., Fettouhi M., Abuilaiwi F.A. Removal of chromium (III) from water by using modified and nonmodified carbon nanotubes. J. Nanomater. 2010;2010:17. [Google Scholar]
- 8.Saputro S., Yoshimura K., Matsuoka S., Takehara K., Aizawa J., Tennichi Y. Speciation of dissolved chromium and the mechanisms controlling its concentration in natural water. Chem. Geol. 2014;364:33–41. [Google Scholar]
- 9.Santhana Krishna Kumar A. Shiuh-Jen Jiang and Wei-Lung Tseng, Effective adsorption of chromium(VI)/Cr(III) from aqueous solution using ionic liquid functionalized multiwalled carbon nanotubes as a super sorbent. J. Mater. Chem. A. 2015;3:7044–7057. [Google Scholar]
- 10.Bulgariu L., Bulgariu D. Prospects and Challenges in Algal Biotechnology. Springer; 2017. Sustainable utilization of marine algae biomass for environmental bioremediation; pp. 179–217. [Google Scholar]
- 11.Lugo-Lugo V., Barrera-Diaz C.E., Bilyeu B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard Mater. 2012;223–224:12. doi: 10.1016/j.jhazmat.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 12.Chan G.Y.S., Kurniawan T.A., Lo W., Babel S. Comparison of low cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006;366:17. doi: 10.1016/j.scitotenv.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Lee S.-M., Laldawngliana C., Tiwari D. Iron oxide nano-particles-immobilized-sand material in the treatment of Cu (II), Cd (II) and Pb (II) contaminated waste waters. Chem. Eng. J. 2012;195:103–111. [Google Scholar]
- 14.Yuan C., Weng C.-H. Electrokinetic enhancement removal of heavy metals from industrial wastewater sludge. Chemosphere. 2006;65:88–96. doi: 10.1016/j.chemosphere.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 15.Michalak I., Chojnacka K. Interactions of metal cations with anionic groups on the cell wall of the macroalga Vaucheria sp. Eng. Life Sci. 2010;10:209–217. [Google Scholar]
- 16.Mata Y., Torres E., Blazquez M., Ballester A., González F., Munoz J. Gold (III) biosorption and bioreduction with the brown alga Fucus vesiculosus. J. Hazard Mater. 2009;166:612–618. doi: 10.1016/j.jhazmat.2008.11.064. [DOI] [PubMed] [Google Scholar]
- 17.Kurniawan T., Babel S. Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere. 2004;54:951–967. doi: 10.1016/j.chemosphere.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Huiting L., Wang S. Kinetic modeling and mechanism of dye adsorption on unburned carbon. Dyes Pigments. 2007;72:7. [Google Scholar]
- 19.Lee T., Park J.-w., Lee J.-H. Waste green sands as reactive media for the removal of zinc from water. Chemosphere. 2004;56:571–581. doi: 10.1016/j.chemosphere.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 20.Feng D., Van Deventer J., Aldrich C. Removal of pollutants from acid mine wastewater using metallurgical by-product slags. Separ. Purif. Technol. 2004;40:61–67. [Google Scholar]
- 21.Alinnor I. Adsorption of heavy metal ions from aqueous solution by fly ash. Fuel. 2007;86:853–857. [Google Scholar]
- 22.Gupta V.K., Jain C., Ali I., Sharma M., Saini V. Removal of cadmium and nickel from wastewater using bagasse fly ash—a sugar industry waste. Water Res. 2003;37:4038–4044. doi: 10.1016/S0043-1354(03)00292-6. [DOI] [PubMed] [Google Scholar]
- 23.Genç-Fuhrman H., Wu P., Zhou Y., Ledin A. Removal of As, Cd, Cr, Cu, Ni and Zn from polluted water using an iron based sorbent. Desalination. 2008;226:357–370. [Google Scholar]
- 24.Ajmal M., Rao R.A.K., Ahmad R., Ahmad J. Adsorption studies on Citrus reticulata (fruit peel of orange): removal and recovery of Ni (II) from electroplating wastewater. J. Hazard Mater. 2000;79:117–131. doi: 10.1016/s0304-3894(00)00234-x. [DOI] [PubMed] [Google Scholar]
- 25.Bishnoi N.R., Bajaj M., Sharma N., Gupta A. Adsorption of Cr (VI) on activated rice husk carbon and activated alumina. Bioresour. Technol. 2004;91:305–307. doi: 10.1016/s0960-8524(03)00204-9. [DOI] [PubMed] [Google Scholar]
- 26.Cechinel M.A.P., de Souza A.A.U. Study of lead (II) adsorption onto activated carbon originating from cow bone. J. Clean. Prod. 2014;65:342–349. [Google Scholar]
- 27.Aman T., Kazi A.A., Sabri M.U., Bano Q. Potato peels as solid waste for the removal of heavy metal copper (II) from waste water/industrial effluent. Colloids Surfaces B Biointerfaces. 2008;63:116–121. doi: 10.1016/j.colsurfb.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Sayyaf H., Iranshahi L., Aseman E. Evaluation and optimization of chromium removal from synthetic aqueous solutions by powdered spirogyra. Int. Arch. Health Sci. 2015;2:57–61. oldiahs.kaums.ac.ir/article-1-52-en.pdf [Google Scholar]
- 29.Cheng J., Yin W., Chang Z., Lundholm N., Jiang Z. Biosorption capacity and kinetics of cadmium (II) on live and dead Chlorella vulgaris. J. Appl. Phycol. 2017;29:211–221. [Google Scholar]
- 30.Ghoneim M.M., El-Desoky H.S., El-Moselhy K.M., Amer A., El-Naga E.H.A., Mohamedein L.I., Al-Prol A.E. Removal of cadmium from aqueous solution using marine green algae, Ulva lactuca. Egypt. J. Aquat. Res. 2014;40:235–242. [Google Scholar]
- 31.Lupea M., Bulgariu L., Macoveanu M. Biosorption of cd (ii) from aqueous solution on marine green algae biomass. Environ. Eng. Manag. J. (EEMJ) 2012;11 [Google Scholar]
- 32.Gupta V.K., Rastogi A. Biosorption of lead (II) from aqueous solutions by non-living algal biomass Oedogonium sp. and Nostoc sp. a comparative study. Colloids Surfaces B Biointerfaces. 2008;64:170–178. doi: 10.1016/j.colsurfb.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Shaban M., Abukhadra M.R., Shahien M., Ibrahim S.S. Novel bentonite/zeolite-NaP composite efficiently removes methylene blue and Congo red dyes. Environ. Chem. Lett. 2017:1–6. [Google Scholar]
- 34.Khedr M., Halim K.A., Soliman N. Synthesis and photocatalytic activity of nano-sized iron oxides. Mater. Lett. 2009;63:598–601. [Google Scholar]
- 35.Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918;40:1361–1403. [Google Scholar]
- 36.Freundlich H. Over the adsorption in solution. J. Phys. Chem. 1906;57:1100–1107. [Google Scholar]
- 37.Foo K., Hameed B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010;156:2–10. [Google Scholar]
- 38.Tempkin M.I., Pyzhev V. Kinetics of ammonia synthesis on protonated iron catalyst. Acta. Physicochem. USSR. 1940;12:217–222. [Google Scholar]
- 39.Mahmoodi N.M. Synthesis of core–shell magnetic adsorbent nanoparticle and selectivity analysis for binary system dye removal. J. Ind. Eng. Chem. 2014;20:2050–2058. [Google Scholar]
- 40.Mahmoodi N.M. Synthesis of amine-functionalized magnetic ferrite nanoparticle and its dye removal ability. J. Environ. Eng. 2013;139:1382–1390. [Google Scholar]
- 41.Mahmoodi N.M. Dendrimer functionalized nanoarchitecture: synthesis and binary system dye removal. J. Taiwan Inst. Chem. Eng. 2014;45:2008–2020. [Google Scholar]
- 42.Xin N., Gu X., Wu H., Hu Y., Yang Z. Application of genetic algorithm-support vector regression (GA-SVR) for quantitative analysis of herbal medicines. J. Chemometr. 2012;26:353–360. [Google Scholar]
- 43.Mahmoodi N.M., Hayati B., Arami M. Kinetic, equilibrium and thermodynamic studies of ternary system dye removal using a biopolymer. Ind. Crops Prod. 2012;35:295–301. [Google Scholar]
- 44.Fan L., Luo C., Sun M., Qiu H., Li X. Synthesis of magnetic β-cyclodextrin–chitosan/graphene oxide as nanoadsorbent and its application in dye adsorption and removal. Colloids Surfaces B Biointerfaces. 2013;103:601–607. doi: 10.1016/j.colsurfb.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 45.Demiral H., Gündüzoğlu G. Removal of nitrate from aqueous solutions by activated carbon prepared from sugar beet bagasse. Bioresour. Technol. 2010;101:1675–1680. doi: 10.1016/j.biortech.2009.09.087. [DOI] [PubMed] [Google Scholar]
- 46.Wu F.-C., Tseng R.-L., Juang R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009;153:1–8. [Google Scholar]
- 47.Sheng P.X., Ting Y.-P., Chen J.P., Hong L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J. Colloid Interface Sci. 2004;275:131–141. doi: 10.1016/j.jcis.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 48.Feng N., Guo X., Liang S. Adsorption study of copper (II) by chemically modified orange peel. J. Hazard Mater. 2009;164:1286–1292. doi: 10.1016/j.jhazmat.2008.09.096. [DOI] [PubMed] [Google Scholar]
- 49.Florido A., Valderrama C.s., Arأ©valo J.A., Casas I., Martأ-nez M.a., Miralles N. Application of two sites non-equilibrium sorption model for the removal of Cu (II) onto grape stalk wastes in a fixed-bed column. Chem. Eng. J. 2010;156:298–304. [Google Scholar]
- 50.Saravanan A., Brindha V., Krishnan S. Studies on the structural changes of the biomass Sargassum sp. on metal adsorption. J. Adv. Bioinf. 2011;2:193–196. https://bipublication.com/files/JABAR-v2i3201104.pdf [Google Scholar]
- 51.Mahmoodi N.M., Mokhtari-Shourijeh Z. Modified poly (vinyl alcohol)-triethylenetetramine nanofiber by glutaraldehyde: preparation and dye removal ability from wastewater. Desalin. Water Treat. 2016;57:20076–20083. [Google Scholar]
- 52.Sharma Y.C. Optimization of parameters for adsorption of methylene blue on a low-cost activated carbon. J. Chem. Eng. Data. 2009;55:435–439. [Google Scholar]
- 53.Hu Z., Chen H., Ji F., Yuan S. Removal of Congo Red from aqueous solution by cattail root. J. Hazard Mater. 2010;173:292–297. doi: 10.1016/j.jhazmat.2009.08.082. [DOI] [PubMed] [Google Scholar]
- 54.Pons M.P., Fuste M.C. Uranium uptake by immobilized cells of Pseudomonas strain EPS 5028. Appl. Microbiol. Biotechnol. 1993;39:661–665. https://link.springer.com/content/pdf/10.1007%2FBF00205071.pdf [Google Scholar]
- 55.Esposito A., Pagnanelli F., Lodi A., Solisio C., Veglio F. Biosorption of heavy metals by Sphaerotilus natans: an equilibrium study at different pH and biomass concentrations. Hydrometallurgy. 2001;60:129–141. [Google Scholar]
- 56.El-Sikaily A., El Nemr A., Khaled A. Copper sorption onto dried red alga Pterocladia capillacea and its activated carbon. Chem. Eng. J. 2011;168:707–714. [Google Scholar]
- 57.Mohan S.V., Rao N.C., Karthikeyan J. Adsorptive removal of direct azo dye from aqueous phase onto coal based sorbents: a kinetic and mechanistic study. J. Hazard Mater. 2002;90:189–204. doi: 10.1016/s0304-3894(01)00348-x. [DOI] [PubMed] [Google Scholar]
- 58.Foo K., Hameed B. Preparation, characterization and evaluation of adsorptive properties of orange peel based activated carbon via microwave induced K2CO3 activation. Bioresour. Technol. 2012;104:679–686. doi: 10.1016/j.biortech.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Sprynskyy M., Buszewski B., Terzyk A.P., Namieśnik J. Study of the selection mechanism of heavy metal (Pb 2+, Cu 2+, Ni 2+, and Cd 2+) adsorption on clinoptilolite. J. Colloid Interface Sci. 2006;304:21–28. doi: 10.1016/j.jcis.2006.07.068. [DOI] [PubMed] [Google Scholar]
- 60.Li Q., Zhai J., Zhang W., Wang M., Zhou J. Kinetic studies of adsorption of Pb (II), Cr (III) and Cu (II) from aqueous solution by sawdust and modified peanut husk. J. Hazard Mater. 2007;141:163–167. doi: 10.1016/j.jhazmat.2006.06.109. [DOI] [PubMed] [Google Scholar]
- 61.Jonasi V., Matina K., Guyo U. Removal of Pb (II) and Cd (II) from aqueous solution using alkaline-modified pumice stone powder (PSP): equilibrium, kinetic, and thermodynamic studies. Turk. J. Chem. 2017;41:748–759. [Google Scholar]
- 62.Kula I., Uğurlu M., Karaoğlu H., Celik A. Adsorption of Cd (II) ions from aqueous solutions using activated carbon prepared from olive stone by ZnCl2 activation. Bioresour. Technol. 2008;99:492–501. doi: 10.1016/j.biortech.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 63.Naiya T.K., Bhattacharya A.K., Das S.K. Adsorption of Cd (II) and Pb (II) from aqueous solutions on activated alumina. J. Colloid Interface Sci. 2009;333:14–26. doi: 10.1016/j.jcis.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 64.Wang J. Science Press; Beijing: 2002. Microbial Immobilization Techniques and Water Pollution Control. [Google Scholar]
- 65.Rahmani A., Mousavi H.Z., Fazli M. Effect of nanostructure alumina on adsorption of heavy metals. Desalination. 2010;253:94–100. [Google Scholar]
- 66.Gundogdu A., Ozdes D., Duran C., Bulut V.N., Soylak M., Senturk H.B. Biosorption of Pb (II) ions from aqueous solution by pine bark (Pinus brutia Ten.) Chem. Eng. J. 2009;153:62–69. [Google Scholar]
- 67.Farhan S.N., Khadom A.A. Biosorption of heavy metals from aqueous solutions by Saccharomyces Cerevisiae. Int. J. Integr. Care. 2015;6:119–130. [Google Scholar]
- 68.Soliman Nk. Effective utilization of Moringa seeds waste as a new green environmental adsorbent for removal of industrial toxic dyes. J Mater Res Technol. 2018 [Google Scholar]
- 69.Naghizadeh A. Regeneration of carbon nanotubes exhausted with humic acid using electro-Fenton technology. Arabian J. Sci. Eng. 2016;41:155–161. [Google Scholar]
- 70.Naghizadeh A., Momeni F., Derakhshani E. Efficiency of ultrasonic process in regeneration of graphene nanoparticles saturated with humic acid. Desalin. Water Treat. 2017;70:290–293. [Google Scholar]
- 71.Derakhshani E., Naghizadeh A. Ultrasound regeneration of multi wall carbon nanotubes saturated by humic acid. Desalin. Water Treat. 2014;52:7468–7472. [Google Scholar]
- 72.Naghizadeh A., Nasseri S., Mahvi A.H., Rashidi A., Nabizadeh R., Kalantary R.R. Fenton regeneration of humic acid-spent carbon nanotubes. Desalin. Water Treat. 2015;54:2490–2495. [Google Scholar]
- 73.Chanzu H.A., Onyari J.M., Shiundu P.M. Biosorption of malachite green from aqueous solutions onto polylactide/spent brewery grains films: kinetic and equilibrium studies. J. Polym. Environ. 2012;20:665–672. [Google Scholar]
- 74.Hameed B. Evaluation of papaya seeds as a novel non-conventional low-cost adsorbent for removal of methylene blue. J. Hazard Mater. 2009;162:939–944. doi: 10.1016/j.jhazmat.2008.05.120. [DOI] [PubMed] [Google Scholar]
- 75.Masoud Moradi A.M.M., Azizi Nahid, Amini Jila, Karimi Kaveh, Sharafi K. Adsorptive removal of phenol from aqueous solutions by copper (cu)-modified scoria powder: process modeling and kinetic evaluation. Desalin.Water Treat. 2015:15. [Google Scholar]
- 76.Gupta V., Nayak A. Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem. Eng. J. 2012;180:81–90. [Google Scholar]
- 77.Nasernejad B., Zadeh T.E., Pour B.B., Bygi M.E., Zamani A. Camparison for biosorption modeling of heavy metals (Cr (III), Cu (II), Zn (II)) adsorption from wastewater by carrot residues. Process Biochem. 2005;40:1319–1322. [Google Scholar]
- 78.Okeola F., Odebunmi E., Nwosu F., Abu T., Mohammed A., Samuel A. Removal of lead and cadmium ions from aqueous solution by adsorption onto jatrophas curcas activated carbon Nig. J. Pure Appl. Sci. (Ankara) 2017;30:2955–2964. [Google Scholar]
- 79.Bishnoi N.R., Kumar R., Kumar S., Rani S. Biosorption of Cr (III) from aqueous solution using algal biomass spirogyra spp. J. Hazard Mater. 2007;145:142–147. doi: 10.1016/j.jhazmat.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 80.Naghizadeh A. Comparison between activated carbon and multiwall carbon nanotubes in the removal of cadmium (II) and chromium (VI) from water solutions. J. Water Supply Res. Technol. - Aqua. 2015;64:64–73. [Google Scholar]
- 81.Demirbaş A. Adsorption of Cr (III) and Cr (VI) ions from aqueous solutions on to modified lignin. Energy Sources. 2005;27:1449–1455. [Google Scholar]
- 82.Alves M.M., Beca C.G., De Carvalho R.G., Castanheira J., Pereira M.S., Vasconcelos L. Chromium removal in tannery wastewaters “polishing” by Pinus sylvestris bark. Water Res. 1993;27:1333–1338. [Google Scholar]
- 83.Azouaou N., Sadaoui Z., Djaafri A., Mokaddem H. Adsorption of cadmium from aqueous solution onto untreated coffee grounds: equilibrium, kinetics and thermodynamics. J. Hazard Mater. 2010;184:126–134. doi: 10.1016/j.jhazmat.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 84.Sharififard H., Nabavinia M., Soleimani M. Evaluation of adsorption efficiency of activated carbon/chitosan composite for removal of Cr (VI) and Cd (II) from single and bi-solute dilute solution. Adv. Environ. Technol. 2017;2:215–227. [Google Scholar]
- 85.McLellan J., Rock C. Pretreating landfill leachate with peat to remove metals. Water, Air, SoilPollut. 1988;37:203–215. https://link.springer.com/content/pdf/10.1007%2FBF00226492.pdf [Google Scholar]
- 86.Wang F.Y., Wang H., Ma J.W. Adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent—bamboo charcoal. J. Hazard Mater. 2010;177:300–306. doi: 10.1016/j.jhazmat.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 87.Senberber F.T., Yildirim M., Mermer N.K., Derun E.M. Adsorption of Cr (III) from aqueous solution using borax sludge. Acta Chim. Slov. 2017;64:654–660. doi: 10.17344/acsi.2017.3534. [DOI] [PubMed] [Google Scholar]
- 88.Tounsadi H., Khalidi A., Abdennouri M., Barka N. Biosorption potential of Diplotaxis harra and Glebionis coronaria L. biomasses for the removal of Cd (II) and Co (II) from aqueous solutions. J. Environ. Chem. Eng. 2015;3:822–830. [Google Scholar]
- 89.Velazquez-Jimenez L.H., Pavlick A., Rangel-Mendez J.R. Chemical characterization of raw and treated agave bagasse and its potential as adsorbent of metal cations from water. Ind. Crops Prod. 2013;43:200–206. [Google Scholar]
- 90.Bellú S., Garcia S., González J.C., Atria A.M., Sala L.F., Signorella S. Removal of chromium (VI) and chromium (III) from aqueous solution by grainless stalk of corn. Separ. Sci. Technol. 2008;43:3200–3220. [Google Scholar]
- 91.Salem N.M., Farhan A.M., Awwad A.M. Biosorption of cadmium (II) from aqueous solutions by Prunus avium leaves. Am. J. Environ. Eng. 2012;2:123–127. [Google Scholar]
- 92.Ding Y., Jing D., Gong H., Zhou L., Yang X. Biosorption of aquatic cadmium (II) by unmodified rice straw. Bioresour. Technol. 2012;114:20–25. doi: 10.1016/j.biortech.2012.01.110. [DOI] [PubMed] [Google Scholar]