Abstract
Testosterone activates singing within days in castrated male songbirds but full song quality only develops after a few weeks. Lesions of the medial preoptic nucleus (POM) inhibit while stereotaxic testosterone implants into this nucleus increase singing rate suggesting that this site plays a key role in the regulation of singing motivation. Testosterone action in the song control system works in parallel to control song quality. Accordingly, systemic testosterone increases POM volume within 1-2 days in female canaries, while the increase in volume of song control nuclei takes at least 2 weeks. The current study tested whether testosterone action is associated with similar differences in latencies in males. Photosensitive castrated male canaries were implanted with testosterone-filled Silastic™ implants and control castrates received empty implants, while simultaneously the photoperiod was switched from short- to long-days. Brains were collected from all subjects two days later. Plasma testosterone was elevated in testosterone-treated but not in controls. HVC volumes were not affected, but testosterone significantly increased the POM volume as identified by the dense group of aromatase-immunoreactive neurons, the number and somal area of these neurons and the fractional area they cover in POM. Testosterone-treated females from a previous experiment had a smaller POM volume in similar conditions suggesting the existence of a stable sex difference potentially affecting singing behavior. Thus testosterone induces male POM growth and aromatase expression in this nucleus within two days without affecting HVC size, further supporting the notion that testosterone increases singing motivation via its action in POM.
Keywords: song control system, HVC, singing motivation, songbirds, medial preoptic nucleus POM, testosterone
1. Introduction
Male songbirds sing at high rates during the breeding season to attract females and defend their territories [1, 2]. As observed for many courtship behaviors, testosterone plays a key role in regulating the probability and intensity of singing [3-5]. Administering testosterone to adult songbirds increases frequency and duration of singing in a number of species (for review see [4, 6] including canaries [7-9]. Testosterone regulates most aspects of song through synergistic effects of its androgenic and estrogenic metabolites (reviewed in [10]).
The songbird brain contains a specialized neural network, called the song control system that controls many aspects of the learning, maintenance and production of song [11]. Testosterone and its metabolites increase the volumes of nuclei in this network [7, 12] by altering various cellular properties in a region-specific manner (reviewed in [13-15]. Despite the profound impact of testosterone on various cellular properties of the song control system and aspects of song behavior, testosterone action within these nuclei does not seem to be responsible for the vernal increase in song rate. Meitzen and colleagues showed that blocking androgen and estrogen receptors in the HVC (previously acronym of High Vocal Center, now used as a full name) of white-crowned sparrows induces a decrease in song stereotypy, but no change in song rate [16]. Similarly, blocking androgen receptors in the HVC or RA (the nucleus robustus arcopallialis) of male canaries affects different aspects of song structure, but does not decrease song rate [17]. Together these studies suggest that testosterone acts in song control nuclei to regulate the quality but not the rate of singing.
The medial preoptic nucleus (POM) is a critical brain site where testosterone acts to activate appetitive aspects of sexual behavior in Japanese quail as well as songbirds [18-20]. Bilateral lesions of POM decrease song rate of male European starlings during the breeding season [21, 22]. Conversely implanting testosterone in the POM of castrated male canaries is sufficient to increase the singing rate, but not the song quality, to the same level as a systemic treatment with testosterone [20, 23]. Testosterone increases the rate of singing within 3-6 days in female or castrated male canaries [9]. Therefore if this behavioral effect is mediated by an action in the POM, testosterone-induced changes in POM should be evident after even shorter latencies. Male quail and adult female canaries implanted with testosterone capsules that increase plasma concentrations of the steroid to concentrations typical of sexually mature males or above, display an increased POM volume one day after the beginning of the treatment [24, 25], while the growth of the song control nuclei HVC, RA and Area X in female canaries takes one to three weeks [25]. Our previous work on female canaries [25] indicated that HVC and POM volumes are approximately twice as large in birds treated with testosterone and exposed to a long-day photoperiod than in birds held in a short-day photoperiod that have low concentrations of testosterone. It is therefore possible to compare directly the growth of these two nuclei following specific treatments. The current study investigated whether, as previously seen in female canaries, there is a rapid increase of POM volume in male canaries following testosterone implantation and whether this increase occurs at latencies that are shorter than the latency of the activation of song production.
2. Materials and Methods
2.1. Subjects
Ten male canaries of the Fife fancy breed were obtained in late March from a colony maintained at the University of Antwerp, Belgium. They were born and had gone through a full breeding cycle in this colony where they were maintained on natural daylight during the months preceding their arrival in our laboratory at the University of Liege, Belgium. All experimental procedures complied with Belgian laws concerning the Protection and Welfare of Animals and the Protection of Experimental Animals, and experimental protocols were approved by the Ethics Committee for the Use of Animals at the University of Liege (Protocol number 926). In all housing situations food (custom made canary mix prepared by and obtained from Moulin Boland, Seraing, Belgium), water, baths, cuttlebone and grit were available ad libitum.
2.2. Experimental procedures
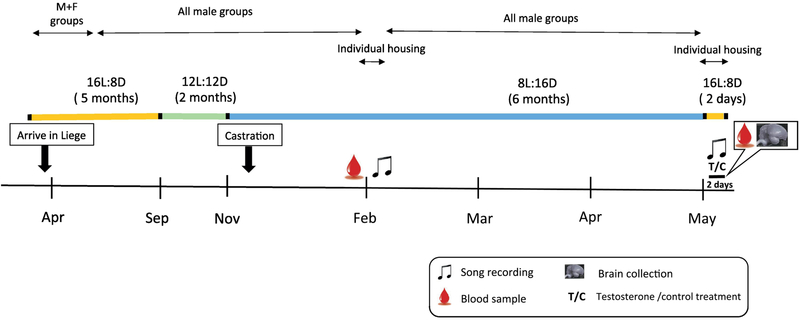
All procedures used in this study are similar to those described in [26] and are only briefly summarized here (See Fig. 1). Upon arrival subjects were exposed to 5 months long day photoperiod (16L:8D), 2 months intermediate photoperiod (12L:12D) and 6 months short photoperiod (8L:16D) in order to progress them through the photostimulated, photoregressed and photosensitive phases. Apart from a brief period of mixed-sex housing soon after arrival and three days of individual housing about 4 months into short day photoperiod, subjects were housed in single sex groups of 5-6 birds per cage. Three weeks after the transfer to 8L:16D (in November) all males were castrated under isoflurane anesthesia following a previously published procedure [8]. Testes were found at that time to be regressed in all subjects.
Figure 1.
Schematic representation of the endocrine and photoperiodic background of the males used in this experiment. The duration of each experimental phase is indicated by the months on the time line and further described in detail in the text but the durations are not strictly proportional to their length in the figure to allow representation of the experimental phase.
In February, birds were weighed, a blood sample (± 150 μl) was collected from the wing vein of all birds to assess their circulating testosterone concentration and their cloacal protuberances, an androgen-dependent structure, was measured (length and width). Testosterone concentrations were low and cloacal protuberance was small in all males. A schematic presentation of all these manipulations preceding the actual experiment can be found in Figure 1.
In May, subjects who were presumably photosensitive at that time, were individually transferred to sound-attenuated boxes for baseline song recordings (2 h of recording each day at light onset). Two days later, each subject received a single subcutaneous 10 mm-long Silastic™ implant inserted via a small incision through the apterium located at the side of the neck and pushed under the skin halfway down the back of the bird. Implants (Dow Corning reference no. 508-004; inner diameter 0.76 mm, outer diameter 1.65 mm) were either filled with crystalline testosterone (n= 4, Fluka Analytical, SigmaAldrich) or kept empty as control (n = 6). Testosterone implants of this size have been shown to strongly activate singing in castrated male canaries [8, 9, 20]. Light in the sound-attenuated boxes was then switched to 14L: 10D to mimic the increase in testosterone circulating concentrations and changes in photoperiod experienced by birds in the spring and singing behavior was recorded for two more days (2 h/day). On the morning of the 2nd day after implantation a 150 μ1 blood sample was collected from the wing vein, subjects were weighed, their cloacal protuberance was measured again, they were deeply anaesthetized with an intramuscular injection of 0.03 ml Nembutal™ (Pentobarbital 60 mg/ml) and intracardially perfused with phosphate-buffered saline (PBS, 1.43 g/L Na2HPO4, 0.48 g/L KH2PO4, 7.2 g/L NaCI) until return flow in the atrium was clear, followed by 4% paraformaldehyde (PFA, 4.3 g/L NaOH, 40 g/L paraformaldehyde, 18.8 g/L NaH2PO4.H20).
The syrinx was extracted and weighed, and the presence of implants was confirmed. The brain was extracted from the skull and post-fixed overnight, in 15 ml PFA. On the next day, brains were cryoprotected in 30% sucrose (15.6 g/L Na2HPO4, 1.5 g/L KH2PO4, 300 g/L sucrose). When they sank to the bottom of the vial they were frozen on dry ice and stored at −80°C until used.
2.3. Testosterone Enzyme Immunoassay (EIA)
Testosterone was assayed in 10 μl aliquots of all samples by an EIA kit (Cayman Chemicals ref. 582701) following manufacturer’s instructions. The details of procedures and validations of the assay were previously published [26]. The samples were diluted 80 times in buffer and measured in two assays with intra-assay coefficients of variation of 11.1 and 14.4%. The minimum and maximum detection limits of the EIA, as determined by the lowest and highest concentration detected in the diluted plasma samples were 4.66 pg/ml and 169.73 pg/ml respectively.
2.4. Brain processing
Brains were cut on a cryostat into 4 series of 30 μm-thick coronal sections. Sections were stored in anti-freeze (0.01M PBS with 10 g/L polyvinylpyrrolidone, 300 g/L sucrose, and 300 ml/L ethylene glycol) at-20°C until further use.
2.4.1. Nissl staining
One series of sections was mounted on Superfrost slides, dried at least overnight, and Nissl-stained with toluidine blue. They were then dehydrated in a series of increasing isopropanol concentrations, 99% ethanol and xylene and coverslipped using Eukitt as a mounting medium.
2.4.2. Aromatase immunohistochemistry
Two series of sections through the preoptic area – from the start of tractus septopallio-mesencephalicus (TSM) rostrally to sections located 240 μm caudally to the anterior commissure (AC) – were stained by immunohistochemistry for aromatase, with the use of a rabbit aromatase antibody kindly provided by Prof. Nobuhiro Harada (Fujita Health University, Toyoake, Japan) as previously described [25, 26].
2.5. Microscopy and image analysis
All Nissl stained sections containing HVC were photographed with a Leica DMRB FL.100 microscope connected to a Leica DFC 480 color camera and HVC volume reconstructed with the ImageJ v1.47v software (National Institutes of Health) as previously described [25, 27]. All sections containing the aromatase-immunoreactive (ARO-ir) cells defining the medial preoptic nucleus (POM) were photographed and the volume of this cell group was measured as previously described [25, 26] using the same microscope, camera and software as for Nissl-stained sections. The first 3 most rostral sections were however excluded from the total volume because in many birds they had been damaged during processing. This however did not change the magnitude of the group difference nor its statistical significance. In addition, like in these previous studies, we quantified in one photomicrograph representing the middle section of the POM in the rostro-caudal axis the number of ARO-ir cells, the percentage of area covered by aromatase staining and the mean somal area of the ARO-ir cells. All analyses were performed in a randomized order on sections that had been coded so that observers were blind to the experimental groups.
2.6. Song analysis
Song recordings were manually analyzed with the help of the Raven Pro 1.5 software. The sound tracks were visualized as spectrograms and scrolling though these spectrograms, we noted the number of songs produced, defined as units of vocal production lasting a minimum of 1 second being preceded and followed by 0.5 seconds of silence.
2.7. Statistical analyses
Data were analyzed by t-tests or repeated measures two-way ANOVA as appropriate that were performed using PRISM 5 or STATISTICA. Testosterone concentrations were log10 transformed before analysis because they displayed a difference in variance between groups while such a difference was not detected in all other sets of data. Effects were considered significant for p<0.05. All data are represented here by their mean ± SEM.
3. Results
3.1. Morphological measures and testosterone
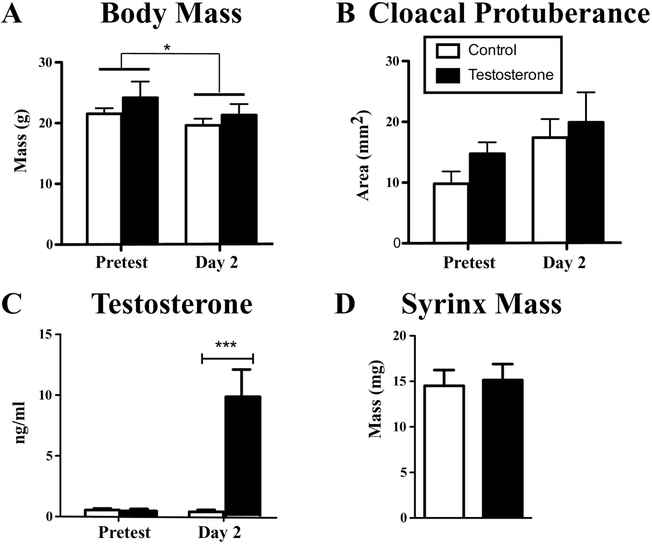
The body mass, cloacal protuberance and plasma testosterone concentrations measured at baseline (February) and at the end of the experiment (May) were compared by two-way repeated-measures ANOVA (Fig. 2). There was over time a slight decrease in body mass in both groups (F1,8 = 8.67, p = 0.019, η 2p = 0.510) but no effect of testosterone treatment (F1,8 = 1.12, p = 0.322, η 2p = 0.122) and no interaction between the two factors (F1,8 = 0.36, p = 0.564, η 2p = 0.042; Fig. 2A). The cloacal protuberance area increased slightly albeit not significantly over time (F1,8 = 3.84, p = 0.086, η 2p = 0.324) but was not affected by testosterone (F1,8 = 1.63, p = 0.238, η2p = 0.169), nor by the interaction between factors (F1,8 = 0.14, p = 0.719, η2p = 0.017; Fig. 2B). Plasma testosterone concentration was significantly affected by the treatment (Fi1,8 = 18.37, p < 0.003, η 2p = 0.697) and its interaction with time (Fi,1,8 = 9.89, p = 0.014, η2p = 0.553) but there was no overall effect of time (Fi,1,8 =2.29, p =0.170, η2p = 0.221). Post-hoc tests indicated that there was no difference between the future control and testosterone-treated birds at baseline, but the testosterone concentrations were, as expected, significantly elevated in the testosterone-treated group after 2 days of treatment (p<0.001, Fig. 2C). A student t-test indicated however that the elevated testosterone did not affect the mass of the syrinx as measured on the day of brain collection (Fig. 2D, t8 = 0.25, p = 0.811, d=0.159).
Figure 2.
Body mass (A), area of the cloacal protuberance (length x width, B) and plasma testosterone concentrations before Log10 transformation (C) measured at the pretest (two months before treatment) and at the time of brain collection after two days of exposure to long days and treatment with Silastic™ implants filled with testosterone or left empty as controls. The mass of the syrinx measured at the time of brain collection is also shown in panel (D). All data are represented by their mean ± SEM. ***= p<0.001.
3.2. HVC volume
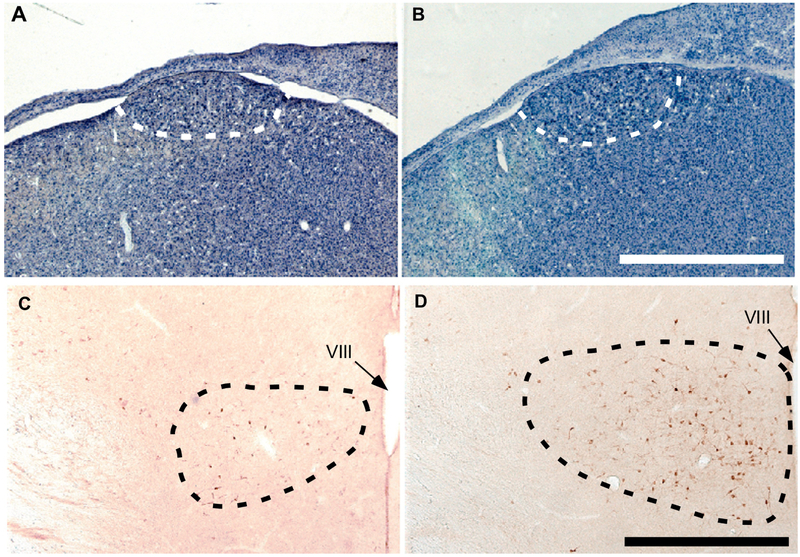
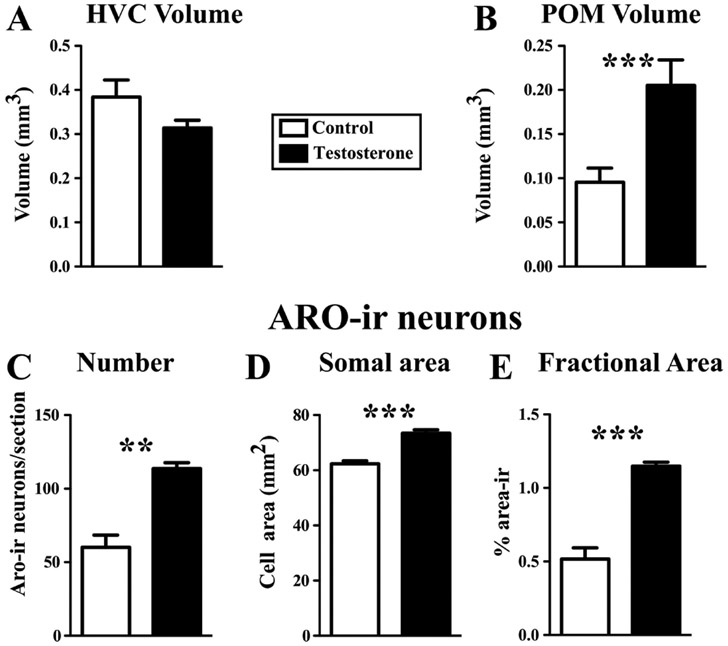
The volume of HVC was not different between the control and testosterone-treated group (see Fig. 3A-B for representative photomicrographs, Fig. 4A for quantitative data) as confirmed by a t-test (t8 = 1.38, p = 0.205, d=0.891).
Figure 3.
Representative photomicrographs illustrating the song control nucleus HVC in Nissl-stained sections (A-B) and the medial preoptic nucleus (POM, C-D) in sections stained by immunohistochemistry for aromatase in a castrated male (A,C) and a castrated male treated for two days with testosterone (B, D). The dotted lines in each panel outline the cytoarchitectonic boundaries of the nuclei. VIII= third ventricle. Magnification bar is equal to 1 mm in A-B and 500 αm in C-D.
Figure 4.
Effects of a two-day exposure to long days and treatment with testosterone on the volume of HVC (A) and of the medial preoptic nucleus, POM (B), and on the number of ARO-ir neurons (C), their average somal area (D) and percentage of the area in POM covered by ARO-ir material (E) quantified in a section located in the middle of the rostro-caudal extent of the POM. Data are means ± SEM. **= p<0.01; ***= p<0.001
3.3. POM volume as defined by aromatase-immunoreactive (ARO-ir) cells
In contrast, POM volume as defined by the cluster of ARO-ir cells was significantly larger in the testosterone-treated than in the control group (Fig. 3C-D for typical photomicrographs, Fig. 4B, t8 = 5.13, p < 0.001 d=3.310). Testosterone treatment also increased the number of ARO-ir neurons counted in the middle section of POM in the rostro-caudal axis (Fig. 4C, t8 = 4.92, p = 0.0012, d=3.176), the somal area of these neurons (Fig. 4D, t8 = 6.66, p < 0.001, d= 4.297) and the fractional area covered by ARO-ir material in that section (Fig. 4E, t8 = 6.50, p < 0.001, d=4.197).
3.4. Singing behavior
One bird from the control group sang at high rates both before and after treatment. This bird had a POM volume that was somewhat higher than the rest of the control group (0.145 mm3) but still well within the 95% confidence interval of the group (mean ± 2 standard deviations: 0.030-0.165 mm3). Two other birds were not singing during the February recordings, but sang at relatively low rates during the May recordings before treatments. One of them, which was in the testosterone-treated group, continued to sing also after treatment at approximately the same rate as before the treatment, the other bird was in the control group and did not sing after treatment onset. All other birds did not sing at all either before or after treatment.
4. Discussion
We show here that treatment with testosterone of photosensitive male canaries of the Fife fancy strain increases within 2 days the volume of the POM and the number and size of ARO-ir cells in this nucleus while having no effect on the volume of HVC, on singing behavior and on the size of the androgen-dependent cloacal protuberance. A similar pattern of results had been previously identified in female canaries [25], and a few comparisons between these two studies deserve additional comments.
Birds had been held in short days for several months and were therefore expected to display a minimal singing activity. They had also been housed in groups prior to recording so that dominant males might have inhibited the vocal activity of the other males in the same cage. The singing activity observed in these males before the treatments thus essentially matched the expected pattern. Based on previous research, two days of treatment with testosterone were also not anticipated to activate a substantial singing activity [9, 20]. The amount of singing observed in these birds was thus fully expected.
Although the area of the cloacal protuberance and the mass of the syrinx are clearly androgen-dependent as demonstrated in a variety of songbird species [28-31], including canaries [32], the size of these two structures did not increase in testosterone-treated subjects as compared to controls. It is likely that more than two days are required for the morphological action of testosterone to become detectable. In female canaries of the same strain, testosterone increased both cloacal protuberance length and syrinx mass after nine days, but had no effect on the size of these organs after only two days of treatment [25]. Similarly, in male quail the size of the cloacal gland is increased by testosterone treatment 7 days post-treatment but not after 2 days [24].
Similarly, the volume of HVC was not increased in testosterone-treated subjects compared to controls despite the fact that an increase in HVC volume following testosterone treatment has been demonstrated in both male and female canaries, but always after longer treatment durations [7, 9, 12, 33, 34]. Testosterone-treatment in female canaries increased HVC volume after 21 days, with marginal increases being already detected after 9 days, but no effect at all was present after 1 or 2 days of treatment [25]. Another study comparing both males and females found no effect of testosterone on HVC volume after one week and suggested a dose-dependent increase in both sexes after 3 weeks, which did not however reach statistical significance presumably because it was superimposed on a negative stress-related trend [9].
Few studies to date have investigated changes in HVC volume following testosterone treatments of short duration. It was demonstrated that in white-crowned sparrows (Zonotrichia Leucophrys), removal of subcutaneous implants releasing testosterone combined with transfer from long days to short days decreases HVC volume within 12 h [35] but the opposite effect - HVC growth following transfer to long days and exposure to testosterone - has only been reported to take place after 7 days of treatment but no shorter latency was analyzed [29]. The present experiment clearly indicates that a period longer than two days is necessary for testosterone to increase HVC volume. These relatively slow changes observed in HVC following testosterone treatment are compatible with testosterone action in HVC modulating song quality [16], an effect that develops slowly after at least one week or more [29]. We did not measure here the volume of the other song control nuclei RA and Area X, because it has been repeatedly shown that these nuclei grow with a slower time course than HVC [25, 29, 36].
In contrast, the POM volume assessed by measuring the dense cluster of ARO-ir cells was significantly increased after only 2 days of exposure to testosterone. Previous studies in Japanese quail (Coturnix japonica) have shown a high positive correlation between changes in POM volume as measured by aromatase immunohistochemistry and by Nissl stain [24, 37, 38]. It was also demonstrated that exposure of castrated quail to testosterone increases POM volume after only one or two days, a neuroanatomical change that immediately precedes the activation of male copulatory behavior [24]. In male canaries, the increase of POM volume after two days of exposure to testosterone is consistent with the role of this nucleus in the control of singing motivation given that castrated males treated with testosterone start singing three to five days following exposure to testosterone [9, 20].
Alward and colleagues showed that implanting testosterone stereotaxically in POM increases the song rate in castrated male canaries to the same level as in subjects systemically exposed to testosterone [20]. However, birds treated with T in POM take longer to begin singing than systemically treated birds. Systemically-treated males already sing at a higher rate than castrated controls after 3 days while this degree of singing activation is only reached after 7 days in birds with testosterone in POM. This suggests that activation of singing requires actions of testosterone in other brain or peripheral structures (e.g., the syrinx) besides the gross morphological changes taking place in the POM and its ARO-ir neurons. Full song quality and song power similarly require testosterone action in other structures such as the song control nuclei HVC and RA and the syrinx (see [39] for review). The aromatase-expressing cells of the POM are however likely to play a substantial role in the control of song motivation given that effects of testosterone on singing behavior are mediated, at least in part, via estrogenic metabolites [10, 34, 40-42] and inhibiting aromatase decreases song rate in a rapid manner in canaries [43].
Comparison of the results of this study with our prior study in female canaries suggests an interesting sex difference in POM neuroendocrine anatomy. The two studies included birds treated with identical doses of testosterone administered for the same short two-day duration and birds were held under the same photoperiod (14L: 10D) during the treatment phase. Interestingly, Silastic™ implants increased plasma testosterone concentrations within 2 days to similar high values in males (9.87±2.23 ng/ml, present study) and females (11.99±1.91 ng/ml in our previous study in females, see [25]). However, the POM volume as determined by the dense cluster of ARO-ir cells ended up being significantly larger in testosterone-treated males (0.22±0.02 mm3) than in females (0.13±0.01 mm3) treated with the same implants for 2 days, while control volumes did not differ much (0.10±0.01 in males vs. 0.08±0.01 mm3 in females). This contrasts with the situation in quail where the sex difference in the volume of POM between sexually mature males and females disappears when birds are gonadectomized and treated with the same amount of exogenous testosterone [18, 44].
Although the sex difference suggested here in testosterone-treated canaries should be confirmed in a future study simultaneously including both sexes, the present results obtained with the same breed of birds, within the same laboratory using the same methods, suggest that this difference cannot be eliminated by equalizing the circulating testosterone concentrations and could thus result from either organizational effects of early sex steroid exposure or from “direct” genetic effects not mediated by sex steroids. This could possibly contribute to explain why it is apparently difficult if not impossible to activate the same number of high quality songs in females as in males by adult treatments with testosterone [9].
Highlights.
Testosterone (T) is known to increase song rate in male canaries after 1-2 weeks
T increased the volume of the medial preoptic nucleus (POM) within 2 days
These changes precede increase in HVC volume or in song rate
T also increased aromatase expression in POM
POM volume in T-treated males is larger than in similarly treated females
Acknowledgments
This work was supported by grants SSTC IAP P7/17 from the Belgian Science Policy to JB and CAC and RO1NS104008 from the National Institute of Neurological disorders and stroke to GFB, JB and CAC. CAC is a senior F.R.S.-FNRS Research Associate. We thank Samar Ghorbanpoor for help with the performance of parts of this experiment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Catchpole CK, Slater PJB Bird song Biological themes and Variations. Cambridge UK: Cambridge University Press; 2008. [Google Scholar]
- [2].Collins S Vocal fighting and flirting: the functions of birdsong In: Marler P, Slabbekoorn H, eds. Nature's Lusic, The Science of Birdsong. Amsterdam: Elsevier; 2004. p. 39–79. [Google Scholar]
- [3].Schlinger BA Sex steroids and their actions on the birdsong system. J.Neurobiol. 1997,33:619–31. [PubMed] [Google Scholar]
- [4].Ball GF, Riters LV, Balthazart J Neuroendocrinology of song behavior and avian brain plasticity: multiple sites of action of sex steroid hormones. Front. Neuroendocrinol. 2002,23:137–78. [DOI] [PubMed] [Google Scholar]
- [5].Ball GF, Balthazart J Neuroendocrine regulation of reproductive behavior in birds. Hormones, Brain and Behavior Online 2010. p. 855–97. [Google Scholar]
- [6].Schlinger BA, Brenowitz EA Neural and hormonal control of birdsong In: Pfaff DW, Joels M, eds. Hormones, brain and behavior. 3rd ed. Oxford: Academic Press; 2017. p. 255–90. [Google Scholar]
- [7].Nottebohm F Testosterone triggers growth of brain vocal control nuclei in adult female canaries. Brain Res. 1980,189:429–36. [DOI] [PubMed] [Google Scholar]
- [8].Sartor JJ, Balthazart J, Ball GF Coordinated and dissociated effects of testosterone on singing behavior and song control nuclei in canaries (Serinus canaria). Hormones and Behavior. 2005,47:467–76. [DOI] [PubMed] [Google Scholar]
- [9].Madison FN, Rouse ML Jr., Balthazart J, Ball GF Reversing song behavior phenotype: Testosterone driven induction of singing and measures of song quality in adult male and female canaries (Serinus canaria). General and comparative endocrinology. 2015,215:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Harding CF Hormonal modulation of singing: hormonal modulation of the songbird brain and singing behavior. Ann N Y Acad Sci. 2004,1016:524–39. [DOI] [PubMed] [Google Scholar]
- [11].Zeigler HP, Marler P Neuroscience of birdsong. Cambridge UK: Cambridge University Press; 2008. [Google Scholar]
- [12].Yamamura T, Barker JM, Balthazart J, Ball GF Androgens and estrogens synergistically regulate the expression of doublecortin and enhance neuronal recruitment in the song system of adult female canaries. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011,31:9649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brenowitz EA Plasticity of the song control system in adult birds In: Zeigler HP, Marler P, eds. Neuroscience of birdsong. Cambridge: Cambridge University Press; 2008. p. 332–49. [Google Scholar]
- [14].Brenowitz EA, Larson TA Neurogenesis in the adult avian song-control system. Cold Spring Harbor perspectives in biology. 2015,7:a019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Balthazart J, Ball GF Endocrine and social regulation of adult neurogenesis in songbirds. Frontiers in neuroendocrinology. 2016,41:3–22. [DOI] [PubMed] [Google Scholar]
- [16].Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ Steroid hormones act transsynaptically within the forebrain to regulate neuronal phenotype and song stereotypy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007,27:12045–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Alward BA, Balthazart J, Ball GF Dissociable Effects on Birdsong of Androgen Signaling in Cortex-Like Brain Regions of Canaries. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2017,37:8612–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Panzica GC, Viglietti-Panzica C, Balthazart J The sexually dimorphic medial preoptic nucleus of quail: A key brain area mediating steroid action on male sexual behavior. Frontiers in neuroendocrinology. 1996,17:51–125. [DOI] [PubMed] [Google Scholar]
- [19].Balthazart J, Ball GF Topography in the preoptic region: Differential regulation of appetitive and consummatory male sexual behaviors. Frontiers in neuroendocrinology. 2007,28:161–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Alward BA, Balthazart J, Ball GF Differential effects of global versus local testosterone on singing behavior and its underlying neural substrate. Proceedings of the National Academy of Sciences of the United States of America. 2013,110:19573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Riters LV, Ball GF Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris). Horm.Behav. 1999,36:276–86. [DOI] [PubMed] [Google Scholar]
- [22].Alger SJ, Riters LV Lesions to the medial preoptic nucleus differentially affect singing and nest box-directed behaviors within and outside of the breeding season in European starlings (Sturnus vulgaris). Behav Neurosci. 2006,120:1326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Alward BA, Madison FN, Parker SE, Balthazart J, Ball GF Pleiotropic Control by Testosterone of a Learned Vocal Behavior and Its Underlying Neuroplasticity(1,2,3). eNeuro. 2016,3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Charlier TD, Ball GF, Balthazart J Rapid action on neuroplasticity precedes behavioral activation by testosterone. Horm Behav. 2008,54:488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shevchouk OT, Ghorbanpoor S, Ball GF, Cornil CA, Balthazart J Testosterone-induced neuroendocrine changes in the medial preoptic area precede song activation and plasticity in song control nuclei of female canaries. The European journal of neuroscience. 2017,45:886–900. [DOI] [PubMed] [Google Scholar]
- [26].Shevchouk OT, Ghorbanpoor S, Smith E, Liere P, Schumacher M, Ball GF, et al. Behavioral evidence for sex steroids hypersensitivity in castrated male canaries. Horm Behav. 2018,103:80–96. [DOI] [PubMed] [Google Scholar]
- [27].Shevchouk OT, Ball GF, Cornil CA, Balthazart J Studies of HVC Plasticity in Adult Canaries Reveal Social Effects and Sex Differences as Well as Limitations of Multiple Markers Available to Assess Adult Neurogenesis. PloS one. 2017,12:e0170938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Luine V, Nottebohm F, Harding CF, McEwen BS Androgen affects cholinergic enzymes in syringeal motor neurons and muscles. Brain Res. 1980,192:89–107. [DOI] [PubMed] [Google Scholar]
- [29].Tramontin AD, Hartman VN, Brenowitz EA Breeding conditions induce rapid and sequential growth in adult avian song control circuits: A model of seasonal plasticity in the brain. J.Neurosci. 2000,20:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tramontin AD, Wingfield JC, Brenowitz EA Androgens and estrogens induce seasonal-like growth of song nuclei in the adult songbird brain. J Neurobiol. 2003,57:130–40. [DOI] [PubMed] [Google Scholar]
- [31].Hall ZJ, MacDougall-Shackleton EA Influence of testosterone metabolites on song-control system neuroplasticity during photostimulation in adult European starlings (Sturnus vulgaris). PloS one. 2012,7:e40060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Appeltants D, Ball GF, Balthazart J Song activation by testosterone is associated with an increased catecholaminergic innervation of the song control system in female canaries. Neuroscience. 2003,121:801–14. [DOI] [PubMed] [Google Scholar]
- [33].Rasika S, Alvarez-Buylla A, Nottebohm F BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999,22:53–62. [DOI] [PubMed] [Google Scholar]
- [34].Fusani L, Metzdorf R, Hutchison JB, Gahr M Aromatase inhibition affects testosterone-induced masculinization of song and the neural song system in female canaries. J Neurobiol. 2003,54:370–9. [DOI] [PubMed] [Google Scholar]
- [35].Thompson CK, Bentley GE, Brenowitz EA Rapid seasonal-like regression of the adult avian song control system. Proceedings of the National Academy of Sciences of the United States of America. 2007,104:15520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Smith GT, Brenowitz EA, Wingfield JC Seasonal changes in the size of the avian song control nucleus HVC defined by multiple histological markers. J.Comp.Neurol. 1997,381:253–61. [DOI] [PubMed] [Google Scholar]
- [37].Balthazart J, Surlemont C, Harada N Aromatase as a cellular marker of testosterone action in the preoptic area. Physiology and Behavior. 1992,51:395–409. [DOI] [PubMed] [Google Scholar]
- [38].Foidart A, de Clerck A, Harada N, Balthazart J Aromatase-immunoreactive cells in the quail brain: Effects of testosterone and sex dimorphism. Physiology and Behavior. 1994,55:453–64. [DOI] [PubMed] [Google Scholar]
- [39].Alward BA, Rouse ML, Balthazart J, Ball GF Testosterone regulates birdsong in an anatomically specific manner. Animal Behaviour. 2017,124:291–8. [Google Scholar]
- [40].Harding CF, Sheridan K, Walters MJ Hormonal specificity and activation of sexual behavior in male zebra finches. Horm.Behav. 1983,17:111–33. [DOI] [PubMed] [Google Scholar]
- [41].Harding CF, Walters MJ, Collado D, Sheridan K Hormonal specificity and activation of social behavior in male red-winged blackbirds. Horm.Behav. 1988,22:402–18. [DOI] [PubMed] [Google Scholar]
- [42].Fusani L, Gahr M Hormonal influence on song structure and organization: the role of estrogen. Neuroscience. 2006,138:939–46. [DOI] [PubMed] [Google Scholar]
- [43].Alward BA, de Bournonville C, Chan TT, Balthazart J, Cornil CA, Ball GF Aromatase inhibition rapidly affects in a reversible manner distinct features of birdsong. Scientific reports. 2016,6:32344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Panzica GC, Viglietti-Panzica C, Calagni M, Anselmetti GC, Schumacher M, Balthazart J Sexual differentiation and hormonal control of the sexually dimorphic medial preoptic nucleus in the quail. Brain Research. 1987,416:59–68. [DOI] [PubMed] [Google Scholar]