Highlights
-
•
Relapsed ATL patients who are not eligible for SCT have limited therapeutic options and a poor prognosis.
-
•
Lenalidomide confers a survival benefit through immunomodulation for patients with relapsed and recurrent ATL.
-
•
The use of low-dose lenalidomide as maintenance therapy may be a new and promising option for these patients after chemotherapy.
-
•
Lenalidomide has potential in the treatment of ATL patients, particularly the elderly who are not eligible for SCT.
Keywords: Lenalidomide, Adult T cell leukemia/ lymphoma (ATL), Maintenance
Abstract
Adult T cell leukemia/lymphoma (ATL) is incurable with conventional chemotherapies, and allogeneic stem cell transplantation (SCT) is the only curative treatment. Direct antitumor effects and antitumor immune responses are important factors that need to be considered in the treatment of ATL. A phase II study reported long overall survival despite short progression-free survival in patients, implying that lenalidomide confers a survival benefit through immunomodulation for patients with ATL. We herein report that low-dose lenalidomide as maintenance therapy maintained a complete remission in a patient with aggressive ATL, whose condition has since remained stable with no recurrence for 24 months.
Adult T-cell leukemia/lymphoma (ATL) is an aggressive peripheral T-cell lymphoma (PTCL) caused by human T-cell lymphotropic virus type I (HTLV-I). Since ATL is resistant to conventional chemotherapeutic agents and there are currently limited treatment options, it has a poor prognosis [1]. There are diverse clinical courses and survival outcomes among patients with acute- and lymphoma-type ATL. Katsuya et al. established a prognostic index (PI) for acute and lymphoma-type ATL (ATL-PI), consisted of Ann Arbor stage, performance status (PS), age, serum albumin, and soluble IL-2 receptor (sIL-2R) [2]. They demonstrated that the ATL-PI were associated with OS, thus it is a promising platform that can be used to determine optimal treatment based on risk stratification or clinical trials. LSG15 is an option and when combined with mogamulizumab, the humanized anti-CC chemokine receptor 4 (CCR4) monoclonal antibody, results in improved responses, however there are no changes in progression-free survival (PFS) or overall survival (OS) [3]. Additional treatment options capable of providing improved response rates and survival for patients with high-risk ATL are urgently needed.
Lenalidomide is an immunomodulator that exhibits anti-proliferative and antineoplastic activities against multiple myeloma. The E3 ubiquitin ligase cereblon has been identified as a direct protein target required for the anti-myeloma and anti-lymphoma effects of lenalidomide as well as thalidomide [4]. Previous clinical trials on lenalidomide monotherapy for patients with recurrent and refractory T-cell lymphoma showed overall response rates of 26 and 22%, respectively [5]. In relapsed and recurrent patients with ATL, the findings of a phase 2 study on lenalidomide showed an overall response rate, median progression-free survival, and median survival time (MST) of 42%, 3.8 months, and 20.3 months, respectively [6]. Based on these findings, lenalidomide was approved for relapsed/refractory ATL in Japan in March 2017. The key for future lenalidomide therapy may be its use in combination with chemotherapy or other agents, such as mogamulizumab, and timing in order to maximally exploit potential synergisms in ATL patients. In our case, low-dose lenalidomide was useful as maintenance therapy to treatment of high risk ATL in a patient who was not eligible for allogeneic stem cell transplantation (SCT).
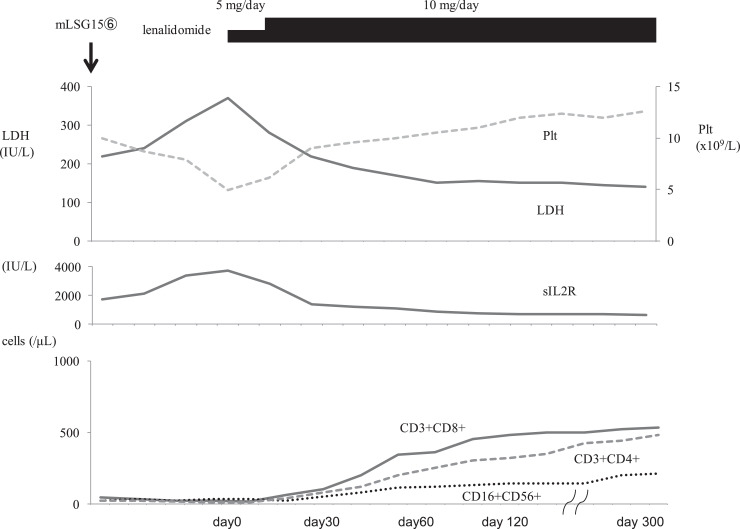
A 70-year-old Japanese female with general fatigue was referred to our hospital. On admission, her temperature was 38.3 °C and blood pressure was 112/64 mmHg. Lymph node enlargement was detected in the bilateral cervical regions. Laboratory tests showed a white blood cell count (WBC) of 95.9 × 109/L, abnormal lymphocytes 43%, red blood cell count (RBC) of 455 × 1010/L, hemoglobin (Hb) concentration of 13.5 g/dL, and platelet count of 18.0 × 109/L. Her serum levels of albumin (3.3 g/dL(normal range:3.9–4.9 g/dL) was decreased, and lactase dehydrogenase (LDH) (909 U/L (normal range: 106–211 IU/L)) and C-reactive protein (CRP) (7.6 mg/dL (normal range: below 0.3 mg/dL)) were elevated. The serum level of soluble IL-2 receptor (sIL-2R) was markedly elevated (34,700 U/ml (normal range: 145–519 U/mL)) and HTLV-1 antibodies were detected. A Southern blot analysis of an 8.25-kb Sac I fragment containing large regions of the HTLV-I genome detected monoclonal bands after EcoRI and Pstl digestions in peripheral blood mononuclear cells. Computed tomography (CT) revealed generalized lymphadenopathy including the mediastinum and abdomen. Bone marrow aspiration showed 52.4% abnormal lymphocytes, and a flow cytometric analysis was positive for CD3, CD4, and CD25, and negative for CD8, CD7, CD30, CD20, and CD19. She was diagnosed with ATL (acute type, ATL-IPI: high risk). She received modified LSG15 chemotherapy with mogamulizumab. After 6 cycles of this treatment, she achieved a complete remission (CR) [7]. CT revealed no lymphadenopathy or organomegaly and bone marrow aspiration showed no abnormal lymphocytes. However, laboratory data showed thrombocytopenia and serum level of LDH and sIL2R were still elevated (Fig. 1). She did not wish for the treatment with SCT. In an effort to avoid relapse, daily low-dose lenalidomide therapy (5 mg/day) was initiated. On day 5 after its initiation, a skin rash associated with lenalidomide was observed. By day 50, platelets began to increase and LDH and sIL2-R decreased. The lenalidomide treatment resulted in long-term increases in the numbers of CD3+ CD8+ cytotoxic T cells, CD3+CD4+T cells and CD56+ CD16 + NK cells (Fig. 1). She has maintained CR for 12 months after the lenalidomide treatment.
Fig. 1.
Clinical course. Plt: platelet count, LDH: lactate dehydrogenase.
Clinical trials have been paramount to the recent advances in ATL treatments, including assessments of chemotherapy and allogeneic SCT. A strategy to treat ATL, stratified by a subtype classification, prognostic factors, and responses to initial treatments as well as response criteria, was recently proposed [7]. However, ATL still has a worse prognosis than other T-cell malignancies. Patients with advanced-phase disease or not eligible for SCT are achieved in low CR rate and in short duration of remission, thus the treatments to prolong the duration of remission and provide relevant benefits in terms of OS, DFS, and quality of life are needed.
According to the dataset of the ATL prognostic index (ATL-PI) project, MST was 8.3 months, while OS at 4 years was 11.4% in patients with acute ATL [8]. In terms of the prognosis of acute ATL with allogeneic HSCT, MST was 14 months and OS at 4 years was 27.8%, while in patients acute ATL without allo HSCT, MST was 6.7 months and OS at 4 years was 6.8% [8]. These findings indicate that allogeneic HSCT contributes to prolonging the survival of patients with acute-type ATL; however, the prognoses of patients without SCT were unfavorable.
It currently remains unclear whether the increased relapse rates observed with combinations of monoclonal antibodies and chemotherapies translate into improvements in OS. Therapies that aim to increase immune responses may be of particular interest in ATL. Lenalidomide reportedly not only exerts direct antitumor effects, but also enhances antitumor immune responses mediated by T cells and NK cells. Previous studies suggested a high degree of immunogenicity of ATL cells caused by HTLV-I-associated or tumor-specific antigens. The possibility of graft-versus-ATL effects after allogeneic SCT also supports the theory of the strong immunogenicity of ATL cells [9].
A correlation has been reported between the development of a skin rash and responses to lenalidomide monotherapy. The skin and subcutaneous tissue disorders, such as rashes, associated with lenalidomide treatment may be regarded as immune-related adverse events and may be manifestations of the underlying provocation of antitumor immune responses to ATL cells. A relationship has not yet been clearly established between the development of a rash and responses in ATL and, thus, further studies are needed.
In this case, lenalidomide triggered increases in the numbers of circulating CD3+CD8+ cytotoxic T cells, CD3+CD4+ T cells and CD16+ CD56+ NK cells. Lenalidomide exerts pleiotropic antitumor effects, including the stimulation of T- and natural killer cell functions. Recently several reports raised the importance of increased no-tumor CD3+CD4+ T cells enhanced reconstitution of normal T cells in ATL [10]. Since the antitumor activity of mogamulizumab is completely dependent on antibody-dependent cell-mediated cytotoxicity, mainly via NK cells as effector cells, the combination of lenalidomide with mogamulizumab has potential in the treatment of ATL. The influence of prior treatments with mogamulizumab on responses remains unclear, and, thus, further investigations are warranted.
To the best of our knowledge, this is the first case report to document a successful treatment outcome with lenalidomide as maintenance therapy in a patient with aggressive ATL. Chemotherapy alone has been largely ineffective, and relapsed ATL patients who are not eligible for SCT have limited therapeutic options and a poor prognosis. The use of low-dose lenalidomide as maintenance therapy may be a new and promising option for these patients after chemotherapy. Moreover, lenalidomide has potential in the treatment of ATL patients, particularly the elderly who are not eligible for SCT, because of its oral dosing and tolerable side effect profile. Further investigations on the use of lenalidomide as maintenance therapy for ATL are needed.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lrr.2019.04.001.
Appendix. Supplementary materials
References
- 1.Vose J., Armitage J., Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J. Clin. Oncol. 2008;26(25):4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 2.Katsuya H., Yamanaka T., Ishitsuka K. Prognostic index for acute- and lymphoma-type adult T-cell leukemia/lymphoma. J. Clin. Oncol. 2012;30(14):1635–1640. doi: 10.1200/JCO.2011.38.2101. [DOI] [PubMed] [Google Scholar]
- 3.Ishida T., Jo T., Takemoto S. Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: a randomized phase II study. Br. J. Haematol. 2015;169(5):672–682. doi: 10.1111/bjh.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fecteau J.F., Corral L.G., Ghia E.M. Lenalidomide inhibits the proliferation of CLL cells via a cereblon/p21(WAF1/Cip1)-dependent mechanism independent of functional p53. Blood. 2014;124(10):1637–1644. doi: 10.1182/blood-2014-03-559591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morschhauser F., Fitoussi O., Haioun C. A phase 2, multicentre, single-arm, open-label study to evaluate the safety and efficacy of single-agent lenalidomide (Revlimid) in subjects with relapsed or refractory peripheral T-cell non-Hodgkin lymphoma: the EXPECT trial. Eur. J. Cancer. 2013;49(13):2869–2876. doi: 10.1016/j.ejca.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 6.Ishida T., Fujiwara H., Nosaka K. Multicenter phase II study of lenalidomide in relapsed or recurrent adult T-cell leukemia/lymphoma: ATLL-002. J. Clin. Oncol. 2016;34(34):4086–4093. doi: 10.1200/JCO.2016.67.7732. [DOI] [PubMed] [Google Scholar]
- 7.Hermine O., Ramos J.C., Tobinai K. A review of new findings in adult T-cell leukemia-lymphoma: a focus on current and emerging treatment strategies. Adv. Ther. 2018;35(2):135–152. doi: 10.1007/s12325-018-0658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsuya H., Ishitsuka K., Utsunomiya A. ATL-prognostic index project. Treatment and survival among 1594 patients with ATL. Blood. 2015;126(24):2570–2577. doi: 10.1182/blood-2015-03-632489. [DOI] [PubMed] [Google Scholar]
- 9.Ishida T., Hishizawa M., Kato K. Impact of graft-versus-host disease on allogeneic hematopoietic cell transplantation for adult T cell leukemia-lymphoma focusing on preconditioning regimens: nationwide retrospective study. Biol. Blood Marrow Transplant. 2013;19(12):1731–1739. doi: 10.1016/j.bbmt.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Shindo T., Kitaura K., Ureshino H. Deep sequencing of the T cell receptor visualizes reconstitution of T cell immunity in mogamulizumab-treated adult T cell leukemia. Oncoimmunology. 2017;7(3) doi: 10.1080/2162402X.2017.1405204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.