Abstract
Aims
Docetaxel has been approved for the treatment of metastatic prostate cancer in combination with prednisone. Since prednisone is known to induce the cytochrome P450 iso‐enzyme CYP3A4, which is the main metabolizing enzyme of docetaxel in the liver, a potential drug–drug interaction may occur. In this prospective randomized pharmacokinetic cross‐over study we investigated docetaxel exposure with concomitant prednisone, compared to docetaxel monotherapy in men with metastatic prostate cancer.
Methods
Patients scheduled to receive at least 6 cycles of docetaxel (75 mg/m2) and who gave written informed consent were randomized to receive either the 1st 3 cycles, or the last 3 consecutive cycles with prednisone (twice daily 5 mg). Pharmacokinetic blood sampling was performed during cycle 3 and cycle 6. Primary endpoint was difference in docetaxel exposure, calculated as area under the curve (AUC0‐inf) and analysed by means of a linear mixed model. Given the cross‐over design the study was powered on 18 patients to answer the primary, pharmacokinetic, endpoint.
Results
Eighteen evaluable patients were included in the trial. Docetaxel concentration with concomitant prednisone (AUC0‐inf 2784 ng*h/mL, 95% confidence interval 2436–3183 ng*h/mL) was similar to the concentration of docetaxel monotherapy (AUC0‐inf 2647 ng*h/mL, 95% confidence interval 2377–2949 ng*h/mL). Exploratory analysis showed no toxicity differences between docetaxel monotherapy and docetaxel cycles with prednisone.
Conclusion
No significant difference in docetaxel concentrations was observed. In addition, we found similar toxicity profiles in absence and presence of prednisone. Therefore, from a pharmacokinetic point of view, docetaxel may be administrated with or without prednisone.
Keywords: docetaxel, drug–drug interaction, metastatic prostate cancer, prednisone
What is already known about this subject
Docetaxel chemotherapy is approved for the treatment of metastatic prostate cancer in combination with prednisone, although the role of prednisone remains controversial
Prednisone is a CYP3A4 inducer, which is the primary enzyme for taxane metabolism
The effect of prednisone on docetaxel pharmacokinetics has never been thoroughly investigated
What this study adds
There is no significant effect of prednisone on docetaxel pharmacokinetics
From pharmacokinetic perspective docetaxel may be administrated without prednisone
The benefits of prednisone use in metastatic prostate cancer patients should outweigh the potential adverse events of long‐term corticosteroid use
1. INTRODUCTION
Docetaxel, a taxane chemotherapeutic agent, was approved by the Food and Drug Administration and European Medicines Agency in 2004 as 1st‐line chemotherapy for metastatic castration‐resistant prostate cancer (mCRPC) as a result of survival benefit obtained in TAX327.1, 2 In that study, mitoxantrone plus prednisone treatment was compared to a 3‐weekly docetaxel (75 mg/m2) regimen in mCRPC patients. Prednisone (5 mg twice daily) was added to docetaxel to equally compare both treatment arms, although the preceding phase 2 trials with docetaxel (36 mg/m2, weekly) in mCRPC had been conducted without prednisone.3, 4 In the final analysis, treatment with docetaxel plus prednisone improved overall survival (OS) with 2.9 months compared to the mitoxantrone group. Subsequently, docetaxel and prednisone became 1st‐line chemotherapy for mCRPC.
After the registration of docetaxel plus prednisone, the role of corticosteroids in the treatment of mCRPC remained controversial. In patients with symptomatic bone metastases corticosteroids may have a favourable palliative effect, and a reduction in docetaxel‐induced toxicity has been suggested.5, 6, 7 However, the effect of prednisone on OS in mCRPC patients remains unclear.6, 8 Of note, prolonged use of corticosteroids may lead to the development of multiple severe toxicities including osteoporosis, adrenal insufficiency, immune suppression, and may exacerbate comorbidities like diabetes.9 These side‐effects of long‐term corticosteroid are a justifiable reason to reconsider the addition of prednisone to the docetaxel regimen.
Recently, 2 large clinical trials, CHAARTED and STAMPEDE, assessed the survival benefit of docetaxel combined with androgen‐deprivation therapy (ADT) in metastatic hormone‐sensitive prostate cancer (mHSPC).10, 11 To avoid long term exposure to steroids, the investigators of the CHAARTED trial decided to administer docetaxel without prednisone, whereas docetaxel was administered with prednisone in the STAMPEDE study. At the time of the initiation of our study, only the results of CHAARTED were available, showing a robust survival benefit of 13.6 months compared to androgen deprivation therapy alone. Toxicity rates were similar to previously published work on docetaxel plus prednisone in mCRPC patients, except for a higher febrile neutropenia rate in CHAARTED without prednisone, as compared to TAX327 where docetaxel was administered with prednisone.1, 12 Likewise, a retrospective trial by Kongsted et al. showed that the toxicity rates of febrile neutropenia and oedema were significantly higher in the docetaxel monotherapy group compared to the docetaxel plus prednisone‐group (for an overview of toxicity rates previously reported on docetaxel with or without prednisone, see Table 1).5
Table 1.
Literature review of docetaxel toxicities with and without prednisone
| Trials | Prednisone | Neutropenia (Gr3–4) | Febrile neutropenia |
|---|---|---|---|
| TAX‐327 | Yes | 32% | 3% |
| Venice | Yes | 7% | <1% |
| Mainsail | Yes | 16% | 5% |
| GETUG‐AFU15 | No | 32% | 8% |
| CHAARTED | No | 12% | 6% |
| STAMPEDE | Yes | 12% | 15% |
| Kongsted et al. | No | ‐ | 25% |
| Yes | ‐ | 10% |
As an underlying mechanism, prednisone could influence docetaxel pharmacokinetics (PK) via the CYP3A4 iso‐enzyme. Glucocorticoids are known as CYP3A inducers, and docetaxel is mainly metabolized in the liver by the cytochrome P450 iso‐enzymes CYP3A4 and CYP3A5.13 Consequently, this potential drug–drug interaction could lead to higher clearance of docetaxel and therefore diminished docetaxel exposure. In this study, we therefore investigated the effects of prednisone on docetaxel PK in patients with metastatic prostate cancer.
2. METHODS
This prospective, randomized, cross‐over PK trial was carried out between September 2016 and February 2018 at the Erasmus MC Cancer Institute in Rotterdam, the Netherlands. The study protocol was approved by the Ethical board of the Erasmus MC, and the study was conducted according to the ethical guidelines of the Declaration of Helsinki. All participants signed informed consent before start of the study. The study was registered at the European Clinical Trials Database (EudraCT 2016–001269‐10) and the Dutch Trial Register (‘www.trailregister.nl’ by NTR‐number NTR6037 or acronym Doc‐Pred).
2.1. Patients
We included patients with histologically confirmed metastatic prostate cancer, both hormone‐sensitive or castration‐resistant, who were scheduled to receive a minimum of 6 cycles of docetaxel chemotherapy. Eligible patients were aged 18 years and older, with an Eastern Cooperative Oncology Group performance status of 0 or 1. Adequate organ function was required, defined by creatinine clearance >60 mL/min, bilirubin levels <1× upper limit of normal (ULN), alanine aminotransferase/aspartate aminotransferase <2.5× ULN, alkaline phosphatase <5× ULN, absolute neutrophil count >1.5 × 109/L and platelets >100 × 109/L. Patients had to be castrated either by continued ADT with gonadotropin releasing hormone analogues or by surgical orchiectomy. It was preferred that ADT started 4 weeks prior to chemotherapy, to reach castration‐levels of testosterone before treatment start. Prior hormonal treatment, such as enzalutamide and abiraterone, was allowed. However, these therapies, including prednisone, had to be stopped at least 6 weeks before the start of this study. Medication or herbal supplements known to induce or inhibit CYP3A pathway were prohibited.
2.2. Study design
Patients received 6 consecutive cycles of 3‐weekly docetaxel (75 mg/m2) and were digitally randomized to receive either the 1st 3 docetaxel cycles or the last 3 cycles with prednisone (cross‐over). Prednisone 5 mg twice daily was administered during 3 consecutive cycles. Prednisone started at day 1 of cycle 1 or cycle 4 and was stopped after the last day of cycle 3 or cycle 6 (depending on randomization arm, A or B respectively). Prednisone dose‐modifications were not allowed during the last week before PK sampling (cycle 3 day 1 and cycle 6 day 1) and patient compliance was assessed through a patient diary. Docetaxel dose‐modifications because of haematological or nonhaematological toxicities were allowed, and schedule modifications were allowed up to 1 week. Dexamethasone is a strong CYP3A4 inducer; its use, as premedication, was restricted to only 12 and 3 hours before docetaxel‐infusion to reduce its influence on docetaxel PK.
2.3. PK sampling
To have maximum inducible effects of prednisone on the CYP‐enzymes and to ensure a sufficient wash‐out period after prednisone, we decided to undertake PK samples during cycle 3 and cycle 6. Hospital admission during the first day of the 3rd and the 6th docetaxel cycle was required to obtain 24‐hour PK blood samples. Blood/plasma samples for determination of docetaxel PK were taken at predefined time points (preinfusion and at 0.5, 0.92, 1.25, 1.5, 2, 3, 4, 6, 8 and 24 hours after the start of docetaxel). Plasma concentrations of docetaxel were measured using a validated liquid chromatography with tandem mass spectrometry method.14 PK parameters were docetaxel concentration, expressed as dose‐corrected area under the curve from preinfusion time‐point to infinity (AUC0‐inf), maximum drug concentration (Cmax), docetaxel half‐life (t1/2) and docetaxel clearance. AUC0‐inf was calculated using a linear PK curve to estimate the residual AUC from the last measurable PK point (24 hours).
2.4. Toxicity
Secondary endpoint was describing toxicity rates during docetaxel monotherapy cycles and docetaxel with prednisone cycles. Standard laboratory control was performed prior to each docetaxel cycle and when indicated according to the physician. Toxicities were scored using the Common Terminology Criteria for Adverse Events (v.4.0) grading. If relevant differences in toxicity rates between the treatment arms occurred, these were analysed by McNemar test.
2.5. Statistical analysis
A difference in systemic exposure to docetaxel of 25% was determined to be clinically relevant and it was assumed that the within‐patient standard deviation in docetaxel PK was 25%. Given a power of 80% and a 2‐sided α of 5%, 18 patients were required to detect a difference.15 Since docetaxel dose‐modifications were allowed, a dose‐correction was applied for all docetaxel concentrations to the standard dose of 75 mg/m2. All docetaxel cycles with prednisone were compared to all docetaxel cycles without prednisone, regardless of the randomization arm. Analyses of the AUC0‐inf and Cmax were performed on log‐transformed values, since these parameters were assumed to follow a lognormal distribution.16 Estimates for the mean differences in (log) AUC0‐inf, Cmax and clearance were obtained using a linear mixed effect model with treatment, sequence and period as fixed effects and subject within sequence as a random effect.17 Variance components were estimated based on restricted maximum likelihood methods and the Kenward–Roger method of computing the denominator degrees of freedom was used. The mean differences and their 95% confidence intervals (CIs) were exponentiated to provide point estimates of the ratio of geometric means and 95% CIs for these ratios, which can be interpreted as relative differences in percentages. Half‐life was analysed by means of the Wilcoxon signed rank test and described with medians and interquartile ranges.
3. RESULTS
3.1. Patients
Twenty‐nine patients were screened, of whom 4 were screen failures and excluded from study participation (Figure 1). We randomized 25 patients to receive either cycles 1–3 with concomitant prednisone, and cycles 4–6 without prednisone (arm A, n = 11), or vice versa (arm B, n = 7). During treatment, 1 patient withdrew consent in arm A, and six patients stopped treatment in arm B due to radiologically confirmed progression (n = 3) or withdrawal of consent (n = 3).
Figure 1.
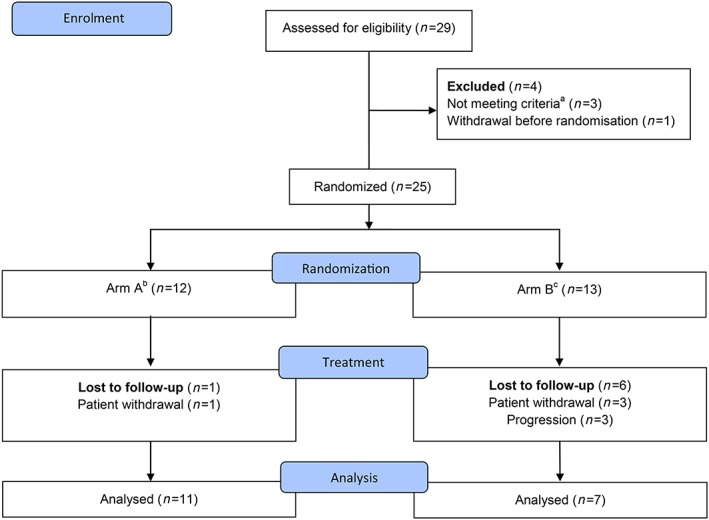
Flowchart. a due to inadequate laboratory values. b Arm A: Three cycles of docetaxel plus prednisone followed by 3 cycles of docetaxel alone. c Arm B: Three cycles of docetaxel alone followed by 3 cycles of docetaxel plus prednisone
Baseline patient and disease characteristics are shown in Table 2. All patients, except 3 mHSPC patients, received the 1st cycle of docetaxel 4 weeks after initiation of ADT, to reach castration levels of testosterone. However, all patients received ADT for >1 month before PK samples during cycle 3 were withdrawn.
Table 2.
Patient and disease characteristics
| Characteristic | n (%) |
|---|---|
| Patients | 18 (100) |
| Age (y), median, IQR | 70 (62–73) |
| BMI (kg/m2), median, IQR | 25.8 (24.6–28.7) |
| WHO performance status | |
| −0 | 8 (44) |
| −1 | 10 (56) |
| Hormone status | |
| ‐Hormone sensitive | 11 (61) |
| ‐Castration resistant | 7 (39) |
| Metastatic stage at screening | |
| ‐M0 | 5 (28) |
| ‐M1a | 4 (22) |
| ‐M1b | 8 (44) |
| ‐M1c | 1 (6) |
| Gleason score at diagnosis | |
| ‐ ≤7 | 4 (22) |
| ‐ >7 | 14 (77) |
| Type of castration | |
| ‐Bilateral orchidectomy | 1 (6) |
| ‐LHRH analogues | 17 (94) |
| Previous therapy | |
| ‐Radical prostatectomy | 1 (6) |
| ‐Radiotherapy prostate | 3 (16) |
| ‐Hormone therapy | |
| Bicalutamide | 6 (33) |
| Enzalutamide | 2 (11) |
| ‐Radium‐223 | 1 (6) |
| ‐Experimental therapy | 1 (6) |
| Lab results at baseline | Median (IQR) |
| ‐PSA, μg/L | 20 (3–87) |
| ‐Hb, mmol/L | 8 (7–10) |
| ‐LDH, U/L | 196 (178–216) |
| ‐AP, U/L | 103 (70–160) |
| ‐Albumin, g/L | 44 (43–46) |
AP = alkaline phosphatase; BMI = body mass index; Hb = haemoglobin; IQR = interquartile range; LDH = lactate dehydrogenase; LHRH = luteinizing hormone releasing hormone; PSA = prostate specific antigen; WHO = World Health Organization.
3.2. PK parameters
The geometric mean exposure of docetaxel was not significantly different (1.9%, 95% CI ‐9.9% till 15.2%, P = .75) during docetaxel with concomitant prednisone treatment (AUC0‐inf of 2784 ng*h/mL, 95% CI 2436–3183 ng*h/mL) compared to docetaxel monotherapy (AUC0‐inf of 2647 ng*h/mL, 95% CI 2377–2949 ng*h/mL). The PK variation, as expressed by coefficient of variation, was slightly higher in the docetaxel with prednisone arm as compared to docetaxel monotherapy (27% and 22% respectively). All PK parameters are shown in Table 3 and were not significantly different for docetaxel with or without prednisone. Additionally, we graphically showed differences in exposure of docetaxel in mCRPC patients (blue line) and mHSPC patients (red line), separately in arm A and arm B; see Figure 2 . We performed a t‐test on the complete patient group (arm A and arm B combined) and found no significant (P = .2) difference between the exposure in mCRPC patients and mHSPC patients. Of note, we found a 13.4% (95% CI 2.1%–23.4%, P = .025) lower exposure of docetaxel over time, independent from randomization or disease setting. This so‐called period effect shows lower measured concentrations of docetaxel in cycle 6 compared to the concentrations in cycle 3, regardless of the addition of prednisone (Figure 2).
Table 3.
Docetaxel pharmacokinetics
| Docetaxel PK parameters | Docetaxel (n = 18) | Docetaxel+prednisone (n = 18) | Relative difference (95% CI) | P‐value |
|---|---|---|---|---|
| AUC0‐inf a geomean ng*h/mL (CV%) | 2647 (22) | 2784 (27) | 1.9% (−9.9 till 15.2) | .75 |
| Cmax a geomean ng/mL (CV%) | 2454 (26) | 2505 (25) | −1.4% (−15.3 till 14.8) | .85 |
| CLa geomean, L/h (CV%) | 55 (26) | 53 (26) | −2.3% (−9.5 till 5–6) | .53 |
| T1/2 b median, h (IQR) | 12.6 (10.6–14.5) | 13.7 (11.3–16.3) | .31 |
AUC0‐inf = Area under curve timepoint zero until infinity; CI = confidence interval; CL = clearance; Cmax = maximum concentration; CV% = coefficient of variation; geomean = geometric mean; T1/2 = half‐life.
= analysed by means of a linear effect model,
= analysed by means of Wilcoxon signed rank test.
Figure 2.
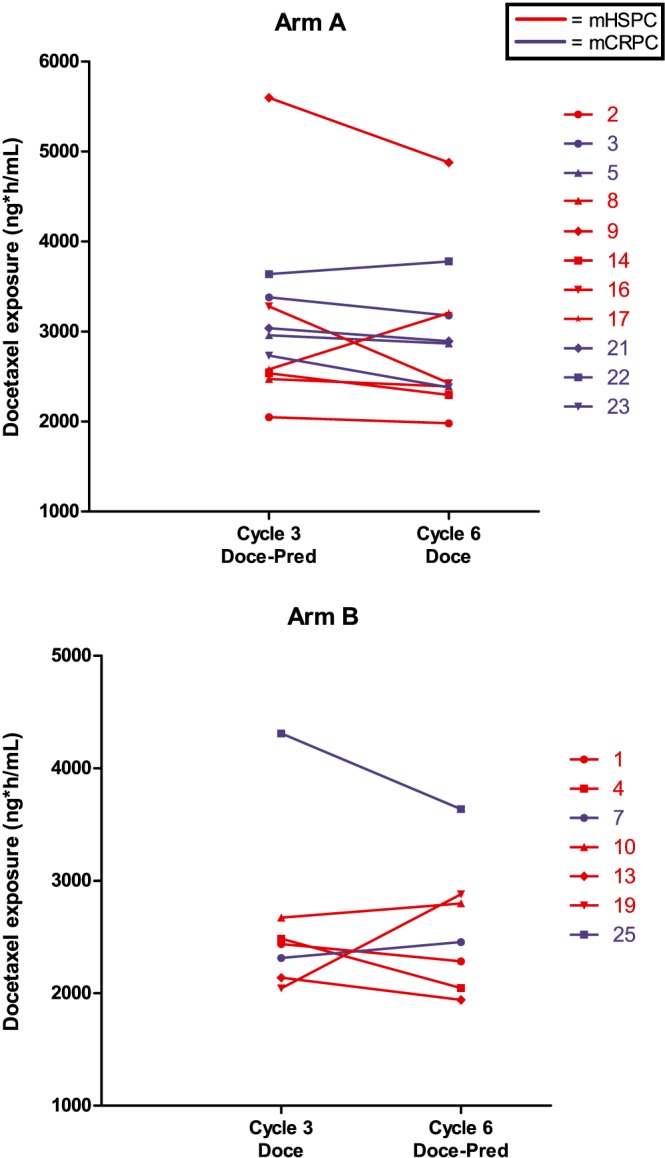
Docetaxel concentration by disease setting. Each line represents a patient for whom the measured docetaxel concentration (geomean AUC0‐inf) during cycle 3 and cycle 6 were connected with a line to visualize the differences in the cycles. In the majority of the patients the measured concentration in cycle 6 is lower than in cycle 3, reflecting the period‐effect observed in this study
3.3. Toxicity
Toxicity rates were similar between the cycles with and without prednisone, see Table 4, except for neutropenia. A nonsignificant trend towards a higher rate of all grade (grade 1–4) neutropenia (n = 12) was observed in patients treated without prednisone as compared to with prednisone (44 vs 22%, P = .22). Seven patients (39%) experienced an episode of grade 3–4 neutropenia. Three febrile neutropenia hospitalizations were observed, 2 of which happened during coadministration of prednisone. There was no difference in the disease setting; toxicity was equally distributed in castration‐resistant and hormone‐sensitive setting (data not shown).
Table 4.
Toxicity with or without prednisone
| Toxicity | All grades | |
|---|---|---|
| With prednisone n (%) | Without prednisone n (%) | |
| Nausea | 3 (17) | 5 (28) |
| Mucositis | 9 (50) | 8 (44) |
| Diarrhea | 5 (28) | 2 (11) |
| Sens PNP | 6 (33) | 6 (33) |
| Fatigue | 12 (67) | 13 (72) |
| Neutropenia | 4 (22) | 8 (44) |
| Febrile neutropenia | 1 (6) | 2 (11) |
| Nail toxicity | 5 (28) | 6 (33) |
| Edema | 0 (0) | 1 (6) |
| Dysgeusia | 1 (6) | 1 (6) |
Toxicity scores with or without prednisone expressed as Common Terminology Criteria for Adverse Events grade. Sens PNP = sensory polyneuropathy.
4. DISCUSSION
In this randomized study, the effects of prednisone on the PK of docetaxel were evaluated. No significant difference in docetaxel exposure with or without the administration of prednisone was observed. This is the 1st randomized PK study investigating the effects of prednisone on the PK of docetaxel. From a pharmacological perspective, we conclude that prednisone did not affect the exposure of the docetaxel regimen.
Glucocorticoids are classified as inducers of the CYP3A enzyme,18 and docetaxel is metabolized primarily by this iso‐enzyme. Previously, an interaction study of docetaxel and prednisone has been published in the Clinical Study Report of docetaxel and no relevant drug–drug interaction was reported.19 However, that PK study was not randomized and included only 2 docetaxel cycles; 1 with prednisone and 1 without, possibly not providing enough time for optimal CYP‐induction by prednisone. Moreover, that study was limited by sparse PK‐sampling (only 6 samples during each cycle) and by limited PK endpoints of docetaxel (clearance only). Therefore, in our study, we used a randomized cross‐over design including 6 cycles of docetaxel (3 cycles in absence and 3 cycles in presence of prednisone), an enriched sampling scheme with more relevant PK endpoints.
Although we corrected for dose reductions due to toxicity over time, we unexpectedly did find a significant period effect in this study. This means that a decrease in docetaxel exposure occurred in the consecutive cycles independent of randomization or treatment. This might be an explanation for the trend towards an overall higher incidence of (febrile) neutropenia seen at the start of chemotherapy cycles. There are a few potential explanations for this phenomenon. First, a time‐dependent induction of CYP3A4 by upregulation of pregnane X receptor due to repetitive docetaxel exposure could occur.20, 21, 22, 23 This phenomenon is called auto‐induction and is previously described with several other agents, e.g. dabrafenib.24 A second possible explanation is an upregulation of ABCB1 (P‐glycoprotein) by docetaxel. P‐glycoprotein is an active drug‐efflux transporter at the cell membrane of hepatocytes, kidney cells and intestine cells. Its upregulation leads to an increased efflux of docetaxel out of the circulation, resulting in decreased plasma concentrations.25 This phenomenon could even lead to PK resistance to the drug.26, 27 This period effect is unlikely to be caused by castration‐levels of the patients, since the maximum induction effect of ADT is reached after approximately 4 weeks, whereas, in our study, patients had received at least 9 weeks of ADT at the time of PK sampling.
Interestingly, we observed no difference in docetaxel‐induced toxicities in the absence or presence of prednisone, except for a nonsignificant difference in neutropenia. Because our study was not powered or designed for toxicity‐related questions, we can only conclude from a PK point of view that prednisone could be safely omitted from the docetaxel regimen.
The major benefit of administering docetaxel without prednisone could be a reduced treatment‐period of prednisone for patients with metastatic prostate cancer. Long‐term corticosteroid use, albeit in low dosage, may contribute to the development of severe toxicities, as mentioned before.9 By excluding prednisone from the initial docetaxel chemotherapy regimen, patients will no longer be unnecessarily exposed to these side‐effects. Especially for those patients in the hormone‐sensitive phase, who usually have a long life expectancy, excluding prednisone will be of relevance to avoid long‐term toxicity with unclear antitumor activity. In this light, Ghatalia et al. found no positive effect on survival nor on cabazitaxel‐induced toxicity in patients with mCRPC.28
Limitations of our study include the administration of the standard premedication dexamethasone, which is another CYP3A inducer. We aimed to minimize the PK effect of dexamethasone on docetaxel by excluding the latest gift of dexamethasone before docetaxel infusion. Strengths of our study are the randomized design with extensive PK sampling at multiple time points.
In conclusion, we found no influence of prednisone on docetaxel PK. Docetaxel is registered with concomitant prednisone in the mCRPC setting. In metastatic hormone‐sensitive disease, the use of prednisone should be supported by other arguments balancing the benefit of prednisone vs the potential long‐term side effects of corticosteroid use.
COMPETING INTERESTS
M.P.L. has received research funding from Sanofi and Astellas. R.d.W. has received research funding (Institutional), and speaker fees from Sanofi, research funding (Institutional) from Bayer, advisory and speaker fees from Merck, advisory fees from Janssen, Roche and Clovis. R.J.v.S. has received speaker fees from Sanofi and Astellas, advisory fees from Janssen and Bayer. R.H.J.M. has received research funding (Institutional) from Sanofi and Astellas. The other authors have no competing interests to declare.
CONTRIBUTORS
B.P.S.B. and K.G.A.M.H., wrote manuscript, performed research, and analyzed data. L.J.v.H., performed research and analyzed data. E.O.‐d.H., analyzed data. P.d.B., analyzed data and contributed to new reagents/analytical tools. P.H., R.J.v.A., and B.H., performed research. M.P.L., performed research and wrote manuscript. R.d.W., R.J.v.S., and R.H.J.M. designed and performed research, and wrote manuscript.
ACKNOWLEDGEMENTS
This work was supported by the Dutch Uro‐Oncology Group (DUOS) [number 16105].
Belderbos BPS, Hussaarts KGAM, van Harten LJ, et al. Effects of prednisone on docetaxel pharmacokinetics in men with metastatic prostate cancer: A randomized drug–drug interaction study. Br J Clin Pharmacol. 2019;85:986–992. 10.1111/bcp.13889
The authors confirm that the PI for this paper is Prof. R. de Wit and that he had direct clinical responsibility for patients.
REFERENCES
- 1. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502‐1512. [DOI] [PubMed] [Google Scholar]
- 2. Berthold DR, Pond GR, de Wit R, Eisenberger M, Tannock IF, TAX 327 Investigators . Survival and PSA response of patients in the TAX 327 study who crossed over to receive docetaxel after mitoxantrone or vice versa. Ann Oncol. 2008;19(10):1749‐1753. [DOI] [PubMed] [Google Scholar]
- 3. Berry W, Dakhil S, Gregurich MA, Asmar L. Phase II trial of single‐agent weekly docetaxel in hormone‐refractory, symptomatic, metastatic carcinoma of the prostate. Semin Oncol. 2001;28:8‐15. [DOI] [PubMed] [Google Scholar]
- 4. Beer TM, Pierce WC, Lowe BA, Henner WD. Phase II study of weekly docetaxel in symptomatic androgen‐independent prostate cancer. Ann Oncol. 2001;12(9):1273‐1279. [DOI] [PubMed] [Google Scholar]
- 5. Kongsted P, Svane IM, Lindberg H, Daugaard G, Sengelov L. Low‐dose prednisolone in first‐line docetaxel for patients with metastatic castration‐resistant prostate cancer: is there a clinical benefit? Urol Oncol. 2015;33(494):e15‐e20. [DOI] [PubMed] [Google Scholar]
- 6. De Santis M, Saad F. Practical guidance on the role of corticosteroids in the treatment of metastatic castration‐resistant prostate cancer. Urology. 2016;96:156‐164. [DOI] [PubMed] [Google Scholar]
- 7. Ndibe C, Wang CG, Sonpavde G. Corticosteroids in the management of prostate cancer: a critical review. Curr Treat Options Oncol. 2015;16(2):6. [DOI] [PubMed] [Google Scholar]
- 8. Venkitaraman R, Lorente D, Murthy V, et al. A randomised phase 2 trial of dexamethasone versus prednisolone in castration‐resistant prostate cancer. Eur Urol. 2015;67(4):673‐679. [DOI] [PubMed] [Google Scholar]
- 9. Morgan CJ, Oh WK, Naik G, Galsky MD, Sonpavde G. Impact of prednisone on toxicities and survival in metastatic castration‐resistant prostate cancer: A systematic review and meta‐analysis of randomized clinical trials. Crit Rev Oncol Hematol. 2014;90(3):253‐261. [DOI] [PubMed] [Google Scholar]
- 10. James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first‐line long‐term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone‐sensitive prostate cancer. N Engl J Med. 2015;373(8):737‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gravis G, Fizazi K, Joly F, et al. Androgen‐deprivation therapy alone or with docetaxel in non‐castrate metastatic prostate cancer (GETUG‐AFU 15): a randomised, open‐label, phase 3 trial. Lancet Oncol. 2013;14(2):149‐158. [DOI] [PubMed] [Google Scholar]
- 13. Pascussi JM, Drocourt L, Gerbal‐Chaloin S, Fabre JM, Maurel P, Vilarem MJ. Dual effect of dexamethasone on CYP3A4 gene expression in human hepatocytes. Sequential role of glucocorticoid receptor and pregnane X receptor. Eur J Biochem. 2001;268(24):6346‐6358. [DOI] [PubMed] [Google Scholar]
- 14. de Graan AJ, Lancaster CS, Obaidat A, et al. Influence of polymorphic OATP1B‐type carriers on the disposition of docetaxel. Clin Cancer Res. 2012;18(16):4433‐4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schoenfeld D . Statistical considerations for a cross‐over study where the outcome is a measurement. http://hedwig.mgh.harvard.edu/sample_size/js/js_crossover_quant.html. Accessed March 22, 2016.
- 16. European Medicines Agency . Guideline on the investigation of bioequivalence. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf Accessed March 22, 2016.
- 17. Jones B, Kenward, M.G . Design and Analysis of Cross‐Over Trials: Chapman&Hall/CRC monographs, Boca Raton, Florida, USA, 2014. [Google Scholar]
- 18. Quattrochi LC, Guzelian PS. Cyp3A regulation: from pharmacology to nuclear receptors. Drug Metab Dispos. 2001;29:615‐622. [PubMed] [Google Scholar]
- 19. Summary of Product Characteristics ‐ Taxotere. In: EMA. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/000073/WC500035264.pdf Accessed August 29, 2018.
- 20. Harmsen S, Meijerman I, Beijnen JH, Schellens JH. Nuclear receptor mediated induction of cytochrome P450 3A4 by anticancer drugs: a key role for the pregnane X receptor. Cancer Chemother Pharmacol. 2009;64(15):35‐43. [DOI] [PubMed] [Google Scholar]
- 21. Istrate MA, Nussler AK, Eichelbaum M, Burk O. Regulation of CYP3A4 by pregnane X receptor: The role of nuclear receptors competing for response element binding. Biochem Biophys Res Commun. 2010;393(4):688‐693. [DOI] [PubMed] [Google Scholar]
- 22. Hilli J, Sailas L, Jyrkkio S, Pyrhonen S, Laine K. NCT01110291: induction of CYP3A activity and lowered exposure to docetaxel in patients with primary breast cancer. Cancer Chemother Pharmacol. 2011;67(6):1353‐1362. [DOI] [PubMed] [Google Scholar]
- 23. Luo G, Cunningham M, Kim S, et al. CYP3A4 induction by drugs: correlation between a pregnane X receptor reporter gene assay and CYP3A4 expression in human hepatocytes. Drug Metab Dispos. 2002;30(7):795‐804. [DOI] [PubMed] [Google Scholar]
- 24. Luke JJ, Ott PA. New developments in the treatment of metastatic melanoma ‐ role of dabrafenib‐trametinib combination therapy. Drug Healthc Patient Saf. 2014;6:77‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harmsen S, Meijerman I, Febus CL, Maas‐Bakker RF, Beijnen JH, Schellens JH. PXR‐mediated induction of P‐glycoprotein by anticancer drugs in a human colon adenocarcinoma‐derived cell line. Cancer Chemother Pharmacol. 2010;66(4):765‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kato T, Mizutani K, Kameyama K, et al. Serum exosomal P‐glycoprotein is a potential marker to diagnose docetaxel resistance and select a taxoid for patients with prostate cancer. Urol Oncol. 2015;33(385):e15‐e20. [DOI] [PubMed] [Google Scholar]
- 27. Shirakawa K, Takara K, Tanigawara Y, et al. Interaction of docetaxel ("Taxotere") with human P‐glycoprotein. Jpn J Cancer Res. 1999;90(12):1380‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghatalia P, Pond GR, Templeton AJ, Sonpavde G. Effect of single‐agent daily prednisone on outcomes and toxicities in metastatic castration‐resistant prostate cancer: pooled analysis of prospective studies. Clin Genitourin Cancer. 2018;16(2):e277‐e287. [DOI] [PubMed] [Google Scholar]


