Abstract
Aims
In previous studies, the histamine‐3 receptor antagonist CEP‐26401 had a subtle effect on spatial working memory, with the best effect seen at the lowest dose tested (20 μg), and a dose‐dependent disruption of sleep. In the current study, 3 low‐dose levels of CEP‐26401 were compared with modafinil and donepezil.
Methods
In this double‐blind, placebo‐ and positive‐controlled, randomized, partial 6‐way cross‐over study, 40 healthy subjects received single doses of placebo, CEP‐26401 (5, 25 or 125 μg) or modafinil 200 mg or donepezil 10 mg. Pharmacokinetic and pharmacodynamic measurements were performed predose and at designated time points postdose.
Results
The main endpoint between‐errors of the spatial working memory‐10‐boxes task only improved for the 125 μg dose of CEP‐26401 with a difference of 2.92 (confidence interval [CI] –1.21 to 7.05), 3.24 (CI –1.57 to 8.04) and 7.45 (CI 2.72 to 12.19) for respectively the 5, 25 and 125 μg dose of CEP‐26401, −1.65 (CI –0.572 to 1.96) for modafinil and − 3.55 (CI –7.13 to 0.03) for donepezil. CEP‐26401 induced an improvement of adaptive tracking, saccadic peak velocity and reaction time during N‐back, but a dose‐related inhibition of sleep and slight worsening of several cognitive parameters at the highest dose. CEP‐26401 significantly changed several subjective visual analogue scales, which was strongest at 25 μg, causing the same energizing and happy feeling as modafinil, but with a more relaxed undertone.
Discussion
Of the doses tested, the 25 μg dose of CEP‐26401 had the most optimal balance between favourable subjective effects and sleep inhibition. Whether CEP‐26401 can have beneficial effects in clinical practice remains to be studied.
Keywords: drug metabolism, neurology, pharmacodynamics, pharmacokinetics, psychopharmacology
What is already known about this subject
Histamine‐3 receptors (H3R) influence several neurotransmitter systems, including acetylcholine, dopamine and norepinephrine.
In preclinical studies, H3R antagonists induced an improvement on several cognitive domains.
The H3R antagonist CEP‐26401 positively influenced spatial working memory with the best effect at the lowest dose.
What this study adds
CEP‐26401 did not have any positive effects on cognitive tests.
CEP‐26401 induced energizing, relaxed and happy feelings in healthy volunteers and a dose‐related improvement of tests measuring attention, reaction time and alertness.
Of the doses tested, the 25 μg dose of CEP‐26401 had the most optimal balance between favourable subjective effects and sleep inhibition.
1. INTRODUCTION
Histamine‐3 receptors (H3Rs) have been suggested as a drug discovery target for many different indications, because of their influence on several neurotransmitter systems.1, 2 The highest levels of this receptor are found in the thalamus, caudate nucleus and cortex.3, 4 High levels of expression are also found in the hypothalamus, hippocampus and olfactory tubercle. H3Rs are located presynaptically and act as inhibitory auto‐ and hetero‐receptors, decreasing the release of histamine and of several important neurotransmitters, such as acetylcholine (ACh), dopamine (DA), γ‐aminobutyric acid (GABA), norepinephrine and serotonin.5, 6, 7 Like all histamine receptors, the H3R is a Gi‐protein coupled receptor which leads to inhibition of the formation of cyclic adenosine monophosphate.8 Also, the β‐ and γ‐ subunits interact with N‐type voltage‐gated calcium channels, to reduce action potential mediated influx of calcium and hence reduce neurotransmitter release.9, 10 H3R antagonists are expected to increase the release of neurotransmitters, including acetylcholine, dopamine and norepinephrine, and are therefore suggested as possible enhancers of cognitive functions in central nervous system (CNS) diseases with cognitive impairments, such as Alzheimer disease, schizophrenia and attention deficit hyperactivity disorder (ADHD).7
In preclinical studies, mainly in mice and rats, positive effects of H3R antagonists were found on working memory, memory consolidation, social memory, spatial orientation and attention and impulsivity.11, 12 These positive effects were also seen in models for negative symptoms of schizophrenia, but not in Alzheimer disease models.11, 12 Human studies have mainly focused on treatment of ADHD and excessive daytime sleepiness. Pitolisant, an H3R inverse agonist, has been shown to improve ADHD symptoms and reduce excessive daytime sleepiness in patients with narcolepsy and obstructive sleep apnoea syndrome.13, 14 The effects of H3R antagonists on cognitive disturbances in Alzheimer disease and schizophrenia were not consistent.15, 16, 17
CEP‐26401 ([6‐[4‐[3‐[(2R)‐2‐methyl‐1‐pyrrolidinyl]propoxy]phenyl]‐3‐(2H)‐pyridazinone hydrochloride]) is an H3R antagonist/inverse agonist that displays high‐affinity H3R binding and potent functional antagonism in both rat and human recombinant cell and native rat brain cortical systems.18, 19, 20, 21, 22 Two clinical studies with orally administered single and multiple doses of CEP‐26401 in healthy volunteers have been performed prior to this study with interesting results on the spatial working memory (SWM) task.23 In this task, several boxes are presented on the screen, in 1 of which a token is to be found. The token never appears in the same box more than once and the test continues until a token had been found in all of the boxes once. Each click on an empty box is counted as an error. Applying a population pharmacokinetic/pharmacodynamic (PK/PD) model, an effect on SWM was found with a maximal decrease of 10.8 errors (clinically relevant improvement of cognitive function) at plasma levels ≤0.01 ng/mL (dose ≤20 μg), but with a maximal increase of 17.6 errors (worsening of cognitive function) at plasma levels ≥0.1 ng/mL (dose ≥80 μg). Sleep was affected in a dose‐related fashion with an increase in time awake after sleep onset to about a 2.4‐fold increase at plasma levels ≥16 ng h/mL (dose ≥50 μg) after single dose. Although data were derived from 2 different studies with parallel‐group designs, where differences between groups and study design may have played a role (i.e. studies were not powered nor specifically designed to detect differences in cognition enhancement), the PK/PD‐model based on these studies consistently indicated the largest cognitive effects at the lowest dose.23 It was therefore of interest to investigate the dose–response relationship of CEP‐26401 on cognition at a dose range below as well as above 20 μg.
The primary objective of this study was to evaluate the dose–response relationships of single doses of CEP‐26401 5, 25 and 125 μg on SWM and a range of other CNS functions in healthy subjects. Secondary objectives were the assessment of the effects of CEP‐26401 on sleep; comparison of the effects of CEP‐26401 with those of positive controls, modafinil and donepezil; and assessment of pharmacokinetics (PK), safety and tolerability of a low dose range of CEP‐26401.
2. METHODS
2.1. Study design
This was a single centre, double‐blind, placebo‐ and positive‐controlled, randomized, partial 6‐way cross‐over study to investigate the pharmacodynamics and pharmacokinetics of CEP‐26401 (5, 25 and 125 μg) following single‐dose administration to healthy male and female subjects. All subjects were informed about study procedures and signed the informed consent form before any study activity took place. All subjects had a screening visit within 4 weeks prior to their 1st study day, followed by 4 treatment periods and a follow up visit. Each study treatment period was separated by a 14‐day wash out.
Within 4 weeks of their 1st check‐in day (day −1), subjects had a training session to familiarize them with the pharmacodynamic tests. After the training session, subjects performed the SWM test (the primary outcome parameter) and test scores were compared with reference values to ensure normal cognitive performance which was an inclusion criterion. Subjects also underwent polysomnography (PSG) during a single habituation night, to get accustomed to this procedure before the effects of study treatment were investigated.
Eligible subjects were admitted to the study centre on study day −1 and their eligibility to participate in the study was confirmed. On the morning of day 1, after a light meal, subjects were randomized to cohorts and underwent baseline assessments. After completion of baseline assessments, subjects received a single oral dose of their randomized treatment after which pharmacodynamic and safety assessments and pharmacokinetic sampling were performed at specified time points. Subjects remained in the study centre during day 1 and until the morning of day 2. Subjects were then released and requested to return to the study centre for check‐in procedures for the next treatment period after a washout period of at least 2 weeks.
All dosed subjects had final procedures and assessments performed approximately 1 week after the last administered dose.
The study was approved by the medical ethical committee (Stichting Bebo, Assen, The Netherlands) and the competent authority (CCMO, The Hague, The Netherlands). The study was conducted according to the Dutch Act on Medical Research Involving Human Subjects (WMO) and in compliance with Good Clinical Practice and the Declaration of Helsinki. The trial was registered in the European Union Clinical Trials Register (2013–001883‐51) and on www.clinicaltrials.gov (NCT01903824). All pharmacological nomenclature nomenclature conforms to BJP's Concise Guide to PHARMACOLOGY 2015/16.24
2.2. Subjects
A total of 40 healthy male and female subjects in an approximate 1:1 ratio, aged 18–50 years (inclusive) with a body mass index of 18.0–30.0 kg/m2 (inclusive), were recruited for this study. Main exclusion criteria were smoking or use of nicotinic products within 3 months before inclusion, alcohol or drug abuse, excessive daily use of caffeine (>800 mg per day), use of medication with CNS effects or PK interactions and irregular diurnal rhythm. Because CEP‐26401 could bind to melanin containing tissues (data on file, Teva Pharmaceuticals), subjects with a dark skin (Fitzpatrick scale 5 or 625) were excluded. Also, subjects had to have a performance score on the spatial working memory test within normal range in order to reduce ceiling effects on cognitive testing.
2.3. Randomization
In this partial 4‐period, 6‐treatment cross‐over study, subjects were 1st randomized to 1 of the 3 cohorts, each with a different combination of treatments (Table 1). Within each cohort, subjects were randomly assigned to a treatment sequence using a Williams design. Each of the cohorts was comprised of 13 or 14 subjects. A total of 40 subjects were enrolled, with the intention of at least 36 subjects completing the entire study, 12 from each cohort. All treatments were administered as a single dose, with 14 days separating each treatment administration. Each subject underwent 4 study periods and received placebo in 1 of these periods. As this was a double dummy design, each subject received on each occasion CEP‐26401 or placebo, modafinil or placebo and donepezil or placebo. Modafinil and donepezil and their matching placebos were over‐encapsulated.
Table 1.
Treatments per cohort
| Placebo | CEP‐26401 | CEP‐26401 | CEP‐26401 | Donepezil HCl | Modafinil | |
|---|---|---|---|---|---|---|
| 5 μg | 25 μg | 125 μg | 10 mg | 200 mg | ||
| Cohort 1 | + | + | + | + | − | − |
| Cohort 2 | + | + | + | − | + | − |
| Cohort 3 | + | + | − | + | − | + |
2.4. Study medication and dosing rationale
2.4.1. CEP‐26401
CEP‐26401 dose levels were determined based on clinical findings from the 2 completed clinical studies with CEP‐26401 and PK/PD modelling.23 A dose of 20 μg was anticipated to have the largest cognition‐enhancing effect in a subset of Cambridge Neuropsychological Test Automated Battery (CANTAB) tests. Because 20 μg was the lowest dose tested in previously completed clinical studies, a dose of 5 μg was chosen to test the possibility of further improvement at lower concentrations. The 25‐μg dose was close to the most active previously tested dose of 20 μg. The high dose of 125 μg would assist in assessing a possible inverted U‐shaped dose–response relationship for cognitive enhancement and in confirming awakening effects during sleep periods. CEP‐26401 and its placebo were administered as an aqueous solution.
2.4.2. Modafinil
Modafinil was selected as a positive control, because it is a CNS‐stimulant compound whose effects include noradrenergic and dopaminergic enhancement, which (among others) are also indirectly produced by H3R antagonists such as CEP‐26401.1, 26 Modafinil is used for the treatment of patients with excessive sleepiness associated with certain disorders and has been studied in ADHD, which may be potential therapeutic areas for H3R antagonists.1, 2, 7, 11 A 200‐mg dose of modafinil was chosen because it has repeatedly demonstrated effects on the SWM task in the CANTAB battery of tests.27, 28 Modafinil also demonstrated statistically significant improvements of other working memory tasks (memory span) that were not studied with CEP‐26401, but were improved in studies with donepezil in healthy subjects.28
2.4.3. Donepezil
A 10‐mg dose of donepezil HCl was selected as an additional positive control. This cholinergic cognitive enhancer is registered for cognitive impairment in patients with mild‐to‐moderate AD.29, 30 As CEP‐26401 also has indirect cholinergic effects, memory disorders are a potential therapeutic indication for this compound.7 If an effect of donepezil can be measured in healthy volunteers, this could provide a benchmark for CEP‐26401 activity related to a registered memory enhancer. Although most studies in healthy subjects have used repeat dose designs or cognitive impairment models, single donepezil HCl doses of 5‐mg have caused small improvements of various aspects of memory and attention.31 None of the tests used were previously employed in CEP‐26401 studies. Therefore, the current study incorporated tests that have shown effects of donepezil HCl, including the n‐back (data on file, CHDR1104, Centre for Human Drug Research Leiden, The Netherlands) and maze learning tasks.32 A dose of 10 mg was chosen in view of the limited effects of the 5 mg dose in previous research, while adverse reactions were still expected to be minimal.31
2.5. Pharmacodynamic methods
Pharmacodynamic tests were performed using 2 different computerized testing platforms. The NeuroCart is a battery of drug‐sensitive tests, developed by the CHDR, for a wide range of CNS domains, including neuropsychological, neurophysiological and subjective measurements, to examine different kinds of CNS‐active drugs. CANTAB is a specific neuropsychological test battery, developed by Cambridge Cognition, UK. Tests were performed predose and at selected time points after drug administration (Figure 1). Measurements were performed in a quiet room with ambient illumination with only 1 subject per session in the same room to minimize distraction. A short test description is given below. More details, including primary and secondary outcome parameters per test, can be found in the supplementary material. The primary parameters are chosen based on their known sensitivity to drug effects.
Figure 1.
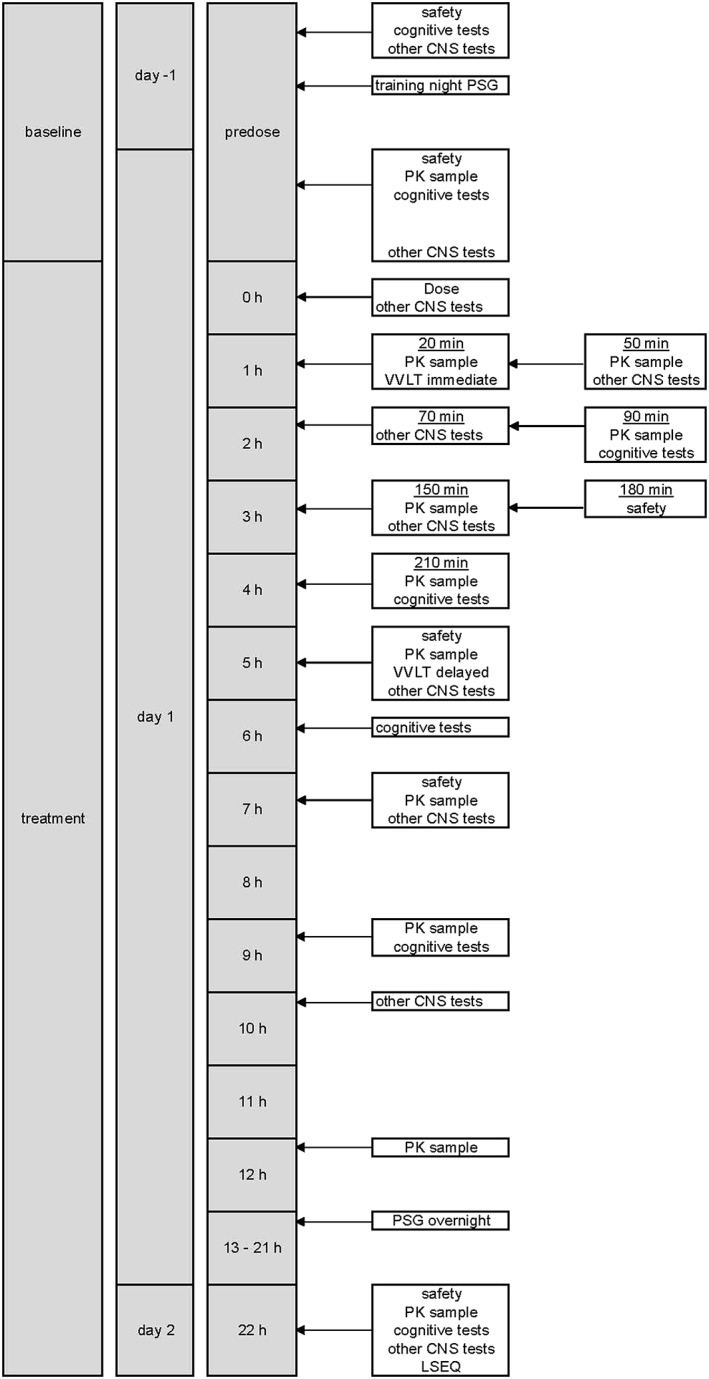
Schedule of assessments. CNS, central nervous system; LSEQ, leeds sleep evaluation questionnaire; PSG, polysomnography; PK, pharmacokinetic; VVLT, visual verbal learning test
2.6. Cognitive tests
2.6.1. SWM
Several boxes were presented on the screen, in 1 of which a token was to be found. The token never appeared in the same box more than once and the test continued until a token had been found in all of the boxes once. The primary outcome parameter on this test was the total of between errors on 10 and 12 box trials. Between errors is the number of times a subject touches a box already found to contain a token.33
2.6.2. Rapid visual information processing
Numbers were presented consecutively on the screen with a speed of 100 numbers per minute. The subject had to press a button if a predefined sequence of even or uneven numbers was seen.34
2.6.3. Stop signal task
The stop signal task (SST) is a classic stop signal response inhibition test. An arrow pointing either to the left or to the right is displayed on the computer screen. Subjects had to indicate in which direction the arrow on the screen pointed, but when an audio tone was presented at the same time, they had to inhibit the response.33
2.6.4. Paired associate learning
Several boxes were presented and automatically opened in a random order. In some of the boxes a pattern was shown. Then patterns were shown and the subject had to indicate which box contained the pattern.33
2.6.5. Visual verbal learning task
The visual verbal learning test contains 3 different subtests that cover basically the whole scope of learning behaviour (i.e. acquisition, consolidation, storage and retrieval). Volunteers performing the visual verbal learning test were presented 30 words in 3 consecutive word trials. Each trial ended with a free recall of the presented words (Immediate Recall). Approximately 30 minutes after start of the 1st trial, the volunteers were asked to recall as many words as possible (delayed recall). Immediately thereafter, the volunteers underwent memory recognition test, which consisted of 15 presented words and 15 new distractors (recognition).35
2.6.6. Maze learning
Subjects had to complete a maze by using trial and error learning to locate a 28‐step pathway (from upper‐left to bottom‐right) that was hidden beneath a 10 × 10 grid of tiles. Individuals had to find the same pathway on five successive trials. Approximately 30 minutes after the start of the 1st trial, the volunteers were asked to identify the same maze again (delayed test, 1 trial). Immediately thereafter, the volunteers underwent the reversed test, which consists of 1 trial of the same maze backwards (from bottom‐right to upper‐left).36
2.6.7. N‐back
This test evaluates working memory and requires buffering and updating consonants, matching, encoding and responding. The N‐back test consists of 3 conditions, with increasing working memory load. Letters were presented consecutively on the screen with a speed of 30 letters per minute. In the 1st condition subjects had to indicate whether the letter on the screen was an X. In the 2nd condition, subjects indicated whether the letter seen was identical to the previous letter. In the 3rd condition, subjects were asked to indicate whether the letter was identical to 2 letters before the letter seen.37, 38, 39
2.6.8. Stroop choice reaction time
The distraction task is a parametric version colour‐word response conflict task.40 The words Left and Right were displayed either at the left or the right side of a computer screen. Response instructions are to respond quickly (by pressing a corresponding button) to the meaning of the word irrespective of its location.
2.7. Subjective measurements: visual analogue scale (VAS) Bowdle, VAS Bond & Lader, VAS Task Enjoyment
Subjective feelings were assessed using classical VASs according to Bowdle and Bond & Lader.41, 42 From these questionnaires, composite scores were derived for internal perception and external perception, originating from the VAS Bowdle. The VAS score for task enjoyment was evaluated by means of a classical VAS (0–10 cm) device, with cut‐off points as follows: 0–1 (no enjoyment), 2–4 (mild enjoyment), 5–7 (moderate enjoyment) and 8–10 (high enjoyment).
2.8. Other CNS tests
2.8.1. Adaptive tracking
Adaptive tracking is a pursuit‐tracking task, measuring attention and eye–hand coordination.43, 44, 45, 46, 47, 48 A circle moves pseudo‐randomly about a screen. The subject must try to keep a dot inside the moving circle by operating a joystick. If this effort is successful, the speed of the moving circle increases. Conversely, the velocity is reduced if the test subject cannot maintain the dot inside the circle. Each test was preceded by 3 training sessions and included 2 baseline measurements.
2.8.2. Eye movements
Both saccadic and smooth pursuit eye movements were measured using a computerized test system to generate a moving dot on the screen, which had to be followed with the eyes by the subject, while the head was stabilized.47, 49, 50
2.8.3. Body sway
The body sway was measured with an apparatus similar to the Wright ataxia meter.51 The body sway meter allows measurement of body movements in a single plane, providing a measure of postural stability. During sway measurements, subjects are instructed to keep their eyes closed for 2 minutes.
2.8.4. Pharmaco‐electroencephalography
Pharmaco‐electroencephalography (EEG) was used to monitor any drug effects, which can be interpreted as evidence of penetration and activity in the brain.52, 53, 54 EEG recordings were made at Fz, Cz, Pz and Oz. For each lead, fast Fourier transform analysis was performed to obtain the sum of amplitudes (power) in the δ‐1 (0.5–2 Hz), δ‐2 (2–4 Hz), θ (4–7.5 Hz), α‐ (7.5–13.5 Hz), β‐ (13.5–35 Hz) and γ‐ (35–48.9 Hz) frequency ranges. The duration of EEG measurements was 64 seconds per session.52, 53, 54
2.9. Measurement of sleep
2.9.1. Polysomnography
The PSG consisted of EEG, electrooculography, electromyography and electrocardiography (ECG) and cardiorespiratory measurements. In PSG, the electromyography is typically recorded from under the chin; since muscles in this area show very dramatic changes associated with the sleep stages. ECG is used for artefact removal.55 PSG data were analysed by The Siesta Group Schlafanalyse GmbH (Vienna, Austria).
2.9.2. Leeds sleep evaluation questionnaire
The Leeds sleep evaluation questionnaire (LSEQ) has 10 questions, the answers for which are captured on a VAS scale. This clinical tool allows test persons to qualitatively assess their sleep. Composite scores were computed for getting to sleep, quality of sleep, awakening following sleep and behaviour following wakening.56, 57
2.10. Assessment of safety
All subjects underwent medical screening before study entry, including medical history, physical examination, ocular pressure measurement, vital signs measurement, 12‐lead ECG, urinalysis, drug screen and safety chemistry and haematology blood sampling. During study periods, safety was monitored based on adverse events (AEs), ocular pressure measurement, vital signs, ECG, safety chemistry and haematology blood sampling, urinalysis, physical examination and concomitant medication usage. In previous studies, CEP‐26401 has been administered to healthy volunteers in doses up to 5 mg.23 In these studies, intraocular pressure emerged as a safety finding of possible concern. In the current study, subjects with intraocular pressure > 22 mmHg were excluded at screening, and pressure was measured repeatedly using an ICare TA01 tonometer (ICare, Finland).
2.11. Pharmacokinetic methods
Venous blood samples were collected via an indwelling catheter before drug administration, and at preselected time points after drug administration (Figure 1). Samples were collected in lithium heparin tubes, centrifuged to obtain plasma and frozen.
CEP‐26401 concentrations in plasma samples were determined by Pharmaceutical Product Development (PPD) (Richmond, Virginia) using ultraperformance liquid chromatography (UPLC) with tandem mass spectrometric detection method that had been validated as per Food and Drug Administration guidelines. The final extracts were injected onto an Acquity UPLC system with chromatographic separation achieved via an Acquity UPLC BEH C18 column (2.1 × 50 mm, 1.7 μm; Waters, Milford, MA, USA). Detection was performed using a Xevo TQ‐S mass spectrometer (Waters) in positive ion‐mode. The assay range is 0.500–250 pg/mL. At the minimum, the method was required to have intra‐ and interday precision (coefficients of variation) for pooled plasma quality control samples of ≤15% except at the lower limit of quantitation, where ≤20% was acceptable. The calculated concentrations (both inter‐ and intraday) were required to be within 15% of nominal at all concentrations except the lower limit of quantitation, where up to 20% deviation from nominal was acceptable. The precision and accuracy of the method exceeded these minimum requirements for assay validation. In addition, stability of the analyte in frozen lithium heparinized human plasma was demonstrated for periods exceeding the storage periods of the samples prior to analysis, as well as under all conditions to which study samples or working solutions were subjected.
The following PK parameters were calculated for CEP‐26401 by noncompartmental methods using WinNonlin software (Enterprise version 5.1.1; Pharsight Corporation, Mountain View, CA, USA): area under the plasma concentration–time curve from time zero to the time of the last measurable concentration (AUC0‐t), maximum observed plasma concentration (Cmax) and time to Cmax (tmax).
2.12. Statistical analysis
For statistical analysis of PD parameters, mixed‐model analyses of covariance (using SAS PROC MIXED) were performed with treatment, treatment period, time and treatment by time as fixed effects, and with subject, subject by treatment and subject by time as random effects, and with the average baseline value per period as covariate, where baseline is defined as the average of the available values obtained prior to dosing. Treatment effects were reported as contrasts where the average of the measurements was calculated within the statistical model up to last time point. Effect sizes for all treatments compared to placebo were calculated as change from baseline. Data were presented with a 95% confidence interval (thus a critical α of 0.05). As this was an exploratory study, no correction for multiple testing was employed.58, 59
2.13. Power calculation
Prestudy power calculations were based on the effects of CEP‐26401 on between errors of the SWM task with 10 boxes, in previous studies with CEP‐26401 in healthy volunteers and PK/PD‐modelling of this data.23 In the study reported in this manuscript, 24 subjects were planned to have a cross‐over comparison between CEP‐26401 5, 25 or 125 μg and placebo. A sample size of 24 would have 80% power to detect a difference in means of 6.6 assuming a standard deviation of differences of 11.0, using a paired t test with a 0.05 2‐sided significance level. Thirty‐six subjects were planned to have a cross‐over comparison between CEP‐26401 5 μg and placebo, which would have 80% power to detect a difference in means of 5.3 under the same assumptions. Modafinil and donepezil were included as active comparator compounds for the effect profile of CEP‐26401 and were each administered to 12 subjects. This sample size would have at least 80% power to detect a difference in means of 12.7 in between errors of the SWM task with 10 boxes, assuming a standard deviation of differences of 11.0, using a paired t‐test with a 0.05 2‐sided significance level. A recent parallel design study showed an average improvement of 7.2 errors on this test, after a single 200‐mg dose of modafinil in adults with ADHD.27 The effects of donepezil on the tests used in this study were unknown at the time this study was planned. Therefore, no formal power calculations could be made to determine sample sizes for the effects of this compound. However, 12 subjects had previously been sufficient to obtain statistically significant effects of donepezil 5 mg in various study designs on working and visual memory, digit span backward, and maze learning in healthy elderly.31, 32 These functional domains were also covered in this study.
2.14. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,60 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.61
3. RESULTS
3.1. Demographics and disposition
A total of 80 subjects were screened for enrolment into this study. Of these, 40 subjects met inclusion criteria and were considered eligible for enrolment into the study. Of the 40 subjects who were not enrolled, 29 were excluded based on enrolment criteria and 11 subjects did not participate for other reasons. Of the 40 subjects enrolled, all received at least 1 dose of study drug and were evaluated for safety. Four subjects withdrew from the study for personal reasons (10%). Of these subjects, 1 subject completed 3 occasions, 1 subject completed 2 occasions and 2 subjects completed 1 occasion. All 4 missed their placebo occasion. Consequently, 36 subjects completed a placebo occasion. The cohorts were similar with regard to age, weight and body mass index (Table 2).
Table 2.
Demographics
| Cohort 1 (n = 13) | Cohort 2 (n = 14) | Cohort 3 (n = 13) | Total (n = 40) | |
|---|---|---|---|---|
| Age (years) | 29.0 (18–48) | 25.4 (19–48) | 26.2 (18–48) | 26.8 (18–48) |
| Sex (n male) | 5 (38%) | 10 (71%) | 7 (54%) | 22 (55%) |
| Weight (kg) | 70.8 (55.5–92.3) | 76.1 (53.8–95.2) | 71.9 (47.6–86.3) | 73.0 (47.6–95.2) |
| BMI (kg/m2) | 23.4 (19.3–29.4) | 23.7 (18.3–28.2) | 23.2 (18.2–28.9) | 23.4 (18.2–29.4) |
For age, weight and body mass index (BMI): mean (range). For sex: number of male subjects (%).
3.2. Pharmacodynamics
3.2.1. Cognitive effects
The most relevant parameters of the cognitive tests are presented in Table 3. A complete overview of summary data of all tests and parameters is provided as supplementary material. After administration of CEP‐26401 in all doses tested, no improvement on any of the cognitive tests could be observed. Of particular interest was the SWM task that showed some evidence of positive effect, which was also observed in the previous phase‐1 studies.23 The number of errors in this task was not different from placebo, after 5 and 25 μg. Similar to the previous findings, performance in the SWM task was statistically significantly worse at the high dose of 125 μg. A slight worsening effect on the paired associate learning (PAL) task was also seen with the 25 and 125 μg doses of CEP‐26401. Accuracy on the 2‐back condition of the N‐back test deteriorated at 125 μg. After administration of modafinil 200 mg, an improvement was observed on rapid visual information processing (RVIP), but no significant effects were observed on other cognitive tests. There were no significant improvements on cognitive tests after administration of donepezil. Detailed results of all parameters are presented in the supplementary material.
Table 3.
Cognitive, subjective and general central nervous system effects compared to placebo, using a mixed‐model analysis of covariance
| CEP‐26401 | CEP‐26401 | CEP‐26401 | Modafinil | Donepezil | |
|---|---|---|---|---|---|
| 5 μg (n = 38) | 25 μg (n = 26) | 125 μg (n = 25) | 200 mg (n = 13) | 10 mg (n = 13) | |
| Spatial working memory—between errors 10 boxes | 2.92 (−1.21 to 7.05) | 3.24 (−1.57 to 8.04) | 7.45 (2.72 to 12.19) | 2.30 (−3.84 to 8.45) | −0.71 (−7.12 to 5.71) |
| P = .4583 | |||||
| P = .8276 | |||||
| P = .1630 | P = .1837 | P = .0024 | |||
| Rapid visual information processing—a prime | 0.00 (−0.00 to 0.01) | 0.00 (−0.00 to 0.01) | 0.00 (−0.00 to 0.01) | 0.01 (0.00 to 0.02) | −0.01 (−0.02 to ‐0.00) |
| Stop signal task—reaction time | −11.57 (−26.28 to 3.15) | −8.79 (−25.46 to 7.89) | −11.39 (−28.07 to 5.28) | −1.79 (−23.12 to 19.55) | 19.77 (−2.32 to 41.86) |
| Paired associate learning—total errors adjusted | 1.78 (−0.47 to 4.02) | 2.69 (0.12 to 5.26) | 2.97 (0.42 to 5.53) | −2.41 (−5.47 to 0.91) | 4.97 (1.51 to 8.42) |
| N—back—0‐back reaction time (ms) | −3.37 (−16.43 to 9.68) | 10.52 (−4.78 to 25.82) | 2.58 (−12.52 to 17.67) | 3.51 (−15.76 to 22.78) | 0.48 (−19.81 to 20.76) |
| N‐back—2‐back accuracy | 0.0 (−0.03 to 0.03) | −0.01 (−0.04 to 0.03) | −0.04 (−0.08 to ‐0.00) | −0.02 (−0.07 to 0.03) | 0.00 (−0.05 to 0.05) |
| N‐back—2‐back reaction time (ms) | −17.65 (−36.66 to 1.36) | −25.04 (−47.34 to −2.73) | −39.25 (−61.10 to −17.40) | −5.97 (−34.32 to 22.38) | −27.72 (−57.26 to 1.82) |
| Vas task enjoyment | 3.21 (0.86 to 5.55) | 3.87 (1.16 to 6.58) | 3.19 (0.49 to 5.89) | 4.64 (1.17 to 8.12) | 0.52 (−3.10 to 4.13) |
| Adaptive tracking (%) | 0.74 (0.06 to 1.43) | 1.08 (0.28 to 1.88) | 1.20 (0.42 to 1.98) | 1.80 (0.80 to 2.81) | 0.49 (−0.57 to 1.54) |
| Saccadic peak velocity (degree/sec) | 4.00 (−2.29 to 10.28) | 6.75 (−0.50 to 13.99) | 16.99 (9.73 to 24.24) | 24.62 (15.32 to 33.92) | 3.06 (−6.92 to 13.04) |
| Body sway (mm) | −16.89 (−46.08 to 12.30) | 3.43 (−30.08 to 36.93) | −28.72 (−61.94 to 4.51) | −54.44 (−97.29 to −11.60) | 24.22 (−20.54 to 68.88) |
| EEG frontal γ frequency | 0.06 (0.01 to 0.12) | 0.07 (0.01 to 0.14) | 0.05 (−0.01 to 0.12) | 0.10 (0.01 to 0.18) | 0.06 (−0.03 to 0.14) |
Mean (confidence interval). Statistically significant differences in bold.
EEG, electroencephalography.
3.2.2. Subjective effects
The 2 lowest doses of CEP‐26401 induced significant improvements on several subscales of the VAS Bond & Lader, which were strongest at the 25 μg dose (Figure 2). Administration of CEP‐26401 5 μg led to feelings of alertness, energy, contentedness, quick‐wittedness, attention, happiness and gregariousness (p < .05). Administration of CEP‐26401 25 μg induced feelings of strength, clear‐headedness, coordination, contentedness, quick‐wittedness, attention, proficiency, happiness, interest and gregariousness. The increase in alertness and energy almost reached significance at this dose of CEP‐26401. Administration of CEP‐26401 125 μg did not lead to any statistically significant changes on the VAS Bond & Lader. All doses of CEP‐26401 induced a significant improvement on the VAS score for task enjoyment. Task enjoyment was also improved by modafinil, which additionally only increased VAS Bond & Lader scores for energy and happiness. Administration of donepezil did not lead to any changes on the VAS Bond & Lader, or task enjoyment, but there was an increase on VAS Bowdle scores of feeling high, change in surroundings and feeling of unreality. These effects in VAS Bowdle were not seen with CEP‐26401 or modafinil.
Figure 2.
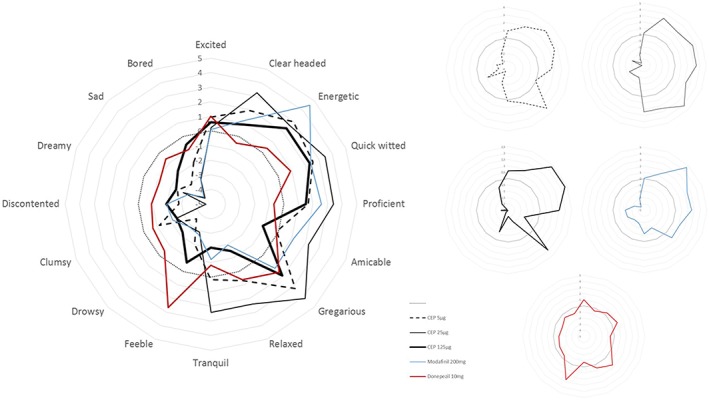
Effect on visual analogue scale Bond & Lader compared to placebo. The order of items corresponds with the order of the questionnaire items
3.2.3. Other CNS performance effects
Dose related improvements of CNS performance were observed after administration of CEP‐26401 on adaptive tracking, saccadic peak velocity and reaction time (during the 2‐back condition of the N‐back task, but not the 0‐back condition; Table 3). There was an increase in frontal γ frequency on the EEG, which was statistically significant for the 2 lowest doses of CEP‐26401. No statistically significant differences were found for other frequency bands of the EEG, finger tapping or body sway after administration of CEP‐26401. Administration of modafinil led to an improvement on adaptive tracking, body sway and saccadic peak velocity and an increase in frontal γ frequency on the EEG, although the latter might be influenced by muscle artefacts. Reaction time and finger tapping were not affected by modafinil. No statistically significant effects of donepezil were seen on any of the parameters (detailed data in supplementary material).
3.2.4. Effects on sleep
CEP‐26401 had an inhibitory, dose dependent effect on all sleep parameters measured during PSG, not with 5 μg but starting at 25 μg and increasing at 125 μg (Table 4). On the subjective assessment of sleep, a similar effect was seen, except for the questions related to awakening following sleep. Modafinil had a significantly inhibitory effect on sleep for sleep efficiency, sleep latency, total sleep time and wake after sleep onset. The subjective scales showed a decrease in the ease of getting to sleep and awakening following sleep. Administration of donepezil led to a slight reduction of frequency of stage shifts; no effects were seen on other parameters of the PSG or on the subjective sleep assessment.
Table 4.
Effects on sleep compared to placebo, using a mixed‐model analysis of covariance
| CEP‐26401 | CEP‐26401 | CEP‐26401 | Modafinil | Donepezil | |
|---|---|---|---|---|---|
| 5 μg (n = 38) | 25 μg (n = 26) | 125 μg (n = 25) | 200 mg (n = 13) | 10 mg (n = 13) | |
| Number of awakenings per night | −1.98 (−4.39 to 0.43) | −3.66 (−6.48 to −0.84) | −3.20 (−5.92 to −0.47) | −1.65 (−.572 to 1.96) | −3.55 (−7.13 to 0.03) |
| Frequency of stage shifts per night | −11.34 (−24.37 to 1.69) | −24.75 (−39.78 to −9.36) | −39.24 (−53.94 to −24.53) | −14.35 (−33.82 to 5.13) | −19.32 (−35.58 to −0.05) |
| REM latency (min) | −3.58 (−19.64 to 12.48) | 15.04 (−3.67 to 33.74) | 44.70 (26.33 to 63.07) | 22.21 (−1.74 to 46.17) | 7.61 (−16.04 to 31.25) |
| Sleep efficiency (%) | −1.48 (−5.58 to 2.62) | −9.04 (−13.58 to −4.24) | −16.01 (−20.65 to −11.38) | −12.13 (−18.28 to −5.98) | −2.61 (−8.71 to 3.48) |
| Sleep latency (minutes) | 3.90 (−8.49 to 16.29) | 7.76 (−6.63 to 22.16) | 20.72 (6.67 to 34.68) | 30.32 (11.89 to 48.76) | 11.44 (−6.74 to 29.63) |
| Total sleep time (minutes) | −13.40 (−38.85 to 12.05) | −44.79 (−74.32 to −15.72) | −70.82 (−99.47 to −42.17) | −59.86 (−97.66 to −22.06) | −35.24 (−72.50 to 2.02) |
| Wake after sleep onset (minutes) | 1.09 (−13.91 to 16.09) | 35.36 (17.68 to 53.05) | 57.56 (40.57 to 74.55) | 29.35 (6.73 to 51.98) | 0.91 (−21.60 to 23.41) |
| LSEQ—getting to sleep (average mm change) | −2.34 (−6.62 to 1.95) | −6.39 (−11.13 to −1.65) | −12.37 (−17.10 to −7.64) | −13.40 (−19.47 to −7.34) | −1.71 (−7.89 to 4.47) |
| LSEQ—quality of sleep (average mm change) | −4.05 (−9.67 to 1.56) | −6.93 (−13.13 to −0.73) | −20.88 (−27.08 to −14.68) | −6.78 (−14.71 to 1.16) | −3.46 (−11.49 to 4.57) |
| LSEQ—awake following sleep (average mm change) | 2.76 (−1.97 to 7.50) | −0.07 (−5.29 to 5.14) | 2.92 (−2.29 to 8.14) | 10.94 (4.16 to 17.71) | −1.32 (−8.09 to 5.46) |
| LSEQ—behaviour following wakening (average mm change) | −0.95 (−5.01 to 3.11) | −3.71 (−8.19 to 0.77) | −5.34 (−9.82 to −0.87) | 0.35 (−5.40 to 6.10) | −4.94 (−10.82 to 0.93) |
Mean (confidence interval). Statistically significant differences in bold. Sleep efficiency is the percentage of time in bed while the subject is asleep. LSEQ, Leeds sleep evaluation questionnaire; REM, rapid eye movement.
3.3. Pharmacokinetics
CEP‐26401 was absorbed with median tmax values of approximately 3.5–5.0 hours (Figure 3, Table 5). After reaching peak plasma levels, CEP‐26401 slowly declined with mean concentrations at the 22‐hour time point representing approximately 60% of Cmax. Systemic exposure to CEP‐26401, (Cmax, AUC0‐t) increased in an approximately dose‐proportional manner across the dose range evaluated. The mean Cmax values for the 5‐, 25‐ and 125‐μg doses were 9.1, 45.4 and 245.4 pg/mL, respectively, and the corresponding mean AUC0‐t values were 152, 743 and 3925 pg h/mL. The coefficient of variation associated with these parameters was between 15 and 20%. Despite 14‐day washout periods, low but quantifiable levels of CEP‐26401 were observed in some of the predose samples from all treatments. This finding was not completely unexpected given the long terminal elimination half‐life observed for CEP‐26401 in previous PK studies23 and in consideration of the sensitivity of the bioanalytical method.
Figure 3.
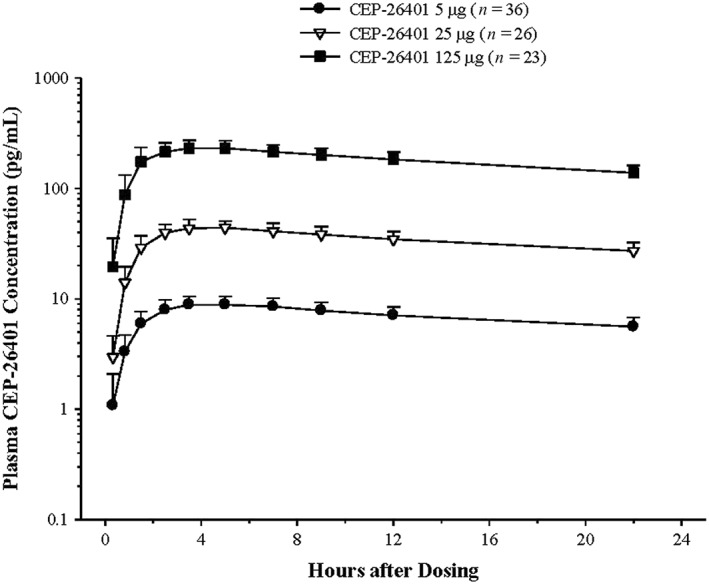
Mean (+standard deviation) plasma concentration–time profiles of CEP‐26401 in subjects administered single oral doses of CEP‐26401 at 5, 25 and 125 μg
Table 5.
Pharmacokinetic parameters of CEP‐26401 in healthy subjects administered Single Oral Doses of CEP‐26401 at 5, 25, and 125 μg
| Parameter | CEP‐26401 5 μg(n = 36) | CEP‐26401 25 μg(n = 26) | CEP‐26401 125 μg(n = 23) |
|---|---|---|---|
| C max , pg/mL | 8.97 (1.770) | 44.83 (7.344) | 242.56 (38.818) |
| t max , h a) | 4.205 (1,300) | 4.453 (1,243) | 3.685 (1,756) |
| AUC 0‐t , pg h/mL | 149.63 (29.168) | 732.93 (126.472) | 3882.36 (612.045) |
Geometric mean (standard deviation).
3.4. Safety
During the 4 double‐blind treatment periods, 27 of 36 (75%) subjects on placebo, 25 of 38 (66%) on CEP‐26401 5 μg, 16 of 26 (62%) on CEP‐26401 25 μg, 16 of 25(64%) on CEP‐26401 125 μg, all 13 (100%) on donepezil 10 mg and 10 of 13 subjects (77%) on modafinil 200 mg reported at least 1 AE (Table 6).
Table 6.
Adverse events occurring in at least 10% of subjects
| MedDRA system organ class MedDRA preferred term | Number (%) of subjects a) | |||||
|---|---|---|---|---|---|---|
| Placebo (n = 36) | CEP‐26401 5 μg (n = 38) | CEP‐26401 25 μg (n = 26) | CEP‐26401 125 μg (n = 25) | Modafinil 200 mg (n = 13) | Donepezil 10 mg (n = 13) | |
| Number of subjects with at least 1 AE | 27 (75) | 25 (66) | 16 (62) | 16 (64) | 10 (77) | 13 (100) |
| Gastrointestinal disorders | ||||||
| Abdominal pain | 1 (3) | 2 (5) | 1 (4) | 2 (8) | 1 (8) | 2 (15) |
| Nausea | 1 (3) | 2 (5) | 3 (12) | 5 (20) | 1 (8) | 12 (92) |
| Vomiting | 0 | 1 (3) | 0 | 0 | 0 | 7 (54) |
| General disorders and administration site conditions | ||||||
| Fatigue | 7 (19) | 9 (24) | 4 (15) | 3 (12) | 1 (8) | 2 (15) |
| Malaise | 1 (3) | 1 (3) | 0 | 0 | 1 (8) | |
| Feeling hot | 0 | 0 | 0 | 1 (4) | 1 (8) | 4 (31) |
| Infections and infestations | ||||||
| Nasopharyngitis | 3 (8) | 2 (5) | 2 (8) | 2 (8) | 2 (15) | 0 |
| Injury, poisoning and procedural complications | ||||||
| Procedural dizziness | 0 | 0 | 0 | 0 | 1 (8) | 2 (15) |
| Nervous system disorders | ||||||
| Headache | 6 (17) | 7 (18) | 6 (23) | 6 (24) | 5 (38) | 3 (23) |
| Somnolence | 10 (28) | 7 (18) | 3 (12) | 1 (4) | 0 | 2 (15) |
| Dizziness | 3 (8) | 3 (8) | 2 (8) | 3 (12) | 0 | 6 (46) |
| Tremor | 0 | 0 | 0 | 0 | 0 | 2 (15) |
| Psychiatric disorders | ||||||
| Hypervigilance | 1 (3) | 0 | 0 | 1 (4) | 3 (23) | 0 |
| Respiratory, thoracic and mediastinal disorders | ||||||
| Oropharyngeal pain | 0 | 3 (8) | 0 | 0 | 2 (15) | 0 |
| Skin and subcutaneous tissue disorders | ||||||
| Hyperhidrosis | 0 | 1 (3) | 1 (4) | 3 (12) | 0 | 3 (23) |
| Vascular disorders | ||||||
| Hot flush | 0 | 0 | 0 | 0 | 0 | 2 (15) |
Number of subjects (%).
MedDRA, Medical Dictionary for Regulatory Activities.
The most important adverse effects, which occurred in at least 10% of subjects and more often with active treatment than after placebo, were as follows: headache at all 3 doses; nausea was more common with CEP‐26401 125 μg and 25 μg than with placebo; CEP‐26401 125 μg was also associated with more dizziness and hyperhidrosis than placebo. CEP‐26401 5 μg was associated with more fatigue than placebo. Somnolence was less frequent with all CEP‐26401 doses. In the modafinil group, headache, hypervigilance, nasopharyngitis and oropharyngeal pain were reported in at least 10% of subjects and more frequently than after placebo. For donepezil: nausea and vomiting; headache; abdominal pain; dizziness and procedural dizziness; feeling hot, hot flush and hyperhidrosis; and tremor all occurred at least in at least 10% of subjects and more frequently than after placebo. In contrast, somnolence and fatigue were reported less often after donepezil than under placebo.
All AEs were mild or moderate, except for 1 subject with severe headache and vomiting after administration of CEP‐26401 5 μg. One subject had an asymptomatic increased intraocular pressure of 23 mmHg on the right eye at 5 hours after administration of CEP‐26401 125 μg, which was normalized at the next measurement at 22 hours after drug administration. Three subjects had an increase in eosinophils during the study. One experienced a progressive rise throughout the study. Two others had eosinophilia at baseline and experienced fluctuations during the study with 1 reaching 23.41% eosinophils (absolute eosinophil count of 1730 × 106/L) before returning to near baseline. A relationship between the eosinophilia and the study drug could not be excluded, but the AEs for these subjects did not seem to point to clinical significance for the eosinophil elevation. There were no clinically significant changes in other laboratory values, vital signs, ECG or physical examination.
No deaths or other serious AEs were observed during this study. During the study, no subjects were withdrawn due to AEs.
4. DISCUSSION
In this study, CEP‐26401 caused significant excitatory effects on a range of drug‐sensitive CNS‐tests including adaptive tracking, saccadic peak velocity, reaction time (during the most demanding 2‐back paradigm of the N‐back task), and frontal EEG γ frequency. As reaction time of the N‐back task did not decrease during the zero‐back condition, this is probably an effect on working memory processing speed, not on sensorimotor speed. The effect on EEG γ frequency might be an artefact as, in awake subjects it is almost impossible to distinguish EEG γ frequency from muscle artefacts. Some of the other effects already reached statistical significance at the 5 μg dose of CEP‐26401, and most were significant with the 125 μg dose. This demonstrates the high potency and stimulatory effects at very low doses of this H3R antagonist.
Despite the significant CNS‐stimulating effects that were demonstrated with the NeuroCart, CEP‐26401 did not have any beneficial effect on cognitive testing, even though this was expected based on previous studies with CEP‐26401 in healthy volunteers.23 At the highest dose of 125 μg there was even some decline at the accuracy of the N‐back task and an increase in total errors on SWM and PAL. As administration of modafinil led to an improvement on RVIP, it is unlikely that the lack of effect of CEP‐26401 on this test is due to inadequate study design or test conditions. As the cholinesterase inhibitor donepezil did not induce any measurable effects on SWM, PAL or SST, it is also possible that this was precluded by ceiling effects in this healthy population or that the tests used were not sensitive enough. The improvement in RVIP after administration of modafinil argues against this explanation, although it is possible that SWM, PAL and SST have a ceiling effect, while RVIP has not. Another possibility is that cognitive enhancement was obscured by AEs of the 10 mg dose in these young subjects. Previous studies at CHDR have shown positive effects of donepezil 10 mg on N‐back and adaptive tracking in healthy elderly volunteers.62 It is possible that a slight, age related cholinergic deficiency in elderly subjects has contributed to the measurability of these effects, and that they tolerate the drug better.63 The current study, however, provides no indication that CEP‐26401 might have cognitive enhancing effects, and does not provide reasons to assume efficacy in cognitive disorders such as AD. This is in contrast with results from several preclinical studies with other H3 antagonists, which demonstrated an effect on working memory, memory consolidation, spatial orientation and attention.11 Also, 2 clinical trials in patients with mild to moderate Alzheimer's disease reported small improvements in attention and memory with the H3R antagonist GSK239512.15, 64 By contrast, it is consistent with a large phase 2 trial with 2 doses of an H3 antagonist in patients with AD, which was aborted prematurely, because futility criteria were met.17 Other trials aimed to improve cognitive impairment associated with schizophrenia with an H3R antagonist, also failed to demonstrate efficacy.16, 65 This study seems to add to the evidence against beneficial cognitive effects of H3R antagonists.
Although not immediately expected, CEP‐26401 had extensive positive effects on several subjective VAS scales, which were significant in 8/16 scales at 5 μg, in 12/16 scales at the 25 μg dose, but in none of the scales at the 125 μg dose. The positive effects were not limited to feelings of energy, happiness and task enjoyment, as was observed after administration of modafinil, but also included feelings of contentedness, proficiency, interest and gregariousness. It is of interest that the 2 lowest doses of CEP‐26401 also produced the lowest number of cognitive AE reports (31–32%)—lower even than placebo (56%) and much lower than modafinil (62%) or donepezil (100%). The subjective energetic and alert feeling is also reflected in the dose dependent improvements on adaptive tracking and saccadic peak velocity, as these indicate an increase in vigilance and motivation. Thus, CEP‐26401 seems to induce the same, subjectively and objectively measured, energizing and happy feeling as modafinil, but with a more relaxed undertone—at least in the low doses used in this study. It is known that modafinil acts on dopaminergic neurons.28 Since CEP‐26401 affects basically the same parameters as modafinil, it could be suggested that it has—at least—indirect influence on this neurotransmitter system. However, since CEP‐26401 has more extensive effects than modafinil, other neurotransmitter systems are probably also involved. This would be consistent with a microdialysis study in rats, where administration of CEP‐26401 led to an increase of both dopamine and acetylcholine.66
As CEP‐26401 is a highly selective H3R antagonist, it inevitably increases the release of histamine via the inhibitory autoreceptors.3, 4 H3R antagonists are also expected to increase the release of noradrenaline via heteroreceptors. The combination of increased levels of both histamine and noradrenaline could very well influence alertness and sleep. This is evident in the effects of CEP‐26401 on sleep. In this study, CEP‐26401 had an inhibitory, dose‐dependent effect on sleep, which was significant for many PSG parameters at the 25‐ and 125‐μg doses of CEP‐26401. Subjective experience of sleep quality, as measured by LSEQ, also decreased in a dose dependent manner, further suggesting a dose‐related disruption of sleep, as was also reported in the previous studies with CEP‐2640123 and also with pitolisant, another H3R antagonist.13 Sleep impairment was also observed for modafinil, although this compound had a more prominent effect on falling asleep and on waking up compared to CEP‐26401.
Although CEP‐26401 did not have the expected positive effect on cognition and cannot be typified as a cognitive enhancer, it may be a useful drug for certain indications that are characterized by a lack of internal drive and energy. The stimulant effects of CEP‐26401 on objective CNS tests (PSG, adaptive tracking, saccadic peak velocity) were generally dose‐dependent, whereas subjective effects were most favourable at a dose of 25 μg, but virtually disappeared after 125 μg. Except for a dose‐dependent inhibitory effect on sleep, CEP‐26401 was well‐tolerated by most study subjects with only 1 patient experiencing severe AEs (an episode of headache and nausea). Based on these observations, the 25‐μg dose of CEP‐26401 has the optimal balance between favourable subjective and stimulatory effects, and inhibitory effects on sleep. The more strong, clear‐headed, well‐coordinated, interested and quick‐witted feeling in combination with a more contented, attentive, proficient, happy and gregarious feeling might give benefit to patients suffering from certain types of mood disorders, such as major depression or dysthymia, negative symptoms of schizophrenia or anxiety disorders, especially social anxiety. The energizing aspects of CEP‐26401 might give extra benefit to elderly patients with mood disorders, because they usually have more apathy, compared to younger patients.67
However, there are also possible challenges with the use of CEP‐26401 in a clinical setting. There appears to be a bell‐shaped response curve which implies a relatively narrow therapeutic window. It remains to be established whether the pleasurable effect might generate abuse potential, especially in already vulnerable, psychiatric patient populations. Stimulant effects may also be undesirable in (unrecognized) bipolar disorder, and the effects may differ in elderly subjects, particularly with cognitive impairment. Also, the effects on sleep cannot be ignored and might constitute a clinically relevant adverse reaction.
This study has several limitations. Despite the performance of many different tests, a correction for multiple testing was not performed. By contrast, both time profile and response pattern on tests expected to be related to each other are consistent, suggesting that the data are trustworthy. The time courses for the repeated tests were also in agreement with the pharmacokinetic time profile. This suggests that the improvements were driven by pharmacological effects, although no PK/PD‐analysis was performed. In general, these consistent observations support the theory that a correction for multiple testing is only necessary in confirmatory studies, studying 1 specific hypothesis without any exploratory objectives.58, 59 The large number of tests on 1 day could induce fatigue or decreased motivation in the subjects. Therefore, drug effects were not compared with baseline, but with the placebo occasion, where fatigue and motivation are expected to play an equal role. The properties of the drug, however, may have helped subjects remain motivated throughout the very intensive study days. Randomization averted decreased motivation over consecutive treatment periods. Although 1 of the objectives of the study was to compare the effects of CEP‐26401 with those of donepezil, this objective could not be met, because donepezil did not have any measurable effects in this study. Therefore, it is impossible to deduce whether the lack of pro‐cognitive effects of CEP‐26401 is caused by a lack of effect on cholinergic neurons or by a lack of sensitivity of the tests used for pro‐cholinergic effects in young, healthy volunteers.
In conclusion, CEP‐26401 had several simulating CNS effects and induced energizing and positive feelings, with a relaxed undertone at the 5 and 25 μg doses, which disappeared at 125 μg. CEP‐26401 caused a dose‐dependent inhibition of sleep, which became symptomatic at the highest dose. It is likely that at least dopaminergic and histaminergic neurons are involved in its effects. It remains to be studied whether CEP‐26401 can have beneficial effects in clinical practice.
COMPETING INTERESTS
Co‐authors N.G., R.Y., M.F., E.H. and O.S. are employees of Research and Development Teva Pharmaceuticals. Y.G.‐S. and A.G. are a former employees of Research and Development Teva Pharmaceuticals. The other authors have no competing interests to declare.
Supporting information
Data S1. Supporting information
Data S2. Supporting information
ACKNOWLEDGEMENTS
We would like to thank Alexei Karamichev, Renate van Rijt and Esther Davidse from CHDR for their contributions to the clinical execution of the study.
Baakman AC, Zuiker R, van Gerven JMA, et al. Central nervous system effects of the histamine‐3 receptor antagonist CEP‐26401, in comparison with modafinil and donepezil, after a single dose in a cross‐over study in healthy volunteers. Br J Clin Pharmacol. 2019;85:970–985. 10.1111/bcp.13885
The authors confirm that the PI for this paper is Prof. Dr J.M.A. van Gerven and that he had direct clinical responsibility for patients.
REFERENCES
- 1. Berlin M, Boyce CW, Ruiz ML. Histamine H3 receptor as a drug discovery target. J Med Chem. 2011;54(1):26‐53. [DOI] [PubMed] [Google Scholar]
- 2. Gemkow MJ, Davenport AJ, Harich S, Ellenbroek BA, Cesura A, Hallett D. The histamine H3 receptor as a therapeutic drug target for CNS disorders. Drug Discov Today. 2009;14(9–10):509‐515. [DOI] [PubMed] [Google Scholar]
- 3. Arrang JM, Garbarg M, Schwartz JC. Auto‐inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302(5911):832‐837. [DOI] [PubMed] [Google Scholar]
- 4. Arrang JM, Garbarg M, Schwartz JC. Autoinhibition of histamine synthesis mediated by presynaptic H3‐receptors. Neuroscience. 1987;23(1):149‐157. [DOI] [PubMed] [Google Scholar]
- 5. Bergquist F, Ruthven A, Ludwig M, Dutia MB. Histaminergic and glycinergic modulation of GABA release in the vestibular nuclei of normal and labyrinthectomised rats. J Physiol. 2006;577(Pt 3):857‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dai H, Fu Q, Shen Y, et al. The histamine H3 receptor antagonist clobenpropit enhances GABA release to protect against NMDA‐induced excitotoxicity through the cAMP/protein kinase A pathway in cultured cortical neurons. Eur J Pharmacol. 2007;563(1–3):117‐123. [DOI] [PubMed] [Google Scholar]
- 7. Esbenshade TA, Browman KE, Bitner RS, Strakhova M, Cowart MD, Brioni JD. The histamine H3 receptor: an attractive target for the treatment of cognitive disorders. Br J Pharmacol. 2008;154(6):1166‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lovenberg TW, Roland BL, Wilson SJ, et al. Cloning and functional expression of the human histamine H3 receptor. Mol Pharmacol. 1999;55(6):1101‐1107. [PubMed] [Google Scholar]
- 9. Lazewska D, Kiec‐Kononowicz K. Recent advances in histamine H3 receptor antagonists/inverse agonists. Expert Opin Ther Pat. 2010;20(9):1147‐1169. [DOI] [PubMed] [Google Scholar]
- 10. Miller TR, Baranowski JL, Estvander BR, et al. A robust and high‐capacity [(35)S]GTPgammaS binding assay for determining antagonist and inverse agonist pharmacological parameters of histamine H(3) receptor ligands. Assay Drug Dev Technol. 2008;6(3):339‐349. [DOI] [PubMed] [Google Scholar]
- 11. Hancock AA, Fox GB. Perspectives on cognitive domains, H3 receptor ligands and neurological disease. Expert Opin Investig Drugs. 2004;13(10):1237‐1248. [DOI] [PubMed] [Google Scholar]
- 12. Witkin JM, Nelson DL. Selective histamine H3 receptor antagonists for treatment of cognitive deficiencies and other disorders of the central nervous system. Pharmacol Ther. 2004;103(1):1‐20. [DOI] [PubMed] [Google Scholar]
- 13. Dauvilliers Y, Bassetti C, Lammers GJ, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double‐blind, randomised trial. Lancet Neurol. 2013;12(11):1068‐1075. [DOI] [PubMed] [Google Scholar]
- 14. Kuhne S, Wijtmans M, Lim HD, Leurs R, de Esch IJ. Several down, a few to go: histamine H3 receptor ligands making the final push towards the market? Expert Opin Investig Drugs. 2011;20(12):1629‐1648. [DOI] [PubMed] [Google Scholar]
- 15. Grove RA, Harrington CM, Mahler A, et al. A randomized, double‐blind, placebo‐controlled, 16‐week study of the H3 receptor antagonist, GSK239512 as a monotherapy in subjects with mild‐to‐moderate Alzheimer's disease. Curr Alzheimer Res. 2014;11(1):47‐58. [DOI] [PubMed] [Google Scholar]
- 16. Haig GM, Bain E, Robieson W, Othman AA, Baker J, Lenz RA. A randomized trial of the efficacy and safety of the H3 antagonist ABT‐288 in cognitive impairment associated with schizophrenia. Schizophr Bull. 2014;40(6):1433‐1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haig GM, Pritchett Y, Meier A, et al. A randomized study of H3 antagonist ABT‐288 in mild‐to‐moderate Alzheimer's dementia. J Alzheimers Dis. 2014;42(3):959‐971. [DOI] [PubMed] [Google Scholar]
- 18. Becknell NC, Dandu RR, Lyons JA, Aimone LD, Raddatz R, Hudkins RL. Synthesis and evaluation of 4‐alkoxy‐[1′‐cyclobutyl‐spiro(3,4‐dihydrobenzopyran‐2,4′‐piperidine)] analogues as histamine‐3 receptor antagonists. Bioorg Med Chem Lett. 2012;22(1):186‐189. [DOI] [PubMed] [Google Scholar]
- 19. Becknell NC, Lyons JA, Aimone LD, et al. Synthesis and evaluation of pyridone‐phenoxypropyl‐R‐2‐methylpyrrolidine analogues as histamine H3 receptor antagonists. Bioorg Med Chem Lett. 2011;21(23):7076‐7080. [DOI] [PubMed] [Google Scholar]
- 20. Dandu RR, Lyons JA, Raddatz R, Huang Z, Aimone LD, Hudkins RL. Synthesis and evaluation of a new series of 1′‐cyclobutyl‐6‐(4‐piperidyloxy)spiro[benzopyran‐2,4′‐piperidine] derivatives as high affinity and selective histamine‐3 receptor (H3R) antagonists. Bioorg Med Chem Lett. 2012;22(6):2151‐2153. [DOI] [PubMed] [Google Scholar]
- 21. Hudkins RL, Becknell NC, Lyons JA, et al. 3,4‐Diaza‐bicyclo[4.1.0]hept‐4‐en‐2‐one phenoxypropylamine analogs of irdabisant (CEP‐26401) as potent histamine‐3 receptor inverse agonists with robust wake‐promoting activity. Eur J Med Chem. 2015;95:349‐356. [DOI] [PubMed] [Google Scholar]
- 22. Knutsen LJS, Aimone LD, Bacon ER, et al. 3,6‐Disubstituted pyridazines as novel analogues of CEP‐26401: CNS penetrant histamine H3 receptor antagonists. Bioorg Med Chem Lett. 2014. (submitted for publication) [Google Scholar]
- 23. Spiegelstein O, Stevens JS, van Gerven JMA, et al. Safety, pharmacokinetics, and pharmacodynamics of CEP‐26401, a high‐affinity histamine‐3 receptor antagonist, following single and multiple dosing in healthy subjects. J Psychopharmacol. 2016;30(10):983‐993. [DOI] [PubMed] [Google Scholar]
- 24. Alexander SP, Kelly E, Marrion N, et al. The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol. 2015;172(24):5729‐5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fitzpatrick TB. The validity and practicality of sun‐reactive skin types I through VI. Arch Dermatol. 1988;124(6):869‐871. [DOI] [PubMed] [Google Scholar]
- 26. Gerrard P, Malcolm R. Mechanisms of modafinil: A review of current research. Neuropsychiatr Dis Treat. 2007;3(3):349‐364. [PMC free article] [PubMed] [Google Scholar]
- 27. Muller U, Rowe JB, Rittman T, Lewis C, Robbins TW, Sahakian BJ. Effects of modafinil on non‐verbal cognition, task enjoyment and creative thinking in healthy volunteers. Neuropharmacology. 2013;64:490‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology (Berl). 2003;165(3):260‐269. [DOI] [PubMed] [Google Scholar]
- 29. Burns A, Rossor M, Hecker J, et al. The effects of donepezil in Alzheimer's disease ‐ results from a multinational trial. Dement Geriatr Cogn Disord. 1999;10(3):237‐244. [DOI] [PubMed] [Google Scholar]
- 30. Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24‐week, double‐blind, placebo‐controlled trial of donepezil in patients with Alzheimer's disease. Donepezil Study Grp Neurol. 1998;50(1):136‐145. [DOI] [PubMed] [Google Scholar]
- 31. Zaninotto AL, Bueno OF, Pradella‐Hallinan M, et al. Acute cognitive effects of donepezil in young, healthy volunteers. Hum Psychopharmacol. 2009;24(6):453‐464. [DOI] [PubMed] [Google Scholar]
- 32. Pietrzak RH, Maruff P, Snyder PJ. Methodological improvements in quantifying cognitive change in clinical trials: an example with single‐dose administration of donepezil. J Nutr Health Aging. 2009;13(3):268‐273. [DOI] [PubMed] [Google Scholar]
- 33. Robbins TW, James M, Owen AM, et al. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. J Int Neuropsychol Soc. 1998;4(5):474‐490. [DOI] [PubMed] [Google Scholar]
- 34. Neale C, Johnston P, Hughes M, Scholey A. Functional Activation during the Rapid Visual Information Processing Task in a Middle Aged Cohort: An fMRI Study. PLoS One. 2015;10(10):e0138994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Haas SL, Franson KL, Schmitt JA, et al. The pharmacokinetic and pharmacodynamic effects of SL65.1498, a GABA‐A alpha2,3 selective agonist, in comparison with lorazepam in healthy volunteers. J Psychopharmacol. 2009;23(6):625‐632. [DOI] [PubMed] [Google Scholar]
- 36. Milner B. Visually‐guided maze learning in man: effects of bilateral hippocampal, bilateral frontal, and unilateral cerebral lesions. Neuropsychologia. 1965;3(4):317‐338. [Google Scholar]
- 37. Lim HK, Juh R, Pae CU, et al. Altered verbal working memory process in patients with Alzheimer's disease: an fMRI investigation. Neuropsychobiology. 2008;57(4):181‐187. [DOI] [PubMed] [Google Scholar]
- 38. Rombouts SA, Barkhof F, Van Meel CS, Scheltens P. Alterations in brain activation during cholinergic enhancement with rivastigmine in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2002;73(6):665‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sweet LH, Rao SM, Primeau M, Durgerian S, Cohen RA. Functional magnetic resonance imaging response to increased verbal working memory demands among patients with multiple sclerosis. Hum Brain Mapp. 2006;27(1):28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643‐662. [Google Scholar]
- 41. Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47(3):211‐218. [Google Scholar]
- 42. Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy‐Byrne PP. Psychedelic effects of ketamine in healthy volunteers: relationship to steady‐state plasma concentrations. Anesthesiology. 1998;88(1):82‐88. [DOI] [PubMed] [Google Scholar]
- 43. Borland RG, Nicholson AN. Comparison of the residual effects of two benzodiazepines (nitrazepam and flurazepam hydrochloride) and pentobarbitone sodium on human performance. Br J Clin Pharmacol. 1975;2(1):9‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Borland RG, Nicholson AN. Visual motor co‐ordination and dynamic visual acuity. Br J Clin Pharmacol. 1984;18(Suppl 1):69S‐72S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gijsman HJ, van Gerven JM, Tieleman MC, et al. Pharmacokinetic and pharmacodynamic profile of oral and intravenous meta‐chlorophenylpiperazine in healthy volunteers. J Clin Psychopharmacol. 1998;18(4):289‐295. [DOI] [PubMed] [Google Scholar]
- 46. van Steveninck AL, Gieschke R, Schoemaker HC, et al. Pharmacodynamic interactions of diazepam and intravenous alcohol at pseudo steady state. Psychopharmacology (Berl). 1993;110(4):471‐478. [DOI] [PubMed] [Google Scholar]
- 47. van Steveninck AL, Schoemaker HC, Pieters MS, Kroon R, Breimer DD, Cohen AF. A comparison of the sensitivities of adaptive tracking, eye movement analysis and visual analog lines to the effects of incremental doses of temazepam in healthy volunteers. Clin Pharmacol Ther. 1991;50(2):172‐180. [DOI] [PubMed] [Google Scholar]
- 48. van Steveninck AL, van Berckel BN, Schoemaker RC, Breimer DD, van Gerven JM, Cohen AF. The sensitivity of pharmacodynamic tests for the central nervous system effects of drugs on the effects of sleep deprivation. J Psychopharmacol. 1999;13(1):10‐17. [DOI] [PubMed] [Google Scholar]
- 49. Baloh RW, Sills AW, Kumley WE, Honrubia V. Quantitative measurement of saccade amplitude, duration, and velocity. Neurology. 1975;25(11):1065‐1070. [DOI] [PubMed] [Google Scholar]
- 50. Bittencourt PR, Wade P, Smith AT, Richens A. Benzodiazepines impair smooth pursuit eye movements. Br J Clin Pharmacol. 1983;15(2):259‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wright BM. A simple mechanical ataxia‐meter. J Physiol. 1971;218(Suppl):27P‐28P. [PubMed] [Google Scholar]
- 52. Cohen AF, Ashby L, Crowley D, Land G, Peck AW, Miller AA. Lamotrigine (BW430C), a potential anticonvulsant. Effects on the central nervous system in comparison with phenytoin and diazepam. Br J Clin Pharmacol. 1985;20(6):619‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van Steveninck AL, Mandema JW, Tuk B, et al. A comparison of the concentration‐effect relationships of midazolam for EEG‐derived parameters and saccadic peak velocity. Br J Clin Pharmacol. 1993;36(2):109‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wauquier A. Aging and changes in phasic events during sleep. Physiol Behav. 1993;54(4):803‐806. [DOI] [PubMed] [Google Scholar]
- 55. Silber MH, Ancoli‐Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3(2):121‐131. [PubMed] [Google Scholar]
- 56. Tarrasch R, Laudon M, Zisapel N. Cross‐cultural validation of the Leeds sleep evaluation questionnaire (LSEQ) in insomnia patients. Hum Psychopharmacol. 2003;18(8):603‐610. [DOI] [PubMed] [Google Scholar]
- 57. Zisapel N, Laudon M. Subjective assessment of the effects of CNS‐active drugs on sleep by the Leeds sleep evaluation questionnaire: a review. Hum Psychopharmacol. 2003;18(1):1‐20. [DOI] [PubMed] [Google Scholar]
- 58. Althouse AD. Adjust for multiple comparisons? It's not that simple. Ann Thorac Surg. 2016;101(5):1644‐1645. [DOI] [PubMed] [Google Scholar]
- 59. Wason JM, Stecher L, Mander AP. Correcting for multiple‐testing in multi‐arm trials: is it necessary and is it done? Trials. 2014;15(1):364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research. 2018;46(D1):D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Alexander SPH, Christopoulos A, Davenport AP, et al. The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology. 2017;174(Suppl 1):S17‐S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Baakman AC, t Hart E, Kay DG, et al. First in human study with a prodrug of galantamine: improved benefit‐risk ratio? Alzheimers Dement (NY). 2016;2(1):13‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011;221(2):555‐563. [DOI] [PubMed] [Google Scholar]
- 64. Nathan PJ, Boardley R, Scott N, et al. The safety, tolerability, pharmacokinetics and cognitive effects of GSK239512, a selective histamine H(3) receptor antagonist in patients with mild to moderate Alzheimer's disease: a preliminary investigation. Curr Alzheimer Res. 2013;10(3):240‐251. [DOI] [PubMed] [Google Scholar]
- 65. Egan F, Zhao X, Gottwald R, et al. Randomized crossover study of the histamine H3 inverse agonist MK‐0249 for the treatment of cognitive impairment in patients with schizophrenia. Schizophr Res. 2013;146(1–3):224‐230. [DOI] [PubMed] [Google Scholar]
- 66. Huang M, Marino MJ, Li Z, Felix AR, Meltzer HY. (Eds.). Histamine 3 receptor antagonists increase dopamine and acetylcholine efflux in rat prefrontal cortex and nucleus accumbens. Neuroscience. 2011. 2011 11/16/2011; Washington DC2011 [Google Scholar]
- 67. Mehta M, Whyte E, Lenze E, et al. Depressive symptoms in late life: associations with apathy, resilience and disability vary between young‐old and old‐old. Int J Geriatr Psychiatry. 2008;23(3):238‐243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information
Data S2. Supporting information


