Abstract
Aims
Reduced nitric oxide (NO) availability may adversely affect renal perfusion and glomerular filtration. The aim of the present study was to characterize in detail the pharmacological effects of VAS203, an inhibitor of NO synthase, on renal haemodynamics in humans.
Methods
This double‐blind, randomized, placebo‐controlled, cross‐over phase‐I‐study comprised 18 healthy men. Renal haemodynamics were assessed with constant‐infusion input‐clearance technique with p‐aminohippurate and inulin for renal plasma flow (RPF) and glomerular filtration rate (GFR), respectively. After baseline measurement, a constant infusion of the tetrahydrobiopterin analogue ronopterin (VAS203, total 10 mg/kg body weight) or placebo was administered at random order for 6 hours additionally. After a wash‐out phase of 28 days, the second course was applied. In parallel, markers of early kidney injury and renal function were assessed repeatedly up to 48 hours after starting VAS203/placebo‐infusion.
Results
VAS203‐infusion resulted in a significant decrease of RPF (P < .0001) and GFR (P < .001) compared to placebo, but magnitude was within the physiological range. RPF and GFR recovered partly 2 hours after end of VAS203‐infusion and was normal at beginning of the second infusion period. Compared to placebo, preglomerular resistance (P < .0001), and to lesser extent postglomerular resistance (P < .0001) increased, resulting in a decrease of intraglomerular pressure (P < .01). No treatment related effect on markers of early kidney injury, and on renal function (P for all >.20) have been observed.
Conclusions
Our phase‐I‐study in healthy humans indicates that VAS203 (10 mg/kg body weight) reduces renal perfusion and glomerular function within the physiological range mainly due to vasoconstriction at the preglomerular site.
Keywords: glomerular filtration rate, nitric oxide synthase, renal haemodynamics, renal plasma flow, VAS203
What is already known about this subject
Although increased nitric oxide plays a crucial role in many pathophysiological processes such as secondary brain damage due to traumatic brain injury or stroke, nitric oxide synthase inhibitors are not in clinical use because of cardiovascular and renal effects.
Pterin analogues may be an alternative. First evidence indicates that the tetrahydrobiopterin analogue ronopterin (VAS203) seems to be a promising tool in reducing secondary damage in traumatic brain injury, but VAS203 also caused a dose‐dependent acute kidney injury.
What this study adds
VAS203‐infusion (10 mg/kg body weight) reduces renal perfusion and glomerular function within the physiological range due to vasoconstriction at the preglomerular site, and these renal haemodynamic changes are reversible, indicating a pharmacodynamic effect.
VAS203 shows no systemic effects on cardiovascular parameters. No treatment related effects on markers of renal function and tubular toxicity are observed at the dose administered.
1. INTRODUCTION
Nitric oxide (NO) plays a crucial role in secondary brain damage due to traumatic brain injury (TBI).1 Although activity of both the neuronal NO synthase (n[NOS]) and endothelial (e)NOS decreases within a short time after TBI, inducible (i)NOS expression and activity being undetectable under physiological conditions in brain tissue is enhanced resulting in an increment of tissue NO.1, 2 In accordance, it was shown that iNOS inhibition is neuroprotective in TBI.3, 4, 5, 6
Since NOS activity depends on the essential cofactor tetrahydrobiopterin, pterin‐binding site antagonists (antipterins) have been developed as NOS inhibitors. One of the most potent is 2‐amino‐5,6,7,8‐tetrahydrobiopterin (VAS203, ronopterin).7 In the presence of competing tetrahydrobiopterin, VAS203 decreases NOS activity to a basal level (20–40% of the fully activated level) and NO production by constitutively expressed eNOS and nNOS is not completely suppressed.8, 9 In contrast, in the case of de novo synthesized iNOS, the cofactor antagonist is incorporated directly into the high affinity cofactor binding site, resulting in more effective in vivo inhibition of iNOS.7, 9, 10 These properties of antipterins suggest that VAS203 might be more suitable as a therapeutic agent.
In an experimental animal model of TBI, VAS203 application led to an improved neurological outcome after experimental TBI.11 In accordance, an explorative phase IIa (the NOSynthase inhibition in TRAumatic brain injury [NOSTRA]) trial comprising 32 patients with moderate and severe TBI revealed an improved functional outcome in VAS203‐treated patients.12
In both the first‐in‐man study and the NOSTRA trial, VAS203 did not cause major safety hepatic, haematological or cardiac toxic effects, but led to acute kidney injury (AKI) in a dose‐dependent manner in few subjects.12
This finding has not been foreseen from animal studies. The potential benefit of VAS203 is related to its selective inhibition of newly synthesized iNOS in TBI, but may also inhibit iNOS in other organs such as the kidney. In renal tissue constitutional nNOS, eNOS and also iNOS are expressed in the healthy state and thus the kidneys are most vulnerable to iNOS inhibition.13
The aim of this phase‐I‐study was to characterize in detail the pharmacological effects of VAS203 on renal haemodynamics in healthy individuals.
2. METHODS
2.1. Study cohort and design
This randomized, double‐blind, cross‐over, single‐centre study was conducted under the legal sponsorship of the Medical Faculty of Friedrich‐Alexander University Erlangen‐Nürnberg, Germany, between August 2015 and May 2016. The study was planned for 16 healthy male participants, who were recruited in the area of Erlangen–Nürnberg, Germany; eligible subjects were enrolled consecutively. Main inclusion criteria were male aged between 18 and 45 years with a body weight between 60 and 100 kg. Main exclusion criteria were a Cockcroft–Gault creatinine clearance <90 mL/min, and any clinically significant diseases. Written informed consent was obtained prior to study inclusion. Participants underwent a screening phase 3–7 days before start of treatment to ensure that they were healthy. Participants were randomized pairwise into 2 groups receiving first VAS203 and placebo, respectively. During the treatment phase, the subjects were hospitalized in the study centre for 2 days (1 night) for each infusion period. In addition, renal function was determined 48 hours after start of infusion. After a 21–35‐day wash‐out period (based on the pharmacokinetic profile of VA203), participants returned for second treatment phase period (cross‐over).
It was prespecified, that after the first pair (i.e. 1 VAS203 and 1 placebo) had received the first infusion and has shown no signs of AKI for 48 hours after start of infusion, the second infusion period can be started after fulfilling the respective wash‐out period. In addition to this first blinded safety interim analysis as well as after conductance of 6 full cross‐over subjects (with both infusion periods), a second interim blinded safety analysis and report was communicated to the legal German authority (Bundesinstitut für Arzneimittel und Medizinprodukte).
Blinded study medication (intravenous infusions) were provided by the local Pharmacy of the University Hospital Erlangen. The study protocol was approved by the Local Ethics Committee (University of Erlangen–Nürnberg) and the study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice guidelines. The study was registered at www.clinicaltrials.gov (ID: NCT02992236).
The financial supporter Vasopharm GmbH, Würzburg, Germany, did not contribute to study conduction or data collection.
2.2. Assessment of renal haemodynamics
Renal haemodynamics were measured using the constant‐infusion input–clearance technique with sodium p‐aminohippurate (PAH; Clinalfa, Basel, Switzerland) and inulin (Inutest, Fresenius, Linz, Austria) for renal plasma flow (RPF) and glomerular filtration rate (GFR), respectively (www.crc‐erlangen.de).14, 15 In brief, after bolus infusion of PAH and inulin over 15 minutes and a subsequent constant infusion over 105 minutes, a steady state between input and renal excretion of the tracer substances is achieved. Duplicate blood samples were collected for the assessment of RPF and GFR. Then VAS203 (10 mg/kg body weight) or placebo (saline) infusion was administered intravenously for 6 hours. Again, blood samples for PAH/inulin concentration measurements were collected 2, 4, 6 and 8 hours after start of VAS203 or placebo infusion. The total duration of PAH/inulin infusion was 10 hours (Figure 1).
Figure 1.
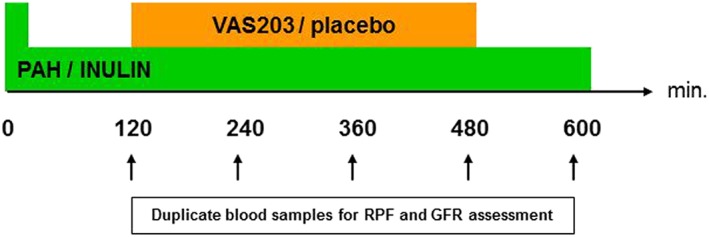
Infusion scheme
PAH was measured according to previously described methods.16 Inulin was measured indirectly by converting inulin to fructose and subsequently measuring fructose by an enzymatic method (r‐biopharm, Darmstadt, Germany). Each blood sample was measured in duplicate with a coefficient variation of <5%.
2.3. Calculation of intraglomerular haemodynamics
Intraglomerular pressure (Pglom) and resistances of the afferent (RA; i.e. preglomerular) and efferent (RE; postglomerular) arterioles were calculated according the model originally established by Gomez,17 which has been discussed by Guidi et al18 and repeatedly applied in previous studies.19, 20
2.4. Online renal safety monitoring
Blood sampling for bedside neutrophil gelatinase‐associated lipocalin (NGAL) determination (Alere Triage NGAL Panel, Köln, Germany) were done 2 hours before, at start, and 2, 4, 6, 8, 10, 24 ± 2 and 48 ± 2 hours after start of infusion of VAS203 or placebo (saline), respectively, and the results were obtained and judged within 15 minutes.
In addition, blood samples for determination of creatinine, urea and cystatin C were collected, immediately transferred to the lab and results were evaluated within 1 hour. The Cockcroft–Gault creatinine clearance for the respective time points was additionally calculated.
2.5. Measurement of renal and tubular markers
At the prespecified time, urine samples were collected and α‐1‐microglobulin, albumin and creatinine were measured centrally at the accredited biochemistry laboratory of the University of Erlangen‐Nürnberg. In addition, NGAL and kidney injury molecule‐1 (KIM‐1) were determined using experimental individual enzyme‐linked immunosorbent assays (R&D Systems Europe, Ltd., Abingdon, UK). Inhibitor of metalloproteinase‐2 (TIMP‐2) and insulin‐like growth factor‐binding protein 7 were determined using a validated sandwich immunoassay (NephroCheck Test; Astute Medical Inc., San Diego, CA, USA).
2.6. Systemic haemodynamics
Brachial blood pressure (BP), representing the indirect arterial BP through the brachial artery, was initially measured in both arms after 5 minutes of rest in a sitting position with an oscillometric device (Dinamap Pro100V2 [Criticon, Norderstedt, Germany] with a printer for documentation) following the current guideline recommendations.21 Subsequent BP measurements were performed on the arm with the higher BP readings, and an average of 3 measurements was taken at the prespecified time points.
2.7. Statistical analyses
The primary objective, RPF at the end of the 6‐hour infusion period between placebo and VAS203, was tested using a paired t test as a linear contrast within a general linear model (GLM) with factors subject, treatment and study period. Because of the long wash‐out period after treatment period 1 relative to the kinetic half‐life of the drug, it was assumed that no unequal carry‐over effects would be present for the parameter RPF. To adjust for possible substantial deviations from the normality assumption Hodges–Lehmann estimates for the treatment effect based on combined data from both periods were calculated. The main difference compared to renal flow is that a potential maximum treatment effect may occur not necessarily after 6 hours after start of infusion but at any time during the infusion period. Therefore, for each subject, each period and each parameter the potential treatment effect was quantified by calculating the area under the curve (AUC). In addition, a recovery was expected at latest towards the end of the observation period; this expectation was quantified by taking the last value of each parameter in each observation period of each patient (i.e. at 8 hours after start of infusion). Accordingly, the other parameters were analysed.
Reported data are based on intention‐to‐treat analysis, comprising all randomized patients for this randomized, double‐blind, cross‐over single‐centre study.
The sample size was calculated for the difference of the RPF between placebo and VAS203 at the end of the 6‐hour infusion period (primary objective). Based on previous studies with NOS inhibitors,22, 23 the standard deviation of RPF was estimated to be 65 mL/min. To show a true difference of RPF between VAS203 and placebo of 50 mL/min (effect size), for α = 0.05 and β = 0.80, the required number of patients was calculated to be 16.
2.8. Nomenclature of target and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,24 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.25
3. RESULTS
In total, 21 subjects entered this cross‐sectional study and 18 subjects were randomized (since based on a pharmacy error 1 subject received the same treatment [placebo] in both study periods; therefore, it was decided to randomize an additional pair of subjects to obtain data from at least 16 subjects who had received cross‐over treatment). The duration of the entire study period was 268 days (August 2015–May 2016). Subjects were aged 31.2 ± 7.9 years, with a normal body mass index (22.9 ± 2.2 kg/m2).
3.1. Renal haemodynamics
A significant decrement of RPF due to VAS203‐infusion was observed at the end of the 6 hours infusion period compared to placebo (541.0 ± 85 mL/min vs 663.3 ± 125 mL/min, P < .0001; Figure 2). The GLM model yielded that the estimated treatment effect between placebo and VAS203 was 122.3 mL/min (95% confidence interval [CI]: 70.3 to 174.2 mL/min). Moreover, the model suggested that there was no different treatment effect on RPF values between study period 1 and study period 2 (−2.5 mL/min [95% CI: –52.5 to 47.6 mL/min]; P = .9231).
Figure 2.
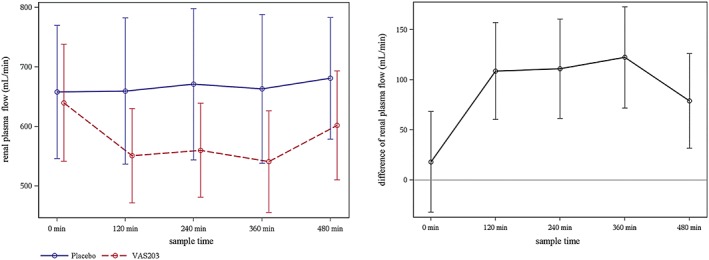
Left: Course of renal plasma flow (mean ± standard deviation) during infusion period. Right: Difference of renal plasma flow (mean ± standard error of the mean) during infusion period
In detail, mean values of RPF remained on a stable level during the entire infusion period of placebo whilst mean RPF values declined after initiating infusion with VAS203, stagnated until end of VAS203 infusion (6 hours) and had started to recover after 8 hours (i.e., 2 hours after stopping VAS203 infusion; Figure 2). In accordance, quantification of the treatment effect by calculation of AUC relative to the corresponding predose level revealed that there was also a significant treatment effect on RPF between active VAS203 and placebo when the entire infusion period was considered (−572.9 ± 406 mL/min vs 35.7 ± 353 mL/min, P < .001).
In order to adjust for possible substantial deviations from the normality assumption, Hodges–Lehmann estimates for the treatment effect based on combined data from both periods were calculated, revealing similar results (data not shown).
With respect to difference in GFR after 6 hours of VAS203 and placebo infusion, the GLM model estimated a significant treatment effect, i.e. a higher GFR level in favour of placebo (11.8 mL/min [95% CI: 5. to 18.1 mL/min], P = .0003, Figure 3). Again, there was no treatment effect detected on GFR levels regarding treatment sequence (i.e. between treatment in study period 1 and treatment in study period 2; −0.7 mL/min [95% CI: –7.0 to 5.7 mL/min] P = .8350).
Figure 3.
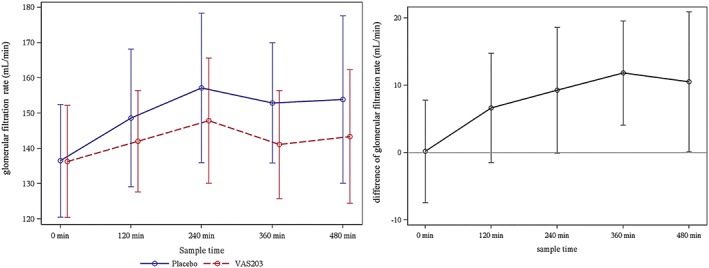
Left: Course of glomerular filtration rate (mean ± standard deviation) during infusion period. Right: Difference of glomerular filtration rate (mean ± standard error of the mean) during infusion period
3.2. Intraglomerular haemodynamics
Detailed data on intraglomerular haemodynamics are given in Table 1. Compared to placebo, there was an increment of RA (GLM model: −952.7 dyn s−1 cm−5 [95% CI: ‐1247.7 to −657.7 dyn s−1 cm−5], P < .0001), and to a lesser extent of RE (GLM model: −423.2 dyn s−1 cm−5 [95% CI: ‐633.7 to −212.7 dyn s−1 cm−5], P < .0001) after 6 hours of VAS203 infusion. Both the observed potential treatment effect of VAS203 on RA and RE was still present after 8 hours, i.e. 2 hours after stopping the infusion, but had started to recover.
Table 1.
Intraglomerular haemodynamics
| R A (dyn/s/cm 5 ) | R E (dyn/s/cm 5 ) | P glom (mmHg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time point | VAS203 | Placebo | P‐value | VAS203 | Placebo | P‐value | VAS203 | Placebo | P‐value |
| 0 min | 1809 ± 572 | 1822 ± 576 | .945 | 2265 ± 329 | 2157 ± 264 | .292 | 64.2 ± 3.2 | 64.2 ± 3.0 | .984 |
| 120 min | 2290 ± 680 | 1541 ± 507 | <.001 | 2820 ± 390 | 2414 ± 544 | .016 | 65.7 ± 2.9 | 66.7 ± 4.4 | .412 |
| 240 min | 2175 ± 676 | 1528 ± 616 | .006 | 2899 ± 412 | 2507 ± 494 | .015 | 65.8 ± 3.3 | 67.8 ± 4.7 | .160 |
| 360 min | 2584 ± 829 | 1631 ± 664 | <.001 | 2885 ± 503 | 2464 ± 450 | .014 | 64.6 ± 3.7 | 66.8 ± 4.2 | .084 |
| 480 min | 2163 ± 732 | 1565 ± 564 | .017 | 2573 ± 381 | 2368 ± 406 | .139 | 64.5 ± 3.7 | 67.1 ± 5.4 | .110 |
Pglom, intraglomerular pressure; RA, resistance of the afferent arterioles; RE, resistance of the efferent arterioles.
In addition, no treatment effect with regard to study sequence was detected (RA P = .8277 and RE P = .4109).
Based on the different amount of increment of RA and RE, the GLM model revealed that Pglom was significantly lower after VAS203 infusion (P < .0091). Again, the treatment effect still persisted after 6 hours, and there was no treatment effect with regard to study sequence was detected (P = .7314).
3.3. Online renal safety monitoring
Bedside NGAL and other laboratory markers of renal function determined at prespecified time points from −2 h before and up to 48 ± 2 h after infusion period are detailed outlined in Table 2.
Table 2.
Laboratory parameters of renal function
| NGAL (μg/mL) | Creatinine (mg/dL) | Cystatin C (mg/dL) | Urea (mg/dL) | |||||
|---|---|---|---|---|---|---|---|---|
| Time point | VAS203 | Placebo | VAS203 | Placebo | VAS203 | Placebo | VAS203 | Placebo |
| −120 min | 69.9 ± 17 | 61.8 ± 16 | 0.89 ± 0.1 | 0.91 ± 0.1 | 0.81 ± 0.1 | 0.82 ± 0.1 | 30.6 ± 8.6 | 30.7 ± 4.6 |
| 0 min | 66.7 ± 20 | 64.2 ± 20 | 0.84 ± 0.1 | 0.86 ± 0.1 | 0.77 ± 0.1 | 0.77 ± 0.1 | 29.4 ± 7.4 | 30.1 ± 3.9 |
| 120 min | 68.1 ± 18 | 66.5 ± 19 | 0.84 ± 0.1 | 0.84 ± 0.1 | 0.77 ± 0.1 | 0.76 ± 0.1 | 29.8 ± 7.1 | 30.9 ± 4.2 |
| 240 min | 63.1 ± 18 | 66.6 ± 18 | 0.94 ± 0.2 | 0.94 ± 0.1 | 0.76 ± 0.1 | 0.74 ± 0.1 | 30.6 ± 6.8 | 31.1 ± 4.3 |
| 360 min | 68.1 ± 20 | 65.6 ± 16 | 0.94 ± 0.1 | 0.91 ± 0.1 | 0.77 ± 0.1 | 0.75 ± 0.1 | 30.8 ± 6.2 | 30.6 ± 4.3 |
| 480 min | 63.4 ± 9.8 | 68.3 ± 18 | 0.92 ± 0.1 | 0.89 ± 0.1 | 0.77 ± 0.1 | 0.75 ± 0.1 | 30.9 ± 6.1 | 30.2 ± 4.4 |
| 600 min | 67.4 ± 16 | 72.1 ± 21 | 0.93 ± 0.1 | 0.91 ± 0.1 | 0.80 ± 0.1 | 0.79 ± 0.1 | 31.3 ± 5.6 | 31.0 ± 4.3 |
| 24 ± 2 h | 70.1 ± 21 | 66.1 ± 21 | 0.92 ± 0.1 | 0.89 ± 0.1 | 0.83 ± 0.1 | 0.83 ± 0.1 | 26.4 ± 4.4 | 27.4 ± 4.2 |
| 48 ± 2 h | 70.8 ± 17 | 69.8 ± 24 | 0.91 ± 0.1 | 0.89 ± 0.1 | 0.81 ± 0.1 | 0.80 ± 0.1 | 29.6 ± 7.5 | 30.1 ± 5.1 |
NGAL, neutrophil gelatinase‐associated lipocalin.
There was no increase of online bedside serum NGAL levels above the normal limit of the test (≥149 μg/l) in either group.
For serum creatinine, with the exception of 1 subject (ID 1) who experienced a minor increase in serum creatinine from 1.13 mg (prior infusion) to 1.27 mg/dL 10 hours after start of VAS203 infusion, serum creatinine was in the normal range (<1.20 mg/dL) in all subjects at any sampling time point regardless of treatment received. In addition, no increment of serum creatinine by >50% from baseline was documented and serum creatinine increased by 0.31 mg/dL (from 0.88 mg/dL to 1.19 mg/dL) only in 1 subject (ID 2) in the placebo phase. However, for this patient, the cystatin C value was within normal range (0.68 mg/dL) at this sampling time point. For cystatin C, values were within normal ranges in all subjects at any sampling time point, with the exception of 1 subject (ID 1) who presented with a minimally elevated cystatin C value of 1.31 mg/dL at visit 5 (screening prior to second infusion period). The subject was discontinued before having received the second infusion due to elevated liver parameters (later on antibody testing established the diagnosis of CMV‐infection).
For urea, no increase by >100% from baseline occurred in either treatment group, Urea levels fluctuated in some individual subjects but were within normal ranges in almost all subjects at any sampling time point regardless of treatment received.
3.4. Renal and tubular markers
Urine markers of tubular function (α1‐microglobulin, albumin, NGAL, KIM‐1 and TIMP‐2) were comparable for active VAS203 and placebo treatment and no relevant changes from baseline were seen within 48 ± 2 h after infusion period in either treatment group. Some minor isolated fluctuations were observed in individual VAS203 treated subjects, mainly at 24 or 48 hours following infusion, but were also found under placebo (data not shown).
3.5. Systemic haemodynamics
No relevant increment in mean brachial BP was detected under VAS203 treatment. In addition, the AUC analysis of brachial BP resulted in similar mean ± standard deviation values for VAS203 and placebo (systolic: 408.3 ± 58.1 vs 371.5 ± 64.9, P = .101; diastolic: 35.7 ± 41.8 vs 214.1 ± 27.8, P = .096).
3.6. Adverse events
In total, 18 adverse events (AE) in 9 subjects during the VAS203 treatment phase and 3 AEs in 3 subjects during the placebo treatment occurred in the intention‐to‐treat population. The most common nonserious AE was headache (5 subjects in VAS203 period and 1 subject in placebo period). All other nonserious AEs were reported once and occurred in single subjects only. All AEs completely resolved without sequela. No serious AE occurred.
4. DISCUSSION
Compared to placebo, intravenous infusion of VAS203 (10 mg/kg body weight) resulted in a statistically significant reduction of RPF (primary objective) with stagnation over the 6‐hour infusion period. Notably, 2 hours after stopping VAS203 infusion RPF had started to recover.
From a subject‐specific point of view, the observed changes (approximately 20%) are of low clinical relevance as changes in the same order of magnitude can also be observed in normal physiological reactions. In an animal model, it was shown that diets high in protein or sucrose resulted in a reduction of effective RPF compared to control chow. Even in apparently healthy participants protein loading by both chicken and beef meals resulted in significant changes of RPF.26 Moreover, also a physiological condition, namely the menstrual cycle, resulted in changes of RPF and GFR.27, 28 Therefore, it has to be assumed that the observed intra‐individual affection of RPF and GFR were clinically not significant and are even evident in normal life.
In more detail, no severe reduction of RPF (≥50%), prespecified as a criterion for study termination, has been occurred.
It is noteworthy to mention, that beside the gold‐standard, namely constant‐infusion input–clearance technique, additional parameters of renal function and biomarkers of AKI were assessed. It was demonstrated that specific markers of kidney damage (e.g. NGAL, KIM‐1) may be elevated prior increment of serum creatinine,29 but also delineate the site of injury based on their specificity.30 In addition, combination of laboratory assessed parameters of renal function (e.g. serum creatinine, cystatin C) with markers of renal damage (as outlined above) may allow the delineation of AKI more precisely.30 Among others, requirements for a clinically applicable biomarker include noninvasive assessment at bedside, or standard clinical laboratory. Therefore, performance (and availability of the result within 15 minutes) of bedside NGAL was implemented in our phase‐I‐study. Utility and accuracy for this point‐of‐care kit was repeatedly shown.31, 32, 33
Bedside and laboratory tests (online bedside serum NGAL, serum creatinine, serum urea and serum cystatin C) did not show any relevant changes from baseline within 48 hours of VAS203‐infusion in either treatment group. Notably, prespecified criteria (increment of serum creatinine ≥50%, urea ≥100% or online bedside NGAL above the normal limit of the test) for premature termination of infusion were not noted in any subject irrespective of treatment period.
In accordance, tubular markers (urine NGAL, α1‐microglobulin, albumin and urine KIM‐1, TIMP‐2) did not show any changes from baseline through the infusion period in either treatment group (VAS203 or placebo). Some minor isolated fluctuations were observed in both treatment periods, mainly at 24 or 48 hours following infusion. There might have been a potential influence of the drinking volume on renal parameters, in particular on urine parameters. Intake of drinking volume was controlled during the infusion period (up to 10 hours), but not through the following time up to 48 hours.
From a pathophysiological point of view, alterations of RPF and GFR seem to be not caused by toxic effects to glomerulus or tubulus, but driven by changes in intraglomerular haemodynamics. By using the Gomez formulas, we observed that VAS203‐infusion mainly increased RA (i.e. resistance at preglomerular site), and to a lesser extent RE (resistance at postglomerular site). Based on this different increment of vasoconstriction, i.e. numerically pronounced increased of RA compared to RE, Pglom was reduced. No meaningful changes were observed during placebo‐infusion. It is worthwhile to mention that no systemic effect on BP of VAS203‐infusion was detected. This implies that the observed intrarenal and global changes are not altered due to concomitant changes in the systemic circulation.
Several limitations of our phase‐I‐study have to be mentioned. Renal haemodynamics in healthy individuals, without having evidence of renal abnormalities, may be less prone to the selective inhibition of the renal constitutional iNOS. Decrement of renal perfusion may have clinical consequences in more vulnerable patients (e.g. volume disturbance and/or impaired cardiac function). Severely ill patients (i.e. TBI) may also have BP changes, that may affect renal perfusion and intraglomerular haemodynamics. Our data cannot be extrapolated to repeated infusion protocols or prolonged infusion applications of VAS203. Moreover, overweight and hence variations in volume of distribution may affect pharmacokinetics of VAS203. Further studies are warranted and, indeed, the European placebo‐controlled, randomized, double‐blind, multicentre NOSTRA III trial (NCT02794168) examines VAS203 (vs placebo) in 220 evaluable patients with sustained acute brain injury, given in addition to best standard of care and under close monitoring of renal function.
In conclusion, our phase‐I‐study in humans indicate a possible mild, transient pharmacodynamic effect of VAS203 (10 mg/kg body weight) on renal perfusion and glomerular function due to vasoconstriction at the preglomerular site. However, the individual affection were clinically not significant and within physiological variation. Moreover, there were no treatment‐related trends seen in the analysis of renal safety parameters or markers of tubular function.
COMPETING INTERESTS
R.E.S. has received advisory honorarium from Vasopharm GmbH and is a DSMP member of the NOSTRA III trial. R.S. and F.T. are employees of Vasopharm GmbH. C.O., A.B. and S.F. have nothing to disclose.
The present work was performed in fulfilment of the requirements for obtaining the degree Dr. med. for N.W.
ACKNOWLEDGEMENTS
We gratefully acknowledge the expert technical assistance of Ortrun Alter, Dorothea Bader‐Schmieder, Ingrid Fleischmann, Kerstin Fröhlich‐Endreß, Ulrike Heinritz, Susanne Muck, Simone Pejkovic and Laura Waldmann.
The study was funded by Vasopharm GmbH.
Ott C, Bosch A, Winzer N, et al. Effects of the nitric oxide synthase inhibitor ronopterin (VAS203) on renal function in healthy volunteers. Br J Clin Pharmacol. 2019;85:900–907. 10.1111/bcp.13870
The authors confirm that the PI for this paper is Roland E. Schmieder and that he had direct clinical responsibility for patients.
REFERENCES
- 1. Cherian L, Hlatky R, Robertson CS. Nitric oxide in traumatic brain injury. Brain Pathol. 2004;14(2):195‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Orihara Y, Ikematsu K, Tsuda R, Nakasono I. Induction of nitric oxide synthase by traumatic brain injury. Forensic Sci Int. 2001;123(2‐3):142‐149. [DOI] [PubMed] [Google Scholar]
- 3. Wada K, Chatzipanteli K, Busto R, Dietrich WD. Role of nitric oxide in traumatic brain injury in the rat. J Neurosurg. 1998;89(5):807‐818. [DOI] [PubMed] [Google Scholar]
- 4. Rinecker M, Plesnila N, Baethmann A, Stoffel M. Secondary growth of a cortical necrosis: effect of NOS inhibition by aminoguanidine post insult. Acta Neurochir. 2003;145(11):977‐981. discussion 981 [DOI] [PubMed] [Google Scholar]
- 5. Louin G, Marchand‐Verrecchia C, Palmier B, Plotkine M, Jafarian‐Tehrani M. Selective inhibition of inducible nitric oxide synthase reduces neurological deficit but not cerebral edema following traumatic brain injury. Neuropharmacology. 2006;50(2):182‐190. [DOI] [PubMed] [Google Scholar]
- 6. Gahm C, Holmin S, Wiklund PN, Brundin L, Mathiesen T. Neuroprotection by selective inhibition of inducible nitric oxide synthase after experimental brain contusion. J Neurotrauma. 2006;23(9):1343‐1354. [DOI] [PubMed] [Google Scholar]
- 7. Werner ER, Pitters E, Schmidt K, Wachter H, Werner‐Felmayer G, Mayer B. Identification of the 4‐amino analogue of tetrahydrobiopterin as a dihydropteridine reductase inhibitor and a potent pteridine antagonist of rat neuronal nitric oxide synthase. Biochem J. 1996;320(Pt 1):193‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pfeiffer S, Gorren AC, Pitters E, Schmidt K, Werner ER, Mayer B. Allosteric modulation of rat brain nitric oxide synthase by the pterin‐site enzyme inhibitor 4‐aminotetrahydrobiopterin. Biochem J. 1997;328(Pt 2):349‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmidt K, Werner‐Felmayer G, Mayer B, Werner ER. Preferential inhibition of inducible nitric oxide synthase in intact cells by the 4‐amino analogue of tetrahydrobiopterin. Eur J Biochem. 1999;259(1‐2):25‐31. [DOI] [PubMed] [Google Scholar]
- 10. Schwarzmaier SM, Terpolilli NA, Dienel A, et al. Endothelial nitric oxide synthase mediates arteriolar vasodilatation after traumatic brain injury in mice. J Neurotrauma. 2015;32(10):731‐738. [DOI] [PubMed] [Google Scholar]
- 11. Terpolilli NA, Zweckberger K, Trabold R, et al. The novel nitric oxide synthase inhibitor 4‐amino‐tetrahydro‐L‐biopterine prevents brain edema formation and intracranial hypertension following traumatic brain injury in mice. J Neurotrauma. 2009;26(11):1963‐1975. [DOI] [PubMed] [Google Scholar]
- 12. Stover JF, Belli A, Boret H, et al. Nitric oxide synthase inhibition with the antipterin VAS203 improves outcome in moderate and severe traumatic brain injury: a placebo‐controlled randomized phase IIa trial (NOSTRA). J Neurotrauma. 2014;31(19):1599‐1606. [DOI] [PubMed] [Google Scholar]
- 13. Mount PF, Power DA. Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiol (Oxf). 2006;187(4):433‐446. [DOI] [PubMed] [Google Scholar]
- 14. Schmieder RE, Gatzka C, Schobel H, Schachinger H, Weihprecht H. Renal hemodynamic response to stress is influenced by ACE‐inhibitors. Clin Nephrol. 1994;42(6):381‐388. [PubMed] [Google Scholar]
- 15. Delles C, Klingbeil AU, Schneider MP, Handrock R, Schaufele T, Schmieder RE. The role of nitric oxide in the regulation of glomerular haemodynamics in humans. Nephrol Dial Transplant. 2004;19(6):1392‐1397. [DOI] [PubMed] [Google Scholar]
- 16. Smith HW, Finkelstein N, Aliminosa L, Crawford B, Graber M. The renal clearances of substituted hippuric acid derivatives and other aromatic acids in dog and man. J Clin Invest. 1945;24(3):388‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gomez DM. Evaluation of renal resistances, with special reference to changes in essential hypertension. J Clin Invest. 1951;30(10):1143‐1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guidi E, Cozzi MG, Minetti EE, Civati G, Busnach G, Brando B. Effect of familial hypertension on glomerular hemodynamics and tubulo‐glomerular feedback after uninephrectomy. Am J Hypertens. 2001;14(2):121‐128. [DOI] [PubMed] [Google Scholar]
- 19. Ott C, Ritt M, Titze SI, Schaufele T, Schmieder RE. Rosuvastatin does not affect intrarenal hemodynamics in patients with hypercholesterolemia. J Nephrol. 2009;22:675‐681. [PubMed] [Google Scholar]
- 20. Ott C, Schneider MP, Raff U, et al. Effects of manidipine vs amlodipine on intrarenal haemodynamics in patients with arterial hypertension. Br J Clin Pharmacol. 2013;75(1):129‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159‐2219. [DOI] [PubMed] [Google Scholar]
- 22. Schmieder RE, Delles C, Mimran A, Fauvel JP, Ruilope LM. Impact of telmisartan versus ramipril on renal endothelial function in patients with hypertension and type 2 diabetes. Diabetes Care. 2007;30(6):1351‐1356. [DOI] [PubMed] [Google Scholar]
- 23. Ott C, Schlaich MP, Schmidt BM, Titze SI, Schaufele T, Schmieder RE. Rosuvastatin improves basal nitric oxide activity of the renal vasculature in patients with hypercholesterolemia. Atherosclerosis. 2008;196(2):704‐711. [DOI] [PubMed] [Google Scholar]
- 24. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS guide to pharmacology in 2018: updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Res. 2018;46(D1):D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alexander SPH, Fabbro D, Kelly E, et al. The Concise Guide to PHARMACOLOGY 2017/18: enzymes. Br J Pharmacol. 2017;174(Suppl 1):S272‐S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simon AH, Lima PR, Almerinda M, Alves VF, Bottini PV, de Faria JB. Renal haemodynamic responses to a chicken or beef meal in normal individuals. Nephrol Dial Transplant. 1998;13(9):2261‐2264. [DOI] [PubMed] [Google Scholar]
- 27. Brochner‐Mortensen J, Paaby P, Fjeldborg P, Raffn K, Larsen CE, Moller‐Petersen J. Renal haemodynamics and extracellular homeostasis during the menstrual cycle. Scand J Clin Lab Invest. 1987;47(8):829‐835. [PubMed] [Google Scholar]
- 28. Chapman AB, Zamudio S, Woodmansee W, et al. Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am J Physiol. 1997;273:F777‐F782. [DOI] [PubMed] [Google Scholar]
- 29. Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008;73(9):1008‐1016. [DOI] [PubMed] [Google Scholar]
- 30. Murray PT, Mehta RL, Shaw A, et al. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th acute dialysis quality initiative consensus conference. Kidney Int. 2014;85(3):513‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dent CL, Ma Q, Dastrala S, et al. Plasma neutrophil gelatinase‐associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care. 2007;11(6):R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Constantin JM, Futier E, Perbet S, et al. Plasma neutrophil gelatinase‐associated lipocalin is an early marker of acute kidney injury in adult critically ill patients: a prospective study. J Crit Care. 2010;25(1):176.e171‐176.e176. [DOI] [PubMed] [Google Scholar]
- 33. Cruz DN, de Cal M, Garzotto F, et al. Plasma neutrophil gelatinase‐associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med. 2010;36(3):444‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]


