Abstract
Osteosarcoma is the most common form of primary malignant bone tumor, with metastasis playing an essential role in determining a patient's prospects for survival. It is essential that new and better molecular targets that respond effectively to therapies and are predictive of the risk of tumor metastasis are identified. We have therefore undertaken the present prospective study to ascertain the clinical significance of circulating tumor cells (CTCs) in osteosarcoma patients. Peripheral blood was obtained from patients both pre- and post-surgery then processed using a CanPatrol™ system, an enrichment technique allowing isolation of CTCs by virtue of their size at baseline. Multiplex RNA in situ hybridization (RNA-ISH) was subsequently conducted to characterize the CTCs based on various molecular markers including MTA1, CD45, EpCAM, CK8, CK19, Vimentin and Twist. MTA1 expression was further validated by immunohistochemistry of the tumor tissue. Besides defining a diagnosis and prognosis for osteosarcoma patients, the correlation between CTC count and their molecular and clinicopathological characteristics was found to assist in the analysis of the response of patients to neoadjuvant chemotherapy. Our results revealed that the number of CTCs was significantly higher at baseline in metastatic patients than in those whose osteosarcomas were localized. The variation was attributed to the neoadjuvant chemotherapy treatment. A cut-off value of 7 CTCs/5 mL was found to effectively distinguish patients who had either a favorable or unfavorable prognosis. Notably, the ratio of mesenchymal CTCs at baseline was found to be higher in metastatic vs. localized osteosarcoma patients. In addition, the expression of MTA1 was higher in mesenchymal CTCs than the other CTC phenotypes. Furthermore, immunohistochemical analysis demonstrated a higher expression of MTA1 in tumor tissues from metastatic osteosarcoma patients. Taken together, our findings conclusively establish that the number and molecular phenotype of CTCs are predictive of tumor metastasis and the response of patients to neoadjuvant chemotherapy.
Keywords: Circulating tumor cells, Metastasis-associated protein 1 (MTA1), Osteosarcoma, Prognosis, Tumor metastasis
1. Introduction
Osteosarcoma is the most common form of primary malignant bone tumor [1], accounting for 2.4% of pediatric tumors and the eighth most common malignancy in adolescents. 15–30% pulmonary metastases are evident on presentation [2]. Current treatment modalities, including complete tumor resection combined with multiagent chemotherapy, have shown limited success [3]. Despite improvements in diagnosis and treatment, 5-year overall survival remains unchanged at 70% for localized and 30% for pulmonary metastatic patients [4], [5], [6], [7]. Pulmonary metastasis and tumor recurrence are the leading causes of death in patients with osteosarcoma and is difficult to treat. To date, a number of markers have been investigated that it has been suggested may predict tumor metastatic potential and prognosis of osteosarcoma patients. However, the majority are derived from the primary tumor or serum and therefore lack the precise information about the cells that are responsible for tumor metastasis [8,9]. Circulating tumor cells (CTCs) are neoplastic and are shed from the primary tumor and thereafter enter the circulation. It has been established that CTCs are a major cause of tumor metastasis and recurrence in many types of tumor [10], [11], [12], [13], [14], [15], [16]. However, their clinical significance in osteosarcoma is unclear. There are few studies of CTCs in osteosarcoma, possibly due to the lack of an effective enrichment system for them [17]. Of the various platforms available, CellSearch from Janssen Diagnostics is the only system approved by the FDA and is appropriate for carcinomas only, based on EpCAM. The practicability of CellSearch has been widely verified in many studies and referred to as the gold standard for CTC enrichment in carcinoma [18], [19], [20]. However, the majority of enrichment systems, including CellSearch, evaluate the cell surface markers of epithelial tumor cells. Osteosarcoma is mesenchymal in origin. Although many makers are described in the literature, they require additional validation and are therefore not used in clinical practice, thus, enrichment of CTCs from peripheral blood using antibody-based detection of cell surface proteins remains unvalidated. Therefore, we utilized the CanPatrol™ System to enrich osteosarcoma CTCs, using a process similar to that reported for a number of other types of tumor [21], [22], [23], [24], [25]. The CanPatrol™ System combines physical filtration and molecular identification to enrich CTCs from peripheral blood [26].
Metastasis associated protein 1 (MTA1) is a component of the nucleosome remodeling and histone deacetylation (NuRD) complex [27], acting both as a transcriptional corepressor or coactivator of various tumor suppressor genes or oncogenes. It performs an essential role in cell survival, tumor invasion, epithelial-mesenchymal transition (EMT) and tumor metastasis [28]. MTA1 has been investigated in a number of tumors, including osteosarcoma, its overexpression having been established as associated with high-risk characteristics of osteosarcoma [29], [30], [31], [32], [33]. Park et al. compared the expression levels of MTA1 protein on primary bone with pulmonary metastatic lesions of osteosarcomas by immunohistochemistry, finding that MTA1 is possibly implicated in the progression of high-grade osteosarcoma, particularly in hematogenous metastasis of osteosarcoma [34,35]. In the present study, we analyzed the relationships between MTA1 expression, CTC phenotype and tumor metastasis through a single-center, prospective study of patients with newly diagnosed osteosarcoma, in order to investigate the clinical significance of CTCs in osteosarcoma. The correlation between CTCs and the clinicopathological characteristics of osteosarcoma patients were investigated to ascertain whether CTCs can serve as a diagnostic and prognostic biomarker in osteosarcoma.
2. Materials and methods
2.1. Patient characteristics
This study prospectively enrolled 30 newly-diagnosed osteosarcoma patients and 10 healthy donors that were attending Xijing Hospital, Xi'an, China, from March 2015 to June 2016. Pathologically, all patients were diagnosed with osteosarcoma. The healthy donors had no history of malignant disease or infection at the time of drawing blood. For this study, written informed consent was obtained from all patients and healthy donors. Parents’ consent was obtained for patients under 18 years of age. The study was approved by the Drug and Clinical Trial Ethics Committee, Xijing Hospital, The Air Force Military Medical University and registered in a public clinical trials management platform (Chinese Clinical Trial Register, www.chictr.org.cn) as ChiCTR-OOC-15005925 (registration date: 28 January 2015).
2.2. Blood sample collection and CTC enrichment by size-based membrane filters
Five mL midstream peripheral blood samples were collected into ethylenediaminetetraacetic acid (EDTA) tubes by venepuncture, then transferred into sample preservative tubes (Surexam Biotech, Guangzhou, Guangdong, China) containing ammonium chloride-based lysing buffer using a tailored connection device (Surexam Biotech, Guangzhou, Guangdong, China) and incubated at room temperature for 30 min. Erythrocytes were removed using a red blood cell lysis buffer (Sigma Aldrich, St. Louis, MO, USA). The sample preservative tubes were centrifuged then the cell pellets suspended in 5 mL PBS (Sigma Aldrich, St. Louis, MO, USA) containing 4% formaldehyde (Sigma Aldrich, St. Louis, MO, USA). Each cell suspension was passed through a filtration system consisting of a calibrated membrane with 8 µm diameter pores (Millipore, Billerica, MA, USA), a manifold vacuum plate with valve settings (Surexam Biotech, Guangzhou, Guangdong, China), an E-Z 96 vacuum manifold (Omega Biotech, Norcross, GA, USA) and a vacuum pump (Auto Science, Tianjin, China). The pump valve was opened to allow a vacuum of 0.08 MPa to maintain the filtering process and after blood filtration, the membrane with isolated CTCs was retained for further experimentation [26].
2.3. CTC classification by multiplex RNA in situ hybridization (RNA-ISH) assay
Three groups of nucleic acid probes were established to examine the expression levels of epithelial and mesenchymal genes in CTCs using a multiplex RNA-in situ hybridization (RNA-ISH) assay. Group A probes comprised four pooled epithelial transcripts (CK8, 18 and 19 and EpCAM), group B probes had two mesenchymal transcripts (Vimentin and Twist) and group C had only a CD45 transcript, used only to distinguish white blood cells from CTCs. The nucleic acid probes were synthesized by Invitrogen (Shanghai, China) with the sequences shown in Table 1. The hybridization assay was conducted as previously reported [26]. Briefly, the cells retained on the filter were permeabilized, digested with protease then subjected to a series of hybridization reactions with a cocktail of probes specific to the genes to be examined, followed by conjugation to branched DNA (bDNA) signal amplification probes. Finally, cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma Aldrich, St. Louis, MO, USA) and the samples analyzed using a fluorescence microscope with a 100x oil objective lens (Olympus BX53, Tokyo, Japan)
Table 1.
Capture probe sequences for the EpCAM, CK8/18/19, vimentin, twist, CD45 and MTA1 genes.
| Genes | Sequences (5′→3′) |
|---|---|
| EpCAM | TGGTGCTCGTTGATGAGTCA |
| AGCCAGCTTTGAGCAAATGA | |
| AAAGCCCATCATTGTTCTGG | |
| CTCTCATCGCAGTCAGGATC | |
| TCCTTGTCTGTTCTTCTGAC | |
| CTCAGAGCAGGTTATTTCAG | |
| CK8 | CGTACCTTGTCTATGAAGGA |
| ACTTGGTCTCCAGCATCTTG | |
| CCTAAGGTTGTTGATGTAGC | |
| CTGAGGAAGTTGATCTCGTC | |
| CAGATGTGTCCGAGATCTGG | |
| TGACCTCAGCAATGATGCTG | |
| CK18 | AGAAAGGACAGGACTCAGGC |
| GAGTGGTGAAGCTCATGCTG | |
| TCAGGTCCTCGATGATCTTG | |
| CAATCTGCAGAACGATGCGG | |
| AAGTCATCAGCAGCAAGACG | |
| CTGCAGTCGTGTGATATTGG | |
| CK19 | CTGTAGGAAGTCATGGCGAG |
| AAGTCATCTGCAGCCAGACG | |
| CTGTTCCGTCTCAAACTTGG | |
| TTCTTCTTCAGGTAGGCCAG | |
| CTCAGCGTACTGATTTCCTC | |
| GTGAACCAGGCTTCAGCATC | |
| Vimentin | GAGCGAGAGTGGCAGAGGAC |
| CTTTGTCGTTGGTTAGCTGG | |
| CATATTGCTGACGTACGTCA | |
| GAGCGCCCCTAAGTTTTTAA | |
| AAGATTGCAGGGTGTTTTCG | |
| GGCCAATAGTGTCTTGGTAG | |
| Twist | ACAATGACATCTAGGTCTCC |
| CTGGTAGAGGAAGTCGATGT | |
| CAACTGTTCAGACTTCTATC | |
| CCTCTTGAGAATGCATGCAT | |
| TTTCAGTGGCTGATTGGCAC | |
| TTACCATGGGTCCTCAATAA | |
| CD45 | TCGCAATTCTTATGCGACTC |
| TGTCATGGAGACAGTCATGT | |
| GTATTTCCAGCTTCAACTTC | |
| CCATCAATATAGCTGGCATT | |
| TTGTGCAGCAATGTATTTCC | |
| TACTTGAACCATCAGGCATC | |
| MTA1 | TTGTCTGTGAGTGGGTTGTG |
| CACCAGGAACTGGTCGATCT | |
| TGGAACAGGGTGATGTCTCG | |
| CTTGTGGAGAGTATCCATGG | |
| CCTTGGAGATGTCGTAGATG | |
| AAAAGGTTGGCCTCTGATGC | |
| ATATTTTTCCAGGGCTTCCT | |
| GAATGTCCGTGAAATCCTTC |
2.4. Detection of MTA1 mRNA expression level in CTCs
MTA1 mRNA expression levels in CTCs were detected using an RNA-ISH assay. A specific probe (sequence shown in Table 1) was used to capture MTA1 mRNA, followed by conjugation to a bDNA signal amplification probe to create a branched structure. The results were analyzed with a fluorescence microscope using a 100x oil objective lens (Olympus BX53, Tokyo, Japan).
2.5. Histopathological and immunohistochemical (IHC) evaluation
The expression of MTA1 in the osteosarcoma tissue was also detected using immunohistochemistry. Four µm sections sliced from paraffin-embedded osteosarcoma tissue of the 30 patients enrolled in the study were mounted on slides. IHC was conducted following a standard protocol. Briefly, the sections were deparaffinized using xylene and rehydrated in a concentration gradient of ethanol. Endogenous peroxidase activity was blocked using 3% hydrogen peroxide in 50% methanol for 10 min at room temperature. The sections were boiled in citrate buffer (0.01 M citric acid, pH 6.0) for 20 min at 95 °C in a microwave oven. The nonspecific binding sites were blocked by incubation in 10% normal goat serum in PBS for 20 min at 37 °C and sections were then incubated with rabbit monoclonal antibody against human MTA1 (1:50 dilution, Cell Signaling Technology, Danvers, MA, USA) overnight at 4 °C. Sections were then incubated with horseradish peroxidase (HRP)-conjugated antibody at room temperature for 30 min, followed by incubation with avidin-biotin complex for 30 min. Finally, each section was developed by the addition of 3,3′-diaminobenzidine tetrahydrochloride (DAB) reagent, counterstained with Mayer's hematoxylin, dehydrated through a concentration gradient of ethanol, then sealed with a coverslip. Appropriate positive and negative controls were tested in parallel. Images were captured using a light microscope.
The immunohistochemical evaluation of MTA1 expression was semiquantitative. The percentage of positive cells and staining intensity were calculated from 5 fields (× 200 magnification) for each section. The percentage of positively-stained cells was scored from 0 to 4 (0: 0% cells stained; 1: 1–25%; 2: 26–50%; 3: 51–75%; 4: 76–100%). The staining intensity of MTA1 was scored as 0 (negative), 1 (weak), 2 (moderate) or 3 (strong). The final evaluation score (0–7) was the addition of the intensity and percentage scores, which was then converted into sum indices −(0–1), + (2–3), ++ (4–5) or +++ (6–7). For statistical analysis, low MTA1 expression was defined as − or +, whereas high MTA1 expression was represented by ++ or +++. Immunohistochemical evaluation was performed by two independent pathologists who were blinded to the clinical and pathologic information.
2.6. Cell lines and cell culture
The HOS cell line (ATCC®, CRL-1543TM, human osteosarcoma-derived) was used in this study. Cells were cultured in RPMI-1640 medium (VWR, Radnor, PA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco/Life Technologies, Carlsbad, CA, USA) and 1% penicillin-streptomycin (Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C in T25 culture flasks (Corning, Manassas, VA, USA) using standard culture conditions (humidified atmosphere of 5% CO2 and 95% air). Cells were subcultured at a ratio of 1:6 then used in experiments after reaching 90% confluence.
2.7. Spiking experiments
The HOS cell line was used in spiking experiment. Firstly, the cells were harvested then washed with PBS containing 2 mM EDTA (Sigma Aldrich, St. Louis, MO, USA). The cells were suspended in RPMI-1640 medium (VWR, Radnor, PA, USA) at a ratio of 1 cell per 2 µL. Then 0, 10, 30, 50 and 100 HOS cells were spiked into 5 mL of blood from healthy donors to analyze the enrichment efficiency and specificity of the CanPatrol™ System. The experiment was repeated 5 times for each rate of cell spiking.
2.8. Statistical analysis
Data are presented either as median and range (or mean ± S.D.) for continuous variables or as frequencies and proportions for categorical variables. The normal distribution of each data set was confirmed using a Kolmogorov-Smirnov test. An unpaired homoscedastic student's t-test was used to assess the statistical significance between ordinal variables. An independent-samples t-test was used to analyze the relationship between CTC count at baseline and tumor metastasis. The relationship between the ratio of mesenchymal CTCs and tumor metastasis was examined using a chi-square test. Progression free survival (PFS) was defined as the time from pathological diagnosis to the date when clinical progression was confirmed or censored at a follow-up appointment. Kaplan-Meier survival plots for PFS were generated based on CTC count after surgery, then compared using log-rank tests. To determine a CTC cut-off level that best predicted rapid progression of the disease, cut-off values of 1–8 CTCs per 5.0 mL of peripheral blood were correlated with PFS, then confirmed using a Cox proportional hazards ratio and 95% confidence intervals (CI). A Fisher's exact test was employed to analyze the relationship between variation in CTC count and response to neoadjuvant chemotherapy, and additionally, the relationship between the MTA1 expression of osteosarcoma tissue and patient clinical characteristics. The expression levels of MTA1 in different phenotypes of CTCs were examined using a chi-square test. All statistical calculations were performed using GraphPad Prism (Version 6.0; La Jolla, CA, USA). P values < 0.05 were considered statistically significant.
3. Results
3.1. Patient demographics, medical history and disease characteristics
A total of 30 osteosarcoma patients diagnosed between March 2015 and June 2016 were prospectively enrolled in the study. The detailed patient demographics and clinicopathological characteristics of the patients are shown in Table 2. After pathological examination and diagnosis all patients received four cycles of first-line neoadjuvant chemotherapy with ifosfamide (12 g/m2) or a combination of cisplatin and adriamycin (45 and 60 mg/m2, respectively), followed by surgery. The patients then received 6–10 cycles of the chemotherapy dose received as neoadjuvant treatment, following surgery. X-ray and chest computed tomography (CT) scans were performed to screen for tumor metastasis and recurrence. At present, the clinical outcome of all 30 patients can be assessed. Median patient follow-up time was 20.5 months and median survival time was 14.5 months.
Table 2.
Patient demographics, medical history and disease characteristics.
| Demographics | Value (N) | (%) |
|---|---|---|
| Gender | ||
| Male | 17 | 56.7% |
| Female | 13 | 43.3% |
| Age | ||
| <18 | 22 | 73.3% |
| ≥18 | 8 | 26.7% |
| Tumor site | ||
| Femur | 18 | 60.0% |
| Tibia | 9 | 30.0% |
| Fibula | 1 | 3.3% |
| Humerus | 2 | 6.7% |
| Pathological type | ||
| Fibroblastic osteosarcoma | 9 | 30.0% |
| Osteoblastic osteosarcoma | 15 | 50.0% |
| Chondroblastic osteosarcoma | 6 | 20.0% |
| Ennecking stage | ||
| Ⅱ | 21 | 70.0% |
| Ⅲ | 9 | 30.0% |
| Metastasis at diagnosis | ||
| Negative | 21 | 70.0% |
| Positive | 9 | 30.0% |
| Postoperative chemotherapy | ||
| Yes | 30 | 100% |
| No | 0 | 0 |
3.2. Detection and classification of CTCs in peripheral blood
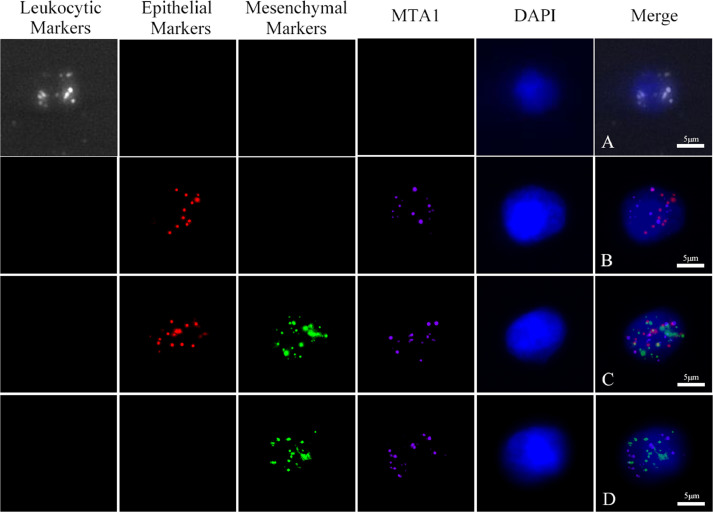
CTCs were classified as epithelial, mesenchymal or biphenotypic (epithelial/mesenchymal), depending on the expression of markers detected by the RNA-ISH assay (Fig. 1). Notably, no CTC phenotype was detected in healthy donor blood, but CTCs were found in the peripheral blood of patients.
Fig. 1.
MTA1, CD45, EpCAM, CK8/18/19, vimentin and twist expression in circulating tumor cells from osteosarcoma patients. Red fluorescence: expression of the epithelial biomarkers EpCAM and CK8/18/19; green: expression of the mesenchymal biomarkers vimentin and twist; purple: expression of MTA1; bright blue fluorescence: expression of the leukocytic biomarker CD45. A: leukocyte; B: epithelial CTC; C: biphenotypic epithelial/mesenchymal CTC; D: mesenchymal CTC. (Magnification: 100X).
3.3. CTC count at baseline and its correlation with clinicopathological characteristics
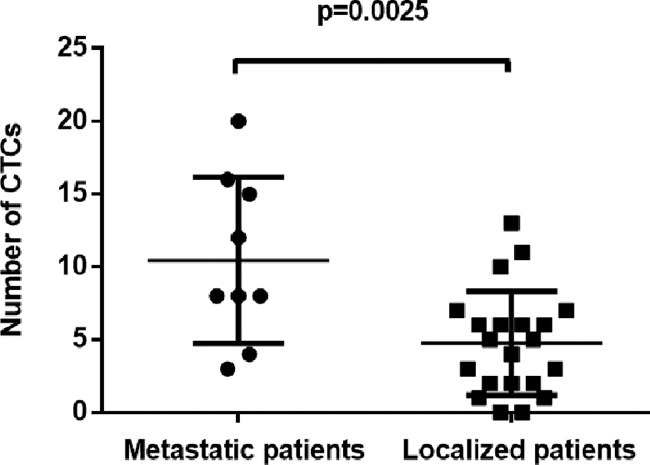
CTCs were detected at baseline in 28 out of the 30 patients. The median number of CTCs in 5 mL of blood at baseline was 6 (range, 0–20) with mean ± S.D. of 6 ± 5. In addition, CTCs were detected significantly more frequently in metastatic vs localized osteosarcoma patients, as shown in Fig. 2 (p = 0.0025). However, no correlation was found between of CTC count and any other clinicopathological characteristic, such as gender, age or tumor site.
Fig. 2.
Number of CTCs in the group of patients with metastasis vs. localized osteosarcoma at baseline (n = 9 and 21, respectively). A statistically significant difference was found between the groups (P = 0.0025, unpaired homoscedastic student's t-test).
3.4. Variation in CTC count and its relationship to the response to neoadjuvant chemotherapy
All patients received neoadjuvant chemotherapy after pathological diagnosis. At present, evaluation of the response to neoadjuvant chemotherapy is based on tumor size, their boundaries, calcification and pain. Positive response includes no increase in tumor size or calcification with clear boundaries and relief from pain, while the reverse outcomes are characterized as a negative response. Amongst the 30 enrolled patients, 21 demonstrated a positive response, with 9 exhibiting negative characteristics in response to neoadjuvant chemotherapy. We also analyzed the variation in CTC count between these time nodes, i.e., at baseline and after neoadjuvant chemotherapy. Our findings reveal that variation in CTC count was significantly related to the response to neoadjuvant chemotherapy. The count decreased in patients who showed a positive response to neoadjuvant chemotherapy (Table 3, p = 0.0016).
Table 3.
Relationship between variation in CTC count and response to neoadjuvant chemotherapy (Fisher's exact test).
| Response to neoadjuvant chemotherapy | Variation of CTC count |
P value | |
|---|---|---|---|
| Increase | Decrease | ||
| Positive | 5 | 16 | 0.0016 |
| Negative | 8 | 1 | |
3.5. CTC count after surgery and its correlation with patient prognosis
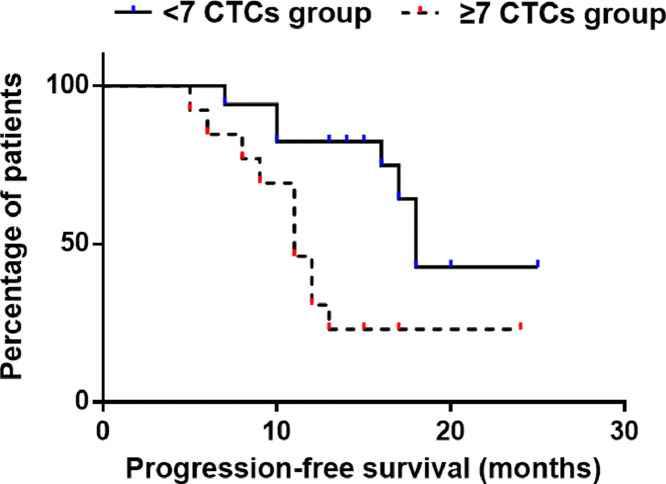
All 30 patients received surgical treatment after neoadjuvant chemotherapy. The median number of CTCs in the peripheral blood of patients after surgery was found to be 5 (range: 0–12) with a mean ± S.D. of 5 ± 3. To establish the optimal threshold of CTCs that most clearly predicted patient prognosis, a Cox proportional hazards ratio was estimated. This analysis revealed that, at a cut-off of 7 CTCs per 5.0 mL of blood, the Cox proportional hazards ratio reached a plateau relative to higher cut-off values. At 7, the log-ranked cut-off hazard ratio was 3.295 with 95% CI of 1.406 to 11.19. Patients with ≥7 CTCs per 5.0 mL after surgery had a median PFS of 11 months compared to 18 in patients with <7 CTCs per 5.0 mL (Fig. 3, P = 0.0116). Thus, a cut-off of 7 CTCs per 5.0 mL after surgery should be adopted as the value to distinguish unfavorable or favorable patient prognosis.
Fig. 3.
Kaplan–Meier plot of progression-free survival in OS patients using a cut-off of 7 CTCs per 5.0 mL peripheral blood. The group with ≥7 CTCs had median progression-free survival of 11 months, whereas the group with <7 CTCs had median progression-free survival of 18 months [p = 0.0116, hazards ratio (log-rank) = 3.295, 95% CI of hazards ratio: 1.406–11.19].
3.6. Ratio of different CTC phenotypes and its correlation with tumor metastasis
Also analyzed was the relationship between the ratio of the different phenotypes of CTCs and tumor metastasis at baseline. As shown in Table 4, the results revealed that the ratio of mesenchymal CTCs in metastatic osteosarcoma patients is higher than that in localized patients (p = 0.0103), suggesting that mesenchymal CTCs possibly perform an important role in tumor metastasis.
Table 4.
Ratios of the different phenotypes of CTCs in patients with metastasis vs. localized osteosarcoma at baseline (Chi-square test).
| Tumor metastasis | Different phenotypes of CTCs |
P value | |||||
|---|---|---|---|---|---|---|---|
| Epithelial |
Biphenotypic epithelial/mesenchymal |
Mesenchymal |
|||||
| n | % | n | % | n | % | ||
| Positive | 6 | 6.4% | 50 | 53.2% | 38 | 40.4% | 0.0103 |
| Negative | 16 | 16.0% | 61 | 61.0% | 23 | 23.0% | |
3.7. Expression of MTA1 in different phenotypes of CTCs
The expression of MTA1 in CTCs from the osteosarcoma patients was measured using RNA-ISH. The results demonstrated that MTA1 was expressed in 72.5% of CTCs. Further analysis indicated that the rates of MTA1 expression in the different phenotypes of CTC were significantly different: 56.6% in epithelial CTCs, 68.8% in biphenotypic (epithelial/mesenchymal) CTCs and 85.5% in mesenchymal CTCs. Overall, the proportion of cells with positive expression of MTA1 was higher in mesenchymal CTCs than in the other two phenotypes (Table 5, p=<0.0001).
Table 5.
Ratios of positive MTA1 expression in different phenotypic CTCs (Chi-square test).
| Phenotype of CTCs | The number of CTCs | Positive expression of MTA1 |
Negative expression of MTA1 |
P value | ||
|---|---|---|---|---|---|---|
| N | n | % | n | % | ||
| Epithelial CTCs | 76 | 43 | 56.6% | 33 | 43.4% | <0.0001 |
| Mesenchymal CTCs | 200 | 171 | 85.5% | 29 | 14.5% | |
| Biphenotypic epithelial/mesenchymal CTCs | 368 | 253 | 68.8% | 115 | 31.2% | |
3.8. MTA1 gene expression levels in osteosarcoma tissues
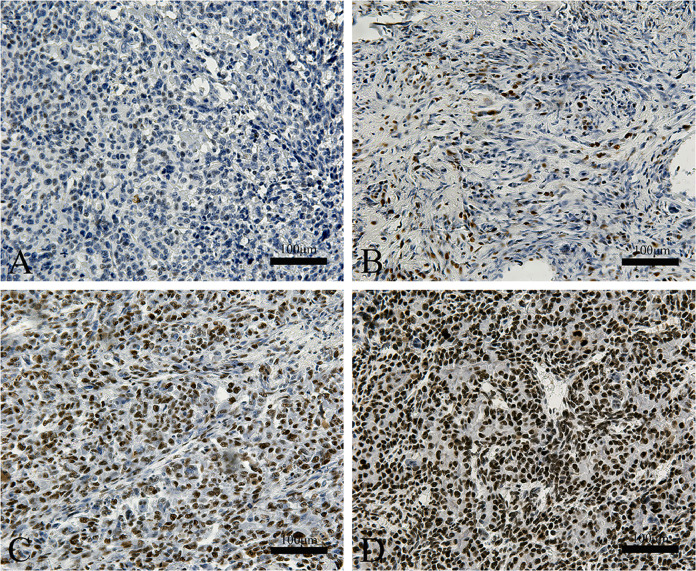
MTA1 gene expression levels in the osteosarcoma tissue of patients was evaluated by immunohistochemistry then correlated with their clinical characteristics. Notably, MTA1 expression was found to be higher in the osteosarcoma tissues of metastatic patients compared to those with localized tumors, thereby indicating that MTA1 is related to osteosarcoma metastasis (Fig. 4, Table 6).
Fig. 4.
Representative photomicrographs of IHC staining for MTA1 in osteosarcoma samples of the enrolled patients. Intensity and percentage scores define the final evaluation score (0–7) of MTA1 expression. (A) —/0–1; (B) +/2–3; (C) ++/4–5; (D) +++/6–7.
Table 6.
Relationship between MTA1 expression and clinical characteristics (Fisher's exact test).
| Characteristics | Low expression |
High expression |
p value | |||
|---|---|---|---|---|---|---|
| N | n | % | n | % | ||
| Gender | 1 | |||||
| Male | 17 | 5 | 29.4% | 12 | 70.6% | |
| Female | 13 | 4 | 30.8% | 9 | 69.2% | |
| Age | 1 | |||||
| <18 | 22 | 7 | 31.8% | 15 | 68.2% | |
| ≧18 | 8 | 2 | 25.0% | 6 | 75.0% | |
| Ennecking stage | 0.0289 | |||||
| Ⅱ | 21 | 9 | 42.9% | 12 | 57.1% | |
| Ⅲ | 9 | 0 | 0 | 9 | 100% | |
| Metastasis at diagnosis | 0.0289 | |||||
| Negative | 21 | 9 | 42.9% | 12 | 57.1% | |
| Positive | 9 | 0 | 0 | 9 | 100% | |
| Response to neoadjuvant | 0.6662 | |||||
| Positive | 21 | 6 | 28.6% | 15 | 71.4% | |
| Negative | 9 | 3 | 33.3% | 6 | 66.6% | |
3.9. Efficiency of tumor cell recovery
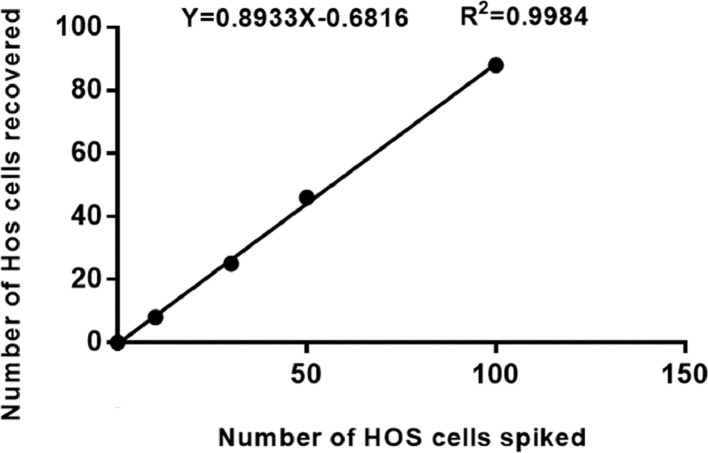
To verify enrichment efficiency, peripheral blood (5 mL) of healthy donors was spiked with HOS cells (0–100) then the CanPatrol™ system used to evaluate enrichment. The experiments were repeated 5 times for each spiked HOS cell number. The results demonstrated that the enrichment process was linear (R2 = 0.998) (Fig. 5), with a mean recovery of 89% at each dilution of cells.
Fig. 5.
Calibration curve obtained using the CanPatrol™ system in the spiking experiment (n = 5) with HOS cells at different dilutions.
4. Discussion
Osteosarcoma is the most prevalent form of malignant bone tumor. Pulmonary metastasis is the principal cause of mortality in patients with osteosarcoma, therefore, identification of metastasis-related factors involved in the process is critical for defining an efficacious therapy [4], [5], [6]. Currently, osteosarcoma is staged by anatomical characteristics which do not include molecular or cytological factors and hence we cannot accurately understand the existence of the tumor state. The detection of pulmonary metastasis generally relies on the examination of images such as those from X-rays, computed tomography (CT), positron emission tomography (PET)-CT or magnetic resonance imaging (MRI), and detection of minimal lesions at an early stage are not trivial when using these methodologies. Several molecular markers have been investigated as diagnostic and prognostic markers for osteosarcoma [36]. However, the majority of currently available markers are derived from the primary tumor which may not reveal information about the cells that are responsible for metastasis. CTCs are tumor cells that are shed from primary or metastatic tumors which circulate in the blood stream. They may lead to a new metastatic lesion and this is a major reason for tumor-related death. In recent years, CTCs have been evaluated as a new diagnostic and prognostic tool for various types of solid tumor [37], [38], [39]. With the development of multidisciplinary studies including materials science, biology, medicine and oncology, the detection technologies of CTCs have developed rapidly [40]. In this study, we have assessed a method for enriching CTCs based on the principle of separation by osteosarcoma cell size, with subsequent multiplex RNA-in situ hybridization (RNA-ISH) for the identification of specific markers, including CD45, EpCAM, CKs, twist and vimentin. CTCs are classified as epithelial, mesenchymal or biphenotypic (epithelial/mesenchymal) by virtue of their specific markers described above. The results of the spiking experiment demonstrated that the CanPatrol™ system could efficiently enrich osteosarcoma cells from peripheral blood. In previous studies, the CTC count has been reported to correlate with tumor metastasis and prognosis in a number of types of tumor [11], [41]. In this study, the results revealed that a significantly greater number of CTCs were detected in metastatic patients than in localized osteosarcoma patients at initial diagnosis (p = 0.0025). The results also demonstrated that the variation in CTC count was related to the patients’ response to neoadjuvant chemotherapy (p = 0.0016). The CTC count decreased significantly after chemotherapy in patients where a positive response to neoadjuvant chemotherapy was observed, demonstrating that variation in CTC count could be a supplementary means of evaluating the effect of neoadjuvant chemotherapy. Moreover, post-surgical CTC count could assist in predicting prognosis. Our results suggest that a cut-off of 7 CTCs per 5.0 mL after surgery could be adopted to distinguish a favorable or unfavorable prognosis. Patients with <7 CTCs per 5.0 mL of peripheral blood had a longer PFS than those with ≧7 CTCs (p = 0.0116).
In addition to validating the significance of CTC count in osteosarcoma, the study suggested that the molecular characteristics of CTCs are also expected to represent diagnostic and prognostic markers. Epithelial to mesenchymal transition (EMT) is a biological process by which epithelial cells lose cell polarity, increase motility and gain invasive properties to become mesenchymal [42]. Multiple studies have demonstrated that EMT enables tumor cells gain invasive properties and metastatic growth characteristics in numerous types of solid tumor including osteosarcoma [43,44]. The major event in EMT is the loss of epithelial markers and the increase in expression of mesenchymal markers, including twist and vimentin [45,46]. In this study, we found that the proportion of mesenchymal CTCs in metastatic patients was significantly higher than in localized patients, suggesting that mesenchymal CTCs may perform an important role in the metastasis of osteosarcoma, which may correlate with EMT in CTCs (p = 0.0103). To exemplify this, we measured MTA1 expression in each CTC. Previous studies have shown that MTA1 is associated with EMT during metastasis [47,48]. In this study, MTA1 expression was detected both in CTCs and in osteosarcoma tissue by RNA-ISH and immunohistochemistry, respectively. The results indicated that the expression of MTA1 in mesenchymal CTCs was significantly higher than in other phenotypes (p<0.0001). In addition, the expression of MTA1 in tumor tissues from metastatic patients was significantly higher than from localized patients (p = 0.0289), a finding similar to that in the published literature. Therefore, we believe that a high expression of MTA1 and EMT in CTCs are associated with osteosarcoma metastasis. It is noteworthy that we detected epithelial CTCs from the osteosarcoma patients, which would not be generally expected for a cancer of a mesenchymal tissue. We hypothesize that this could be related to mesenchymal to epithelial transition (MET), the inverse of EMT. However, this hypothesis requires further validation.
In conclusion, the present study demonstrated that circulating tumor cells can be utilized as a novel diagnostic and prognostic biomarker in osteosarcoma. However, there are limitations to the immediate clinical applicability. Firstly, due to the relatively small sample size from a single center, further studies are required with a greater number of patients from multiple centers to validate our conclusions. Secondly, follow-up time should also be extended to determine the relationship between CTC number and overall survival (OS). In addition, patient blood samples were only collected at baseline, pre- and post-surgery. Future studies should focus on collecting blood samples at several time points through the course of therapy, such as both before and after each cycle of chemotherapy to determine how the number and phenotype of CTCs change over time and in response to each chemotherapy cycle.
Acknowledgments
Acknowledgments
The authors would like to thank Surexam Biotech, Guangzhou, China, for technical support.
Conflict of interest
The authors stated that there are no conflicts of interest regarding the publication of this article.
References
- 1.Jemal A., Bray F., Center M.N., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ottaviani G., Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 3.Ando K., Heymann M.F., Stresing V., Mori K., Rédini F., Heymann D. Current therapeutic strategies and novel approaches in osteosarcoma. Cancers (Basel) 2013;5:591–616. doi: 10.3390/cancers5020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ando K., Heymann M.F., Stresing V., Mori K., Rédini F., Heymann D. Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr. Opin. Oncol. 2007;19:41–46. doi: 10.1097/CCO.0b013e328122d73f. 3. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe N. Adjuvant chemotherapy in osteosarcoma: an odyssey of rejection and vindication. Cancer Treat Res. 2009;152:219–237. doi: 10.1007/978-1-4419-0284-9_11. [DOI] [PubMed] [Google Scholar]
- 6.Chou A.J., Geller D.S., Gorlick R. Therapy for osteosarcoma: where do we go from here? Paediatr. Drugs. 2008;10:315–327. doi: 10.2165/00148581-200810050-00005. [DOI] [PubMed] [Google Scholar]
- 7.Brown H.K., Schiavone K., Gouin F., Heymann M.F., Heymann D. Biology of bone sarcomas and new therapeutic developments. Calcif. Tissue Int. 2018;102:174–195. doi: 10.1007/s00223-017-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang L., Asatrian G., Dry S.M., James A.W. Circulating tumor cells in sarcomas: a brief review. Med. Oncol. 2015;32:430. doi: 10.1007/s12032-014-0430-9. [DOI] [PubMed] [Google Scholar]
- 9.Salah S., Ahmad R., Sultan I., Yaser S., Shehadeh A. Osteosarcoma with metastasis at initial diagnosis: current outcomes and prognostic factors in the context of a comprehensive cancer center. Mol. Clin. Oncol. 2014;2:811–816. doi: 10.3892/mco.2014.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Budd G.T., Cristofanilli M., Ellis M.J., Stopeck A., Borden E., Miller M.C., Matera J., Repollet M., Doyle G.V., Terstappen L.W., Hayes D.F. Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin. Cancer Res. 2006;12:6403–6409. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H., Gao P P., Xiao X., Heger M., Geng L., Fan B., Yuan Y., Huang C., Chen G., Liu Y., Hu Y., Yu X., Wu S., Wang L., Wang Z. A liquid biopsy-based method for the detection and quantification of circulating tumor cells in surgical osteosarcoma patients. Int. J. Oncol. 2017:3095. doi: 10.3892/ijo.2017.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y., Gong J., Zhang Q., Lu Z., Gao J., Li Y., Cao Y., Shen L. Dynamic monitoring of circulating tumour cells to evaluate therapeutic efficacy in advanced gastric cancer. Br. J. Cancer. 2016;114:138–145. doi: 10.1038/bjc.2015.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anantharaman A., Friedlander T., Lu D., Krupa R., Premasekharan G., Hough J., Edwards M., Paz R., Lindquist K., Graf R., Jendrisak A., Louw J., Dugan L., Baird S., Wang Y., Dittamore R., Paris P.L. Programmed death-ligand 1 (PD-L1) characterization of circulating tumor cells (CTCs) in muscle invasive and metastatic bladder cancer patients. BMC Cancer. 2016;16:744. doi: 10.1186/s12885-016-2758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connor A.A., McNamara K., Al-Sukhni E., Diskin J., Chan D., Ash C., Lowes L.E., Allan A.L., Zogopoulos G.C.A., Moulton C.A., Gallinger S. Central, but not peripheral, circulating tumor cells are prognostic in patients undergoing resection of colorectal cancer liver metastases. Ann Surg Oncol. 2015;23:2168–2175. doi: 10.1245/s10434-015-5038-6. [DOI] [PubMed] [Google Scholar]
- 15.Markiewicz A., Książkiewicz M., Wełnicka-Jaśkiewicz M., Seroczyńska B., Skokowski J., Szade J., Żaczek A.J. Mesenchymal phenotype of CTC-enriched blood fraction and lymph node metastasis formation potential. PLoS One. 2014;9:93901. doi: 10.1371/journal.pone.0093901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchetti A., Del Grammastro M., Felicioni L., Malatesta S., Filice G., Centi I., De Pas T., Santoro A., Chella A., Brandes A.A., Venturino P., Cuccurullo F., Crinò L., Buttitta F. Assessment of EGFR mutations in circulating tumor cell preparations from NSCLC patients by next generation sequencing: toward a real-time liquid biopsy for treatment. PLoS One. 2014;9 doi: 10.1371/journal.pone.0103883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chalopin A., Tellez-Gabriel M., Brown H.K., Vallette F., Heymann M.F., Gouin F., Heymann D. Isolation of circulating tumor cells in a preclinical model of osteosarcoma: effect of chemotherapy. J. Bone Oncol. 2018;12:83–90. doi: 10.1016/j.jbo.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andree K.C., van Dalum G., Terstappen L.W. Challenges in circulating tumor cell detection by the CellSearch system. Mol. Oncol. 2016;10:395–407. doi: 10.1016/j.molonc.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beije N., Jager A., Sleijfer S. Circulating tumor cell enumeration by the CellSearch system: the clinician's guide to breast cancer treatment? Cancer Treat Rev. 2015;41:144–150. doi: 10.1016/j.ctrv.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Riethdorf S., Fritsche H., Müller V., Rau T., Schindlbeck C., Rack B., Janni W., Coith C., Beck K., Jänicke F., Jackson S., Gornet T., Cristofanilli M., Pantel K. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin. Cancer Res. 2017;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 21.Zhong G.X., Feng S.D., Shen R., Wu Z.Y., Chen F., Zhu X. The clinical significance of the Ezrin gene and circulating tumor cells in osteosarcoma. Onco Targets Ther. 2017;10:527–533. doi: 10.2147/OTT.S125589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin X.R., Zhu L.Y., Qian K., Feng Y.G., Zhou J.H., Wang R.W., Bai L., Deng B., Liang N., Tan Q.Y. Circulating tumor cells in early stage lung adenocarcinoma: a case series report and literature review. Oncotarget. 2017;8:23130–23141. doi: 10.18632/oncotarget.15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan X., Ma F., Liu S., Wu S., Xiao R., Yuan L., Sun X., Yi Z., Yang H., Xu B. Analysis of the hormone receptor status of circulating tumor cell subpopulations based on epithelial-mesenchymal transition: a proof-of-principle study on the heterogeneity of circulating tumor cells. Oncotarget. 2016;7:65993–66002. doi: 10.18632/oncotarget.11787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y.K., Hu B.S., Li Z.L., He X., Li Y., Lu L.G. An improved strategy to detect the epithelial-mesenchymal transition process in circulating tumor cells in hepatocellular carcinoma patients. Hepatol. Int. 2016;10:640–646. doi: 10.1007/s12072-016-9732-7. [DOI] [PubMed] [Google Scholar]
- 25.Li T.T., Liu H., Li F.P., Hu Y.F., Mou T.Y., Lin T., Yu J., Zheng L., Li G.X. Evaluation of epithelial-mesenchymal transitioned circulating tumor cells in patients with resectable gastric cancer: relevance to therapy response. World J. Gastroenterol. 2015;21:13259–13267. doi: 10.3748/wjg.v21.i47.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu S., Liu S., Liu Z., Huang J., Pu X., Li J., Yang D., Deng H., Yang N., Xu J. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marzook H., Deivendran S., George B., Reshmi G., Santhoshkumar T.R., Kumar R., Pillai M.R. Cytoplasmic translocation of MTA1 coregulator promotes de-repression of SGK1 transcription in hypoxic cancer cells. Oncogene. 2017;36:5263–5273. doi: 10.1038/onc.2017.19. [DOI] [PubMed] [Google Scholar]
- 28.Ma K., Fan Y., Dong X., Dong D., Guo Y., Wei X., Ning J., Geng Q., Wang C., Hu Y., Li M., Niu W., Li E., Wu Y. MTA1 promotes epithelial to mesenchymal transition and metastasis in non-small-cell lung cancer. Oncotarget. 2017;8:38825–38840. doi: 10.18632/oncotarget.16404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deivendran S., Marzook H., Santhoshkumar T.R., Kumar R R., Pillai M.R. Metastasis-associated protein 1 is an upstream regulator of DNMT3a and stimulator of insulin-growth factor binding protein-3 in breast cancer. Sci. Rep. 2017;7:44225. doi: 10.1038/srep44225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C., Gao L., Cai Y., Liu H., Gao D., Lai J., Jia B., Wang F., Liu Z. Inhibition of tumor growth and metastasis by photoimmunotherapy targeting tumor-associated macrophage in a sorafenib-resistant tumor model. Biomaterials. 2016;84:1–12. doi: 10.1016/j.biomaterials.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 31.Dhar S., Kumar A., Gomez C.R., Akhtar I., Hancock J.C., Lage J.M., Pound C.R., Levenson A.S. MTA1-activated Epi-microRNA-22 regulates E-cadherin and prostate cancer invasiveness. FEBS Lett. 2017;591:924–933. doi: 10.1002/1873-3468.12603. [DOI] [PubMed] [Google Scholar]
- 32.Sun X., Xu Y., Zhang S., Li X., Wang Y., Zhang Y., Zhao X., Li Y., Wang Y. MicroRNA-183 suppresses the vitality, invasion and migration of human osteosarcoma cells by targeting metastasis-associated protein 1. Exp. Ther. Med. 2018;15:5058–5064. doi: 10.3892/etm.2018.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park H.R., Jung W.W., Kim H.S., Bacchini P., Bertoni F., Park Y.K. Overexpression of metastatic tumor antigen in osteosarcoma: comparison between conventional high-grade and central low-grade osteosarcoma. Cancer Res. Treat. 2005;37:360–364. doi: 10.4143/crt.2005.37.6.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park H.R., Cabrini R.L., Araujo E.S., Paparella M.L., Brandizzi D., Park Y.K. Expression of ezrin and metastatic tumor antigen in osteosarcomas of the jaw. Tumori. 2009;95:81–86. doi: 10.1177/030089160909500113. [DOI] [PubMed] [Google Scholar]
- 35.Yang J., Gao T., Tang J., Cai H., Lin L., Fu S. Loss of microRNA-132 predicts poor prognosis in patients with primary osteosarcoma. Mol. Cell Biochem. 2013;381:9–15. doi: 10.1007/s11010-013-1677-8. [DOI] [PubMed] [Google Scholar]
- 36.Giuliano M., Giordano A., Jackson S., Hess K.R., De Giorgi U., Mego M., Handy B.C., Ueno N.T., Alvarez R.H., De Laurentiis M., De Placido S., Valero V., Hortobagyi G.N., Reuben J.M., Cristofanilli M. circulating tumor cells as prognostic and predictive markers in metastatic breast cancer patients receiving first-line systemic treatment. Breast Cancer Res. 2011;13:67. doi: 10.1186/bcr2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin J., Wang Y., Yin H., Chen W., Jin G., Ma H., Dai J., Chen J., Jiang Y., Wang H., Liu Z., Hu Z., Shen H. Circulating tumor cells enriched by the depletion of leukocytes with bi-antibodies in non-small cell lung cancer: potential clinical application. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen S.J., Punt C.J., Iannotti N., Saidman B.H., Sabbath K.D., Gabrail N.Y., Picus J., Morse M., Mitchell E., Miller M.C., Doyle G.V., Tissing H., Terstappen L.W., Meropol N.J. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 39.Shen Z., Wu A., Chen X. Current detection technologies for circulating tumor cells. Chem. Soc. Rev. 2017;46:2038–2056. doi: 10.1039/c6cs00803h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banys-Paluchowski M., Krawczyk N., Meier-Stiegen F., Fehm T. Circulating tumor cells in breast cancer-current status and perspectives. Crit. Rev. Oncol. Hematol. 2016;97:22–29. doi: 10.1016/j.critrevonc.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Kong D., Li Y., Wang Z., Sarkar F.H. Cancer stem cells and epithelial-to-mesenchymal transition (EMT)-phenotypic cells: are they cousins or twins? Cancers (Basel) 2011;3:716–729. doi: 10.3390/cancers30100716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai J., Qin L., Chen Y., Wang H., Lin G., Li X., Liao H., Fang H. Matrix stiffness regulates epithelial-mesenchymal transition via cytoskeletal remodeling and MRTF-A translocation in osteosarcoma cells. J. Mech. Behav. Biomed. Mater. 2019;90:226–238. doi: 10.1016/j.jmbbm.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Zeng Q., Li Z., Zhao X., Guo L., Yu C., Qin J., Zhang S., Zhang Y., Yang X. Ubiquitinspecific protease 7 promotes osteosarcoma cell metastasis by inducing epithelialmesenchymal transition. Oncol. Rep. 2019;41:543–551. doi: 10.3892/or.2018.6835. [DOI] [PubMed] [Google Scholar]
- 44.Wang X., Liang X., Liang H., Wang B. SENP1/HIF-1alpha feedback loop modulates hypoxia-induced cell proliferation, invasion and EMT in human osteosarcoma cells. J. Cell Biochem. 2017;119:1819–1826. doi: 10.1002/jcb.26342. [DOI] [PubMed] [Google Scholar]
- 45.Wang S., Zhang D., Han S., Gao P., Liu C., Li J., Pan X. Fibulin-3 promotes osteosarcoma invasion and metastasis by inducing epithelial to mesenchymal transition and activating the Wnt/beta-catenin signaling pathway. Sci. Rep. 2017;7:6215. doi: 10.1038/s41598-017-06353-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Tuncay Cagatay S., Cimen I., Savas B., Banerjee S. MTA-1 expression is associated with metastasis and epithelial to mesenchymal transition in colorectal cancer cells. Tumour Biol. 2013;34:1189–1204. doi: 10.1007/s13277-013-0662-x. [DOI] [PubMed] [Google Scholar]
- 47.Deng L., Tang J., Yang H., Cheng C., Lu S., Jiang R., Sun B. MTA1 modulated by miR-30e contributes to epithelial-to-mesenchymal transition in hepatocellular carcinoma through an ErbB2-dependent pathway. Oncogene. 2017;36:3976–3985. doi: 10.1038/onc.2016.491. [DOI] [PubMed] [Google Scholar]
- 48.Kim S.S., Park Y.K. Significance of MTA1 in the molecular characterization of osteosarcoma. Cancer Metastasis Rev. 2014;33:981–991. doi: 10.1007/s10555-014-9523-3. [DOI] [PubMed] [Google Scholar]