Abstract
Background
The Sabes study, a treatment as prevention intervention in Peru, tested the hypothesis that initiating antiretroviral therapy (ART) early in HIV infection when viral load is high, would markedly reduce onward HIV transmission among high-risk men who have sex with men (MSM) and transgender women (TW). We investigated the potential population-level benefits of detection of HIV early after acquisition and rapid initiation of ART.
Methods
We designed a transmission dynamic model to simulate the HIV epidemic among MSM and TW in Peru, calibrated to data on HIV prevalence and ART coverage from 2004 to 2011. We assessed the impact of an intervention starting in 2018 in which up to 50% of the new infections were diagnosed within three months of acquisition and initiated on ART within 1 month of diagnosis. We estimated the impact of the intervention over 20 years using the cumulative prevented fraction of new HIV infections compared to scenarios without intervention.
Findings
Our model suggests that only 19% of the infected MSM and TW are virally suppressed in 2018 and 35%–40% of the new HIV infections are transmitted from contacts with acutely-infected partners. An intervention reaching 10% of all acutely infected MSM and TW is projected to prevent 13.3% [Uncertainty interval: 11.9%–14.3%] of the new infections over 20 years and reduce HIV incidence in 2038 by 24%. Reaching 50% of all acutely infected MSM and TW will increase the prevalence of viral suppression in 2038 to 59% and prevent 41% of expected infections over 20 years. Reaching 50% of the high-risk MSM and TW in acute phase would reduce HIV incidence in 2038 by 60% and prevent 36% of new infections between 2018 and 2038.
Conclusions
Early detection of HIV infections and rapid initiation of ART among MSM is desirable as it would increase the effectiveness of the HIV prevention program in Peru. Targeting high-risk MSM and TW will be highly efficient.
Keywords: Mathematical model, Antiretroviral therapy, HIV incidence, HIV prevention
1. Introduction
Despite a number of HIV prevention interventions and the scale-up of antiretroviral therapy (ART), HIV incidence remains unabated, with nearly two million new adult cases per year (UNAIDS, 2016). Stagnant incidence rates may be in part due to the failure to detect and treat acute HIV infection (AHI) (Dehne et al., 2016; Rutstein et al., 2017). Data from clinical trials, phylogenetic research, and mathematical models support the importance of AHI in propagating the epidemic—the likelihood of transmission during AHI is higher due to the presence of very high viral load (up to 10-fold higher than set-point) and high infectivity of founder viruses (Carlson et al., 2014; Fiebig et al., 2003; Ma et al., 2009; Pilcher et al., 2007). Furthermore, because AHI occurs soon after acquisition, this period of high infectivity often occurs before an individual knows s/he is infected (Brenner et al., 2007; Eaton, Hallett, & Garnett, 2011; Pines et al., 2016; Steward et al., 2009). (Hollingsworth, Anderson, & Fraser, 2008) Phylogenetic studies have demonstrated the importance of AHI in driving HIV epidemics, estimating that individuals with AHI are a source of infection for 10–50% of all transmissions (Brenner et al., 2007, 2011; Brown et al., 2009; Chibo, Kaye, & Birch, 2011; English et al., 2011; Frange et al., 2012; Hollingsworth, Pilcher, Hecht, Deeks, & Fraser, 2015; Kouyos et al., 2010; Pao et al., 2005). It is increasingly clear that efforts to identify and treat individuals with acute or early HIV infection will be critical to bring about HIV epidemic control.
A public health approach to detection of and treatment during AHI is necessary, especially in concentrated HIV epidemics such as in Peru, where the HIV epidemic is concentrated in men who have sex with men (MSM) and transgender women (TW) (Rutstein et al., 2017). Although HIV prevalence in the general population is estimated to be below 0.2% (Report on, 2012), HIV prevalence has been reported to be up to 22% and 30% among MSM and TW, respectively (Caceres & Mendoza, 2009; Sanchez et al., 2007; Silva-Santisteban et al., 2012; Tabet et al., 2002). It is estimated that AHI accounts for 22–29% of onward HIV transmission in Peru (Goodreau et al., 2012).
We conducted the Sabes study (“¿Sabes?” in Spanish means “Do you know?”): enrollment occurred between July 2013 and September 2015; follow-up was completed in 2017. A treatment-as-prevention (TasP) intervention, Sabes tested the hypothesis that intervening early (within 3 months of HIV acquisition) when viral load is high, would markedly reduce onward HIV transmission among high-risk populations of MSM and TW in Lima, Peru (Lama et al., 2018). Newly HIV-infected (HIV+) MSM and TW in Lima were identified by frequent testing and rapidly linked to care by peer health navigators – a modification of the role of peer health educators long used in Peru to increase HIV testing. Overall, 3,337 subjects were screened for HIV; 2,685 (80.5%) were negative and 2,109 began monthly testing for HIV infection using both serologic assays and HIV RNA testing. This intervention proved that frequent HIV testing and rapid linkage to care are feasible in high-risk populations. We identified 311 individuals shortly after HIV acquisition, 256 of whom were diagnosed within 3 months of HIV acquisition. The time since HIV acquisition for the remaining 54 participants could not be demonstrated to be 3 months or less (Lama et al., 2018). Ninety percent of eligible participants were linked to the study clinic within 6 days.
The Sabes study showed the feasibility of a treatment-as-prevention strategy employing frequent HIV testing and rapid linkage to care among MSM and TW in Lima. However, it could not measure the impact of this intervention on HIV incidence over time. Mathematical modeling provides a useful framework for projecting potential population-level benefits of HIV prevention programs (de Montigny et al., 2018; Dimitrov et al., 2011, 2016; Eaton et al., 2014; Selinger et al., 2019; van de Vijver et al., 2013; Vickerman et al., 2010; Wood et al., 2018). In this paper we evaluate the effectiveness of detecting early HIV infection and rapidly initiating ART, tested in Sabes, by estimating the number and proportion of new infections averted over 20 years, as well as the reduction in HIV incidence among MSM and TW due to the intervention. Our analysis may be of public-health significance not only for Peru but also for other settings where Sabes-like interventions are considered.
2. Methods
2.1. Model
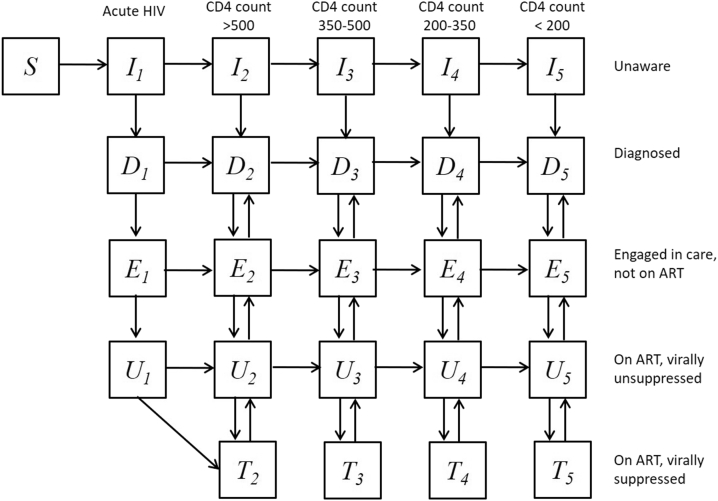
We developed a compartmental mathematical model (see model diagram, Fig. 1) to simulate the HIV epidemic among MSM and TW in Peru and assess the benefits from the intervention tested in Sabes. The individuals in the simulated population are divided into groups by risk using number of sexual partners as a surrogate (high: ≥5 male and/or TW partners in 3 months, low: <5 male and/or TW partners in 3 months) but also by age (15–24 years, 25–34 years, and 35–49 years) and sexual role during anal intercourse (insertive, receptive and versatile). The population is additionally stratified by HIV infection status and CD4 cells per mm3 (acute HIV infection, CD4 > 500, CD4 350-500, CD4 200-350, and CD4<200). Infected individuals are assigned to compartments by treatment status (not diagnosed, diagnosed but not on ART, not virally suppressed on ART, and virally suppressed on ART). Complete model description including model equations is provided in the Supplement.
Fig. 1.
Flow diagram of the model. Simulated population is stratified in compartments by HIV infection status and progression towards AIDS during five disease phases. Infected individuals are additionally stratified by treatment status as undiagnosed (compartments I), diagnosed who are not in care (compartments D), diagnosed in care not in treatment (compartment E), diagnosed on ART but virally unsuppressed (compartment U) and diagnosed on ART virally suppressed (compartment T). MSM who become sexually active join the susceptible compartment (S) at constant rate. Population is additionally stratified by age, HIV risk and sexual positioning which is not shown in the diagram. A complete description of the model including the expressions for the forces of infections (λ) is presented in the Supplement.
2.2. Model parameterization
The model is parameterized with epidemiological data representative of the HIV epidemic among MSM and TW in Peru. Demographic and sexual behavior characteristics including average number of partners per year, frequency of sex acts, proportion of acts protected by condoms, and lifetime duration of sexual activity are estimated from published data (see Table 1). The mixing between age and risk subgroups is informed from data collected in Sabes. The number of partners used in the analysis (see Table S1 in the Supplement) for the subgroups by age and risk are the average annual numbers based on reported partnerships over 3-month period by Sabes participants in each subgroup. We balance the overall number of partnerships between population subgroups by continually updating the fraction of partners someone with a given risk, role and age has from the other subgroups. Complete list of fixed parameter values used in the analysis is presented in the Supplement, Table S1.
Table 1.
Key behavioral, epidemic and intervention parameters used in the analysis.
| Description | Value (range) | Ref |
|---|---|---|
| MSM population size at the start of the simulation in 2004 | 400,000 | Chow et al. (2016) |
| HIV acquisition risk per sexual act with infected partner in asymptomatic HIV stage (CD4 >200) | 0.1%–0.2% | (Baggaley et al., 2010; Jin et al., 2010) |
| Relative infectiousness of acutely infected MSM compared to MSM with CD4>200 | 26 | Hollingsworth et al. (2008) |
| Multiplier of HIV acquisition risk per sexual act with infected partner with 12CD4<200 | 3 | calculated (Hollingsworth et al., 2008) |
| Average number of sexual acts per year in main partnerships | 40–60 | assumed |
| Average number of sexual acts per year in short-term partnerships | 2–5 | assumed |
| Average number of sexual partners per year of low risk MSM | 1.3–1.7 | SABES data |
| Average number of sexual partners per year of high risk MSM | 51–58 | SABES data |
| Proportion of sex acts protected by condom | 50% | (Ministry of Health of Per, 2006; Sanchez et al., 2007) |
| Efficacy of condom in preventing HIV transmission per anal act | 70%–90% | Smith, Herbst, Zhang, and Rose (2015) |
| Reduction of HIV infectiousness of virally-suppressed MSM on ART | 100% | (Bavinton et al., 2018; Rodger et al., 2016) |
| Reduction of HIV infectiousness of virally-unsuppressed MSM on ART | 30%–70% | assumed |
| Average time to initiate ART for newly diagnosed MSM who meet eligibility criteria at the time | 6–10 months | estimated to fit cascade |
| Annual ART drop rate | 6%–8% | Rebeiro et al. (2013) |
2.3. Model calibration
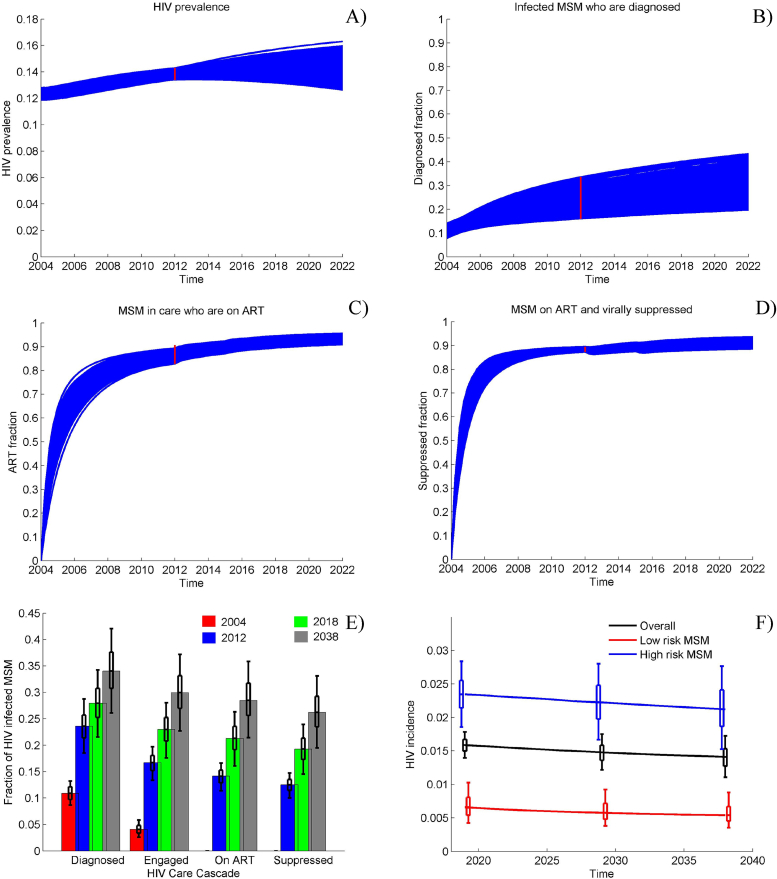
In order to calibrate the model, we fit its outputs to the HIV prevalence and the treatment cascade among MSM and TW in Peru (Table 2) using a calibration procedure described in the Supplement. Monte Carlo filtering is used to select 1000 parameter sets closely matching the 2011–2012 epidemiological data (Beyrer et al., 2011; Chow et al., 2016) and used in the analysis (see Fig. 2A–D). Complete list of parameter ranges used in the calibration procedure is presented in the Supplement, Table S2. Posterior distributions of the model parameters resulting from the calibration procedure are shown in Fig. S1.
Table 2.
Calibration targets used in the analysis.
| Parameter | Description | Range | Reference |
|---|---|---|---|
| HIV prevalence | Percent of MSM population who have HIV in 2011 | 13.4% −14.3% | Beyrer et al. (2011) |
| HIV diagnosed | Percent of infected MSM who are diagnosed with HIV in 2012 | 16%–33.6% | Chow et al. (2016) |
| Engaged in care | Percent of diagnosed MSM who are engaged in care in 2012 | 53.2%–75.5% | Chow et al. (2016) |
| On ART | Percent of infected MSM engaged in care who are on ART in 2012 | 82.8%–90.7% | Chow et al. (2016) |
| On ART, virally suppressed | Percent of infected MSM on ART, virally suppressed in 2012 | 87.3%–89.5% | Chow et al. (2016) |
Fig. 2.
Model Calibration Main reference scenario dynamics of A) HIV prevalence among MSM in Peru; B) fraction of HIV + MSM who are diagnosed; C) ART coverage among MSM in care; D) percentage of all MSM on ART who are virally suppressed; E) care cascade from 2004 to 2038 presented as fractions of all infected being diagnosed, engaged in care, on ART and virally suppressed; F) HIV incidence among high- and low-risk MSM from 2004 to 2038. Initially, ART is offered to infected individuals with CD4 < 200 cells per mm3 only, later expanded to individuals with CD4 < 350 at the end of 2011 and to individuals with CD4 < 500 at the end of 2014. Universal access to ART is introduced in 2018.1000 epidemic simulations are selected to meet the HIV prevalence and care cascade targets (red bars in panels A–D) in 2012.
2.4. Assumptions
We have modeled the acute infection period as the three months after HIV acquisition, which includes both the seronegative interval (Fiebig stages 1 and 2) and the early post-seroconversion period. In the absence of intervention, we assume that the AHI is not detected due to its short duration and the standard use of serologic assays; therefore, ART is not initiated during AHI. We assume those who are virally suppressed are not infectious, and MSM who are not virally suppressed, but on ART, are 30–70% less infectious relative to those who are not on ART. Individuals on ART may interrupt treatment and reinitiate. Universal access to ART by all HIV-infected MSM after 2018 is assumed in the main reference scenario with no changes in the calibrated rates of HIV diagnosis and ART initiation rates for each CD4 group maintained unchanged until 2038. Alternatively, we explored a more optimistic reference scenario, assuming elevated rates of HIV diagnosis after 2018 (up to three times the HIV diagnosis rate prior to 2018) leading to an improved care cascade, to study the importance of the background epidemic conditions for the impact of the Sabes intervention.
Susceptible MSM who become sexually active join the community at a constant rate, corresponding to the estimated population growth among the MSM population in Peru (Instituto Nacional de Est, 2013). The rates at which individuals acquire HIV infection depend on the annual number of partners per susceptible person, the number of sex acts per partnership, the fraction of sex acts protected by condoms, and the HIV acquisition risk per receptive and insertive anal intercourse with an HIV infected partner obtained from meta-analyses of the published data (Baggaley, White, & Boily, 2010; Jin et al., 2010).
2.5. Simulations
The model is used to simulate HIV epidemics without intervention to provide a reference scenario for the evaluation of the impact of the intervention. We evaluated the effectiveness of the intervention over the 2018–2038 period by comparing the intervention to the reference scenario. In the main reference scenario (starting in 2004), we assume that ART is initially offered to infected individuals with CD4 < 200 cells per mm3 only, with eligibility expanded to individuals with CD4 < 350 at the end of 2011 and to individuals with CD4 < 500 at the end of 2014. We assumed that ART was offered to all HIV-infected persons, regardless of CD4 count, in 2018.
The Sabes intervention included several components to promote early diagnosis and treatment. These included the following: 1) monthly HIV testing, 2) use of HIV RNA tests as well as serologic assays, 3) use of peer health navigators to facilitate rapid linkage to care and 4) immediate ART initiation regardless of CD4 count. In the intervention scenarios, we simulated “Sabes like” intervention by assuming that different proportions of acutely infected MSM were diagnosed, linked to care and initiated ART within 1 month of diagnosis. We also model interventions targeting only acutely infected MSM from the high-risk group, which constitutes an estimated 56% of the total MSM population in Peru.
2.6. Intervention impact
The impact of HIV programs are often evaluated over 20–30 years of intervention in published modeling analyses and effectiveness studies. Here we project the effectiveness of “Sabes like” intervention for each scenario over 20 years of intervention using the following effectiveness metrics: i) cumulative fraction of HIV infections prevented; ii) reduction in HIV incidence and iii) reduction in HIV prevalence due to the enhanced ART program. All metrics are compared across intervention coverage levels for 1000 simulations using the preselected sets of epidemic parameters identified in the calibration procedure.
2.7. Sensitivity analysis
The influence of key parameters on the effectiveness metrics (cumulative fraction of HIV infections prevented and reduction in HIV incidence) is studied in a multivariate sensitivity analysis. Partial rank correlation coefficients (PRCC) are calculated for each parameter-outcome pair as robust sensitivity measure for nonlinear but monotonic relationship between input and outcomes (Blower & Dowlatabadi, 1994; Marino, Hogue, Ray, & Kirschner, 2008). The analysis is based on 1000 simulations identified in the calibration procedure. Only one parameter from highly correlated pairs is included in the analysis.
3. Results
In the main reference scenario, our analysis estimates that the proportion of HIV infected MSM and TW who are diagnosed will rise to 28% by 2018, with the proportion of virally suppressed reaching 19.3% (Fig. 2E). The HIV incidence in 2018 is expected to be 1.6 per 100 person-years (pyrs) among all MSM in Peru (Fig. 2F), with substantially higher incidence in high-risk MSM (2.3/100 pyrs) compared to low-risk MSM (0.7/100 pyrs). Maintaining constant rates of ART initiation, 131,000 new HIV infections are expected over the 20-year period 2018–2038 in the absence of the Sabes intervention, with approximately 50,000 of them transmitted by infected MSM in early (acute) phase. Our model projects that the HIV incidence will remain practically unchanged and that approximately 80,000 MSM will be living with HIV in Peru in 2038, a 24.6% increase over 2018, with a small improvement in the care cascade expected (26.2% of the infected MSM being virally suppressed). In comparison, the more optimistic reference scenario, which triples the HIV diagnosis rate after 2018, will result in a significantly improved care cascade, with the diagnosed fraction of infected MSM reaching 55% and the virally suppressed fraction reaching 42% in 2038 (see Supplement, Fig. S1). As a result, overall HIV incidence is projected to decrease to 1/100 pyrs and the number of new infections over 20 years to drop by approximately 20,000 compared to the main reference scenario.
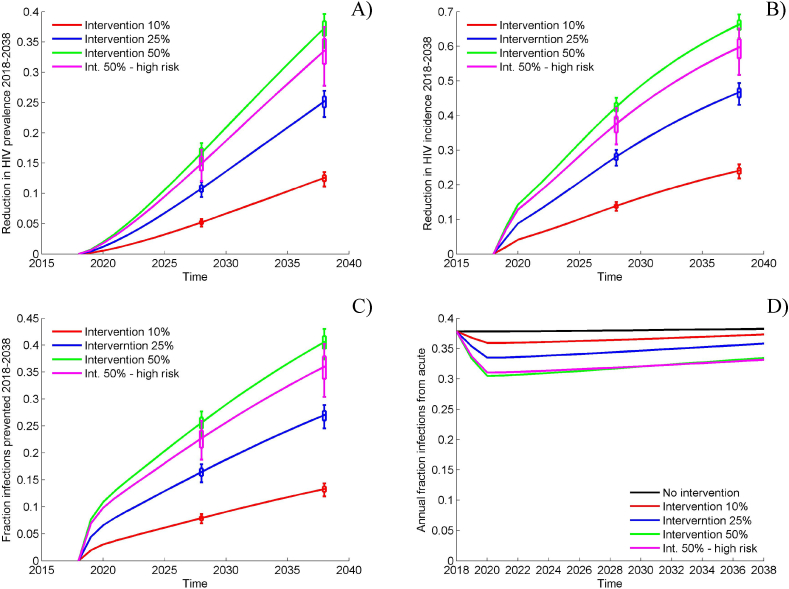
Under the assumptions of the main reference scenario, we modeled the addition of the Sabes intervention and assumed that 10% of the acutely infected MSM were diagnosed, linked to HIV care and initiated ART within 1 month of diagnosis. This intervention resulted in a projected reduction in the number of new HIV infections of 13.3% [UI: 11.9%–14.3%] over 20 years while reducing the HIV prevalence by 12.6% and HIV incidence by more than 24% by 2038 (Fig. 3). Reaching 25% and 50% of all acutely infected MSM in Peru regardless of their risk level is expected to reduce HIV incidence in 2038 by more than 45% and 65% and prevent 27% and 41% of the new infections between 2018 and 2038, respectively. At this scale, the intervention is expected to alter the care cascade substantially. HIV diagnosis and ART initiation of 50% of the acutely infected MSM beginning in 2018 will increase the diagnosed proportion of HIV + MSM in 2038 to 68% and the overall prevalence of viral suppression to 59% compared to 26% in absence of intervention (Supplement, Fig. S3A).
Fig. 3.
Projected impact of the Sabes intervention in the main analysis. A) Effectiveness in terms of cumulative fraction of infections prevented; B) Effectiveness in terms of reduction in HIV incidence; C) Effectiveness in terms of reduction in HIV prevalence; D) Estimated contribution of the acute infections to the HIV epidemic. The impact indicators are calculated using the reference scenario without intervention as a baseline. Box plots reflect estimated variation (interquartile range and 90% uncertainty interval [UI]) over 1000 epidemic simulations selected in the calibration procedure while the solid lines represent the median effectiveness estimates.
Our analysis suggests that an intervention targeting high-risk MSM who constitute 56% of the total MSM population (see Supplement, Fig. S4). Will be highly efficient. Reaching 50% of the high-risk MSM during acute HIV infection is projected to have impact comparable to non-targeted intervention at potentially lower cost. It is expected to reduce HIV incidence in 2038 by 60% and prevent 36% of the new infections between 2018 and 2038.
Our model projects that between 35% and 40% of the annual number of HIV infections among MSM in Peru result from contacts with acutely infected partners (Fig. 3D). This proportion is not affected significantly by the modeled intervention. It is reduced by less than five percent over 20 years when 50% of the acutely infected are diagnosed and initiate ART within 1 month of diagnoses.
The alternative optimistic reference scenario, which assumed elevated rates of HIV diagnosis after 2018, is expected to influence the course of the HIV epidemic (see Supplement, Figs. S1E and F). However, the Sabes intervention is projected to have a comparable impact under both scenarios. Analysis of the correlation between the pre-intervention conditions and intervention effectiveness suggests that better viral suppression at the start of the intervention will result in larger reduction in HIV incidence after 20 years (Supplement, Fig. S3B).
The partial rank correlation coefficients (PRCC) between parameters varied in the analysis and the effectiveness metrics are presented in the Supplement, Fig. S5. They suggest that assumptions about the rate of achieving viral suppression and ART efficacy for virally unsuppressed MSM are most important for both, infections prevented and reduction in HIV incidence due to the intervention. Not surprisingly, increased rate of sexual activity which results in elevated HIV risk is associated with smaller fraction of prevented infections.
4. Discussion
Published reports suggest that the HIV epidemic in Peru is concentrated in MSM and TW with high HIV prevalence, comparable to the prevalence observed in USA or among some heterosexual populations of sub-Saharan Africa. Ongoing incidence among MSM and TW is estimated to contribute to more than 95% of the new infections in Peru in 2016 (Wirtz et al., 2013). Additional evidence suggests that current HIV testing and treatment programs among MSM and TW in Peru are not effective in reducing HIV transmission due to suboptimal levels of linkage to care and viral suppression among HIV-infected MSM and TW (Chow et al., 2016).
In this study we assessed the effectiveness of a novel HIV prevention strategy, tested in the Sabes study, based on seeking, testing, treating and retaining HIV-infected MSM with recent infections (Lama et al., 2018). Our modeling analysis demonstrated that early detection of HIV infections among MSM and TW in Peru is desirable as it would increase the effectiveness of the HIV prevention program, especially if a high proportion of acutely infected MSM are diagnosed and rapidly initiated on ART. If the intervention could identify half of the acutely infected MSM and TW and initiate ART rapidly, we could expect a 3-fold decrease in HIV incidence and more than a 40% reduction in the number of HIV infections over 20 years. Moreover, we project that interventions targeting high-risk MSM with large number of sex partners will be more efficient due to disproportionate number of infections attributed to transmission from this group. An intervention reaching 50% of the high-risk MSM and TW, which results in 28% overall coverage, is expected to have an impact comparable to a non-targeted intervention with 50% coverage.
In addition to the analysis of the impact of enhanced testing and treatment our model allowed us to estimate the population attributable fraction (PAF) associated with acute HIV infections. PAF measures the contribution of a risk factor to a disease. (World Health Organization (WHO)) The PAF of acute HIV is the proportional reduction in annual HIV transmissions that would occur if exposure to HIV from acutely infected partners was completely eliminated. Mathematical models have a long history of estimating the PAF associated with acute HIV, using estimates which vary considerably between studies (Miller, Rosenberg, Rutstein, & Powers, 2010). Our analysis suggests that approximately a third of the annual new infections among MSM and TW in Peru could be attributed to transmission during acute HIV, which was higher than modeling estimates representative for MSM communities in Australia (19%) (Wilson, Hoare, Regan, & Law, 2009) and high risk behavior MSM in Amsterdam (25%) (Xiridou, Geskus, de Wit, Coutinho, & Kretzschmar, 2004) but lower than modeling estimates in the UK (48%) (Phillips et al., 2013) and Detroit, Michigan, US (45%) (Volz et al., 2013), where all transmissions occurring during the first year after infection were included. Our acute PAF estimate is also high compared to another modeling analysis for MSM in Lima, Peru (Goodreau et al., 2012) and the difference can be attributed to shorter and less infectious acute period assumed in the prior study.
What are the key drivers of intervention impact? A substantial part of the intervention effectiveness could be explained by the rapid ART initiation among acutely infected MSM and TW, which eliminates the HIV transmission mostly after the acute phase is over and leads to significant improvement of the overall HIV care cascade over 20 years. Additional benefits from improved life expectancy and quality are not considered here but should be important for cost-benefit analyses preceding implementation of such programs. Although targeting acutely infected MSM, the simulated intervention resulted in relatively small decrease in the acute PAF over time. Assuming that 50% of the acutely infected MSM and TW are identified and accounting for the 1-month delay in their ART initiation, suggests that less than 20% of the infections attributed to acutely infected MSM and TW, would be prevented which is clearly demonstrated by the insignificant drop in PAF over time. As a result, acute PAF reduction has a little contribution to the intervention impact even in the most aggressive intervention scenarios.
A key question is the feasibility of the intervention scenarios simulated in the analysis assuming that up to 50% of MSM with acute HIV infection will be consistently diagnosed and rapidly initiated on ART over prolonged periods of time. Of the 2685 uninfected MSM and TW screened in Sabes, 78.5% agreed to enroll and 62% of those still returning to retest after 12 months (Lama et al., 2018). The majority of the infections identified in the study (79%) were categorized as acquired within 3 months of the time of infection. These results suggest that 50% is a reasonable upper bound for the potential coverage of “Sabes like” intervention. The additional 20% drop in retention during the second year of follow up in Sabes indicated that 25%–30% coverage may be a more realistic target for a sustainable intervention over extended time periods.
This modeling study has some limitations. First, our calibration targets for the HIV care cascade among MSM in Peru were informed by data which was predominately obtained from the capital city, Lima, due to limited data on other regions in Peru. However, scenarios exploring alternative care cascades showed insignificant differences in effectiveness estimates. Second, the sexual mixing matrix between different age and risk subgroups were based on data from Sabes study participants, which may not be representative for the entire MSM population. Alternative scenarios assuming proportional mixing demonstrated no influence on the reported results. Third, pre-exposure prophylaxis (PrEP) was not included in the model given that it is not currently included in the prevention and treatment guidelines provided by the Peruvian Ministry of Health, and to date, the proportion of people accessing PrEP has been negligible. Fourth, we were not able to separate MSM and TW based on the lack of power to identify differences between these populations in Sabes. Finally, our analysis does not provide a sense of efficiency of the intervention, which may be important for benefits to be considered in the context of the effort and resources needed for their realization. Such analysis will require more detailed data on the number of tests needed to achieve the targeted intervention coverage as well as the cost to keep newly diagnosed MSM and TW on treatment. It is planned as a future research direction of our team. Questions about the asymptotic behavior of the model as dynamical system and particularly about existence and stability of equilibria may be theoretically interesting but remained beyond the scope of this paper. These questions may be studied by existing methodologies (Guo, Li, & Shuai, 2012) but obtaining practical results may be hindered by the extreme complexity of the model.
In the Sabes study we demonstrated that early HIV detection among MSM in Peru is feasible; herein we have outlined its importance for the success of future HIV prevention programs. Early HIV detection is expected to increase ART coverage substantially and therefore increase the prevalence of viral suppression among HIV-infected MSM. Further analyses which optimize treatment-as-prevention strategies will be warranted with the expected expansion of available HIV prevention tools, including daily and long-acting PrEP, and preventive HIV vaccines.
Acknowledgements
The Sabes study was funded by National Institute on Drug Abuse (RO1 DA032106; PI: Ann Duerr). We acknowledge ART drug donation from Gilead Sciences Inc. and Merck & Co Inc for this study.
Handling Editor: J. Wu
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.idm.2019.04.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Baggaley R.F., White R.G., Boily M.C. HIV transmission risk through anal intercourse: Systematic review, meta-analysis and implications for HIV prevention. International Journal of Epidemiology. 2010;39(4):1048–1063. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavinton B.R., Pinto A.N., Phanuphak N., Grinsztejn B., Prestage G.P., Zablotska-Manos I.B. Viral suppression and HIV transmission in serodiscordant male couples: An international, prospective, observational, cohort study. The Lancet HIV. 2018;5(8):e438–e447. doi: 10.1016/S2352-3018(18)30132-2. [DOI] [PubMed] [Google Scholar]
- Beyrer C.W., Andrea L., Walker D., Johns B., Sifakis F., Baral Stefan D. The World Bank; 2011. The global HIV epidemics among men who have sex with men (MSM) [Google Scholar]
- Blower S.M., Dowlatabadi H. Sensitivity and uncertainty analysis of complex-models of disease transmission - an hiv model, as an example. International Statistical Review. 1994;62(2):229–243. [Google Scholar]
- Brenner B.G., Roger M., Routy J.-P., Moisi D., Ntemgwa M., Matte C. High rates of forward transmission events after acute/early HIV-1 infection. The Journal of Infectious Diseases. 2007;195(7):951–959. doi: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- Brenner B.G., Roger M., Stephens D., Moisi D., Hardy I., Weinberg J. Transmission clustering drives the onward spread of the HIV epidemic among men who have sex with men in Quebec. The Journal of Infectious Diseases. 2011;204(7):1115–1119. doi: 10.1093/infdis/jir468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.E., Gifford R.J., Clewley J.P., Kucherer C., Masquelier B., Porter K. Phylogenetic reconstruction of transmission events from individuals with acute HIV infection: Toward more-rigorous epidemiological definitions. The Journal of Infectious Diseases. 2009;199(3):427–431. doi: 10.1086/596049. [DOI] [PubMed] [Google Scholar]
- Caceres C.F., Mendoza W. The national response to the HIV/AIDS epidemic in Peru: Accomplishments and gaps--a review. Journal of Acquired Immune Deficiency Syndromes. 2009;51(Suppl 1):S60–S66. doi: 10.1097/QAI.0b013e3181a66208. [DOI] [PubMed] [Google Scholar]
- Carlson J.M., Schaefer M., Monaco D.C., Batorsky R., Claiborne D.T., Prince J. Selection bias at the heterosexual HIV-1 transmission bottleneck. Science. 2014;345 doi: 10.1126/science.1254031. (6193), 1254–031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibo D., Kaye M., Birch C. HIV transmissions during seroconversion contribute significantly to new infections in men who have sex with men in Australia. AIDS Research and Human Retroviruses. 2011;28(5):460–464. doi: 10.1089/AID.2011.0137. [DOI] [PubMed] [Google Scholar]
- Chow J.Y., Konda K.A., Borquez A., Caballero P., Silva-Santisteban A., Klausner J.D. Peru's HIV care continuum among men who have sex with men and transgender women: Opportunities to optimize treatment and prevention. International Journal of STD & AIDS. 2016;27(12):1039–1048. doi: 10.1177/0956462416645727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehne K.L., Dallabetta G., Wilson D., Garnett G.P., Laga M., Benomar E. HIV prevention 2020: A framework for delivery and a call for action. The Lancet HIV. 2016;3(7):e323–e332. doi: 10.1016/S2352-3018(16)30035-2. [DOI] [PubMed] [Google Scholar]
- Dimitrov D.T., Boily M.C., Baggaley R.F., Masse B. Modeling the gender-specific impact of vaginal microbicides on HIV transmission. Journal of Theoretical Biology. 2011;288:9–20. doi: 10.1016/j.jtbi.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D.T., Masse B.R., Donnell D. PrEP adherence patterns strongly impact individual HIV risk and observed efficacy in randomized clinical trials. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2016;72(72):444–451. doi: 10.1097/QAI.0000000000000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton J.W., Menzies N.A., Stover J., Cambiano V., Chindelevitch L., Cori A. Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. The Lancet Global Health. 2014;2(1):e23–e34. doi: 10.1016/S2214-109X(13)70172-4. [DOI] [PubMed] [Google Scholar]
- Eaton J.W., Hallett T.B., Garnett G.P. Concurrent sexual partnerships and primary HIV infection: A critical interaction. AIDS and Behavior. 2011;15(4):687–692. doi: 10.1007/s10461-010-9787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English S., Katzourakis A., Bonsall D., Flanagan P., Duda A., Fidler S. Phylogenetic analysis consistent with a clinical history of sexual transmission of HIV-1 from a single donor reveals transmission of highly distinct variants. Retrovirology. 2011;8(1):54. doi: 10.1186/1742-4690-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig E.W., Wright D.J., Rawal B.D., Garrett P.E., Schumacher R.T., Peddada L. Dynamics of HIV viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17(13):1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- Frange P., Meyer L., Deveau C., Tran L., Goujard C., Ghosn J. Recent HIV-1 infection contributes to the viral diffusion over the French territory with a recent increasing frequency. PLoS One. 2012;7(2):e31695. doi: 10.1371/journal.pone.0031695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodreau S.M., Carnegie N.B., Vittinghoff E., Lama J.R., Sanchez J., Grinsztejn B. What drives the US and Peruvian HIV epidemics in men who have sex with men (MSM)? PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Li M., Shuai Z. Global dynamics of a general class of multistage models for infectious diseases. SIAM Journal on Applied Mathematics. 2012;72(1):261–279. [Google Scholar]
- Hollingsworth T.D., Anderson R.M., Fraser C. HIV-1 transmission, by stage of infection. Journal of Infectious Diseases. 2008;198(5):687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- Hollingsworth T.D., Pilcher C.D., Hecht F.M., Deeks S.G., Fraser C. High transmissibility during early HIV infection among men who have sex with men—san Francisco, California. The Journal of Infectious Diseases. 2015;211(11):1757–1760. doi: 10.1093/infdis/jiu831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto Nacional de Estadística e Informática - INEI/Perú . 2013. Perú Encuesta Demográfica y de Salud Familiar - ENDES 2012. Retrieved from Lima, Perú: http://dhsprogram.com/pubs/pdf/FR284/FR284.pdf. [Google Scholar]
- Jin F.Y., Jansson J., Law M., Prestage G.P., Zablotska I., Imrie J.C.G. Per-contact probability of HIV transmission in homosexual men in Sydney in the era of HAART. AIDS. 2010;24(6):907–913. doi: 10.1097/QAD.0b013e3283372d90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouyos R.D., von Wyl V., Yerly S., Böni J., Taffé P., Shah C. Molecular epidemiology reveals long-term changes in HIV type 1 subtype B transmission in Switzerland. The Journal of Infectious Diseases. 2010;201(10):1488–1497. doi: 10.1086/651951. [DOI] [PubMed] [Google Scholar]
- Lama J.R., Brezak A., Dobbins J.G., Sanchez H., Cabello R., Rios J. Design strategy of the Sabes study: Diagnosis and treatment of early HIV infection among men who have sex with men and transgender women in Lima, Peru, 2013-2017. American Journal of Epidemiology. 2018;187(8):1577–1585. doi: 10.1093/aje/kwy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z.-M., Stone M., Piatak M., Schweighardt B., Haigwood N.L., Montefiori D. High specific infectivity of plasma virus from the pre-ramp-up and ramp-up stages of acute simian immunodeficiency virus infection. Journal of Virology. 2009;83(7):3288–3297. doi: 10.1128/JVI.02423-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino S., Hogue I.B., Ray C.J., Kirschner D.E. A methodology for performing global uncertainty and sensitivity analysis in systems biology. Journal of Theoretical Biology. 2008;254(1):178–196. doi: 10.1016/j.jtbi.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W.C., Rosenberg N.E., Rutstein S.E., Powers K.A. The role of acute and early HIV infection in the sexual transmission of HIV. Current Opinion in HIV and AIDS. 2010;5(4):277–282. doi: 10.1097/COH.0b013e32833a0d3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health of Peru . 2006. A step forward in the fight against AIDS: The first two years of universal access to antiretroviral treatment in Peru. Lima, Peru. [Google Scholar]
- de Montigny S., Adamson B.J.S., Mâsse B.R., Garrison L.P., Kublin J.G., Gilbert P.B. Projected effectiveness and added value of HIV vaccination campaigns in South Africa: A modeling study. Scientific Reports. 2018;8(1):6066. doi: 10.1038/s41598-018-24268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao D., Fisher M., Hue S., Dean G., Murphy G., Cane P.A. Transmission of HIV-1 during primary infection: Relationship to sexual risk and sexually transmitted infections. AIDS. 2005;19(1):85–90. doi: 10.1097/00002030-200501030-00010. [DOI] [PubMed] [Google Scholar]
- Phillips A.N., Cambiano V., Nakagawa F., Brown A.E., Lampe F., Rodger A. Increased HIV incidence in men who have sex with men despite high levels of ART-induced viral suppression: Analysis of an extensively documented epidemic. PLoS One. 2013;8(2):e55312. doi: 10.1371/journal.pone.0055312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilcher C.D., Joaki G., Hoffman I.F., Martinson F.E., Mapanje C., Stewart P.W. Amplified transmission of HIV-1: Comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS. 2007;21(13):1723–1730. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines H.A., Wertheim J.O., Liu L., Garfein R.S., Little S.J., Karris M.Y. Concurrency and HIV transmission network characteristics among MSM with recent HIV infection. AIDS. 2016;30(18):2875–2883. doi: 10.1097/QAD.0000000000001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiro P., Althoff K.N., Buchacz K., Gill J., Horberg M., Krentz H. Retention among North American HIV-infected persons in clinical care, 2000-2008. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2013;62(3) doi: 10.1097/QAI.0b013e31827f578a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger A.J., Cambiano V., Bruun T., Vernazza P., Collins S., van Lunzen J. Sexual activity without condoms and risk of hiv transmission in serodifferent couples when the hiv-positive partner is using suppressive antiretroviral therapy. Journal of the American Medical Association. 2016;316(2):171–181. doi: 10.1001/jama.2016.5148. [DOI] [PubMed] [Google Scholar]
- Rutstein S.E., Ananworanich J., Fidler S., Johnson C., Sanders E.J., Sued O. Clinical and public health implications of acute and early HIV detection and treatment: A scoping review. Journal of the International AIDS Society. 2017;20(1):21579. doi: 10.7448/IAS.20.1.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J., Lama J.R., Kusunoki L., Manrique H., Goicochea P., Lucchetti A. HIV-1, sexually transmitted infections, and sexual behavior trends among men who have sex with men in Lima, Peru. Journal of Acquired Immune Deficiency Syndromes. 2007;44(5):578–585. doi: 10.1097/QAI.0b013e318033ff82. [DOI] [PubMed] [Google Scholar]
- Selinger C., Bershteyn A., Dimitrov D.T., Adamson B.J.S., Revill P., Hallett T.B. Targeting and vaccine durability are key for population-level impact and cost-effectiveness of a pox-protein HIV vaccine regimen in South Africa. Vaccine. 2019;37(16):2258–2267. doi: 10.1016/j.vaccine.2019.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Santisteban A., Raymond H.F., Salazar X., Villayzan J., Leon S., McFarland W. Understanding the HIV/AIDS epidemic in transgender women of Lima, Peru: Results from a sero-epidemiologic study using respondent driven sampling. AIDS and Behavior. 2012;16(4):872–881. doi: 10.1007/s10461-011-0053-5. [DOI] [PubMed] [Google Scholar]
- Smith D.K., Herbst J.H., Zhang X., Rose C.E. Condom effectiveness for HIV prevention by consistency of use among men who have sex with men in the United States. Jaids Journal of Acquired Immune Deficiency Syndromes. 2015;68(3):337–344. doi: 10.1097/QAI.0000000000000461. [DOI] [PubMed] [Google Scholar]
- Steward W.T., Remien R.H., Higgins J.A., Dubrow R., Pinkerton S.D., Sikkema K.J. Behavior change following diagnosis with acute/early HIV infection-a move to serosorting with other HIV-infected individuals. The NIMH multisite acute HIV infection study: III. AIDS and Behavior. 2009;13(6):1054–1060. doi: 10.1007/s10461-009-9582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabet S., Sanchez J., Lama J., Goicochea P., Campos P., Rouillon M. HIV, syphilis and heterosexual bridging among Peruvian men who have sex with men. AIDS. 2002;16(9):1271–1277. doi: 10.1097/00002030-200206140-00010. [DOI] [PubMed] [Google Scholar]
- UNAIDS . 2012. UNAIDS report on the global AIDS epidemic. Retrieved from http://www.unaids.org/en/resources/documents/2012/20121120_UNAIDS_Global_Report_2012. [Google Scholar]
- UNAIDS . 2016. Prevention gap report.http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf Retrieved from. [Google Scholar]
- Vickerman P., Ndowa F., O'Farrell N., Steen R., Alary M., Delany-Moretlwe S. Using mathematical modelling to estimate the impact of periodic presumptive treatment on the transmission of sexually transmitted infections and HIV among female sex workers. Sexually Transmitted Infections. 2010;86:163–168. doi: 10.1136/sti.2008.034678. [DOI] [PubMed] [Google Scholar]
- van de Vijver D.A.M.C., Nichols B.E., Abbas U.L., Boucher C.A.B., Cambiano V., Eaton J.W. Preexposure prophylaxis will have a limited impact on HIV-1 drug resistance in sub-saharan Africa: A comparison of mathematical models. AIDS. 2013;27:2943–2951. doi: 10.1097/01.aids.0000433237.63560.20. [DOI] [PubMed] [Google Scholar]
- Volz E.M., Ionides E., Romero-Severson E.O., Brandt M.-G., Mokotoff E., &Koopman J.S. HIV-1 transmission during early infection in men who have sex with men: A phylodynamic analysis. PLoS Medicine. 2013;10(12) doi: 10.1371/journal.pmed.1001568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D.P., Hoare A., Regan D.G., Law M.G. Importance of promoting HIV testing for preventing secondary transmissions: Modelling the Australian HIV epidemic among men who have sex with men. Sexual Health. 2009;6(1):19–33. doi: 10.1071/sh08081. [DOI] [PubMed] [Google Scholar]
- Wirtz A.L., Walker D.G., Bollinger L., Sifakis F., Baral S., Johns B. Modelling the impact of HIV prevention and treatment for men who have sex with men on HIV epidemic trajectories in low- and middle-income countries. International Journal of STD & AIDS. 2013;24(1):18–30. doi: 10.1177/0956462412472291. [DOI] [PubMed] [Google Scholar]
- Wood D., Lancaster K.E., Boily M.-C., Powers K.A., Donnell D., Cohen M.S. Recruitment of female sex workers in HIV prevention trials: Can efficacy endpoints be reached more efficiently? JAIDS Journal of Acquired Immune Deficiency Syndromes. 2018;77(4):350–357. doi: 10.1097/QAI.0000000000001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) Metrics: Population attributable fraction (PAF) http://www.who.int/healthinfo/global_burden_disease/metrics_paf/en/ Retrieved from.
- Xiridou M., Geskus R., de Wit J., Coutinho R., Kretzschmar M. Primary HIV infection as source of HIV transmission within steady and casual partnerships among homosexual men. AIDS. 2004;18(9):1311–1320. doi: 10.1097/00002030-200406180-00010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.