Abstract
Timely placement of an arteriovenous (AV) vascular access (native AV fistula [AVF] or prosthetic AV graft [AVG]) is necessary to limit the use of tunneled central venous catheters (TCVC) in patients with end-stage kidney disease (ESKD) treated with hemodialysis (HD). National guidelines recommend placement of AVF as the AV access of first choice in all patients to improve patient survival. The benefits of AVF over AVG are less certain in the older adults, as age-related biological changes independently modulate patient outcomes. This manuscript describes the rationale, study design and protocol for a randomized controlled pilot study of the feasibility and effects of AVG-first access placement in older adults with no prior AV access surgery. Fifty patients age ≥65 years, with incident ESKD on HD via TCVC or advanced kidney disease facing imminent HD initiation, and suitable upper extremity vasculature for initial placement of an AVF or AVG, will be randomly assigned to receive either an upper extremity AVG-first (intervention) or AVF-first (comparator) access. The study will establish feasibility of randomizing older adults to the two types of AV access surgery, evaluate relationships between measurements of preoperative physical function and vascular access development, compare vascular access outcomes between groups, and gather longitudinal assessments of upper extremity muscle strength, gait speed, performance of activities of daily living, and patient satisfaction with their vascular access and quality of life. Results will assist with the planning of a larger, multicenter trial assessing patient-centered outcomes.
Keywords: arteriovenous access; Fistula; Graft; Hemodialysis; Older patients; Word count; abstract; 240; Text: 4,526; Figures: 1; Tables: 4
1. Introduction
Each year, more than 600,000 people in the United States receive life-saving hemodialysis (HD) treatments for end-stage kidney disease (ESKD), more than a third of whom are adults 65 years of age or older [1]. Timely placement of an arteriovenous (AV) vascular access (native AV fistula [AVF] or prosthetic AV graft [AVG]) is necessary to avoid (or limit) the use of tunneled central venous catheters (TCVC) for HD. Several retrospective studies have assessed the relationship between the type of HD vascular access with access complications and patient survival. The lowest access complication rate and mortality rate were seen with native, autologous AVF; whereas, the highest access complication rates and mortality rate were seen with TCVC [[2], [3], [4], [5], [6]]. Intermediate results were seen with AVG. These findings led national committees to promote ‘Fistula First Catheter Last’ guidelines that recommend placement of AVF as the access of first choice in all patients on HD [7]. However, the studies that formed the basis for these guidelines were not representative of older populations. The demographics of the dialysis population have continuously changed over the last two decades, such that the proportion of patients ≥65 years old has increased more than two-fold in the last two decades [1]. In a more contemporary national cohort of patients with incident ESKD who started HD with a TCVC, subsequent placement of either AVF or AVG was associated with similar mortality hazard in the whole cohort (hazard ratio [HR], 0.98, 95% CI, 0.93–1.02; P = 0.349); in patients older than 80 years with albumin level >4.0 g/dl, AVF creation was associated with higher mortality hazard compared with AVG creation (HR, 1.22, 95% CI, 1.04–1.43; P = 0.013) [8].
It is challenging for observational studies to determine whether the type of vascular access placed directly affect clinical outcomes or whether there is a selection bias whereby the choice of vascular access reflects comorbidities that impacted outcomes. For example, the decision to place an AVF may reflect a healthier clinical status in ways that cannot be captured with sophisticated statistical analyses (e.g., perceived better prognosis, less severe comorbidities) [9,10]. These confounding factors can ultimately impact achievement of a usable AVF and affect patient survival. Additionally, the pathophysiologic mechanism underlying the purported link between the vascular access and survival on HD has not been elucidated. Vascular access-related infectious complications have been proposed as the primary mechanism responsible for the excess mortality observed with TCVC or AVG, contrasted with AVF [11]. Recent studies revealed that only 2.3% of deaths in patients on HD are access-related, and mediation analyses indicated that vascular access complications cannot adequately explain the association between access type and mortality [10,12]. In a study with prospective monthly collection of infection data in 177,875 prevalent and 11,290 incident patients on HD, vascular access infection rates were identical in patients with AVFs and AVGs [13]. This highlights the need for clinicians to continually re-evaluate data on vascular access outcomes to choose the optimal HD vascular access. Age also modulates TCVC-related infectious complications. In a 3-year retrospective study of 464 incident and prevalent patients on HD, we found that the likelihood of TCVC-related bacteremia was 67% lower in patients >75 years of age compared to younger patients; these analyses adjusted for comorbidities, catheter lock solutions, catheter location, and immunosuppressive medications [14].
Outcomes of HD vascular access may need to be considered separately in older populations. Compared with AVGs, AVFs fail more often and necessitate longer maturation times and more subsequent procedures to aid development and patency [[15], [16], [17], [18]]. Specifically, in patients 66 years of age and older, placement of AVF was accompanied by repeat vascular access creation or TCVC insertion in 44% of the patients, while vascular access reinterventions occurred in 33.7% of those who underwent AVG placement (P < 0.001) [18]. This exposes older patients to time-consuming procedures that may negatively affect upper extremity strength and erode quality of life. Prosthetic AVGs have nearly double primary patency rates (80–90% vs. 40–60%) and three times shorter intervals to cannulation (44 vs. 97 days) than native AVFs [[19], [20], [21]]. These properties may render AVGs as a superior “catheter-sparing” strategy compared to AVFs. Even though AVFs are thought to offer longer cumulative patency (i.e., total time in use for HD until the access is abandoned), vascular access survival analyses that include primary AVF failures (i.e., surgically created AVFs that never developed or were never successfully used for HD) show similar AVG and AVF cumulative survival or superior survival of AVGs in the initial 18 months after creation [22,23]. Considering that older patients experience shorter survival on dialysis, seamless transitions from a TCVC to an AV access is critical, rather than pursuing access strategies with slower and unpredictable maturation. Moreover, hemodynamic perturbations and local inflammation that occur following placement of an AV access can have a negative impact on hand function [24]; consequently, patient's independence level can dramatically change postoperatively. This manuscript describes the design and methodology of a randomized pilot trial to evaluate feasibility of graft-first AV access placement and relationships between initial AV access approach and outcomes important to health and quality of life in older patients. Outcomes include muscle strength as well as vascular access outcomes in patients 65 years of age and older on HD.
2. Materials and methods
2.1. Research objectives and hypotheses
This single center study, performed at the Wake Forest School of Medicine (WFSM), focuses on older patient population with advanced kidney disease and no prior vascular access surgery. It will randomly allocate patients ≥65 years of age, with ESKD initiated on HD via a TCVC within 90 days prior to screening or with advanced kidney disease expected to require chronic HD initiation within 90 days of screening, to either graft-first or fistula-first AV access placement. Patient-centered functional outcomes (upper extremity strength, independence and quality of life) and vascular access outcomes (AV access primary failure incidence rate, AV access primary patency rate, time to successful AV access cannulation, TCVC-free HD days) will be compared. The scientific premise is that graft-first placement will yield higher rates of functional access, the need for fewer postoperative procedures to aid access development, and faster transition from TCVC to AV access use. These will translate into higher patient satisfaction in the graft-first group. The first hypothesis is that AV access placement will have adverse consequences on upper extremity function, mediated by the degree of preoperative muscle strength and frailty. We anticipate that the fistula-first strategy will have a greater negative impact on upper extremity physical function than the graft-first strategy. The second hypothesis is that the success rate of AV access approach (i.e., primary AV access patency) will correlate with patient-reported outcomes for quality of life. Data from this pilot study will guide the formulation of the study design and implementation plan for a subsequent multicenter study comparing AVG-first to AVF-first strategy in older adults with advanced kidney disease, with the overarching objective of identifying strategies that decrease AV access failure rates and improve patients’ quality of life.
2.2. Overview of study design
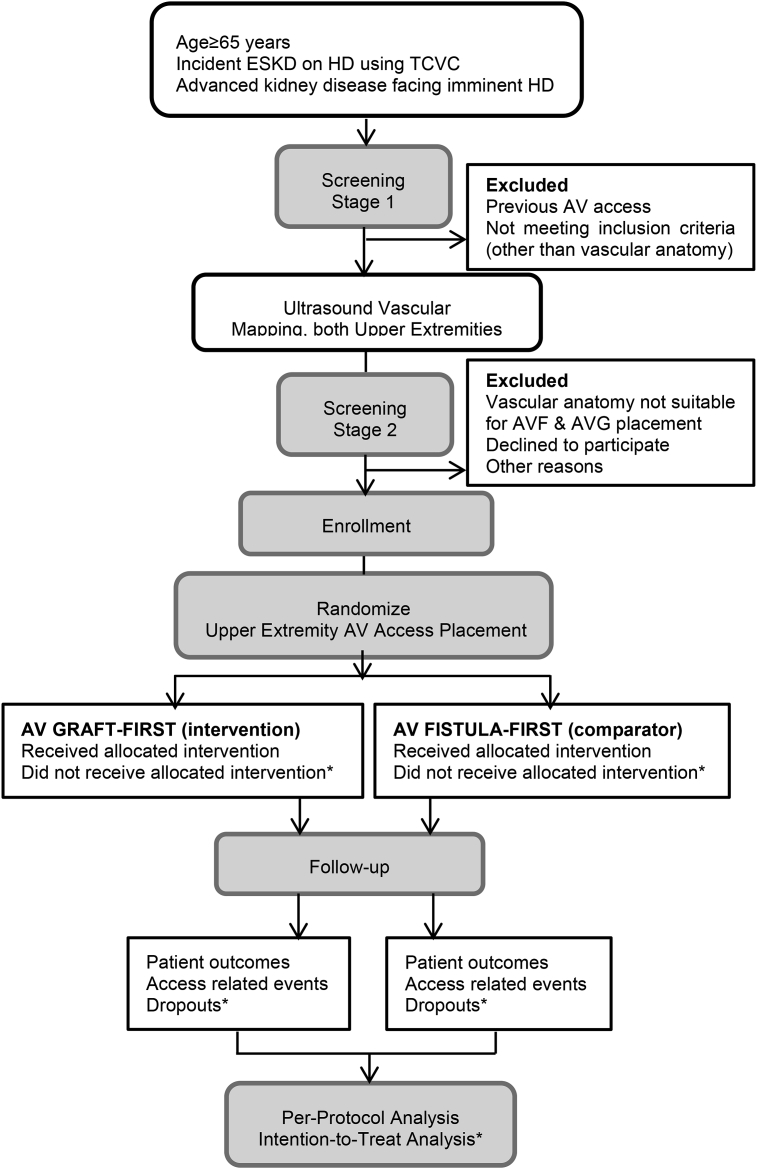
This pilot study has a randomized parallel-arm design with a 12-month planned enrollment of 50 patients (25 per arm). Screening for potential participants includes two stages (Fig. 1). All patients ≥65 years old with incident ESKD in the first 90 days of receipt of chronic HD via a TCVC or with advanced kidney disease will be screened for eligibility in stage one. Of these, only those who did not have a previous AV access surgery and are being considered for AV access placement by the patient's primary nephrologist will advance to the second screening stage. The second stage depends on surgical candidacy for placement of an upper extremity AV access. Ultrasound vascular mapping of both upper extremities to assess vascular anatomy is routinely performed preoperatively at our institution. Arterial diameter ≥2 mm and vein diameter ≥2.5 mm at the site of AV anastomosis is applied to indicate suitability for AVF surgical creation [7]. To complete the eligibility criteria, patients must have proper anatomy (in either upper extremity) for placement of AVF (forearm or upper arm) and AVG (forearm or upper arm). The study has been approved by the WFSM Institutional Review Board and will be carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Fig. 1.
Study flow diagram.
2.3. Participant eligibility
Final eligibility will be based on the inclusion and exclusion criteria listed in Table 1. After written informed consent is obtained, participants will be randomized with 1:1 allocation to surgical placement of an AVG or AVF. Allocation to index AV access surgery will be done using randomly permuted blocks of varying sizes to maintain balance between the groups.
Table 1.
Eligibility criteria.
| Inclusion criteria |
|---|
|
| Exclusion criteria |
|
Abbreviations: ESKD, end-stage kidney disease; HD, hemodialysis; TCVC, tunneled central venous catheter; AV, arteriovenous; AVF, arteriovenous fistula; AVG, arteriovenous graft.
2.4. Participant recruitment
Potential participants will be identified through screening of electronic clinical records, including those of outpatient dialysis facilities, nephrology outpatient clinics, and nephrology inpatient service. The study will be performed across 15 outpatient dialysis units affiliated with a large tertiary academic medical center in North Carolina. Once all inclusion and exclusion criteria are met, eligible participants will be approached and the study's objectives explained. They will then be asked if they wish to participate. Interactions between study personnel and participants will take place in traditional care settings (dialysis units, hospital, physician offices).
2.5. Specific aims
2.5.1. Specific aim 1
To establish feasibility of randomizing initial AV access placement to an upper extremity AVG-first (intervention) or AVF-first (comparator) strategy in patients ≥65 years of age. Feasibility will be tested as: (1) proportion of eligible patients recruited, (2) proportion of participants who receive AV access placement as randomized, (3) adherence to study-related assessments, and (4) proportion of participants with successful follow-up.
2.5.2. Specific aim 2
To determine effects of AV access placement on participant grip strength, physical activity and quality of life. The outcomes of interest are changes in grip strength, self-reported ability to perform activities of daily living (ADLs) and instrumental ADLs (IADLs), patient satisfaction with AV access and health-related quality of life.
2.5.2.1. Sub-aim 2
To explore relationships between preoperative upper limb muscle strength, frailty, and functional status with primary AV access outcome.
2.5.3. Specific aim 3
To compare AV access-related outcomes between the two AV access strategies. The primary outcome is the incidence rate of AV access primary failure. Secondary outcomes will include: i) access primary patency, ii) time to successful AV access cannulation, iii) access cumulative patency, and iv) rate of AV access salvage procedures required to attain or maintain access patency.
2.6. Trial interventions
Eligibility for anesthesia and surgical placement of an AV access will be determined as part of standard of care for each patient. In clinical practice, this considers the patient's prognosis (e.g., unlikely to recover kidney function, medically stable to undergo surgical placement of AV access) and treatment goals (e.g., HD is the intended long-term therapeutic modality). Surgical suitability for placement of AV access, AVF and AVG, will be determined by the vascular surgeon. The vascular surgeon will use the results of the ultrasound vascular mapping of both upper extremities to make this determination.
Based on randomization, participants will undergo an attempt at AVG or AVF creation by an experienced vascular surgeon. All AV grafts will be of expandable polytetrafluoroethylene (ePTFE) material. The standardization of the type of material used for AV shunt creation eliminates graft outcome variability by type of conduit. Although the use of ePTFE grafts might limit external validity, this type of conduit is, at present, the most commonly used material for AVG creation.
Given suitable vasculature, preference will be given to placement of distal (forearm) over proximal (arm) AV access in both study groups. This preserves vascular access sites for future use. The protocol requires that the graft and fistula placement surgery occur within the initial 90 days post-randomization. Location of graft placement will also take into account preservation of autologous options by using opposite arm when vein suitable for an AVF is only present on one side.
Following surgical placement of the index AV access, the vascular access will be evaluated within the first 6–8 postoperative hours. The nature of the vascular access thrill, bruit, pulse, and/or signs of upper arm ischemia will be documented. Three to four weeks after the index AVG is placed or four weeks after the index AVF is placed, the index vascular access will be evaluated by an interventional nephrologist to assess AV access development and suitability for cannulation (Table 2, Schedule of Assessments).
Table 2.
Schedule of assessments.
| Assessment | Enrollment | Week 1 after AV access placement ( ±7 days) | Week 3 after AVG or Week 4 after AVF placement | Week 4 of AV access cannulation ( ±7 days) | Week 12 & 24 of AV access use ( ±7 days) | Week 24 after AV access placement if AV access primary failure ( ±7 days) |
|---|---|---|---|---|---|---|
| Grip strength | ✓ | ✓ | Evaluation by Interventional Nephrologist to assess development and cannulation suitability | ✓ | ✓ | ✓ |
| VDS or APS | ✓ | ✓ | ✓ | ✓ | ||
| SF-VAQ | ✓ | ✓ | ✓ | ✓ | ||
| ADLs and IADLs | ✓ | ✓ | ✓ | ✓ | ||
| KDQOL-SF 1.3. | ✓ | ✓ | ✓ | ✓ |
Abbreviations: AV, arteriovenous; AVF, arteriovenous fistula; AVG, arteriovenous graft; VDS, Verbal Descriptor Scale; APS, Abbey Pain Scale; SF-VAQ, Short-form vascular access questionnaire; ADLs, Activities of daily living; IADLs, Instrumental ADLs; KDQOL-SF, Kidney Disease Quality of Life Short-form.
2.7. Data collection and study measures
2.7.1. Data collection
Baseline demographic and clinical characteristics obtained at enrollment include sociodemographic characteristics (age, sex, self-reported race, marital status, educational level, living arrangement [home, assisted living facility, nursing home]), health behaviors (smoking and alcohol consumption [no, formerly, or yes]), ESKD etiology, date of HD commencement, pre-dialysis care (first nephrology clinic visit and number of clinic visits prior to the first HD date), comorbidity, frailty, and medications (lipid lowering drugs, anticoagulants, antiplatelet agents) [25,26]. Comorbidity will be estimated using the Charlson Comorbidity Index with diagnosis codes extracted from the electronic medical record [27]. Frailty at baseline will be assessed using an electronic Frailty Index (eFI), which is based on the model of deficit accumulation and has been adapted to data derived from the electronic medical record. Specifically, the eFI incorporates data on chronic disease, clinical signs and symptoms, functional impairment, laboratory results, medications, and vital signs. The eFI is then estimated as the proportion of deficits each patient has out of the total set of possible deficits, producing a score between 0 and 1. Higher eFI scores indicate increasing frailty, with scores >0.20 generally indicating frailty [28,29]. Characteristics of upper extremity vasculature and surgery for index AV access will be recorded, such as arterial and venous diameters on preoperative ultrasound mapping, AV access date of placement, and location of AV anastomosis.
Results of routine monthly blood work (per standard care) at outpatient dialysis units will be recorded, including measurements of dialysis adequacy, anemia management, and bone-mineral metabolism. Hospital admissions with primary admission diagnoses and length of stay will be recorded during the study period. All deaths that occur during the study period will be recorded and classified into six categories (cardiovascular, sudden death at home, infectious, malignancy-related, elective withdrawal from dialysis, and other), sub-classified as to whether death was vascular access-related. The cause of death will be determined by the patient's primary nephrologist and/or primary physician according to usual practice.
2.7.2. Vascular access outcomes
Vascular access outcomes definitions are summarized in Table 3 [30]. Access outcomes will be evaluated as time-to-event and as rate per 1,000 access days to allow cross-study comparison. The denominator ‘access days’ would be the number of days the access (index fistula, index graft, or TCVC) was in place from surgical placement or study enrollment to the end of access use or reaching a censoring event. Time-to-event for AV access-related events will be calculated from the date of index AV access placement. Time-to-event for TCVC-related events will be calculated from the date of participant enrollment.
Table 3.
Vascular access outcomes (according to the recommendations of the Committee on Reporting Standards for Arteriovenous Accesses, Society for Vascular Surgery and American Association for Vascular Surgery).
| AV access outcome | Definition |
|---|---|
| Access primary failure | Permanent failure of the fistula or graft before hemodialysis suitability. This includes inadequate maturation, thrombosis, failure of first and subsequent cannulations, and other complications leading to nonfunctional fistula or graft. |
| Successful cannulation | The AV access became the primary vascular access for hemodialysis (the fistula or graft access has been cannulated with two 16- or 15-gauge needles for ≥3 consecutive dialysis sessions and the TCVC was removed). |
| Suitability for hemodialysis | Fistula or graft use with two needles and maintenance of blood flow ≥300 ml/min for ≥75% of dialysis sessions over a continuous 4-week. The maturation criteria can be satisfied at any time within 6 months of fistula or graft creation surgery. |
| Unassisted access maturation | Criteria for fistula or graft suitable for hemodialysis (based on the above criteria) are met before any endovascular or secondary surgical procedure to facilitate maturation. |
| Assisted access maturation | Satisfaction of the criteria for fistula or graft suitability for hemodialysis after a procedure to facilitate maturation (e.g., angioplasty, stent placement, surgical revision, ligation of accessory veins). |
| Access primary patency | Procedure-free access survival defined as the time from index fistula or graft creation to the first of one of the following events: access thrombosis; any procedure designed to facilitate, maintain, or re-establish patency. |
| Access assisted primary patency | Interval of time from index fistula or graft creation until the first of one of the following events: access thrombosis, censoring event, or study end. This period includes all procedures (surgical or endovascular) designed to maintain the functionality of the dialysis vascular access as long as access patency was not lost. |
| Access cumulative patency | Interval of time from index fistula or graft creation until access abandonment, censoring event, or study end. This period include access primary patency period and access assisted primary patency period. |
| Post-procedure primary patency | Interval of time from the first procedure designed to maintain the functionality of the index dialysis vascular access until the first of one of the following events: access thrombosis; any procedure designed to facilitate, maintain, or re-establish patency; censoring event; or study end. |
| Noninfectious complications | Stenosis, thrombosis, hand ischemia, aneurysm, pseudoaneurysm, infiltration. |
| Infectious complications | Fistula or graft cellulitis, abscess, bacteremia. |
| Access procedures | Angioplasty, stent placement, surgical revision, ligation of accessory veins, superficialization of vein. |
| Other clinical outcomes | TCVC placement, new AV access surgical creation, AV access–related hospitalization or death. |
| Limb ischemia following AV access placement | |
| Symptoms | Paresthesia, pain, hand stiffness, ulceration and tissue loss in the limb with AV access. |
| Physical examination | Diminished or absent radial pulse, pallor, diminished sensation, and, in advanced stages or severe cases, ulceration and gangrene. |
| Grade 1, mild | Cool extremity with few symptoms but steal demonstrable by flow augmentation with access occlusion. |
| Grade 2, moderate | Intermittent ischemia only during dialysis/claudication. |
| Grade 3, severe |
Ischemic pain at rest/tissue loss. |
| TCVC access outcome |
Examples |
| Infectious complications | Catheter exit site infection, tunnel infection, bacteremia. |
| Non-infectious complications | Catheter malposition, mechanical dysfunction, catheter migration, venous thrombosis, pneumothorax, heamothorax, arterial puncture. |
Abbreviations: AV, arteriovenous; TCVC, tunneled central venous catheter.
Outcomes of the index AV access will include incidence rate of AV access primary failure; time to successful AV access cannulation and access cumulative patency from surgical placement; rate of access procedures (e.g., angioplasty, stent placement, surgical revision, ligation of accessory veins and superficialization of vein), infectious complications (cellulitis, abscess and bacteremia) and rate of non-infectious complications (bleeding, stenosis, thrombosis, arterial steal syndrome, nerve injury, seroma, aneurysm, pseudoaneurysm and infiltration) from surgical placement; and proportion of AV accesses that achieved suitability for HD, unassisted access maturation, and assisted access maturation. The incidence rate of AV access primary failure will be calculated as the ratio of the number of AV accesses that did not attain successful cannulation by the sixth month after surgical placement over the number of AV accesses placed during the study period (multiplied by 100 for percentage) [31]. Lack of successful cannulation (i.e., inability to remove the catheter and use the AV access as the main access for hemodialysis) by the sixth month after AV access placement surgery will encompass various etiologies of AV access primary failure (e.g., inadequate maturation, thrombosis, failure of first and subsequent cannulations, or infection leading to nonfunctional fistula or graft). The proportion of AV accesses without primary failure that demonstrate suitability for HD, unassisted access maturation, and assisted access maturation; and time to successful cannulation will be assessed at 12 months after surgical placement.
Outcomes related to TCVC will include date of insertion, date of removal as a result of index AV access becoming the primary vascular access, infectious complications (e.g., catheter exit site infection, tunnel infection and catheter-related bacteremia) and non-infectious complications (e.g., catheter malposition, mechanical dysfunction, catheter migration, venous thrombosis, pneumothorax, arterial puncture and hemothorax). The proportion of time when HD was delivered via index AV access (i.e., TCVC-free HD) from placement of the index AV access to reaching a censoring event ([number of days with HD delivered via index AV access/number of days of HD delivered via any vascular access] x 100) will be calculated.
2.7.3. Measurements
Study specific assessments will be completed to evaluate participant's upper extremity muscle strength, physical activity, level of independence, satisfaction with AV access, and health-related quality of life.
2.7.3.1. Grip strength
Upper extremity strength will be measured with the grip strength test using a hand-held dynamometer. Grip strength will be tested on a non-dialysis day or pre-dialysis during a dialysis day. Participants will adopt the standard testing position (seated with shoulder and forearm of the test arm in a neutral position and the elbow flexed at 90°) and the maximum force will be recorded twice for each hand. The mean of the two results will be used for statistical analyses. Changes in grip strength will be analyzed before and after AV access placement, and between the extremity with and without AV access. A cut-off point <16 kg in women and <26 kg in men will define muscle weakness [32].
2.7.3.2. Level of independence and health-related quality of life
The level of functional independence will be assessed using ADLs and IADLs [33,34]. The ADL instrument covers six items (toileting, feeding, dressing, grooming, physical ambulation, and bathing) and the IADL instrument IADLs covers eight domains (using a telephone, shopping, food preparation, housekeeping, laundry, transportation, taking medications, handling finances). These assessments will rate patient's ability to perform ADLs and IADLs in a real-world setting over the prior 24–48 h, based on self-report, collateral information, and/or direct observation. Performance on these domains is graded by level of assistance needed, with higher scoring denoting more independence. Participants will be asked to complete the Kidney Disease Quality of Life Short Form Questionnaire (KDQOL-SF) version 1.3, a self-administered survey consisting of a generic core (Short Form-36 [SF-36]) and an 11-item kidney disease-specific scale [35]. The domains measured by the SF-36 include physical domains (physical functioning, role limitations due to physical health, general health perceptions and pain) and mental domains (energy/fatigue, social functioning, emotional wellbeing and role limitations due to emotional problems). The domains targeted specifically for patients with kidney disease include work status, quality of social interactions, burden of kidney disease, social support, cognitive function, sexual function, sleep, effects of kidney disease (overall health), and symptoms/problem list. The scores are aggregated and transformed linearly to a 0- to 100-range. Higher scores indicate better status.
2.7.3.3. Patient-reported views on AV access outcomes
Pain at the AV access site during access cannulation will be assessed using the Verbal Descriptor Scale (VDS) and Abbey Pain Scale (APS). The VDS evaluates pain as absent, mild, moderate and severe [36]. The APS is a scale used for people who cannot verbalize their pain and the study staff will score the pain scale by observing the participant's vocalization, facial expression, and body language [37]. Pain experienced during AV access cannulation as an average score obtained at 3 consecutive access cannulation sessions (initial assessment performed during first cannulation) will be analyzed. Patients' satisfaction with the vascular access will be assessed with the Short-Form Vascular Access Questionnaire (SF-VAQ) [38].
The aforementioned assessments will be conducted at pre-specified time-points as shown in Table 2. All assessments will be conducted on a non-dialysis day or before HD on the day of scheduled dialysis.
2.8. Data analysis plan
2.8.1. General considerations
Trial outcomes are summarized in Table 4. This pilot study will be considered successful based on attaining the following feasibility outcomes: (1) ≥70% of eligible patients are recruited, (2) ≥50% to 70% of participants with ESKD on HD undergo placement of index AV access within 90–180 days of enrollment, respectively, (3) ≥80% patients adhere to all study-specific assessments (grip strength, level of independence, satisfaction with vascular access, and health-related quality of life), absent of a condition that precludes assessment, and (4) ≥70% of participants who underwent AV access placement will have a follow-up duration of ≥12 months after index access placement. Progress from pilot to large scale trial will be considered as: i) continue the study without modifications (feasible as is) if all four feasibility criteria are met; ii) continue with protocol modifications (feasible with modifications) (e.g., change in frequency of study-specific assessments if high rate of participant refusal or inability to complete study-specific assessments); iii) stop the main study (not feasible) if none of the four feasibility criteria was met. The results of recruitment rate will be used to determine the number of centers needed to participate for a future large scale trial, with an enrollment period of two years and follow-up period of three years from placement of index AV access.
Table 4.
Trial outcomes.
| Variable | Feasibility outcomes |
|---|---|
| Exclusion of screened people Patients declining to participate |
Proportion of people meeting inclusion criteria who ultimately consent to randomization |
| Patients ineligible for AV access placement Late AV access surgery (>180 days from randomization) |
Proportion of participants who receive the assigned AV access surgery within 90 and 180 days of randomization |
| Sociodemographic parameters Charlson comorbidity index Electronic Frailty Index (eFI) Grip strength |
Vascular Access Outcomes
|
| Type of first AV access strategy |
Clinical and Quality of Life Consequences
|
Abbreviations: ESKD, end-stage kidney disease; HD, hemodialysis; TCVC, tunneled central venous catheter; AV, arteriovenous; AVF, arteriovenous fistula; AVG, arteriovenous graft.
For Aim 1, the proportion of people meeting each of the feasibility and protocol adherence endpoints with accompanying 95% confidence intervals (CI) will be estimated using skew-corrected score tests with a continuity correction. Participant characteristics and reasons for protocol violations will be described, along with calculated rates of dropout. For Aims 2 and 3, outcomes will be analyzed as continuous variables using analysis of covariance (ANCOVA) within the robust regression framework to limit the effect of potential outliers and influential points given the small sample size. We will report the percent-point difference in changes in grip strength (100*[preoperative grip strength – postoperative grip strength]/preoperative grip-strength) for each group. The mean and 95% confidence interval (CI) for TCVC-free days, following index AV access placement, will be estimated in each arm. Kaplan-Meier curves will be generated for time-to-event analyses to help evaluate the cumulative incidence of vascular access-related outcomes The participants will be followed for 12 months following index AV access placement. Participants who undergo placement of AV access as randomized will be censored at transfer to a non-WFSM affiliated dialysis unit, transfer to peritoneal dialysis, transplantation, death and end of study period. Lack of AV access placement within 12 months after enrollment will be logged as dropout event. Causes of dropout (e.g., medical reasons, patient shift in preference or refusal for AV access creation, transfer to a non-WFSM affiliated dialysis unit, transfer to peritoneal dialysis, transplantation, and death) will be recorded. Dropouts will be analyzed as randomized in the intention-to-treat (ITT) analysis. Inadvertent creation of a different type of AV access than as randomized (i.e., placement of AVF in a participant randomized to graft-first strategy) will be logged as protocol violation and not included in the final analysis. Missing data will be handled under the missing at random (MAR) assumptions in compliance with an ITT protocol [39]. We will pay close attention to variables with higher rates of missingness, relative to others or display missingness patterns that deviate from the MAR assumptions since they could inform and guide the development of the larger trial. Results from the pilot analyses are likely to have high variability, but will provide valuable insight that will be considered in the design of a larger trial in the future.
2.8.2. Power estimation
Sample size and statistical power were estimated for Aim 2. Assuming a significance level of 5% and a one-sided test as advocated for pilot studies, a sample size of 25 individuals per arm provides 73% power to detect a change of ∼0.6σ (corresponding to 21percentage-point difference) between the two study arms, whenever the correlation between pre- and post-randomization grip strength measures is ≥ 0.45 [40]. Based on a standard deviation of 13 reported by Vermeulen et al. and a pre- and post-randomization grip strength correlation of 0.45, a sample size of 25 individuals per group will provide error margins of 1.50, 1.31 and 1.17 kg for vascular access effect on grip strength with significance levels of 10, 15 and 20%, respectively [41].
2.9. Safety
This study poses moderate risk to participants. Complications related to placement of an AV access—such as access thrombosis and infection with/without systemic infection—will be closely tracked. All vascular access-related clinical events will be adjudicated (based on standards of care) by the patient's nephrologist and treating physicians. Events will be recorded electronically and reported to the principal investigator (PI) monthly. An independent Medical Safety Officer (MSO), unblinded to treatment assignments, will review the following vascular access-related events on a quarterly basis: access primary failure (i.e., permanent access failure before hemodialysis suitability), access infection (local and systemic), and arterial steal syndrome. All concerns regarding the frequency of AV access-related complications will be reported to the WFSM Institutional Review Board. In addition, the PI, MSO, and members of the research group will carefully consider amendments (including halting the trial if necessary).
3. Discussion
This is the first randomized trial that will assess fistula-first against graft-first vascular access approach in a population on HD. ‘Fistula First’ guidelines are based on retrospective analyses performed more than 20 years ago, when older patients comprised less than 15% of the ESKD population [2,3]. Based on contemporary national registry data, older adults comprise more than 30% of incident patients with ESKD on HD, have 3 times higher prevalence of advanced chronic kidney disease, and 4 times higher incidence of dialysis-requiring acute kidney injury [42]. Older patients have multiple comorbidities, polypharmacy, undertreated conditions, and a high prevalence of physical disabilities [[43], [44], [45]]. The prognosis for older adults on HD is poor, with 25–60% mortality rate in the first year on HD, compared with 7–12% mortality in younger patients [[46], [47], [48]]. Following dialysis initiation, patients ≥65 years old experience substantial declines in their functional abilities, with most physical deterioration taking place in the initial 3 months on dialysis [49].
Despite awareness of evolving functional impairments in older patients after dialysis initiation, little emphasis has been placed on preventing the physical consequences brought on by vascular access placement. Unsuccessful and/or repetitive painful procedures to place an AVF—which have higher rates of yielding a nonfunctional AV access compared to an AVG—could induce loss of muscle mass in the involved limb. This could erode the remaining function and quality of life by subjecting patients to costly, recurrent and painful procedures requiring multiple physician visits, hospitalizations and time away from loved ones [50,51]. Collectively, the burden of comorbidities and age-related biological changes may prevent achievement of a functional AVF and could supersede potential benefits of an AVF. Thus, a “Fistula or Graft First” approach appears more rational considering the multidimensional needs of older patients on HD. In this context, we and others reason that maintaining physical ability and self-sufficiency in older patients with ESKD is the most important factor in choice of vascular access [52,53]. This pilot study will yield valuable data related to participant recruitment, study dropout and the conduct of study assessments to inform operational requirements for a large scale trial.
Funding
The study is supported by grant R03AG060178-01 from the National Institute on Aging, NIH, Bethesda, MD,USA .
Ethics approval
The study has been approved by the Wake Forest School of Medicine Institutional Review Board (IRB00050577). The study is registered at Clinicaltrials.gov (NCT03545113).
Acknowledgements
The authors thank the participants and the National Institute on Aging (NIA) representatives Basil A. Eldadah, MD, PhD, Susan Zieman, MD, PhD, and Marcel E. Salive, MD, MPH for their invaluable contributions to the trial.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.conctc.2019.100357.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.U.S. Renal data system 2015 . USRDS 2015 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. 2015. Chapter 1: incidence, prevalence, patient characteristics, and treatment modalities.https://www.usrds.org/2015/view/v2 01.aspx [Google Scholar]
- 2.Dhingra R.K., Young E.W., Hulbert-Shearon T.E., Leavey S.F., Port F.K. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int. 2001;60(4):1443–1451. doi: 10.1046/j.1523-1755.2001.00947.x. [DOI] [PubMed] [Google Scholar]
- 3.Pastan S., Soucie J.M., McClellan W.M. Vascular access and increased risk of death among hemodialysis patients. Kidney Int. 2002;62(2):620–626. doi: 10.1046/j.1523-1755.2002.00460.x. [DOI] [PubMed] [Google Scholar]
- 4.Ishani A., Collins A.J., Herzog C.A., Foley R.N. Septicemia, access and cardiovascular disease in dialysis patients: the USRDS Wave 2 study. Kidney Int. 2005;68(1):311–318. doi: 10.1111/j.1523-1755.2005.00414.x. [DOI] [PubMed] [Google Scholar]
- 5.Bradbury B.D., Fissell R.B., Albert J.M., Anthony M.S., Critchlow C.W., Pisoni R.L., Port F.K., Gillespie B.W. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin. J. Am. Soc. Nephrol. 2007;2(1):89–99. doi: 10.2215/CJN.01170905. [DOI] [PubMed] [Google Scholar]
- 6.Lee T., Thamer M., Zhang Q., Zhang Y., Allon M. Vascular access type and clinical outcomes among elderly patients on hemodialysis. Clin. J. Am. Soc. Nephrol. 2017;12(11):1823–1830. doi: 10.2215/CJN.01410217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.KDOQI Clinical practice guidelines and clinical practice recommendations for vascular access. Am. J. Kidney Dis. 2006;48(Suppl 1):S176–S247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Yuo T.H., Chaer R.A., Dillavou E.D., Leers S.A., Makaroun M.S. Patients started on hemodialysis with tunneled dialysis catheter have similar survival after arteriovenous fistula and arteriovenous graft creation. J. Vasc. Surg. 2015:1–8. doi: 10.1016/j.jvs.2015.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown R.S., Patibandla B.K., Goldfarb-Rumyantzev A.S. The survival benefit of "fistula first, catheter last" in hemodialysis is primarily due to patient factors. J. Am. Soc. Nephrol. 2017;28(2):645–652. doi: 10.1681/ASN.2016010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinn R.R., Oliver M.J., Devoe D., Poinen K., Kabani R., Kamar F., Mysore P., Lewin A.M., Hiremath S., Macrae J., James M.T., Miller L., Hemmelgarn B.R., Moist L.M., Garg A.X., Chowdhury T.T., Ravani P. The effect of predialysis fistula attempt on risk of all-cause and access-related death. J. Am. Soc. Nephrol. 2017;28(2):613–620. doi: 10.1681/ASN.2016020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nassar G.M., Ayus J.C. Infectious complications of the hemodialysis access. Kidney Int. 2001;60(1):1–13. doi: 10.1046/j.1523-1755.2001.00765.x. [DOI] [PubMed] [Google Scholar]
- 12.Ravani P., Quinn R., Oliver M., Robinson B., Pisoni R., Pannu N., Macrae J., Manns B., Hemmelgarn B., James M., Tonelli M., Gillespie B. Examining the association between hemodialysis access type and mortality: the role of access complications. Clin. J. Am. Soc. Nephrol. 2017;12(6):955–964. doi: 10.2215/CJN.12181116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.C S., R F. Presented at ASN Kidney Week; San Diego, CA: 2012. Vascular access and infections from dialysis claims. October 30-November 4, 2012. [Google Scholar]
- 14.Murea M., James K.M., Russell G.B., Byrum G.V., III, Yates J.E., Tuttle N.S., Bleyer A.J., Burkart J.M., Freedman B.I. Risk of catheter-related bloodstream infection in elderly patients on hemodialysis. Clin. J. Am. Soc. Nephrol. 2014;9(4):764–770. doi: 10.2215/CJN.07710713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lok C.E., Allon M., Moist L., Oliver M.J., Shah H., Zimmerman D. Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I) J. Am. Soc. Nephrol. 2006;17(11):3204–3212. doi: 10.1681/ASN.2006030190. [DOI] [PubMed] [Google Scholar]
- 16.Peterson W.J., Barker J., Allon M. Disparities in fistula maturation persist despite preoperative vascular mapping. Clin. J. Am. Soc. Nephrol. 2008;3(2):437–441. doi: 10.2215/CJN.03480807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson A.I., Leake A., Schmieder G.C., Biuckians A., Stokes G.K., Panneton J.M., Glickman M.H. Should fistulas really be first in the elderly patient? J. Vasc. Access. 2009;10(3):199–202. doi: 10.1177/112972980901000311. [DOI] [PubMed] [Google Scholar]
- 18.Woo K., Goldman D.P., Romley J.A. Early failure of dialysis access among the elderly in the era of fistula first, clin. J. Am. Soc. Nephrol. 2015;10(10):1791–1798. doi: 10.2215/CJN.09040914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leake A.E., Yuo T.H., Wu T., Fish L., Dillavou E.D., Chaer R.A., Leers S.A., Makaroun M.S. Arteriovenous grafts are associated with earlier catheter removal and fewer catheter days in the United States Renal Data System population. J. Vasc. Surg. 2015;62(1):123–127. doi: 10.1016/j.jvs.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Lee T., Thamer M., Zhang Y., Zhang Q., Allon M. Outcomes of elderly patients after predialysis vascular access creation. J. Am. Soc. Nephrol. 2015;26(12):3133–3140. doi: 10.1681/ASN.2014090938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murea M., Brown W.M., Divers J., Moossavi S., Robinson T.W., Bagwell B., Burkart J.M., Freedman B.I. Vascular access placement order and outcomes in hemodialysis patients: a longitudinal study. Am. J. Nephrol. 2017;46(4):268–275. doi: 10.1159/000481313. [DOI] [PubMed] [Google Scholar]
- 22.Lok C.E., Sontrop J.M., Tomlinson G., Rajan D., Cattral M., Oreopoulos G., Harris J., Moist L. Cumulative patency of contemporary fistulas versus grafts (2000-2010) Clin. J. Am. Soc. Nephrol. 2013;8(5):810–818. doi: 10.2215/CJN.00730112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee T., Barker J., Allon M. Comparison of survival of upper arm arteriovenous fistulas and grafts after failed forearm fistula. J. Am. Soc. Nephrol. 2007;18(6):1936–1941. doi: 10.1681/ASN.2006101119. [DOI] [PubMed] [Google Scholar]
- 24.Rehfuss J.P., Berceli S.A., Barbey S.M., He Y., Kubilis P.S., Beck A.W., Huber T.S., Scali S.T. The spectrum of hand dysfunction after hemodialysis fistula placement. Kidney Int Rep. 2017;2(3):332–341. doi: 10.1016/j.ekir.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clegg A., Bates C., Young J., Ryan R., Nichols L., Ann Teale E., Mohammed M.A., Parry J., Marshall T. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–360. doi: 10.1093/ageing/afw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Iorio B., Cillo N., Cirillo M., De Santo N.G. Charlson Comorbidity Index is a predictor of outcomes in incident hemodialysis patients and correlates with phase angle and hospitalization. Int. J. Artif. Organs. 2004;27(4):330–336. doi: 10.1177/039139880402700409. [DOI] [PubMed] [Google Scholar]
- 27.Quan H., Sundararajan V., Halfon P., Fong A., Burnand B., Luthi J.C., Saunders L.D., Beck C.A., Feasby T.E., Ghali W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 28.Paper abstract. J. Am. Geriatr. Soc. 2018;66(S2):S1–S369. doi: 10.1111/jgs.15376. [DOI] [PubMed] [Google Scholar]
- 29.Pajewski N.L., Wells B.J., Williamson J.D., Callahan K.E. Frailty screening using the electronic health record within a medicare accountable care organization. J Gerontol A Biol Sci Med Sci. Jan 21, 2019:1–7. doi: 10.1093/gerona/glz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shenoy S., Allon M., Beathard G., Brouwer-Maier D., Dember L.M., Glickman M., Lee C., Litchfield T., Lok C., Huber T., Roy-Chaudhury P., Work J., West M., Wasse H. Clinical trial end points for hemodialysis vascular access: background, rationale, and definitions. Clin. J. Am. Soc. Nephrol. 2018;13(3):490–494. doi: 10.2215/CJN.13321216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee T., Mokrzycki M., Moist L., Maya I., Vazquez M., Lok C.E. Standardized definitions for hemodialysis vascular access. Semin. Dial. 2011;24(5):515–524. doi: 10.1111/j.1525-139X.2011.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Studenski S.A., Peters K.W., Alley D.E., Cawthon P.M., McLean R.R., Harris T.B., Ferrucci L., Guralnik J.M., Fragala M.S., Kenny A.M., Kiel D.P., Kritchevsky S.B., Shardell M.D., Dam T.T., Vassileva M.T. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol. A Biol. Sci Med Sci. 2014;69(5):547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz S., Ford A.B., Moskowitz R.W., Jackson B.A., Jaffe M.W. Studies of illness in the aged. the index of adl: a standardized measure of biological and psychosocial function. J. Am. Med. Assoc. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 34.Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontol. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 35.Hays R.D., Kallich J.D., Mapes D.L., Coons S.J., Carter W.B. Development of the kidney disease quality of life (KDQOL) instrument. Qual. Life Res. 1994;3(5):329–338. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 36.Herr K.A., Garand L. Assessment and measurement of pain in older adults. Clin. Geriatr. Med. 2001;17(3):457–478. doi: 10.1016/s0749-0690(05)70080-x. (vi) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldt K.S. The checklist of nonverbal pain indicators (CNPI) Pain Manag. Nurs. Times. 2000;1(1):13–21. doi: 10.1053/jpmn.2000.5831. [DOI] [PubMed] [Google Scholar]
- 38.Kosa S.D., Bhola C., Lok C.E. Measuring patient satisfaction with vascular access: vascular access questionnaire development and reliability testing. J. Vasc. Access. 2015;16(3):200–205. doi: 10.5301/jva.5000339. [DOI] [PubMed] [Google Scholar]
- 39.Sued M., Yohai V.J. Robust location estimation with missing data. Can. J. Stat./La Revue Canadienne de Statistique. 2013;41(1):111–132. [Google Scholar]
- 40.Cocks K., Torgerson D.J. Sample size calculations for pilot randomized trials: a confidence interval approach. J. Clin. Epidemiol. 2013;66(2):197–201. doi: 10.1016/j.jclinepi.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Vermeulen J., Neyens J.C., Spreeuwenberg M.D., van Rossum E., Hewson D.J., de Witte L.P. Measuring grip strength in older adults: comparing the grip-ball with the Jamar dynamometer. J. Geriatr. Phys. Ther. 2001;38(3):148–153. doi: 10.1519/JPT.0000000000000034. 2015. [DOI] [PubMed] [Google Scholar]
- 42.U.S. Renal Data System 2015 . USRDS 2015 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Vol. 2. 2015. End-stage renal disease (ESRD) in the United States. Chapter 1: incidence, prevalence, patient characteristics and treatment modalities.https://www.usrds.org/2015/view/v1 01.aspxhttps://www.usrds.org/2015/view/v1 01.aspx [Google Scholar]
- 43.Winchester J.F. Special clinical problems in geriatric patients. Semin. Dial. 2002;15(2):116–120. doi: 10.1046/j.1525-139x.2002.00036.x. [DOI] [PubMed] [Google Scholar]
- 44.Berger J.R., Hedayati S.S. Renal replacement therapy in the elderly population. Clin. J. Am. Soc. Nephrol. 2012;7(6):1039–1046. doi: 10.2215/CJN.10411011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh P., Germain M.J., Cohen L., Unruh M. The elderly patient on dialysis: geriatric considerations. Nephrol. Dial. Transplant. 2014;29(5):990–996. doi: 10.1093/ndt/gft246. [DOI] [PubMed] [Google Scholar]
- 46.Kurella M., Covinsky K.E., Collins A.J., Chertow G.M. Octogenarians and nonagenarians starting dialysis in the United States. Ann. Intern. Med. 2007;146(3):177–183. doi: 10.7326/0003-4819-146-3-200702060-00006. [DOI] [PubMed] [Google Scholar]
- 47.Canaud B., Tong L., Tentori F., Akiba T., Karaboyas A., Gillespie B., Akizawa T., Pisoni R.L., Bommer J., Port F.K. Clinical practices and outcomes in elderly hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin. J. Am. Soc. Nephrol. 2011;6(7):1651–1662. doi: 10.2215/CJN.03530410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson B.M., Zhang J., Morgenstern H., Bradbury B.D., Ng L.J., McCullough K.P., Gillespie B.W., Hakim R., Rayner H., Fort J., Akizawa T., Tentori F., Pisoni R.L. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014;85(1):158–165. doi: 10.1038/ki.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurella Tamura M., Covinsky K.E., Chertow G.M., Yaffe K., Landefeld C.S., McCulloch C.E. Functional status of elderly adults before and after initiation of dialysis. N. Engl. J. Med. 2009;361(16):1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murea M., Burkart J. Finding the right hemodialysis vascular access in the elderly: a patient-centered approach. J. Vasc. Access. 2016;17(5):386–391. doi: 10.5301/jva.5000590. [DOI] [PubMed] [Google Scholar]
- 51.Monroy-Cuadros M., Yilmaz S., Salazar-Banuelos A., Doig C. Risk factors associated with patency loss of hemodialysis vascular access within 6 months. Clin. J. Am. Soc. Nephrol. 2010;5(10):1787–1792. doi: 10.2215/CJN.09441209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murea M., Satko S. Looking beyond "fistula first" in the elderly on hemodialysis. Semin. Dial. 2016;29(5):396–402. doi: 10.1111/sdi.12481. [DOI] [PubMed] [Google Scholar]
- 53.Iyasere O., Brown E.A. Mortality in the elderly on dialysis: is this the right debate? Clin. J. Am. Soc. Nephrol. 2015;10(6):920–922. doi: 10.2215/CJN.03650415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.