Abstract
Dendritic cells (DCs) are the most potent antigen-presenting cells and are the key initiator of tumor-specific immune responses. These characteristics are exploited by DC therapy, where DCs are ex vivo loaded with tumor-associated antigens (TAAs) and used to induce tumor-specific immune responses. Unfortunately, clinical responses remain limited to a proportion of the patients. Tumor characteristics and the immunosuppressive tumor microenvironment (TME) of the tumor are likely hampering efficacy of DC therapy. Therefore, reducing the immunosuppressive TME by combining DC therapy with other treatments could be a promising strategy. Initially, conventional cancer therapies, such as chemotherapy and radiotherapy, were thought to specifically target cancerous cells. Recent insights indicate that these therapies additionally augment tumor immunity by targeting immunosuppressive cell subsets in the TME, inducing immunogenic cell death (ICD), or blocking inhibitory molecules. Therefore, combining DC therapy with registered therapies such as chemotherapy, radiotherapy, or checkpoint inhibitors could be a promising treatment strategy to improve the efficacy of DC therapy. In this review, we evaluate various clinical applicable combination strategies to improve the efficacy of DC therapy.
Keywords: dendritic cell-based therapy, chemotherapy, radiotherapy, checkpoint inhibitors, immunotherapy, tumor microenvironment, regulatory T cells, myeloid-derived suppressor cells, immunogenic cell death, macrophages
Main Text
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) capable of inducing a potent immune response through the presentation of exogenous antigens.1 Immature DCs, efficient at engulfing and processing antigens, reside in the periphery, and they mature upon encounter with danger-associated molecular patterns (DAMPs) and pattern-associated molecular patterns (PAMPs).2 Upon encounter with these danger signals, DCs upregulate co-stimulatory molecules (CD80, CD86, and CD40) and chemokine receptors (e.g., CCR7), produce pro-inflammatory cytokines, and migrate to the lymph node to activate T cells.3 T cell activation is induced by antigen presentation (signal 1), co-stimulation (signal 2), and the secretion of pro-inflammatory cytokines (signal 3).4 In contrast, antigen presentation in the absence of signals 2 and 3 induces tolerance.5, 6 In a tumor setting, both tumor cells and immunosuppressive cells in the tumor microenvironment (TME) can hamper anti-tumor immune responses.7
In DC therapy production, DCs are loaded and matured ex vivo to circumvent the initial immunosuppressive influence of the TME and tumor cells on endogenous DC maturation. In addition, the administration of autologous DCs could induce and improve in vivo tumor-specific immune response. It is believed that DC therapy has not yet reached its full potential.8, 9, 10 The rather limited clinical efficacy of DC therapy can be dependent on DC therapy-related aspects, such as the choice of antigen, method of loading, or type of DCs used. Next to that, active immunosuppression by the tumor and the TME could also hamper the immune-activating potential of the administered DCs and suppress the function and infiltration of activated T cells.11, 12, 13
Therefore, targeting these immunosuppressive features of the TME using FDA-approved treatment modalities, such as chemotherapy, radiotherapy, or more recently developed checkpoint inhibitors (CIs), in combination with DC therapy could improve DC therapy efficacy1, 7, 8, 12, 14, 15, 16, 17 (Figure 1). In this review, we discuss the immunological barriers that DC therapy faces and potential synergistic immunomodulating treatment modalities. In addition, we review clinical trials that have combined DC therapy with additional treatments. Data regarding these conducted clinical trials were found using a search string of relevant terms, as described in the Supplemental Information.
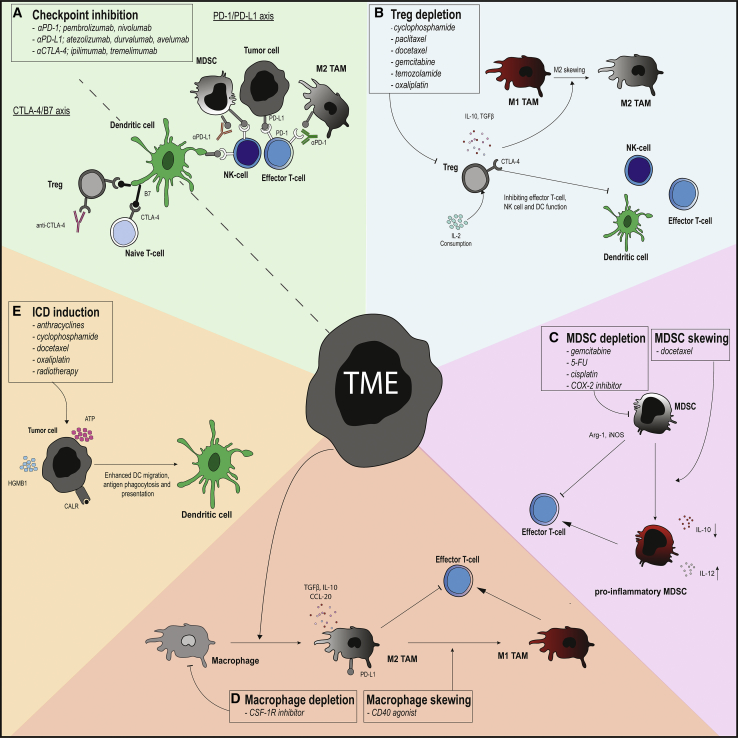
Figure 1.
Targeting the TME with Conventional Treatment Modalities
(A) Inhibitory molecules (PD-(L)1, CTLA-4) inhibit T-cell effector, dendritic cell and natural killer (NK)-cell function, and T-cell activation in the lymphnode. Checkpoint inhibitors targeting (PD-(L)1, CTLA-4) can reinvigorate the anti-tumor immune response induced by dendritic cell (DC) therapy by blocking PD-(L)1 signaling in the tumor and CTLA-4 in the lymph node. (B) Regulatory T cells (Tregs) exert their immunosuppressive mechanisms through inhibitory molecules (CTLA-4), secretion of immunosuppressive cytokines (interleukin [IL]-10, TGFβ), and IL-2 consumption, thereby inhibiting NK-cells, T cells, and DCs and skewing tumor-associated macrophages (TAMs) in a unfavorable M2 phenotype. Tregs can be depleted with several chemotherapeutics (cyclophosphamide, paclitaxel, docetaxel, gemcitabine, temozolamide, and oxaliplatin). (C) Myeloid-derived suppressor cells (MDSCs) can exert their immunosuppressive function by relieving Arginase 1 (Arg1) and inducible nitric oxide synthase (iNOS) to deprive T cells of metabolites. MDSCs can be depleted by chemotherapeutics gemcitabine, 5-FU, cisplatin, and docetaxel and skewed into a M1 phenotype by docetaxel. (D) M2 TAMs secrete IL-10 and transforming growth factor β (TGF-β) and are involved in tissue remodeling, wound healing, and tumor progression. M2 TAMs can be depleted by CSF-1R and skewed into an M1 phenotype by CD40 agonists. (E) Immunogenic cell death (ICD) is characterized by secretion of ATP and high mobility group box 1 (HGMB-1) and expression of Calreticulin (CRT) on the cell surface, which stimulates DC phagocytosis, antigen presentation, and migration. ICD can be induced by chemotherapeutics, cyclophosphamide, oxaliplatin, paclitaxel, docetaxel and anthracyclines, and radiotherapy.
Immunosuppressive Mechanisms of the TME and Tumor Cells that Hamper the Efficacy of DC Therapy
Both tumor cells and immunosuppressive immune cells in the TME hamper the effectivity of DC therapy through various mechanisms, such as the expression of inhibitory molecules, secretion of inhibitory cytokines or enzymes, induction of tolerogenic cell death, and creation of a dense extracellular matrix.18, 19 Tumor cells recruit immunosuppressive immune cells, fibroblasts,20 and endothelial cells to the TME through the secretion of growth factors, chemokines, and cytokines, thereby hampering the infiltration of DCs and other pro-inflammatory cells into the TME.21, 22 Moreover, fibroblasts and immunosuppresive immune cells interact synergistically with each other to maximize the immunosuppressive character of the TME.
Tolerogenic and Immunogenic Cell Death
Cancer cell death can be tolerogenic or immunogenic depending on the stimulus of apoptosis.23 Immunogenic cancer cell death leads to the secretion of DAMPs, attracts pro-inflammatory cells, and subsequently elicits a tumor-specific immune response (Box S1). Non-immunogenic cell death of malignant cells occurs without secretion of pro-inflammatory DAMPs. Tumor cells undergo non-immunogenic cell death through chemo-attraction of immunosuppressive phagocytes and induction of immunosuppressive phagocytosis.24 Tumor cells actively impair DC maturation through the secretion of immunosuppressive cytokines, leading to the presentation of tumor-associated antigens (TAAs) by immature DCs. Presentation of antigens by immature DCs induces T cell anergy and activation of TAA-specific regulatory T cells (Tregs), resulting in TAA-specific tolerance.1, 25, 26, 27
Tregs
Tregs are recruited to the TME through CCR4 chemokine signaling, and they expand in the TME upon transforming growth factor β (TGF-β) and interleukin (IL)-10 exposure.28 Tregs enable tumor progression by suppressing tumor-specific immune responses. In general, Tregs induce immunosuppression directly through cell-cell contact via inhibitory receptors, such as programmed cell death 1 (PD-1) or cytotoxic T-lymphocyte associated protein 4 (CTLA-4), or indirectly through the secretion of immunosuppressive cytokines, such as IL-10 and TGF-β, or IL-2 consumption of pore-forming proteins, such as granzyme and perforin.29 Via these mechanisms, Tregs suppress a wide array of pro-inflammatory immune cells that can be induced upon DC therapy, such as CD8+ T cells, CD4+ T cells, natural killer (NK) cells, NK T cells, and B cells. Additionally, Tregs can also suppress macrophages and DCs, thereby hampering the induction of an initial anti-tumor immune response.8, 14 Moreover, tumor-infiltrating Tregs have a higher affinity to TAAs derived from self-antigens presented on tumor cells than CD8+ T cells, thereby affecting the activation of TAA-specific CD8+ T cells in the TME.22 The Treg functions create a hostile and competitive environment for DC therapy-induced tumor-specific CD8+ T cells, hindering their cytotoxic functions.
Myeloid-Derived Suppressor Cells
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells with immunosuppressive effects. MDSCs are one of the most abundant immune cells in the TME, and they are attracted to the tumor site by chemokines secreted by the tumor cells.30, 31, 32 MDSCs comprise two subsets: monocytic MDSC (mMDSC) and polymorphonuclear MDSC (pMDSC).33, 34 mMDSCs tend to be more immunosuppressive than pMDSCs, as they are capable of both antigen-dependent and antigen-independent inhibition of T cell responses.18, 35 The hypoxic environment of the TME induces the release of hypoxia-inducible factor 1-alpha (HIF-1α), which causes mMDSCs to upregulate the enzymes arginase 1 (Arg1) and inducible nitric oxide synthase (iNOS) that break down L-arginase.35 Two products of this enzymatic reaction, urea and nitric oxide (NO), induce T cell depletion and inhibit T cell function.18, 31, 36, 37 Moreover, mMDSCs attract Tregs through C-C motif chemokine ligand 2 (CCL4) and CCL5 production, secrete IL-10,38 and upregulate PD-L1 on their cell surface, which inhibits tumor-specific T cell cytotoxicity.39 Furthermore, HIF-1α induces differentiation of mMDSCs into tumor-associated macrophages (TAMs) through the downregulation of phosphorylated signal transducer and activator of transcription 3 (pSTAT3), indicating that the TME can influence both immune cell function and differentiation.40, 41
TAMs
Monocytes are derived from the bone marrow and are recruited to the TME through CCL2 signaling, where these cells can differentiate into macrophages. Phenotypically, macrophages can broadly be divided into two subtypes: a pro-inflammatory M1 phenotype and an immunosuppressive M2 phenotype. Differentiation of M1 macrophages is induced by pro-inflammatory cytokines such as interferon (IFN)-γ and bacterial components such as lipopolysaccharide (LPS). M1 macrophages secrete pro-inflammatory cytokines, interleukins such as IL-12, and tumor necrosis factor α (TNF-α), leading to inflammation.19 Macrophages are skewed into an M2 phenotype, through the secretion of immunosuppressive cytokines by tumor cells or immune cells in the TME, and they inhibit CD8+ T cell function.41 TAMs display an M2-like phenotype and secrete IL-10, prostaglandin E2 (PGE2), and chemokines to attract and induce Tregs.18, 41 Moreover, TAMs express iNOS and Arg1, and they upregulate PD-L1 on their cell surface, which inhibits CD8+ T cell function.18, 42 These mechanisms hamper DC therapy-induced anti-tumor immunity.
DC Therapy
Different DC Therapy Strategies
DC therapy aims at eliciting a tumor-specific immune response, by in vitro loading DCs in vitro with tumor antigens and additional maturation stimuli. DC therapy can be historically divided into three categories: first-, second-, and next-generation DC therapy.1 In first- and second-generation DC therapy, monocyte-derived DCs (moDCs) were used. moDCs are generated from monocytes upon culture with granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4. moDCs have been shown to promote T cell differentiation and CD8+ T cell activation.43 In first-generation DC therapy, moDCs were loaded with a tumor lysate, TAAs, or synthetic peptides without additional maturation stimuli. Not surprisingly, without a proper maturation stimulus, clinical results were disappointing, with a tumor regression rate of 3.3%.44
In second-generation DC therapy, moDCs were additionally matured after loading these immature moDCs, using maturation cocktails, including IL-6, TNF, IL-1β, PGE2, and polyinosinic:polycytidylic acid (poly(I:C)).45 Maturing these tumor antigen-loaded moDCs significantly improved clinical results, with overall response rates (ORRs) of 8%–15%, depending on the tumor type.9 Median overall survival (OS) was increased by 20% in multiple clinical trials with second-generation DC therapy, which is the threshold for clinical relevance.9, 45, 46 Furthermore, the IMPACT trial showed an increase in median OS of 3.9 months for castration-resistant prostate cancer patients treated with sipuleucel-T (DC therapy) compared to the placebo group, leading to FDA approval in 2010.47
In next-generation DC therapy, naturally occurring DCs (nDCs), such as plasmacytoid DCs (pDCs) and conventional DCs (cDCs), are used for vaccination. cDCs can be divided into two main subtypes: cDC1 and cDC2.48 cDC1s are superior in cross-presenting antigens and, thereby, inducing CD8+ T cell activation. Recent studies have shown that cDC1s are critically important for anti-tumor immune responses and that their presence in the TME positively correlates to OS and clinical responses upon PD-1 monoclonal antibody (mAb) in melanoma.49, 50, 51 Classically, cDC2s mainly activate CD4+ T cells. The characterization of human cDC2 function remains difficult, as this subset is very heterogeneous, shares markers such as CD11b and CD172a with macrophages and moDCs, and has functional overlap with cDC1s.50 pDCs in the TME produce type 1 IFN, which attracts NK, B, and T cells.52 However, pDCs have questionable antigen-presenting skills, as CD123+ pDCs were found to be contaminated with pre-cDCs.53 Naturally occurring DCs can, therefore, be selected based on their superior functional properties, and they can be obtained without an additional culture period, leading to reduced production costs.1
DCs can also be targeted in vivo using Toll-like receptor (TLR) ligands, intra-tumoral injection of TriMix mRNA, or attenuated viral agents (virotherapy).54, 55, 56 Furthermore, FMS-like tyrosine kinase 3 ligand (FLT3L) injection increases cDC proliferation and infiltration into the tumor site, which enhanced PD-L1 mAb efficacy in a melanoma mouse model.57, 58 Clinical trials exploiting next-generation DC therapy are currently being performed, and the coming years will indicate whether the use of nDCs further improves the clinical efficacy of DC therapy.59, 60 Currently, clinical studies comparing the effectivity of different DC subtypes for vaccination purposes are lacking and are urgently needed to determine which DC subset would induce the most effective anti-tumor immune response.61
Immune Monitoring
Immunological responses induced by DC therapy are generally measured by IFN-γ enzyme-linked immunospots (ELISPOTs), tetramer analysis, co-cultures with lysate-loaded DCs, and delayed type hypersensitivity (DTH) skin tests. Correlating these immunological parameters to clinical response remains challenging, as not all patients show increased IFN-γ production in an ELISPOT upon DC therapy, and even positive ELISPOTs can be encountered before DC therapy.62, 63 Furthermore, tumor-specific IFN-γ production by peripheral blood mononuclear cells (PBMCs), as determined by an ELISPOT analysis, often does not correlate with clinical outcome or allergic reaction measured by the DTH skin test.62, 64, 65 However, in some studies, immunological parameters have been correlated with clinical parameters in both hematological and solid malignancies.59, 66, 67
DTH skin tests have been shown to correlate with clinical outcome in DC therapy trials in melanoma and colorectal cancer patients.68, 69, 70 Furthermore, in mesothelioma patients treated with DC therapy, two patients with a negative DTH skin test had progressive disease and had the shortest OS,71 indicating that the DTH skin test could correlate with clinical outcomes. Additionally, in pancreatic cancer patients treated with Wilms tumor protein 1 (WT1) I-II peptide-loaded DC therapy, positive DTH skin tests correlated with longer progression-free survival (PFS) and OS than negative DTH skin tests.72 However, all other studies combining DC therapy and chemotherapy did not show any correlation between DTH skin testing and clinical outcome.62, 64, 65, 72, 73, 74 The lack of correlation between DTH skin tests with clinical outcome could be dependent on the timing of the DTH skin test, the evaluation of response to the skin test, and the lack of a negative control. The classification of a positive DTH skin test varies from 2- to 5-mm erythema, while one could argue that induration is a more important parameter.75 Also timing of DTH skin testing varies between studies or is not mentioned.
Combination Strategies to Optimize DC Therapy
Combining CIs with DC Therapy
Inhibitory molecules that hamper anti-tumor immune responses, such as PD-1, PD-L1, and CTLA-4, can be expressed on both tumor cells and various immune cells.76, 77 Blocking these inhibitory molecules has been shown to restore tumor-specific T cell activity.76 There are many other inhibitory and even co-stimulatory molecules identified that could function as potential targets for immunotherapy, such as lymphocyte activation gene-3 (LAG-3), B and T lymphocyte attenuator (BTLA), programmed death-1 homolog (PD-1H), T cell immunoglobulin (TIM-3), T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT), glucocorticoid-induced TNF receptor (GITR), and NK cell inhibitory receptor NKG2A.78, 79, 80, 81, 82 The efficacy of these co-inhibitory and co-stimulatory molecules is currently being investigated in preclinical and/or clinical studies, and it is, therefore, not addressed in this review.
CTLA-4 blockage inhibits T cell activation in the lymph node, whereas blocking the PD-1 and PD-L1 axis mainly inhibits the effector function of activated T cells in the TME.83 Anti-CTLA-4 (ipilimumab and durvalumab), anti-PD-L1 (atezolizumab, durvalumab, and avelumab), and anti-PD-1 (nivolumab and pembrolizumab) are registered for the treatment of solid tumors, because of striking clinical effects. The efficacy of these CIs, especially PD-(L)1 mAb, often depends on and correlates with PD-L1 expression in the TME, mutational burden, and the number of tumor-infiltrating lymphocytes (TILs).13, 84, 85, 86, 87 High ORRs of 57% are reported in immunogenic cancers such as melanoma, which is ascribed to a high mutational burden and high numbers of TILs.88, 89 In tumors with lower mutational burden (e.g., mesothelioma), ORRs remain between 9% and 25%,90 likely due to the relative low frequency of TILs. DC therapy induces the infiltration of tumor-specific CD8+ T cells and upregulates PD-1 expression on these TILs, which could render tumors with low TIL numbers susceptible to anti-PD-(L)1 treatment.91, 92, 93, 94
It is likely that the limited efficacy of DC therapy trials is in part due to inhibitory signaling in the TME and lymph node. Additional administration of CIs can block inhibitory signaling on tumor cells and immunosuppressive cells in the TME. CIs could even inhibit the signaling of these inhibitory molecules on the DCs administered during DC therapy, as DCs express PD-1, PD-L1, and PD-L2.95 Expression of PD-1 and its ligands likely limits the induction of tumor-specific immune responses, as high PD-L1 expression on DCs suppresses CD4+ and CD8+ T cell proliferation and promotes Treg proliferation in various diseases, including cancer.8, 96, 97, 98, 99, 100, 101 This suggests that combining DC therapy with CIs can result in a two-sided synergy by targeting not only tumor cells and immunosuppressive cells in the TME but also DCs administered during DC therapy and even T cells induced by DC therapy (Figure 1A). Different DC subtypes differentially express these inhibitory molecules, suggesting that specific CIs should be used in combination with certain DC subsets.102 The rationale for using CIs in combination with DC therapy is further supported by the finding that the addition of a PD-1 mAb to ex vivo-cultured autologous T cells and DCs of patients with myeloma improved IFN-γ production while limiting Treg expansion.103 Furthermore, combining DC therapy with systemic PD-1 blockade in mice bearing intracranial glioma tumors improved survival compared to both single treatments.91 In addition, PD-1 blockage on DCs, administered in a breast tumor-bearing mouse model, that were subsequently systemically treated with anti-PD-1 mAb reduced tumor growth and increased survival compared to untreated mice.104
Clinical Trials Combining CIs with DC Therapy
Until now, three clinical studies combined DC therapy with CI treatment. In all of these studies, CTLA-4 mAb has been used. Clinical responses were retrospectively observed in patients with stage III and IV melanoma that were treated with ipilimumab upon disease progression, after receiving at least 3 bi-weekly vaccinations with gp100 and tyrosinase-loaded DCs.105 Especially patients with stage III melanoma responded well, with an OS rate of 51% after 2 years. The presence of tumor-specific T cells obtained from DTH skin biopsies did not correlate to OS in patients with stage III and IV melanoma. Ipilimumab-related adverse events were not increased in patients pretreated with DC vaccination (58%) compared to patients treated with ipilimumab monotherapy (61%–70%).106
In a clinical phase I, dose escalation trial, patients with stage IIIc or IV melanoma received three bi-weekly intradermal vaccinations with MART-1-loaded DCs and concurrent systemic treatment with a dose escalation of tremelimumab (3, 6, and 10 mg/kg), a CTLA-4-blocking mAb.107 Four of 16 patients developed a clinical response upon treatment, of which 2 patients developed a complete response (CR) and 2 patients developed a partial response (PR). This indicates that response rates upon combination therapy are promising, compared to response rates to tremelimumab monotherapy (7%–10% ORR)107, 108, 109, 110 and DC vaccination monotherapy (15% ORR).9 Remarkably, responses were also observed in patients treated with only 3 mg/kg tremelimumab, achieving plasma levels of 30 μg/mL, which is below the target level determined by prospective clinical trials.108 This suggests that these results are not solely the effect of tremelimumab or DC therapy alone, indicating a synergistic effect. However, immunological analysis was inconclusive, with a minority of patients showing a response to tetramer or ELISPOT analysis.108
A phase II, open-label, single-arm clinical trial combined ipilimumab treatment with DCs electroporated with Trimix-mRNA (CD40L, CD70, constitutive active TLR4) and mRNA encoding for MAGE-A3, MAGE-C2, tyrosinase3, or gp100 in patients with stage III and IV melanoma.111 Radiological responses were assessed with immune-related response criteria (irRCs), which showed an ORR of 38%; 20% of the responding patients showed a CR and 18% had a PR. A disease control rate of 51% at 6-month follow-up was observed, and the ORR was better than ORRs observed in patients treated with ipilimumab as monotherapy.112 Furthermore, the number of CRs was similar to clinical trials investigating combination therapy of ipilimumab and nivolumab.106 Immunological analysis showed an overall increase of CD4+ and CD8+ T cells in the peripheral blood and a positive tumor antigen-specific ELISPOT analysis in two of ten patients.111
Combining different CIs often leads to increased toxicity, such as dermatologic toxicity, colitis, or pneumonitis,113, 114 whereas combining DC therapy with CIs does not increase the immune-related adverse event profile of CI monotherapy. Furthermore, ORRs, PFS, and OS in clinical trials investigating combination therapy consisting of DC therapy and CTLA-4 mAb treatment are promising, as compared to clinical trials that investigated these therapies as monotherapy. However, phase III, randomized, controlled clinical trials are needed to determine the efficacy improvement of combining DC therapy and CI to either treatment modality alone. Furthermore, combining PD-(L)1-targeting CIs with DC therapy still needs to be evaluated in clinical trials. Immunological analysis was inconclusive in all studies and did not correlate with clinical outcome. Concurrent intensive immunological analysis of blood and tumor material could provide proof of principle, expand current knowledge, and possibly lead to objectifiable immunological parameters for immunotherapy. Currently, many phase I-II trials are being conducted that combine PD-(L)1 mAb with DC therapy.56, 115 However, to date, there are no clinical phase III trials being conducted to observe the synergy of CI treatment in combination with DC therapy (https://clinicaltrials.gov/).
Combining Chemotherapy with DC Therapy
Apart from specifically targeting cancerous cells, it is becoming apparent that chemotherapy can also actively influence the immune system by depletion of specific cell types, such as Tregs and MDSCs, and by induction of immunogenic cell death (ICD). Furthermore, chemotherapy can skew immunomodulatory cells in a more pro-inflammatory subset. Depletion of Tregs12 and MDSCs116 in the TME after chemotherapy treatment was already observed in preclinical and clinical studies.11, 12, 17, 23, 117, 118 Such immunological changes were even associated with clinical response.118, 119, 120, 121, 122 Furthermore, ICD-inducing chemotherapy, such as anthracyclines, has a suboptimal result in immunodeficient mice.23, 123 This indicates that chemotherapy treatment can affect the immunosuppressive TME by cell-specific depletion and improve immune responses by the induction of ICD, thereby proving beneficial when combined with DC therapy.
In most clinical trials, DC therapy was administered in combination with registered chemotherapy treatment. Therefore, studies that combined treatment of chemotherapeutics with DC therapy were not often designed to improve DC therapy efficacy. Consequently, most of the immunological parameters, such as IFN-γ ELISPOTs, tetramer analysis, and DTH skin tests, determined immunological response to DC therapy rather than the immunological effects induced by chemotherapy.62, 63, 65, 72, 124, 125 Consequently, monitoring of immunomodulatory effects and, therefore, objectifying the attributable effect of chemotherapy on DC therapy is difficult to achieve. Clinical trials that investigated treatment with DC therapy in combination with chemotherapy in esophageal, prostate, and pancreatic cancers; mesothelioma; glioblastoma; and melanoma are summarized in Table 1.
Table 1.
Overview of Clinical Trials Combining moDC Therapy with Chemotherapy
| Disease | Loading Material for DCs | Maturation Cocktail | CTX (+ Other Additional Treatments) | Immunomodulatory Effecta | Immunological Rationaleb | CTX Immune Readoutc | DC Therapy Immune Readout d | n | CO | CO Corresponding to IR | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glioblastoma | autologous tumor lysate | TNF-a, IFN-a and POLI I:C | RTX + TMZ 75 mg/m2TMZ 200 mg/m2 | Treg depletion | none | none | 8/25 pos ELISPOT | 31 | PFS 12.7m, OS 23.4m |
no | 63 |
| Pancreatic cancer | WT1 peptide | OK-432 and PGE2 | S1 or S1 + gemcitabine dose not stated | Treg depletion MDSC depletion | none | none | 7/8 pos ELISPOT | 8 | 4 PD 4 alive 2 years post-treatment |
pos ELISPOT correlated to 2-year OS | 125 |
| Pancreatic cancer | WT1-I, -II, I/II peptide | OK-432 and PGE2 | gemcitabine 1000 mg/m2 | Treg depletion, MDSC depletion | none | none | 4/11 pos DTH | 10 | 7 SD, 3 PD | pos DTH positively correlated with PFS | 72 |
| Glioblastoma | autologous tumor lysate | monocyte-derived conditioned medium (MCM) | TMZ 150-200 mg | Treg depletion | none | none | 2/9 pos ELISPOT 0 pos DTH |
14 | 2 PR, 3 SD, 4 PD median OS 23m | no | 62 |
| Esophageal cancer | WT1 peptide | OK-432 andPG-E2 | DTX 50 mg/m2 | Treg depletion, MDSC skewing, ICD induction |
non-specific immune enhancement | none | 5/8 pos ELISPOT 3/7 pos DTH 3/7 pos tetramer 5/8 pos HLA |
10 | 10 PD | no | 65 |
| Melanoma | WT1 peptide, gp100 tyrosinase, MAGE-A3 or MAGE-A2 | Matured with OK-432 and PG-E2 | carboplatin (AUC 5) and paclitaxel (175 mg/m2) | Treg depletion | Treg depletion and decrease IL-10 and TGF-β secretion | none | 4/9 pos ELISPOT | 9 | 1 PR, 4 SD, 5 PD OS 12m PFS 2,3m |
no | 124 |
| Prostate cancer | PSA, PAP mRNA | TNF-a IL-1B, IL-6, PGE2 | DTX 75mg/m2 | Treg depletion, MDSC skewing, ICD induction |
MDSC depletion and ICD | DC + DTX: decrease in MDSC | 9/18 pos ELISPOT 5/18 pos DTH |
19 DTX 21 DTX + DC | no difference in PFS and OS | decreasing levels of MDSC were correlated to better PFS | 64 |
| Melanoma | p53, survivin, and hTERT mRNA | TNF-a IL-1B, IL-6, PGE2 | cyclophosphamide 50 mg | Treg depletion, ICD induction | Treg depletion | CD4+ T-cell depletion | 6/17 pos ELISPOT | 22 | 9 SD, 13 PD OS 10,4m PFS 3,1m |
no | 127 |
| Mesothelioma | autologous tumor lysate | PGE2 TNF-a IL-1B IL-6 | cyclophosphamide 2x50 mg | Treg depletion, inducing ICD | Treg depletion | Treg depletion | 8/10 pos DTH | 10 | 1 CR, 4 SD, NA 3,PD 2 | no | 71 |
| Prostate cancer | killed LNCaP prostate cancer cells | poly I:C | 50 mg cyclophosphamide, 75 mg/m2 DTX | Treg depletion, MDSC skewing, ICD induction |
Treg depletion, enhancement of T and NK cell activation | Treg depletion | increased CD8+ T-cells, increased PSA- specific IFN- γ production | 24 | OS 19m | no | 132 |
| Melanoma | autologous pulsed DC | TNF-a IL-1B, IL-6 PGE2 | TMZ 75mg/m2 (IL-2)3,000,000 IU/day | Treg depletion | Treg depletion | Treg depletion | 9/17 pos DTH | 17 | 1 PR, 6 SD, 10 PD | no | 74 |
| Melanoma | autologous tumor lysate or survivan, hTERT, p53 | TNF-a IL-1b IL-6 PGE2 | IL-2, cyclophosphamide and a COX-2 inhibitor | Treg depletion, MDSC depletion | Treg depletion, MDSC depletion |
Treg increase, MDSC depletion | 8/17 pos DTH at baseline 1/17 pos DTH after vaccination |
28 | 16 SD, 12 PD PFS 4,5m |
no | 73 |
DC, dendritic cell; CTX, chemotherapy; CO, clinical outcome; IR, immunological readout; n, number; LNCaP, androgen-sensitive human prostate adenocarcinoma cell line; WT, Wilms tumor; MAGE, melanoma-associated antigen; PSA, prostate specific antigen; PAP, prostate acidic phosphatase; p53, tumor protein p53; hTERT, telomerase reverse transcriptase; gp100, glycoprotein 100; RTX, radiotherapy; S-1, Tegafur/gimeracil/oteracil; TMZ, temozolomide; DTX, docetaxel; COX, cyclooxygenase; IL, interleukin; Treg: regulatory T cell; MDSC, myeloid derived suppressor cell; ICD, immunogenic cell death; PFS, progression free survival; m, months; OS, overall survival; PR, partial response; SD, stable disease; PD, progressive disease; ELISPOT, enzyme-linked immunospot assay; DTH, delayed type hypersensitivity skin test; pos, positive; AUC, area under the curve; NA, not applicable because of non-measurable lesions; TGF- β, transforming growth factor β; OK-432, penicillin-killed and lyophilized preparations of a low virulence strain (Su) of Streptococcus pyogenes.
The hypothesized immunomodulatory effect of chemotherapeutics as described in preclinical studies and reviews.
The immunological rationale for the use of the chemotherapeutic agent described in the respective article.
The results of the immunological analysis done to evaluate immunomodulatory effects of chemotherapeutics.
The results of the immunological analysis done to evaluate immunomodulatory effects of DC therapy.
Targeting of the Immunosuppressive Environment by Chemotherapy
Tregs
Various chemotherapeutics, such as cyclophosphamide, paclitaxel, docetaxel, gemcitabine, temozolamide (TMZ), and oxaliplatin, are capable of Treg depletion in clinical and preclinical settings.12, 17, 117, 118, 126 Cyclophosphamide is the best known and studied chemotherapeutic agent with the capability of depleting Tregs. Four clinical studies evaluated the immunological and clinical effects of potentially Treg-depleting chemotherapeutics in combination with DC therapy, in which 3 studies used cyclophosphamide64, 71, 73 and one study used TMZ.74 Two other studies evaluated only clinical effects of potentially Treg-depleting chemoteherapeutics, cyclophosphamide and paclitaxel, in combination with DC therapy.
In a phase I clinical trial, melanoma patients were treated with six biweekly injections of DCs electroporated with mRNA encoding p53, survivin, and hTER and concurrent low-dose cyclophosphamide (2 × 50 mg/day biweekly).127 The OS was 10.4 months and 9 of 22 patients had stable disease (SD). Tregs as well as total CD4+ T cells were depleted upon cyclophosphamide treatment, questioning whether cyclophosphamide induced specific depletion of Tregs.127 However, another clinical trial in mesothelioma patients that also combined concurrent low-dose cyclophosphamide (2 × 50 mg/day biweekly) with three biweekly injections of DCs loaded with tumor lysate did show selective depletion of Tregs.71 Unfortunately, depletion of Tregs was not correlated with a better clinical outcome. However, detailed analysis of naive Tregs (nTregs, CD45RA+ FoxP3int) and activated Tregs (aTregs, CD45RA− FoxP3hi) showed a positive correlation between the pretreatment levels of nTregs and OS.128 In addition, results from this clinical study are quite promising, with patients still alive up to 6 years after diagnosis.
Another phase II clinical study combined DC therapy loaded with tumor lysate or peptides (surviving, telomerase and p53) with consecutive IL-2 (2 million international units [mIUs]/day for 5 days) with metronomic cyclophosphamide (2 × 50 mg/day biweekly) and a Cox-2 inhibitor (200 mg daily) in melanoma patients.73 Melanoma patients treated with this combination therapy also showed increased numbers of Tregs after four vaccinations, indicating that cyclophosphamide was not able to counteract the effect of IL-2 on Tregs, as IL-2 has the potency to increase Treg numbers.129 In contrast to Tregs, mMDSCs were significantly decreased after four vaccinations, indicating that the combination treatment depletes mMDSCs. However, the changes observed in Treg numbers and mMDSC frequency did not correlate with clinical outcome. Clinical results were significantly improved compared to a previous trial where DC therapy was only combined with IL-2.130
Combining neoadjuvant TMZ (75 mg/m2/day for 14 days) treatment followed by autologous tumor lysate-loaded DC therapy with consecutive IL-2 (3 mIU/day for 5 days) in 17 melanoma patients, also significantly depleted Tregs, although this did not correlate to clinical outcome. One patient showed a PR and six patients had SD.74
In another study, patients with metastatic castration-resistant prostate cancer were treated with neoadjuvant metronomic cyclophosphamide (50 mg/day for 1 week) followed by LNCaP- (androgen-sensitive human prostate adenocarcinoma cells) loaded DC therapy and docetaxel (75 mg/m2 every 3 weeks). The predicted OS was 11.3 months, whereas in this study an OS of 19 months was observed, suggestive of a synergistic effect upon the combination of these treatments.131, 132
In a clinical trial in patients with stage IV melanoma treated with DCs loaded with a multi-peptide (WT1, gp100, tyrosinase, and MAG-E3 or MAGE-A2) combined with paclitaxel (175 mg/m) and carboplatin (area under the curve 5), an OS of up to 24 months was observed.124 Unfortunately, Treg numbers were not assessed in these clinical trials. Taken together, these clinical trials indicate that chemotherapy is capable of depleting Tregs and inducing promising clinical responses in combination with DC therapy. Alterations in Treg numbers upon treatment could not be correlated with clinical outcome, although nTreg frequencies at baseline were predictive of clinical response.71, 73 However, further research is needed to determine whether this is also observed in other malignancies and combination therapies (Figure 1B).
MDSCs
Numerous chemotherapeutics, such as gemcitabine, 5-FU, cisplatin, and docetaxel, have been shown to specifically deplete MDSCs.12, 73, 117, 118 Furthermore, docetaxel possibly improves immunostimulatory effects of total MDSCs by skewing them into a more favorable pro-inflammatory and migratory phenotype (CCR2, CCR5, CX3CR1, and CCR7) rather than an immunosuppressive phenotype.133 This indicates that chemotherapeutics not only deplete immunoregulatory cells but also can change the phenotype of immunosuppressive cells. In contrast to the above-described chemotherapeutics, cyclophosphamide increases the amount of specific pMDSCs, but not mMDSCs, in the peripheral blood of mice and human.134 The increase in pMDSCs induced by cyclophosphamide can be counteracted by the addition of chemotherapeutics targeting MDSCs, as combining cyclophosphamide and gemcitabine treatment decreased both Treg and GR1high MDSC numbers and reduced tumor growth.135
One study in patients with stage IV pancreatic ductal adenocarcinoma treated with WT1-loaded DC therapy and gemcitabine (1,000 mg/m2 three times every 28 days) showed that combining these treatments is safe and feasible.72 They also found a positive correlation between DTH skin testing and PFS. Unfortunately, MDSC numbers were not assessed in this study, so it remains inconclusive whether gemcitabine administration in combination with DC therapy affected MDSC numbers in these patients.
In a clinical study in patients with metastasized adenocarcinoma of the prostate, docetaxel (75 mg/m2 every 3 weeks) monotherapy was compared to combined treatment of docetaxel with DCs transfected with mRNAs encoding PAP (prostate acidic phosphatase) and PSA (prostate-specific antigen).64 There was no significant difference in OS and PFS between both treatment arms. Patients with decreased MDSC frequencies in cryopreserved PBMCs upon treatment had a longer PFS as compared to patients with increasing frequencies of MDSCs upon treatment. A decrease of MDSCs in cryopreserved PBMCs was only observed 6 weeks after the start of combination therapy, but not upon docetaxel monotherapy, which suggests that docetaxel monotherapy is not sufficient to decrease MDSCs in peripheral blood.64 Paradoxically, a preclinical study observed a decrease in total MDSC numbers upon treatment with docetaxel monotherapy.133 Additionally, docetaxel treatment skewed total MDSCs toward a more pro-inflammatory phenotype in a preclinical study (Figure 1C).133 Characterization of MDSCs remains challenging due to a lack of specific markers and the need to assess these cell populations in freshly isolated blood, as especially pMDSCs are lost upon cryopreservation.33 In addition, most clinical trials focus on evaluating MDSC numbers rather than MDSC characteristics, leading to a lack of evidence for the phenotypical switch of MDSCs upon docetaxel treatment in humans.
ICD
Various chemotherapeutics, such as cyclophosphamide, oxaliplatin, paclitaxel, docetaxel, and anthracyclines, are able to induce ICD (Figure 1E). The antineoplastic effects of these chemotherapeutics are also dependent on ICD.136, 137, 138, 139 Current monitoring of ICD occurs via vaccination assays that are not applicable in clinical trials.139 This limits the possibility for evaluation of ICD-induced synergistic effects. Consequently, ICD is not often used as a rationale for combining chemotherapy and DC therapy (Table 1). A phase III clinical study in patients with metastatic castration-resistant prostate cancer comparing docetaxel treatment combined with DC therapy and docetaxel monotherapy has finished; accrual end results are awaited (ClinicalTrials.gov: NCT02111577). Hopefully, immunological data will lead to a better understanding of the immunomodulatory effects of docetaxel. A phase III trial in glioma patients evaluates the additional effect of DC therapy to current treatment consisting of TMZ and radiotherapy (ClinicalTrials.gov: NCT03548571). The addition of a DC monotherapy arm to both these studies could have revealed the synergistic effect of docetaxel and TMZ on DC therapy.
Combining Radiotherapy with DC Therapy
Radiotherapy has been used as local tumor treatment for the last century. Recently, radiotherapy was also found to affect non-radiated tumor lesions, which is called the abscopal effect. This suggests systemic effects of radiotherapy that can be explained by the upregulation of radiation-induced double-stranded DNA in the cytosol, which serves as a DAMP for the instigation of ICD. Subsequently, the secretion of type I IFNs by tumor cells will attract cDC1s to the tumor site, which can engulf the released tumor antigens and initiate an immune response.16, 140 Therefore, radiation-induced ICD can act as in situ vaccination.
Apart from inducing ICD, radiation induces the upregulation of adhesion molecules on the vascular endothelium of tumor cells, which enables T cell infiltration141 (Figure 1E). Furthermore, a non-lethal radiotherapy dosage increases surface expression of first apoptosis signal (Fas) ligand, carcinoembryonic antigen, and major histocompatibility complex I (MHCI) on tumor cells, enabling tumor-specific CD8+ T cells to recognize the tumor cells and exert their cytotoxic effects.142 Together, these immunomodulatory effects are hypothesized to be responsible for the abscopal effect.143 However, the theoretical immunomodulatory effect of radiotherapy lacks clinical support, as a recent review found only 46 reported cases of abscopal effect from 1969 to 2004.144 This could be dependent on the irradiation dose used, radiation schedule, and lack of additional immunostimulation. This is supported by a recent study in breast and colorectal tumor-bearing mice, where different radiation doses were compared in combination with CI. Here they found that repetitive radiation at a low dose (5–8 Gy) was more effective than a high (20-Gy) single dose.145, 146 High-dose radiation is thought to indirectly downregulate cytosolic double-stranded DNA (dsDNA) and, thus, inhibit radiation-dependent ICD.16
Clinical Trials Combining DC Therapy with Radiotherapy
In a phase I clinical trial, 14 patients with advanced hepatoma received immature DC therapy followed by 8-Gy radiotherapy. The clinical results varied, with 2 PRs, 4 minor responses, 3 SD, and 4 PD.147 Seven of ten immunologically evaluated patients developed an IFN-γ ELISPOT response upon treatment, which did not correlate with clinical response.
In an observational study, patients with esophageal cancer receiving autologous tumor lysate-loaded DC therapy in combination with concurrent radiotherapy (60 Gy) were compared to radiotherapy alone. The 2-year survival was significantly improved upon combination therapy (67.8%) as compared to single radiotherapy treatment (33.3%).148 Additionally, in patients with metastatic solid tumors, radiotherapy (35 Gy) combined with in situ DC therapy using GM-CSF administration, induced an abscopal effect in 11 of 41 patients, which was significantly higher compared to abscopal effects induced by radiotherapy alone.149
These data suggest that regional radiotherapy can act synergistically when combined with DC therapy. The synergy likely depends on the release of tumor antigen that boosts the anti-tumor immune response, and it should be further investigated in ongoing clinical trials. A recently registered trial for patients with metastatic melanoma will evaluate the additional effect of different immunostimulatory agents, including radiotherapy (24–32 Gy), to autologous tumor lysate-loaded DC therapy (ClinicalTrials.gov: NCT01973322).150 Additionally, the effect of sipuleucel-T therapy combined with stereotactic ablative body radiation in patients with metastatic castration-resistant prostate cancer will be observed in a single-arm study (ClinicalTrials.gov: NCT01818986). These studies will allow further investigation into the exact immunological mechanism of action of radiotherapy combined with DC therapy.
Other Combination Strategies: Targeting TAMs
Immunoinhibitory TAMs are abundant in many solid tumors.151, 152 These TAMs can be either depleted via colony-stimulating factor 1 receptor (CSF-1R) blockade or skewed into an immunostimulatory M1 phenotype by CD40 agonistic mAb.153, 154, 155, 156, 157, 158, 159 CSF-1R blockade increased the efficacy of chemotherapy in pancreatic tumor-bearing mice.160 In glioblastoma tumor-bearing mice, TAM depletion by CSF-1R monotherapy induced tumor reduction and increased survival.161 In contrast, in a mesothelioma mouse model, CSF-1R kinase inhibitor PLX3397 (pexidartinib)-mediated TAM depletion as monotherapy did not improve survival.153 However, when pexidartinib treatment was combined with DC therapy, improved survival was observed when compared to both monotherapies, which was accompanied by increased numbers of proliferating T cells and effector T cells.153
These studies indicate that TAM depletion can improve the immunosuppressive character of the TME and, thereby, act synergistically when combined with DC therapy. In addition to TAM depletion, skewing TAMs toward an immunostimulatory M1 phenotype using CD40 mAb may even hold more clinical potential, as CD40 mAb-activated macrophages in pancreatic cancer infiltrate the tumor and facilitate tumor stroma depletion.162 Especially in tumors with dense stroma, targeting the stroma indirectly through macrophage skewing could facilitate and improve tumor-specific T cell infiltration upon DC therapy (Figure 1D).
Future Perspectives
Currently, most clinical trials that combine DC therapy with other treatments, such as chemotherapy, radiotherapy, or CI, often lack immunological rationale. This is likely due to already existing registrations of these treatments based on other rationales, leading to the mandatory use of certain therapies in specific dosages and schedules. In an experimental setting, a lack of consistency in dosing and schedule of treatments complicates the comparison of both immunological responses and clinical efficacy between different studies. For example, a “metronomic” dosage of cyclophosphamide varies from 2 × 50 mg/day to 100 mg/day between different studies,71, 127 whereas Treg depletion is dependant on the dose and schedule of chemotherapy.29 Separate studies should be performed to analyze the immunomodulatory effects of chemotherapy, radiotherapy, targeted therapies, and immunotherapies alone. During these studies, adequate reports of the used methods and analyzation strategies are necessary to facilitate the standardization of type and timing of immunomonitoring assays.163 This will create the possibility to replicate and subsequently compare the immunomodulatory effects between studies, and, finally, it leads to a better understanding of the immunomodulatory effects of monotherapies.115 With this knowledge, specific therapies with the right dosing can be combined with DC therapy based on the TME characteristics (Figure 1).
Variations in DC therapy, in terms of antigen loading, maturation, use of different DC subsets, dosage per injection, and the interval or total amount of vaccinations, make it difficult to compare clinical studies with each other. Adequate immunomonitoring in DC therapy is mandatory to create the possibility to compare studies and evaluate the effects of different antigen-loading methods, maturation cocktails, and administration schedules or injection sites. Additionally, registration of DC therapies will enable the investigation of clinical efficacy of DC therapy in combination with other treatments as compared to DC monotherapy. This will inevitably lead to an increase in the number of randomized trials and rapid release and approval for registration of immunomodulatory therapies in combination with DC therapy. To date, sipuleucel-T is the only registered DC therapy.47 Phase III trials that could eventually lead to the registration of DC therapy are ongoing for melanoma (ClinicalTrials.gov: NCT02993315), glioblastoma (ClinicalTrials.gov: NCT03548571), mesothelioma (ClinicalTrials.gov: NCT03610360), and colorectal cancer (ClinicalTrials.gov: NCT02503150).
Hopefully, trials combining DC therapy with other therapies, based on a solid rationale and performed with adequate immunomonitoring and uniformity in administration schedules, will lead to the registration of already existing treatment modalities for new purposes. In this way, chemotherapeutics, radiotherapy, CIs, or other targeted therapies can be used as off-the-shelf, affordable immunomodulating agents to support DC therapy in a personalized manner. To accomplish this, intensive cooperation between clinicians and basic scientists will be needed.
Other targets for cancer therapy, such as additional co-inhibitory molecules, co-stimulatory molecules, or even targeted therapies such as indoleamine 2,3-dioxygenase (IDO), are upcoming. These targets could all hypothetically be used as immunomodulators in the future. However, we have to be cautious as some of these therapies are not yet found to be effective in phase III trials in humans.164 In the process of proving clinical efficacy of these drugs, immunomonitoring data should already be obtained in an earlier stage, whereby the immunomodulatory effects of these therapies are known before registration.
Conclusions
Apart from improving DC therapy itself, influencing the immunosuppressive character of the TME by targeting immune cells, such as Tregs, MDSCs, or TAMs, with already registered therapies could improve response rates upon DC therapy. To accomplish this, phase III clinical trials are urgently required that investigate clinical efficacy upon DC therapy combined with other treatments and registration of DC therapy for multiple malignancies. Additionally, elucidating the underlying immunological mechanism of these synergistic effects upon combination therapy will further boost the combination of DC therapy with other therapies. A better understanding will also lead to personalized combination therapy, wherein DC therapy will be combined with other therapies based on composition of the TME, the expression of inhibitory molecules on the surface of tumor and immunosuppressive cells, and tumor mutational burden (Figure 1).
Author Contributions
R.A.B. wrote the paper, contributed to the conception of the work and the interpretation of data, drafted the paper, and has approved the submitted version. J.G.J.V.A. and H.V. contributed to the conception of the work and the interpretation of data, drafted the paper and substantively revised it, and approved the submitted version.
Conflicts of Interest
J.G.J.V.A. reports receiving commercial research grants from Amphera and Roche; holds ownership interest (including patents) in Amphera BV; and is a consultant/advisory board member for Amphera, Boehringer Ingelheim, Bristol-Myers Squibb, Eli-Lilly, MSD, and Roche. No potential conflicts of interest were disclosed by the other authors.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omto.2019.03.007.
Supplemental Information
References
- 1.Garg A.D., Coulie P.G., Van den Eynde B.J., Agostinis P. Integrating Next-Generation Dendritic Cell Vaccines into the Current Cancer Immunotherapy Landscape. Trends Immunol. 2017;38:577–593. doi: 10.1016/j.it.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Nace G., Evankovich J., Eid R., Tsung A. Dendritic cells and damage-associated molecular patterns: endogenous danger signals linking innate and adaptive immunity. J. Innate Immun. 2012;4:6–15. doi: 10.1159/000334245. [DOI] [PubMed] [Google Scholar]
- 3.Sabado R.L., Balan S., Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017;27:74–95. doi: 10.1038/cr.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mempel T.R., Henrickson S.E., Von Andrian U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 5.Nurieva R., Thomas S., Nguyen T., Martin-Orozco N., Wang Y., Kaja M.K., Yu X.Z., Dong C. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 2006;25:2623–2633. doi: 10.1038/sj.emboj.7601146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutz M.B., Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 7.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Versteven M., Van den Bergh J.M.J., Marcq E., Smits E.L.J., Van Tendeloo V.F.I., Hobo W., Lion E. Dendritic Cells and Programmed Death-1 Blockade: A Joint Venture to Combat Cancer. Front. Immunol. 2018;9:394. doi: 10.3389/fimmu.2018.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anguille S., Smits E.L., Lion E., van Tendeloo V.F., Berneman Z.N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15:e257–e267. doi: 10.1016/S1470-2045(13)70585-0. [DOI] [PubMed] [Google Scholar]
- 10.Boudreau J.E., Bonehill A., Thielemans K., Wan Y. Engineering dendritic cells to enhance cancer immunotherapy. Mol. Ther. 2011;19:841–853. doi: 10.1038/mt.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fecek R.J., Storkus W.J. Combination strategies to enhance the potency of monocyte-derived dendritic cell-based cancer vaccines. Immunotherapy. 2016;8:1205–1218. doi: 10.2217/imt-2016-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kershaw M.H., Devaud C., John L.B., Westwood J.A., Darcy P.K. Enhancing immunotherapy using chemotherapy and radiation to modify the tumor microenvironment. OncoImmunology. 2013;2:e25962. doi: 10.4161/onci.25962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dammeijer F., Lau S.P., van Eijck C.H.J., van der Burg S.H., Aerts J.G.J.V. Rationally combining immunotherapies to improve efficacy of immune checkpoint blockade in solid tumors. Cytokine Growth Factor Rev. 2017;36:5–15. doi: 10.1016/j.cytogfr.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Kersten K., Salvagno C., de Visser K.E. Exploiting the Immunomodulatory Properties of Chemotherapeutic Drugs to Improve the Success of Cancer Immunotherapy. Front. Immunol. 2015;6:516. doi: 10.3389/fimmu.2015.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen R., Deng X., Wu H., Peng P., Wen B., Li F., Li F. Combined immunotherapy with dendritic cells and cytokine-induced killer cells for malignant tumors: a systematic review and meta-analysis. Int. Immunopharmacol. 2014;22:451–464. doi: 10.1016/j.intimp.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez-Ruiz M.E., Perez-Gracia J.L., Rodríguez I., Alfaro C., Oñate C., Pérez G., Gil-Bazo I., Benito A., Inogés S., López-Diaz de Cerio A. Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann. Oncol. 2018;29:1312–1319. doi: 10.1093/annonc/mdy089. [DOI] [PubMed] [Google Scholar]
- 17.Emens L.A., Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol. Res. 2015;3:436–443. doi: 10.1158/2326-6066.CIR-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aras S., Zaidi M.R. TAMeless traitors: macrophages in cancer progression and metastasis. Br. J. Cancer. 2017;117:1583–1591. doi: 10.1038/bjc.2017.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gkretsi V., Stylianou A., Papageorgis P., Polydorou C., Stylianopoulos T. Remodeling Components of the Tumor Microenvironment to Enhance Cancer Therapy. Front. Oncol. 2015;5:214. doi: 10.3389/fonc.2015.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devaud C., John L.B., Westwood J.A., Darcy P.K., Kershaw M.H. Immune modulation of the tumor microenvironment for enhancing cancer immunotherapy. OncoImmunology. 2013;2:e25961. doi: 10.4161/onci.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaudhary B., Elkord E. Regulatory T Cells in the Tumor Microenvironment and Cancer Progression: Role and Therapeutic Targeting. Vaccines (Basel) 2016;4:28. doi: 10.3390/vaccines4030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroemer G., Galluzzi L., Kepp O., Zitvogel L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 24.Garg A.D., Agostinis P. Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses. Immunol. Rev. 2017;280:126–148. doi: 10.1111/imr.12574. [DOI] [PubMed] [Google Scholar]
- 25.Green D.R., Ferguson T., Zitvogel L., Kroemer G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson T.A., Choi J., Green D.R. Armed response: how dying cells influence T-cell functions. Immunol. Rev. 2011;241:77–88. doi: 10.1111/j.1600-065X.2011.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonso R., Flament H., Lemoine S., Sedlik C., Bottasso E., Péguillet I., Prémel V., Denizeau J., Salou M., Darbois A. Induction of anergic or regulatory tumor-specific CD4+ T cells in the tumor-draining lymph node. Nat. Commun. 2018;9:2113. doi: 10.1038/s41467-018-04524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka A., Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–118. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J., Waxman D.J. Immunogenic chemotherapy: Dose and schedule dependence and combination with immunotherapy. Cancer Lett. 2018;419:210–221. doi: 10.1016/j.canlet.2018.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber R., Fleming V., Hu X., Nagibin V., Groth C., Altevogt P., Utikal J., Umansky V. Myeloid-Derived Suppressor Cells Hinder the Anti-Cancer Activity of Immune Checkpoint Inhibitors. Front. Immunol. 2018;9:1310. doi: 10.3389/fimmu.2018.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar V., Patel S., Tcyganov E., Gabrilovich D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016;37:208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umansky V., Blattner C., Gebhardt C., Utikal J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines (Basel) 2016;4:E36. doi: 10.3390/vaccines4040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bronte V., Brandau S., Chen S.H., Colombo M.P., Frey A.B., Greten T.F., Mandruzzato S., Murray P.J., Ochoa A., Ostrand-Rosenberg S. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostrand-Rosenberg S., Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marvel D., Gabrilovich D.I. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J. Clin. Invest. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greten T.F., Manns M.P., Korangy F. Myeloid derived suppressor cells in human diseases. Int. Immunopharmacol. 2011;11:802–807. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong Y.Y., Fuchsberger M., Xiang S.D., Apostolopoulos V., Plebanski M. Myeloid derived suppressor cells and their role in diseases. Curr. Med. Chem. 2013;20:1437–1444. doi: 10.2174/0929867311320110006. [DOI] [PubMed] [Google Scholar]
- 38.Hart K.M., Byrne K.T., Molloy M.J., Usherwood E.M., Berwin B. IL-10 immunomodulation of myeloid cells regulates a murine model of ovarian cancer. Front. Immunol. 2011;2:29. doi: 10.3389/fimmu.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu C., Redd P.S., Lee J.R., Savage N., Liu K. The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. OncoImmunology. 2016;5:e1247135. doi: 10.1080/2162402X.2016.1247135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostrand-Rosenberg S., Fenselau C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J. Immunol. 2018;200:422–431. doi: 10.4049/jimmunol.1701019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeNardo D.G., Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019 doi: 10.1038/s41577-019-0127-6. Published online February 4, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L., Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J. Hematol. Oncol. 2017;10:58. doi: 10.1186/s13045-017-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heystek H.C., Mudde G.C., Ohler R., Kalthoff F.S. Granulocyte-macrophage colony-stimulating factor (GM-CSF) has opposing effects on the capacity of monocytes versus monocyte-derived dendritic cells to stimulate the antigen-specific proliferation of a human T cell clone. Clin. Exp. Immunol. 2000;120:440–447. doi: 10.1046/j.1365-2249.2000.01225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmed M.S., Bae Y.-S. Dendritic cell-based therapeutic cancer vaccines: past, present and future. Clin. Exp. Vaccine Res. 2014;3:113–116. doi: 10.7774/cevr.2014.3.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anguille S., Smits E.L., Bryant C., Van Acker H.H., Goossens H., Lion E., Fromm P.D., Hart D.N., Van Tendeloo V.F., Berneman Z.N. Dendritic Cells as Pharmacological Tools for Cancer Immunotherapy. Pharmacol. Rev. 2015;67:731–753. doi: 10.1124/pr.114.009456. [DOI] [PubMed] [Google Scholar]
- 46.Turnis M.E., Rooney C.M. Enhancement of dendritic cells as vaccines for cancer. Immunotherapy. 2010;2:847–862. doi: 10.2217/imt.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kantoff P.W., Higano C.S., Shore N.D., Berger E.R., Small E.J., Penson D.F., Redfern C.H., Ferrari A.C., Dreicer R., Sims R.B., IMPACT Study Investigators Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 48.Collin M., Bigley V. Human dendritic cell subsets: an update. Immunology. 2018;154:3–20. doi: 10.1111/imm.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barry K.C., Hsu J., Broz M.L., Cueto F.J., Binnewies M., Combes A.J., Nelson A.E., Loo K., Kumar R., Rosenblum M.D. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 2018;24:1178–1191. doi: 10.1038/s41591-018-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Böttcher J.P., Bonavita E., Chakravarty P., Blees H., Cabeza-Cabrerizo M., Sammicheli S., Rogers N.C., Sahai E., Zelenay S., Reis e Sousa C. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell. 2018;172:1022–1037.e14. doi: 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spranger S., Dai D., Horton B., Gajewski T.F. Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell. 2017;31:711–723.e4. doi: 10.1016/j.ccell.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinto A., Rega A., Crother T.R., Sorrentino R. Plasmacytoid dendritic cells and their therapeutic activity in cancer. OncoImmunology. 2012;1:726–734. doi: 10.4161/onci.20171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villani A.C., Satija R., Reynolds G., Sarkizova S., Shekhar K., Fletcher J., Griesbeck M., Butler A., Zheng S., Lazo S. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356:eaah4573. doi: 10.1126/science.aah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Lint S., Renmans D., Broos K., Goethals L., Maenhout S., Benteyn D., Goyvaerts C., Du Four S., Van der Jeught K., Bialkowski L. Intratumoral Delivery of TriMix mRNA Results in T-cell Activation by Cross-Presenting Dendritic Cells. Cancer Immunol. Res. 2016;4:146–156. doi: 10.1158/2326-6066.CIR-15-0163. [DOI] [PubMed] [Google Scholar]
- 55.Prestwich R.J., Errington F., Diaz R.M., Pandha H.S., Harrington K.J., Melcher A.A., Vile R.G. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum. Gene Ther. 2009;20:1119–1132. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saxena M., Bhardwaj N. Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment. Trends Cancer. 2018;4:119–137. doi: 10.1016/j.trecan.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karsunky H., Merad M., Cozzio A., Weissman I.L., Manz M.G. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J. Exp. Med. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salmon H., Idoyaga J., Rahman A., Leboeuf M., Remark R., Jordan S., Casanova-Acebes M., Khudoynazarova M., Agudo J., Tung N. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity. 2016;44:924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bol K.F., Aarntzen E.H., Hout F.E., Schreibelt G., Creemers J.H., Lesterhuis W.J., Gerritsen W.R., Grunhagen D.J., Verhoef C., Punt C.J. Favorable overall survival in stage III melanoma patients after adjuvant dendritic cell vaccination. Oncoimmunology. 2015;5:e1057673. doi: 10.1080/2162402X.2015.1057673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Butterfield L.H. Dendritic cells in cancer immunotherapy clinical trials: are we making progress? Front. Immunol. 2013;4:454. doi: 10.3389/fimmu.2013.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huber A., Dammeijer F., Aerts J.G.J.V., Vroman H. Current State of Dendritic Cell-Based Immunotherapy: Opportunities for in vitro Antigen Loading of Different DC Subsets? Front. Immunol. 2018;9:2804. doi: 10.3389/fimmu.2018.02804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hunn M.K., Bauer E., Wood C.E., Gasser O., Dzhelali M., Ancelet L.R., Mester B., Sharples K.J., Findlay M.P., Hamilton D.A., Hermans I.F. Dendritic cell vaccination combined with temozolomide retreatment: results of a phase I trial in patients with recurrent glioblastoma multiforme. J. Neurooncol. 2015;121:319–329. doi: 10.1007/s11060-014-1635-7. [DOI] [PubMed] [Google Scholar]
- 63.Inogés S., Tejada S., de Cerio A.L., Gállego Pérez-Larraya J., Espinós J., Idoate M.A., Domínguez P.D., de Eulate R.G., Aristu J., Bendandi M. A phase II trial of autologous dendritic cell vaccination and radiochemotherapy following fluorescence-guided surgery in newly diagnosed glioblastoma patients. J. Transl. Med. 2017;15:104. doi: 10.1186/s12967-017-1202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kongsted P., Borch T.H., Ellebaek E., Iversen T.Z., Andersen R., Met Ö., Hansen M., Lindberg H., Sengeløv L., Svane I.M. Dendritic cell vaccination in combination with docetaxel for patients with metastatic castration-resistant prostate cancer: A randomized phase II study. Cytotherapy. 2017;19:500–513. doi: 10.1016/j.jcyt.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 65.Matsuda T., Takeuchi H., Sakurai T., Mayanagi S., Booka E., Fujita T., Higuchi H., Taguchi J., Hamamoto Y., Takaishi H. Pilot study of WT1 peptide-pulsed dendritic cell vaccination with docetaxel in esophageal cancer. Oncol. Lett. 2018;16:1348–1356. doi: 10.3892/ol.2018.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anguille S., Van de Velde A.L., Smits E.L., Van Tendeloo V.F., Juliusson G., Cools N., Nijs G., Stein B., Lion E., Van Driessche A. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood. 2017;130:1713–1721. doi: 10.1182/blood-2017-04-780155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kyte J.A., Aamdal S., Dueland S., Sæbøe-Larsen S., Inderberg E.M., Madsbu U.E., Skovlund E., Gaudernack G., Kvalheim G. Immune response and long-term clinical outcome in advanced melanoma patients vaccinated with tumor-mRNA-transfected dendritic cells. OncoImmunology. 2016;5:e1232237. doi: 10.1080/2162402X.2016.1232237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.López M.N., Pereda C., Segal G., Muñoz L., Aguilera R., González F.E., Escobar A., Ginesta A., Reyes D., González R. Prolonged survival of dendritic cell-vaccinated melanoma patients correlates with tumor-specific delayed type IV hypersensitivity response and reduction of tumor growth factor beta-expressing T cells. J. Clin. Oncol. 2009;27:945–952. doi: 10.1200/JCO.2008.18.0794. [DOI] [PubMed] [Google Scholar]
- 69.de Vries I.J., Bernsen M.R., Lesterhuis W.J., Scharenborg N.M., Strijk S.P., Gerritsen M.J., Ruiter D.J., Figdor C.G., Punt C.J., Adema G.J. Immunomonitoring tumor-specific T cells in delayed-type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J. Clin. Oncol. 2005;23:5779–5787. doi: 10.1200/JCO.2005.06.478. [DOI] [PubMed] [Google Scholar]
- 70.Lesterhuis W.J., de Vries I.J., Schuurhuis D.H., Boullart A.C., Jacobs J.F., de Boer A.J., Scharenborg N.M., Brouwer H.M., van de Rakt M.W., Figdor C.G. Vaccination of colorectal cancer patients with CEA-loaded dendritic cells: antigen-specific T cell responses in DTH skin tests. Ann. Oncol. 2006;17:974–980. doi: 10.1093/annonc/mdl072. [DOI] [PubMed] [Google Scholar]
- 71.Cornelissen R., Hegmans J.P.J.J., Maat A.P.W.M., Kaijen-Lambers M.E., Bezemer K., Hendriks R.W., Hoogsteden H.C., Aerts J.G. Extended Tumor Control after Dendritic Cell Vaccination with Low-Dose Cyclophosphamide as Adjuvant Treatment in Patients with Malignant Pleural Mesothelioma. Am. J. Respir. Crit. Care Med. 2016;193:1023–1031. doi: 10.1164/rccm.201508-1573OC. [DOI] [PubMed] [Google Scholar]
- 72.Koido S., Homma S., Okamoto M., Takakura K., Mori M., Yoshizaki S., Tsukinaga S., Odahara S., Koyama S., Imazu H. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clin. Cancer Res. 2014;20:4228–4239. doi: 10.1158/1078-0432.CCR-14-0314. [DOI] [PubMed] [Google Scholar]
- 73.Ellebaek E., Engell-Noerregaard L., Iversen T.Z., Froesig T.M., Munir S., Hadrup S.R., Andersen M.H., Svane I.M. Metastatic melanoma patients treated with dendritic cell vaccination, Interleukin-2 and metronomic cyclophosphamide: results from a phase II trial. Cancer Immunol. Immunother. 2012;61:1791–1804. doi: 10.1007/s00262-012-1242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ridolfi L., Petrini M., Granato A.M., Gentilcore G., Simeone E., Ascierto P.A., Pancisi E., Ancarani V., Fiammenghi L., Guidoboni M. Low-dose temozolomide before dendritic-cell vaccination reduces (specifically) CD4+CD25++Foxp3+ regulatory T-cells in advanced melanoma patients. J. Transl. Med. 2013;11:135. doi: 10.1186/1479-5876-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rogers A.S., Ellenberg J.H., Douglas S.D., Henry-Reid L., Peralta L., Wilson C.M., Adolescent Medicine HIV/AIDS Research Network Performance of antigens used in detecting delayed-type hypersensitivity in adolescents infected with the human immunodeficiency virus. Clin. Diagn. Lab. Immunol. 2001;8:273–278. doi: 10.1128/CDLI.8.2.273-278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baumeister S.H., Freeman G.J., Dranoff G., Sharpe A.H. Coinhibitory Pathways in Immunotherapy for Cancer. Annu. Rev. Immunol. 2016;34:539–573. doi: 10.1146/annurev-immunol-032414-112049. [DOI] [PubMed] [Google Scholar]
- 77.Vreeland T.J., Clifton G.T., Herbert G.S., Hale D.F., Jackson D.O., Berry J.S., Peoples G.E. Gaining ground on a cure through synergy: combining checkpoint inhibitors with cancer vaccines. Expert Rev. Clin. Immunol. 2016;12:1347–1357. doi: 10.1080/1744666X.2016.1202114. [DOI] [PubMed] [Google Scholar]
- 78.Torphy R.J., Schulick R.D., Zhu Y. Newly Emerging Immune Checkpoints: Promises for Future Cancer Therapy. Int. J. Mol. Sci. 2017;18:E2642. doi: 10.3390/ijms18122642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.André P., Denis C., Soulas C., Bourbon-Caillet C., Lopez J., Arnoux T., Bléry M., Bonnafous C., Gauthier L., Morel A. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell. 2018;175:1731–1743.e13. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruggeri L., Urbani E., André P., Mancusi A., Tosti A., Topini F., Bléry M., Animobono L., Romagné F., Wagtmann N., Velardi A. Effects of anti-NKG2A antibody administration on leukemia and normal hematopoietic cells. Haematologica. 2016;101:626–633. doi: 10.3324/haematol.2015.135301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knee D.A., Hewes B., Brogdon J.L. Rationale for anti-GITR cancer immunotherapy. Eur. J. Cancer. 2016;67:1–10. doi: 10.1016/j.ejca.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 82.Wang B., Zhang W., Jankovic V., Golubov J., Poon P., Oswald E.M., Gurer C., Wei J., Ramos I., Wu Q. Combination cancer immunotherapy targeting PD-1 and GITR can rescue CD8+ T cell dysfunction and maintain memory phenotype. Sci. Immunol. 2018;3:eaat7061. doi: 10.1126/sciimmunol.aat7061. [DOI] [PubMed] [Google Scholar]
- 83.Topalian S.L., Drake C.G., Pardoll D.M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tumeh P.C., Harview C.L., Yearley J.H., Shintaku I.P., Taylor E.J., Robert L., Chmielowski B., Spasic M., Henry G., Ciobanu V. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fennell D.A., Kirkpatrick E., Cozens K., Nye M., Lester J., Hanna G., Steele N., Szlosarek P., Danson S., Lord J. CONFIRM: a double-blind, placebo-controlled phase III clinical trial investigating the effect of nivolumab in patients with relapsed mesothelioma: study protocol for a randomised controlled trial. Trials. 2018;19:233. doi: 10.1186/s13063-018-2602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Snyder A., Makarov V., Merghoub T., Yuan J., Zaretsky J.M., Desrichard A., Walsh L.A., Postow M.A., Wong P., Ho T.S. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yi M., Jiao D., Xu H., Liu Q., Zhao W., Han X., Wu K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol. Cancer. 2018;17:129. doi: 10.1186/s12943-018-0864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Espinosa E., Márquez-Rodas I., Soria A., Berrocal A., Manzano J.L., Gonzalez-Cao M., Martin-Algarra S., Spanish Melanoma Group (GEM) Predictive factors of response to immunotherapy-a review from the Spanish Melanoma Group (GEM) Ann. Transl. Med. 2017;5:389. doi: 10.21037/atm.2017.08.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Rutkowski P., Grob J.J., Cowey C.L., Lao C., Schadendorf D., Ferrucci P.F., Smylie M. Updated results from a phase III trial of nivolumab (NIVO) combined with ipilimumab (IPI) in treatment-naive patients (pts) with advanced melanoma (MEL) (CheckMate 067) J. Clin. Oncol. 2016;34(Suppl 15):9505. [Google Scholar]
- 90.Lievense L.A., Sterman D.H., Cornelissen R., Aerts J.G. Checkpoint Blockade in Lung Cancer and Mesothelioma. Am. J. Respir. Crit. Care Med. 2017;196:274–282. doi: 10.1164/rccm.201608-1755CI. [DOI] [PubMed] [Google Scholar]
- 91.Antonios J.P., Soto H., Everson R.G., Orpilla J., Moughon D., Shin N., Sedighim S., Yong W.H., Li G., Cloughesy T.F. PD-1 blockade enhances the vaccination-induced immune response in glioma. JCI Insight. 2016;1:e87059. doi: 10.1172/jci.insight.87059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liau L.M., Prins R.M., Kiertscher S.M., Odesa S.K., Kremen T.J., Giovannone A.J., Lin J.W., Chute D.J., Mischel P.S., Cloughesy T.F., Roth M.D. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin. Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 93.Chao T., Xiaowen W., Zhiqi L., Qi Y., Zixiao Y., Kun F., Hoon D.S.B., Wei H. A Systemic Review of Clinical Trials on Dendritic-Cells Based Vaccine Against Malignant Glioma. J. Carcinog. Mutagen. 2015;6:222. [Google Scholar]
- 94.Motamedi M., Arab S., Moazzeni S.M., Khamis Abadi M., Hadjati J. Improvement of a dendritic cell-based therapeutic cancer vaccine with components of Toxoplasma gondii. Clin. Vaccine Immunol. 2009;16:1393–1398. doi: 10.1128/CVI.00199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Packard R.R.R., Lichtman A.H., Libby P. Innate and adaptive immunity in atherosclerosis. Semin. Immunopathol. 2009;31:5–22. doi: 10.1007/s00281-009-0153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Curiel T.J., Wei S., Dong H., Alvarez X., Cheng P., Mottram P., Krzysiek R., Knutson K.L., Daniel B., Zimmermann M.C. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 97.Ge W., Ma X., Li X., Wang Y., Li C., Meng H., Liu X., Yu Z., You S., Qiu L. B7-H1 up-regulation on dendritic-like leukemia cells suppresses T cell immune function through modulation of IL-10/IL-12 production and generation of Treg cells. Leuk. Res. 2009;33:948–957. doi: 10.1016/j.leukres.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 98.Schneider T., Hoffmann H., Dienemann H., Schnabel P.A., Enk A.H., Ring S., Mahnke K. Non-small cell lung cancer induces an immunosuppressive phenotype of dendritic cells in tumor microenvironment by upregulating B7-H3. J. Thorac. Oncol. 2011;6:1162–1168. doi: 10.1097/JTO.0b013e31821c421d. [DOI] [PubMed] [Google Scholar]
- 99.Gibbons R.M., Liu X., Harrington S.M., Krco C.J., Kwon E.D., Dong H. B7-H1 signaling is integrated during CD8(+) T cell priming and restrains effector differentiation. Cancer Immunol. Immunother. 2014;63:859–867. doi: 10.1007/s00262-014-1563-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song S., Yuan P., Wu H., Chen J., Fu J., Li P., Lu J., Wei W. Dendritic cells with an increased PD-L1 by TGF-β induce T cell anergy for the cytotoxicity of hepatocellular carcinoma cells. Int. Immunopharmacol. 2014;20:117–123. doi: 10.1016/j.intimp.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 101.Francisco L.M., Salinas V.H., Brown K.E., Vanguri V.K., Freeman G.J., Kuchroo V.K., Sharpe A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saxena M., Balan S., Roudko V., Bhardwaj N. Towards superior dendritic-cell vaccines for cancer therapy. Nat. Biomed. Eng. 2018;2:341–346. doi: 10.1038/s41551-018-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rosenblatt J., Glotzbecker B., Mills H., Vasir B., Tzachanis D., Levine J.D., Joyce R.M., Wellenstein K., Keefe W., Schickler M. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J. Immunother. 2011;34:409–418. doi: 10.1097/CJI.0b013e31821ca6ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ge Y., Xi H., Ju S., Zhang X. Blockade of PD-1/PD-L1 immune checkpoint during DC vaccination induces potent protective immunity against breast cancer in hu-SCID mice. Cancer Lett. 2013;336:253–259. doi: 10.1016/j.canlet.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 105.Boudewijns S., Westdorp H., Koornstra R.H.T., Aarntzen E.H., Schreibelt G., Creemers J.H., Punt C.J., Figdor C.G., de Vries I.J., Gerritsen W.R., Bol K.F. Immune-related adverse events of dendritic cell vaccination correlate with immunologic and clinical outcome in stage III and IV melanoma patients. J. Immunother. 2016;39:241–248. doi: 10.1097/CJI.0000000000000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D., Schadendorf D., Dummer R., Smylie M., Rutkowski P. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ribas A., Comin-Anduix B., Chmielowski B., Jalil J., de la Rocha P., McCannel T.A., Ochoa M.T., Seja E., Villanueva A., Oseguera D.K. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin. Cancer Res. 2009;15:6267–6276. doi: 10.1158/1078-0432.CCR-09-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ribas A., Antonia S., Sosman J., Kirkwood J.M., Redman B., Gajewski T.F., Pavlov D., Bulanhagui C., Gomez-Navarro J., Camacho L.H. Results of a phase II clinical trial of 2 doses and schedules of CP-675,206, an anti-CTLA4 monoclonal antibody, in patients (pts) with advanced melanoma. J. Clin. Oncol. 2007;25(Suppl 18):3000. [Google Scholar]
- 109.Ribas A., Kefford R., Marshall M.A., Punt C.J., Haanen J.B., Marmol M., Garbe C., Gogas H., Schachter J., Linette G. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 2013;31:616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Camacho L.H., Antonia S., Sosman J., Kirkwood J.M., Gajewski T.F., Redman B., Pavlov D., Bulanhagui C., Bozon V.A., Gomez-Navarro J., Ribas A. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J. Clin. Oncol. 2009;27:1075–1081. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 111.Wilgenhof S., Corthals J., Heirman C., van Baren N., Lucas S., Kvistborg P., Thielemans K., Neyns B. Phase II Study of Autologous Monocyte-Derived mRNA Electroporated Dendritic Cells (TriMixDC-MEL) Plus Ipilimumab in Patients With Pretreated Advanced Melanoma. J. Clin. Oncol. 2016;34:1330–1338. doi: 10.1200/JCO.2015.63.4121. [DOI] [PubMed] [Google Scholar]
- 112.Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shoushtari A.N., Friedman C.F., Navid-Azarbaijani P., Postow M.A., Callahan M.K., Momtaz P., Panageas K.S., Wolchok J.D., Chapman P.B. Measuring Toxic Effects and Time to Treatment Failure for Nivolumab Plus Ipilimumab in Melanoma. JAMA Oncol. 2018;4:98–101. doi: 10.1001/jamaoncol.2017.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Friedman C.F., Proverbs-Singh T.A., Postow M.A. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016;2:1346–1353. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 115.van Willigen W.W., Bloemendal M., Gerritsen W.R., Schreibelt G., de Vries I.J.M., Bol K.F. Dendritic Cell Cancer Therapy: Vaccinating the Right Patient at the Right Time. Front. Immunol. 2018;9:2265. doi: 10.3389/fimmu.2018.02265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bracci L., Schiavoni G., Sistigu A., Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen G., Emens L.A. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol. Immunother. 2013;62:203–216. doi: 10.1007/s00262-012-1388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Galluzzi L., Buqué A., Kepp O., Zitvogel L., Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell. 2015;28:690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 119.Senovilla L., Vitale I., Martins I., Tailler M., Pailleret C., Michaud M., Galluzzi L., Adjemian S., Kepp O., Niso-Santano M. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337:1678–1684. doi: 10.1126/science.1224922. [DOI] [PubMed] [Google Scholar]
- 120.Denkert C., von Minckwitz G., Brase J.C., Sinn B.V., Gade S., Kronenwett R., Pfitzner B.M., Salat C., Loi S., Schmitt W.D. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J. Clin. Oncol. 2015;33:983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 121.Salgado R., Denkert C., Campbell C., Savas P., Nuciforo P., Aura C., de Azambuja E., Eidtmann H., Ellis C.E., Baselga J. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015;1:448–454. doi: 10.1001/jamaoncol.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ruffell B., Affara N.I., Coussens L.M. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ghiringhelli F., Apetoh L. The interplay between the immune system and chemotherapy: emerging methods for optimizing therapy. Expert Rev. Clin. Immunol. 2014;10:19–30. doi: 10.1586/1744666X.2014.865520. [DOI] [PubMed] [Google Scholar]
- 124.Fukuda K., Funakoshi T., Sakurai T., Nakamura Y., Mori M., Tanese K., Tanikawa A., Taguchi J., Fujita T., Okamoto M. Peptide-pulsed dendritic cell vaccine in combination with carboplatin and paclitaxel chemotherapy for stage IV melanoma. Melanoma Res. 2017;27:326–334. doi: 10.1097/CMR.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 125.Yanagisawa R., Koizumi T., Koya T., Sano K., Koido S., Nagai K., Kobayashi M., Okamoto M., Sugiyama H., Shimodaira S. WT1-pulsed dendritic cell vaccine combined with chemotherapy for resected pancreatic cancer in a phase I study. Anticancer Res. 2018;38:2217–2225. doi: 10.21873/anticanres.12464. [DOI] [PubMed] [Google Scholar]
- 126.Ghiringhelli F., Menard C., Puig P.E., Ladoire S., Roux S., Martin F., Solary E., Le Cesne A., Zitvogel L., Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Borch T.H., Engell-Noerregaard L., Zeeberg Iversen T., Ellebaek E., Met Ö., Hansen M., Andersen M.H., Thor Straten P., Svane I.M. mRNA-transfected dendritic cell vaccine in combination with metronomic cyclophosphamide as treatment for patients with advanced malignant melanoma. OncoImmunology. 2016;5:e1207842. doi: 10.1080/2162402X.2016.1207842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Noordam L., Kaijen M.E.H., Bezemer K., Cornelissen R., Maat L.A.P.W.M., Hoogsteden H.C., Aerts J.G.J.V., Hendriks R.W., Hegmans J.P.J.J., Vroman H. Low-dose cyclophosphamide depletes circulating naïve and activated regulatory T cells in malignant pleural mesothelioma patients synergistically treated with dendritic cell-based immunotherapy. OncoImmunology. 2018;7:e1474318. doi: 10.1080/2162402X.2018.1474318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nelson A., Cunha C., Nishimura M.I., Iwashima M. Activated human Foxp3+ regulatory T cells produce membrane-bound TNF. Cytokine. 2018;111:454–459. doi: 10.1016/j.cyto.2018.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Trepiakas R., Berntsen A., Hadrup S.R., Bjørn J., Geertsen P.F., Straten P.T., Andersen M.H., Pedersen A.E., Soleimani A., Lorentzen T. Vaccination with autologous dendritic cells pulsed with multiple tumor antigens for treatment of patients with malignant melanoma: results from a phase I/II trial. Cytotherapy. 2010;12:721–734. doi: 10.3109/14653241003774045. [DOI] [PubMed] [Google Scholar]
- 131.Halabi S., Lin C.-Y.Y., Kelly W.K., Fizazi K.S., Moul J.W., Kaplan E.B., Morris M.J., Small E.J. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2014;32:671–677. doi: 10.1200/JCO.2013.52.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Podrazil M., Horvath R., Becht E., Rozkova D., Bilkova P., Sochorova K., Hromadkova H., Kayserova J., Vavrova K., Lastovicka J. Phase I/II clinical trial of dendritic-cell based immunotherapy combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 2015;6:18192–18205. doi: 10.18632/oncotarget.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kodumudi K.N., Woan K., Gilvary D.L., Sahakian E., Wei S., Djeu J.Y. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin. Cancer Res. 2010;16:4583–4594. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wesolowski R., Duggan M.C., Stiff A., Markowitz J., Trikha P., Levine K.M., Schoenfield L., Abdel-Rasoul M., Layman R., Ramaswamy B. Circulating myeloid-derived suppressor cells increase in patients undergoing neo-adjuvant chemotherapy for breast cancer. Cancer Immunol. Immunother. 2017;66:1437–1447. doi: 10.1007/s00262-017-2038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tongu M., Harashima N., Monma H., Inao T., Yamada T., Kawauchi H., Harada M. Metronomic chemotherapy with low-dose cyclophosphamide plus gemcitabine can induce anti-tumor T cell immunity in vivo. Cancer Immunol. Immunother. 2013;62:383–391. doi: 10.1007/s00262-012-1343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fucikova J., Kralikova P., Fialova A., Brtnicky T., Rob L., Bartunkova J., Spísek R. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71:4821–4833. doi: 10.1158/0008-5472.CAN-11-0950. [DOI] [PubMed] [Google Scholar]
- 137.Schiavoni G., Sistigu A., Valentini M., Mattei F., Sestili P., Spadaro F., Sanchez M., Lorenzi S., D’Urso M.T., Belardelli F. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2011;71:768–778. doi: 10.1158/0008-5472.CAN-10-2788. [DOI] [PubMed] [Google Scholar]
- 138.Apetoh L., Ghiringhelli F., Tesniere A., Criollo A., Ortiz C., Lidereau R., Mariette C., Chaput N., Mira J.P., Delaloge S. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol. Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 139.Kepp O., Senovilla L., Vitale I., Vacchelli E., Adjemian S., Agostinis P., Apetoh L., Aranda F., Barnaba V., Bloy N. Consensus guidelines for the detection of immunogenic cell death. OncoImmunology. 2014;3:e955691. doi: 10.4161/21624011.2014.955691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sánchez-Paulete A.R., Teijeira A., Cueto F.J., Garasa S., Pérez-Gracia J.L., Sánchez-Arráez A., Sancho D., Melero I. Antigen cross-presentation and T-cell cross-priming in cancer immunology and immunotherapy. Ann. Oncol. 2017;28(Suppl 12):xii44–xii55. doi: 10.1093/annonc/mdx237. [DOI] [PubMed] [Google Scholar]
- 141.Lugade A.A., Moran J.P., Gerber S.A., Rose R.C., Frelinger J.G., Lord E.M. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 142.Garnett C.T., Palena C., Chakraborty M., Tsang K.Y., Schlom J., Hodge J.W. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–7994. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 143.Galluzzi L., Kepp O., Kroemer G. Immunogenic cell death in radiation therapy. OncoImmunology. 2013;2:e26536. doi: 10.4161/onci.26536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Abuodeh Y., Venkat P., Kim S. Systematic review of case reports on the abscopal effect. Curr. Probl. Cancer. 2016;40:25–37. doi: 10.1016/j.currproblcancer.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 145.Reits E.A., Hodge J.W., Herberts C.A., Groothuis T.A., Chakraborty M., Wansley E.K., Camphausen K., Luiten R.M., de Ru A.H., Neijssen J. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dewan M.Z., Galloway A.E., Kawashima N., Dewyngaert J.K., Babb J.S., Formenti S.C., Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chi K.H., Liu S.J., Li C.P., Kuo H.P., Wang Y.S., Chao Y., Hsieh S.L. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J. Immunother. 2005;28:129–135. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 148.Wang C., Pu J., Yu H., Liu Y., Yan H., He Z., Feng X. A Dendritic Cell Vaccine Combined With Radiotherapy Activates the Specific Immune Response in Patients With Esophageal Cancer. J. Immunother. 2017;40:71–76. doi: 10.1097/CJI.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 149.Golden E.B., Chhabra A., Chachoua A., Adams S., Donach M., Fenton-Kerimian M., Friedman K., Ponzo F., Babb J.S., Goldberg J. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16:795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- 150.de Rosa F., Ridolfi L., Ridolfi R., Gentili G., Valmorri L., Nanni O., Petrini M., Fiammenghi L., Granato A.M., Ancarani V. Vaccination with autologous dendritic cells loaded with autologous tumor lysate or homogenate combined with immunomodulating radiotherapy and/or preleukapheresis IFN-α in patients with metastatic melanoma: a randomised “proof-of-principle” phase II study. J. Transl. Med. 2014;12:209. doi: 10.1186/1479-5876-12-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sawa-Wejksza K., Kandefer-Szerszeń M. Tumor-Associated Macrophages as Target for Antitumor Therapy. Arch. Immunol. Ther. Exp. (Warsz.) 2018;66:97–111. doi: 10.1007/s00005-017-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Noy R., Pollard J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dammeijer F., Lievense L.A., Kaijen-Lambers M.E., van Nimwegen M., Bezemer K., Hegmans J.P., van Hall T., Hendriks R.W., Aerts J.G. Depletion of Tumor-Associated Macrophages with a CSF-1R Kinase Inhibitor Enhances Antitumor Immunity and Survival Induced by DC Immunotherapy. Cancer Immunol. Res. 2017;5:535–546. doi: 10.1158/2326-6066.CIR-16-0309. [DOI] [PubMed] [Google Scholar]
- 154.Wiehagen K.R., Girgis N.M., Yamada D.H., Smith A.A., Chan S.R., Grewal I.S., Quigley M., Verona R.I. Combination of CD40 agonism and CSF-1R blockade reconditions tumor-associated macrophages and drives potent antitumor immunity. Cancer Immunol. Res. 2017;5:1109–1121. doi: 10.1158/2326-6066.CIR-17-0258. [DOI] [PubMed] [Google Scholar]
- 155.Ries C.H., Cannarile M.A., Hoves S., Benz J., Wartha K., Runza V., Rey-Giraud F., Pradel L.P., Feuerhake F., Klaman I. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 156.Vonderheide R.H., Bajor D.L., Winograd R., Evans R.A., Bayne L.J., Beatty G.L. CD40 immunotherapy for pancreatic cancer. Cancer Immunol. Immunother. 2013;62:949–954. doi: 10.1007/s00262-013-1427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vonderheide R.H., Burg J.M., Mick R., Trosko J.A., Li D., Shaik M.N., Tolcher A.W., Hamid O. Phase I study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumors. OncoImmunology. 2013;2:e23033. doi: 10.4161/onci.23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vonderheide R.H., Glennie M.J. Agonistic CD40 antibodies and cancer therapy. Clin. Cancer Res. 2013;19:1035–1043. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vonderheide R.H., Nathanson K.L. Immunotherapy at large: the road to personalized cancer vaccines. Nat. Med. 2013;19:1098–1100. doi: 10.1038/nm.3317. [DOI] [PubMed] [Google Scholar]
- 160.Mitchem J.B., Brennan D.J., Knolhoff B.L., Belt B.A., Zhu Y., Sanford D.E., Belaygorod L., Carpenter D., Collins L., Piwnica-Worms D. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Beatty G.L., Chiorean E.G., Fishman M.P., Saboury B., Teitelbaum U.R., Sun W., Huhn R.D., Song W., Li D., Sharp L.L. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Beyranvand Nejad E., Welters M.J., Arens R., van der Burg S.H. The importance of correctly timing cancer immunotherapy. Expert Opin. Biol. Ther. 2017;17:87–103. doi: 10.1080/14712598.2017.1256388. [DOI] [PubMed] [Google Scholar]
- 164.PipelineReview.com Incyte and Merck Provide Update on Phase 3 Study of Epacadostat in Combination with KEYTRUDA® (pembrolizumab) in Patients with Unresectable or Metastatic Melanoma. 2018. https://pipelinereview.com/index.php/2018040767784/Antibodies/Incyte-and-Merck-Provide-Update-on-Phase-3-Study-of-Epacadostat-in-Combination-with-KEYTRUDA-pembrolizumab-in-Patients-with-Unresectable-or-Metastatic-Melanoma.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.