Abstract
Background
Antimicrobial resistance is a major public health threat internationally but, particularly in India. A primary contributing factor to this rise in resistance includes unregulated access to antimicrobials. Implementing antimicrobial stewardship programs (ASPs) in the acute hospital setting will help curb inappropriate antibiotic use in India. Currently, ASPs are rare in India but are gaining momentum. This study describes ASP implementation in a large, academic, private, tertiary care center in India.
Methods
An ASP was established in February 2016 consisting of an administrative champion, hospitalist, microbiologist, intensivist, and pharmacists. Antimicrobial stewardship program interventions included postprescriptive audit and establishment of institutional guidelines. The ASP tracked appropriate drug selection including loading dose, maintenance dose, frequency, route, duration of therapy, de-escalation, and compliance with ASP recommendations. Defined daily dose (DDD) of drugs and cost of antimicrobials were compared between the pre-implementation phase (February 2015–January 2016) and post-implementation phase (February 2016–January 2017).
Results
Of 48 555 patients admitted during the post-implementation phase, 1020 received 1326 prescriptions for restricted antibiotics. Antibiotic therapy was appropriate in 56% (742) of the total patient prescriptions. A total of 2776 instances of “inappropriate” antimicrobial prescriptions were intervened upon by the ASP. Duration (806, 29%) was the most common reason for inappropriate therapy. Compliance with ASP recommendations was 54% (318). For all major restricted drugs, the DDD/1000 patient days declined, and there was a significant reduction in mean monthly cost by 14.4% in the post-implementation phase.
Conclusions
Implementation of a multidisciplinary antibiotic stewardship program in this academic, large, Indian hospital demonstrated feasibility and economic benefits.
Keywords: antimicrobial resistance, antimicrobial stewardship, appropriateness, defined daily dose
Antimicrobial resistance (AMR) is a major public health problem in India, and the nation’s infectious diseases burden is among the highest in the world [1]. Public health systems in India have struggled to keep up with rapid economic growth and urbanization. Antibiotic availability without prescription and unregulated use are major drivers of resistance [1]. India led all nations in antibiotic consumption from 2000 to 2010 [2]. Stakeholders including the Indian government have recognized AMR as a major problem. From 2017 to 2021, India is implementing the National Action Plan on Antimicrobial Resistance to combat AMR and improve antibiotic use by doctors, consumers, and healthcare institutions [3]. The plan follows a call by the World Health Organization to member states to combat AMR [4]. Antimicrobial stewardship programs (ASPs) have been shown to improve antibiotic use and patient outcomes [5–7]. Currently, ASPs are rare and unstructured in India. However, the concept of supporting an ASP in acute care hospitals to curb AMR is gaining momentum [8]. We describe implementation of an ASP in a large, private tertiary care center in Southern India.
METHODS
Study Design, Setting, and Population
This was a single-center, quasi-experimental study done at an academic tertiary care referral center in the state of Kerala from February 2015 to January 2017 evaluating the impact of a new ASP. The 1300-bed hospital has 254 intensive care unit (ICU) beds with 13 ICUs (including surgical and medical units) and admits a high census of morbidly ill patients requiring critical care. The institutional Ethics Committee approved this study.
Deploying a Formal Antimicrobial Stewardship Program as the Intervention
In the preintervention period, “justification forms” stating indication for use of (colistin, polymyxin B, tigecycline, meropenem, ertapenem, doripenem, fosfomycin, vancomycin, aztreonam, and linezolid) were required to be completed within 24 hours by the treating doctor. These forms were submitted to the infection control team. The infection control team comprised a microbiologist, internal medicine physician, trained infection control nurses, and a medical administrator/physician. On receiving the justification forms, the infection control nurses collected relevant patient information and presented it to the team in a biweekly meeting for review of appropriateness. Feedback was provided via e-mail to the treating doctor based on the review by the infection. When indicated, de-escalation was recommended.
In February 2016, a formal ASP was created and included a physician/hospitalist, intensivist, microbiologists, clinical pharmacists, and an administrative champion. The ASP team reviewed and adapted content from the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, and the Centers for Disease Control and Prevention [9–11] for guiding principles of antibiotic stewardship. The ASP focused on postprescriptive audit with feedback and intervention and development of institutional guidelines. Updated institutional antibiograms were disseminated to all providers and were accessible on the hospital intranet. A list of “restricted” antimicrobials was generated based on previous antibiogram data (this included polymyxins B and E [colistin], carbapenems, glycopeptides, aztreonam, tigecycline, linezolid, fosfomycin, echinocandins, lipids/liposomal amphotericin B, and voriconazole). Double anaerobic coverage was targeted as a stewardship target as was appropriate dosing of polymyxins. To standardize dosing for colistin and polymyxin B, the ASP team established guidelines for loading dose and maintenance dose based on creatinine clearance [12, 13].
Every weekday morning, data on patients who were receiving restricted antibiotics were obtained through the electronic medical record. Data abstracted from the electronic medical record included demographics, clinician notes, laboratory investigations, microbiology tests, imaging results, and drug details. The ASP team discussed these cases, the appropriateness of therapy for each case based on definitions stated in Table 1. The team defined appropriateness using the “5 Rs” or Right drug, Right indication, Right dose, Right frequency, and Right duration. References for appropriateness included Infectious Diseases Society of America practice guidelines [14–16], stewardship guidelines [17], and standard treatment recommendations of antimicrobial therapy [18–21].
Table 1.
Definition of Parameters Used for Assessing Appropriateness
| Parameter | Definition |
|---|---|
| Right indication [18] | When the prescribed antimicrobial is the most appropriate selection in terms of the pathogen, if known, and the site of infection (eg, prescribing polymyxin B instead of colistin for multidrug-resistant Klebsiella pneumoniae urinary tract infection is considered inappropriate because polymyxin B does not achieve optimal concentrations in the urine) |
| Right drug [10] | When the antimicrobial is the narrowest and the most effective option (eg, prescribing meropenem instead of ceftriaxone for pan-sensitive Escherichia coli in blood is considered inappropriate in a hemodynamically stable patient) |
| Right dose | When the loading dose and maintenance dose of the prescribed antimicrobial is appropriate and accurate for the patient’s diagnosis as per standard recommendations [19] (antimicrobials that required a loading dose for this study included colistin, tigecycline, polymyxin B, and caspofungin) |
| Right frequency [38] | When the frequency of the prescribed antimicrobial dose is appropriate for the patient’s diagnosis as per standard recommendations |
| Right duration | When the prescribed antimicrobial has been administered for the correct duration based on the patient’s diagnosis as per standard recommendations [19–21] |
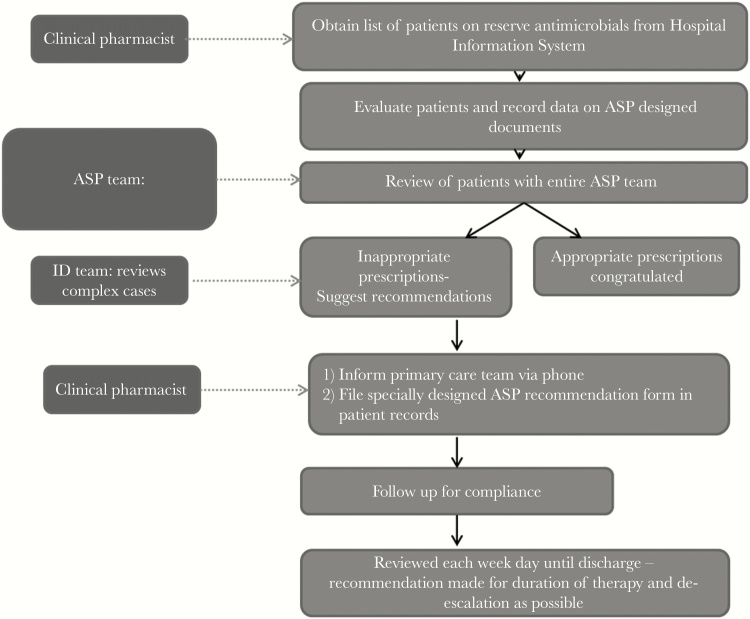
The ASP team reviewed clinical charts every weekday. Appropriate use was encouraged with positive feedback to providers. Inappropriate use was discussed with providers and coupled with a stewardship recommendation, which was filed in the patient’s record and discussed with the care providers through phone or e-mail (Figure 1).
Figure 1.
Antimicrobial stewardship program (ASP) work flow for audit and review. ID, infectious diseases.
Outcomes
The pre-implementation phase was February 2015–January 2016 and post-implementation phase of ASP was February 2016–January 2017. The primary outcome was the cost of consumption of all the restricted antimicrobials audited by ASP team. The cost data was based on purchasing costs by hospital pharmacy. The hospital pharmacy negotiated the cost of antibiotics individually with the pharmaceutical retailer and sold it to the patients at the negotiated prices. All patients were required to buy antibiotics from the hospital pharmacy. Medications from outside pharmacies were not administered, as per hospital policy. The cost data for our analysis was obtained from the hospital pharmacy sales. Secondary outcomes included defined daily dose (DDD) per 1000 patient days to compare the consumption of antimicrobials [17] and compliance to recommendations filed by the ASP team. Data on de-escalation of antimicrobials based on microbiology results were also captured.
Statistical Analysis
Descriptive statistics were used to summarize the key outcome data before and after the implementation of ASP. Categorical variables were analyzed by χ2 tests and continuous variables using Student’s t test. SPSS version 17 (SPSS Inc., Chicago, IL) was used for all statistical analysis.
RESULTS
Demographics and Clinical Characteristics
A total of 48 555 patients were admitted during the post-implementation period, from February 1, 2016 to January 31, 2017. A total of 4613 (10%) patients received at least 1 antibiotic during their inpatient stay during this period. There were 1326 patient prescriptions for restricted antibiotics for 1020 unique patients during this period. The general characteristics of the patient cohort including the types of primary care team are described in Table 2. Bloodstream infection (n = 395, 30%) was the most common focus of infection that required antimicrobial therapy, followed by urinary tract (n = 308, 23%) and skin and soft tissue infection (n = 307, 23%). Culture specimens were sent before antibiotic administration for 85% of the cohort. Seven percent of the cases had no positive cultures. The mortality per 1000 inpatients improved from 31.6 in the pre-implementation phase to 28.9 in the post-implementation phase. The average length of stay decreased from 6.6 days before establishment of ASP team to 6.4 days in the post-implementation phase.
Table 2.
General Characteristics of the Patient Cohort
| General Characteristics (N = 1326) | |||
|---|---|---|---|
| Age (median and range) | 55 (1–92) | ||
| Male gender (N, %) | 892 (67%) | ||
| Departments | Total Number of Prescriptions | Appropriate N = 742 (56%) |
Inappropriate N = 584 (44%) |
| General Medicine | 231 (17%) | 129 (56%) | 102 (44%) |
| Medical Specialties | 549 (41%) | 304 (55%) | 245 (45%) |
| Cardiology | 44 (3%) | 28 (64%) | 16 (36%) |
| Endocrinology | 126 (9%) | 69 (55%) | 57 (45%) |
| Nephrology | 99 (7%) | 53 (54%) | 46 (46%) |
| Neurology and Stroke | 63 (5%) | 28 (44%) | 35 (56%) |
| Gastroenterology | 80 (6%) | 43 (54%) | 37 (46%) |
| Medical Oncology | 106 (8%) | 68 (64%) | 38 (36%) |
| Dermatology/Geriatrics/Psychiatry/Physical Medicine/Rheumatology/ Pulmonology | 31 (2%) | 15 (48%) | 16 (52%) |
| General Surgery | 22 (2%) | 14 (64%) | 8 (36%) |
| Surgical Specialties | 401(30%) | 222 (55%) | 179 (45%) |
| Cardiovascular and Thoracic Surgery | 59 (4%) | 36 (61%) | 23 (39%) |
| Gastrointestinal Surgery | 135 (10%) | 82 (61%) | 53 (39%) |
| Neuro Surgery | 108 (8%) | 60 (56%) | 48 (44%) |
| Plastic surgery | 28 (2%) | 11 (39%) | 17 (61%) |
| Urology | 34 (3%) | 13 (38%) | 21 (62%) |
| ENT/Gynecology/Head and Neck Surgery/Ophthalmology/ Orthopedics | 37 (3%) | 20 (54%) | 17 (46%) |
| Pediatrics | 123 (9%) | 73 (59%) | 50 (41%) |
| Pediatrics | 36 (3%) | 19 (53%) | 17 (47%) |
| Neonatology | 26 (2%) | 14 (54%) | 12 (46%) |
| Pediatric surgery | 61 (5%) | 40 (66%) | 21 (34%) |
| Types of infection | |||
| Blood stream infections | 395 (30%) | 300 (76%) | 95 (24%) |
| Skin and soft tissue infection | 307 (23%) | 213 (69%) | 94 (31%) |
| Urinary tract infection | 308 (23%) | 224 (73%) | 84 (27%) |
| Pneumonia | 218 (16%) | 155 (71%) | 63 (29%) |
| Intra-abdominal infection | 58 (4%) | 43 (74%) | 15 (26%) |
| Central nervous system infection | 36 (3%) | 31 (86%) | 5 (14%) |
| Others (Infective endocarditis, septic arthritis, endophthalmitis, otitis media) | 5 (0.4%) | 4 (80%) | 1 (20%) |
| Appropriateness of sending cultures | 1326 | 1123 (85%) | 203 (15%) |
| Specimen | |||
| Blood | 470 (35%) | - | - |
| Urine | 416 (31%) | - | - |
| Respiratory secretions | 261 (20%) | - | - |
| Body fluids | 57 (4%) | - | - |
| Cerebrospinal fluid | 37 (3%) | - | - |
| Skin and soft tissue infection | 277 (21%) | - | - |
| Other | 24 (2%) | - | - |
| No culture sent | 95 (7%) | - | - |
Appropriateness of Antibiotic Therapy
Antibiotic therapy was determined to be appropriate for 56% (742) of the total patient prescriptions during the ASP implementation period. Appropriate prescriptions for restricted antimicrobials were present in 76% (300 of 395) of bloodstream infections, 73% (224 of 308) of urinary tract infections, and 69% (213 of 307) of skin and soft tissue infections.
A total of 2776 instances of “inappropriate” antimicrobial prescriptions were recognized and intervened upon by the ASP team during the implementation period. The distribution of inappropriateness in antibiotic therapy is depicted in Table 3. Eight hundred six instances of inappropriate duration (29%) in antimicrobial therapy accounted for the most common reason for inappropriateness. Inappropriateness in loading dose was also common with 272 (38%) instances observed among 710 antibiotic prescriptions that required a loading dose to be administered.
Table 3.
Reasons for Inappropriate Antimicrobial Therapy
| Episodes of Inappropriate Antimicrobial Treatment (N = 2776) | ||
|---|---|---|
| N | % | |
| Inappropriate indication | 514 | 19 |
| Inappropriate drug | 483 | 17 |
| Inappropriate dose | 606 | 22 |
| →Inappropriate loading dose* | 272 | 38 |
| →Inappropriate maintenance dose | 417 | 15 |
| Inappropriate frequency | 367 | 13 |
| Inappropriate duration | 806 | 29 |
*Number of patients for which loading dose was required = 710 (26%).
Compliance With Antimicrobial Stewardship Program Recommendations
Compliance with ASP recommendations was achieved among 318 (54%) of 584 total recommendations during the implementation period. The department of medical oncology (66%) and pediatrics (65%) recorded high rates of compliance to the ASP guidelines, whereas low compliance rates were noted for the departments of pediatric surgery (33%) and neonatology (17%).
Of the 490 prescriptions for which de-escalation of treatment was recommended, only 41% (201) complied with the recommendation. De-escalation of therapy was observed to be highest in the department of neonatology (60%), whereas the department of gastrointestinal surgery (20%) recorded the lowest rate of antibiotic de-escalation.
Impact of Antimicrobial Stewardship Program on Defined Daily Dose of Drugs
The total patient days for preintervention and postintervention period were found to be 308 040 and 311 640 days, respectively. By antibiotic class, the DDD per 1000 patient days declined in the postintervention period for polymyxins (34.03 to 28.16), echinocandins (1.8 to 1.6), linezolid (41.9 to 36.1), amphotericin B (13.9 to 12), whereas it increased for the carbapenems (Table 4). Specifically for colistin, the DDD per 1000 patient days was reduced from 33.2 during pre-implementation phase to 25.18 in post-implementation phase (Table 5). The increase in DDD of carbapenems was predominantly due to an increase in meropenem utilizations (13.3 to 71.2).
Table 4.
Defined Daily Dose of Drugs by Class Before and After ASP Implementation
| Drug | Pre-consumption DDD for 1000 Patient Days | Post-consumption DDD for 1000 Patient Days |
|---|---|---|
| Polymyxins | 34.03 | 28.16 |
| Carbapenems | 15.86 | 73.51 |
| Echinocandins | 1.84 | 1.64 |
Abbreviations: ASP, antimicrobial stewardship program; DDD, defined daily dose.
Table 5.
Defined Daily Dose Before and After ASP Implementation
| Drug | Preconsumption DDD for 1000 Patient Days | Postconsumption DDD for 1000 Patient Days |
|---|---|---|
| Colistin | 33.22 | 25.18 |
| Polymyxin B | 0.81 | 2.98 |
| Vancomycin | 5.4 | 6.65 |
| Amphotericin B | 13.99 | 12.01 |
| Anidulafungin | 0.39 | 0.74 |
| Caspofungin | 1.45 | 0.69 |
| Doripenem | 0.63 | 0.57 |
| Ertapenem | 1.89 | 1.72 |
| Tigecycline | 3.85 | 5.17 |
| Micafungin | 0 | 0.21 |
| Fosfomycin | 0 | 2.45 |
| Linezolid | 41.99 | 36.13 |
| Meropenem | 13.34 | 71.22 |
| Aztreonam | 0 | 0.011 |
Abbreviations: ASP, antimicrobial stewardship program; DDD, defined daily dose.
Impact of Antimicrobial Stewardship Program on Cost of Consumption
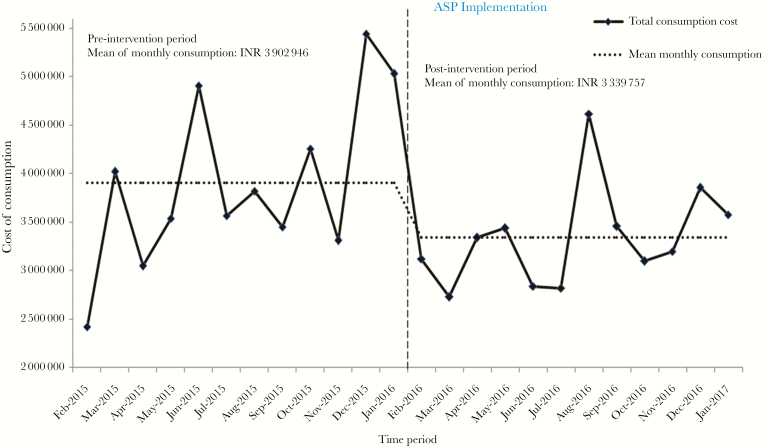
The mean monthly cost for restricted drugs significantly dropped by 14.4% in the post-implementation phase of ASP in comparison with the pre-implementation phase (P = .03) (Figure 2). The total cost of consumption for colistin significantly decreased from Indian Rupees (INR) 28 349 685 (US $442 964) during the pre-implementation period of ASP to INR 22 975 459 (US $358 992) during the post-implementation period (P = .01). Similar declines in cost of consumption were also observed for amphotericin B, doripenem, and linezolid during the post-implementation period of ASP, although they were not statistically significant.
Figure 2.
Aggregate cost of consumption of reserve antimicrobials before and after antimicrobial stewardship program (ASP). *, The exchange rate for 1 dollar was 63.86 Indian Rupees (INR) during analysis.
DISCUSSION
A multidisciplinary ASP was successfully deployed in this academic tertiary hospital in India. Evaluation of the program revealed a decreasing mean monthly cost of consumption of restricted antimicrobials and a decreasing trend of DDD of colistin. The savings were all transferred to patients because the predominant patient population pays “out of pocket”. Other studies in areas with novel implementation of ASP have also shown cost-savings and reduced antibiotic consumption [7, 22].
Our data on DDD of antimicrobials revealed changes in antimicrobial consumption trends that could be explained by the interventions of the ASP team, changes to the hospital formulary, and physician prescribing habits. Polymyxin B was added to the formulary in the post-implementation period. Colistin’s common side effects of nephrotoxicity and its relative expense in India make polymyxin B preferred by physicians in our center and likely contributed to its rise in DDD during the post-implementation period, whereas the DDD of colistin decreased. A similar opposing trend was also observed among carbapenems. Surgeons at our center tended to use several classes of broad-spectrum antibiotics empirically, but the ASP encouraged them to use meropenem based on institutional antibiogram data, possibly leading to the increase in DDD of this class while other classes decreased. The decrease in DDD of caspofungin with concomitant increase in anidulafungin was likely due to preferential use in patients who underwent liver transplantation and patient with signs of hepatotoxicity, preventing use of caspofungin.
This study helped identify ASP targets for the future and what outcomes to study in the future to improve patient care. Regulative measures such as written justification forms for restricted antimicrobials without further interaction, which were in place before the ASP was created, were largely ineffective. The reasons for ineffectiveness were multifactorial, including significant delays in reviewing the antibiotic justification forms, absence of interaction with the prescribing doctor within an actionable time frame after feedback, and lack of monitoring of compliance with feedback and duration of therapy. This signals that messaging in stewardship is important and is consistent with stewardship literature [5, 23]. Compliance with ASP recommendations was also surprisingly high, compared with published rates of compliance [24], indicating that future directed ASP targets could have an even higher impact. Other literature has also shown that surgeons often are slow to adapt to standard ASP strategies [25–27], so this is a potential future targeted ASP intervention. Another future target identified from this work is de-escalation of therapy. More than half of the time, prescribers did not narrow antimicrobials when it was possible to do so, despite culture data being available. De-escalation and duration are common stewardship targets and impact cost, antimicrobial consumption, and patient outcomes [28–30]. Despite having institutional guidelines for loading doses of meropenem and the polymyxins, 38% of the audited prescriptions were inappropriate in terms of dosing. This continues to be an ongoing target for our ASP, especially given the increasingly resistant Gram-negative infections seen in the patient population at this institution and broadly in Indian hospitals.
Because AMR has been such a significant threat in India, stakeholders are interested in understanding gaps in training that could be worsening the problem. The Indian Council of Medical Research (ICMR) identified lack of trained clinical pharmacists as a gap in improving antimicrobial stewardship practices in India in 2015 [31]. Clinical pharmacists have an established role within hospitals as promoters of evidence-based medicine and cost-effective prescribing [32]. Having a clinical pharmacy training program in this institution facilitated the inclusion and mentoring of trained graduates in the ASP, which was novel. With clinical pharmacists driving ASP worldwide, our experience also confirms the success of a multidisciplinary model involving clinical pharmacists who utilize their expertise to optimize antimicrobial treatment to promote rational prescriptions and reduce inappropriate prescriptions [31, 33, 34]. Mandating multidisciplinary ASPs in acute care hospitals would be a wise next step for policy in India and would mirror antimicrobial stewardship policy work in the United States [1, 35, 36].
This study has several limitations. First, the quasi-experimental nature of this study has inherent limitations. The infection control team had no new initiatives during the intervention period, which decreased this effect. Second, there are no standardized appropriateness measures for antimicrobial use. We used available best practice published work to come up with the definition of appropriateness used by our ASP and replicated measures that have been done in other stewardship work [37]. Third, this is the experience of a single institution that can limit generalizability, but it adds to the limited published work of implementation of an ASP in an environment where resistant Gram-negatives are part of the common ecological flora, and more work from these types of hospitals, particularly in India, are needed. Fourth, even before deployment of the formal ASP structure, restrictions for selected antimicrobials were in place, and, thus, the impact of the ASP interventions might have been greater, if data were available from a time before restrictions were in place and if we had more measures across time to help strengthen statistical findings.
CONCLUSIONS
In conclusion, this work demonstrated successful implementation of a multidisciplinary ASP team in a large hospital in South India, with economic benefits. The ASP effectively implemented several stewardship interventions including postprescriptive auditing and feedback and identified multidisciplinary stewardship champions. Our preliminary results with an ASP in India are encouraging, but a national effort to initiate, implement, and maintain ASPs in acute care hospitals needs to be studied in India. Clinical pharmacists were critical to the success of this ASP and were uniquely empowered in our center, which is an uncommon model in India. Further work empowering academic pharmacists to take part in antimicrobial stewardship in acute care inpatient hospitals in India should be undertaken.
Acknowledgments
We acknowledge all of the physicians and nurses from our hospital for their support. We also acknowledge the Hospital Information System team and hospital pharmacy for assistance in extracting data on antimicrobial consumption.
Funding. The study has not received any financial support from any external funding agencies.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Laxminarayan R, Chaudhury RR. Antibiotic resistance in India: drivers and opportunities for action. PLoS Med 2016; 13:e1001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14:742–50. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization, Ministry of Health and Family Welfare. National Action Plan on Antimicrobial Resistance (NAP-AMR) 2017–2021. 2017; 1–57 (April). Available at: http://www.searo.who.int/india/topics/antimicrobial_resistance/nap_amr.pdf. Accessed 6 June 2018. [Google Scholar]
- 4. World Health Organization. Global Action Plan on Antimicrobial Resistance. World Health Organization; 2015. Available at: http://www.wpro.who.int/entity/drug_resistance/resources/global_action_plan_eng.pdf. Accessed 7 June 2018. [Google Scholar]
- 5. Davey P, Marwick CA, Scott CL, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2017; 2:CD003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention (CDC). Vital signs: preventing Clostridium difficile infections. Morb Mortal Wkly Rep 2012; 61:157–62. [PubMed] [Google Scholar]
- 7. Lee CF, Cowling BJ, Feng S, et al. Impact of antibiotic stewardship programmes in Asia: a systematic review and meta-analysis. J Antimicrob Chemother 2018; 73:844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kakkar M, Walia K, Vong S, et al. Antibiotic resistance and its containment in India. BMJ 2017; 358:j2687. [DOI] [PubMed] [Google Scholar]
- 9. Pollack LA, Srinivasan A. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin Infect Dis 2014; 59(Suppl_3):S97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barlam TF, Cosgrove SE, Abbo LM, et al. Executive summary: implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:1197–202. [DOI] [PubMed] [Google Scholar]
- 11. Pope SD, Dellit TH, Owens RC, Hooton TM. Results of survey on implementation of Infectious Diseases Society of America and Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Infect Control Hosp Epidemiol 2009; 30:97–8. [DOI] [PubMed] [Google Scholar]
- 12. Nation RL, Garonzik SM, Thamlikitkul V, et al. Dosing guidance for intravenous colistin in critically-ill patients. Clin Infect Dis 2017; 65:565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sandri AM, Landersdorfer CB, Jacob J, et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 2013; 57:524–31. [DOI] [PubMed] [Google Scholar]
- 14. Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:503–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patterson TF, Thompson GR 3rd, Denning DW, et al. Executive summary: practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 17. Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44:159–77. [DOI] [PubMed] [Google Scholar]
- 18. Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc 2011; 86:156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gilbert D, Eliopoulos G, Chambers H, Saag M, Pavia A.. The Sanford Guide to Antimicrobial Therapy 2017. 47th ed. Sperryville, VA: Antimicrobial Therapy; 2017. [Google Scholar]
- 20. National Institute of Health and Care Guidance. Pneumonia in Adults: Diagnosis and Management. Available at: https://www.nice.org.uk/guidance/cg191. Accessed December 2014. [Google Scholar]
- 21. National Institute of Health and Care Guidance. Urinary Tract Infections in Adults. Available at: https://www.nice.org.uk/guidance/qs90. Accessed June 2015. [Google Scholar]
- 22. Alawi MM, Darwesh BM. A stepwise introduction of a successful antimicrobial stewardship program. Experience from a tertiary care university hospital in Western, Saudi Arabia. Saudi Med J 2016; 37:1350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luther VP, Shnekendorf R, Abbo LM, et al. Antimicrobial stewardship training for infectious diseases fellows: program directors identify a curriculum need. Clin Infect Dis 2018; 67:1285–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Foolad F, Huang AM, Nguyen CT, et al. A multicentre stewardship initiative to decrease excessive duration of antibiotic therapy for the treatment of community-acquired pneumonia. J Antimicrob Chemother 2018; 73:1402–7. [DOI] [PubMed] [Google Scholar]
- 25. Duane TM, Zuo JX, Wolfe LG, et al. Surgeons do not listen: evaluation of compliance with antimicrobial stewardship program recommendations. Am Surg 2013; 79:1269–72. [PubMed] [Google Scholar]
- 26. Sartelli M, Duane TM, Catena F, et al. Antimicrobial stewardship: a call to action for surgeons. Surg Infect (Larchmt) 2016; 17:625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Çakmakçi M. Antibiotic stewardship programmes and the surgeon’s role. J Hosp Infect 2015; 89:264–266. [DOI] [PubMed] [Google Scholar]
- 28. Avdic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis 2012; 54:1581–7. [DOI] [PubMed] [Google Scholar]
- 29. Nilholm H, Holmstrand L, Ahl J, et al. An Audit-based, infectious disease specialist-guided antimicrobial stewardship program profoundly reduced antibiotic use without negatively affecting patient outcomes. Open Forum Infect Dis 2015; 2:ofv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel PK, Srinivasan A. Moving antibiotic stewardship from theory to practice. J Hosp Med 2017; 12:382–3. [DOI] [PubMed] [Google Scholar]
- 31. Walia K, Ohri VC, Mathai D; Antimicrobial Stewardship Programme of ICMR Antimicrobial stewardship programme (AMSP) practices in India. Indian J Med Res 2015; 142:130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perez A, Doloresco F, Hoffman JM, et al. Economic evaluations of clinical pharmacy services: 2001–2005. Pharmacotherapy 2009; 29:128. [DOI] [PubMed] [Google Scholar]
- 33. Waters CD. Pharmacist-driven antimicrobial stewardship program in an institution without infectious diseases physician support. Am J Health Syst Pharm 2015; 72:466–8. [DOI] [PubMed] [Google Scholar]
- 34. Li Z, Cheng B, Zhang K, et al. Pharmacist-driven antimicrobial stewardship in intensive care units in East China: a multicenter prospective cohort study. Am J Infect Control 2017; 45:983–9. [DOI] [PubMed] [Google Scholar]
- 35. Nain VK, Khurana GS, Singh S, et al. Antibiotic resistance pattern in bacterial isolates obtained from different water samples of Delhi region. DU J Undergrad Res Innov 2015; 1:219–27. [Google Scholar]
- 36. Chandy SJ, Mathai E, Thomas K, et al. Antibiotic use and resistance: perceptions and ethical challenges among doctors, pharmacists and the public in Vellore, South India. Indian J Med Ethics 2013; 10:20–7. [DOI] [PubMed] [Google Scholar]
- 37. Sikkens JJ, Gerritse SL, Peters EJ, Kramer MH, van Agtmael MA. The ‘morning dip’ in antimicrobial appropriateness: circumstances determining appropriateness of antimicrobial prescribing. J Antimicrob Chemother 2018; 73:1714–20. [DOI] [PubMed] [Google Scholar]
- 38. Doron S, Davidson LE. Antimicrobial stewardship. Mayo Clin Proc 2011; 86:1113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]