1. BACKGROUND
Optimal dosing of vancomycin in neonates remains a matter of debate despite the frequent use of vancomycin in neonatal intensive care units (NICUs).1, 2 The optimal use of vancomycin requires knowledge of its pharmacokinetics (PK) and pharmacodynamics (PD) properties in neonates. The ratio of 24‐hour area under the concentration versus time curve to minimal inhibitory concentration (AUC24/MIC) was shown to be the best PK/PD surrogate marker of vancomycin efficacy.3 The target of AUC24/MIC ≥ 400 (where AUC24 and MIC are expressed as mg h/L and mg/L, respectively) is most commonly used in neonatal vancomycin PK/PD studies. However, it was originally defined in adult methicillin‐resistant Staphylococcus aureus (MRSA) pneumonia4 and was never validated in neonatal Staphylococci septicaemia. Moreover, this AUC24/MIC target level of 400 is based on total vancomycin concentration, while Smits et al recently demonstrated that the fraction unbound (FU) of vancomycin is much higher in neonates (median 0.9) compared with that in adults (median 0.6).5 Using PK/PD simulation, we have explored the impact of this recent finding on vancomycin dosing in neonates.
It is important to first introduce two different concepts for vancomycin dosing optimization in neonates.
The traditional “total drug target approach” aimed at achieving similar total vancomycin exposure in neonates as in adults, irrespective of the differences in protein binding, and thus targeted an AUC24/MIC ≥ 400 for optimal dosing in neonates.
A novel “unbound drug target approach,” based on the recent findings of Smits et al,5 aimed at achieving similar unbound vancomycin exposure in neonates as in adults and thus targeted an AUC24/MIC ≥ 267 for optimal dosing in neonates. This 267 target level is based on the following: (i) The total AUC24/MIC of 400 (part of the drug which can be measured) corresponds to an unbound AUC24/MIC of 240 (part of the drug which is pharmacologically active), assuming a median vancomycin FU of 0.6 in adults; (ii) this unbound AUC24/MIC of 240 finally corresponds to a total AUC24/MIC of 267 in neonates based on the median vancomycin FU of 0.9 in neonates.
2. HOW DOES THIS NEW “UNBOUND DRUG TARGET APPROACH” INFLUENCE THE PK/PD TARGET ATTAINMENT RATES IN 249 PRETERM NEONATES THAT HAVE BEEN ENROLLED IN A PREVIOUS PHARMACOKINETIC STUDY?
This large group of preterm neonates is a realistic representation of the distribution of demographic characteristics of neonates undergoing vancomycin treatment in NICUs. In this population, the overall median (range) gestational age, post‐natal age, and bodyweight were 29 weeks (23‐34), 11 days (1‐27), and 1200 g (415‐2630), respectively. These neonates received vancomycin as a 60‐minute infusion at a dose of 15 mg/kg once or twice a day according to their post‐natal age and serum creatinine value.6
Vancomycin PK parameters and exposure profiles of these 249 neonates were estimated using a PK model which was previously developed during a population PK meta‐analysis of vancomycin in neonates.7 Briefly, this two‐compartment model was built based on a dataset of 4894 vancomycin concentrations from 1631 neonates. In this PK model, bodyweight, post‐menstrual age (PMA), and serum creatinine value were the significant covariates for clearance.
In the selected cohort, population PK parameters (mean ± SD) obtained from this PK model were Vd (sum of V1 and V2) = 0.54 ± 0.10 L/kg and CL = 0.06 ± 0.02 L/h kg at steady state. Vancomycin AUC24 values (mean ± SD) were 446.4 ± 161.3 mg h/L at steady state. According to the traditional “total drug target approach,” the AUC24/MIC ≥ 400 target was achieved by only 54.2% of the neonates (for an MIC of 1 mg/L). However, with the “unbound drug target approach,” the AUC24/MIC ≥ 267 target was achieved by 91.2% of the neonates (for an MIC of 1 mg/L).
3. FINALLY, HOW DOES THIS NEW FINDING BASED ON THE “UNBOUND DRUG TARGET APPROACH” WILL GUIDE THE OPTIMAL USE OF VANCOMYCIN AS AN INTERMITTENT INFUSION IN THESE PRETERM NEONATES?
To answer that question, Monte Carlo simulations (n = 100) were performed, using the software package NONMEM, to generate vancomycin pharmacokinetic profiles for different dosing regimens in the aforementioned cohort. The simulation population was divided into three subgroups by age (97 neonates with a PMA of less than 30 wk, 105 neonates with a PMA of 30‐34 wk, and 47 neonates with a PMA of 34 wk or more), on the basis of visual inspection of the plot of vancomycin clearance versus PMA. The neonatal maintenance dose of vancomycin was simulated on a milligram‐per‐kilogram basis for the different age groups.
For each vancomycin dosing optimization approach, the target attainment rates as a function of dose and PMA groups for an MIC of 1 mg/L are shown in Figure 1. Maintenance doses required for 90% probability of target attainment (PTA) of AUC24/MIC ≥ 400 at steady state for MIC 1 mg/L were 15 mg/kg twice daily (BID) for neonates with a PMA of less than 30 weeks and 15 mg/kg three times daily (TID) for neonates with a PMA of 30 weeks or more. This dosing regimen resulted in vancomycin trough concentrations of 19 mg/L (fifth to 95th percentile: 7.7‐39.8) at steady state. Considering the “unbound drug target approach,” a lower dosing regimen of 10 mg/kg BID for neonates with a PMA of less than 30 weeks and 10 mg/kg TID for neonates with a PMA of 30 weeks or more was sufficient to achieve 90% PTA at steady state. This dosing regimen resulted in vancomycin trough concentrations of 12.7 mg/L (fifth to 95th percentile: 5.1‐26.5) at steady state.
Figure 1.
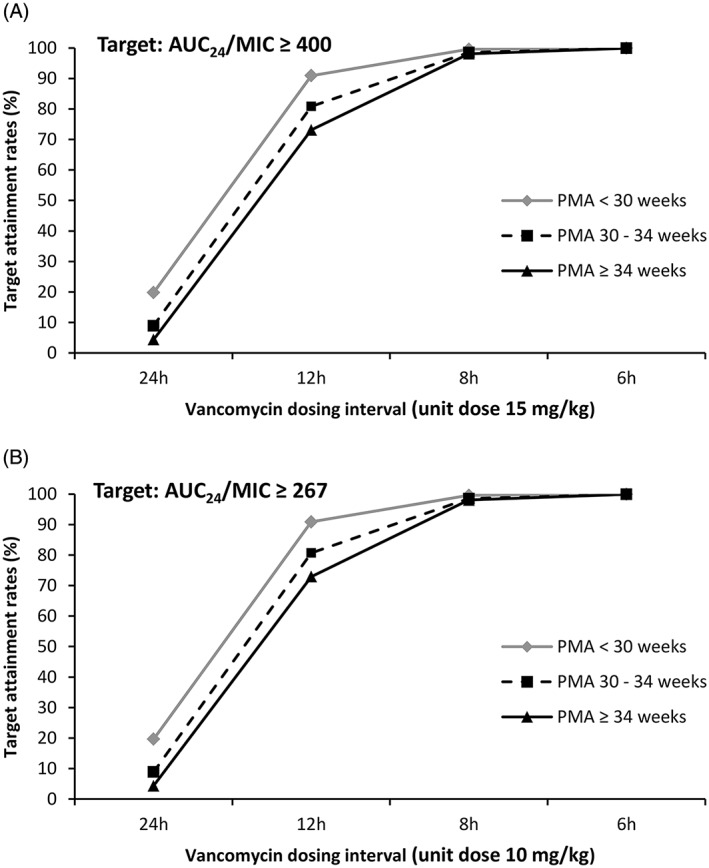
Vancomycin target attainment rates at steady state, for 100 simulated trials, in 249 neonates. For each level of AUC24/MIC target, the target attainment rates for an MIC value of 1 mg/L are presented as a function of dose and post‐menstrual age (PMA) group. A, Traditional dosing optimization approach based on total vancomycin concentrations. B, New dosing optimization approach based on unbound vancomycin concentrations
4. CONCLUSION
This editorial illustrates that appropriate dose adjustments in neonates need to account for maturation processes, which may affect not just pharmacokinetic parameters such as clearance but also protein binding of drugs. Due to maturational changes in vancomycin protein binding and as previously mentioned by other authors,8, 9 it is not reasonable to assume a similar AUC24/MIC target level for vancomycin dosing optimization in neonates and adults. As a consequence, we have to be aware that the higher unbound vancomycin fraction in neonates will impact the dose. As a proof of concept, simulations were here based on a fixed unbound vancomycin fraction. However, this unbound fraction might be variable within and between neonates. Further clinical and bacteriological studies are needed to validate a vancomycin PK/PD target based on unbound concentrations in neonates.
Leroux S, van den Anker JN, Smits A, Pfister M, Allegaert K. Maturational changes in vancomycin protein binding affect vancomycin dosing in neonates. Br J Clin Pharmacol. 2019;85:865–867. 10.1111/bcp.13899
REFERENCES
- 1. Zhao W, Kaguelidou F, Biran V, et al. External evaluation of population pharmacokinetic models of vancomycin in neonates: the transferability of published models to different clinical settings. Br J Clin Pharmacol. 2013;75(4):1068‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Linder N, Lubin D, Hernandez A, Amit L, Ashkenazi S. Duration of vancomycin treatment for coagulase‐negative Staphylococcus sepsis in very low birth weight infants. Br J Clin Pharmacol. 2013;76(1):58‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jacqz‐Aigrain E, Zhao W, Sharland M, van den Anker JN. Use of antibacterial agents in the neonate: 50 years of experience with vancomycin administration. Semin Fetal Neonatal Med. 2013;18(1):28‐34. [DOI] [PubMed] [Google Scholar]
- 4. Moise‐Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925‐942. [DOI] [PubMed] [Google Scholar]
- 5. Smits A, Pauwels S, Oyaert M, et al. Factors impacting unbound vancomycin concentrations in neonates and young infants. Eur J Clin Microbiol Infect Dis. 2018;37(8):1503‐1510. [DOI] [PubMed] [Google Scholar]
- 6. Allegaert K, Anderson BJ, van den Anker JN, Vanhaesebrouck S, de Zegher F. Renal drug clearance in preterm neonates: relation to prenatal growth. Ther Drug Monit. 2007;29(3):284‐291. [DOI] [PubMed] [Google Scholar]
- 7. Jacqz‐Aigrain E, Leroux S, Thomson A, et al. Population pharmacokinetic meta‐analysis of individual data to design the first randomized efficacy trial of vancomycin in neonates and young infants. J Antimicrob Chemother. 2019; In Press. [DOI] [PubMed] [Google Scholar]
- 8. Padari H, Oselin K, Tasa T, Metsvaht T, Lõivukene K, Lutsar I. Coagulase negative staphylococcal sepsis in neonates: do we need to adapt vancomycin dose or target? BMC Pediatr. 2016;16(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stockmann C, Sammons H, Starkey E, Constance JE, Sherwin CMT. Unanswered questions regarding optimal pediatric vancomycin use. Ther Drug Monit. 2016;38(3):419‐420. [DOI] [PubMed] [Google Scholar]


