Abstract
Dried jujube (Ziziphus jujuba) was incubated at high temperature and humidity for 96 hr in blacking process and sampled every 12 hr. Results showed that the saccharose reduced from 195.6 to 3.1 g/kg rapidly in 24 hr. The total acid content was mild with 8.82 g/kg and increased to 23.45 g/kg by 177.21% with thermal processing for 96 hr. The contents of total polyphenols were enhanced during 0–48 hr processing, and the amount of the compound increased with treatment by 50.99%. The total reducing sugar increased 29.79% on 60 hr. cAMP was decreased with aging and ripening by 65.85%. 5‐HMF was keep growing to 3.52 g/kg. The volatile component had great change in black jujube fruits compared to untreated jujubes, especially treated in 12 hr. The results indicated that backing pretreatment can facilitate the generation of functional food materials and support the development of this nutrition product.
Keywords: blacking process, compositions, jujube, volatile component
1. INTRODUCTION
Jujube (Ziziphus jujuba Mill.) belongs to the Rhamnaceae family and was originally grown in the mountains, hills, or plains at altitudes below 1,700 m in subtropical and tropical regions of Asia, and it has also been cultivated in Europe and in the Americas in recent years (Gao, Wu, & Wang, 2013). The jujube is cultivated in China on a total of 1.5 million hectares to produce 400,000 tons of fruit, which makes China output account for 98% of the total output worldwide. Jujube fruits have a huge potential for development and economic benefits as part of the economic forest. Jujube fruit is well known for its high nutritional content (Li, Fan, Ding, & Ding, 2007), including common sugars (Chen et al., 2015), acids, and vitamins (Wojdyło, Figiel et al., 2016). Natural bioactive substances that have antioxidant effects, such as triterpenic acid (Gong, Zhao, Liu, Hu, & Gao, 2014; Guo et al., 2015), phenolic compounds (Gao et al., 2012; Wojdyło, Carbonell‐Barrachina, Carbonell‐Barrachina, Legua, & Hernández, 2016), cyclic adenosine monophosphate (cAMP) (Bai et al., 2016; Ji et al., 2017), and flavonoids, (Chen et al., 2013) have also been found in recent studies (Gao et al., 2011).
Jujube fruits have been recognized as a nutritious food and have important uses in the diet and in traditional medicine in China (Wojdyło, Figiel et al., 2016). Jujube as a raw material is used in traditional products, such as mud, candied dates, and moon cake. In recent years, new products have also been developed and applied, such as drinks like milk and wine. However, jujube fruits are not applicable for children and elderly men, especially those with high blood sugar (Wang, Cheng, Cao, & Jiang, 2013).
With similar processing as black garlic, black jujube is a processed jujube product that is prepared via heat treatment of the raw jujube at high temperature and in a high humidity environment for natural aging for a certain time after the finished product. When jujube undergoes heat treatment, various physicochemical changes occur in the color, aroma, flavor, and nutrient content. Heat treatment, in particular, brings about nonenzymatic browning reactions, such as the caramelization reaction, Maillard reaction, and other chemical oxidation reactions. Nonenzymatic browning reactions are associated with the formation of strong antioxidant compounds because jujube contains sugar and amino acids (Kim, Kim, Kim, Park, & Lee, 2013). Black jujubes enhance the antioxidant activity via DPPH radical scavenging, which occurs for a long time during aging. (Park et al., 2012). The sugar content is reduced for the specific population in the black jujube during the aging process. However, information on the change in biological activities and chemical constituents is lacking in the literature. In this study, we monitored the changes in sugar, total acid, pentahydroxymethyl furfural (5′‐HMF), total phenols, cAMP, and volatile components during the process of jujube aging to support the development of low‐sugar and nutrition products.
2. MATERIALS AND METHODS
2.1. Reagents and materials
We obtained 3′,5′‐cyclic AMP and 5′‐HMF from Ziqibio Co., Ltd. (Shanghai, China), fructose, glucose, and sucrose were purchased from Shanghai Yuanye Bio‐Technology Co., Ltd. (Shanghai, China). Other reagents were of analytical grade. Acetonitrile and methanol were both of HPLC grade and purchased from Merck (Darmstadt, Germany). The dry jujubes were varieties of Ningyang jujube provided by the market (Taian, China).
The high‐performance liquid chromatography (HPLC) was performed on a LC‐20A system Shimadzu (Kyoto, Japan), equipped with diode array detector (SPD‐20A) and refractive index detector (RID‐10A). Data acquisitions were performed using Lab Solution software. IMS instrument (FlavourSpec®) from Gesellschaft für Analytische Sensorsysteme mbH (G.A.S., Dortmund, Germany) equipped with a heated splitless injector and automatic sampler unit (CTC‐PAL, CTC Analytics AG, Zwingen, Switzerland) was utilized for instrumental aroma analysis. The IMS was equipped with a FS‐SE‐54‐CB‐1 column (15 m × 0.53 mm). IMS data were acquired and identified unknown compounds using GCxIMS Library Search software, and then analyzed by Laboratory Analytical Viewer supplied by G.A.S.
2.2. Sample preparation
The treatment of jujube refers to the production method of black garlic. The dry jujubes were cleaned and soaked in water for one hour and sealed with water (600 g/400 ml); then, the entire jujube was placed in a constant temperature and humidity incubator. The jujube was aged and fermented in an 80% humidity environment at 75°C for 96 hr. 500 g jujubes were taken out every 12 hr and then homogenized after removing the nucleus. All the samples mentioned below were obtained from the homogenized jujubes.
2.3. The measurement method of color difference
Taking the raw jujube as a comparison, the color difference was measured by the color difference meter for the jujube samples of each period. The color difference was represented by the values of ΔE*ab. L* represents brightness, a* and b* represent chromaticity. L1*, a1*, and b1* were the measured values of untreated jujubes. L2*, a2*, and b2* were the measured values of treated jujubes of each period.
2.4. Determination of total acid content
The total acid content was analyzed using the national standard of GB/T 12456‐2008. The samples (20 g) were homogenized with 80°C boiled water and placed in a boiling water bath for 30 min (shaken 2–3 times). The volume was adjusted to 250 ml with water after it was cooled down. The supernatant was filtered, added 50 ml of water, and then titrated with 0.1 M of sodium hydroxide solution to a pH of 8.3 in a magnetic stirrer.
2.5. Analysis of sugar
The measurement method for fructose, glucose, and sucrose was according to the China National Standard protocol GB 5009.8‐2016. Two grams of homogenized samples were weighed in a beaker with 40 mL of water and allowed to mix in a magnetic stirrer for 10 min. 5 mL of zinc acetate (219 g/L) and 5 mL of potassium ferrocyanide (106 g/L) was added. Then the mixed sample was transferred into bottle and injected water to 100ml. The mixtures were extracted via ultrasonic for 30 min. Finally, the supernatant was reconstituted and transferred into vials after filtering through a 0.45‐μm nylon filter. The mixtures were extracted via ultrasonic for 30 min. Finally, the supernatant was reconstituted and transferred into vials after filtering through a 0.45‐μm nylon filter.
A sample of 10 µl was injected into an Inertsil NH2 column (250 × 4.6 mm, 5 µm; Shimadzu) and detected using a refractive index detector in 40°C. The elution was carried out using acetonitrile and water (70:30, v/v) at a flow rate of 1.0 ml/min.
The determination method for the reducing sugar was completely in reference to the national standard (GB 5009.7‐2016, China).
2.6. Determination of the total phenolic content
The total phenolic extracts were analyzed using the Folin−Ciocalteu phenol reagent method, which was modified on the basis of the reported literature (Gao et al., 2012). A one‐gram sample was dissolved in a 50 ml ethanol solution (70%), then socked for 10 min and extracted via ultrasonification for 30 min at 66°C.
A total of 750 µl of the Folin−Ciocalteu reagent and 750 µl of deionized water were added to 200 μl of the supernatant of the sample extracts. The mixture was added to 1.5 ml of sodium carbonate (10%, w/v) and brought to a 10 ml volume by deionized water and allowed to stand for 10 min at 75°C before measured on a spectrophotometer at an absorbance of 760 nm. A mixture of Folin−Ciocalteu phenol reagents and deionized water was used as the blank.
2.7. Analysis of cAMP
The cAMP content was determined based on an assay method (Kou et al., 2015) with some modifications. The samples (2 g) were extracted for 30 min with 100 ml of boiled and distilled water using an ultrasonic washing unit and then centrifuged for 5 min at 1,509.3 g; the supernatant was filtered through a 0.45‐μm syringe filter.
The compounds were eluted in an Inertsil ODS‐3 column (250 × 4.6 mm; 5 μm) at a flow rate of 1 ml/min with a mobile phase of a methanol–potassium dihydrogen phosphate solution (0.05 M) mixture (10:90, v/v). The flow rate was 1 ml/min, and the detection temperature was 30°C. The detection wavelength was 254 nm (5‐nm bandwidth), and the injection volume was 10 µl.
2.8. 5‐HMF analysis
The 5 g samples were homogenized with 10.0 ml of methanol; then, the mixture was stirred with 30.0 ml of water with mixing using a magnetic stirrer for 10 min. The phase was transferred into a 50 ml bottle and fixed with water and extracted via ultrasonic 30 min after it was fully mixed. The supernatant was filtered through a 0.45‐μm syringe filter prior to HPLC.
A total of 10 μl were injected onto the HPLC column. The analytes were separated in the IntertSustain C18 column (250 × 4.6 mm, 5 μm; Shimadzu) at 35°C. The mobile phase was a methanol–water mixture (2:98, v/v). The absorbance wavelength for determination was 282 nm.
2.9. Detection of volatile components
Nitrogen of 99.99% purity was used as the drift and carrier gas. Drift gas flow was set at a constant flow of 150 ml/min. Initial carrier gas flow of 2 ml/min, holding 2 min, increased to 100 ml/min at 20 min. The samples were incubated in headspace volume at 40°C lasting 10 min. 1 g jujube sample was transferred into a 20 ml headspace vial, and then incubated in headspace volume at 40 °C for 25 min. 500 μl headspace was injected by a syringe temperature of 45°C into the heated injector in splitless mode. The analytes were separated at 40 °C column and then ionized in the IMS ionization chamber of 45°C.
A GC–IMS analysis results in a blue picture as shown in Figure 1. It is a two‐dimensional map in which X axis represents the drift time and Y axis represents the retention time. The red vertical line on the left side is the reactive ion (RIP) in the blue background. Each point on entire spectrum represents a volatile organic compound. The color represents the normalized concentration of the substance, white indicates less concentration, and red indicates greater concentration. The darker the color, the greater the concentration. Qualitative data were obtained through NIST database of retention index and IMS migration time database based on GC×IMS Library Search from G.A.S. IMS migration time database was based on GC×IMS Library Search software. Forty‐three qualitative compounds are marked in Figure 1. The information of CAS number, formula, molecular weight (MW), retention index (RI), retention time (RT), migration time (MT) were showed in Table 1.
Figure 1.

Comparison on the color of raw and black jujubes (96 hr)
Table 1.
The information of 43 qualitative substances
| Compound | CAS# | Formula | MW | RI | RT [s] | DT [RIPrel] | |
|---|---|---|---|---|---|---|---|
| 1 | Maltol | C118718 | C6H6O3 | 126.1 | 1,093.4 | 793.841 | 1.2103 |
| 2 | Furaneol | C3658773 | C6H8O3 | 128.1 | 1,071.9 | 722.677 | 1.2019 |
| 3 | Ethyl hexanoate | C123660 | C8H16O2 | 144.2 | 1,008.8 | 548.599 | 1.8025 |
| 4 | Ethyl hexanoate | C123660 | C8H16O2 | 144.2 | 1,008.4 | 547.504 | 1.3475 |
| 5 | 1‐Octen‐3‐ol | C3391864 | C8H16O | 128.2 | 986.6 | 501.521 | 1.1585 |
| 6 | Benzaldehyde | C100527 | C7H6O | 106.1 | 954.2 | 445.685 | 1.4665 |
| 7 | Benzaldehyde | C100527 | C7H6O | 106.1 | 954.9 | 446.779 | 1.1515 |
| 8 | 2‐Furanmethanol, 5‐methyl‐ | C3857258 | C6H8O2 | 112.1 | 956.9 | 450.064 | 1.5687 |
| 9 | Methyl hexanoate | C106707 | C7H14O2 | 130.2 | 928 | 405.176 | 1.6835 |
| 10 | Methyl hexanoate | C106707 | C7H14O2 | 130.2 | 928.7 | 406.271 | 1.2901 |
| 11 | Gamma‐Butyrolactone | C96480 | C4H6O2 | 86.1 | 908.3 | 377.259 | 1.0852 |
| 12 | Gamma‐Butyrolactone | C96480 | C4H6O2 | 86.1 | 907.1 | 375.603 | 1.3025 |
| 13 | Ethyl pentanoate | C539822 | C7H14O2 | 130.2 | 905.5 | 373.395 | 1.2752 |
| 14 | Ethyl pentanoate | C539822 | C7H14O2 | 130.2 | 903.9 | 371.186 | 1.6824 |
| 15 | Heptanal | C111717 | C7H14O | 114.2 | 901.8 | 368.426 | 1.6967 |
| 16 | Heptanal | C111717 | C7H14O | 114.2 | 901.4 | 367.874 | 1.3584 |
| 17 | 2,5‐dimethylpyrazine | C123320 | C6H8N2 | 108.1 | 905.5 | 373.395 | 1.5028 |
| 18 | 2‐Heptanone | C110430 | C7H14O | 114.2 | 893 | 356.833 | 1.6316 |
| 19 | 2‐Heptanone | C110430 | C7H14O | 114.2 | 893 | 356.833 | 1.2635 |
| 20 | Ethyl 3‐methylbutanoate | C108645 | C7H14O2 | 130.2 | 853 | 316.533 | 1.2635 |
| 21 | Ethyl 3‐methylbutanoate | C108645 | C7H14O2 | 130.2 | 853 | 316.533 | 1.6563 |
| 22 | 2‐Hexen‐1‐ol | C2305217 | C6H12O | 100.2 | 848.3 | 312.117 | 1.1828 |
| 23 | 2‐Hexen‐1‐ol | C2305217 | C6H12O | 100.2 | 848.3 | 312.117 | 1.5145 |
| 24 | Butyl acetate | C123864 | C6H12O2 | 116.2 | 810 | 278.413 | 1.2392 |
| 25 | Butyl acetate | C123864 | C6H12O2 | 116.2 | 810.8 | 279.025 | 1.6177 |
| 26 | Ethyl butanoate | C105544 | C6H12O2 | 116.2 | 819.5 | 286.37 | 1.556 |
| 27 | Ethyl butanoate | C105544 | C6H12O2 | 116.2 | 819.1 | 286.064 | 1.2178 |
| 28 | 2‐Hexanol | C626937 | C6H14O | 102.2 | 793.4 | 264.947 | 1.5636 |
| 29 | 2‐Hexanol | C626937 | C6H14O | 102.2 | 791.9 | 263.723 | 1.2869 |
| 30 | 2‐Hexanone | C591786 | C6H12O | 100.2 | 782.3 | 256.378 | 1.4995 |
| 31 | 2‐Hexanone | C591786 | C6H12O | 100.2 | 783.1 | 256.99 | 1.1914 |
| 32 | Methyl 2‐methylbutanoate | C868575 | C6H12O2 | 116.2 | 771.7 | 249.033 | 1.5309 |
| 33 | 3‐methylbutanol | C123513 | C5H12O | 88.1 | 730.8 | 222.407 | 1.3322 |
| 34 | Ethyl propanoate | C105373 | C5H10O2 | 102.1 | 708.7 | 209.247 | 1.4542 |
| 35 | 2‐Ethylfuran | C3208160 | C6H8O | 96.1 | 703.3 | 206.186 | 1.3295 |
| 36 | Pentanal | C110623 | C5H10O | 86.1 | 693.5 | 200.678 | 1.423 |
| 37 | 2‐Pentanone | C107879 | C5H10O | 86.1 | 683.2 | 195.892 | 1.3694 |
| 38 | 3‐methylbutanal | C590863 | C5H10O | 86.1 | 648.1 | 182.764 | 1.4068 |
| 39 | Ethyl Acetate | C141786 | C4H8O2 | 88.1 | 605.9 | 168.195 | 1.3348 |
| 40 | 2‐Butanone | C78933 | C4H8O | 72.1 | 586.8 | 161.951 | 1.2441 |
| 41 | Ethanol | C64175 | C2H6O | 46.1 | 446.1 | 122.727 | 1.0477 |
| 42 | Acetone | C67641 | C3H6O | 58.1 | 492.8 | 134.574 | 1.1141 |
| 43 | 2‐Propanol | C67630 | C3H8O | 60.1 | 503.6 | 137.456 | 1.1796 |
3. RESULTS AND DISCUSSION
3.1. The change of color
As shown in Figure 1, the color and form of the jujubes have great changes through blacking process. Table 2 shows the values of the color difference of the sample in the process. It could be seen that the data of 12 hr and the original date are significantly different, while the data of 12–96 hr are relatively close. It indicated that the color of the sample changes most obviously at 12 hr, after which the color of the treatment does not change much.
Table 2.
The change of ΔL, Δa, Δb, and ΔE in the process of jujube blacking
| 时间/hr | ΔL | Δa | Δb | ΔE |
|---|---|---|---|---|
| 0 | −0.43 | −1.95 | −0.69 | 2.12 |
| 12 | −1.69 | −9.68 | −2.07 | 10.04 |
| 24 | −1.47 | −9.45 | −2.01 | 9.77 |
| 36 | −1.01 | −8.76 | −1.87 | 9.02 |
| 48 | −1.55 | −9.69 | −1.96 | 10.01 |
| 60 | −1.70 | −10.41 | −2.16 | 10.77 |
| 72 | −1.53 | −10.02 | −2.14 | 10.36 |
| 84 | −0.74 | −8.20 | −1.85 | 8.44 |
| 96 | −0.96 | −8.84 | −1.92 | 9.10 |
3.2. The change of total acid contents during the thermal processing of black jujube
Figure 2a presents the changes in the total acid contents during the aging period of the jujube fruits. The total acid content of dried jujube was mild with 8.82 g/kg and increased to 23.45 g/kg by 177.21% with thermal processing for 96 hr (Table 3). The rate of acid formation was different, and it was assumed to be related to nonenzymatic browning due to early color changes when the acidity increased faster. The acid increase was partially associated with the production of carboxylic acids during the browning reactions, which have been reported to be generated via the oxidation of the aldehyde in aldohexose (Sang, Cho, Yong, Lee, & Park, 2014). It also has been established that a variation in organic acids may occur during the Maillard reaction due to the co‐existence of amino and carbonyl groups.
Figure 2.
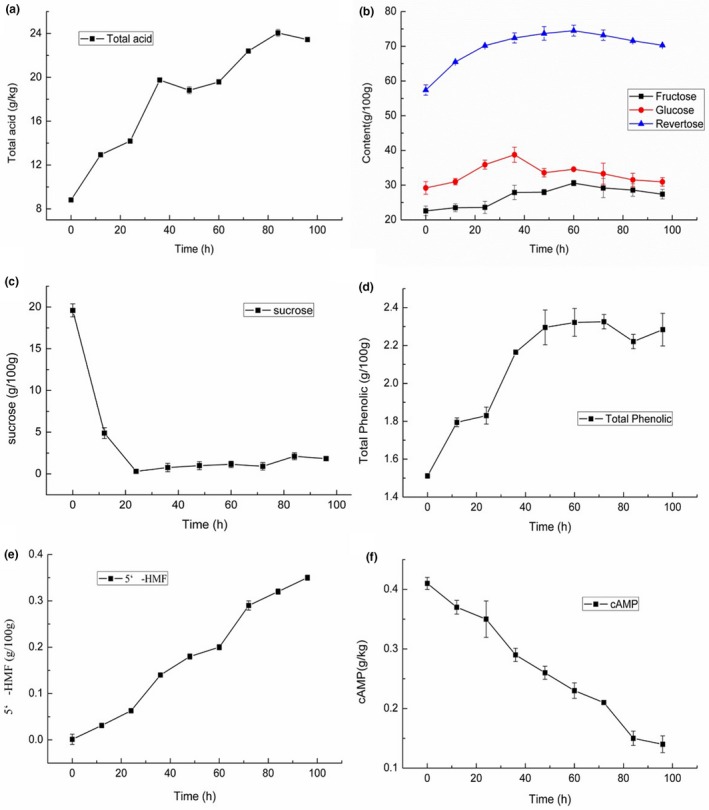
The changing tendency of total acid (a), sugar (b), sucrose (c), total phenolic (d), 5‐HMF (e), and cAMP (f) in aging process
Table 3.
Contents (dw, mean ± SD, n = 3) of the ingredients in samples with different aging stages
| Analytes (g/kg) | Aging stages (hr) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 12 | 24 | 36 | 48 | 60 | 72 | 84 | 96 | |
| Fructose | 226.3 ± 13.7 | 235.1 ± 11.4 | 236.7 ± 17.5 | 278.6 ± 20.8 | 280.3 ± 7.8 | 306.3 ± 7.8 | 291.7 ± 27.6 | 286.2 ± 17.9 | 274.1 ± 13.6 |
| Glucose | 292.4 ± 18.3 | 310.2 ± 8.9 | 359.0 ± 12.7 | 387.7 ± 21.7 | 335.8 ± 12.2 | 346.3 ± 0.9 | 332.9 ± 30.2 | 315.2 ± 19.3 | 309.5 ± 12.2 |
| Sucrose | 195.6 ± 7.8 | 48.9 ± 6.4 | 3.1 ± 0.8 | 7.6 ± 5.1 | 9.9 ± 4.9 | 11.5 ± 3.9 | 9.1 ± 1.5 | 21.1 ± 4.1 | 18.3 ± 1.9 |
| Reducing sugar | 574.3 ± 15.4 | 655.2 ± 1.2 | 701.9 ± 10.2 | 723.5 ± 14.4 | 737.2 ± 19.8 | 745.0 ± 15.5 | 731.7 ± 15.0 | 715.6 ± 1.1 | 703.0 ± 5.8 |
| Total acid | 8.82 ± 0.02 | 12.94 ± 0.15 | 14.19 ± 0.01 | 19.76 ± 0.01 | 18.82 ± 0.32 | 19.59 ± 0.03 | 22.4 ± 0.06 | 24.05 ± 0.32 | 23.05 ± 0.02 |
| Total phenolic | 15.1 ± 1.3 | 17.9 ± 0.2 | 18.3 ± 0.4 | 21.6 ± 0.1 | 23.0 ± 0.9 | 23.2 ± 0.7 | 23.3 ± 0.4 | 22.2 ± 0.4 | 22.8 ± 0.9 |
| 5′‐HMF | 0.01 ± 0.11 | 0.29 ± 0.01 | 0.64 ± 0.02 | 1.38 ± 0.03 | 1.81 ± 0.06 | 2.05 ± 0.06 | 2.89 ± 0.01 | 3.23 ± 0.06 | 3.52 ± 0.06 |
| cAMP | 0.41 ± 0.01 | 0.37 ± 0.01 | 0.35 ± 0.03 | 0.29 ± 0.01 | 0.26 ± 0.01 | 0.23 ± 0.01 | 0.21 ± 0.01 | 0.15 ± 0.01 | 0.14 ± 0.02 |
3.3. Sugar content during the blackening process
The changes in sugar contents in black jujube at different aging stages are presented in Figure 2. As shown in Figure 2b, increasing trends were found for the contents of total reducing sugar, glucose, and fructose during pre‐period aging. Fructose, glucose, and total reducing sugar increased by 35.40% on 60 hr, 32.76% on 36 hr, and 29.79% on 60 hr, respectively. Otherwise, their content began to decrease during the later period of aging. The sucrose content was exactly opposite to that of the reducing sugar during aging, and it rapidly reduced from 195.6 to 3.1 g/kg in 24 hr (Table 3) and then changed slowly (Figure 2c). Because sucrose is decomposed into monosaccharides or disaccharides, which have reducibility, the results could indicate that sucrose may be converted to reducing sugar with sample aging. The relevance between the trends of sucrose and reducing sugar was analyzed, and the correlation coefficient was −0.934; the results showed a bilateral significant correlation. At the end of aging, the content of reducing sugar, which is one of the reactants of the Maillard reaction, decreased because of continued consumption. In addition, the total amount of glucose and fructose accounted for 83.0%–90.1% of the reducing sugar during the aging process. This confirmed that fructose and glucose are the main contents of the reducing sugar.
3.4. Content of functional components during the blackening process
Changes in the chemical functional composition, including HMF, total phenolic, and cAMP, were studied during the aging process. The contents of total polyphenols were constantly enhanced during pre‐period processing (0–4 8 hr), the amount of the compound increased with treatment by 50.99% compared to raw jujube, and it then remained basically the same (48–96 hr) (Figure 2d). An increase in these properties in black garlic compared with those in raw garlic due to heating at high temperatures has also been reported (Nencini, Menchiari, Franchi, & Micheli, 2011; Shin et al., 2008). The increasing content of polyphenol was considered to be caused by the release of these compounds to the outside or by the conversion of some other ingredients into polyphenols during the heat processing of aging jujube (Kim et al., 2013).
As shown in Figure 2e and Table 3, HMF that almost nonexistent in raw jujube rapidly increased to 3.5 g/kg by the blacking process of 96 hr. HMF mainly formed via the Maillard reaction, which is regarded as the most important contaminant that is present in heat‐induced products, especially in bakery food (Capuano & Fogliano, 2011). The results could be associated with the fact that HMF is produced at high temperature.
The raw samples had higher contents of cAMP (4.1 g/kg DW) compared to the black samples in which the trend of contents decreased as shown in Figure 2f and dramatically dropped to 1.4 g/kg DW by 65.85% (Table 1). This confirms that cAMP was consumed during nonenzymatic browning during the process.
3.5. Change in the aroma ingredients
The content of volatile organic compounds between samples during 0–96 hr blackening was compared using Gallery Plot. The result automatically generated a fingerprint map in Figure 3 arranged from top to bottom according to processing time. Each row represents a sample, and each column represents a substance. As shown in Figure 4, the flavor change of jujube is obvious. The content of benzaldehyde, γ‐butyrolactone, ethyl hexanoate, 1‐octene‐3‐ol, methyl hexanoate, ethyl valerate, heptaldehyde, 2‐heptanone, ethyl 3‐methylbutanoate, 2‐hexen‐1‐ol, ethyl butyrate, butyl acetate, 3‐methylbutanol, valeraldehyde, pentanone, and other substances gradually decreased until disappearing in the process of red frame region. The material in the green frame area is slowly produced during the fermentation process and increases with time, such as acetone, 2‐acetylfuran, and some indeterminate compounds.
Figure 3.
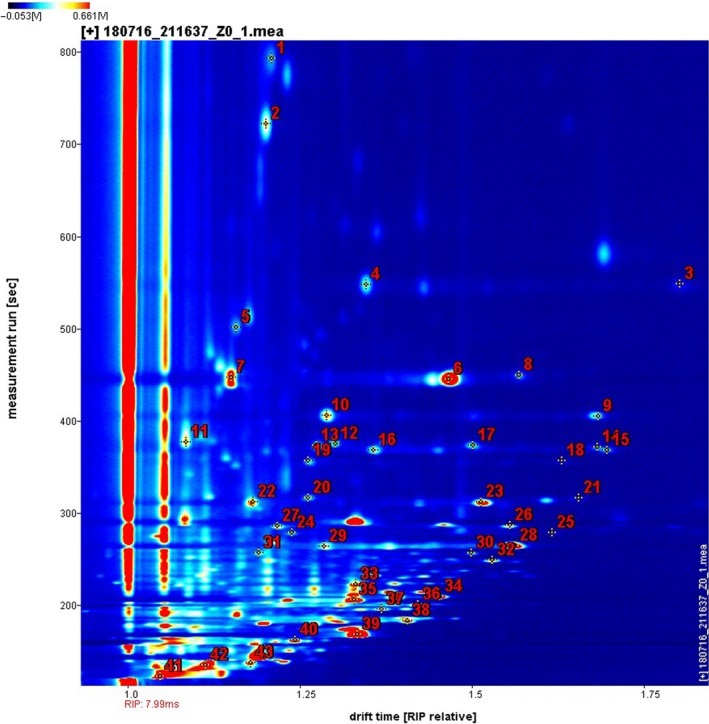
Gas phase ion mobility spectrogram of the sample
Figure 4.

The Gallery Plot of volatile components in black jujubes
The result of PAC analysis on ingredients data was shown in Figure 5. The volatile components of raw jujube (Z0) were obviously different from processed jujube (Z12‐Z108). The components of Z60 and Z72 were very similar, and the same situation occurs in Z84 and Z96. It demonstrated that the volatile components change slowly in the later period, and there is a significant difference.
Figure 5.
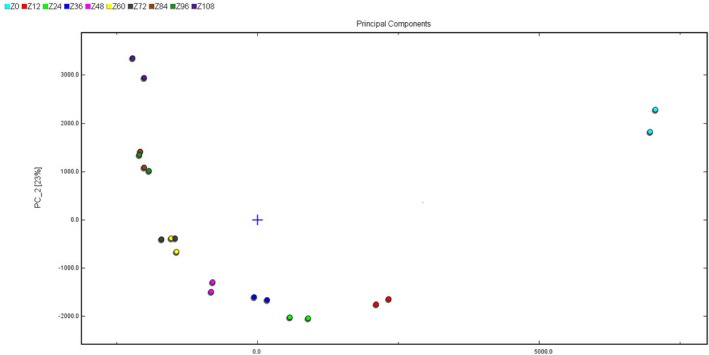
The PAC analysis of jujube samples
4. CONCLUSIONS
In this study, black jujube was prepared via high temperature aging from untreated jujube, and the contents of sugar, acid, total phenols acid, 5′‐HMF, cAMP, and volatile ingredients in jujube were monitored. The results revealed that black jujube is richer in reducing sugar, 5′‐HMF and phenolic, which leads to a corresponding increase in antioxidant properties, but it lacks cAMP.
It shown that both colorinternal composition and internal composition changes in the high temperature and high humidity processing environment. A quantitative analysis of the contents of reducing sugar, total phenols, and 5′‐HMF present in black jujube is considered as great importance for evaluating the effect of black jujube aging. This result is of interest to nutritionists and consumers as new jujube products will be developed to enhance the health functionality of jujubes
ETHICAL STATEMENTS
This study does not involve any human or animal testing.
CONFLICT OF INTEREST
The authors declare that they do not have any conflict of interest.
ACKNOWLEDGMENTS
This work was financially supported by the Shandong Province Key Research and Development Funds (2016GNC113015) and Shandong Province major application of technological innovation projects.
Sun X, Gu D, Fu Q, et al. Content variations in compositions and volatile component in jujube fruits during the blacking process. Food Sci Nutr. 2019;7:1387–1395. 10.1002/fsn3.973
REFERENCES
- Bai, L. , Zhang, H. , Liu, Q. , Zhao, Y. , Cui, X. , Guo, S. , … … N. (2016). Chemical characterization of the main bioactive constituents from fruits of Ziziphus jujuba . Food Function, 7(6), 2870 10.1039/C6FO00613B [DOI] [PubMed] [Google Scholar]
- Capuano, E. , & Fogliano, V. (2011). Acrylamide and 5‐hydroxymethylfurfural (hmf): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT ‐ Food Science and Technology, 44(4), 793–810. 10.1016/j.lwt.2010.11.002 [DOI] [Google Scholar]
- Chen, J. , Li, Z. , Maiwulanjiang, M. , Zhang, W. L. , Zhan, J. Y. X. , Lam, C. T. W. , … … K. W. (2013). Chemical and biological assessment of Ziziphus jujuba fruits from China: Different geographical sources and developmental stages. Journal of Agricultural and Food Chemistry, 61, 7315–7324. [DOI] [PubMed] [Google Scholar]
- Chen, Q. , Bi, J. , Wu, X. , Yi, J. , Zhou, L. , & Zhou, Y. (2015). Drying kinetics and quality attributes of jujube (Zizyphus jujuba Miller) slices dried by hot‐air and short and medium‐wave infrared radiation. LWT ‐ Food Science and Technology, 64, 759–766. 10.1016/j.lwt.2015.06.071 [DOI] [Google Scholar]
- Gao, Q. H. , Wu, C. S. , & Wang, M. (2013). The jujube (Ziziphus Jujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. Journal of Agricultural and Food Chemistry, 61, 3351–3363. [DOI] [PubMed] [Google Scholar]
- Gao, Q. H. , Wu, P. T. , Liu, J. R. , Wu, C. S. , Parry, J. W. , & Wang, M. (2011). Physico‐chemical properties and antioxidant capacity of different jujube (Ziziphus jujuba, mill.) cultivars grown in loess plateau of china. Scientia Horticulturae, 130(1), 67–72. 10.1016/j.scienta.2011.06.005 [DOI] [Google Scholar]
- Gao, Q. H., Wu, C. S., Wang, M., … L. J. (2012). Effect of drying of jujubes (Ziziphus jujuba Mill.) on the contents of sugars, organic acids, α‐tocopherol, β‐carotene, and phenolic compounds. Journal of Agricultural & Food Chemistry, 60(38), 9642–9648. [DOI] [PubMed] [Google Scholar]
- Gong, S. J. , Zhao, Z. H. , Liu, M. J. , Hu, F. , & Gao, Y. R. (2014). Determination and comparison of triterpene acid, ascorbic acid and b‐complex vitamins of main jujube processing products. Journal of Food Agriculture Environment, 12(2), 169–173. [Google Scholar]
- Guo, S. , Duan, J. A. , Qian, D. , Tang, Y. , Wu, D. , Su, S. , … Zhao, Y. (2015). Content variations of triterpenic acid, nucleoside, nucleobase, and sugar in jujube (Ziziphus jujuba) fruit during ripening. Food Chemistry, 167, 468–474. 10.1016/j.foodchem.2014.07.013 [DOI] [PubMed] [Google Scholar]
- Ji, X. , Qiang, P. , Yuan, Y. , Jing, S. , Xie, X. , & Min, W. (2017). Isolation, structures and bioactivities of the polysaccharides from jujube fruit ( Ziziphus jujuba, mill.): A review. Food Chemistry, 227, 349–357. 10.1016/j.foodchem.2017.01.074 [DOI] [PubMed] [Google Scholar]
- Kim, J. E. , Kim, M. A. , Kim, J. S. , Park, D. C. , & Lee, S. P. (2013). Enhancing the organoleptic and functional properties of jujube by a quick aging process. Preventive Nutrition and Food Science, 18, 50–59. 10.3746/pnf.2013.18.1.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou, X. , Chen, Q. , Li, X. , Li, M. , Kan, C. , Chen, B. , … … Z. (2015). Quantitative assessment of bioactive compounds and the antioxidant activity of 15 jujube cultivars. Food Chemistry, 173, 1037–1044. 10.1016/j.foodchem.2014.10.110 [DOI] [PubMed] [Google Scholar]
- Li, J. W. , Fan, L. P. , Ding, S. D. , & Ding, X. L. (2007). Nutritional composition of five cultivars of Chinese jujube. Food Chemistry, 103, 454–460. 10.1016/j.foodchem.2006.08.016 [DOI] [Google Scholar]
- Nencini, C. , Menchiari, A. , Franchi, G. G. , & Micheli, L. (2011). In vitro antioxidant activity of aged extracts of some Italian Allium species. Plant Foods for Human Nutrition, 66, 11–16. 10.1007/s11130-010-0204-2 [DOI] [PubMed] [Google Scholar]
- Park, H.‐J. , Lee, S.‐H. , Kim, H.‐Y. , Jang, G.‐Y. , Hwang, I.‐G. , Woo, K.‐S. , … Jeong, H.‐S. (2012). Changes in chemical components and antioxidant activity of dried jujube with different aging temperatures and durations. Journal of the Korean Society of Food Science Nutrition, 41(5), 591–597. 10.3746/jkfn.2012.41.5.591 [DOI] [Google Scholar]
- Sang, E. B. , Cho, S. Y. , Yong, D. W. , Lee, S. H. , & Park, H. J. (2014). Changes in s ‐allyl cysteine contents and physicochemical properties of black garlic during heat treatment. LWT ‐ Food Science and Technology, 55(1), 397–402. 10.1016/j.lwt.2013.05.006 [DOI] [Google Scholar]
- Shin, J. H. , Choi, D. J. , Lee, S. J. , Cha, J. Y. , Kim, J. G. , & Sung, N. J. (2008). Changes of physicochemical components and antioxidant activity of garlic during its processing. Journal of Life Science, 18, 1123–1131. 10.5352/JLS.2008.18.8.1123 [DOI] [Google Scholar]
- Wang, C. , Cheng, D. , Cao, J. , & Jiang, W. (2013). Antioxidant capacity and chemical constituents of Chinese jujube (Ziziphus jujuba, mill.) at different ripening stages. Food Science and Biotechnology, 22(3), 639–644. 10.1007/s10068-013-0125-6 [DOI] [Google Scholar]
- Wojdyło, A. , Carbonell‐Barrachina, Á. A. , Legua, P. , & Hernández, F. (2016). Phenolic composition, ascorbic acid content, and antioxidant capacity of Spanish jujube (Ziziphus jujube mill.) fruits. Food Chemistry, 201, 307–314. 10.1016/j.foodchem.2016.01.090 [DOI] [PubMed] [Google Scholar]
- Wojdyło, A. , Figiel, A. , Legua, P. , Lech, K. , Carbonellbarrachina, Á. A. , & Hernández, F. (2016). Chemical composition, antioxidant capacity, and sensory quality of dried jujube fruits as affected by cultivar and drying method. Food Chemistry, 207, 170–179. 10.1016/j.foodchem.2016.03.099 [DOI] [PubMed] [Google Scholar]


