Abstract
Background
Glucocorticoids are widely used in the treatment of several pediatric diseases with undisputed disease-related benefits. Perinatal exposure to high levels of glucocorticoids can have long-term adverse cerebral effects. In adults, glucocorticoid treatment has been associated with smaller volumes of subcortical grey matter structures. Recently, we observed smaller total brain volumes in children and adolescents treated with glucocorticoid during childhood compared to healthy controls. The possible long-term effects of glucocorticoid treatment during childhood on subcortical brain volume and microstructure remain unknown.
Method
We examined 30 children and adolescents, who had previously been treated with glucocorticoids for nephrotic syndrome or rheumatic disease, and 30 healthy volunteers. Patients and healthy control groups were matched by age, gender, and level of parent education. Participants underwent 3 T magnetic resonance (MR) brain imaging. T1-weighted and diffusion-weighted images were acquired. Volume and mean diffusivity (MD) measures were extracted for hippocampus, amygdala, nucleus accumbens, caudate nucleus and putamen. Multiple linear regression analyses were used to assess differences between patients and controls, and to evaluate possible dose-response relationships. A priori, we expected patients to display lower hippocampal and amygdala volumes.
Results
While children previously treated with glucocorticoids displayed smaller right hippocampal volumes than controls, this difference did not survive correction for multiple comparisons. Furthermore, patients as compared to controls showed lower right hippocampal MD, which remained when correcting for global changes in MD. The longer the time between the glucocorticoid treatment termination and MR-scan, the more right hippocampal MD values resembled that of healthy controls. Patient and controls did not differ in amygdala volume or MD. Analyses of the nucleus accumbens, caudate nucleus and putamen only revealed smaller putamen volumes in patients compared to controls, which remained significant when controlling for total brain volume.
Conclusion
The results suggest that extra-cerebral diseases during childhood treated with glucocorticoids may be associated with reduced subcortical grey matter volumes and lower right hippocampal mean diffusivity later in life. Our findings warrant replication and elaboration in larger, preferably prospective and longitudinal studies. Such studies may also allow disentangling disease-specific effects from possible glucocorticoid treatment effects.
Keywords: Glucocorticoid treatment, Brain structure, Subcortical, MRI, Childhood, Adolescence
Abbreviations: AMD, adjusted mean difference; DW, diffusion weighted; DWI, diffusion weighted imaging; HPA, hypothalamus pituitary adrenal; MD, mean diffusivity; MRI, magnetic resonance imaging; ROI, region of interest; TBV, total brain volume; VCI, verbal comprehension index
Highlights
-
•
Previous glucocorticoid treatment linked to lower right hippocampal mean diffusivity
-
•
This effect was most prominent in children and adolescents with nephrotic syndrome
-
•
Right hippocampal mean diffusivity normalised with longer time since treatment
-
•
Smaller putamen volumes in patients were driven by children with rheumatic disease
1. Introduction
Glucocorticoids have profound anti-inflammatory and immunosuppressive effects and are successfully used in the treatment of a number of pediatric diseases (Coutinho and Chapman, 2011; Gravanis and Margioris, 2001). Along the targeted effects of glucocorticoid treatment, glucocorticoids can profoundly affect many parts of the body. Detrimental side-effects on the developing brain have been identified (Damsted et al., 2011). In the brain, the effects of glucocorticoids are mediated by the mineralocorticoid and glucocorticoid receptors by both genomic, epigenomic and rapidly induced non-genomic mechanisms (Gray et al., 2017; Reul and de Kloet, 1985). Mineralocorticoid receptors are especially abundant in hippocampus and amygdala, while glucocorticoid receptors are more widely distributed throughout the human brain (Cao-Lei et al., 2013; de Kloet, 2014; Klok et al., 2011; Reul and de Kloet, 1985). Furthermore, both hippocampus and amygdala play an important role in regulating endogenous cortisol levels (Dedovic et al., 2009), and are regarded as cerebral target structures for glucocorticoids (Joels et al., 2009).
The potentially harmful effects of exogenous glucocorticoids in the brain have so far primarily been studied in relation to exposure perinatally or in adulthood. Perinatal exposure to glucocorticoids has been linked to structural brain changes and neurological and behavioural problems in offspring later in life, including cortical thinning, cerebral palsy, abnormal neurological examination and affective problems (Davis et al., 2013; Doyle et al., 2014; Reynolds, 2013). Furthermore, a post-mortem study of preterm-born children found that children prenatally exposed to glucocorticoids had a lower density of hippocampal neurons than non-exposed children (Tijsseling et al., 2012), an observation that is consistent with findings in animal models (Noorlander et al., 2008; Uno et al., 1990). Glucocorticoid treatment for extra-cerebral diseases in adulthood has been related to smaller amygdala and hippocampal volumes measured during treatment (Brown et al., 2004; Brown et al., 2008). The observed smaller hippocampal volumes may, at least partially, be due to a lower density of healthy neurons as suggested by lower hippocampal N-acetyl aspartate ratios measured with magnetic resonance spectroscopy (Brown et al., 2004). Hippocampal and amygdala atrophy has also been found in adults and children with elevated levels of endogenous glucocorticoids due to Cushing's disease (Merke et al., 2005; Patil et al., 2007). Moreover, magnetic resonance spectroscopy studies in cured Cushing's disease patients revealed lower hippocampal N-acetyl aspartate and higher glutamate and glutamine levels suggestive of decreased neuronal density and increased glial proliferation (Resmini et al., 2013).
While most studies investigated the impact of glucocorticoids on limbic structures, glucocorticoid treatment may impact other subcortical brain structures as well (Cao-Lei et al., 2013). Notably, exposure to glucocorticoids in the neonatal period has recently been associated with smaller white matter, putamen, caudate nucleus, and globus pallidus volumes in adolescents born extremely premature (<28 weeks), while differences in hippocampus or amygdala volumes were not observed (Cheong et al., 2014).
In contrast to pre- and perinatal glucocorticoid exposure, studies investigating the long-term effects of exogenous glucocorticoid exposure later in childhood are sparse. This is despite the widespread use of glucocorticoids in the treatment of multiple pediatric diseases (Damsted et al., 2011), and the fact that the brain continues to mature throughout childhood and adolescence as evidenced by in vivo magnetic resonance imaging (MRI) studies (Brown et al., 2012; Jernigan et al., 2011; Lebel et al., 2017). Hippocampal and amygdala volumes increase during late childhood and level off during adolescence. Caudate nucleus, putamen and nucleus accumbens appear to decrease in volume during late childhood and adolescence into early adulthood (Ostby et al., 2009; Wierenga et al., 2014). Studies employing diffusion-weighted imaging (DWI) to assess brain tissue microstructure revealed that subcortical grey matter structures undergo developmental changes, as indexed by decreasing mean diffusivity (MD) (Lebel et al., 2008). MD reflects the magnitude of water diffusion and is negatively proportional to the amount of cell membranes and cellular structures (Beaulieu, 2002). Notably, caudate nucleus MD has been reported to decrease well into adulthood, while putamen and globus pallidus MD appear to level off to adult levels in late adolescence (Lebel et al., 2008). Thus, both volumetric and microstructural maturation occurs in subcortical regions during childhood and adolescence, which could potentially be affected by glucocorticoid exposure in this period of life.
The present study aimed to investigate the possible long-term effects of previous glucocorticoid treatment during childhood on the volume and microstructure of subcortical grey matter structures. To this end, we compared child and adolescent patients, previously treated with glucocorticoids for nephrotic syndrome or rheumatic disease, with healthy controls. We included these two different extra-cerebral pediatric diseases with the aim to control for potential disease-related effects. In previous work, we observed that these patients had lower verbal intellectual abilities (Holm et al., 2015) and smaller total brain volumes as compared to healthy controls (Holm et al., 2018). Furthermore, the children treated with glucocorticoid showed sex-dependent differences in endogenous cortisol profiles compared to healthy controls, with girls having higher cortisol awakening response (CAR) and boys having higher cortisol levels during the day (Vestergaard et al., 2017). In the present study, our primary hypothesis was that patients, as compared to healthy controls, would have smaller hippocampal and amygdala volumes independent of differences in total brain volume. Secondary, we expected that patients would differ from controls in hippocampal and amygdala MD. Furthermore, we examined if patients differed from controls in putamen, caudate nucleus, or nucleus accumbens volume or MD. Finally, within patients we examined for possible doses-response associations between glucocorticoid treatment variables and subcortical brain structure volume or MD.
2. Material and methods
The participants in the present study are the same as in our recent study investigating the effects on glucocorticoid treatment on global structural brain measures (Holm et al., 2018). Therefore, if identical, information on participants, clinical characteristics and background variables is presented between quotation marks followed by the reference to the previous article.
2.1. Participants
“A total of 30 children and adolescents previously treated with glucocorticoid because of either rheumatic disease or nephrotic syndrome, recruited from outpatient pediatric clinics, and 30 matched healthy controls, recruited from public schools, were included in the present study. Patient and control groups were matched on average age, gender, and parent education. The patients were between 7.0 and 16.1 years of age and consisted of 8 boys and 22 girls. The controls were between 7.0 and 15.6 years of age and consisted of 11 boys and 19 girls. The subjects were part of a larger cohort, which has been described in detail elsewhere (Holm et al., 2018). Of the initial thirty-eight included patients, seven did not complete MRI scanning and one was excluded because of poor image quality due to motion artefacts. Thus, 30 patients with good-quality T1-weighted scans were included in the current study. Of the initially forty-two included healthy controls, five participants did not complete scanning and one control was excluded because of motion artefacts. Thus, good-quality scans were available for 36 healthy controls. To ensure that group averages for included patients were matched to controls on age, gender, and parent education, we excluded five boys and one girl with a mean age of 11.8 years and mean parental education of 17.1 years. Exclusion was done blinded to the MRI data and before any processing of MRI images or statistical analyses. There were no significant differences in age, gender, or parental education between the initial included subjects and the subjects with good-quality MRI scan (all P values ≥0.30).
Only children and adolescents without neurological or psychiatric diseases or preterm birth were included. Controls were required to be healthy and without previous systemic glucocorticoid treatment. Only patients without current glucocorticoid treatment at the time of the study were included. The study was ethically approved by the Scientific Ethical Committee, Capital Region, Denmark (H-KF-01- 131/03 and addendum of June 2009). Written informed consent was obtained from parents of all participants” (Holm et al., 2018). The study was conducted in accordance with the Helsinki declaration.
2.2. Clinical characteristics
“Patients' medical charts were reviewed, and diagnosis and glucocorticoid treatment data were collected” (Holm et al., 2018). “The study included 11 patients with nephrotic syndrome and 19 with rheumatic disease. The group of children with nephrotic syndrome included idiopathic nephrotic syndrome (n = 5), nephrotic syndrome associated with Henoch-Schönlein purpura (n = 4), and nephrotic syndrome associated with glomerulonephritis (n = 2). The group of children with rheumatic disease included systemic juvenile idiopathic arthritis (n = 1), polyarticular juvenile idiopathic arthritis (n = 3), oligoarticular juvenile idiopathic arthritis (n = 8), enthesitis-related juvenile idiopathic arthritis (n = 2), juvenile dermatomyositis (n = 3), mixed connective tissue disease (n=1), and systemic lupus erythematosus (n = 1).” (Holm et al., 2018). Treatment variables are presented in Table 2. “The primary treatment variable was cumulative prednisolone equivalent glucocorticoid dose (mg/kg). In addition, median daily dose (mg/kg/day), median treatment age, and time since treatment (i.e., time elapsed from treatment termination to assessment) were registered. Glucocorticoids were administered orally. However, five patients (one with nephrotic syndrome and four with rheumatic disease) received intravenous glucocorticoid high-dose therapy (pulse therapy) in addition to oral glucocorticoid treatment, which constituted between 36 and 64% of their cumulative dose” (Holm et al., 2018). Twelve patients received non-glucocorticoid treatment at the time of assessment, which included TNF-α-inhibitors (n= 7), methotrexate (n= 3), anti-hypertensive drugs (n = 2), interleukin-1-inhibitor (n = 1), and non-steroid anti-inflammatory drugs (n = 1).
Table 2.
Glucocorticoid treatment variables.
| Treatment variables | All patients | Rheumatic disease | Nephrotic syndrome | p§ | Girls | Boys | p# |
|---|---|---|---|---|---|---|---|
| Median treatment age years | 7.0 (1.9–12.3) | 6.9 1.9–12.3) | 7.1 (4.5–10.5) | .40 | 7.1 (1.9–12.3) | 7.0 (5.1–9.8) | .99 |
| Cumulative dose mg/kg | 157.8 (20.8–580.5) | 102.8 (20.8–580.5) | 298.8 (118.6–514.2) | .03 | 146.6 (20.8–580.5) | 237.5 (73.5–514.2) | .50 |
| Median daily dose mg/kg/day | 0.2 (0.1–2.1) | 0.2 (0.1–1.1) | 0.6 (0.1–2.1) | .03 | 0.2 (0.1–1.1) | 0.6 (0.1–2.1) | .05 |
| Treatment duration years | 1.0 (0.1–5.7) | 1.1 (0.1–5.7) | 0.8 (0.3–3.9) | .50 | 0.8 (0.1–5.7) | 1.1 (0.5–1.8) | .95 |
| Time since treatment years | 4.0 (0.2–8.9) | 3.6 (0.2–6.8) | 4.6 (0.6–8.9) | .55 | 3.4 (0.2–6.8) | 5.0 (2.4–8.9) | .02 |
Values are presented as median (range). Glucocorticoid treatment variables are expressed as prednisolone equivalents. Mann Whitney U tests were used to compare § Rheumatic group versus Nephrotic group and # Girls versus Boys. P values <.05 are displayed in italic bold.
2.3. Background variables
“The assessment of background and cognitive variables are described in detail elsewhere (Holm et al., 2015). Height, weight, head circumference, and pubertal development (Tanner staging) were measured. Current and previous physical activity, stressful life events, and behavioral problems were assessed by questionnaires” (Holm et al., 2018). Verbal intellectual ability was estimated using the verbal comprehension index (VCI) based on the information and vocabulary subtests from the Wechsler Intelligence Scale for Children III (Prifitera and Saklofoske, 1998). Endogenous cortisol levels were measured by collecting saliva samples using oral swabs (Salimetrics Oral Swab, Salimetrics, Pennsylvania, USA) on two consecutive regular schooldays. Measures of interest were CAR and daily cortisol levels assessed by calculating area under the curve, both expressed as averages of the two sample days. For a detailed description of how measures were calculated see (Vestergaard et al., 2017).
2.4. Image acquisition
Structural MRI of participants was obtained on a 3 T Siemens Magnetom Trio magnetic resonance scanner (Siemens, Erlangen, Germany) using an eight-channel head coil (Invivo, Florida, USA). High-resolution whole brain 3D T1-weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE) images were acquired (TE = 3.04 ms, TR = 1550 ms, matrix = 256 × 256 mm, 192 sagittal slices, no gap, 1 mm3 voxels). Whole brain DWI was acquired using a twice-refocused balanced spin-echo sequence to minimize distortions by eddy currents (Reese et al., 2003). We acquired 10 non-DW images (b = 0) and 61 DW images, which were encoded along independent collinear diffusion gradient orientations (TR = 8200 ms, TE = 100 ms, FOV = 220 × 220 mm2, matrix = 96 × 96, GRAPPA (GeneRalized Autocalibrating Partial Parallel Acquisition): factor 2, 48 lines, 61 perpendicular slices, no gap, 2.3 mm3 voxels). A gradient echo field map was obtained to correct for B0 field distortions (Andersson et al., 2001) (TR = 530 ms, TE[1] = 5.19 ms and TE[2] = 7.65 ms, FOV = 256 × 256 mm2, matrix = 128 × 128, 47 perpendicular slices with no gap, 2 × 2 × 3 mm3 voxels). Both the DWI and the gradient field map scans were oriented parallel to the anterior-posterior commissure line. All images were inspected for quality. All T1-weighted images were evaluated by a neuroradiologist and deemed without clinical pathology.
2.5. Image preprocessing
2.5.1. Preprocessing of T1-weighted images
T1-weighted images were processed using the VBM5.1 toolbox implemented in SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8) running in Matlab R2013b. Initially, images were gradient unwarped to correct for non-linear spatial distortions in the scanner gradients (Jovicich et al., 2006). After a 6 parameter rigid transformation to MNI-space, grey and white matter and CSF were segmented with VBM5.1 using default settings and the Hidden Markov Random Field method (Cuadra et al., 2005). A brain mask generated using the graph-cut mask function was applied to the segmented tissue images to remove extra-cerebral tissue voxels. Tissue segmentations were visually inspected to ensure quality before warping the grey and white matter images with DARTEL (Diffeomorphic Anatomic Registration Through Exponentiated Lie algebra) to achieve high-dimensional inter-individual alignment (Ashburner, 2007). Subsequently, the individual DARTEL deformation flow fields were applied to individual masked brain tissue images in order to bring them into a common “DARTEL” space.
2.5.2. Pre-processing of diffusion weighted images
DW images were pre-processed using pipelines implemented in Matlab, using SPM8 routines as well as FSL 5.0.1 (http://www.fmrib.ox.ac.uk/). For each individual, DW images were co-registered to the average of all b0 images (no reslicing). Subsequently, DWI images were corrected for geometric distortions due to B0 field inhomogeneity and gradient non-linearity (Andersson et al., 2001; Jovicich et al., 2006) and transformed into MNI orientation (no scaling) using a single tri-linear interpolation. The diffusion orientations were adjusted to account for any rotation applied during registration (Leemans and Jones, 2009). To exclude non-brain voxels DW images were masked with a brain mask that was created by applying the Brain Extraction Tool (BET) implemented in FSL (Smith, 2002) on each non-DW image and taking the median of the resulting masks. The diffusion tensor was fitted using a least-squares-fit by non-linear optimization employing a Levenburg-Marquardt algorithm (Jones and Basser, 2004) and constrained to be positive definite by fitting its Cholesky decomposition implemented in Camino (Cook et al., 2006). Fractional anisotropy (FA) and mean diffusivity (MD) images were calculated. MD was calculated as the mean of diffusivities along three perpendicular axes (λ1, λ2, λ3) (Basser and Jones, 2002). FA images were used to calculate the best possible (non-linear) registration between MD images and individual T1-weighted anatomical images as part of the procedure to extract individual ROI MD (see below).
2.5.3. Grey matter regions-of-interest
Participants' T1-weighted images were warped into “DARTEL” space using individual DARTEL flow fields. Next, resulting images were averaged to create a T1-weighted study template, on which grey matter regions-of-interest (ROIs) were delineated using FSLView (www.fsl.fmrib.ox.ac.uk/fsl/fslview). Using the inverse DARTEL flow fields ROIs were transformed into each individual's T1-weighted image native space and edited to ensure their fit while blinded to subject identity.
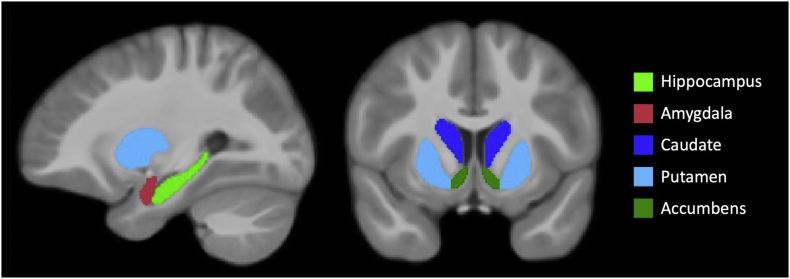
The left and right hippocampi and amygdalae were drawn using previously described guidelines (Entis et al., 2012; Maller et al., 2006). The hippocampal ROI included the cornu ammonis, dentate gyrus, alveus, and part of subiculum, while the fimbria and parahippocampal gyrus were excluded. For the striatal ROIs, striatal cell bridges were not included. The accumbens was delineated on the first axial slice where the caudate nucleus and putamen joined inferiorly and was not allowed to extend posteriorly beyond the anterior commissure. In coronal view, the accumbens was separated from the caudate nucleus by a straight line connecting the most inferior part of the frontal horn of the lateral ventricle to the most inferior part of the internal capsule. The border between accumbens and putamen was drawn as a straight vertical line from the most inferior part of the internal capsule. The caudate nucleus was delineated posteriorly until the posterior commissure. The ROIs are shown in Fig. 1.
Fig. 1.
Regions-of-interest.
Regions-of-interest overlaid on the average of warped T1-weighted images.
To extract individual ROI MD, we used the following procedure to minimize white matter and CSF partial volume effects and maximizing sampling within grey matter tissue. Firstly, ROIs were eroded using a 1 mm Gaussian kernel, thereby excluding an approximately one-voxel thick layer from the outer border of the ROI. Secondly, for each individual, the FA image was warped to the T1-weighted image in native space using the linear FLIRT and non-linear FNIRT registration algorithms in FSL. The resulting deformation fields were then applied to the MD images and the anatomical fit was visually inspected. Thirdly, using the inverse deformation the eroded ROIs residing in T1-weighted native space were transformed to diffusion-weighted native image space using tri-linear interpolation and binarized using a threshold of 0.5. The anatomical match was visually inspected. Fourthly, the binary ROIs were multiplied by the participant's perpendicular diffusivity image, and then thresholded at a perpendicular diffusivity of <0.0011, in order to exclude any remaining CSF partial volume voxels. Finally, mean MD values were extracted from the final ROIs for each subject. To estimate individual global MD within grey and white matter, mean MD was calculated within a whole brain mask created by thresholding individual perpendicular diffusivity images <0.0009 to exclude CSF and CSF partial volume voxels.
To obtain estimates of total brain volume (TBV) for use in the statistical analyses, we used the automated calculated TBV within FreeSurfer, a freely available software package for structural and functional image analyses (https://surfer.nmr.mgh.harvard.edu). Processing of individual T1-weighted images with FreeSurfer followed standard procedures and is described in detail elsewhere (Holm et al., 2018). The pial and grey-white matter surfaces generated by FreeSurfer were visually inspected and corrected following standard guidelines where necessary. The TBV included supra- and infratentorial volumes of grey and white matter, while ventricles, brain-stem and choroid plexus were excluded (www.freesurfer.net/fswiki/MorphometryStats).
2.6. Statistics
The statistical analyses for demographic and background variables are largely the same as in our previous work (Holm et al., 2018). Therefore, wherever information is the same it is presented between quotation marks, followed by the reference to the article in which the information can be found. SPSS 20 (SPSS, IBM Corp., USA) was used for statistical analyses.
2.6.1. Demographic and background variables
“A χ2-test tested for possible gender differences. The Shapiro–Wilk test was used to test whether continuous variable distributions deviated from a normal distribution. Independent samples t-tests tested for group differences in normally distributed variables, including age and parent education. Group differences in the background, behavioral, and cognitive variables, height, weight, head circumference, verbal comprehension index (VCI), perceptual organization index, Tanner stage, physical activity, stressful life events, behavioral problems, and pattern recognition memory were evaluated using multiple linear regression with age and gender as control variables. The lifetime stressful life event (SLE) questionnaire includes two statements regarding somatic disease (i.e., “my child had a medical diagnosis” and “my child was severely ill or injured”), which patients and controls are expected to differ on. Therefore, we report stressful life events with and without these disease-related questions. Within patients, Mann–Whitney U tests tested for potential gender- and disease-group differences in treatment variables” (Holm et al., 2018). CAR and the daily cortisol levels were evaluated using multiple linear regression with age and gender as control variables.
2.6.2. Subcortical brain measures
Potential differences between patients and controls in ROI volume or ROI MD were evaluated using multiple linear regression analyses. All assumptions for linear regression were fulfilled. Adjusted mean differences (AMD) are reported i.e. difference between groups in ROI volumes and ROI MD after controlling for all other predictors in the regression model.
Our primary hypothesis was that patients would have smaller left and right hippocampal and amygdala volumes compared to controls and that this difference would be independent of previously observed group differences in whole brain volume. Secondary, we expected that patients as compared to controls would differ in hippocampus and amygdala MD. ROI volume or ROI MD were used as dependent variables, and group (controls versus patients), age and gender were used as independent prediction variables. The anatomic specificity of observed associations was assessed by adding TBV in models predicting ROI volume, and global MD in models predicting ROI MD. Hippocampus and amygdala volume and MD were analyzed unilaterally since study findings suggest lateralized relations between glucocorticoids and limbic structures (Cerqueira et al., 2008; Madsen et al., 2012). Bilateral measures were used for testing group differences in putamen, caudate nucleus or nucleus accumbens volume or MD. Finally, we explored group by gender interactions to evaluate potential gender specific differences between patients and controls controlling for age and global measures. Pertinent to a significant interaction, we tested group differences for each gender separately.
2.6.3. Associations with glucocorticoid treatment variables
Contingent on finding significant group differences in ROI volume or ROI MD, adjusted for global measures, we investigated for associations between ROI volume or ROI MD and treatment variables in patients, controlling for age, sex and global measure. First, we tested if ROI volume or ROI MD was associated with cumulative glucocorticoid dose. Second, we tested for associations with median daily dose, treatment duration, median treatment age, or time since treatment. Third, we tested for a cumulative dose by median treatment age interaction, controlling for age, sex and global measure, cumulative dose, median treatment age, and time since treatment. The cumulative dose by median treatment age interaction tested whether the effect of glucocorticoid treatment differed depending on the age at which children received treatment.
2.6.4. Planned follow-up analyses
To evaluate if observed group differences were present in each of the disease groups, we contrasted rheumatic disease patients and nephrotic syndrome patients separately with healthy controls, controlling for age, gender, and TBV or global MD.
Since patients had significant lower VCI scores than controls (see under results), we investigated the possible relationship between VCI and those volume and MD measures for which controls and patients significantly differed from each other. Firstly, VCI was predicted with brain measure controlling for group, age, gender and global measure. Secondly, we tested for possible group by VCI interactions on ROI volume and ROI MD, controlling group, age, gender, VCI and global measure.
For the primary hypotheses concerning left and right hippocampal and amygdala volume, we used a Bonferroni corrected p-value of 0.013 (α = 0.05/4). Since this, to our knowledge, is the first study to investigate the possible long-term impact of glucocorticoid treatment during childhood on subcortical grey matter structures, we were interested to identify potential group differences and associations that can be tested in future studies. Therefore, in all subsequent analyses we considered effects with p-values lower than 0.05 of interest.
3. Results
Results on demographic data and background variables (except CAR and daily cortisol levels) are the same as presented in our previous article on global brain measures (Holm et al., 2018). These results will be presented between quotation marks followed by the reference to the previous article and the page at which the information can be found.
3.1. Missing data
There was missing data for measures of CAR and daily cortisol level (one female patient with rheumatic disease), current physical activity (two female patients with rheumatic disease and one male control), previous physical activity (two female patients with rheumatic disease) and DWI scans (two controls: one boy, one girl, and three female patients with rheumatic disease).
3.2. Demographic data and background variables
Demographic variables are presented in Table 1. Age, gender, and parental education were not significantly different between controls and all patients or between controls and the separate disease groups. Furthermore, comparisons between the two patient groups revealed no significant differences in age (t = 0.12, p = .91), gender (p = .08), or parent education (t = 0.54, p = .59).
Table 1.
Demographic variables.
| Controls |
All patients |
Nephrotic syndrome |
Rheumatic disease |
|
|---|---|---|---|---|
| (n = 30) | (n = 30) | (n = 11) | (n = 19) | |
| Gender (boys/girls) | 11/19 | 8/22 | 5/6 | 3/16 |
| p-Value | NA | p = .405 | p = .609 | p = .115 |
| Age (years) | ||||
| Mean (SD) | 11.9 (2.4) | 12.5 (2.2) | 12.6 (2.2) | 12.4 (2.3) |
| Range | 7.0–15.6 | 7.0–16.1 | 8.2–16.1 | 7.0–15.6 |
| p-Value | NA | t = −0.99, p = .327 | t = −0.820, p = .417 | t = −0.809, p = .424 |
| Parental education (years) | ||||
| Mean (SD) | 14.5 (1.6) | 14.0 (2.0) | 14.25 (2.7) | 13.8 (1.5) |
| Range | 11.0–16.5 | 9.0–17.0 | 9.0–17.0 | 11.5–17.0 |
| p-Value | NA | t = 1.00, p = .320 | t = 0.282, p = .780 | t = 1.356, p = .182 |
SD = standard deviation. All p-values are derived from comparisons against controls. Gender differences were tested with Chi Square tests. Group differences in age and parent education were tested with independent samples t-tests. Patients (n = 27) and controls (n = 28) for whom diffusion weighted images were obtained did not differ in age (t = −1.3, p = .19), gender (p = .63), or parental education (t = 1.1, p = .29).
“Patients had significantly lower weight and height in comparison with control. Head circumference, although lower, was not significantly different between groups. In agreement with our findings in the larger cohort, patients had significantly lower VCI compared with that in the controls, whereas there was no difference regarding the perceptual organization index, memory performance, or behavioural problems (Holm et al., 2015). Patients and controls did not significantly differ in pubertal development, physical activity, or stressful life events in the preceding year. In accordance with our previous findings in a larger cohort (Holm et al., 2015), the number of stressful life events during the child's entire lifetime was higher in patients compared with that in controls; however, after exclusion of disease-related questions, this difference was no longer present” (Holm et al., 2018, Table 2, page 807). Patients and controls did not significantly differ in CAR (Patients: Mean (standard deviation (SD)) = 0.33 (0.03); Controls: Mean (SD) = 0.30 (0.02); β = 0.13, p = .36) or daily cortisol (in nmol/l/min; Patients: Mean (SD) = 4.01 (0.38); Controls: mean (SD) = 3.87 (0.24); β = 0.02, p = .91).
3.3. Treatment variables
Treatment variables and the results of statistical analyses comparing children with nephrotic syndrome with children with rheumatic disease and comparing boys and girls are presented in Table 2. Children with nephrotic syndrome received significant higher cumulative dose and median daily dose of glucocorticoids than children with rheumatic disease. Disease groups did not differ on any of the other treatment variables. Additionally, patient boys had significantly longer time since treatment and received higher median daily doses than patient girls. Boys and girls did not differ in the remaining treatment variables. Disease group by gender interaction effects were absent (p-values >.11).
3.4. Subcortical brain measures
Measures of ROI volume and ROI MD for controls and patients and patient groups separately are presented in Table 3. Results of multiple regression analyses of volume and MD are presented in Table 4 and Table 5, respectively.
Table 3.
Volume and MD for ROIs in controls, patients and the separate disease groups.
| Measure | Hemisphere | Controls | All patients | Rheumatic disease | Nephrotic syndrome |
|---|---|---|---|---|---|
| Hippocampal volume | Right | 2352 ± 276 | 2186 ± 196 | 2188 ± 212 | 2182 ± 176 |
| Left | 2265 ± 300 | 2181 ± 217 | 2178 ± 204 | 2214 ± 245 | |
| Hippocampal MD | Right | 903 ± 16 | 892 ± 18 | 893 ± 13 | 890 ± 24 |
| Left | 877 ± 17 | 872 ± 22 | 873 ± 22 | 870 ± 22 | |
| Amygdala volume | Right | 1234 ± 113 | 1193 ± 128 | 1190 ± 127 | 1198 ± 135 |
| Left | 1197 ± 110 | 1162 ± 109 | 1154 ± 115 | 1177 ± 101 | |
| Amygdala MD | Right | 839 ± 21 | 838 ± 16 | 837 ± 15 | 840 ± 17 |
| Left | 827 ± 23 | 821 ± 20 | 819 ± 21 | 823 ± 19 | |
| Putamen volume | Bilateral | 8721 ± 808 | 8156 ± 695 | 8053 ± 607 | 8305 ± 814 |
| Putamen MD | 797 ± 13 | 793 ± 16 | 778 ± 16 | 801 ± 14 | |
| Caudate nucleus volume | Bilateral | 7637 ± 920 | 7404 ± 844 | 7282 ± 684 | 7582 ± 1045 |
| Caudate nucleus MD | 798 ± 22 | 793 ± 19 | 786 ± 16 | 802 ± 20 | |
| Accumbens volume | Bilateral | 505 ± 61 | 493 ± 58 | 490 ± 47 | 498 ± 74 |
| Accumbens MD | 835 ± 20 | 831 ± 30 | 810 ± 32 | 837 ± 27 | |
| Total brain volume (cm3) | NA | 1264 ± 108 | 1201 ± 104 | 1185 ± 112 | 1230 ± 88 |
| Whole brain MD | NA | 800 ± 16 | 796 ± 14 | 792 ± 11 | 801 ± 15 |
MD: mean diffusivity. Values are mean ± standard deviation. Volumes are given as mm3 if not stated otherwise and MD is given as 10−6 m2/s. Volume measures included 30 controls, 19 patients with rheumatic disease, and 11 with nephrotic syndrome. MD measures included 28 controls, 16 patients with rheumatic disease, and 11 with nephrotic syndrome.
Table 4.
Results for multiple regression analyses of ROI volume.
| ROI | Model |
Group |
Age |
Gender |
TBV |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2adj | F | p | β | p | β | p | β | p | β | p | ||
| Right hippocampus | 1 | 0.09 | 3.0 | .04 | −0.31 | .018a | −0.05 | .69 | −0.17 | .19 | ||
| 2 | 0.2 | 4.7 | .003 | −0.21 | .10 | −0.08 | .50 | −0.08 | .54 | 0.37 | .005 | |
| Left hippocampus | 1 | −0.02 | 0.62 | .60 | −0.15 | .28 | 0.09 | .51 | −0.06 | .67 | ||
| 2 | 0.15 | 3.61 | .01 | −0.02 | .87 | 0.5 | .68 | 0.06 | .65 | 0.46 | .001 | |
| Right amygdala | 1 | 0.05 | 2.11 | .11 | −0.15 | .24 | 0.08 | .56 | −0.25 | .06 | ||
| 2 | 0.45 | 12.93 | 2*10−7 | 0.03 | .76 | 0.02 | .84 | −0.08 | .44 | 0.68 | 4*10−8 | |
| Left amygdala | 1 | 0.06 | 2.12 | .10 | −0.16 | .22 | 0.16 | .20 | −0.21 | .12 | ||
| 2 | 0.43 | 11.91 | 5*10−7 | 0.02 | .84 | 0.11 | .27 | −0.04 | .69 | 0.65 | 1*10−7 | |
| Putamen | 1 | 0.17 | 5.15 | .003 | −0.39 | .002b | −0.04 | .74 | −0.21 | .09 | ||
| 2 | 0.58 | 21.37 | 5*10−10 | −0.20 | .026c | −0.10 | .27 | −0.04 | .70 | 0.68 | 8*10−10 | |
| Caudate nucleus | 1 | 0.00 | 1.00 | .40 | −0.20 | .128 | −0.06 | .64 | 0.05 | .68 | ||
| 2 | 0.58 | 21.53 | 10*10−11 | 0.02 | .84 | −0.13 | .14 | 0.26 | .005 | 0.81 | 3*10−12 | |
| Nucleus accumbens | 1 | 0.03 | 1.59 | .20 | −0.13 | .31 | −0.05 | .70 | −0.23 | .08 | ||
| 2 | 0.29 | 7.06 | .0001 | 0.02 | .87 | −0.10 | .40 | −0.09 | .44 | 0.56 | 2*10−5 | |
In models 1 (Degrees of freedom: = 3, 56), group, age and gender were used as independent variables. In models 2 (Degrees of freedom = 4, 55), group, age, gender and total brain volume (TBV) were used as independent variables. Negative β- values reflect lower volume in patients compared to controls or lower volume in girls compared to boys. Analyses included 30 patients and 30 controls. ROI = Region-of-interest. R2adj = adjusted R-squared. P values <.05 are displayed in italic bold. Effect sizes are expressed as squared semi-partial correlations (Sr2), which represent the proportion of the outcome variance that is associated uniquely with the predictor.
Sr2(model 1, right hippocampus, group) = 0.09.
Sr2(model 1, putamen, group) = 0.15.
Sr2(model 2, putamen, group) = 0.04.
Table 5.
Results for multiple regression analyses of ROI mean diffusivity (MD).
| ROI | Model |
Group |
Age |
Gender |
TBV |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2adj | F | p | β | p | β | p | β | p | β | p | ||
| Right hippocampus | 1 | 0.16 | 4.49 | .007 | −0.26 | .044a | −0.32 | .015 | −0.10 | .42 | ||
| 2 | 0.24 | 5.35 | .001 | −0.28 | .027b | −0.02 | .90 | 0.08 | .52 | 0.42 | .014 | |
| Left hippocampus | 1 | 0.07 | 2.45 | .07 | −0.8 | .57 | −0.33 | .016 | −0.01 | .96 | ||
| 2 | 0.10 | 2.51 | .05 | −0.08 | .53 | −0.13 | .48 | 0.01 | .95 | 0.29 | .12 | |
| Right amygdala | 1 | 0.32 | 9.54 | 4*10−5 | 0.11 | .36 | −0.61 | 3*10−6 | −0.18 | .12 | ||
| 2 | 0.33 | 7.60 | 7*10−5 | 0.10 | .39 | −0.47 | .005 | −0.17 | .14 | 0.19 | .23 | |
| Left amygdala | 1 | 0.20 | 4.27 | .009 | −0.08 | .54 | −0.34 | .011 | −0.30 | .021 | ||
| 2 | 0.15 | 3.31 | .018 | −0.08 | .53 | −0.25 | .17 | −0.29 | .026 | 0.13 | .47 | |
| Putamen | 1 | 0.03 | 1.56 | .21 | −0.12 | .38 | −0.22 | .12 | 0.10 | .47 | ||
| 2 | 0.45 | 11.96 | 7*10−7 | 0.15 | .16 | 0.42 | .006 | 0.15 | .15 | 0.89 | 1*10−7 | |
| Caudate nucleus | 1 | 0.21 | 5.80 | .002 | −0.05 | .67 | −0.47 | .0004 | 0.12 | .35 | ||
| 2 | 0.44 | 11.68 | 9*10−7 | −0.07 | .50 | 0.01 | .95 | 0.15 | .14 | 0.67 | 2*10−5 | |
| Nucleus accumbens | 1 | −0.03 | 0.54 | .66 | −0.06 | .68 | −0.12 | .40 | 0.09 | .52 | ||
| 2 | 0.12 | 2.92 | .030 | −0.07 | .58 | 0.27 | .14 | 0.12 | .35 | 0.56 | .003 | |
In models 1(Degrees of freedom: = 3, 56), group, age and gender were used as independent variables. In models 2 (Degrees of freedom: = 3, 56), group, age, gender and global MD were used as independent variables. Negative β-values reflect lower MD in patients compared to controls or lower MD in girls compared to boys. Analyses included 28 controls and 27 patients. ROI = Region-of-interest, M = Model, R = Right, L = Left. P values <.05 are displayed in italic bold. Effect sizes are expressed as squared semi-partial correlations (Sr2), which represent the proportion of the outcome variance that is associated uniquely with the predictor.
Sr2(model 1, right hippocampus, group) = 0.07.
Sr2(model 2, right hippocampus, group) = 0.07.
3.4.1. Primary analyses
3.4.1.1. Hippocampal and amygdala volume
Patients had smaller right hippocampal volumes compared to controls (AMD = −153.3 mm3; 95% CI = −280 – -27). This difference was not statistically significant when correcting for multiple comparisons. (α = 0.05/4 = 0.013). Nevertheless, we corrected for TBV to evaluate if smaller hippocampal volumes would show some anatomical specificity i.e. if the effect remained or became more outspoken. However, neither was the case (AMD = −102.8 mm3; 95% CI = −226–21). Groups did not differ in left hippocampal volume. We did not find any significant group by gender interaction effects (right: β = 0.06, p = .63 and left: β = 0.10, p = .44).
Patients and controls did not significantly differ in left or right amygdala volume without or with correction for TBV. There was some indication for a group by gender interaction for both left (β = 0.22, p = .035) and right amygdala (β = 0.19, p = .066) volumes. These interactions were due to patient boys having smaller volumes than healthy control boys, while patient and control girls did not differ from each other.
3.4.2. Secondary analyses
3.4.2.1. Hippocampal and amygdala MD
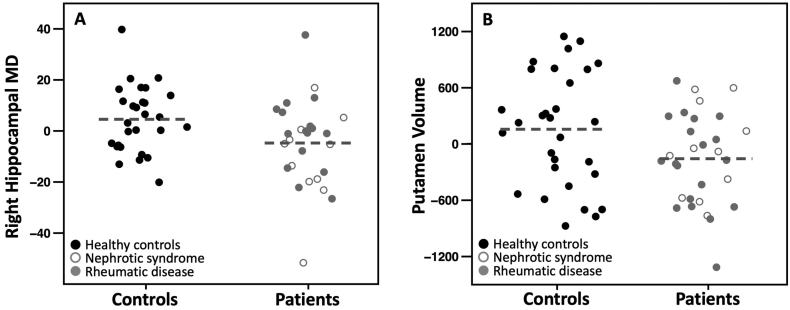
Patients had lower right hippocampal MD as compared to controls (AMD = −9.3 × 10−6 m2/s; 95% CI = −18 × 10−6 to −0.3 × 10−6). This group difference remained when controlling for global MD (AMD = −9.7 × 10−6 m2/s; 95% CI = −18 × 10−6 to −1 × 10−6; Fig. 2A). Patients and controls did not statistically differ in left hippocampal MD. Group by gender interactions were absent (right hippocampus: β = 0.23, p = .68, left hippocampus: β = −0.06, p = .67).
Fig. 2.
Group differences in ROI MD and volume.
Partial regression plots for differences between controls and patients in (A) right hippocampal mean diffusivity adjusted for age, gender and global MD, and (B) bilateral putamen volume adjusted for age, gender and total brain volume. The horizontal dashed lines represent the group mean. MD: mean diffusivity. Note that the partial regression plots display the model residuals and thus render values arbitrary.
Patients and controls did not significantly differ in left or right amygdala MD without or with correction for global MD. Group by gender interactions were absent (left amygdal: β = 0.12, p = .39 and right amygdala: β = 0.15, p = .22).
3.5. Explorative analyses
3.5.1. Putamen, caudate nucleus and nucleus accumbens volume
Patients had significantly smaller putamen volume as compared to controls (AMD = −675 mm3; 95% CI = −1091 to −259) and this group difference remained after controlling for TBV (AMD = −353 mm3; 95% CI = −662 – -44; Fig. 2B). We did not find a significant group by gender interaction (β = 0.04, p = .69).
Patients and controls did not significantly differ in caudate nucleus or nucleus accumbens volumes with or without correction for TBV. Likewise, no significant group by gender interactions were observed (caudate nucleus: β = −0.07, p = .46; nucleus accumbens: β = 0.02, p = .90).
3.5.2. Putamen, caudate nucleus and nucleus accumbens MD
Patients and controls did not significantly differ in putamen, caudate nucleus or nucleus accumbens MD. We did not find any significant group by gender interactions (putamen: β = 0.004, p = .97, caudate nucleus: β = 0.05, p = .63; nucleus accumbens: β = 0.12, p = .38).
3.6. Planned follow-up analyses
3.6.1. Right hippocampal MD
3.6.1.1. Associations with glucocorticoid treatment variables within patients
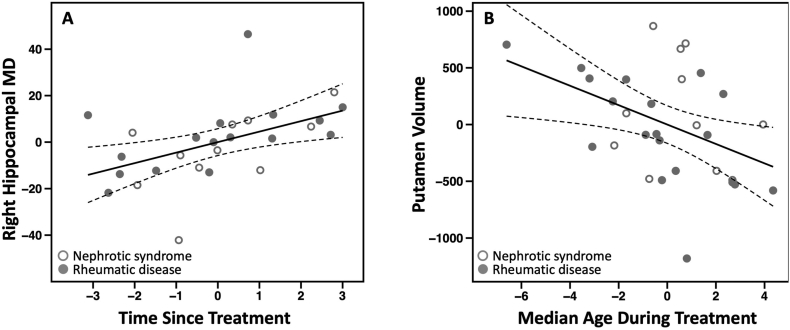
Within patients, right hippocampal MD was not significantly associated with cumulative dose (β = −0.27, p = .17). However, we found a positive association between right hippocampal MD and the time elapsed since termination of treatment (β = 0.57, p = .016, Fig. 3A), suggesting that the longer time since patients received glucocorticoids the more they resembled the control group. Median daily dose, treatment duration, median treatment age, or the cumulative dose by median treatment age interaction were not significantly associated with right hippocampal MD (p's > 0.1). Excluding the five individuals who received pulse therapy did not affect the above findings.
Fig. 3.
Associations between treatment variables and ROI MD and volume.
Partial regression plots with solid lines representing the linear model slope and dashed lines representing the 95% confidence intervals. A) Association between right hippocampal MD and the time elapsed since termination of glucocorticoid treatment, adjusted for age, gender, and global MD. B) Association between putamen volume and the median treatment age adjusted for age, gender, and total brain volume. Note that the partial regression plots render values to be arbitrary.
3.6.1.2. Comparing disease groups separately to healthy controls
Follow-up analyses investigating for group differences in right hippocampal MD for each disease group separately, controlling for age, gender and global MD, revealed that children with nephrotic syndrome (β = −0.39; p = .005; AMD = −16.4 × 10−6; 95% CI = −27 × 10−6 to −5 × 10−6) but not children with rheumatic disease (β = −0.17; p = .23; AMD = −2.8 × 10−6; 95% CI = −7 × 10−6–2 × 10−6) showed lower right hippocampal MD as compared to controls. Nevertheless, the observed positive association between time since treatment and right hippocampal MD was present in both patient groups (nephrotic syndrome: β = 0.48, p = .052 and rheumatic disease: β = 0.58, p = .059).
3.6.1.3. Associations with verbal comprehension index
VCI was still significantly lower in patients than controls after controlling for right hippocampal MD, and there was no apparent association between right hippocampal MD and VCI (Group: β = −0.46, p = .001; Right hippocampal MD: β = 0.13, p = .38; Age: β = 0.17, p = .33; Gender: β = 0.05, p = .67; Global MD: β = −0.02, p = .91). Moreover, we found no significant group by VCI interaction effect on right hippocampal MD (β = 0.41, p = .36).
3.6.2. Putamen volume
3.6.2.1. Associations with glucocorticoid treatment variables within patients
Putamen volume was not associated with cumulative dose (β = −0.19, p = .14), median daily dose, treatment duration, nor time since treatment (p's > 0.26). However, putamen volume was negatively associated with median treatment age (β = −0.31, p = .03; Fig. 3B). Putamen volume was not associated with cumulative dose by median treatment age (p > .7). Excluding individuals who received pulse therapy did not affect the above findings.
3.6.2.2. Comparing disease groups separately to healthy controls
Analyses revealed that the observed group difference in putamen volume was present in children with rheumatic disease (β = −0.25; p = .02; AMD = −446 mm3; 95% CI = −818 – -74), but not in children with nephrotic syndrome (β = −0.13; p = .26; AMD = −247 mm3; 95% CI = −686–193). Furthermore, the observed negative association between median treatment age and putamen volume was present in the children with rheumatic disease (β = −0.44, p = .015), while absent in children with nephrotic disease (β = 0.08, p = .84).
3.6.2.3. Associations with verbal comprehension index
VCI was still significantly lower in patients than controls after controlling for putamen volume, and there was no apparent relationship between putamen volume and VCI (Group: β = −0.48, p = .0004; Putamen volume: β = 0.07, p = .70; Age: β = 0.22, p = .07; Gender: β = 0.06, p = .63; TBV; β = 0.18, p = .30). There was no group by VCI interaction (β = −0.01, p = .99).
4. Discussion
To the best of our knowledge the present study is the first to describe a possible relationship between extra-cerebral diseases treated with glucocorticoids during childhood and adolescence, and differences in limbic and striatal grey matter structure volume and microstructure in children and adolescents. Contrary to our expectations, patients did not differ from controls in hippocampal or amygdala volumes, as the initially observed smaller right hippocampal volumes in patients as compared to healthy did not survive correction for multiple comparisons. Furthermore, exploring if the uncorrected group difference showed anatomical specificity, by correcting for TBV, revealed this likely not to be the case. Nevertheless, patients displayed lower right hippocampal MD than healthy controls, a difference that remained when accounting for whole brain MD. Although lower right hippocampal MD was most prominent in children with nephrotic syndrome, who had received the highest cumulative and median daily glucocorticoid dose, possible disease-specific effects cannot be excluded. Interestingly, we found that the right hippocampal MD in patients reached values comparable to those of healthy controls the more time had elapsed since glucocorticoid treatment. This relationship was observed in both disease groups separately. Patients did not differ from controls in amygdalae MD. Finally, putamen volume was smaller in patients as compared to controls, a difference that was mainly driven by the children with rheumatic disease. Notably, putamen volume was smaller the older the children were when receiving glucocorticoid treatment.
The general lack of studies investigating the effect of glucocorticoid treatment in childhood and adolescence for extra-cerebral diseases on limbic and striatal grey matter structures makes it difficult to assess the validity and significance of our findings. Our observation of smaller right hippocampal volumes in patients as compared to healthy controls did not survive correction for multiple comparisons. Exploring if this difference might show anatomical specificity, by correcting for TBV, revealed that this likely was not the case. The latter would align with our previous finding in the same cohort with patients displaying significant smaller total brain volumes as compared to healthy controls (Holm et al., 2018). Overall, our failure to observe hippocampal volume differences may be rooted in e.g. insufficient statistical power, lack of anatomical specificity, and/or a lack of signal in hippocampal volume due to relatively low median daily glucocorticoid doses in combination with relative lengthy time since treatment. Nevertheless, our observation warrants further investigation given the available evidence suggesting that postnatal treatment with glucocorticoids affects hippocampal volume. In a study of preterm born children (gestational age at birth <30 weeks), scanned at term-equivalent age (38–42 weeks' postmenstrual age), postnatal exposure to glucocorticoids was found to predict smaller right and left hippocampal volumes corrected for intracranial volume (Thompson et al., 2008). Moreover, adult patients with asthma or rheumatic diseases receiving long-term glucocorticoid treatment had smaller hippocampal volumes bilaterally as compared to patients with asthma or rheumatic diseases with minimal lifetime corticosteroid exposure (Brown et al., 2004). However, it is not clear whether this difference in volume is anatomically specific since the authors did not correct for either intracranial volume or TBV. Furthermore, in a recent study it was found that adult women treated with glucocorticoids due to congenital adrenal hyperplasia (CAH) had lower right hippocampal volumes (as well as smaller volumes in left and right thalami, cerebellum, and brainstem), corrected for TBV, as compared to healthy controls (Webb et al., 2018).
Our finding of lower right hippocampal MD in children previously treated with glucocorticoids compared to healthy controls seems to contrast with a recent report of increased MD in hippocampus as well as in multiple white matter fiber tracts, uncorrected for whole brain tissue MD, in women treated with glucocorticoids for CAH (Webb et al., 2018). However, studies are difficult to compare given the differences in stage of development, the type of disease studied, demographic characteristics of the study populations, and the applied methodologies. Grey matter MD is thought to be affected by cell density as in studies of tumour tissue lower MD was related to higher cell density (Beaulieu, 2009; Chenevert et al., 2006; Gibbs et al., 2009). This suggests that lower right hippocampal MD in children previously treated with glucocorticoids may reflect an increase in the number of cellular elements. Such an interpretation, however, seems counter-intuitive given glucocorticoids' known anti-proliferative effects (Kino, 2015; Numakawa et al., 2017). Furthermore, in a MR-spectroscopy study lower hippocampal N-acetyl aspartate ratio, representative of lower hippocampal neural cell densities, was observed in adults chronically treated with glucocorticoids for extra-cerebral diseases (Brown et al., 2004). Moreover, in animal models of perinatal glucocorticoid treatment, reduced neurogenesis occurred both shortly after the exposure as well as in the long-term (Kanagawa et al., 2006; Noorlander et al., 2008). Neuron density, however, is not the only factor that can influence tissue MD. Factors such as level of myelination, cell sizes, number of dendrites, number of glial cells, inflammation or extracellular properties also play a role (Alexander et al., 2007; Beaulieu, 2002). Although speculatively, our finding of lower right hippocampal MD but not lower hippocampal volumes, may well reflect an increase in the number of glia cells (Chetty et al., 2014). In support for this notion, a MR-spectroscopy study in adults with previously elevated endogenous glucocorticoid levels, due to Cushing's syndrome, reported lower hippocampal N-acetyl aspartate and higher glutamate and glutamine levels, suggestive of respectively reduced neuronal density and increased glial proliferation (Resmini et al., 2013). Our observation that right hippocampal MD became more similar to that of healthy controls the more time had elapsed after termination of glucocorticoid treatment, suggests that this effect is, at least partly, reversible. The functional implications of our findings remain to be clarified. Since lower right hippocampal MD was more prominent in children with nephrotic syndrome than in those with rheumatic disease, we cannot rule out disease-specific effects. However, compared to the rheumatic group, the children with nephrotic syndrome had generally received higher glucocorticoid doses and the association between right hippocampal MD and time since glucocorticoid treatment was present in both disease-groups separately.
Given the widespread distribution of the glucocorticoid receptor throughout the brain (Cao-Lei et al., 2013) and its affinity of exogenous glucocorticoids such as prednisolone, we also investigated for possible group differences in striatal grey matter structures. Children previously treated with glucocorticoids had significantly smaller putamen volumes than healthy controls. Although preliminary, this finding seems to align with findings of a recent study of preterm born children treated with dexamethasone in neonatal life, who had smaller caudate nucleus and putamen volumes, as compared to preterm born children, who had not received dexamethasone treatment, 18 years after exposure (Cheong et al., 2014). Moreover, a study in preterm fetal sheep found that exposure to glucocorticoids aggravated the neuronal injury following asphyxia in the hippocampus, but also in the putamen (Koome et al., 2013). However, smaller putamen volumes were most prominent in children with rheumatic diseases and the observed negative association between the median treatment age and putamen volume was absent in the children with nephrotic disorder. Since the children with rheumatic disease received lower cumulative and median daily dose of glucocorticoids than the children with nephrotic syndrome, our findings suggest that the observed smaller putamen volume may more likely be a disease-specific effect rather than due to the glucocorticoid treatment by itself. Furthermore, we may have lacked the statistical power to find smaller putamen volumes in patients with nephrotic syndrome given the lower number of patients with nephrotic syndrome in our study. Finally, it might also be that potential disease-specific findings are, at least partly, reflective of an uneven gender distribution in the two disease groups, with more girls among the children with rheumatic disease.
Overall, the present study is limited due to the relatively small sample sizes, as well as an uneven gender distribution in the two disease groups, with more girls among the children with rheumatic disease. Thus, we cannot exclude if the lack of specific hippocampal volume differences is due to a lack of statistical power. Moreover, by the very nature of our cross-sectional design we are not able to infer causality of previous glucocorticoid treatment, nor are we able to infer possible disease-related mechanism. Unfortunately, this inability to differentiate disease-specific effects from glucocorticoid treatment effects befalls most cross-sectional studies in patient populations. Furthermore, some participants also received other drugs such as TNFα-inhibitors or methotrexate. The most dominant non-glucocorticoid treatment at the time of assessment concerned TNFα-inhibitors. Interestingly, these have recently been found to prevent pharmacological neurotoxicity in the developing brain in rodents (Chen et al., 2016). Unfortunately, given the small number of patients receiving additional drug treatment, we were not able to address this issue in the current study and therefore cannot exclude the possibility that additional treatments may have influenced our results. Finally, an ideal control group would consist of patients with comparable disease histories, who had not been treated with glucocorticoids. However, it not possible to identify such a disease control group due to differences in disease mechanisms, course of illness, illness severity, and treatment. By including the two non-cerebral disease groups, we reasoned that effects common to both groups, specifically associations with glucocorticoid treatment variables, would be more reflective of glucocorticoid treatment than disease-specific effects.
In conclusion, we revealed for the first time that extra-cerebral diseases during childhood treated with glucocorticoids may be associated with reduced subcortical grey matter volumes and lower right hippocampal mean diffusivity later in life. While our results should be considered preliminary, they warrant replication and elaboration in larger, preferably prospective and longitudinal studies. Such studies would also allow disentangling disease-specific effects from glucocorticoid treatment effects.
Financial support
All phases of this study were kindly supported by The Danish Council for Independent Research (grant number: 09-071546), The Lundbeck Foundation (grant number: R48-A4968), and The Ville Heise Foundation. Funding sources were not involved in any phases of the study (i.e. study design; collection, analysis and interpretation of data; writing of the manuscript; decision to submit the article for publication).
Financial disclosure
Hartwig R. Siebner has received honoraria as speaker from Novartis Denmark and Genzyme, Denmark, as consultant from Genzyme, Denmark, as senior editor from Elsevier Publishers, Amsterdam, The Netherlands and royalties as co-editor of a book from Springer Publishing, Stuttgart, Germany. Hartwig R. Siebner is clinical professor with special focus on precision medicine at the Institute for Clinical Medicine, University of Copenhagen. The professorship is sponsored by the Lundbeck Foundation.
The remaining authors have no financial disclosures to declare.
Conflict of interest
The authors declare no potential conflict of interest.
Acknowledgements
Our greatest acknowledgement is directed toward all participating children and their parents. Furthermore, The Danish Research Council for Independent Research, The Lundbeck Foundation, and The Ville Heise Foundation are acknowledged for kindly funding the study.
References
- Alexander A.L., Lee J.E., Lazar M., Field A.S. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L., Hutton C., Ashburner J., Turner R., Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Jones D.K. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 2002;15:456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. (doi: papers://D8F752D0-1E23-4EB3-9F6A-9A0C8E776B87/Paper/p37) [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The biological basis of diffusion anisotropy. In: Johansen-Berg H., Behrens T.E., editors. Diffusion MRI - From Quantitative Measurement to In-vivo Neuroanatomy. Elsevier; London: 2009. [Google Scholar]
- Brown E.S., Woolston J., Frol A., Bobadilla L., Khan D.A., Hanczyc M., Rush A.J., Fleckenstein J., Babcock E., Cullum C.M. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biol. Psychiatry. 2004;55:538–545. doi: 10.1016/j.biopsych.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Brown E.S., Woolston D.J., Frol A.B. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol. Psychiatry. 2008;63:705–709. doi: 10.1016/j.biopsych.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T.T., Kuperman J.M., Chung Y., Erhart M., McCabe C., Hagler D.J., Jr., Venkatraman V.K., Akshoomoff N., Amaral D.G., Bloss C.S., Casey B.J., Chang L., Ernst T.M., Frazier J.A., Gruen J.R., Kaufmann W.E., Kenet T., Kennedy D.N., Murray S.S., Sowell E.R., Jernigan T.L., Dale A.M. Neuroanatomical assessment of biological maturity. Curr. Biol. 2012;22:1693–1698. doi: 10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lei L., Suwansirikul S., Jutavijittum P., Meriaux S.B., Turner J.D., Muller C.P. Glucocorticoid receptor gene expression and promoter CpG modifications throughout the human brain. J. Psychiatr. Res. 2013;47:1597–1607. doi: 10.1016/j.jpsychires.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Cerqueira J.J., Almeida O.F., Sousa N. The stressed prefrontal cortex. Left? Right! Brain Behav. Immun. 2008;22:630–638. doi: 10.1016/j.bbi.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Chen B., Deng X., Wang B., Liu H. Etanercept, an inhibitor of TNF-a, prevents propofol-induced neurotoxicity in the developing brain. Int. J. Dev. Neurosci. 2016;55:91–100. doi: 10.1016/j.ijdevneu.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Chenevert T.L., Sundgren P.C., Ross B.D. Diffusion imaging: insight to cell status and cytoarchitecture. Neuroimag. Clin. N Am. 2006;16:619–632. doi: 10.1016/j.nic.2006.06.005. viii-ix. [DOI] [PubMed] [Google Scholar]
- Cheong J.L., Burnett A.C., Lee K.J., Roberts G., Thompson D.K., Wood S.J., Connelly A., Anderson P.J., Doyle L.W. Association between postnatal dexamethasone for treatment of bronchopulmonary dysplasia and brain volumes at adolescence in infants born very preterm. J. Pediatr. 2014;164:737–743. doi: 10.1016/j.jpeds.2013.10.083. e731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty S., Friedman A.R., Taravosh-Lahn K., Kirby E.D., Mirescu C., Guo F., Krupik D., Nicholas A., Geraghty A., Krishnamurthy A., Tsai M.K., Covarrubias D., Wong A., Francis D., Sapolsky R.M., Palmer T.D., Pleasure D., Kaufer D. Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Mol. Psychiatry. 2014;19:1275–1283. doi: 10.1038/mp.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P.A., Bai Y., Nedjati-Gilani S., Seunarine K.K., Hall M.G., Parker G.J., Alexander D.C. 2006. Camino: Open-Source Diffusion-MRI Reconstruction and Processing 14th Scientific Meeting of the International Society for Magnetic Resonance in Medicine, Seattle, WA, USA; p. 2759. [Google Scholar]
- Coutinho A.E., Chapman K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadra M.B., Cammoun L., Butz T., Cuisenaire O., Thiran J.P. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Trans. Med. Imaging. 2005;24:1548–1565. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- Damsted S.K., Born A.P., Paulson O.B., Uldall P. Exogenous glucocorticoids and adverse cerebral effects in children. Eur. J. Paediatr. Neurol. 2011;15:465–477. doi: 10.1016/j.ejpn.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Davis E.P., Sandman C.A., Buss C., Wing D.A., Head K. Fetal glucocorticoid exposure is associated with preadolescent brain development. Biol. Psychiatry. 2013;74:647–655. doi: 10.1016/j.biopsych.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet E.R. From receptor balance to rational glucocorticoid therapy. Endocrinology. 2014;155:2754–2769. doi: 10.1210/en.2014-1048. [DOI] [PubMed] [Google Scholar]
- Dedovic K., Duchesne A., Andrews J., Engert V., Pruessner J.C. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47:864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Doyle L.W., Ehrenkranz R.A., Halliday H.L. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst. Rev. 2014 doi: 10.1002/14651858.CD001146.pub4. Cd001146. [DOI] [PubMed] [Google Scholar]
- Entis J.J., Doerga P., Barrett L.F., Dickerson B.C. A reliable protocol for the manual segmentation of the human amygdala and its subregions using ultra-high resolution MRI. Neuroimage. 2012;60:1226–1235. doi: 10.1016/j.neuroimage.2011.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs P., Liney G.P., Pickles M.D., Zelhof B., Rodrigues G., Turnbull L.W. Correlation of ADC and T2 measurements with cell density in prostate cancer at 3.0 tesla. Investig. Radiol. 2009;44:572–576. doi: 10.1097/RLI.0b013e3181b4c10e. [DOI] [PubMed] [Google Scholar]
- Gravanis A., Margioris A.N. Pharmacology of glucocorticoids: an overview. In: Margioris A.N., Chrousos G.P., editors. Adrenal Disorders. Humana Press; Totowa, NJ: 2001. pp. 59–70. [Google Scholar]
- Gray J.D., Kogan J.F., Marrocco J., McEwen B.S. Genomic and epigenomic mechanisms of glucocorticoids in the brain. Nat. Rev. Endocrinol. 2017;13:661–673. doi: 10.1038/nrendo.2017.97. [DOI] [PubMed] [Google Scholar]
- Holm S.K., Vestergaard M., Madsen K.S., Baare W.F., Hammer T.B., Born A.P., Siebner H.R., Paulson O.B., Uldall P.V. Children and adolescents previously treated with glucocorticoids display lower verbal intellectual abilities. Acta Paediatr. 2015;104:784–791. doi: 10.1111/apa.13010. [DOI] [PubMed] [Google Scholar]
- Holm S.K., Madsen K.S., Vestergaard M., Paulson O.B., Uldall P., Siebner H.R., Born A.P., Baare W.F.C. Total brain, cortical, and white matter volumes in children previously treated with glucocorticoids. Pediatr. Res. 2018;83:804–812. doi: 10.1038/pr.2017.312. [DOI] [PubMed] [Google Scholar]
- Jernigan T.L., Baare W.F., Stiles J., Madsen K.S. Postnatal brain development: structural imaging of dynamic neurodevelopmental processes. Prog. Brain Res. 2011;189:77–92. doi: 10.1016/B978-0-444-53884-0.00019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M., Krugers H.J., Lucassen P.J., Karst H. Corticosteroid effects on cellular physiology of limbic cells. Brain Res. 2009;1293:91–100. doi: 10.1016/j.brainres.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Basser P.J. "Squashing peanuts and smashing pumpkins": how noise distorts diffusion-weighted MR data. Magn. Reson. Med. 2004;52:979–993. doi: 10.1002/mrm.20283. [DOI] [PubMed] [Google Scholar]
- Jovicich J., Czanner S., Greve D., Haley E., van der Kouwe A., Gollub R., Kennedy D., Schmitt F., Brown G., Macfall J., Fischl B., Dale A. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kanagawa T., Tomimatsu T., Hayashi S., Shioji M., Fukuda H., Shimoya K., Murata Y. The effects of repeated corticosteroid administration on the neurogenesis in the neonatal rat. Am. J. Obstet. Gynecol. 2006;194:231–238. doi: 10.1016/j.ajog.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Kino T. Stress, glucocorticoid hormones, and hippocampal neural progenitor cells: implications to mood disorders. Front. Physiol. 2015;6:230. doi: 10.3389/fphys.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok M.D., Alt S.R., Irurzun Lafitte A.J., Turner J.D., Lakke E.A., Huitinga I., Muller C.P., Zitman F.G., de Kloet E.R., Derijk R.H. Decreased expression of mineralocorticoid receptor mRNA and its splice variants in postmortem brain regions of patients with major depressive disorder. J. Psychiatr. Res. 2011;45:871–878. doi: 10.1016/j.jpsychires.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Koome M.E., Davidson J.O., Drury P.P., Mathai S., Booth L.C., Gunn A.J., Bennet L. Antenatal dexamethasone after asphyxia increases neural injury in preterm fetal sheep. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lebel C., Treit S., Beaulieu C. A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR Biomed. 2017 doi: 10.1002/nbm.3778. [DOI] [PubMed] [Google Scholar]
- Leemans A., Jones D.K. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009;61:1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Madsen K.S., Jernigan T.L., Iversen P., Frokjaer V.G., Knudsen G.M., Siebner H.R., Baare W.F. Hypothalamic-pituitary-adrenal axis tonus is associated with hippocampal microstructural asymmetry. Neuroimage. 2012;63:95–103. doi: 10.1016/j.neuroimage.2012.06.071. [DOI] [PubMed] [Google Scholar]
- Maller J.J., Reglade-Meslin C., Anstey K.J., Sachdev P. Sex and symmetry differences in hippocampal volumetrics: before and beyond the opening of the crus of the fornix. Hippocampus. 2006;16:80–90. doi: 10.1002/hipo.20133. [DOI] [PubMed] [Google Scholar]
- Merke D.P., Giedd J.N., Keil M.F., Mehlinger S.L., Wiggs E.A., Holzer S., Rawson E., Vaituzis A.C., Stratakis C.A., Chrousos G.P. Children experience cognitive decline despite reversal of brain atrophy one year after resolution of Cushing syndrome. J. Clin. Endocrinol. Metab. 2005;90:2531–2536. doi: 10.1210/jc.2004-2488. [DOI] [PubMed] [Google Scholar]
- Noorlander C.W., Visser G.H., Ramakers G.M., Nikkels P.G., de Graan P.N. Prenatal corticosteroid exposure affects hippocampal plasticity and reduces lifespan. Dev. Neurobiol. 2008;68:237–246. doi: 10.1002/dneu.20583. [DOI] [PubMed] [Google Scholar]
- Numakawa T., Odaka H., Adachi N. Actions of brain-derived neurotrophic factor and glucocorticoid stress in neurogenesis. Int. J. Mol. Sci. 2017:18. doi: 10.3390/ijms18112312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y., Tamnes C.K., Fjell A.M., Westlye L.T., Due-Tonnessen P., Walhovd K.B. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J. Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil C.G., Lad S.P., Katznelson L., Laws E.R., Jr. Brain atrophy and cognitive deficits in Cushing's disease. Neurosurg. Focus. 2007;23 doi: 10.3171/foc.2007.23.3.13. [DOI] [PubMed] [Google Scholar]
- Prifitera A., Saklofoske D. Academic Press Elsevier Science; San Diego, USA: 1998. WISC-III Clinical Use and Interpretation: Scientist Practitioner Perspectives. [Google Scholar]
- Reese T.G., Heid O., Weisskoff R.M., Wedeen V.J. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn. Reson. Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Resmini E., Santos A., Gomez-Anson B., Lopez-Mourelo O., Pires P., Vives-Gilabert Y., Crespo I., Portella M.J., de Juan-Delago M., Webb S.M. Hippocampal dysfunction in cured Cushing's syndrome patients, detected by (1) H-MR-spectroscopy. Clin. Endocrinol. 2013;79:700–707. doi: 10.1111/cen.12224. [DOI] [PubMed] [Google Scholar]
- Reul J.M., de Kloet E.R. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Reynolds R.M. Programming effects of glucocorticoids. Clin. Obstet. Gynecol. 2013;56:602–609. doi: 10.1097/GRF.0b013e31829939f7. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D.K., Wood S.J., Doyle L.W., Warfield S.K., Lodygensky G.A., Anderson P.J., Egan G.F., Inder T.E. Neonate hippocampal volumes: prematurity, perinatal predictors, and 2-year outcome. Ann. Neurol. 2008;63:642–651. doi: 10.1002/ana.21367. [DOI] [PubMed] [Google Scholar]
- Tijsseling D., Wijnberger L.D., Derks J.B., van Velthoven C.T., de Vries W.B., van Bel F., Nikkels P.G., Visser G.H. Effects of antenatal glucocorticoid therapy on hippocampal histology of preterm infants. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno H., Lohmiller L., Thieme C., Kemnitz J.W., Engle M.J., Roecker E.B., Farrell P.M. Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus. Brain Res. Dev. Brain Res. 1990;53:157–167. doi: 10.1016/0165-3806(90)90002-g. [DOI] [PubMed] [Google Scholar]
- Vestergaard M., Holm S.K., Uldall P., Siebner H.R., Paulson O.B., Baare W.F.C., Madsen K.S. Glucocorticoid treatment earlier in childhood and adolescence show dose-response associations with diurnal cortisol levels. Dev. Psychobiol. 2017;59:1010–1020. doi: 10.1002/dev.21559. [DOI] [PubMed] [Google Scholar]
- Webb E.A., Elliott L., Carlin D., Wilson M., Hall K., Netherton J., Reed J., Barrett T.G., Salwani V., Clayden J.D., Arlt W., Krone N., Peet A.C., Wood A.G. Quantitative brain MRI in congenital adrenal hyperplasia: in vivo assessment of the cognitive and structural impact of steroid hormones. J. Clin. Endocrinol. Metab. 2018;103:1330–1341. doi: 10.1210/jc.2017-01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga L., Langen M., Ambrosino S., van Dijk S., Oranje B., Durston S. Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. Neuroimage. 2014;96:67–72. doi: 10.1016/j.neuroimage.2014.03.072. [DOI] [PubMed] [Google Scholar]