Hepatitis B virus (HBV) is a major global health concern, chronically infecting millions of patients and contributing to a rising burden of liver disease. The viral genome forms the basis for chronic infection and has been shown to be subject to regulation by epigenetic mechanisms, such as posttranslational modification of histone proteins. Here, we confirm and expand on previous results by adapting a high-resolution technique for analysis of histone modifications for use with patient-derived fine-needle liver biopsy specimens. Our work highlights that the situation in vivo is more complex than predicted by current in vitro models, for example, by suggesting a novel, noncanonical role of the histone modification H3K9me3 in the HBV life cycle. Importantly, enabling the use of fine-needle liver biopsy specimens for such high-resolution analyses may facilitate further research into the epigenetic regulation of the HBV genome.
KEYWORDS: epigenetics, hepatitis B virus, histone methylation, histones, liver biopsies, splicing, transcription, viral integration
ABSTRACT
Covalently closed circular DNA (cccDNA) forms the basis for replication and persistence of hepatitis B virus (HBV) in the chronically infected liver. We have previously shown that viral transcription is subject to regulation by posttranslational modifications (PTMs) of histone proteins bound to cccDNA through analysis of de novo HBV-infected cell lines. We now report the successful adaptation of this chromatin immunoprecipitation sequencing (ChIPseq) approach for analysis of fine-needle patient liver biopsy specimens to investigate the role of histone PTMs in chronically HBV-infected patients. Using 18 specimens from patients in different stages of chronic HBV infection, our work shows that the profile of histone PTMs in chronic infection is more nuanced than previously observed in in vitro models of acute infection. In line with our previous findings, we find that the majority of HBV-derived sequences are associated with the activating histone PTM H3K4me3. However, we show a striking interpatient variability of its deposition in this patient cohort correlated with viral transcription and patient HBV early antigen (HBeAg) status. Unexpectedly, we detected deposition of the classical inhibitory histone PTM H3K9me3 on HBV-DNA in around half of the patient biopsy specimens, which could not be linked to reduced levels of viral transcripts. Our results show that current in vitro models are unable to fully recapitulate the complex epigenetic landscape of chronic HBV infection observed in vivo and demonstrate that fine-needle liver biopsy specimens can provide sufficient material to further investigate the interaction of viral and host proteins on HBV-DNA.
IMPORTANCE Hepatitis B virus (HBV) is a major global health concern, chronically infecting millions of patients and contributing to a rising burden of liver disease. The viral genome forms the basis for chronic infection and has been shown to be subject to regulation by epigenetic mechanisms, such as posttranslational modification of histone proteins. Here, we confirm and expand on previous results by adapting a high-resolution technique for analysis of histone modifications for use with patient-derived fine-needle liver biopsy specimens. Our work highlights that the situation in vivo is more complex than predicted by current in vitro models, for example, by suggesting a novel, noncanonical role of the histone modification H3K9me3 in the HBV life cycle. Importantly, enabling the use of fine-needle liver biopsy specimens for such high-resolution analyses may facilitate further research into the epigenetic regulation of the HBV genome.
INTRODUCTION
Chronic infection with hepatitis B virus (HBV) is estimated to affect around 250 million to 350 million people worldwide, with up to 2 billion having been exposed to HBV (1; see https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b). Progression from acute to chronic HBV infection (CHB) is variable and age dependent: adults generally clear HBV, but younger patients mainly progress to chronicity, especially after perinatal infection. Patients with CHB often have a poor prognosis linked to persistent infection, ongoing liver injury, and the development of cirrhosis and/or hepatocellular carcinoma (2). Available therapies suppress viral replication but fail to completely eliminate the virus or the risk of liver disease (3). The natural progression of CHB is considered to evolve through four clinical stages, which can be distinguished by the occurrence of liver inflammation and the presence of HBV early antigen (HBeAg) in the serum (3). The first stage of CHB, immune-tolerant CHB, is marked by the presence of HBeAg and high viral loads, with limited or no liver inflammation. Immune-active CHB manifests by the onset of hepatic inflammation, which can transition to HBeAg-negative (HBeAg−) CHB upon the loss of HBeAg from the circulation (1, 3). In some HBeAg− patients, viral loads can drop to the level of detection, coinciding with a lack of hepatic inflammation despite a persistence of HBV surface antigen (HBsAg) in the serum. However, even in these patients with inactive CHB, spontaneous clearance of HBV is rare (3). Some studies have suggested integration of the HBV genome into the host genome as one possible cause for the transition of CHB to HBeAg− disease stages (4, 5), but this integration event cannot support viral replication, and thus, the covalently closed circular DNA (cccDNA) minichromosome in the nucleus of infected cells forms the basis for viral replication and persistence (6). cccDNA is bound to viral proteins as well as histones, which are subject to posttranslational modifications (PTMs) and known regulators of gene expression (7–12). Our previous studies in de novo HBV-infected cell cultures demonstrated that histone PTMs are involved in regulation of viral transcription. However, unlike host chromatin, HBV-DNA in those cells is mainly marked by histone PTMs linked to active transcription, such as trimethylation of lysine 4 of the H3 protein (H3K4me3), while lacking histone PTMs frequently linked to inhibition of transcription, such as H3K9me3 or H3K27me3 (7, 12). These findings were replicated in vivo, although the scope of the previous study was limited to a single patient liver biopsy specimen obtained after surgical resection, which did not allow for the assessment of the histone PTMs on HBV-DNA across disease stages (12). In the present work, we adapted our chromatin immunoprecipitation (ChIP) sequencing (ChIPseq) assay for the analysis of fine-needle liver biopsy specimens, which are more commonly available from routine diagnostic procedures from a wide range of patients (13). As a proof of concept, we successfully analyzed the association between histone PTMs and HBV-DNA in a small cohort of 18 patients across different stages of CHB. Consistent with our previous work on in vitro models, we observed conservation across patients and viral genotypes of the HBV-specific histone PTM pattern marked by strong deposition of H3K4me3 and the absence of H3K27me3. This study reveals for the first time interindividual differences in the deposition of histone PTMs on HBV-DNA during chronic infection, particularly a reduced deposition of H3K4me3 in samples from patients in HBeAg− stages of CHB. Surprisingly, we also observed the presence of canonically inhibitory H3K9me3 on HBV-derived sequences that was not linked to reduced levels of viral transcripts in vivo. Together, these results indicate that current in vitro models cannot fully recapitulate the complexity of the histone PTMs on HBV-DNA in vivo and enable further research using fine-needle liver biopsy specimens.
RESULTS
Patient clinical and virological characteristics.
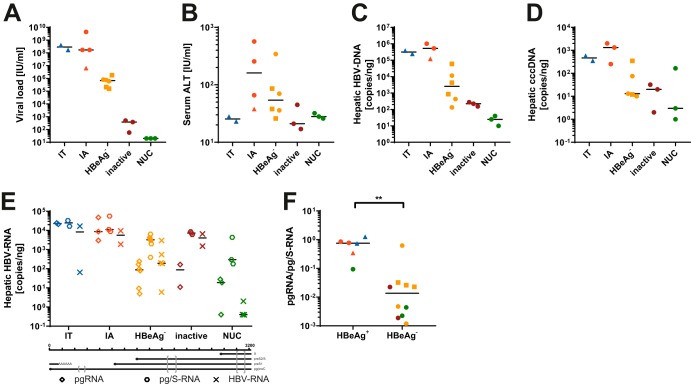
A total of 18 fine-needle liver biopsy specimens were analyzed (Table 1; see also Table S1 in the supplemental material). This cohort included patients across the four major stages of CHB as well as patients under therapy with nucleos(t)ide analogs (NUCs). Patients under therapy received NUCs for at least 6 months prior to biopsy. Patient c809 has a history of nonresponse to interferon treatment and received a combination of two NUCs for 5 years that was reduced to a single NUC 1 month prior to biopsy. Consistent with reported clinical observations, serum viral loads were higher in patients with HBeAg-positive (HBeAg+) stages of CHB than in patients with HBeAg− stages of CHB and undetectable in patients receiving NUCs (Fig. 1A) (3). As expected, serum alanine aminotransferase (ALT) levels were elevated in patients with immune-active CHB and HBeAg− CHB (Fig. 1B) (3). Total intrahepatic viral DNA as well as cccDNA levels were typically lower in HBeAg− stages of CHB, as expected (Fig. 1C and D). We analyzed hepatic viral RNA levels using up to three different primer pairs located in different loci of the viral genome, depending on material availability: the preC/pregenomic RNA (pgRNA) locus, the HBs locus (pg/S-RNA [preC/pgRNA and transcripts from preS1 and preS2/S promoters]), and the HBx locus (HBV-RNA [all HBV-derived transcripts]) (Table 2 and Fig. 1E). Analysis showed that pg/S-RNA levels were similar between groups, with only a slight reduction observed in patients with HBeAg− stages of CHB and patients under NUC therapy. In contrast, pgRNA levels were reduced in patients undergoing NUC therapy as well as in those in HBeAg− stages of CHB, resulting in a reduction of the pgRNA-to-pg/S-RNA ratio in those patients (Fig. 1F). Total HBV-RNA levels were mostly comparable to pgRNA levels except for in patients with inactive CHB, where pgRNA levels were lower. The higher levels of pg/S-RNA than “total” HBV-RNA levels in some samples may be due to viral integrants that have been suggested to be a major source of HBsAg-encoding transcripts, especially in HBeAg− stages of CHB (4, 5). In these events, the junction between viral and host genomes usually lies within the direct repeat 1 (DR1) region of the viral genome (5, 14), which is upstream of the primer pair used for total HBV-DNA determination (Table 2).
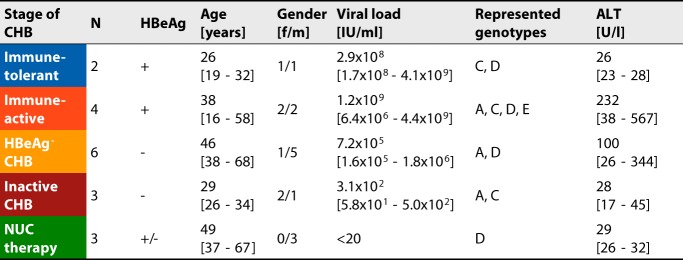
TABLE 1.
Cohort characteristicsa
Shown is an overview of the clinical parameters of the patient cohorts grouped according to disease stage. Numerical values are indicated as means [ranges]. ALT, alanine aminotransferase; f, female; HBeAg, HBV early antigen; m, male.
FIG 1.
Patient characteristics. Shown are scatter plots comparing different experimental and clinical data between subcohorts. Color coding is according to disease stage (Table 1). Squares indicate samples with low-quality sequencing results after both H3K4me3-ChIP as well as H3-ChIP (samples c162, c317, and c687), and triangles indicate samples with low quality after total H3-ChIP (samples c23, c537, and c770). Shown are viral loads (A), serum ALT levels (B), hepatic HBV-DNA levels (C), hepatic cccDNA levels (D), hepatic HBV-RNA levels determined by the different primer pairs indicated at the bottom (E), and comparison of the ratio of pgRNA to pg/S-RNA based on HBeAg status (F). The P value was determined by a Mann-Whitney test. **, P < 0.01. IT, immune-tolerant CHB; IA, immune-active CHB.
TABLE 2.
Primersa
| Target | Forward primer (5′–3′) | HBV positions (genotype C) | Reverse primer (5′–3′) | HBV positions (genotype C) |
|---|---|---|---|---|
| Analysis of ChIP samples | ||||
| Actin TSS | TAG AAG TCG CAG GAC CAC ACT | NA | TGG GTA GGT TTG TAG CCT TCA T | NA |
| Nanog TSS | TGG GTT TGT CTT CAG GTT CTG | NA | CTA CTG ACC CAC CCT TGT GAA | NA |
| Sat2 | CAT CGA ATG GAA ATG AAA GGA GTC | NA | ACC ATT GGA TGA TTG CAG TCA A | NA |
| HBV_1 | TTA ACA GGC CTA TTG ATT GGA AA | 965–984 | TCA ACG CAG GAT AAC CAC ATT | 1034–1049 |
| DNA quantification | ||||
| HBV-DNA | CCA AAA TTC GCA GTC CCC AAC | 304–324 | GAG GCA TAG CAG CAG GAT GAA G | 406–427 |
| RNA quantification | ||||
| pgRNA | GAG TGT GGA TTC GCA CTC CTC | 2268–2288 | CGA GGC GAG GGA GTT CTT CT | 2378–2397 |
| pg/S-RNA | CCC GTT TGT CCT CTA ATT CC | 467–486 | GTC CGA AGG TTT GGT ACA GC | 567–585 |
| HBV-RNA | GTG CAC TTC GCT TCA CCT CT | 1579–1598 | TTG ACA TTG CTG AGA GTC CAA | 1666–1686 |
| IMAP | TTT TCA GCT CCC AAG TGT CC | NA | GCC GAG AGC AGG TAG CAG T | NA |
Experimental design.
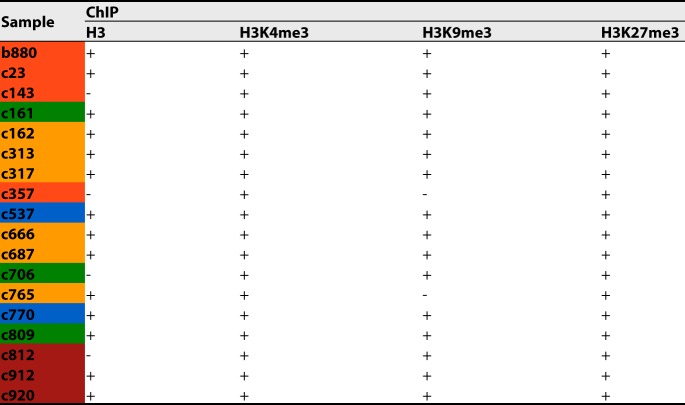
Our previously established ChIPseq protocol was limited by the amount of cells (typically 8 × 106) necessary to obtain sufficient amounts of chromatin (12). While this allowed a proof-of-concept analysis of liver tissue obtained by surgical resection, fine-needle liver biopsy specimens are a more widely available clinical specimen from CHB patients across all stages of disease. However, they yield only a small number of cells for analysis, only a fraction of which are infected hepatocytes. In this case, only around 2.5 × 105 cells were available for ChIPseq, too few to allow reliable identification of mononucleosome-containing bands after ultracentrifugation. Furthermore, processing such small amounts of material is challenging due to a higher risk of sample loss. To overcome these limitations, the pieces of fine-needle liver biopsy specimens were diluted in uninfected HepG2 cells expressing the HBV entry receptor human sodium taurocholate cotransporting polypeptide (hNTCP) (HepG2-hNTCP cells), increasing the total cell count to aid in the accuracy of the downstream processes. For all samples, ChIP was performed for the histone PTMs H3K4me3 and H3K27me3, linked to active and inactive transcription, respectively, to allow comparison to data from our previous study (7, 12). Next, we used the remaining samples (16/18) to investigate the deposition of H3K9me3. Finally, we performed ChIP for total H3 (total H3-ChIP) (14/18) to determine whether samples with low read counts after H3K4me3-ChIP contained chromatinized HBV-DNA above the limit of detection of our assay. An overview of the ChIPs performed for each sample is shown in Table 3.
TABLE 3.
ChIP overviewa
Shown is an overview of the ChIPs performed (+) for each liver biopsy specimen. − indicates that ChIP was not performed due to a lack of material. Color coding indicates the disease stage according to Table 1.
Quality control of the experimental procedure.
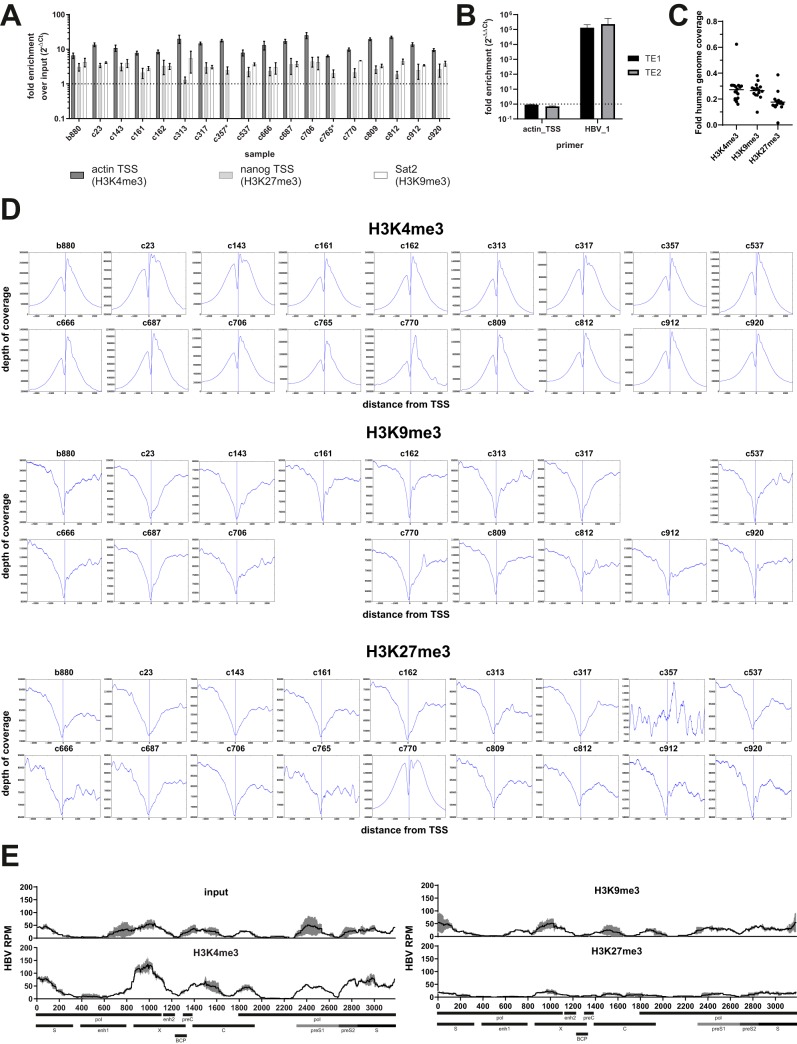
ChIPs were validated by quantitative PCR (qPCR) for known genomic sites linked to histone PTMs (Fig. 2A), and samples were subsequently subjected to sequencing library construction and HBV-specific target enrichment. Target enrichment relies on using HBV-specific probes covering the entire HBV genome in a tiling fashion (Table S2) for the pulldown of the HBV-derived parts of the library, followed by PCR-based amplification using primers binding to the adapter segments attached during library construction. This enables reliable enrichment of HBV-derived reads, typically by a factor of 104 to 105 (Fig. 2B). We previously showed that this procedure does not bias for individual parts of the HBV genome since it maintains patterns that can be observed using higher-throughput sequencing technologies without target enrichment (12). We also sought to verify the integrity of the constructed libraries and the quality of the ChIP by analyzing the distribution of reads on the human genome after ChIP for the different histone PTMs. Due to the used sequencing approach and library pooling, human genome coverage was low (on average 0.24× [0.02× to 0.62×]). We thus chose to average all covered transcriptional start sites (TSS) rather than focus on individual TSS. As expected, H3K4me3 was clearly enriched around human TSS (Fig. 2C), while the deposition of H3K9me3 and H3K27me3 was reduced (15). Exceptions were noted for H3K27me3-ChIPs for samples c357 and c770. The reason for these differences is unclear and may reflect technical issues with these two libraries. Thus, data on H3K27me3 from these two samples were excluded from further analysis. Finally, we sought to validate that the adapted ChIPseq workflow yields results comparable to those of our previous study (12). We thus analyzed chromatin from primary human hepatocytes (PHH) infected in vitro with cell culture-derived HBV and reproduced the previously described phenotype of strong deposition of H3K4me3 on cccDNA concomitant with a lack of H3K9me3 and H3K27me3 (Fig. 2D).
FIG 2.
Control of the ChIPseq workflow. (A) Bar diagram showing results of a qPCR to control for the success of ChIP for histone PTMs. Fold enrichments over the input after qPCR for actin TSS (H3K4me3-ChIP), nanog TSS (H3K27me3-ChIP), and Sat2 (H3K9me3-ChIP) are indicated. No H3K9me3-ChIP could be performed for samples c357 and c765 (marked with *). Data are means of results from two independent experiments with three technical replicates each. (B) Bar diagram showing the efficacy of HBV-specific target enrichment (TE). Library pools (4 nM) with and without target enrichment were subjected to qPCR using the indicated primer pairs (Table 4). Data were first normalized to background amplification in the respective no-template control (ΔCT) before comparing nonenriched versus enriched samples (ΔΔCT). The dashed line indicates the level without target enrichment. (C) Diagrams showing the distribution of reads mapping to the human genome around known TSS. Data are averaged across TSS and grouped according to histone PTMs. Vertical lines indicate positions of the TSS. (D) Exemplary ChIPseq results obtained after infection of primary human hepatocytes (PHH) with cell culture-derived HBV as described previously (12). Cells were collected at 12 days postinfection and subjected to ChIPseq for H3K4me3, H3K9me3, and H3K27me3. HBV-derived reads per position were normalized to 106 total reads to enable comparison between libraries. Results are indicated as means from two independent sequencing runs (black lines), with error bars (SD) in gray. Select genomic loci are indicated at the bottom.
Detection of HBV-derived sequences from liver biopsy specimens.
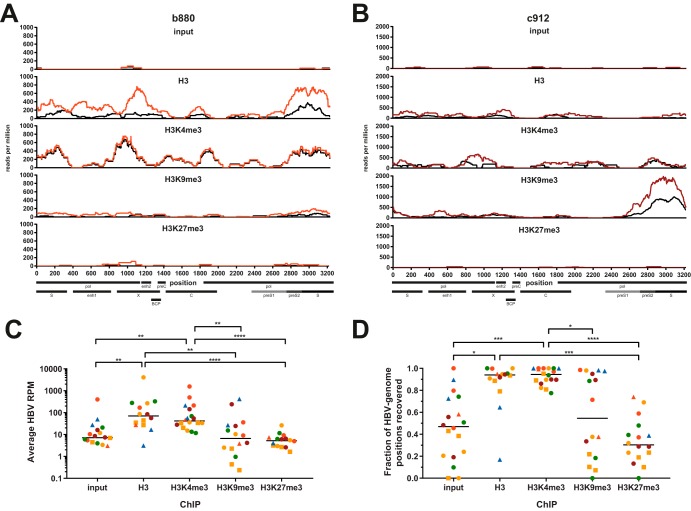
HBV-derived reads were readily detectable after H3K4me3-ChIP and ChIP for total H3, confirming that fine-needle liver biopsy specimens can yield sufficient viral DNA for detection by our ChIPseq assay (Fig. 3A and B, colored tracks). Surprisingly, HBV-derived reads were detectable in a subset of patients after ChIP for H3K9me3. These results were confirmed by constructing and analyzing independent libraries from the same samples (Fig. 3A and B, black tracks). Differences were mostly observed in the amounts of reads between replicates, likely a consequence of slight differences in the amounts of the rare HBV-DNA template during the PCR-based amplification or enrichment steps. However, since the observed patterns were consistent between replicates, the average from the two replicates was used for further analysis. In contrast to H3K4me3, HBV-derived reads were sparse in input material and following ChIP for H3K27me3 in all patients, except for the input from sample c357 (Fig. 3A to C). It is unclear why this sample from a patient infected with HBV genotype E showed such a large amount of HBV-derived reads in the input material, and a lack of material precluded repeating the experiment. Since HBV-derived reads were so rare in most input samples, we chose not to calculate a fold-enrichment-over-input metric for individual samples, as it would be strongly biased by minor absolute changes in the amounts of reads recovered in the input. HBV-derived reads were highly enriched following both H3- and H3K4me3-ChIP compared to the input, H3K9me3-ChIP, and H3K27me3-ChIP (Fig. 3C). This is consistent with de novo infection of PHH with HBV genotype D (Fig. 2D) and the results of our previous study using in vitro models of HBV infection (12). As expected, the fraction of the HBV genome that could be recovered following H3K27me3-ChIP was low compared to that with H3- or H3K4me3-ChIP, confirming the lack of recruitment of this PTM to HBV-DNA (Fig. 3D). For H3K9me3, a high proportion (>70%) of the HBV genome could be recovered in about half of the tested patients but not the others. Nevertheless, average read counts were at low levels in all but two samples (Fig. 3C), indicating that a low level of deposition of H3K9me3 may occur in a fraction of CHB patients in vivo but that strong deposition may occur only sporadically.
FIG 3.
Reproducible detection of HBV-derived reads from liver biopsy specimens. (A and B) Sequencing tracks showing the distributions of HBV-derived reads across the HBV genome for samples b880 (A) and c912 (B). Colored and black tracks depict sequencing results from two independently constructed libraries expressed as reads per million (RPM), i.e., HBV-specific reads per position normalized to 106 total reads. The ChIP performed is indicated above the sequencing tracks. Select genomic features and open reading frames (ORFs) are indicated at the bottom. BCP, basal core promoter; enh, enhancer; pol, polymerase. (C and D) Average HBV RPM (C) and percentage of genomic positions recovered by sequencing (D) under each ChIP condition. One data point each for the input, H3K9me3, and H3K27me3 is not displayed in panel C since zero HBV-derived reads were recovered. H3K27me3-ChIP results from samples c357 and c770 were excluded from panels C and D due to poor quality of reads mapping to the human genome. Color coding throughout the figure indicates the disease stage according to Table 1. Squares indicate samples with low-quality sequencing results after both H3K4me3-ChIP as well as H3-ChIP (samples c162, c317, and c687), and triangles indicate samples with low quality after total H3-ChIP (samples c23, c537, and c770). All P values were calculated by a Kruskal-Wallis test followed by Dunn’s multiple-comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Quality of HBV-derived sequencing tracks.
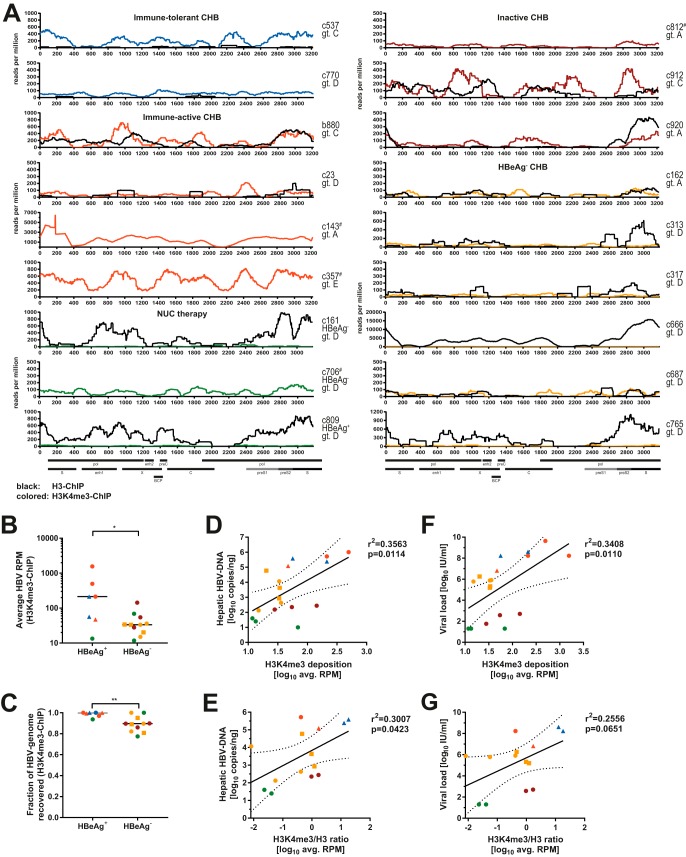
Since HBV-derived reads were highly enriched after ChIP for H3 and H3K4me3, we next analyzed the distributions of H3 and H3K4me3 on the HBV genome in more detail (Fig. 4A). HBV read counts were normalized to the total reads within each library. Clear sequencing tracks covering the HBV genome were obtained for most patients after ChIP for at least one of the two markers. In samples c162, c317, and c687, both tracks were of relatively low quality, indicating that in these samples, the amount of HBV-derived sequences in the material was likely at or below the limit that allows genome-wide recovery. While total HBV-derived read counts were quantifiable and on scale with those of the remaining samples, we excluded these samples from all localization-specific analyses. For samples c23, c537, and c770, HBV-derived sequences could be readily observed after ChIP for H3K4me3, but only a few reads were recovered after ChIP for total H3. This may indicate a failure of the H3-ChIP in these samples and potentially also samples c162, c317, and c687. We were unable to predict the performance of our assay based on any of the available parameters (Table S1), especially since clear sequencing data could be obtained from the samples with the lowest hepatic cccDNA and HBV-DNA levels (samples c706 and c809). Nevertheless, we obtained sufficient sequencing information to reliably analyze the distribution of histones and histone PTMs in 57% (total H3) and 83% (H3K4me3) of samples tested.
FIG 4.
Deposition of H3 and H3K4me3 on HBV-DNA in vivo. (A) Sequencing tracks comparing results after H3-ChIP (black) and H3K4me3-ChIP (colored according to disease stage [Table 1]) for all analyzed liver biopsy specimens. The sample identifier, viral genotype, as well as HBeAg status for patients under nucleos(t)ide (NUC) therapy are indicated. # marks samples for which no ChIP for total H3 could be performed (samples c143, c357, c706, and c812). Lines represent the averages of data from two independently constructed libraries expressed as RPM. Select genomic features and ORFs are indicated at the bottom. Viral sequences recovered from sample c143 aligned similarly well to genotypes A and D, with higher read counts after alignment to genotype A (55). The sharp peak consistently observed in this sample coincides with a shift from a genotype A-like sequence to a genotype D-like sequence. (B and C) Average HBV RPM across the genome (B) and percentage of genomic positions recovered by sequencing (C) displayed according to HBeAg status after H3K4me3-ChIP. P values were calculated by a Mann-Whitney test. *, P < 0.05; **, P < 0.01. (D and E) Correlation of average read counts after H3K4me3-ChIP and hepatic HBV-DNA (D) and ratio of reads recovered after H3K4me3-ChIP to those recovered after H3-ChIP (E). (F) Similar to panel D but for serum viral loads. (G) Similar to panel E but for serum viral loads. Color coding is according to disease stage (Table 1). For linear regressions, Pearson correlation coefficients and P values are indicated, and the dashed lines indicate the 95% confidence interval of regression. Squares indicate samples with low-quality sequencing results after both H3K4me3-ChIP as well as H3-ChIP (samples c162, c317, and c687), and triangles indicate samples with low quality after total H3-ChIP (samples c23, c537, and c770).
Heterogeneity of H3K4me3 deposition on HBV-DNA.
Comparison of the different H3K4me3 profiles revealed a striking heterogeneity between samples. For example, while most samples from HBeAg+ CHB patients exhibited a broad deposition of H3K4me3 across the HBV genome, as seen in vitro (12), individual specimens showed a mostly localized deposition of H3K4me3 (e.g., sample c23) or generally low levels of H3K4me3 deposition (e.g., sample c770). Furthermore, the relative intensities of the different peaks varied between samples. We observed significantly less H3K4me3 deposition, and genome coverage in general, in most samples from patients with HBeAg− CHB (Fig. 4B and C). Comparing the deposition of H3K4me3 to that of total H3, we observed comparable results in several samples (b880, c313, c912, and c920), indicating that, here, most histones associated with HBV-DNA carry H3K4me3, even though consecutive ChIPs would be required to prove this. Surprisingly, we observed several samples in which HBV-DNA was linked to histones without H3K4me3 (samples c161, c666, c765, and c809). This demonstrates that HBV-DNA in vivo can lack deposition of H3K4me3, in contrast to observations by us and others in in vitro models (12, 16, 17).
Correlates of H3K4me3 deposition on HBV-DNA.
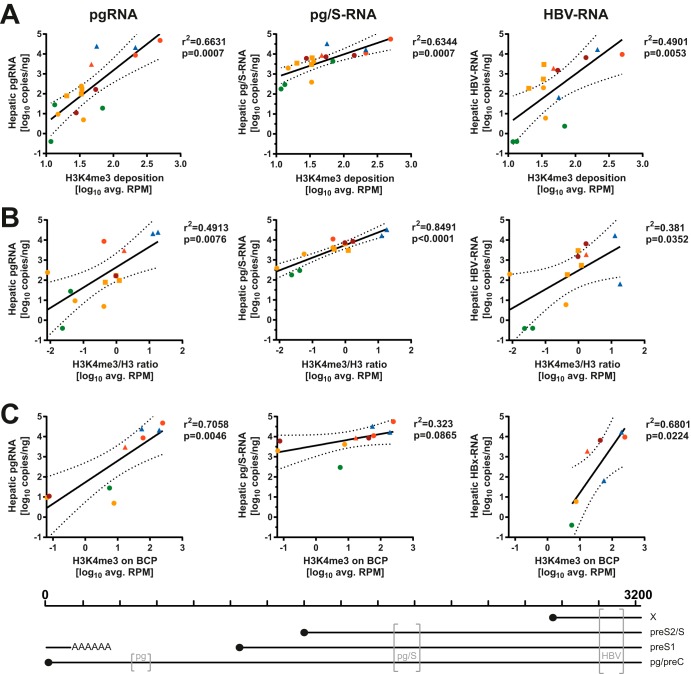
We analyzed the observed patterns of H3K4me3 deposition to look for any correlates with patient clinical and virological parameters. Most described correlations maintained statistical significance even if values derived from patients with low-quality sequences after H3K4me3-ChIP (samples c162, c317, and c687) and/or H3-ChIP (c23, c537, and c770) were excluded from the analyses (data not shown). Independent of HBeAg status, there were no obvious effects of hepatic inflammation (immune-tolerant and inactive CHB versus immune-active and HBeAg− CHB) or viral genotype on the deposition of H3K4me3 (Fig. 4A). The average HBV read count after H3K4me3-ChIP was weakly correlated with the amount of total HBV-DNA in the liver, but the significance of this finding was strongly reduced when the ratio of H3K4me3/total H3 was used (Fig. 4D and E). Similar observations were made for serum viral loads (Fig. 4F and G), even excluding patients with artificially reduced viral loads due to NUC treatment (data not shown). Since H3K4me3 is linked to active transcription of HBV in vitro (12, 18), we hypothesized that this may also be reflected in our biopsy specimens. Therefore, we statistically analyzed the average read counts observed after ChIP with the hepatic viral RNA levels (Fig. 1E and Table S1). Indeed, there is a positive correlation between the average HBV read count after H3K4me3-ChIP and the amounts of hepatic pgRNA, pg/S-RNA, as well as HBV-RNA (Fig. 5A). When the levels of H3K4me3 were normalized to the total H3 level, this correlation was even more pronounced for pg/S-RNA (Fig. 5B) and maintained when all six samples with low-quality results after total H3-ChIP were excluded. We next investigated whether H3K4me3 deposition on the basal core promoter (BCP) correlates with the levels of pgRNA, since this is the only transcript whose levels can be determined individually by reverse transcription-qPCR (RT-qPCR) due to the organization of the HBV genome (19). Indeed, the average read count mapping to the BCP was well correlated with the measured levels of pgRNA, while correlations with HBV-RNA levels were weaker, and the association with pg/S-RNA levels failed to reach statistical significance (Fig. 5C). This observation may indicate epigenetic regulation of pgRNA transcription by H3K4me3 deposition on the BCP, especially since hepatic cccDNA levels were only loosely correlated with pgRNA levels (data not shown) (r2 = 0.3388; P = 0.0290). While levels of viral transcripts clearly correlated with H3K4me3 deposition, this observation did not extend to the observable protein level as determined by the percentage of HBV core antigen (HBcAg)+ or HBsAg+ cells by immunohistochemistry; however, this readout may only partially reflect levels of protein production in infected cells and was performed on a separate biopsy cylinder (Table S1). Serum HBsAg levels were unavailable in this cohort.
FIG 5.
H3K4me3 deposition on HBV-DNA correlates with viral transcription. Average HBV RPM after H3K4me3-ChIP (A), ratios of H3K4me3 RPM/total H3 RPM (B), and average HBV RPM mapping to the BCP (C) are displayed in relation to viral transcripts determined by primers in the core locus (pgRNA) (left), S locus (pg/S-RNA) (middle), and X locus (HBV-RNA) (right). Pearson correlation coefficients and P values for the linear regression are displayed, and the dashed lines indicate the 95% confidence interval of the linear regression. Color coding indicates disease stage according to Table 1. Squares indicate samples with low-quality sequencing results after both H3K4me3-ChIP as well as H3-ChIP (samples c162, c317, and c687), and triangles indicate samples with low quality after total H3-ChIP (samples c23, c537, and c770). Samples marked by squares were excluded in panel C to not bias localized analysis with low-quality sequencing results. The positions of the primer pairs (gray brackets) relative to the HBV genome and the transcripts measured by each pair are indicated at the bottom.
H3K9me3 can be deposited on HBV-DNA in vivo.
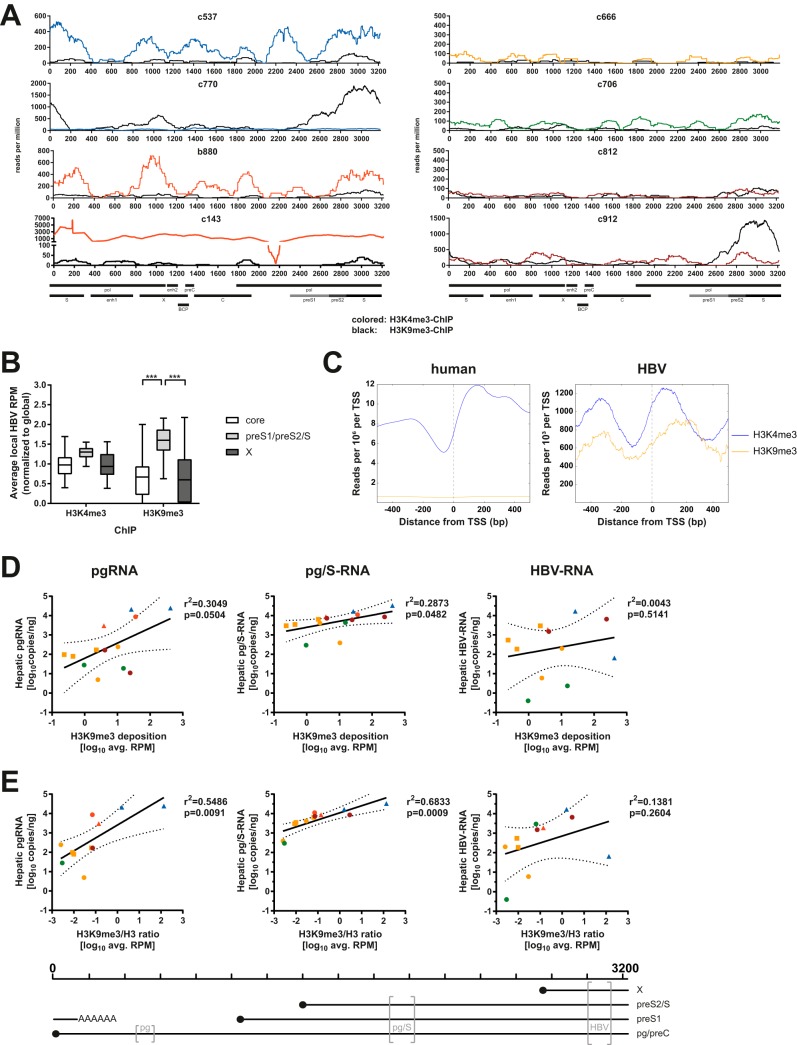
We next explored whether HBV-DNA may be shifted toward a heterochromatin state in patients with inactive CHB, as has been suggested by others looking at H3K9me3 on HBV-DNA (17). As discussed above, almost the entire HBV genome could be recovered after H3K9me3-ChIP in around half of the tested samples (Fig. 3D and Fig. 6A), and far less could be recovered in the others (Fig. 3D and data not shown), showing that deposition of inhibitory histone PTM can occur during the course of CHB in vivo. In contrast to literature findings (17), deposition of H3K9me3 did not differentiate samples from inactive carrier patients from samples from others in our cohort (Fig. 3D). In most samples with deposition of H3K9me3, a consistently low level was observed across the genome (e.g., samples b880, c537, and c812). In two samples, the deposition of H3K9me3 surpassed that of H3K4me3 locally (sample c912) or globally (samples c770). Interestingly, H3K9me3 deposition tended to be localized to the HBs locus, which is in contrast to patterns observed after ChIP for H3K4me3 (Fig. 6B). Comparing the pattern of deposition of histone PTMs around viral TSS to that for human TSS showed similarities but also differences between the two (Fig. 6C). In both cases, deposition of H3K4me3 was observed on the +1 nucleosome downstream of the TSS, as previously described (12). For HBV, H3K4me3 deposition appeared more pronounced upstream of the TSS. H3K9me3 appeared to be deposited in a pattern similar to that for H3K4me3 around the TSS on the viral genome, which was not observed for the human genome, where H3K9me3 mostly marks widespread areas of heterochromatin and thus does not enrich around TSS (7, 15). We thus sought to assess the impact of H3K9me3 on viral transcript levels. Surprisingly, we observed a weak but positive association between H3K9me3 deposition and levels of pgRNA as well as pg/S-RNA but not total HBV-RNA (Fig. 6D). These correlations were retained when analyzing the ratio of H3K9me3 to total H3 (Fig. 6E), and those for pg/S-RNA remained even if samples with low-quality sequencing data following total H3-ChIP were removed (data not shown). This indicates that H3K9me3 can be deposited on HBV-DNA in vivo but does not appear to be linked to lower levels of viral transcripts.
FIG 6.
Deposition of H3K9me3 on HBV-DNA in vivo and viral transcription. (A) Sequencing tracks comparing results after H3K9me3-ChIP (black) and H3K4me3-ChIP (colored according to disease stage [Table 1]) for samples with >70% HBV genome coverage after H3K9me3-ChIP (Fig. 1D). Lines represent the averages of data from two independently constructed libraries expressed as RPM. Select genomic features and ORFs are indicated at the bottom. (B) Box-and-whisker plot of average local HBV RPM after ChIP for H3K4me3 and H3K9me3, split according to genomic locus. Local averages were normalized to the global average to account for different HBV contents between libraries. Samples with low read counts after ChIP for both H3K4me3 as well as total H3 were excluded from the analysis to not bias the localized analysis with low-quality sequencing results. Bars extend to minimum and maximum values, boxes indicate quartiles, and central lines indicate the median. P values were calculated by two-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test. ***, P < 0.001. (C) Read distribution around TSS analyzed for HBV (left) and human (right) genomes. Reads were mapped in a 1,000-bp window around annotated TSS in either genome and averaged across all analyzed samples. (D and E) Average HBV RPM after H3K9me3-ChIP (D) and ratios of H3K9me3 RPM/total H3 RPM (E) in relation to viral transcripts determined by primers in the core locus (pgRNA) (left), S locus (pg/S-RNA) (middle), and X locus (HBV-RNA) (right). Pearson correlation coefficients and P value for the linear regression are displayed, and dashed lines indicate the 95% confidence interval of the linear regression. Color coding indicates disease stage according to Table 1. Squares indicate samples with low-quality sequencing results after both H3K4me3-ChIP as well as H3-ChIP (samples c162, c317, and c687), and triangles indicate samples with low quality after total H3-ChIP (samples c23, c537, and c770). The positions of the primer pairs (gray brackets) relative to the HBV genome and the transcripts measured by each pair are indicated at the bottom.
Integration of HBV-DNA and chromatin structure.
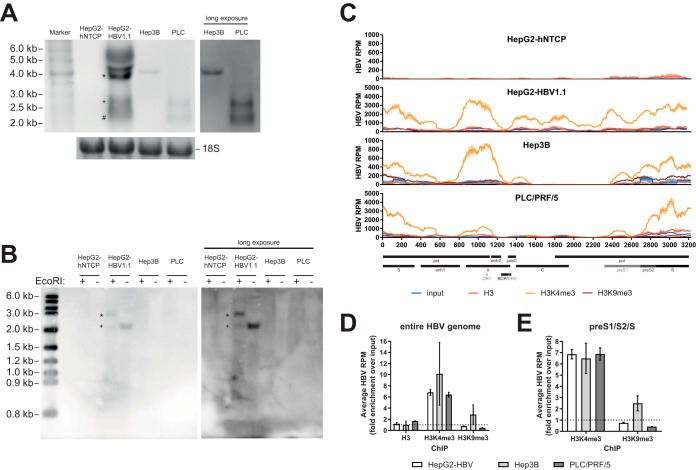
H3K9me3 has also been shown to silence sequences derived from endogenous retroviruses in the human genome (20). Since our ChIPseq approach is unable to differentiate integrated HBV sequences from cccDNA, we analyzed whether H3K9me3 may mark HBV-DNA integrated into the host genome. To this end, several cell lines with known integrated HBV sequences were used: Hep3B, PLC/PRF/5, as well as HepG2-HBV1.1 (12, 21, 22). Hep3B and PLC/PRF/5 are derived from patients with HBV-related hepatocellular carcinoma and known to produce HBsAg (21). HepG2-HBV1.1, a HepG2-based cell line with an integrated 1.1× HBV genotype D genome capable of producing infectious viral particles, was generated in-house and included as a control containing both HBV integrates as well as cccDNA (12). Additionally, we used HepG2-hNTCP cells as negative controls. As expected, HBV-derived transcripts were detected in HepG2-HBV1.1, Hep3B, and PLC/PRF/5 but not HepG2-hNTCP cells (Fig. 7A); cccDNA was detected only in HepG2-HBV1.1 cells (Fig. 7B, ∼2.1-kb band [+]; ∼3.2 kb after linearization by EcoRI [*]). Despite these differences, ChIPseq analysis of these cell lines revealed a consistent pattern of histone PTMs that matched the phenotype that we previously described in in vitro models (12) with a strong deposition of H3K4me3 (Fig. 7C and D). Under all conditions, Hep3B and PLC/PRF/5 cells lacked coverage of the C region, in line with literature findings and the suspected origin of integrated HBV-DNA from double-stranded linear DNA species (4, 5, 14, 23). A low level of deposition of H3K9me3 was observed on the preS1/preS2/S locus of Hep3B cells, even though it was exceeded by H3K4me3 deposition (Fig. 7E), and transcripts from this region were below the level of detection by Northern blotting in this cell line (∼2.1-kb and ∼2.4-kb transcripts) (Fig. 7A). Thus, H3K9me3 can be deposited on integrated HBV-DNA but is not obligatorily linked to chromosomal integration.
FIG 7.
Analysis of histone PTMs in cell lines with integrated HBV-DNA. (A) Northern blot of 10 μg total RNA per lane. The inset on the right shows a longer exposure of the same blot for Hep3B and PLC/PRF/5 (PLC) cells. The bottom inset shows methylene blue staining of 18S rRNA as a loading control. *, pregenomic RNA (∼3.5 kb); +, preS1 mRNA (∼2.4 kb); #, S mRNA (∼2.1 kb). Representative images from three independent experiments are shown. (B) Southern blot of 50 μg Hirt DNA per lane. All samples were denatured at 85°C prior to incubation at 37°C for 1 h with or without EcoRI, as indicated. The right panel shows a longer exposure of the same blot. *, linearized cccDNA (∼3.2 kb); +, cccDNA (∼2.1 kb). Representative images from two independent experiments are shown. (C) HBV-derived reads in the input, H3-ChIP, H3K4me3-ChIP, and H3K9me3-ChIP samples for four different cell lines. Select genomic features and ORFs are indicated at the bottom. (D) Fold enrichment over the input of averaged HBV RPM for HepG2-HBV1.1, Hep3B, and PLC/PRF/5 cells after the different ChIPs. Data are averaged from two independent sequencing runs. The dashed line indicates the level of input. (E) Bar diagram of average fold enrichment of HBV RPM over the input for the preS1/preS2/S locus after ChIP for H3K4me3 and H3K9me3. The dashed line indicates the level of input.
DISCUSSION
Epigenetic regulation has emerged as an important component in the life cycle of HBV in recent years and may open novel avenues for therapy of CHB (24). Indeed, we and others have demonstrated that interference with the activity of enzymes involved in the deposition or removal of histone PTMs can transcriptionally modulate HBV (11, 12, 25, 26). However, most reported studies with access to patient samples rely on ChIP-qPCR, limiting the resolution of histone PTM mapping (11, 26). Using a modified version of our previously reported ChIPseq assay, we show for the first time high-resolution mapping of histone PTMs on HBV-DNA from fine-needle liver biopsy specimens, which are the most commonly available clinical sample from HBV-infected liver of patients (12, 13). Applying this technology to a small cohort of patient-derived liver biopsy specimens, we find that despite the limited amount of material available (around 2.5 × 105 cells/sample), our technique allowed the identification of reproducible, high-quality sequencing results from the majority of samples tested.
Consistent with our previous work and other studies, we find that HBV-DNA is highly associated with histone PTMs linked to active transcription, in this case H3K4me3 (11, 12, 27). In our previous study, we observed a shared pattern of H3K4me3 deposition between different in vitro models as well as one patient-derived liver specimen obtained by surgical resection (12). While this pattern was preserved in some patients with HBeAg+ CHB, the majority of the analyzed CHB patient cohort showed differences in histone PTM deposition from the in vitro samples, more closely resembling acute HBV infection (28). Compared to human TSS, H3K4me3 was also strongly deposited upstream of viral TSS, but the significance of these findings is unclear, especially given the small size of the genome as well as its complex organization (19). We were able to observe a clear correlation of the deposition of H3K4me3 with levels of viral transcripts and could even show signs of a link of pgRNA levels to H3K4me3 deposition on the BCP. Furthermore, we observed only a loose correlation of hepatic cccDNA levels with hepatic pgRNA levels, further supporting the hypothesis of epigenetic regulation of pgRNA transcription. We also observed a weak association of hepatic HBV-DNA levels and serum viral loads with deposition of H3K4me3, which failed to reach statistical significance. This is likely due to the multiple other factors impacting these parameters further downstream of viral transcription, such as splicing, nuclear export, or stability of viral mRNAs, or the relative fitness of the individual virus infecting the patient (29, 30).
A surprising finding in the setting of CHB was the lack of H3K4me3 deposition in several patients with HBeAg− CHB. While a low HBV-DNA content in some patient biopsy samples resulted in read counts at the limit of detection of our assay, HBV-derived reads were still readily detectable after ChIP for total H3 in around half of the samples with low H3K4me3 deposition, confirming a lack of H3K4me3 deposition. The sporadic technical failures of H3-ChIP in some samples (e.g., samples c537 and c770) may mean that this phenotype could be even more widespread. The phenotype of low H3K4me3 levels relative to total H3 levels was observed in two out of three patients under NUC therapy. The third patient could not be analyzed for total H3 but showed a generally robust deposition of H3K4me3. The only HBeAg+ patient showing a lack of H3K4me3 was receiving NUCs (patient c809), yet the mechanism of action of NUCs as reverse transcriptase inhibitors makes direct interference with histone PTMs unlikely. Of note, patient c809 has a history of interferon-based therapy, which was shown to reduce levels of H3K4me3 on cccDNA in vitro and may thus be more likely to affect the epigenetic status of HBV-DNA (12, 25). While these results hint at differences in the epigenetic regulation of cccDNA between HBeAg+ and HBeAg− CHB, our data can only partially support such a hypothesis.
Recent studies have suggested that viral integration into the host genome is especially common in patients with HBeAg− stages of CHB (4, 5) and our techniques, as well as most other previously reported techniques for analysis of viral epigenetics in vivo, cannot readily distinguish HBV-derived sequences originating from integrated or episomal genome copies. Thus, a distinct histone PTM signature on integrated HBV-DNA could explain differences observed between the different stages of the disease. Our results using cell lines with known integration of viral DNA show a strong deposition of H3K4me3 on integrated HBV-DNA, comparable to previous results from cccDNA in vitro (12, 21, 22). However, since these cell lines were actively selected for HBV protein production (31), we cannot exclude that they were concomitantly selected for retaining the highly active histone PTM pattern that may not be associated with most viral integrants in vivo. Indeed, the extremely high read counts observed in sample c666 after ChIP for total H3 but not H3K4me3 could be explained by the presence of a clonal expansion of cells carrying integrated HBV, if this integration event should indeed lack H3K4me3. Due to the short nature of the mononucleosome-derived reads, we failed to robustly detect or quantify HBV/human chimeric reads as signs of viral integration in our data set using previously reported in silico tools (32, 33), and thus, further studies will be required to elucidate the interplay of viral epigenetics and integration in HBV.
Independent of disease stage, we observed no deposition of the inhibitory PTM H3K27me3 on HBV-DNA, consistent with our observations in vitro (12). Contrary to previous observations, however, we observed deposition of H3K9me3 on HBV-DNA in vivo, a histone PTM frequently associated with transcriptional silencing, heterochromatin assembly, as well as the suppression of endogenous retroviruses (20, 34, 35). Deposition of H3K9me3 occurred across the genome in around half of the samples analyzed but mostly at levels far below those of H3K4me3. In two samples, we observed a very strong and highly localized deposition of H3K9me3 at the HBs locus. To the best of our knowledge, this is the first description of the localized deposition of this PTM on HBV-DNA in vivo. These different patterns are unlikely to be due to cross-reactivity of the commercially available antibody used (Abcam), which has been widely used and was shown to not recognize similar histone PTMs such as H3K9me or H3K9me2 or unmodified H3K9. In vitro, H3K9me3 was deposited in a similarly localized fashion on the preS1/preS2/S locus of the integrated HBV-DNA in Hep3B cells, coinciding with low levels of transcripts derived from this locus. While this is in line with literature reports on the silencing of endogenous retroviruses by H3K9me3, it was not observed in other cell lines carrying integrated HBV-DNA or in other in vitro models (20). Furthermore, H3K9me3 was associated with HBV-DNA in a patient with immune-tolerant CHB (patient c770), a disease stage that has not been associated with significant levels of viral integration thus far (5). H3K9me3 was previously reported on cccDNA in vitro in the context of HBx deficiency, concomitant with a loss of histone PTMs linked to active transcription (16). Only one of the tested biopsy specimens (c770) showed a similar phenotype of strong H3K9me3 deposition and low deposition of H3K4me3. Furthermore, the lack of H3K4me3 was not always accompanied by deposition of H3K9me3 (e.g., sample c666), making a link to HBx activity alone an unlikely explanation for these samples. A recent study suggested elevated levels of H3K9me3 on HBV-DNA in patients with inactive CHB relative to those with HBeAg+ CHB (17); however, both low-level deposition as well as localized deposition of H3K9me3 were observed independent of disease stage in our cohort.
To our surprise, we observed a positive correlation between the amounts of viral transcripts and H3K9me3 deposition. This appears to be in contrast to the canonical role of H3K9me3 as a marker for transcriptionally silenced heterochromatin (7). While other copies of the viral genome present at the same time could be the source of the measured transcript, this does not easily explain the observed positive correlation. Of note, H3K9me3 has also been seen in association with the TSS of actively transcribed genes as well as linked to maintaining low-level transcription as part of bivalent chromatin domains marked by H3K4me3/H3K9me3 and H3K9me3/H3K36me2/3 (36, 37). We were able to detect both H3K4me3 and H3K9me3 on HBV-DNA in the same samples, although consecutive ChIP experiments would be necessary to prove that these PTMs indeed occur on the same nucleosomes. H3K9me3 was detectable on nucleosomes surrounding the TSS of HBV-DNA, in contrast to the pattern on the human genome. However, due to the extremely compact nature of the viral genome and the occurrence of several TSS (38), this proximity to the TSS may simply be coincidental. H3K9me3 has also been linked to exon inclusion in alternatively spliced genes (39). It is tempting to speculate that one role of H3K9me3 in the viral life cycle may be to reduce cotranscriptional splicing of HBV-derived transcripts, as it is crucial for a productive viral infection that HBV transcripts remain unspliced (29, 40, 41). Deposition of H3K9me3 correlated with HBV transcripts measured by pgRNA and especially pg/S-RNA primer pairs, both of which are disrupted by either of the major splice sites in the HBV genome (data not shown) (29). In contrast, total HBV-RNA includes all spliced transcripts and did not correlate with H3K9me3 deposition. While forming a tempting basis for speculation, further studies will be required to prove or disprove this hypothesis, especially since the primer pairs used would exclude only some, but not all, spliced transcripts, and levels of spliced transcripts could not be measured directly. Spliced HBV transcripts have been linked to viral immune evasion (42, 43); thus, factors affecting splicing of viral transcripts may be more readily detectable in vivo than in vitro.
In sum, we were able to establish a technique allowing for the first time a robust characterization of histone PTMs on HBV-DNA using the limited amount of material available after fine-needle liver biopsy, further enabling investigation of the epigenetic modulation of HBV-DNA in vivo. We find a reduced deposition of H3K4me3 on HBV-DNA in samples derived from HBeAg− stages of CHB and show that deposition of H3K4me3 in vivo is linked to levels of viral transcripts. Surprisingly, H3K9me3 can associate with HBV-DNA in vivo, and its deposition is positively correlated with viral transcript levels. Due to the available amount of material, we were limited in the number of experiments that we could perform and focused on H3K4me3 and H3K9me3, but it will be important to perform additional studies including other histone PTMs linked to active transcription, especially histone acetylations, which have been linked to viral replication in vivo (11). These studies could also clarify whether HBV-DNA in patients with HBeAg− stages of CHB is specifically depleted of H3K4me3 or whether this is observed for multiple PTMs linked to active transcription.
While there are some limitations to our technique, such as the addition of carrier chromatin prohibiting the analysis of the host epigenome, as well as the work with in vivo samples in general, such as the inability to distinguish between sequences derived from cccDNA and those derived from integrated viral DNA, we believe that this technique may prove valuable to determine biological correlates of response or nonresponse to epigenetic-based therapies for use as a potential biomarker (24). Even higher-quality sequencing results better suited for in-depth analyses can be obtained using larger samples obtained by surgical resection (12); however, sample availability, especially for HBeAg+ disease stages or during clinical trials, is limited compared to the availability of fine-needle liver biopsy specimens. Using our technique, it will be interesting to investigate correlates of response in patients receiving interferon, for which an effect on histone PTMs could be demonstrated in vitro (12, 25). Finally, follow-up experiments using our assay on material from patients who spontaneously resolved CHB may help elucidate whether this is due to elimination of infected cells or epigenetic silencing of persisting viral genomes. While these samples are rare and were not available in our cohort, their analysis may lead to a better understanding of what is required to cure CHB in vivo. Future studies should substitute the input material for a ChIP with nonspecific antibody to enable control of ChIP for total H3, determine the levels of spliced viral transcripts, and try to monitor the burden of viral integration to be able to assess its impact on histone PTMs on HBV-DNA.
Altogether, we established a technique for the analysis of the interactions of HBV-DNA with histone PTMs and other proteins in vivo and demonstrate its utility using a small cohort of fine-needle liver biopsy specimens to enable future studies using these samples.
MATERIALS AND METHODS
Patient samples.
Fine-needle liver biopsy specimens were collected prospectively from HBsAg+ patients presenting at the University Hospital Basel for diagnostic liver biopsy after written informed consent in accordance with the Declaration of Helsinki. The use of biopsy material was approved by the local ethics committee (Ethikkommission Nordwest- und Zentralschweiz, Basel, Switzerland). The biopsy specimens were acquired using a coaxial needle biopsy technique that allows single-stick, multiple-pass biopsies (BioPince; Peter Pflugbeil GmbH, Zorneding, Germany). After acquisition of the biopsy specimen, one cylinder was sent to the local pathology institute for routine diagnostic histological and immunohistochemical analyses, including staining for HBcAg and HBsAg. The other cylinder was snap-frozen and stored at −80°C for later processing. After thawing, material was split between experiments, as follows: 50% for ChIPseq, 25% for RNA extraction, and 25% for DNA extraction. Patient characteristics are listed in Table S1 in the supplemental material, and cohort characteristics are listed in Table 1. Patient clinical data were determined as described previously (44). The stage of chronic HBV infection in HBeAg+ patients was determined according to the extent of inflammation in the liver biopsy specimen (METAVIR grading of ≤1 and ISHAK grading of ≤3 for immune-tolerant CHB; METAVIR grading of >1 or ISHAK grading of >3 for immune-active CHB) (3). HBeAg− patients with viral loads of ≤2,000 IU/ml as well as an absence of histological inflammation (METAVIR grading of 0 and ISHAK grading of ≤3) qualified as having inactive CHB, whereas those with viral loads of >2,000 IU/ml or the presence of moderate to severe hepatic necroinflammation (METAVIR grading of >0 or ISHAK grading of >3) were considered to have HBeAg− CHB (3).
Nucleic acid extraction from biopsy specimens.
Total liver RNA was isolated from frozen biopsy tissue using TRIzol reagent (Ambion, Austin, TX) and DNase treated using the DNA-free DNA removal kit (Ambion) according to the manufacturer’s instructions. Low-molecular-weight DNA (containing cccDNA) and total DNA were isolated from one piece of biopsy tissue. Briefly, the frozen liver tissue was homogenized in homogenization buffer (50 mM Tris-HCl [pH 8.0], 1 mM EDTA, 0.2% Nonidet P-40 [NP-40], 0.15 M NaCl). Ninety percent and 10% of the homogenate were subsequently used for low-molecular-weight DNA (i.e., cccDNA) and total DNA extraction, respectively. Low-molecular-weight DNA was extracted by a modified Hirt supernatant protocol (45, 46), followed by exonuclease V (NEB, Ipswich, MA) digestion, as described previously (44, 47). For total DNA isolation, the homogenate was digested with proteinase K, followed by organic extraction, as described previously (44, 47). DNA and RNA were subjected to SYBR green real-time quantitative PCR (qPCR) and RT-qPCR, respectively, using the primers listed in Table 2, as previously described (44).
Cell culture.
The cell lines Hep3B and PLC/PRF/5 were obtained from the ATCC (Manassas, VA) and cultured according to the supplier’s instructions. HepG2 cells expressing human sodium taurocholate cotransporting polypeptide (hNTCP) (HepG2-hNTCP) and HepG2-HBV1.1 cells carrying an integrated 1.1-mer HBV genotype D genome were generated and cultured as described previously (12). Primary human hepatocytes were obtained from Thermo Fisher Scientific (Waltham, MA) and cultured according to the manufacturer’s instructions. Cells were infected with cell culture-derived HBV as described previously (12).
Chromatin isolation.
Biopsy specimens were thawed in prewarmed 0.05% trypsin–EDTA (Thermo Fisher Scientific, Waltham, MA) and mixed with 8 × 106 uninfected HepG2-hNTCP cells. Cells were fixed in freshly prepared 1% formaldehyde in phosphate-buffered saline (PBS) (both from Thermo Fisher Scientific) for 5 min prior to quenching with 125 mM glycine in PBS. Pelleted cells were either used directly for analysis or snap-frozen and stored at −80°C until use. Next, cells were washed with cold lysis buffer (PBS with 0.1% Triton X-100, 0.1% NP-40, 1 mM dithiothreitol [DTT], 50 ng/ml trichostatin A [TSA] to inhibit histone deacetylases, and 1× EDTA-free protease inhibitor [Roche, Basel, Switzerland]) and lysed in the same buffer for 10 min on ice. Nuclei were pelleted, resuspended in digestion buffer (H2O with 50 mM Tris-HCl [pH 7.5], 4 mM MgCl2, 1 mM CaCl2, 10% glycerol, 50 ng/ml TSA, and 1× EDTA-free protease inhibitor [Roche]), and digested with 600 IU/ml micrococcal nuclease (MNase; Thermo Fisher Scientific) for 12 min at 37°C. Digestion was stopped by the addition of 10 mM EDTA. Nuclei were pelleted at 6,500 × g, and supernatants were collected. The pellet was resuspended in digestion buffer with 10 mM EDTA and 300 mM NaCl and mildly sonicated using a W 375 sonicator (Qsonica, Newtown, CT) at 50% duty cycle and power setting 3. Nuclei were again pelleted, and supernatants were combined and mixed with an equal amount of sucrose buffer (H2O with 50 mM Tris-HCl [pH 7.5], 50 mM NaCl, 5 mM EDTA, 0.01% NP-40, 50 ng/ml TSA, and 1× EDTA-free protease inhibitor [Roche]). Samples were concentrated using Amicon Ultra-4 100-kDa centrifugal filter units (Millipore-Sigma, Merck KGaA, Darmstadt, Germany) and spun on a 5 to 30% continuous sucrose gradient in sucrose buffer for 4 h at 40,000 × g at 4°C using an SW41Ti rotor (Beckman Coulter Inc., Brea, CA). Chemicals were obtained from Millipore-Sigma unless noted otherwise. Mononucleosome-containing fractions were identified by agarose gel electrophoresis, pooled, and concentrated to ∼500 μl prior to the addition of 100 ng/μl bovine serum albumin (BSA; Thermo Fisher Scientific). Mononucleosomes were either stored at −20°C or used immediately for ChIP.
ChIPseq.
Antibodies (Table 4) were bound to 20 μl/ChIP Dynabeads protein G (Thermo Fisher) in ChIP buffer (H2O with 20 mM Tris-HCl [pH 8.0], 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, and 200 μg/ml BSA) at 4°C, added to 2 μg of chromatin in ChIP buffer, and allowed to bind overnight. Samples were washed six times in LiCl wash buffer (H2O with 150 mM LiCl, 50 mM Tris-HCl [pH 8.0], 1 mM EDTA, 1% NP-40, and 0.7% sodium deoxycholate) and eluted in 100 μl elution buffer (H2O with 1% SDS and 100 mM NaHCO3). Cross-links were reversed by digestion with proteinase K (Thermo Fisher) for 5 h at 65°C and then digested for another 30 min with DNase-free RNase A (Thermo Fisher) at 37°C. DNA was purified using the QIAquick PCR purification kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. A total of 0.5 ng of DNA was used for qPCR to determine the success of ChIP for H3K4me3, H3K27me3, and H3K9me3 using Fast SYBR green master mix (Thermo Fisher) and actin TSS-, nanog TSS-, and Sat2-specific primers, respectively (Table 2 and Fig. 2A), on a StepOnePlus qPCR machine (Thermo Fisher). One nanogram of DNA was used for sequencing library construction with the Kapa hyper prep kit (Roche) according to the manufacturer’s instructions. For barcoding, 2 μl of TruSeq RNA adapter stocks were used (Illumina Inc., San Diego, CA). Up to 6 libraries were pooled per sequencing run, and 500 ng of the pooled library was subjected to HBV-specific target enrichment using the xGen lockdown reagent hybridization and wash kit (Integrated DNA Technologies, Skokie, IL) according to the manufacturer’s instructions. A custom set of xGen lockdown probes with a length of 60 bp tiling the entire HBV genomes of genotypes A to D was used for target enrichment (Integrated DNA Technologies) (Table S2) together with Dynabeads MyOne T-270 streptavidin (Thermo Fisher). The pulldown was amplified for 12 or 8 PCR cycles (biopsy specimens versus de novo-infected cells/cell lines) (Kapa HiFi HotStart ReadyMix; Roche), and the product was cleaned up using Agencourt AMPure XP beads (Beckman Coulter Inc.). The eluted material was used to dilute an aliquot of the original library to 20 nM. The success of target enrichment was controlled by qPCR as outlined above, using actin TSS- and HBV_1-specific primers (Table 2). Typically, 104- to 105-fold target enrichment was observed (data not shown). For most samples, two independent libraries were constructed and subjected to HBV-specific target enrichment. Only single libraries were constructed for most samples from the input and H3K27me3-ChIP, due to low read counts and material constraints. Sequencing was performed using an Illumina MiSeq system with a MiSeq v3 reagent kit for 2x76-bp paired-end reads (Illumina Inc.).
TABLE 4.
Antibodies
| Target | Clone | Host | Manufacturer | Catalog no. |
|---|---|---|---|---|
| H3 | Polyclonal | Rabbit | Abcam (Cambridge, UK) | ab1791 |
| H3K4me3 | Polyclonal | Rabbit | Abcam | ab8580 |
| H3K9me3 | Polyclonal | Rabbit | Abcam | ab8898 |
| H3K27me3 | C36B11 | Rabbit | Cell Signaling Technology (Danvers, MA) | 9733S |
Bioinformatic analysis.
Since no clinical genotyping data were available, raw sequencing data in FASTQ format were aligned to 1.1-mer HBV reference genomes, of genotypes A to H (Table 5), shifted by 500 nucleotides (nt) compared to the EcoRI site using bowtie2 v2.3.0 (48). The no-discordant, no-mixed, and no-unal options were used, and the insert size was limited to between 120 and 200 bp (12). The resulting SAM file with the highest number of aligned reads was used for further analysis and is designated by genotype in Table S1 in the supplemental material. The best-matched genotype typically aligned twice the number of reads compared to the second best genotype. Using a custom script, paired reads were merged, and the gap between the two mapped reads was represented as “N” with low read quality to represent the entire sequence recovered by sequencing. This file was converted to BEDGRAPH format via BAM using bedtools v2.19.1 and samtools v1.7, respectively (49, 50). A custom script was used to wrap around reads in the repeated segment of the 1.1-mer reference genome to the beginning of the 1.0-mer reference genome, again shifted by 500 nt relative to the EcoRI site. HBV-derived reads were normalized to total read counts in the library (alignment to a reference genome combining human [hg38], HBV [respective genotype], and phiX sequences) (Table 5), and read counts per position per 106 total reads (reads per million [RPM]) were saved in WIG format. A custom python script was used to average the read counts and determine coverage and RPM for the entire genome and subregions (core, preS1/S2/S, and X). WIG files were visualized using GraphPad Prism 7.04 (GraphPad Software Inc., La Jolla, CA).
TABLE 5.
Reference genomesa
| Genome | GenBank accession no. or assembly no. | Description |
|---|---|---|
| HBV genotype A | EU594392.1 | |
| HBV genotype B | EF473976.1 | |
| HBV genotype C | EU410079.1 | |
| HBV genotype D | EU787447.1 | |
| HBV genotype E | AM494708.1 | |
| HBV genotype F | AY179734.1 | |
| HBV genotype G | AP007264.1 | |
| HBV genotype H | AY090460.1 | |
| HBV genotype D/ayw | V01460.1 | For de novo-infected samplesb |
| Human (hg38) | GRCh38.p10 | GenBank assembly |
| phiX | NC_001422.1 | NCBI reference sequence |
Representative HBV reference sequences were selected from a set of genomes used by the World Health Organization to inform the selection of a broad serotyping panel (57). For each genotype, reference genomes were ranked by the number of annotated proteins in the genome, and the reference genome with the most annotated proteins was selected as the representative.
See reference 12.
For TSS profiling, each HBV genome (Table 5) was concatenated with the human genome to create a pseudometagenomic FASTA sequence file, and a bowtie2 index was built from this metagenome sequence (48). The annotations for each HBV genome were combined with the human genome annotations as well into new, dual-species GFF files. Each pair of FASTQ sequence files was aligned to its corresponding HBV genotype’s pseudometagenome using bowtie2 with the same parameters as the ones indicated above, and the results were captured in BAM files (48). The resulting BAM files were merged into a single BAM file for sample replicates, and the merged BAM files were sorted by chromosome and genomic position and indexed for downstream processing using samtools (50). TSS proximity plots were generated using a custom-written python script using the HTSeq v0.9.1, NumPy v1.14.0, and Matplotlib v1.5.3 modules and run using the python v2.7.15 interpreter (51–53). Briefly, providing BAM files and the GFF annotations for the metagenomes to which sequences were aligned, TSS profiles were built for each sample. Profiles were built first by parsing the GFF annotation file to capture the first base of the first exon for each transcript. Viral transcripts and human transcripts were tabulated separately, and the statistics for each were computed independently, as the relative abundances of human and viral reads were skewed by the HBV genome enrichment process during library preparation. With the TSS identified genome-wide, alignments in the provided BAM files were parsed, and a normalized count was added across a user-provided interval representing the size of the insert (200 bp) for each position in a window of user-provided width around the TSS. The normalized count represents the number of aligned reads at that position per 103 reads. This depth-normalized count was divided by the number of different TSS represented in the profile and averaged over the number of samples.
Southern blotting.
Southern blotting was performed on Hirt-extracted DNA as described previously (54). DNA was loaded after heat denaturation at 85°C for 5 min and incubation at 37°C for 1 h in the presence or absence of 1 U/μl EcoRI (New England Biolabs, Ipswich, MA). The TurboBlotter kit (GE Healthcare, Little Chalfont, UK) and the DIG Northern starter kit (Roche, Basel, Switzerland) were used to develop the blot according to the manufacturers’ instructions. An RNA probe was generated from pGEM3Z-HBV linearized with ScaI (New England Biolabs); hybridization was performed at 50°C. Images were recorded on an Azure c600 imaging system (Azure Biosystems, Dublin, CA).
Northern blotting.
Total RNA was isolated using TRIzol reagent (Thermo Fisher) according to the manufacturer’s instructions. Gel electrophoresis and blotting were performed using a NorthernMax kit (Thermo Fisher) and a TurboBlotter kit (GE Healthcare). The blot was developed as described above for Southern blotting but using a hybridization temperature of 68°C.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism 7.04 (GraphPad Software Inc., La Jolla, CA). Horizontal lines in scatter plots indicate median values. In bar graphs, means ± standard deviations (SD) are displayed. All P values were calculated in a two-tailed manner and to a significance level of 95%. Tests are indicated in the figure legends.
Accession number(s).
Raw and processed files were deposited in the Gene Expression Omnibus (GEO) database under accession number GSE113879.
Supplementary Material
ACKNOWLEDGMENTS
We thank all patients for participation in the study. Furthermore, we thank Benjamin Madej for help with computational analysis. We thank Don E. Ganem for critical revision of the manuscript.
Financial support for M.-A.M., M.H.H., and S.F.W. was provided by Swiss National Science Foundation grants 310030B_14708 and 310030_166202. D.T.B., M.M.H., P.S.-C., and T.F. were funded by Novartis Institutes for Biomedical Research.
D.T.B., M.M.H., and P.S.-C. are employees of Novartis Institutes for Biomedical Research. T.F. is a postdoctoral fellow at Novartis Institutes for Biomedical Research.
M.M.H. and T.F. designed the study and wrote the manuscript. M.H.H. and S.F.W. obtained patient samples and helped with data interpretation. M.-A.M. determined levels of viral nucleic acids and collected patient clinical information. T.F. performed ChIPseq experiments and analysis. D.T.B. designed the probe sets and compiled viral reference genomes. P.S.-C. performed bioinformatic data analysis. All authors reviewed the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.02036-18.
REFERENCES
- 1.Trépo C, Chan HLY, Lok A. 2014. Hepatitis B virus infection. Lancet 384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 2.McMahon BJ. 2009. The natural history of chronic hepatitis B virus infection. Hepatology 49:S45–S55. doi: 10.1002/hep.22898. [DOI] [PubMed] [Google Scholar]
- 3.Terrault NA, Lok ASF, McMahon BJ, Chang K-M, Hwang JP, Jonas MM, Brown RS, Bzowej NH, Wong JB. 2018. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tu T, Budzinska MA, Shackel NA, Urban S. 2017. HBV-DNA integration: molecular mechanisms and clinical implications. Viruses 9:E75. doi: 10.3390/v9040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wooddell CI, Yuen M-F, Chan HL-Y, Gish RG, Locarnini SA, Chavez D, Ferrari C, Given BD, Hamilton J, Kanner SB, Lai C-L, Lau JYN, Schluep T, Xu Z, Lanford RE, Lewis DL. 2017. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci Transl Med 9:eaan0241. doi: 10.1126/scitranslmed.aan0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nassal M. 2015. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 64:1972–1984. doi: 10.1136/gutjnl-2015-309809. [DOI] [PubMed] [Google Scholar]
- 7.Bannister AJ, Kouzarides T. 2011. Regulation of chromatin by histone modifications. Cell Res 21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bock CT, Schranz P, Schröder CH, Zentgraf H. 1994. Hepatitis B virus genome is organized into nucleosomes in the nucleus of the infected cell. Virus Genes 8:215–229. doi: 10.1007/BF01703079. [DOI] [PubMed] [Google Scholar]
- 9.Bock CT, Schwinn S, Locarnini S, Fyfe J, Manns MP, Trautwein C, Zentgraf H. 2001. Structural organization of the hepatitis B virus minichromosome. J Mol Biol 307:183–196. doi: 10.1006/jmbi.2000.4481. [DOI] [PubMed] [Google Scholar]
- 10.Levrero M, Pollicino T, Petersen J, Belloni L, Raimondo G, Dandri M. 2009. Control of cccDNA function in hepatitis B virus infection. J Hepatol 51:581–592. doi: 10.1016/j.jhep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Pollicino T, Belloni L, Raffa G, Pediconi N, Squadrito G, Raimondo G, Levrero M. 2006. Hepatitis B virus replication is regulated by the acetylation status of hepatitis B virus cccDNA-bound H3 and H4 histones. Gastroenterology 130:823–837. doi: 10.1053/j.gastro.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Tropberger P, Mercier A, Robinson M, Zhong W, Ganem DE, Holdorf M. 2015. Mapping of histone modifications in episomal HBV cccDNA uncovers an unusual chromatin organization amenable to epigenetic manipulation. Proc Natl Acad Sci U S A 112:E5715–E5724. doi: 10.1073/pnas.1518090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mani H, Kleiner DE. 2009. Liver biopsy findings in chronic hepatitis B. Hepatology 49:S61–S71. doi: 10.1002/hep.22930. [DOI] [PubMed] [Google Scholar]
- 14.Staprans S, Loeb DD, Ganem D. 1991. Mutations affecting hepadnavirus plus-strand DNA synthesis dissociate primer cleavage from translocation and reveal the origin of linear viral DNA. J Virol 65:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. 2007. High-resolution profiling of histone methylations in the human genome. Cell 129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Rivière L, Gerossier L, Ducroux A, Dion S, Deng Q, Michel M-L, Buendia M-A, Hantz O, Neuveut C. 2015. HBx relieves chromatin-mediated transcriptional repression of hepatitis B viral cccDNA involving SETDB1 histone methyltransferase. J Hepatol 63:1093–1102. doi: 10.1016/j.jhep.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Ren J-H, Hu J-L, Cheng S-T, Yu H-B, Wong VKW, Law BYK, Yang Y-F, Huang Y, Liu Y, Chen W-X, Cai X-F, Tang H, Hu Y, Zhang W-L, Liu X, Long Q-X, Zhou L, Tao N-N, Zhou H-Z, Yang Q-X, Ren F, He L, Gong R, Huang A-L, Chen J. 2018. SIRT3 restricts HBV transcription and replication via epigenetic regulation of cccDNA involving SUV39H1 and SETD1A histone methyltransferases. Hepatology 68:1260–1276. doi: 10.1002/hep.29912. [DOI] [PubMed] [Google Scholar]
- 18.Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. 2007. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 130:77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glebe D, Bremer CM. 2013. The molecular virology of hepatitis B virus. Semin Liver Dis 33:103–112. doi: 10.1055/s-0033-1345717. [DOI] [PubMed] [Google Scholar]
- 20.Groh S, Schotta G. 2017. Silencing of endogenous retroviruses by heterochromatin. Cell Mol Life Sci 74:2055–2065. doi: 10.1007/s00018-017-2454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aden DP, Fogel A, Plotkin S, Damjanov I, Knowles BB. 1979. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature 282:615–616. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- 22.Alexander JJ, Bey EM, Geddes EW, Lecatsas G. 1976. Establishment of a continuously growing cell line from primary carcinoma of the liver. S Afr Med J 50:2124–2128. [PubMed] [Google Scholar]
- 23.Jain S, Chang T-T, Chen S, Boldbaatar B, Clemens A, Lin SY, Yan R, Hu C-T, Guo H, Block TM, Song W, Su Y-H. 2015. Comprehensive DNA methylation analysis of hepatitis B virus genome in infected liver tissues. Sci Rep 5:10478. doi: 10.1038/srep10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong X, Kim ES, Guo H. 2017. Epigenetic regulation of hepatitis B virus covalently closed circular DNA: implications for epigenetic therapy against chronic hepatitis B. Hepatology 66:2066–2077. doi: 10.1002/hep.29479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu F, Campagna M, Qi Y, Zhao X, Guo F, Xu C, Li S, Li W, Block TM, Chang J, Guo J-T. 2013. Alpha-interferon suppresses hepadnavirus transcription by altering epigenetic modification of cccDNA minichromosomes. PLoS Pathog 9:e1003613. doi: 10.1371/journal.ppat.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Chen J, Wu M, Zhang X, Zhang M, Yue L, Liu J, Li B, Shen F, Wang Y, Bai L, Protzer U, Levrero M, Yuan Z. 2017. PRMT5 restricts hepatitis B virus replication via epigenetic repression of cccDNA transcription and interference with pgRNA encapsidation. Hepatology 66:398–415. doi: 10.1002/hep.29133. [DOI] [PubMed] [Google Scholar]
- 27.Belloni L, Allweiss L, Guerrieri F, Pediconi N, Volz T, Pollicino T, Petersen J, Raimondo G, Dandri M, Levrero M. 2012. IFN-α inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Invest 122:529–537. doi: 10.1172/JCI58847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allweiss L, Dandri M. 2016. Experimental in vitro and in vivo models for the study of human hepatitis B virus infection. J Hepatol 64:S17–S31. doi: 10.1016/j.jhep.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Sommer G, Heise T. 2008. Posttranscriptional control of HBV gene expression. Front Biosci 13:5533–5547. [DOI] [PubMed] [Google Scholar]
- 30.Villet S, Billioud G, Pichoud C, Lucifora J, Hantz O, Sureau C, Dény P, Zoulim F. 2009. In vitro characterization of viral fitness of therapy-resistant hepatitis B variants. Gastroenterology 136:168.e2–176.e2. doi: 10.1053/j.gastro.2008.09.068. [DOI] [PubMed] [Google Scholar]
- 31.Knowles BB, Howe CC, Aden DP. 1980. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science 209:497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q, Jia P, Zhao Z. 2015. VERSE: a novel approach to detect virus integration in host genomes through reference genome customization. Genome Med 7:2. doi: 10.1186/s13073-015-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shieh F-S, Jongeneel P, Steffen JD, Lin S, Jain S, Song W, Su Y-H. 2017. ChimericSeq: an open-source, user-friendly interface for analyzing NGS data to identify and characterize viral-host chimeric sequences. PLoS One 12:e0182843. doi: 10.1371/journal.pone.0182843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SIS. 2001. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292:110–113. doi: 10.1126/science.1060118. [DOI] [PubMed] [Google Scholar]
- 35.Hyun K, Jeon J, Park K, Kim J. 2017. Writing, erasing and reading histone lysine methylations. Exp Mol Med 49:e324. doi: 10.1038/emm.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumura Y, Nakaki R, Inagaki T, Yoshida A, Kano Y, Kimura H, Tanaka T, Tsutsumi S, Nakao M, Doi T, Fukami K, Osborne TF, Kodama T, Aburatani H, Sakai J. 2015. H3K4/H3K9me3 bivalent chromatin domains targeted by lineage-specific DNA methylation pauses adipocyte differentiation. Mol Cell 60:584–596. doi: 10.1016/j.molcel.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 37.Mauser R, Kungulovski G, Keup C, Reinhardt R, Jeltsch A. 2017. Application of dual reading domains as novel reagents in chromatin biology reveals a new H3K9me3 and H3K36me2/3 bivalent chromatin state. Epigenetics Chromatin 10:45. doi: 10.1186/s13072-017-0153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altinel K, Hashimoto K, Wei Y, Neuveut C, Gupta I, Suzuki AM, Dos Santos A, Moreau P, Xia T, Kojima S, Kato S, Takikawa Y, Hidaka I, Shimizu M, Matsuura T, Tsubota A, Ikeda H, Nagoshi S, Suzuki H, Michel M-L, Samuel D, Buendia MA, Faivre J, Carninci P. 2016. Single-nucleotide resolution mapping of hepatitis B virus promoters in infected human livers and hepatocellular carcinoma. J Virol 90:10811–10822. doi: 10.1128/JVI.01625-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saint-André V, Batsché E, Rachez C, Muchardt C. 2011. Histone H3 lysine 9 trimethylation and HP1γ favor inclusion of alternative exons. Nat Struct Mol Biol 18:337–344. doi: 10.1038/nsmb.1995. [DOI] [PubMed] [Google Scholar]
- 40.Terré S, Petit MA, Bréchot C. 1991. Defective hepatitis B virus particles are generated by packaging and reverse transcription of spliced viral RNAs in vivo. J Virol 65:5539–5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosmorduc O, Petit MA, Pol S, Capel F, Bortolotti F, Berthelot P, Brechot C, Kremsdorf D. 1995. In vivo and in vitro expression of defective hepatitis B virus particles generated by spliced hepatitis B virus RNA. Hepatology 22:10–19. [PubMed] [Google Scholar]
- 42.Chen J, Wu M, Wang F, Zhang W, Wang W, Zhang X, Zhang J, Liu Y, Liu Y, Feng Y, Zheng Y, Hu Y, Yuan Z. 2015. Hepatitis B virus spliced variants are associated with an impaired response to interferon therapy. Sci Rep 5:16459. doi: 10.1038/srep16459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duriez M, Mandouri Y, Lekbaby B, Wang H, Schnuriger AA, Redelsperger F, Guerrera CI, Lefevre M, Fauveau V, Ahodantin J, Quetier I, Chhuon C, Gourari S, Boissonnas A, Gill U, Kennedy P, Debzi N, Sitterlin D, Maini MK, Kremsdorf D, Soussan P, Boissonas A, Gill U, Kennedy P, Debzi N, Sitterlin D, Maini MK, Kremsdorf D, Soussan P. 2017. Alternative splicing of hepatitis B virus: a novel virus/host interaction altering liver immunity. J Hepatol 67:687–699. doi: 10.1016/j.jhep.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meier MA, Suslov A, Ketterer S, Heim MH, Wieland SF. 2017. Hepatitis B virus covalently closed circular DNA homeostasis is independent of the lymphotoxin pathway during chronic HBV infection. J Viral Hepat 24:662–671. doi: 10.1111/jvh.12689. [DOI] [PubMed] [Google Scholar]
- 45.Hirt B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol 26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 46.Zhang YY, Summers J. 2000. Low dynamic state of viral competition in a chronic avian hepadnavirus infection. J Virol 74:5257–5265. doi: 10.1128/JVI.74.11.5257-5265.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wieland SF, Spangenberg HC, Thimme R, Purcell RH, Chisari FV. 2004. Expansion and contraction of the hepatitis B virus transcriptional template in infected chimpanzees. Proc Natl Acad Sci U S A 101:2129–2134. doi: 10.1073/pnas.0308478100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinlan AR, Hall IM. 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliphant TE. 2015. Guide to NumPy, 2nd ed CreateSpace Independent Publishing Platform, Austin, TX. [Google Scholar]
- 52.Anders S, Pyl PT, Huber W. 2015. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hunter JD. 2007. Matplotlib: a 2D graphics environment. Comput Sci Eng 9:90–95. doi: 10.1109/MCSE.2007.55. [DOI] [Google Scholar]
- 54.Cai D, Nie H, Yan R, Guo J, Block TM, Guo H. 2013. A Southern blot assay for detection of hepatitis B virus covalently closed circular DNA from cell cultures. Methods Mol Biol 1030:151–161. doi: 10.1007/978-1-62703-484-5_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang J, Xing K, Deng R, Wang J, Wang X. 2006. Identification of hepatitis B virus putative intergenotype recombinants by using fragment typing. J Gen Virol 87:2203–2215. doi: 10.1099/vir.0.81752-0. [DOI] [PubMed] [Google Scholar]
- 56.Panjaworayan N, Roessner SK, Firth AE, Brown CM. 2007. HBVRegDB: annotation, comparison, detection and visualization of regulatory elements in hepatitis B virus sequences. Virol J 4:136. doi: 10.1186/1743-422X-4-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chudy M, Hanschmann KM, Kress J, Nick S, Campos R, Wend U, Gerlich W, Nübling CM. 2012. First WHO International Reference Panel containing hepatitis B virus genotypes A-G for assays of the viral DNA. J Clin Virol 55:303–309. doi: 10.1016/j.jcv.2012.08.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.