Transmission activation, i.e., a viral response to the presence of vectors on infected hosts that regulates virus acquisition and thus transmission, is an only recently described phenomenon. It implies that viruses contribute actively to their transmission, something that has been shown before for many other pathogens but not for viruses. However, transmission activation has been described so far for only one virus, and it was unknown whether other viruses also rely on transmission activation. Here we present evidence that a second virus uses transmission activation, suggesting that it is a general transmission strategy.
KEYWORDS: transmission, insect vector, interaction, plant viruses
ABSTRACT
Cauliflower mosaic virus (CaMV; family Caulimoviridae) responds to the presence of aphid vectors on infected plants by forming specific transmission morphs. This phenomenon, coined transmission activation (TA), controls plant-to-plant propagation of CaMV. A fundamental question is whether other viruses rely on TA. Here, we demonstrate that transmission of the unrelated turnip mosaic virus (TuMV; family Potyviridae) is activated by the reactive oxygen species H2O2 and inhibited by the calcium channel blocker LaCl3. H2O2-triggered TA manifested itself by the induction of intermolecular cysteine bonds between viral helper component protease (HC-Pro) molecules and by the formation of viral transmission complexes, composed of TuMV particles and HC-Pro that mediates vector binding. Consistently, LaCl3 inhibited intermolecular HC-Pro cysteine bonds and HC-Pro interaction with viral particles. These results show that TuMV is a second virus using TA for transmission but using an entirely different mechanism than CaMV. We propose that TuMV TA requires reactive oxygen species (ROS) and calcium signaling and that it is operated by a redox switch.
IMPORTANCE Transmission activation, i.e., a viral response to the presence of vectors on infected hosts that regulates virus acquisition and thus transmission, is an only recently described phenomenon. It implies that viruses contribute actively to their transmission, something that has been shown before for many other pathogens but not for viruses. However, transmission activation has been described so far for only one virus, and it was unknown whether other viruses also rely on transmission activation. Here we present evidence that a second virus uses transmission activation, suggesting that it is a general transmission strategy.
INTRODUCTION
Transmission is an obligatory step in the life cycle of parasites but it is also an Achilles’s heel, because parasites must leave the comparably comfortable environment of the host they are installed in and face a potentially adverse environment during the passage to a new host. Some pathogens rely on resistant dormant states such as spores to persist in the “wild” until they reach a new host passively, e.g., carried by the wind. Most pathogens, however, actively use vectors for transmission, and they can manipulate both hosts and vectors in an impressive number of ways, all potentially increasing transmission (1–4). In the most sophisticated cases, pathogens “use exquisitely controlled mechanisms of environmental sensing and developmental regulation to ensure their transmission” (5). This concept, implying active contribution of the pathogen, is widely accepted for eukaryotic parasites (for example, plasmodium, schistosoma, wuchereria, and dicrocoelium), which developed fascinating transmission cycles to control and adapt vector-host or primary-secondary host interactions for their propagation (4). We have recently discovered a remarkable phenomenon for a virus. Cauliflower mosaic virus (CaMV; family Caulimoviridae) responds to the presence of aphid vectors on infected host plants by forming transmission morphs at the exact time and location of the plant-aphid contact (6). This process, coined transmission activation, or TA (7), is characterized by the formation of transmission complexes between CaMV virus particles and the transmission helper component (HC), the CaMV protein P2, which mediates vector binding (1). P2 and virus particles are spatially separated in infected cells, since the cell’s pool of P2 is retained in specific cytoplasmic inclusions called transmission bodies (TBs) while most virus particles are contained in another type of viral inclusion, the virus factories (8, 9). In such cells, aphid punctures trigger instant disruption of TBs, and the liberated P2 relocalizes onto microtubules. Simultaneously, the virus factories release virus particles that associate with P2 on the microtubules (10) to form P2/virus particle complexes, which is the virus form that aphid vectors can acquire and transmit. TA is transient; P2 reforms a new TB (6) and the virus particles return to virus factories (10) after aphid departure. TA implies that CaMV passes, induced by yet unknown mechanisms, from a nontransmissible to a transmissible state. It has been suggested that this phenomenon exists to economize host resources and to invest energy in transmission only when relevant, i.e., in the presence of vectors (7). Whether this hypothesis is true, inhibiting TA inhibits transmission, pointing to the importance of TA for CaMV. A fundamental question that arises is whether TA, which is reminiscent of the active transmission strategies employed by eukaryotic parasites, is exclusive to CaMV or whether it might be a general phenomenon in the virus world. Therefore, we studied transmission of the turnip mosaic virus (TuMV; family Potyviridae), which is entirely unrelated to CaMV but uses also an HC for aphid transmission. The HC of TuMV and of other potyviruses is the viral protein helper component protease (HC-Pro). It is a multifunctional protein that, other than its HC function, bears no structural, functional, or other similarity with P2. Our results show that TuMV is a second virus relying on TA for transmission but using a totally different mechanism.
RESULTS AND DISCUSSION
Signaling molecules modify TuMV transmission by aphids.
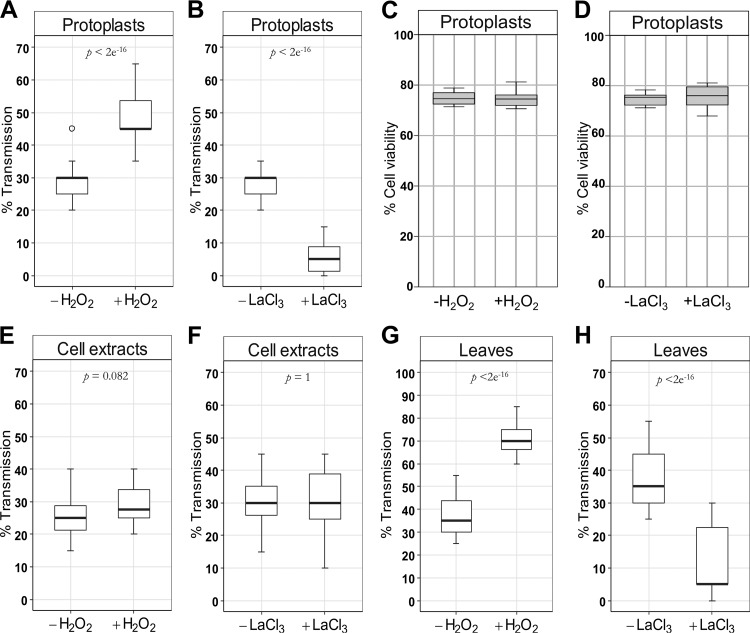
TA requires a signaling cascade that connects the initial recognition of the presence of aphids, most likely via a yet unknown elicitor, with a cellular response that is hijacked by the virus. Since TA is fast for CaMV and likely also for TuMV, which uses the same transmission mode, reactive oxygen species (ROS) or calcium are good signaling candidates. We therefore tested the effect of the ROS signaling compound hydrogen peroxide (H2O2) and of a general inhibitor of calcium signaling, lanthanum(III) chloride (LaCl3), on TuMV transmission, using infected protoplasts as virus source (6, 11). Aphid transmission tests performed with H2O2-treated protoplasts showed a drastic increase of TuMV transmission (Fig. 1A), whereas treatment of protoplasts with LaCl3 caused a strong reduction of transmission (Fig. 1B). This effect was not due to modified cell viability (Fig. 1C and D). Furthermore, transmission increase by H2O2 and inhibition by LaCl3 were clearly biological effects requiring living cells, since no effect was observed when the experiments were repeated using cell extracts, i.e., dead cells (Fig. 1E and F). The same control experiments indicated also that H2O2 and LaCl3 did not modify aphid feeding behavior, which might have been an alternative explanation for the observed differences in transmission rates. Taken together, our data show that TuMV transmission can be artificially enhanced or inhibited.
FIG 1.
Effect of H2O2 and LaCl3 on TuMV transmission by aphids. Turnip protoplasts were incubated for 5 min with 2 mM H2O2 (A) or 1 mM LaCl3 (B) and then employed in transmission assays. (C and D) Cell viability of protoplasts was measured to determine if the altered transmission rates were due to modified viability. Cell extracts from protoplasts were treated with H2O2 (E) or LaCl3 (F) and used in transmission assays. Leaves on intact plants were sprayed with 20 mM H2O2 (G) or 10 mM LaCl3 (H) and then employed in transmission assays. Means from the infected test plants (horizontal black bars in the box plots) were calculated from a pool of three independent experiments in which a total of 360 test plants were used per condition. Each experiment had 6 repetitions for each condition and 20 test plants per repetition (see Data Set S1 in the supplemental material for raw data). P values were obtained by generalized linear models (see Materials and Methods). The box plots here and in the other figures present medians with upper and lower quartiles, the ends of the whiskers present lowest and highest datum still within 1.5 interquartile range (IQR) of the lower and higher quartile, respectively, and the circles show outliers.
The protoplast system is a useful but simplified biological system, because the cells are individualized and not in their natural symplasmic context in a tissue. Hence, we sought to validate the protoplast results by using leaves on intact infected plants as virus source. We applied H2O2 or LaCl3 to leaves by spraying treatment (12) and used these plants for aphid transmission assays. To rule out any interference, only one leaf of the same developmental stage was sprayed on each plant, and different plants were used for each condition. H2O2 treatment increased significantly and LaCl3 treatment decreased significantly the plant-to-plant transmission rates of TuMV (Fig. 1G and H). This confirmed the results obtained with protoplasts and showed that TuMV TA is observed similarly in intact plants. Compared to those in the protoplast experiments, higher H2O2 and LaCl3 concentrations were required to observe significant effects. This was probably due to the dilution of these substances during leaf penetration. Combined, these results suggest that the TA phenomenon exists for TuMV, like for CaMV, and that calcium and ROS signaling might be important for TA of TuMV.
The increase in virus transmission correlates with the formation of HC-Pro/TuMV transmissible complexes.
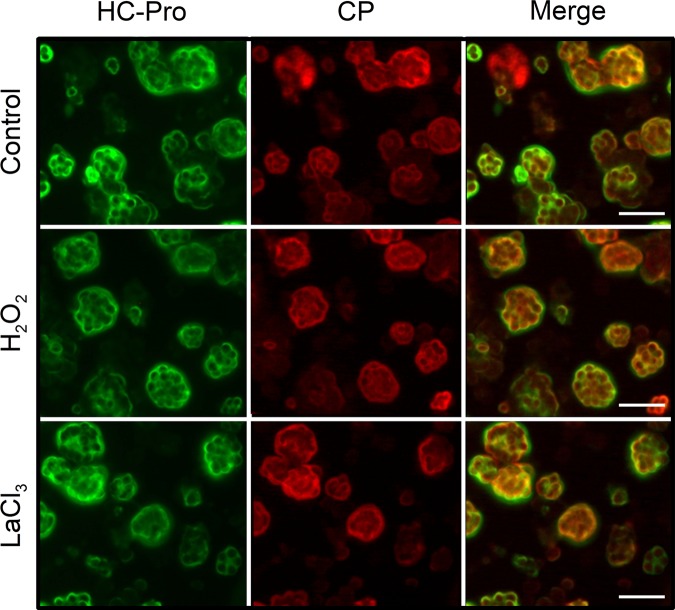
Next, we wanted to know how TuMV TA manifests itself in infected cells. TA of CaMV is characterized by the relocalization of CaMV particles and CaMV helper protein P2 from viral inclusions to microtubules (6, 10). Therefore, we performed immunofluorescence experiments on TuMV-infected protoplasts with antibodies directed against HC-Pro and the viral capsid protein CP to determine whether H2O2 and LaCl3 induced the relocalization of TuMV virus particles and/or HC-Pro. In untreated cells, HC-Pro and CP localized in the cytoplasm as reported for other potyviruses (13, 14). Treatment with H2O2 and LaCl3 did not induce any visible rearrangement of HC-Pro or of CP (Fig. 2). Thus, TA of TuMV is not characterized by the redistribution of HC-Pro and/or virus particles within infected cells.
FIG 2.
Immunofluorescence of turnip protoplasts infected with TuMV. TuMV-infected protoplasts were treated as indicated and double labeled against HC-Pro (green, left) and viral capsid protein CP (red, middle). Merge (right) represents superposition of HC-Pro and CP labels, with colabeling appearing in yellow. Control, untreated protoplasts; H2O2, incubation with 2 mM H2O2 for 15 min, LaCl3, incubation with 1 mM LaCl3 for 15 min. Scale bars, 50 μm.
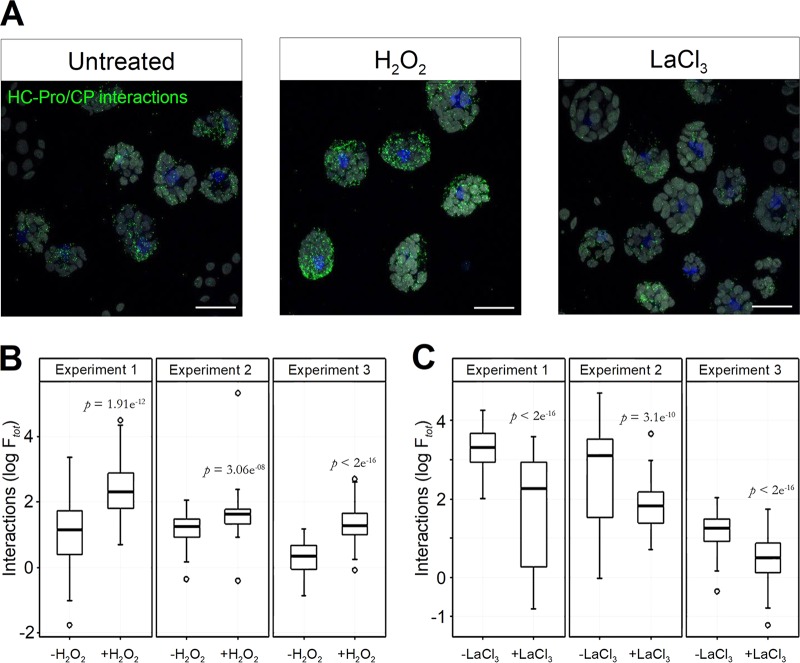
We thus hypothesized that HC-Pro and virus particles, both evenly distributed in the cytoplasm, could pass from a nonassociated state to an associated state, i.e., to transmissible HC-Pro-virion complexes, upon TA. To visualize such complexes in situ, we resorted to the Duolink technique (15), an antibody-based version of the proximity ligation assay allowing detection of intermolecular interactions. Duolink performed with HC-Pro and CP antibodies showed that H2O2 treatment indeed increased the number and intensity of HC-Pro/CP interaction spots (Fig. 3A and B), indicative of binding of HC-Pro to virus particles. Interestingly, incubation of protoplasts with LaCl3 decreased the number of transmissible complexes (Fig. 3C). Thus, the increase and decrease of HC-Pro/CP interactions, triggered by application of ROS or of a calcium channel blocker, respectively, correlated with an increase and decrease of transmission (compare Fig. 1 and 3).
FIG 3.
In situ Duolink proximity ligation assay on turnip protoplasts infected with TuMV. (A) Untreated control protoplasts or protoplasts incubated with either H2O2 or LaCl3 were processed by Duolink for detection of HC-Pro/TuMV particle interactions using HC-Pro and CP antibodies and corresponding Duolink probes. Interactions are visible as green fluorescing spots. Nuclei were counterstained with DAPI (blue), and chloroplast autofluorescence is presented in gray to reveal the cell lumen. Scale bars, 20 μm. Quantitative analysis of the Duolink signal shows that H2O2 increased (B) and LaCl3 decreased (C) HC-Pro/CP interactions. The box plots present data from three independent experiments using between 56 and 115 protoplasts for each condition. The y axes show HC-Pro/CP interactions, presented as total fluorescence intensity (Ftot). P values were obtained by generalized linear models (see Materials and Methods).
Transmission activation of TuMV is characterized by formation of cysteine bridges between HC-Pro molecules.
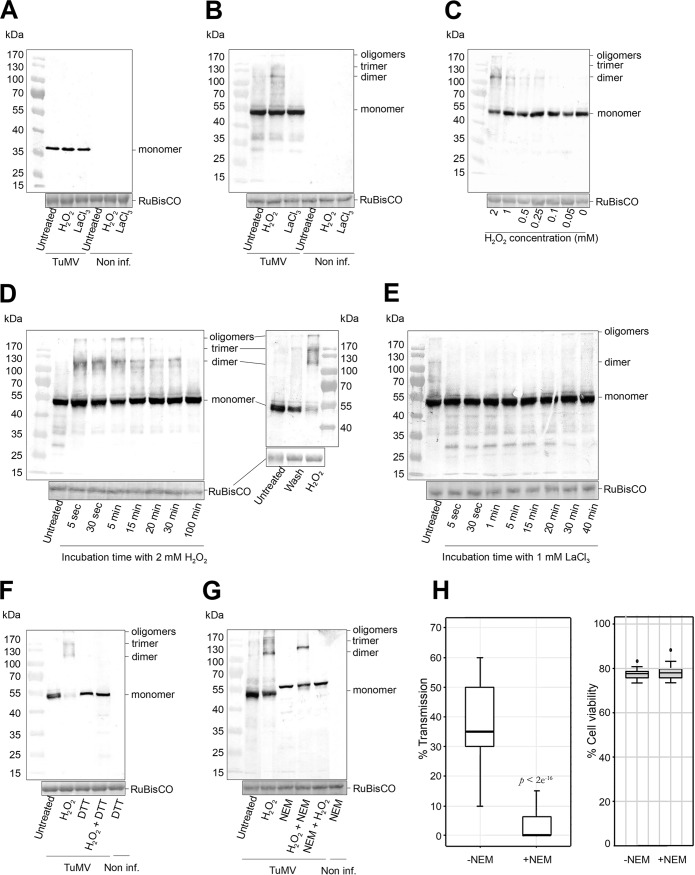
We wanted to understand how HC-Pro and virus particles could rapidly transit from “free” to virus-associated forms. Since ROS such as H2O2 change directly or indirectly the cellular redox potential, the formation of HC-Pro/TuMV transmissible complexes might be controlled by the redox state of HC-Pro and CP, both of which contain cysteine residues that can form disulfide bridges under oxidizing conditions. Therefore, we performed nonreducing SDS-PAGE/Western blotting to detect HC-Pro and CP migration profiles altered by intramolecular or intermolecular cysteine disulfide bridges. H2O2 and LaCl3 did not modify the migration profile of CP (Fig. 4A). However, H2O2 treatment increased the amount of oligomeric HC-Pro and especially of its dimeric form (Fig. 4B) that was previously reported to be active in transmission (16–18). LaCl3 treatment had the inverse effect and decreased the amount of HC-Pro oligomers (Fig. 4B). The effect of H2O2 was concentration dependent and clearly visible using physiologic H2O2 concentrations (0.25 mM) (Fig. 4C). Thus, the increase in transmission induced by H2O2 correlated not solely with formation of HC-Pro/TuMV complexes but also with the appearance of HC-Pro oligomers held together by intermolecular cysteine bridges.
FIG 4.
Nonreducing SDS-PAGE/Western blotting of HC-Pro and CP from TuMV-infected turnip protoplasts. The samples were lysed in a buffer without reducing agents to conserve the disulfide bridges. H2O2 and LaCl3 treatments did not modify the migration profile of the capsid protein (CP) (A), whereas they induced (H2O2) or inhibited (LaCl3) formation of HC-Pro oligomers (B). The concentration range and the kinetics of H2O2 incubation show that HC-Pro oligomerization was induced by a minimum concentration of 0.25 mM (C) and that it was rapid and reversible, either by extended H2O2 treatment (left) or by washing protoplasts (right) (D). (E) Inhibition of HC-Pro oligomerization by LaCl3 was also rapid and reversible. HC-Pro oligomers were formed by intermolecular disulfide bridges, because incubation of protoplasts with DTT, either alone or after H2O2 treatment, abolished HC-Pro oligomers (F), and treatment with NEM before but not after previous incubation with H2O2 prevented their formation (G). (H, left) Transmission tests using NEM-treated protoplasts show a drastic diminution of TuMV transmission. Transmission tests were performed three times using 320 plants per condition and analyzed by generalized linear models as described in Fig. 1. (Right) Protoplast viability assays show that NEM treatment did not change cell viability under the conditions used. TuMV, samples of TuMV-infected protoplasts; Non inf., samples of noninfected protoplasts; LaCl3, treatment with 1 mM LaCl3 for 5 min; H2O2, treatment with 2 mM H2O2 for 5 min; wash, H2O2 was removed by centrifugation and resuspension of protoplasts in fresh medium; DTT, treatment with 5 mM DTT for 30 min; NEM, treatment with 3 mM NEM for 20 min. Equal loading of lanes is shown by Ponceau S red staining of the large RuBisCO subunit. A precolored ladder and the molecular masses in kilodaltons are indicated at one side of each blot. P value in panel H obtained by generalized linear models from three independent experiments.
To have a biological significance, HC-Pro oligomerization should be completed within the duration of an aphid puncture, i.e., within seconds. Kinetics of formation and breakup of HC-Pro oligomers showed that both occurred within 5 s of incubation with H2O2 and LaCl3, respectively (Fig. 4D and E). The effects of both treatments were transient, because HC-Pro oligomers disappeared (H2O2) or reappeared (LaCl3) after ∼30 min of incubation. Furthermore, the removal of H2O2 by washing the protoplasts showed the reversibility of HC-Pro oligomerization (Fig. 4D). Induction of HC-Pro oligomers by H2O2 was not restricted to TuMV or to turnip hosts, because experiments with lettuce protoplasts infected with another potyvirus, lettuce mosaic virus (LMV), yielded similar results (not shown).
To better establish that the formation of disulfide bridges between HC-Pro monomers contributes to oligomerization, infected protoplasts were treated with the disulfide bond-reducing agent dithiothreitol (DTT) or with N-ethylmaleimide (NEM) that does not break existing disulfide bridges but prevents the formation of new ones by blocking free thiols. Figure 4F shows that DTT treatment abolished the appearance of H2O2-induced HC-Pro oligomers in an SDS-PAGE/Western blot. This confirmed that oligomerization of HC-Pro requires the establishment of intermolecular disulfide bridges. NEM treatment blocked the appearance of HC-Pro oligomers in SDS-PAGE/Western blots when applied before the H2O2 treatment, but NEM did not prevent their appearance when applied after H2O2 treatment (Fig. 4G). This is a further confirmation of the involvement of disulfide bridges in HC-Pro oligomerization. Note that NEM treatment caused a mobility shift of HC-Pro. This might have been due to disulfide shuffling during the denaturation of the samples as reported for papillomavirus (19). To establish a direct role of intermolecular HC-Pro disulfide bonds in TuMV transmission, we performed transmission assays. Because of the toxicity of NEM, we did not use plants as virus source but resorted to the protoplast system where exposure of aphids (and the experimenter) to the substance is minimized by confining it in the protoplast medium. The NEM treatment reduced virus transmission drastically (Fig. 4H, left) but did not affect protoplast viability (Fig. 4H, right), suggesting that de novo formation of intermolecular HC-Pro disulfide bonds is required for the formation of transmissible complexes and thus for aphid acquisition of TuMV.
Model of TuMV transmission activation.
In this study, we demonstrate that TA exists for a second virus, TuMV. TuMV TA was induced by the ROS H2O2 and inhibited by the calcium channel blocker LaCl3. ROS and calcium signaling are both important in early perception of parasites, including insects (20), and recently, aphid punctures were described to induce rapid calcium elevations around feeding sites (21). Since ROS and calcium signaling are often interconnected (22, 23), TuMV TA likely hijacks an early step of at least one of these pathways. The initial eliciting event remains unknown. It might be a direct effect of aphid saliva-contained ROS or ROS-producing peroxidases (24) that are injected into cells during feeding activity. Alternatively, an aphid or aphid-induced plant factor might interact in a classic pathogen-associated molecular pattern (PAMP)-triggered immunity reaction with a pattern recognition receptor (PRR) (25) that prompts calcium- and ROS-mediated downstream events. Interestingly, a recent study has demonstrated that the red clover necrotic mosaic virus (RCNMV) requires ROS for replication (26). The authors proposed that plant viruses may have evolved a complex mechanism to manipulate the ROS-generating machinery of plants to improve their infectivity, or, transferred to this case, transmission.
TA of TuMV manifests itself by the creation of HC-Pro intermolecular disulfide bridges, driven by oxidation of the cellular redox potential. We propose that oxidation of HC-Pro induces a functional switch rendering HC-Pro able to interact with virus particles and form transmissible complexes (Fig. 5). Functional switching (moonlighting) by the redox-driven modification of disulfide bridges has been reported for other proteins and is operated by conformation changes affecting the secondary, tertiary, or quaternary structure of proteins (27–29). Why would there be such a switch? HC-Pro is a multifunctional protein involved not only in aphid transmission (30) but also in pathogenicity (31), viral movement (32), and suppression of plant RNA silencing (33–35). One (or more) functional switch could assist to coordinate these multiple functions by allowing interaction with virions and formation of transmissible complexes only when transmission is possible, i.e., when the aphids puncture cells. This would help to economize finite plant resources as proposed earlier (7).
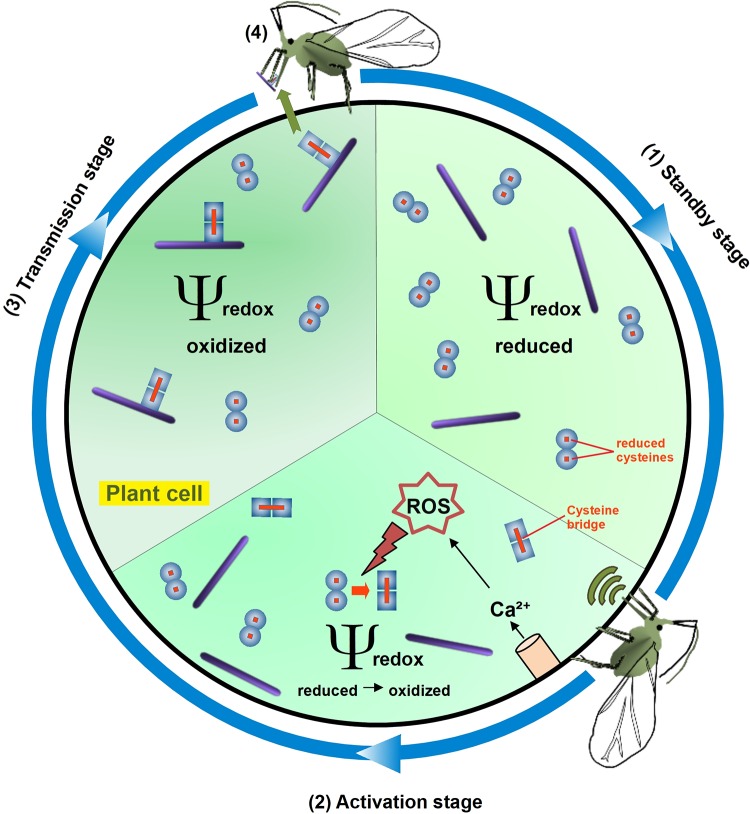
FIG 5.
Model of TuMV acquisition by aphids. For simplicity, aphids, viral components, and the plant cell are not drawn to scale. (1) Before the arrival of aphid vectors, the redox potential of the cytosol of TuMV-infected cells has “normal” values, i.e., it is reduced. Consequently, the cytosolic HC-Pro protein (blue circles) is in a reduced form (the red points in HC-Pro present reduced cysteines) and contains no intermolecular disulfide bridges. This form of HC-Pro is presumably not associated with virus particles (purple lines). It is likely but remains to be confirmed whether reduced HC-Pro is dimeric as presented here. (2) When an aphid feeds on a leaf infected with TuMV, an unknown elicitor is recognized by the plant cell and induces the opening of calcium channels (pink cylinder) and triggers directly or indirectly ROS production in the cell. During this activation stage, the ROS in the cytoplasm increases (red lightning) the redox potential of the cell cytoplasm and oxidizes one or more HC-Pro cysteines. This oxidation generates disulfide bridges (red lines) between different HC-Pro molecules. The intermolecular disulfide bridges either induce oligomerization of a portion of HC-Pro or change the conformation of a part of existing oligomers, presented by the transition of the circles to squares. For simplicity, higher HC-Pro forms are not shown. (3) Whatever the case, oxidation of a fraction of HC-Pro results in a functional switch of the protein, and the oxidized tertiary or quaternary conformation allows interaction between HC-Pro and TuMV particles and the formation of TuMV transmissible complexes, symbolized by square HC-Pro aligned with a virion. Now the infected cell is switched into transmission mode, and this stage allows efficient acquisition of TuMV. (4) The aphid acquires transmissible complexes and transmits the TuMV during the next puncture on another plant. After vector departure, the redox potential of cell cytoplasm lowers again and HC-Pro is reduced. This changes its conformation and induces dissociation of the transmissible complexes, leaving HC-Pro free to fulfill its other functions during infection. The aphid drawing is modified from reference 43, published under open CC3.0 license.
Unfortunately, we cannot provide empirical proof that the aphid punctures directly trigger TuMV TA. In contrast to CaMV, where TA was directly visible using qualitative immunofluorescence observation of P2 and virus particle networks (the characteristic manifestation of CaMV TA) in cells in contact with aphid saliva sheaths, TuMV TA cannot be revealed by a qualitative analysis. The quantitative Duolink approach we used to demonstrate TuMV HC-Pro/CP interactions in protoplasts required an enormous number of cells for analysis and statistical validation. Identifying a comparable number of cells in tissue and in contact with aphid stylets is barely feasible. The same restrictions apply to electron microscopy techniques to localize HC-Pro on virus particles by immunogold labeling. Thus, the proof of aphid implication in TA of TuMV remains indirect, for the time being.
Nonetheless, we here demonstrate TA for a second virus, TuMV, different from CaMV, suggesting that transmission activation might be a more general phenomenon. The great phylogenetic distance between TuMV and CaMV makes it likely that the phenomenon of TA arose independently for the two viruses during evolution. An obvious question is whether yet other viruses use TA for their transmission.
MATERIALS AND METHODS
Plants, viruses, and inoculation.
Turnip plants (Brassica rapa cv. Just Right) and lettuce (Lactuca sativa cv. Mantilla and Trocadero) were grown in a greenhouse at 24°C day/15°C night with a 14-h day/10-h night photoperiod. Two-week-old turnip plants were mechanically inoculated with wild-type TuMV strain C42J (36), and 2-week-old lettuce plants were inoculated with lettuce mosaic virus (LMV) strain E (37). Plants were used for experiments at 14 days postinoculation (dpi).
Isolation of protoplasts.
Protoplasts from turnip leaves were obtained by enzymatic digestion as described (6).
Preparation of infected cell extracts.
TuMV-infected turnip protoplasts were sedimented and resuspended in SAKO buffer (500 mM KPO4 and 10 mM MgCl2; pH 8.5) (38). Then, sucrose was added to a final concentration of 15%, and the suspension was vortexed to homogenize the protoplasts.
Drug treatments and cell viability assay.
For drug treatments of protoplasts, the following substances were added from stock solutions for the indicated times to 500 μl of protoplast suspension: 1 mM LaCl3 (5 min), 2 mM H2O2 (5 min), 3 mM NEM (20 min), and 5 mM DTT (30 min). Protoplasts were incubated at room temperature with gentle stirring (5 rpm). Fifteen minutes after the treatments, protoplast viability was determined with the fluorescein diacetate (FDA) test (39). For drug treatments of plants, one leaf per plant was sprayed with 10 mM LaCl3, 20 mM H2O2, or water, and the leaf, still attached to the plant, was used for transmission experiments after the applied solutions had evaporated.
Aphid transmission tests.
A nonviruliferous clonal Myzus persicae colony was reared under controlled conditions (22°C day/18°C night with a photoperiod of 14-h day/10-h night) on eggplant. The transmission tests using protoplasts were performed as described (6), with an acquisition access period of 15 min and transferring 10 aphids to each test plant. For plant-to-plant transmission tests, an acquisition time of 2 min was used and only one aphid was transferred on each turnip plant for inoculation. Infected plants were identified by visual inspection for symptoms 3 weeks after inoculation.
Antisera.
The following primary antibodies were used: commercial rabbit anti-TuMV (Sediag, Bretenière, France) and mouse and rabbit anti-HC-Pro (recognizing HC-Pro from different potyviruses, produced against the conserved peptide SEIKMPTKHHLVIGNSGDPKYIDLP by Proteogenix (Schiltigheim, France) and Eurogentec (Seraing, Belgium), respectively. The following secondary antibodies were used: Alexa Fluor 488 and Alexa Fluor 594 anti-rabbit and anti-mouse conjugates (Thermo Fisher Scientific, Waltham, MA) for immunofluorescence, anti-rabbit IgG conjugated to alkaline phosphatase (Sigma-Aldrich, St. Louis, MO) for Western blotting and corresponding Minus and Plus probes (Sigma-Aldrich, St. Louis, MO) for Duolink.
Immunofluorescence.
Protoplasts were fixed with 1% glutaraldehyde and processed as described (6). The primary and secondary antibodies were used at 1:100 and 1:200 dilutions, respectively.
Western blotting.
Drug treatments of protoplasts were stopped by lysing protoplasts in nonreducing 2× Laemmli buffer (vol/vol) (40) except where indicated otherwise. Optionally, oligomer formation was stabilized by incubating protoplasts with 3 mM NEM for 20 min before lysis. This step yielded sharper oligomer bands. Samples were then resolved by 10% SDS-PAGE. Proteins were transferred to nitrocellulose membranes and incubated with primary and secondary antibodies as described (6), except that TuMV-specific primary antibodies (1:1,000 dilution) were used. Antigens were then revealed by the nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (NBT/BCIP) reaction. Equal protein charges on the membranes were verified by coloring the RuBisCO with Ponceau S red.
Duolink proximity ligation assay.
In situ protein/protein interactions were detected by proximity ligation assay using the Duolink kit (Sigma-Aldrich, St. Louis, MO). Protoplasts were isolated from healthy or infected (14 dpi) turnip leaves and fixed with 3% paraformaldehyde in 100 mM cacodylate buffer (pH 7.2) or 100 mM phosphate buffer (pH 7.4). The fixed protoplasts were immobilized on l-polylysine-coated slides. Antibody incubation with rabbit anti-TuMV and mouse anti-HC-Pro, ligation, and probe amplification were performed according to the manufacturer’s instructions. The slides were mounted with Duolink in situ mounting medium with DAPI (4′,6-diamidino-2-phenylindole; Sigma-Aldrich, St. Louis, MO).
Microscopy.
Immunolabeled protoplasts were observed with an Olympus BX60 epifluorescence microscope (Olympus Corp., Tokyo, Japan) equipped with green fluorescent protein (GFP) and Texas Red narrow-band filters, and images were acquired with a color camera. Duolink images were acquired with a Zeiss LSM700 confocal microscope (Zeiss, Oberkochen, Germany) operated in sequential mode. DAPI was exited with the 405-nm laser, and fluorescence was collected from 405 to 500 nm; Duolink probes and chlorophyll were excited with the 488-nm laser, and fluorescence was collected from 490 to 540 nm (Duolink signal) or from 560 to 735 nm (chlorophyll autofluorescence). Raw images were processed using ZEN or ImageJ software. Quantification of Duolink interactions was performed on maximum intensity projections with the Analyze_Spots_Per_Protoplast macro for ImageJ, developed for this experiment (41).
Statistical analysis.
Statistics and box plots were calculated with R software version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria). Transmission rates and cell viability were analyzed with generalized linear models (GLM). Quasibinomial distributions were used in order to take overdispersion into account, and P values were corrected with the Holm method (42) to account for multiple comparisons.
Analyzing the Duolink experiments required the calculation of the total fluorescence intensity (Ftot) of labeled foci as
where n is the number of labeled foci, the average size of a focus, the average fluorescence intensity of a focus, and A the size of the protoplast. Ftot was log transformed (to normalize the distribution) and analyzed with linear models using “treatment” and “replicate” as categorical explanatory variables.
Supplementary Material
ACKNOWLEDGMENTS
We thank Takii Europe for providing turnip seeds. We also thank Albin Teulet for help with the box plots and Sophie Le Blaye for plant care.
All authors declare that there is no conflict of interest.
Our work is financed by the INRA SPE department, Agence Nationale de la Recherche (ANR), grant 12-BSV7-005-01 awarded to M.D., and grant RGP0013/2015 from the Human Frontier Science Program (HFSP), awarded to M.D. E.B. is supported by CIFRE PhD fellowship number 2015/1115, financed by the Association Nationale Recherche Technologie (anrt), Semences Innovation Protection Recherche et Environnement (SIPRE), and Fédération Nationale des Producteurs de Plants de Pomme de Terre (FN3PT).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.01822-18.
REFERENCES
- 1.Blanc S, Drucker M, Uzest M. 2014. Localizing viruses in their insect vectors. Annu Rev Phytopathol 52:403–425. doi: 10.1146/annurev-phyto-102313-045920. [DOI] [PubMed] [Google Scholar]
- 2.Dáder B, Then C, Berthelot E, Ducousso M, Ng JCK, Drucker M. 2017. Insect transmission of plant viruses: multilayered interactions optimize viral propagation. Insect Sci 24:929–946. doi: 10.1111/1744-7917.12470. [DOI] [PubMed] [Google Scholar]
- 3.Kuno G, Chang G-JJ. 2005. Biological transmission of arboviruses: reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin Microbiol Rev 18:608–637. doi: 10.1128/CMR.18.4.608-637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefèvre T, Adamo SA, Biron DG, Missé D, Hughes D, Thomas F. 2009. Invasion of the body snatchers: the diversity and evolution of manipulative strategies in host-parasite interactions. Adv Parasitol 68:45–83. doi: 10.1016/S0065-308X(08)00603-9. [DOI] [PubMed] [Google Scholar]
- 5.Matthews KR. 2011. Controlling and coordinating development in vector-transmitted parasites. Science 331:1149–1153. doi: 10.1126/science.1198077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinière A, Bak A, Macia J-L, Lautredou N, Gargani D, Doumayrou J, Garzo E, Moreno A, Fereres A, Blanc S, Drucker M. 2013. A virus responds instantly to the presence of the vector on the host and forms transmission morphs. Elife 2:e00183. doi: 10.7554/eLife.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drucker M, Then C. 2015. Transmission activation in non-circulative virus transmission: a general concept? Curr Opin Virol 15:63–68. doi: 10.1016/j.coviro.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Drucker M, Froissart R, Hébrard E, Uzest M, Ravallec M, Espérandieu P, Mani J-C, Pugnière M, Roquet F, Fereres A, Blanc S. 2002. Intracellular distribution of viral gene products regulates a complex mechanism of cauliflower mosaic virus acquisition by its aphid vector. Proc Natl Acad Sci U S A 99:2422–2427. doi: 10.1073/pnas.042587799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinoza AM, Medina V, Hull R, Markham PG. 1991. Cauliflower mosaic virus gene II product forms distinct inclusion bodies in infected plant cells. Virology 185:337–344. doi: 10.1016/0042-6822(91)90781-6. [DOI] [PubMed] [Google Scholar]
- 10.Bak A, Gargani D, Macia J-L, Malouvet E, Vernerey M-S, Blanc S, Drucker M. 2013. Virus factories of cauliflower mosaic virus are virion reservoirs that engage actively in vector transmission. J Virol 87:12207–12215. doi: 10.1128/JVI.01883-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinière A, Macia J-L, Bagnolini G, Jridi C, Bak A, Blanc S, Drucker M. 2011. VAPA, an innovative “virus-acquisition phenotyping assay” opens new horizons in research into the vector-transmission of plant viruses. PLoS One 6:e23241. doi: 10.1371/journal.pone.0023241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng H-C, Walker GP. 30 May 2018. Sieve element occlusion provides resistance against Aphis gossypii in TGR-1551 melons. Insect Sci doi: 10.1111/1744-7917.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baunoch DA, Das P, Hari V. 1990. Potato virus Y helper component protein is associated with amorphous inclusions. J Gen Virol 71:2479–2482. doi: 10.1099/0022-1317-71-10-2479. [DOI] [PubMed] [Google Scholar]
- 14.Riedel D, Lesemann D-E, Maiß E. 1998. Ultrastructural localization of nonstructural and coat proteins of 19 potyviruses using antisera to bacterially expressed proteins of plum pox potyvirus. Arch Virol 143:2133–2158. doi: 10.1007/s007050050448. [DOI] [PubMed] [Google Scholar]
- 15.Söderberg O, Gullberg M, Jarvius M, Ridderstråle K, Leuchowius K-J, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson L-G, Landegren U. 2006. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods 3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 16.Plisson C, Drucker M, Blanc S, German-Retana S, Le Gall O, Thomas D, Bron P. 2003. Structural characterization of HC-Pro, a plant virus multifunctional protein. J Biol Chem 278:23753–23761. doi: 10.1074/jbc.M302512200. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Ferrer V, Boskovic J, Alfonso C, Rivas G, Llorca O, López-Abella D, López-Moya JJ. 2005. Structural analysis of tobacco etch potyvirus HC-pro oligomers involved in aphid transmission. J Virol 79:3758–3765. doi: 10.1128/JVI.79.6.3758-3765.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thornbury DW, Hellmann GM, Rhoads RE, Pirone TP. 1985. Purification and characterization of potyvirus helper component. Virology 144:260–267. doi: 10.1016/0042-6822(85)90322-8. [DOI] [PubMed] [Google Scholar]
- 19.Modis Y, Trus BL, Harrison SC. 2002. Atomic model of the papillomavirus capsid. EMBO J 21:4754–4762. doi: 10.1093/emboj/cdf494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zebelo SA, Maffei ME. 2015. Role of early signalling events in plant-insect interactions. J Exp Bot 66:435–448. doi: 10.1093/jxb/eru480. [DOI] [PubMed] [Google Scholar]
- 21.Vincent TR, Avramova M, Canham J, Higgins P, Bilkey N, Mugford ST, Pitino M, Toyota M, Gilroy S, Miller AJ, Hogenhout SA, Sanders D. 2017. Interplay of plasma membrane and vacuolar ion channels, together with BAK1, elicits rapid cytosolic calcium elevations in Arabidopsis during aphid feeding. Plant Cell 29:1460–1479. doi: 10.1105/tpc.17.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeworutzki E, Roelfsema MRG, Anschütz U, Krol E, Elzenga JTM, Felix G, Boller T, Hedrich R, Becker D. 2010. Early signaling through the Arabidopsis pattern recognition receptors FLS2 and EFR involves Ca-associated opening of plasma membrane anion channels. Plant J 62:367–378. doi: 10.1111/j.1365-313X.2010.04155.x. [DOI] [PubMed] [Google Scholar]
- 23.Lachaud C, Da Silva D, Amelot N, Béziat C, Brière C, Cotelle V, Graziana A, Grat S, Mazars C, Thuleau P. 2011. Dihydrosphingosine-induced programmed cell death in tobacco BY-2 cells is independent of H2O2 production. Mol Plant 4:310–318. doi: 10.1093/mp/ssq077. [DOI] [PubMed] [Google Scholar]
- 24.Miles PW. 1999. Aphid saliva. Biol Rev 74:41–85. doi: 10.1017/S0006323198005271. [DOI] [Google Scholar]
- 25.Jones JDG, Dangl JL. 2006. The plant immune system. Nature 444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 26.Hyodo K, Hashimoto K, Kuchitsu K, Suzuki N, Okuno T. 2017. Harnessing host ROS-generating machinery for the robust genome replication of a plant RNA virus. Proc Natl Acad Sci U S A 114:E1282–E1290. doi: 10.1073/pnas.1610212114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan SW, George RA, Haworth NL, Feng LL, Liu JY, Wouters MA. 2009. Conformational changes in redox pairs of protein structures. Protein Sci 18:1745–1765. doi: 10.1002/pro.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mou Z, Fan W, Dong X. 2003. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113:935–944. doi: 10.1016/S0092-8674(03)00429-X. [DOI] [PubMed] [Google Scholar]
- 29.Zaffagnini M, Fermani S, Costa A, Lemaire SD, Trost P. 2013. Plant cytoplasmic GAPDH: redox post-translational modifications and moonlighting properties. Front Plant Sci 4:450. doi: 10.3389/fpls.2013.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Govier DA, Kassanis B. 1974. A virus induced component of plant sap needed when aphids acquire potato virus Y from purified preparations. Virology 61:420–426. doi: 10.1016/0042-6822(74)90278-5. [DOI] [PubMed] [Google Scholar]
- 31.Atreya CD, Atreya PL, Thornbury DW, Pirone TP. 1992. Site-directed mutations in the potyvirus HC-Pro gene affect helper component activity, virus accumulation, and symptom expression in infected tobacco plants. Virology 191:106–111. doi: 10.1016/0042-6822(92)90171-K. [DOI] [PubMed] [Google Scholar]
- 32.Cronin S, Verchot J, Haldeman-Cahill R, Schaad MC, Carrington JC. 1995. Long-distance movement factor: a transport function of the potyvirus helper component proteinase. Plant Cell 7:549–559. doi: 10.1105/tpc.7.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anandalakshmi R, Marathe R, Ge X, Herr JM, Mau C, Mallory A, Pruss G, Bowman L, Vance VB. 2000. A calmodulin-related protein that suppresses posttranscriptional gene silencing in plants. Science 290:142–144. doi: 10.1126/science.290.5489.142. [DOI] [PubMed] [Google Scholar]
- 34.Brigneti G, Voinnet O, Li WX, Ji LH, Ding SW, Baulcombe DC. 1998. Viral pathogenicity determinants are suppressors of transgene silencing in Nicotiana benthamiana. EMBO J 17:6739–6746. doi: 10.1093/emboj/17.22.6739. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Kasschau KD, Carrington JC. 1998. A counterdefensive strategy of plant viruses: suppression of posttranscriptional gene silencing. Cell 95:461–470. doi: 10.1016/S0092-8674(00)81614-1. [DOI] [PubMed] [Google Scholar]
- 36.Ohshima K, Tomitaka Y, Wood JT, Minematsu Y, Kajiyama H, Tomimura K, Gibbs AJ. 2007. Patterns of recombination in turnip mosaic virus genomic sequences indicate hotspots of recombination. J Gen Virol 88:298–315. doi: 10.1099/vir.0.82335-0. [DOI] [PubMed] [Google Scholar]
- 37.Revers F, Yang SJ, Walter J, Souche S, Lot H, Le Gall O, Candresse T, Dunez J. 1997. Comparison of the complete nucleotide sequences of two isolates of lettuce mosaic virus differing in their biological properties. Virus Res 47:167–177. doi: 10.1016/S0168-1702(96)01411-6. [DOI] [PubMed] [Google Scholar]
- 38.Sako N, Ogata K. 1981. Different helper factors associated with aphid transmission of some potyviruses. Virology 112:762–765. doi: 10.1016/0042-6822(81)90322-6. [DOI] [PubMed] [Google Scholar]
- 39.Widholm JM. 1972. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol 47:189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]
- 40.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 41.Baecker V. 2016. Analyse spots per protoplast. ImageJ-macros. MRI’s Redmine. http://dev.mri.cnrs.fr/projects/imagej-macros/wiki/Analyse_Spots_Per_Protoplast.
- 42.Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70. [Google Scholar]
- 43.Bak A, Martinière A, Blanc S, Drucker M. 2013. Early interactions during the encounter of plants, aphids and arboviruses. Plant Signal Behav 8:e24225. doi: 10.4161/psb.24225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.