Humans may experience repeated infections caused by the same serotype of respiratory syncytial virus (RSV), in contrast to infections with most other viruses, indicating that immune memory responses to RSV are defective. However, the effects of any residual but nonprotective immunity on responses to RSV vaccines are not clear. This study demonstrates that a VLP vaccine candidate containing a stabilized prefusion F protein can robustly stimulate protective immunity in animals previously infected with RSV, while a second RSV infection or a postfusion F-containing VLP cannot. This result shows that a properly constructed immunogen can be an effective vaccine in animals previously infected with RSV. The results also suggest that the defect in RSV memory is not in the induction of that memory but rather in its activation by a subsequent RSV infection.
KEYWORDS: F protein, virus-like particles, neutralizing antibodies, respiratory syncytial virus
ABSTRACT
Most individuals are infected with respiratory syncytial virus (RSV) by age two, but infection does not result in long-term protective immunity to subsequent infections. Previous RSV infection may, however, impact responses to an RSV vaccine. The goal of these studies was to explore the effect of previous RSV infection on murine antibody responses to RSV F and G protein-containing virus-like particles (VLP), comparing responses to those resulting from VLP immunization of RSV-naive animals. These studies showed that after RSV infection, immunization with a single dose of VLPs containing a conformation-stabilized prefusion F protein stimulated high titers of neutralizing antibodies (NA), while an immunization with post-F-containing VLPs or a second RSV infection only weakly stimulated NA, even though total anti-F protein IgG antibody levels in both VLP-immunized animals were similar. Furthermore, single pre-F or post-F VLP immunization of animals previously infected (primed) with RSV resulted in total anti-F antibody titers that were 10- to 12-fold higher than titers after a VLP prime and boost of RSV-naive animals or after two consecutive RSV infections. The avidities of serum antibodies as well as numbers of splenic B cells and bone marrow cells after different immunization protocols were also assessed. The combined results show that RSV infection can quite effectively prime animals for the production of protective antibodies that can be efficiently activated by a pre-F VLP boost but not by a post-F VLP boost or a second RSV infection.
IMPORTANCE Humans may experience repeated infections caused by the same serotype of respiratory syncytial virus (RSV), in contrast to infections with most other viruses, indicating that immune memory responses to RSV are defective. However, the effects of any residual but nonprotective immunity on responses to RSV vaccines are not clear. This study demonstrates that a VLP vaccine candidate containing a stabilized prefusion F protein can robustly stimulate protective immunity in animals previously infected with RSV, while a second RSV infection or a postfusion F-containing VLP cannot. This result shows that a properly constructed immunogen can be an effective vaccine in animals previously infected with RSV. The results also suggest that the defect in RSV memory is not in the induction of that memory but rather in its activation by a subsequent RSV infection.
INTRODUCTION
Respiratory syncytial virus (RSV) is an agent of acute viral respiratory disease, which is particularly serious for three different populations. The virus is a common cause of severe acute viral lower respiratory tract disease in infants and young children worldwide (1). In the United States, RSV infections frequently result in hospitalization, and in developing countries the infections cause significant mortality rates (1, 2). In the elderly, RSV infections cause morbidity and mortality that rivals that of influenza infections (3–6). Mortality due to RSV infection in stem cell transplant patients is estimated between 6% and 80% (7, 8). RSV infections also produce significant morbidity in normal adult populations (9). Despite the importance of RSV infections in human health, there are no vaccines available, although many candidates have been evaluated in preclinical and clinical studies over several decades.
One major issue that has complicated management of RSV disease and vaccine development is the failure of natural RSV infection to protect from subsequent infections. Thus, humans may experience repeated RSV infections caused by the same virus serogroup over several years or even within the same season (9–11), indicating that human memory responses to RSV infection are defective (11).
A significant related problem is that most people have been infected with RSV by 2 years of age (12). Any preexisting immunity, while poorly protective, may well impact immune responses to a vaccine. Successful vaccine candidates must stimulate high titers of neutralizing antibody in the context of preexisting immunity. Results of studies of efficacy of vaccine candidates in naive animal models may not directly bear on human responses, which will virtually always be in the context of previous infection.
Furthermore, in early studies, the role of RSV F protein conformation in vaccine candidates for stimulation of protective antibody responses was not appreciated. Like many viral fusion proteins, the RSV F protein is folded into a metastable, prefusion conformation which, upon fusion activation, refolds into a structurally very different postfusion conformation (13–17). The prefusion form of F protein is most effective in inducing optimally neutralizing antibodies (17, 18). However, virtually all early candidates contained only the postfusion form of F protein (19). Indeed, there are still ongoing clinical trials using the postfusion form of the F protein (20).
We have developed novel virus-like particle (VLP) vaccine candidates for RSV (21–24). Properties of VLPs make them ideal vaccine candidates for many pathogens. VLPs do not need the addition of adjuvant for potent immune responses, in contrast to soluble proteins (25). Because production of VLPs does not require viral replication, different conformational forms of antigens, such as a stabilized prefusion F protein or a stabilized postfusion F protein, can be assembled into VLPs, in contrast to attenuated viruses, which must remain infectious. VLPs are also safer as vaccines for many populations than infectious, attenuated, or vector viruses, since they do not contain a genome and do not produce a spreading infection. We have recently reported that immunization with a VLP vaccine candidate containing a stabilized prefusion F protein resulted in robust neutralization titers, in both naive mice and cotton rats (23, 24), and higher titers than those after immunization with VLPs containing the postfusion F protein.
Using prefusion F- and postfusion F-containing VLPs, we assessed the influence of previous RSV infections on neutralizing antibody production in mice. We have reported that, in RSV-experienced mice, a single injection of a prefusion F-containing VLP stimulated high titers of neutralizing antibodies compared to those for postfusion F-containing VLPs or a second RSV infection (26). Here, we further characterized the immune response after VLP immunization of RSV-experienced mice by examining the durability of neutralizing antibody responses as well as levels, avidity, and durability of total anti-F protein IgG antibodies and the numbers of F protein-specific bone marrow-associated long-lived plasma cells and splenic memory B cells. Responses were compared to those of naive mice immunized with a prime and boost with VLPs. Our results show that immune responses to VLP immunization are different in RSV-experienced mice than in RSV-naive mice. Furthermore, our results suggest that RSV infection can effectively prime animals for the production of high titers of protective antibodies that can be efficiently induced by a pre-F VLP boost but not by a post-F VLP boost or a second RSV infection.
RESULTS
Infection and immunization.
Two different VLPs, based on the Newcastle disease virus (NDV) core proteins M and NP and containing the RSV G and F protein ectodomains, were used as immunogens. The RSV glycoproteins were assembled into these VLPs by constructing chimera protein genes composed of ectodomains of the G or F glycoproteins (from RSV, strain A2) fused to the transmembrane (TM) and cytoplasmic (CT) domains of the NDV HN protein or NDV F glycoprotein, respectively, as previously described (22, 26). Both VLPs contained the same RSV G protein ectodomain as an H/G chimera protein and in the same amounts (26). The VLPs differed in the conformation of assembled F protein. One VLP contained the ectodomain of the mutation-stabilized prefusion RSV F protein (DS-Cav1) described by McLellan et al. (18) as an RSV pre-F/NDV F chimera protein (Pre-F/F), while the other VLP contained the ectodomain of the mutation-stabilized postfusion F protein as an RSV post-F/NDV F chimera protein (Post-F/F) (16, 27). The levels of Pre-F/F and Post-F/F proteins in the two VLPs were identical (26). The generation, purification, and characterization of these VLPs, abbreviated here as Pre-F VLPs (VLP-H/G+Pre-F/F) and Post-F VLPs (VLP-H/G+Post-F/F), have been previously described (23, 24, 26).
To assess properties of antibody responses in VLP-immunized mice previously infected with RSV (strain A2), three groups of five mice each were primed by intranasal infection with RSV (Fig. 1A). After 14 weeks, one group was immunized (boosted) with Pre-F VLPs, another group boosted with Post-F VLPs, and a third group received a second RSV infection. Serum samples were acquired from 0 to 17 weeks postboost. Mice were then challenged with RSV at 21 weeks and sacrificed 4 days later (Fig. 1A). This experiment was carried out two separate times for a total of 10 animals per group.
FIG 1.
Experimental protocol. Shown is the timing of RSV, strain A2, infections and VLP immunizations in RSV-primed (infected) (A) and VLP-primed (B) animals. VLP boosts of RSV-primed animals and VLP-primed animals were at 14 weeks postpriming. Upward arrows indicate times of acquisition of serum samples. The first bleed (t = 0 weeks postboost) was at the time of VLP boosts. At week 21 (RSV primed) or week 15 (VLP primed), animals were infected with RSV, and 4 days later they were sacrificed and terminal bleeds and lungs harvested.
For comparison, a set of RSV-naive mice received a VLP prime followed by a VLP boost, after 14 weeks, with either Pre-F VLPs or Post-F VLPs (Fig. 1B). Serum samples were obtained from these mice 0 to 15 weeks postboost. This experiment was done three separate times for a total of 15 animals per group.
Neutralization titers in RSV-primed and VLP-primed animals.
Since neutralizing antibody (NA) is a key indicator of an effective vaccine, the levels and durability of NA after different immunization protocols were determined. NA titers were measured in an in vitro plaque reduction assay, using pooled sera obtained at different times after the VLP boosts. In RSV-experienced (primed) mice, Pre-F VLP immunization resulted in significantly higher neutralization titers than Post-F VLP immunization (6.4-fold higher titers at 4 weeks postboost) and 13-fold higher titers than after RSV prime and boost immunization (Fig. 2A and C). The Post-F VLP boost stimulated NA titers slightly but still significantly more so than a second RSV infection (Fig. 2C). The results indicate that RSV infection can quite effectively prime for protective NA, since Pre-F VLPs can robustly boost these antibodies while Post-F VLPs cannot efficiently boost them. Importantly, a second RSV infection also cannot boost these NA antibodies effectively, suggesting that RSV infections are defective in activating the primed NA.
FIG 2.
Neutralizing antibody titers. Neutralizing antibody titers with time after boosts of RSV-primed (set 1) and VLP-primed (set 2) animals are shown in panels A and B, respectively. Aliquots of sera from each group of animals acquired at each time point after the VLP or RSV boost were pooled. The experiment was accomplished twice for the RSV-primed animals (10 animals/group) and three times for the VLP-primed animals (15 animals/group). Serum from each experiment at each time point was pooled separately and titers of each pool determined separately. The NA titers are the reciprocal of the dilution of sera resulting in a 50% reduction of virus titer in a plaque reduction assay. The results are averages from 4 to 6 separate determinations on each pool. Since the results from each of the pools from equivalent groups were not statistically different, the results were combined. The averages and standard deviations are shown. Note the change in y axis scales to visualize lower titers. (C) Titers in each group of animals in the two sets of mice at week 4 after the VLP boost with statistically significant differences in values for between groups within a set and between groups in the two sets of animals. The significance of results at 17 weeks postboost is indicated in panel A. There were no significant differences at late times in VLP-primed animals. (D) RSV, strain B, neutralization titers using sera from animals primed with either RSV or with pre-F VLPs, boosted with pre-F VLPs, and harvested at 4 weeks postboost. Results are from pools of sera from two separate experiments, and the assay with each pool was accomplished twice. *, P = 0.05; **, P = 0.005; ***, P = 0.0005.
Neutralizing antibody titers in all groups declined after week 4 postboost, and there was no significant change in titers after 6 weeks postboost. However, titers in Pre-F VLP-boosted animals remained 4-fold higher than those in Post-F VLP- or RSV-boosted mice (Fig. 2A). Importantly, sera from mice boosted with Pre-F VLPs retained neutralizing antibody titers of 3,000 (dilution of sera resulting in 50% reduction in virus titers), levels significantly above previously defined minimum protective levels (serum dilution of 256 to 512) (18, 28, 29).
In VLP-primed animals at 4 weeks postboost, the Pre-F VLP boost resulted in higher NA titers than Post-F VLP boost (1.7-fold), and Post-F VLP boost resulted in significantly higher NA titers (5-fold) than RSV-primed RSV boosts (Fig. 2B and C). However, titers in both groups of VLP-primed animals declined with time to nearly equivalent levels by week 15, indicating that the conformation of F protein had little effect on the long-term NA titers in VLP-immunized, RSV-naive mice.
Figure 1C also compares NA titers at 4 weeks postboost between the two sets of animals, RSV-primed and VLP-primed animals. Pre-F VLP immunization of RSV-primed animals resulted in somewhat higher titers than Pre-F VLP immunization of VLP-primed animals, but the differences were not significant. However, Post-F VLP immunization of RSV-primed animals resulted in 2-fold lower titers than Post-F VLP boosts of Post-F VLP-primed animals, suggesting that RSV priming was not as effective in priming for Post-F VLP-activated protective antibodies as Post-F VLP priming.
The experiments shown in Fig. 2A to C were accomplished using RSV, strain A2. To determine if the sera resulting from the A2-derived Pre-F VLP immunization could induce cross-reactive protection to RSV, strain B, sera obtained 4 weeks after Pre-F VLP immunization of RSV (A2)-primed animals or VLP-primed animals were used in a plaque reduction assay using RSV strain B (WV/14617/85; ATCC VR-1400). Figure 2D shows that the RSV (A2)-primed sera neutralize strain B significantly more effectively that the VLP-primed sera. Both sera were less effective in neutralizing strain B than strain A2. Using RSV-primed sera, the NA titer of RSV, strain B, is 4-fold lower than the titer using strain A virus but 7-fold lower than titers obtained with VLP-primed sera. However, NA titers of RSV strain B are still at or above previously defined minimal protective levels, demonstrating good cross-strain protection with A2 Pre-F VLPs.
Total anti-F IgG titers after VLP immunization of RSV-primed and VLP-primed animals.
The significant differences in NA titers in RSV-primed animals after Pre-F or Post-F VLP boosts or after a second RSV infection could be due to differences in the total levels of anti-F protein antibodies stimulated in these animals. The total anti-pre-F and anti-post-F IgG binding proteins in the sera of two groups of RSV-primed, VLP-boosted mice were measured by enzyme-linked immunosorbent assay (ELISA) (Fig. 3A and B). The level (in nanograms per milliliter) of both pre-F (Fig. 3A) or post-F (Fig. 3B) binding antibodies after either VLP boost was similar at 1 to 4 weeks. Thus, the differences in NA titers in Pre-VLP- and Post-F VLP-boosted mice at 4 weeks cannot be due to differences in the total levels of anti-F IgG at that time point. This result is consistent with the hypothesis that both VLPs can significantly boost anti-F antibodies primed by RSV infection but that the specificities of the antibodies activated by the two different VLPs in RSV-primed animals are different.
FIG 3.
Titers of anti-F protein IgG. (A and B) The level (ng/ml) of anti-F protein antibodies, determined by ELISA in pooled sera, that binds to the soluble prefusion or postfusion F protein, with time after VLP boosts of RSV-primed animals or a second RSV infection. The values shown are averages with standard deviations from 4 to 5 separate determinations on each of two pools of sera from two different experiments. Values obtained with the two pools of sera from each time point were not statistically significantly different and therefore were combined. (C and D) Levels (ng/ml) of pre-F binding or post-F binding antibodies with time in pools of sera from VLP-primed, VLP-boosted animals. The values shown are averages, with standard deviations, from 4 to 5 separate determinations on each of three pooled sera from three different experiments. Values obtained with each of the three pools of sera were not statistically significantly different and therefore were combined. (E and F) Level (ng/ml) of pre-F (E) or post-F (F) binding antibodies at 4 weeks postboost (the time of maximal NA titers) are shown for clear presentation of statistically significant differences between groups and between the sets of mice. (G and H) Statistically significant differences, at 4 weeks postboost, between pre-F and post-F protein binding antibodies within each set of animals. There were no significant differences between VLP-primed animals at late times postboost. *, P = 0.05; **, P = 0.005; ***, P = 0.0005; ****, P = 0.00005. Only significant differences are indicated.
In contrast, the level (in nanograms per milliliter) of anti-F protein IgG in RSV-primed, RSV-boosted animals was much lower than that in the VLP-boosted animals (Fig. 3A, B, E, and F). Thus, the low levels of NA titers in these animals may be, at least in part, due to the significantly lower total anti-F IgG.
By 17 weeks, the RSV-primed, Pre-F VLP-immunized animals had slightly but significantly higher levels of pre-F binding IgG than the Post-F VLP-immunized animals (Fig. 3A), while levels of post-F binding IgG activated by either VLP were the same (Fig. 3B). This result suggests some differences in durability of prefusion F binding antibodies activated by the two different VLPs. Pre-F and post-F proteins share common epitopes. In addition, the pre-F protein also contains epitopes unique to the prefusion protein. Antibodies specific to these unique epitopes may account for the increased durability of the IgG activated by Pre-F VLPs.
In VLP-primed animals, the IgG levels that bound soluble pre-F and post-F targets after VLP prime and boost are shown in Fig. 3C and D, respectively. Both VLPs stimulated similar levels of IgG that bound to the pre-F targets, and both stimulated the same levels of antibodies that bound to post-F targets (Fig. 3C and D) at all times after immunization. The levels of anti-F binding antibodies after two sequential RSV infections were similar to those in the VLP-primed animals (Fig. 3C, D, and H). The total anti-F IgG levels declined minimally after week 4 in both groups of VLP-primed animals.
To further illustrate results from our different immunization protocols, Fig. 3E and F compare antibody binding between the two sets of mice, RSV-primed and VLP-primed, using the same target. Using either target, the titers of IgG after VLP prime and boosts are 11 (Fig. 3E)- and 8 (Fig. 3F)-fold lower than levels generated after RSV priming and a single VLP boost (Fig. 3E and F). These results suggest that RSV infections prime for total anti-F antibody responses more effectively than VLP priming.
Figure 3G and H compare the IgG titers specific for pre-F target or post-F target within each set of animals. Within each set, the levels of IgG that bound post-F target were higher than levels that bound to pre-F target. However, the differences were more pronounced in the RSV-primed animals. Taken together, these data indicate that prior exposure to RSV as well as the conformation of the immunogen affect the quantity of anti-RSV antibodies produced.
Avidity of anti-F protein antibodies.
The neutralizing activity of a monoclonal antibody relates not only to the specificity of the antibody but also to the affinity of the antibody to its target. While the affinity of polyclonal antibodies cannot be accurately measured, the avidity, or the strength of the binding of a polyclonal antibody population to a target antigen, can be assessed by measuring the sensitivity of antigen-antibody complexes to increasing concentrations of urea. Complexes with higher-avidity polyclonal antibodies are more resistant to urea than those containing lower-avidity antibodies (30, 31). Higher avidity correlates with increased affinity maturation of the population of antibodies in the sera.
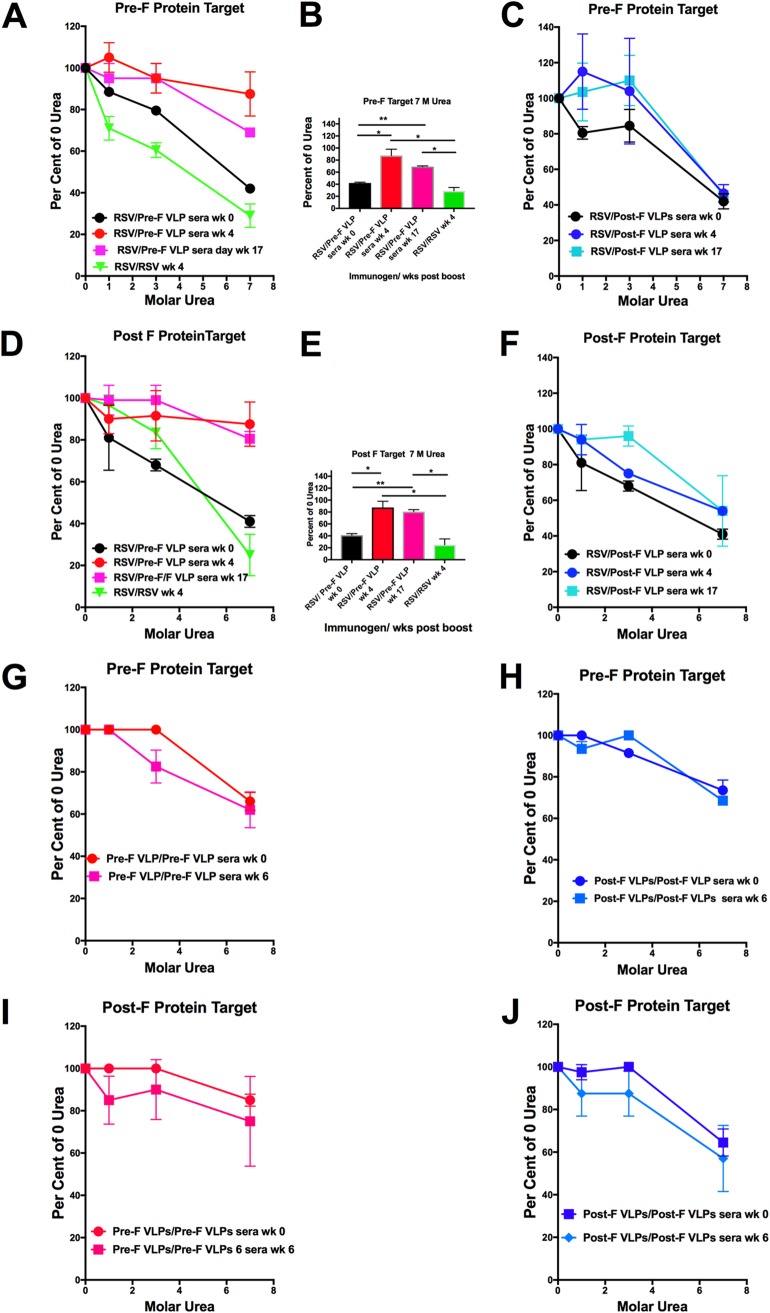
To evaluate the degree of affinity maturation of the polyclonal antibodies stimulated by VLP immunization in RSV-primed or VLP-primed animals, the antibody avidities in sera from the different groups of mice specific to the prefusion F target and to the post-F target were assessed in sera harvested at different times postboost. Avidities of sera from RSV-primed, Pre-F VLP-boosted or RSV-primed/RSV-boosted animals harvested at weeks 0, 4, and 17 postboost are shown in Fig. 4A and D. After a Pre-F VLP boost, the avidities of anti-pre-F (Fig. 4A) or anti-post-F (Fig. 4D) binding antibodies increase significantly by 4 weeks after the boost. Furthermore, the avidities of these antibodies are considerably higher after Pre-F VLP immunization than after two sequential RSV infections. The statistical significance of differences in results at 7 M urea are shown in Fig. 4B and E.
FIG 4.
Avidities of anti-F protein antibodies. (A to F) Results with sera from RSV-primed animals. Shown is the stability in increasing concentrations of urea of anti-F antibody-prefusion F protein complexes (A and C) or anti-F antibody-postfusion F protein complexes (D and F) in sera from RSV-primed animals at 0, 4, and 17 weeks after the VLP boost. (A and D) Results using sera from pre-F VLP-boosted animals. (C and F) Results using sera from post-F VLP-boosted animals. (B and E) Results using pre-F VLP sera at 7 M urea in order to illustrate statistically significant differences between groups (*, P = 0.05; **, P = 0.005; ***, P = 0.0005). There were no statistical differences between the values obtained in sera from post-F VLP sera. The results are averages with standard deviations from four separate determinations. RSV/RSV wk 0 indicates RSV/pre-F VLPs at week 0 and RSV/Post-F VLPs at week 0. (G to J) Results with sera from VLP-primed, VLP-boosted animals obtained at 0 and 6 weeks postboost from pre-F VLP (G and I) or post-F VLP (H and J) sera. There were no statistically significant differences between groups of animals at the two time points.
In contrast, Post-F VLP immunization of RSV-primed animals did not result in an increase in avidities with time after the boost (Fig. 4C and F). Indeed, the avidities of both the pre-F and post-F binding antibodies at 4 and 17 weeks postboost were very similar to avidities in the week 0 serum. These combined results suggest that the higher NA titers after a Pre-F VLP boost compared to a Post-F VLP boost can be explained, in part, by differences in avidities of the populations of antibodies.
Avidity measures of antibodies stimulated after VLP prime and boost protocols yielded quite different results. We assessed the avidities of the sera from these VLP-primed animals at 0 and 6 weeks postboost. In contrast to results in RSV-primed animals, there were no statistically significant differences in avidities of sera at 6 weeks compared to 0 weeks (Fig. 3G to J), a result consistent with either a larger proportion of lower-affinity antibodies induced by the VLP prime immunization or a higher proportion of primary responses after the VLP boosts. While we were unable to assess avidity at the 4-week time point due to a limitation in serum samples, we know that affinity maturation is stable by 4 weeks postboost.
Levels of anti-F antibody-secreting bone marrow cells.
The bone marrow is the primary site of long-lived antibody-secreting plasma cells that are the source of prolonged levels of serum antibodies. Figure 5 compares the levels of these cells, measured in enzyme-linked immunosorbent spot (ELISpot) assays, in the different groups of animals at 15 to 17 weeks after the VLP boosts of the RSV- or VLP-primed animals. The results of two separate experiments are shown. While the absolute numbers of cells were different in the two experiments, the relative ratios of the cells between groups are very similar.
FIG 5.
Bone marrow secreting anti-F protein antibodies. At weeks 21 and 15 postboost, bone marrow cells secreting anti-F antibodies that bound soluble prefusion F (A and C) or soluble postfusion F (B and D) were prepared and quantified by ELISpot assay as described in Materials and Methods. Each panel shows results of duplicate assays for the five animals in each group of RSV-primed, VLP-boosted animals or VLP-primed, VLP-boosted animals. The experiment was repeated twice with two different sets of animals. Panels A and B show results from experiment 1, while panels C and D show results from experiment 2. Statistically significant differences are indicated. *, P = 0.05; **, P = 0.005; ***, P = 0.0005; ****, P = 0.00005.
In RSV-primed animals, there were higher numbers of cells secreting anti-pre-F binding antibodies in the Pre-F VLP-immunized animals than those in the Post-F VLP-immunized animals. This result is consistent with the larger amount of anti-pre-F binding antibodies in sera from Pre-F VLP-immunized animals than Post-F VLP-immunized animals at 17 weeks postboost (Fig. 3A). The levels of post-F antibody-secreting cells were, however, similar after Pre-F or Post-F VLP immunization, a result consistent with the finding of equivalent quantities of anti-post F binding antibodies in the sera from these animals at late times (Fig. 3B). Both VLP-boosted animals contained much higher numbers of anti-F-secreting bone marrow cells than the RSV-primed/boosted animals. These results are consistent with the much higher levels of anti-F IgG serum antibodies in the VLP-boosted animals than the RSV-boosted animals at late times after the boosts (Fig. 3A and B).
In both groups of VLP-primed animals as well as the RSV-primed/boosted animals, the numbers of bone marrow cells secreting anti-F antibodies are similar. In one experiment, there was a slight increase in numbers of cells specific for pre-F antibodies in animals primed and boosted with Pre-F VLPs, but the difference was not observed in the other experiment, indicating it is not a consistent result. The combined results are consistent with the finding of very similar amounts of total anti-F IgG serum antibodies in both groups of VLP-primed animals and RSV-primed/boosted animals at 15 weeks postboost (Fig. 3C and D).
A comparison of RSV-primed and VLP-primed animals shows that RSV priming resulted in much higher numbers of anti-F-secreting bone marrow cells than VLP priming, a result that correlates with the finding of an approximately 10-fold difference in levels of total anti-F serum antibodies between the RSV- and VLP-primed groups of animals at late times postboost (Fig. 3E and F).
Levels of anti-F antibody-secreting splenic B cells.
Activation of antigen-specific memory B cells in spleens is an important component of protection from recurrent infections by a pathogen. Memory responses to natural RSV infections are defective, since individuals may experience repeated RSV infections with the same strain of virus throughout life, although the severity of the infections is usually less with repeated infections (9–11, 32). Thus, the numbers of anti-F protein antibody-specific memory B cells in spleens after the immunization protocols relative to RSV infection are an important component in the evaluation of a vaccine candidate in both naive and RSV-experienced animals. Accordingly, at late times after the VLP boosts, the numbers of anti-F protein-secreting B cells in spleens were quantified by ELISpot assay using soluble pre-F or soluble post-F proteins as targets. This experiment was accomplished twice, and results from each experiment are shown in Fig. 6. Again, the absolute numbers of B cells differ between the two experiments, but the relative levels between groups are similar.
FIG 6.
Splenic B cells secreting anti-F protein antibodies. At weeks 21 and 15 postboost, splenic B cells secreting antibodies that bound soluble prefusion F (A and C) or soluble postfusion F (B and D) were prepared and quantified by ELISpot assay as described in Materials and Methods. Panels show results of duplicate assays for the five animals in each group of RSV-primed, VLP-boosted animals or VLP-primed, VLP-boosted animals. The experiment was repeated twice with two different sets of animals. Panels A and B show results from experiment 1, while panels C and D show results from experiment 2. Statistically significant differences are indicated. *, P = 0.05; **, P = 0.005; ***, P = 0.0005; ****, P = 0.00005.
In RSV-primed animals, a Pre-F VLP immunization resulted in increased numbers of both pre-F and post-F-specific B cells compared to those for Post-F VLP immunization. Boosts with either VLP resulted in significantly higher numbers of anti-pre- and anti-post F-specific B cells compared to those of a second RSV infection. These results indicate that VLP boosts of RSV-infected animals is far superior to RSV infections in activating F protein memory.
In VLP-primed animals of experiment 1, after a Pre-F VLP or a Post-F VLP prime and boost, the numbers of anti-F protein antibody-secreting B cells specific for either pre-F or post-F protein were similar to each other and to numbers of cells after RSV prime and boost. In the other experiment, the Pre-F VLP prime/boost resulted in slightly higher levels of F-specific spleen cells than the post-F VLP prime/boost animals, and both VLP-immunized animals had somewhat higher levels of these cells than RSV-primed and -boosted animals.
The combined results indicate that RSV priming followed by a VLP boost results in numbers of F-specific memory B cells superior to those of VLP prime and boosts. The results also suggest that, in RSV-primed animals, the Pre-F VLPs are better at stimulating memory B cells than the Post-F VLPs. Furthermore, the results indicate that RSV boosts of RSV-primed animals do not result in high levels of F-specific splenic B cells.
RSV replication upon RSV challenge of RSV-primed or VLP-primed mice.
We next determined if there were differences in protection of animals from RSV challenge at extended times after the VLP boosts of the RSV-primed or VLP-primed animals. At 21 weeks after single VLP boosts, RSV-primed animals were RSV challenged and 4 days later were sacrificed. At 15 weeks postboost, VLP-primed animals were RSV challenged and then sacrificed 4 days later. Lung titers were determined to assess any RSV replication after challenge. Figure 7 shows that there was no detectable virus in the lungs of any of the animals in any of the groups except in sham-vaccinated animals, a result that was expected, since even a single RSV infection results in protection from RSV replication in mice (21, 22).
FIG 7.

Protection from RSV challenge. Animals were infected with RSV at 21 (RSV primed) or 15 (VLP primed) weeks postboost, and lungs were harvested 4 days later. The virus titers in the lungs of each animal were determined as previously described (23, 26). RSV infection of animals, sham immunized, served as positive controls. PFU per gram of lung tissue are shown for each mouse in each group of animals. RSV primed, 10 animals/group; VLP primed, 15 animals/group. RSV titers in lungs of each animal were determined.
DISCUSSION
While most people have been infected with RSV by age 2 (12), the resulting immune responses are poorly protective. However, residual immunity could impact responses to vaccine candidates, a topic that has not been extensively investigated. Thus, we explored the effects of prior RSV infection on murine responses to VLP vaccine candidates and compared them to responses to immunization in VLP-primed, RSV-naive animals. For these studies, we used two different RSV VLP vaccine candidates, one with a stabilized RSV prefusion F protein (18) and one with a stabilized postfusion F protein (16, 27), allowing us to compare the role of F protein conformation in immune responses in RSV-primed and VLP-primed animals. We found that responses to VLPs in RSV-infected (primed) animals were different from those in VLP-primed animals.
The most surprising observation was that, contrary to expectations, a single injection of Pre-F VLPs into RSV-primed animals resulted in a very significant increase in the NA titers. These results were in dramatic contrast to the very weak induction of NA titers in animals receiving a second RSV infection. These results suggest that RSV infection can quite effectively prime memory responses specific for antibodies that bind sites that stimulate the most potent neutralizing antibodies (33). These memory cells then could be robustly activated by the Pre-F VLPs but not by a second RSV infection. This result is consistent with the defective protective memory to RSV infections in humans, which fails to protect from subsequent infections. Less surprising was the relatively weak induction of NA by immunization with the Post-F VLPs. Post-F and pre-F proteins do share many epitopes, but these shared epitopes do not stimulate very potent NA (33); thus, Post-F VLPs may activate only antibodies to these shared epitopes, which will not result in potent, high titers of neutralizing antibodies.
The responses to VLP boosts in the VLP-primed animals were somewhat different from those in the RSV-primed animals. First, two immunizations with Pre-F VLPs were required to generate NA titers similar to a single immunization of RSV-primed animals with Pre-F VLPs. This result is not surprising, since memory to pre-F-specific epitopes must be primed and then activated by the Pre-F VLP. However, Post-F VLP-primed and -boosted animals had significantly higher NA titers than Post-F VLP boosts of RSV-experienced animals. A possible explanation for this finding is that Post-F VLPs can prime and, thus, activate upon a boost different sets of memory B cells than an RSV infection. A correlate to this hypothesis is that the F protein in Post-F VLPs exposes different epitopes to the immune system than RSV infection. Alternatively, the different routes of priming, intranasal versus intramuscular, may account in part for the more effective responses to the Post-F VLP in VLP-primed mice than in RSV-primed mice.
To begin to understand these results, the properties of antibodies activated by the VLPs and by RSV infection in RSV-primed or in VLP-primed animals were characterized and compared. A possible explanation for the differences in NA after Pre-F or Post-F VLP immunization of RSV-primed animals or in VLP-primed animals was that the levels of total anti-F antibodies were different. However, in RSV-primed animals, the total anti-F antibody levels at 4 weeks postboost, the time of maximal NA titers, were the same after Pre-F or Post-F VLP boosts. Thus, differences in NA titers were not due to total amounts of antibody but likely due to different populations of antibodies activated by the two VLPs. The levels of total anti-F antibodies activated after the second RSV infection were, however, 10-fold lower than levels activated by the VLPs, suggesting defects in activation of anti-F protein memory B cells by the second infection. The smaller amounts of total antibodies could account, in part, for the lower NA titers in these animals. Again, this result is consistent with the failure of the second RSV infection to activate anti-F protein memory. The lower quantities of total anti-F protein antibodies in RSV prime/boost animals also correlate with the differences in the levels of bone marrow-associated anti-F protein-secreting antibodies.
The levels of total anti-pre-F or anti-post-F binding antibodies in VLP-primed animals were the same for Pre-F or Post-F VLP boosts or a second RSV infection. Thus, quantities of total anti-F antibodies cannot account for the differences in NA titers in these animals. Rather, the specificities of the antibodies are likely different.
Another difference between RSV- and VLP-primed animals was that the levels of total anti-F antibodies in RSV-primed, VLP-boosted animals were 10-fold higher than levels in the VLP-primed and -boosted animals even at late times. Levels of total anti-F protein antibody after a second RSV infection were also 10-fold lower than those in RSV-primed, VLP-boosted animals and comparable to levels in VLP-primed, VLP-boosted animals. These results suggest that RSV infection can prime for higher numbers of memory B cells than VLP priming but VLPs are better able to activate these memory B cells than a second RSV infection. Consistent with this hypothesis is the finding that the numbers of anti-F protein-specific B cells in spleens of RSV-primed animals at late times after Pre-F VLP immunization were higher than those after a second RSV infection. Furthermore, the numbers of F protein-specific splenic B cells in VLP-primed, VLP-immunized mice are lower than those in RSV-primed, VLP-immunized animals, which can also account for lower total anti-F IgG in VLP-primed animals.
The potency of neutralizing monoclonal antibodies is also related to the affinity of the binding of the antibody to its epitope. Boosts serve to promote affinity increases with time. Since the affinity of polyclonal antibodies cannot be measured, the avidity or the stability of antigen-antibody complexes in increasing concentrations of urea can be used to assess the overall affinity maturation with time of a polyclonal antibody population after a boost. The antibodies resulting from the Pre-F VLP boost demonstrated increased avidity with time after the boost, peaking at 4 weeks postboost and remaining unchanged to 17 weeks postboost. The pre-F and the post-F binding antibodies after the Pre-F VLP boost underwent similar increases in avidity, consistent with activation of pre-F antibodies that bound to epitopes unique and in common with post-F protein. What was surprising and puzzling was that the Post-F VLP boost did not result in antibodies that increased in avidity after the boost. A second RSV infection also did not result in an increase in avidity, indicating defects in the secondary immune responses after the second RSV infection. These combined results suggest that Post-F VLPs activate lower-affinity antibodies than the Pre-F VLPs, antibodies specific to epitopes shared by the pre- and post-F proteins. Alternatively, compared to Post-F VLP immunization, Pre-F VLP immunization of RSV-primed animals may stimulate a larger proportion of memory cells, resulting in increased selection and antibody affinity maturation, while Post-F VLP immunization may stimulate a different population of memory cells or more naive B lymphocytes, thereby generating less affinity maturation.
Also surprising was that the antibodies activated with either VLP in VLP-primed animals did not undergo measurable increases in avidity. While the explanation for this result is unclear, it is possible that VLP prime immunization does not result in long-lasting memory responses, responses that wain by 100 days postpriming. If so, then the VLP boosts at 100 days stimulate a larger proportion of primary immune responses. This possibility is consistent with the lower levels of both bone marrow-associated anti-F antibody-secreting cells and F-specific splenic B cells compared to the levels in RSV-primed animals.
Conclusions.
Previous RSV infection effectively primes animals for good protective responses, as indicated by the observation that these responses can be robustly activated upon Pre-F VLP immunization but not by a second RSV infection. This result suggests that the defect in RSV memory is not in induction of antibody responses but failure to activate preexisting memory upon a second RSV infection. Post-F VLPs also do not activate high titers of NA in RSV-primed animals. However, both VLPs stimulate equivalent levels of total anti-F antibodies, consistent with activation by the Pre-F VLPs of high-affinity antibodies targeting sites unique to the prefusion F protein and the activation by Post-F VLPs of only poorly neutralizing antibodies that bind to epitopes on both pre-F and post-F proteins.
In summary, the results indicate that immune responses to a vaccine candidate in previously RSV-infected mice compared to those in VLP-primed mice are different with respect to NA titers, total anti-F IgG, antibody avidity, bone marrow-associated anti-F antibody-secreting plasma cells, and splenic anti-F protein memory B cells. These findings suggest that vaccine candidates need to be tested in preclinical studies in animals that are previously infected with RSV in order to accurately assess their potential.
MATERIALS AND METHODS
Cells, virus, and plasmids.
ELL-0 (ATCC UMNSAH DF-1, East Lansing strain 0), Vero cells, and Hep2 cells, obtained from the American Type Culture Collection, were grown in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with penicillin, streptomycin (Invitrogen), and 5% (Vero cells) or 10% fetal calf serum (Invitrogen). Expi293F, obtained from ThermoFisher/Invitrogen, was grown in Expi293 medium (ThermoFisher/Gibco/Invitrogen). RSV, A2 strain, was obtained from Robert Finberg. RSV, strain B (WV/14617/85; ATCC VR-1400), was obtained from the American Type Culture Collection.
VLPs containing the RSV F and G proteins are formed with the Newcastle disease virus (NDV) core proteins NP and M. The cDNAs encoding the NDV NP and M protein have been previously described (34). The RSV F and G proteins were incorporated into these VLPs by constructing chimera protein genes composed of ectodomains of the G or F glycoproteins (from RSV, strain A2) fused to the transmembrane (TM) and cytoplasmic (CT) domains of the NDV HN protein or NDV F glycoprotein, respectively, as previously described (22, 26). These NDV domains specifically interact with the NDV NP and M protein, resulting in efficient incorporation of the chimera proteins into VLPs.
The construction, expression, and incorporation of the chimera protein NDVHN/RSVG (H/G) into VLPs have been previously described (22). The construction, expression, and incorporation into VLPs of the stabilized prefusion F protein (Pre-F/F; DS-Cav1) to generate VLP-H/G+Pre-F/F (Pre-F VLPs) and the stabilized postfusion F protein to create VLP-H/G+Post-F/F (Post-F VLPs) have been previously described (23, 26).
The constructions of genes encoding the soluble pre-F protein, the soluble post-F protein, and the soluble G protein used as targets in ELISA and ELISpot assay were previously described (26).
Polyacrylamide gel electrophoresis, silver staining, and Western blot analysis.
Proteins were resolved on 8% Bis-Tris gels (NuPage; ThermoFisher/Invitrogen). Silver staining of proteins in the polyacrylamide gels was accomplished as recommended by the manufacturer (ThermoFisher/Pierce). Quantifications of NP, M, different forms of F/F proteins, H/G protein, and soluble pre-F, post-F, and soluble G proteins were accomplished after their separation in polyacrylamide gels followed by silver staining or by Western bloting of the proteins, as well as protein standards, as previously described (23, 35). For Western analysis, proteins in the polyacrylamide gels were transferred to polyvinylidene difluoride membranes using dry transfer (iblot; ThermoFisher/Invitrogen). Proteins were detected in the blots using anti-RSV HR2 peptide antibody, anti-NDV F tail antibody, or anti-RSV G protein antibody as previously described (23, 26, 36, 37).
Antibodies.
RSV F monoclonal antibody clone 131-2 A (MAB8599; Millipore) was used in RSV plaque assays. Monoclonal antibody (MAb) 1112, MAb 1200, MAb 1243 (generous gifts of J. Beeler) (38), MAb D25 (18), and the MAb motavizumab (39) (generous gifts of J. McLellan) were used to vallidate F protein conformations and for ELISA analysis of VLPs and soluble F proteins. Anti-RSV F protein HR2 antibody and anti-NDV F-tail antibody, used for Western blotting to quantify F protein levels, are polyclonal antibodies specific to the HR2 domain of the RSV F protein or the cytoplasmic tail of NDV F protein (21, 40). Anti-RSV G protein antibody used to measure VLP G protein levels is a polyclonal antibody raised against a peptide containing G protein amino acids 180 to 198 (ThermoFisher). Secondary antibodies against goat, mouse, and rabbit IgG were purchased from Sigma.
VLP preparation, purification, and characterization.
For preparations of VLPs to be used as immunogens (Pre-F VLPs and Post-F VLPs), ELL-0 cells growing in T-150 flasks were transfected with cDNAs encoding the NDV M protein, NP, and the chimera proteins H/G and either Pre-F/F or Post-F/F as previously described (24). At 24 h posttransfection, heparin (Sigma) was added to the cells at a final concentration of 10 μg/ml to inhibit rebinding of released VLPs to cells. At 72, 96, and 120 h posttransfection, cell supernatants were collected and VLPs purified by sequential pelleting and sucrose gradient fractionation as previously described (21, 35, 41). Concentrations of proteins in the purified VLPs were determined by silver-stained polyacrylamide gels and by Western analysis using marker proteins for standard curves (24, 37). The conformation of F protein in the VLP preparations was verified by reactivity to MAbs (26). The characterization of purified preparations of Pre-F VLPs and Post-F VLPs has been previously published (24, 26).
Preparation of soluble F protein.
Expi293F cells were transfected with pCAGGs vector-containing sequences encoding the soluble pre-F protein or the soluble post-F protein. At 5 to 6 days posttransfection, total cell supernatants were collected and cell debris removed by centrifugation. Soluble polypeptides were then purified on columns using the His tag and then the Strep tag as previously described (18, 24, 26, 39). Our soluble pre-F protein, which retains the foldon sequence to stabilize trimers, efficiently binds AM14, a trimer-specific antibody (42).
Quantification of soluble and VLP-associated F and G proteins.
Determinations of amounts of RSV F protein in VLPs or in soluble F protein preparations were accomplished by Western blotting using anti-HR2 antibody for detection and comparing the signals obtained with a standard curve of purified F proteins as previously described (24, 35).
Preparation of RSV, RSV plaque assays, and antibody neutralization.
RSV, strain A2, was grown in Hep2 cells, and RSV plaque assays were accomplished on Vero cells as previously described (23). Antibody neutralization assays in a plaque reduction assay have been previously described (23). Neutralization titer was defined as the reciprocal of the dilution of serum that reduced the virus titer by 50%.
Animals, animal immunization, and RSV challenge.
Four-week-old female BALB/c mice from Taconic Laboratories (BALB-F) were housed (groups of 5) under pathogen-free conditions in microisolator cages at the University of Massachusetts Medical Center animal quarters. Female mice were used in order to assess the potential of VLPs for maternal immunization. Protocols requiring open cages were accomplished in biosafety cabinets. BALB/c mice were immunized by intramuscular inoculation of 30 μg total VLP protein (5 μg F protein) in 0.05 ml of TNE (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA) containing 10% sucrose. For infections with RSV, the animals were lightly anesthetized with isoflurane and then infected by intranasal inoculation of 50 μl of RSV (1 × 107 PFU/ml). All animal procedures and infections were performed in accordance with the University of Massachusetts Medical School IACUC and IBC approved protocols.
ELISA protocols.
For determination of anti-F protein antibody titers, blood was obtained from immunized animals by tail vein nicks and centrifuged in BD Microtainer serum separator tubes (ThermoFisher) to remove blood cells. Wells of microtiter plates (ThermoFisher/Costar) were coated with either purified soluble prefusion F protein or soluble postfusion F protein (25 ng/well) and incubated for 24 h at 4°C. Wells were then incubated in phosphate-buffered saline (PBS)–2% bovine serum albumin (BSA) for 16 h. Different dilutions of sera, in 0.05% Tween 20 and 2% BSA, were added to each well and incubated for 2 h at room temperature. After six washes in PBS, sheep anti-mouse antibody coupled to horseradish peroxidase (HRP; A5906; Sigma) was added in 50 μl PBS–2% BSA and incubated for 1.5 h at room temperature. Bound HRP was detected by adding 50 μl TMB (3,3′5,5′-tetramethylbenzidin; 34028; ThermoFisher) and incubating for 5 to 20 min at room temperature until blue color developed. The reaction was stopped with 50 μl 2 N sulfuric acid. Color was read in a SpectraMax Plus plate reader (Molecular Devices) using SoftMax Pro software. Amounts of IgG (nanograms per milliliter) bound to the wells were calculated using a standard curve generated using defined amounts of purified IgG.
Avidity of polyclonal antibodies.
Wells of microtiter plates were coated with purified soluble pre-F or post-F polypeptides as described for ELISA (25 ng/well). Wells were incubated in PBS containing 2% BSA overnight at 40°C. After extensive washing of the microtiter plates, aliquots of dilutions of pooled sera, in PBS containing 2% BSA and 0.05% Tween 20, from week 0, 4, 6, 15, or 17 were added to duplicate wells and incubated for 2 h at room temperature. Wells were then incubated in PBS containing 2% BSA and 0, 1, 3, or 7 M urea for 10 min at room temperature. After extensive washing with PBS, bound antibody was detected with secondary antibody as described for ELISA.
ELISpot assays.
Bone marrow cells were prepared as previously described (24, 43).
Total splenocytes, prepared from disrupted spleens as previously described (43, 44), were resuspended at a concentration of 10 × 106 to 20 × 106 cells/ml in complete medium (RPMI) containing 10% serum, penicillin-streptomycin, glutamine, and β-mercaptoethanol (β-ME) (5 × 10−5 M). B cell numbers were determined by fluroscence-activated cell sorting analysis using anti-CD19 and anti-B220 antibodies.
Wells of ELISpot plates (Millipore) were coated overnight with purified soluble pre-F protein or soluble post-F protein (25 ng/well in PBS). Wells were washed and blocked for 1 h in compete medium. Four-fold serial dilutions of bone marrow cells or spleen cells were added in triplicate to precoated wells and incubated at 37°C for 6 h. Plates were washed and blocked overnight in PBS containing 1% BSA. Wells were incubated with biotinylated anti-mouse IgG (1/2,000 dilution; Southern), washed, incubated with streptavidin AP (1/4,000 dilution; Southern) diluted in PBS–1% BSA, washed, and then developed with BCIP-NBT (5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium). Spots were counted using a CTL Immunospot S5 analyzer.
Statistical analysis.
Statistical analyses (Student's t test) of data were accomplished using GraphPad Prism 7 software.
ACKNOWLEDGMENTS
This work was supported by NIH/NIAID grant AI 114809 and by the Hood Foundation.
We thank Judy Beeler and Jason McLellan for monoclonal antibodies. We thank Jorge Blanco for helpful comments and editing the manuscript.
L.M.C. and M.R.S. performed the in vitro experiments; T.G.M. and M.R.S. did the animal infections, immunizations, and serum, spleen, and bone marrow harvest; T.M. analyzed the data and wrote the manuscript; T.G.M. and M.R.S. edited the manuscript.
REFERENCES
- 1.Shi T, McAllister DA, O'Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, Alassani I, Ali A, Antonio M, Awasthi S, Awori JO, Azziz-Baumgartner E, Baggett HC, Baillie VL, Balmaseda A, Barahona A, Basnet S, Bassat Q, Basualdo W, Bigogo G, Bont L, Breiman RF, Brooks WA, Broor S, Bruce N, Bruden D, Buchy P, Campbell S, Carosone-Link P, Chadha M, Chipeta J, Chou M, Clara W, Cohen C, de Cuellar E, Dang D-A, Dash-Yandag B, Deloria-Knoll M, Dherani M, Eap T, Ebruke BE, Echavarria M, de Freitas Lázaro Emediato CC, Fasce RA, Feikin DR, Feng L, Gentile A, Gordon A, Goswami D, Goyet S, Groome M, Halasa N, Hirve S, Homaira N, Howie SRC, Jara J, Jroundi I, Kartasasmita CB, Khuri-Bulos N, Kotloff KL, Krishnan A, Libster R, Lopez O, Lucero MG, Lucion F, Lupisan SP, Marcone DN, McCracken JP, Mejia M, Moisi JC, Montgomery JM, Moore DP, Moraleda C, Moyes J, Munywoki P, Mutyara K, Nicol MP, Nokes DJ, Nymadawa P, da Costa Oliveira MT, Oshitani H, Pandey N, Paranhos-Baccalà G, Phillips LN, Picot VS, Rahman M, Rakoto-Andrianarivelo M, Rasmussen ZA, Rath BA, Robinson A, Romero C, Russomando G, Salimi V, Sawatwong P, Scheltema N, Schweiger B, Scott JAG, Seidenberg P, Shen K, Singleton R, Sotomayor V, Strand TA, Sutanto A, Sylla M, Tapia MD, Thamthitiwat S, Thomas ED, Tokarz R, Turner C, Venter M, Waicharoen S, Wang J, Watthanaworawit W, Yoshida L-M, Yu H, Zar HJ, Campbell H, Nair H. 2017. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karron RA. 2008. Respiratory syncytial virus and parainfluenza virus vaccines, p 1146 In Plotkin SA, Orenstein WA, Offit P (ed), Vaccines, 5th ed, vol 6 Saunders-Elsevier, Philadelphia, PA. [Google Scholar]
- 3.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 4.Falsey AR, Walsh EE. 2000. Respiratory syncytial virus infection in adults. Clin Microbiol Rev 13:371–384. doi: 10.1128/CMR.13.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han LL, Alexander JP, Anderson LJ. 1999. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis 179:25–30. doi: 10.1086/314567. [DOI] [PubMed] [Google Scholar]
- 6.Raboni SM, Nogueira MB, Tsuchiya LRV, Takahashi GA, Pereira LA, Pasquini R, Siqueira MM. 2003. Respiratory tract viral infections in bone marrow transplant patients. Transplant 76:142–146. doi: 10.1097/01.TP.0000072012.26176.58. [DOI] [PubMed] [Google Scholar]
- 7.Ison MG. 2009. Respiratory syncytial virus and other respiratory viruses in the setting of bone marrow transplantation. Curr Opin Oncol 21:171–176. doi: 10.1097/CCO.0b013e328324bc1c. [DOI] [PubMed] [Google Scholar]
- 8.Shah JN, Chemaly RF. 2011. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood 117:2755–2763. doi: 10.1182/blood-2010-08-263400. [DOI] [PubMed] [Google Scholar]
- 9.Hall CB, Long CE, Schnabel KD. 2001. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis 33:792–796. doi: 10.1086/322657. [DOI] [PubMed] [Google Scholar]
- 10.Power UF. 2008. Respiratory syncytial virus (RSV) vaccines–two steps back for one leap forward. J Clin Virol 41:38–44. doi: 10.1016/j.jcv.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Hall CB, Simoes EAF, Anderson LJ. 2013. Clinical and epidemiologic features of respiratory syncytial virus, p 39–58. In Anderson LJ, Graham BS (ed), Challenges and opportunities for respiratory syncytial virus vaccines, vol 372 Springer, Heidelberg, Germany. [Google Scholar]
- 12.Glezen W, Taber LH, Frank AL, Kasel JA. 1986. RIsk of primary infection and reinfection with respiratory syncytial virus. Arch Pediatr Adolesc Med 140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 13.Jardetzky TS, Lamb RA. 2004. A class act. Nature 427:307–308. doi: 10.1038/427307a. [DOI] [PubMed] [Google Scholar]
- 14.Lamb RA, Parks GD. 2007. Paramyxoviridae: the viruses and their replication, p 1450–1496. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Strauss SE (ed), Fields virology, 5th ed, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 15.Swanson KA, Settembre EC, Shaw CA, Dey AK, Rappuoli R, Mandl CW, Dormitzer PR, Carfi A. 2011. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci U S A 108:9619–9624. doi: 10.1073/pnas.1106536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLellan JS, Yang Y, Graham BS, Kwong PD. 2011. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J Virol 85:7788–7796. doi: 10.1128/JVI.00555-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, Kumar A, Modjarrad K, Zheng Z, Zhao M, Xia N, Kwong PD, Graham BS. 2013. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GBE, Yang Y, Zhang B, Chen L, Srivatsan S, Zheng A, Zhou T, Graepel KW, Kumar A, Moin S, Boyington JC, Chuang G-Y, Soto C, Baxa U, Bakker AQ, Spits H, Beaumont T, Zheng Z, Xia N, Ko S-Y, Todd J-P, Rao S, Graham BS, Kwong PD. 2013. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342:592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham BS, Modjarrad K, McLellan JS. 2015. Novel antigens for RSV vaccines. Curr Opin Immunol 35:30–38. doi: 10.1016/j.coi.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuzil KM. 2016. Progress toward a respiratory syncytial virus vaccine. Clin Vaccine Immunol 23:186–188. doi: 10.1128/CVI.00037-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGinnes LW, Gravel KA, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Schmidt MR, Morrison TG. 2011. Assembly and immunological properties of Newcastle disease virus-like particles containing the respiratory syncytial virus F and G proteins. J Virol 85:366–377. doi: 10.1128/JVI.01861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murawski MR, McGinnes LW, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Heaton PM, Fraire AE, Morrison TG. 2010. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice with no evidence of immunopathology. J Virol 84:1110–1123. doi: 10.1128/JVI.01709-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cullen LM, Blanco JCG, Morrison TG. 2015. Cotton rat immune responses to virus-like particles containing the pre-fusion form of respiratory syncytial virus fusion protein. J Transl Med 13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGinnes L, Schmidt MR, Kenward SA, Woodland RT, Morrison TG. 2015. Murine immune responses to virus-like particle-associated pre- and postfusion forms of the respiratory syncytial virus F protein. J Virol 89:6835–6847. doi: 10.1128/JVI.00384-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachmann MF, Jennings GT. 2010. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 26.Cullen LM, Schmidt MR, Morrison TG. 2017. The importance of RSV F protein conformation in VLPs in stimulation of neutralizing antibody titers in mice previously infected with RSV. Hum Vaccin Immunother 13:2814–2823. doi: 10.1080/21645515.2017.1329069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swanson K, Wen X, Leser GP, Paterson RG, Lamb RA, Jardetzky TS. 2010. Structure of the Newcastle disease virus F protein in the post-fusion conformation. Virology 402:372–379. doi: 10.1016/j.virol.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piedra PA, Cron SG, Jewell A, Hamblett N, McBride R, Palacio MA, Ginsberg R, Oermann CM, Hiatt PW. 2003. Immunogenicity of a new purified fusion protein vaccine to respiratory syncytial virus: a multi-center trial in children with cystic fibrosis. Vaccine 21:2448–2460. doi: 10.1016/S0264-410X(03)00098-7. [DOI] [PubMed] [Google Scholar]
- 29.Piedra PA, Jewell AM, Cron SG, Atmar RL, Paul GW. 2003. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 21:3479–3482. doi: 10.1016/S0264-410X(03)00355-4. [DOI] [PubMed] [Google Scholar]
- 30.Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang H-Y, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP. 2009. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med 15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polack FP, Hoffman SJ, Crujeiras G, Griffin DE. 2003. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat Med 9:1209–1213. doi: 10.1038/nm918. [DOI] [PubMed] [Google Scholar]
- 32.Hall CB. 2001. Respiratory syncytial virus and parainfluenza virus. N Engl J Med 344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 33.Ngwuta JO, Chen M, Modjarrad K, Joyce MG, Kanekiyo M, Kumar A, Yassine HM, Moin SM, Killikelly AM, Chuang G-Y, Druz A, Georgiev IS, Rundlet EJ, Sastry M, Stewart-Jones GBE, Yang Y, Zhang B, Nason MC, Capella C, Peeples ME, Ledgerwood JE, McLellan JS, Kwong PD, Graham BS. 2015. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med 7:309ra162. doi: 10.1126/scitranslmed.aac4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pantua H, McGinnes LW, Leszyk J, Morrison TG. 2005. Characterization of an alternate form of the NDV fusion glycoprotein. J Virol 79:11660–11670. doi: 10.1128/JVI.79.18.11660-11670.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGinnes LW, Morrison TG. 2013. Current protocols in microbiology. John Wiley & Sons, Inc, Hoboken, NJ. [Google Scholar]
- 36.Blanco JCG, Pletneva LM, McGinnes-Cullen L, Otoa RO, Patel MC, Fernando LR, Boukhvalova MS, Morrison TG. 2018. Efficacy of a respiratory syncytial virus vaccine candidate in a maternal immunization model. Nat Commun 9:1904–1914. doi: 10.1038/s41467-018-04216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGinnes LW, Morrison TG. 2013. Newcastle disease virus-like particles: preparation. purification, quantification, and incorporation of foreign glycoproteins Curr Protoc Microbiol 30:Unit 18.2. doi: 10.1002/9780471729259.mc1802s30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beeler JA, van Wyke Coelingh K. 1989. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J Virol 63:2941–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLellan JS, Chen M, Kim A, Yang Y, Graham BS, Kwong PD. 2010. Structural basis of respiratory syncytial virus neutralization by motavizumab. Nat Struct Mol Biol 17:248–250. doi: 10.1038/nsmb.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGinnes LW, Reitter JN, Gravel K, Morrison TG. 2003. Evidence for mixed membrane topology of the Newcastle disease virus fusion protein. J Virol 77:1951–1963. doi: 10.1128/JVI.77.3.1951-1963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGinnes LW, Pantua H, Laliberte JP, Gravel KA, Jain S, Morrison TG. 2010. Assembly and biological and immunological properties of Newcastle disease virus-like particles. J Virol 84:4513–4523. doi: 10.1128/JVI.01931-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilman MSA, Moin SM, Mas V, Chen M, Patel NK, Kramer K, Zhu Q, Kabeche SC, Kumar A, Palomo C, Beaumont T, Baxa U, Ulbrandt ND, Melero JA, Graham BS, McLellan JS. 2015. Characterization of a prefusion-specific antibody that recognizes a quaternary, cleavage-dependent epitope on the RSV fusion glycoprotein. PLoS Pathog 11:e1005035. doi: 10.1371/journal.ppat.1005035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt MR, McGinnes LW, Kenward SA, Willems KN, Woodland RT, Morrison TG. 2012. Long term and memory immune responses in mice against Newcastle disease virus-like particles containing respiratory syncytial virus glycoprotein ectodomains. J Virol 86:11654–11662. doi: 10.1128/JVI.01510-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt MR, McGinnes-Cullen LW, Kenward SA, Willems KN, Woodland RT, Morrison TG. 2014. Modification of the respiratory syncytial virus F protein in virus-like particles impacts generation of B cell memory. J Virol 88:10165–10176. doi: 10.1128/JVI.01250-14. [DOI] [PMC free article] [PubMed] [Google Scholar]