Abstract
BACKGROUND
Over 400000 Americans annually undergo spinal fusion surgeries, yet up to 40% of these procedures result in pseudoarthrosis even with iliac crest autograft, the current “gold standard” treatment. Tissue engineering has the potential to solve this problem via the creation of bone grafts involving bone-promoting growth factors (e.g., bone morphogenetic protein 2). A broad assessment of experimental growth factors is important to inform future work and clinical potential in this area. To date, however, no study has systematically reviewed the investigational growth factors utilized in preclinical animal models of spinal fusion.
AIM
To review all published studies assessing investigational growth factors for spinal fusion in animal models and identify promising agents for translation.
METHODS
We conducted a systematic review of the literature using PubMed, Embase, Cochrane Library, and Web of Science databases with searches run on May 29th, 2018. The search query was designed to include all non-human, preclinical animal models of spinal fusion reported in the literature without a timespan limit. Extracted data for each model included surgical approach, level of fusion, animal species and breed, animal age and sex, and any other relevant characteristics. The dosages/sizes of all implant materials, spinal fusion rates, and follow-up time points were recorded. The data were analyzed and the results reported in tables and text. PRISMA guidelines were followed for this systematic review.
RESULTS
Twenty-six articles were included in this study, comprising 14 experimental growth factors: AB204 (n = 1); angiopoietin 1 (n = 1); calcitonin (n = 3); erythropoietin (n = 1); basic fibroblast growth factor (n = 1); growth differentiation factor 5 (n = 4), combined insulin-like growth factor 1 + transforming growth factor beta (n = 4); insulin (n = 1); NELL-1 (n = 5); noggin (n = 1); P-15 (n = 1); peptide B2A (n = 2); and secreted phosphoprotein 24 (n = 1). The fusion rates of the current gold standard treatment (autologous iliac crest bone graft, ICBG) and the leading clinically used growth factor (BMP-2) ranged widely in the included studies, from 0-100% for ICBG and from 13%-100% for BMP-2. Among the identified growth factors, calcitonin, GDF-5, NELL-1, and P-15 resulted in fusion rates of 100% in some cases. In addition, six growth factors - AB204, angiopoietin 1, GDF-5, insulin, NELL-1, and peptide B2A - resulted in significantly enhanced fusion rates compared to ICBG, BMP-2, or other internal control in some studies. Large heterogeneity in animal species, fusion method, and experimental groups and time points was observed across the included studies, limiting the direct comparison of the growth factors identified herein.
CONCLUSION
Several promising investigational growth factors for spinal fusion have been identified herein; directly comparing the fusion efficacy and safety of these agents may inform clinical translation.
Keywords: Spinal fusion, Growth factor, Pseudoarthrosis, Systematic review
Core tip: This is the first study to systematically review all the published investigational growth factors utilized in preclinical animal models of spinal fusion. Among the identified growth factors, calcitonin, GDF-5, NELL-1, and P-15 resulted in fusion rates of 100% in some studies. In addition, six growth factors - AB204, angiopoietin 1, GDF-5, insulin, NELL-1, and peptide B2A - resulted in significantly enhanced fusion rates compared to autologous iliac crest bone graft, BMP-2, or other internal controls in some cases. Directly comparing the fusion efficacy and safety of these growth factors may inform the development of clinically translatable materials for spinal fusion.
INTRODUCTION
Over 400000 Americans undergo spinal fusion surgeries each year, with the number increasing yearly alongside a growing and aging population[1-3]. However, pseudoarthrosis, or failed fusion, rates are reported to be as high as 40% in primary spinal fusion surgery and up to 60% in revision cases, even when the “gold standard” treatment of grafting bone from the patient’s own iliac crest is used[4,5]. When this happens, patients often suffer from significant pain and disability, and remaining treatment options are limited.
Tissue engineering has the potential to solve the problem of pseudoarthrosis by promoting site-specific de novo bone generation. Classically, tissue engineering involves using a scaffold, cells, and growth factors to generate living tissues. At present, the only tissue engineered product involving a growth factor that is FDA-approved for spinal fusion is a collagen sponge delivered with recombinant human bone morphogenetic protein 2 (rhBMP-2) (INFUSE Bone Graft, Medtronic). However, this product is associated with significant complications[6,7], which are thought to arise from the supraphysiologic therapeutic dose of rhBMP-2 required for effective bone formation[8]. In addition to rhBMP-2, recombinant human parathyroid hormone (rhPTH) and recombinant human BMP-7 (rhBMP-7) have been studied clinically in spinal fusion[9-11].
Given the potential impact of tissue engineering to advance spinal fusion, many biomaterials and bioactive agents have been investigated. However, no study to date has systematically reviewed the experimental growth factors investigated for spinal fusion in preclinical animal models. Considering the efficacy and widespread use of recombinant growth factors (i.e., rhBMP-2 and rhPTH) to optimize spinal fusion, a broad assessment of experimental growth factors is essential to inform future work and clinical potential in this area[9,12]. The present study aims to systematically review all published translational animal models assessing investigational growth factors for spinal fusion and identify promising agents for translation.
MATERIALS AND METHODS
Electronic literature search
A systematic review of the literature using PubMed, Embase, Cochrane Library, and Web of Science databases was performed with searches run on May 29th, 2018, along with a review of the bibliographies of the examined articles. The search query was designed to include all non-human, preclinical animal spinal fusion models reported in the literature without a timespan limit (Table 1). PRISMA guidelines were followed for this systematic review[13].
Table 1.
Search terms across 4 databases to identify experimental growth factors in animal models of spinal fusion
| Databases | Search terms |
| PubMed (1693) | (spinal fusion [mesh] OR spine fusion*[tw] OR spinal fusion*[tw] OR “spondylosyndesis”[tw]) AND (animals [mesh:noexp] OR “chordata”[mesh:noexp] OR (“vertebrates”[mesh] NOT “humans”[mesh]) OR “animals, domestic”[mesh] OR “animals, exotic”[mesh] OR “animals, genetically modified”[mesh] OR “animals, laboratory”[mesh] OR “animals, outbred strains”[mesh] OR “animals, wild”[mesh] OR “animals, zoo”[mesh] OR “mice”[tw] OR “mouse” [tw] OR murine*[tw] OR “rat”[tw] OR “rats”[tw] OR rabbit*[tw] OR “leporine”[tw] OR ovine*[tw] OR sheep*[tw] OR goat*[tw] OR “caprine”[tw] OR “porcine”[tw] OR “pig” [tw] OR “pigs”[tw] OR “swine”[tw] OR “cow”[tw] OR “cows”[tw] OR “bovine”[tw] OR “horse”[tw] OR “horses”[tw] OR equine*[tw] OR “canine”[tw] OR “feline”[tw] OR “animal”[tw] OR “animals” [tw] OR “dog”[tw] OR “dogs”[tw] OR “cat”[tw] OR “cats”[tw] OR monkey*[tw] OR “non human primate”[tw] OR “non human primates”[tw] OR “simian”[tw] OR “ape”[tw] OR “apes”[tw] OR “gorilla”[tw] OR “gorillas”[tw] OR “piscine”[tw] OR “fish”[tw] OR “fishes”[tw] OR “goose”[tw] OR “geese”[tw] OR “fowl”[tw] OR “poultry”[tw] OR “chicken”[tw] OR “chickens”[tw]) |
| Embase (1709) | |
| Cochrane Library (52) | |
| Web of Science (1352) | |
| Total Results (4806) |
Inclusion/Exclusion criteria
Inclusion criteria were original studies involving the implantation/administration of one or more identifiable, quantifiable, experimental growth factors (i.e., not BMPs or PTH) in an animal model of spinal fusion in the English language. Growth factors were defined as peptide-based molecules that function to regulate cell division/survival.
Our exclusion criteria were studies that involved the implantation of (1) scaffolds without growth factors; (2) BMPs or PTH; (3) non-peptide-based agents; and (4) cells, platelet-rich plasma, or other processed blood products that could confound effects of the growth factors.
All potentially eligible studies meeting the inclusion criteria were determined by 2 reviewers (Cottrill E and Lessing N). A third reviewer (Ahmed AK) served as a referee, resolving any discrepancies. Articles that met predetermined criteria for exclusion were not included in the study.
Data extraction
Extracted data for each animal model included surgical approach (e.g., posterolateral or anterior), level of fusion, animal species and breed, animal age and sex, and any other relevant characteristics of the animals (e.g., ovariectomized or genetically mutated). For each animal group studied, dosages/sizes of all implant materials, including growth factor and scaffold, were recorded. Spinal fusion rates as assessed by manual palpation, the “gold standard” technique[14,15], or alternatively other methods (e.g., micro- computed tomography (CT), plain radiographs, and histological analysis), were extracted, along with the associated follow-up time points.
RESULTS
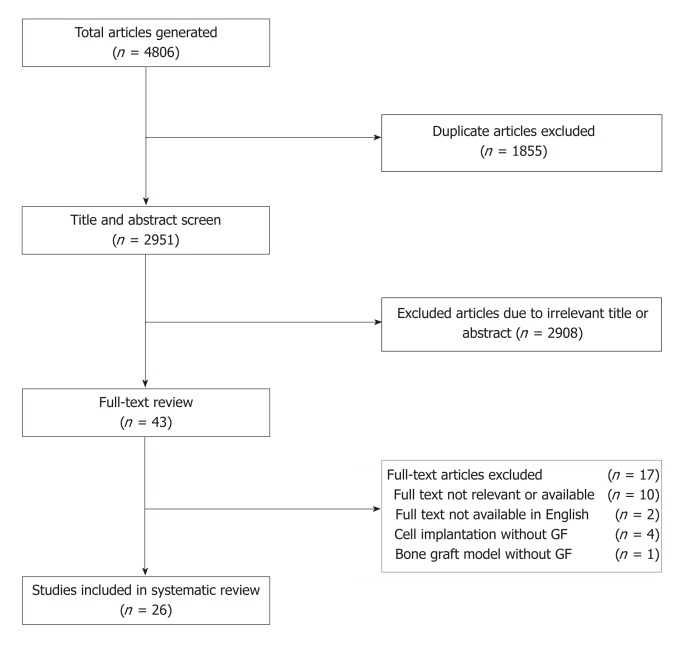
The literature search identified 4806 total articles. Following the predetermined exclusion criteria, 26 articles assessing experimental growth factors in vertebrate animal models of spinal fusion were included in this review (Figure 1). Among the included studies, 14 experimental growth factors have been described: AB204 (n = 1); angiopoietin 1 (n = 1); calcitonin (n = 3); erythropoietin (EPO) (n = 1); basic fibroblast growth factor (bFGF) (n = 1); growth differentiation factor 5 (GDF-5) (n = 4), combined insulin-like growth factor 1 (IGF-1) + transforming growth factor beta (TGF- β) (n = 4); insulin (n = 1); NELL-1 (n = 5); noggin (n = 1); P-15 (n = 1); peptide B2A (n = 2); and secreted phosphoprotein 24 (SPP24) (n = 1). Descriptions of the growth factors are provided in Table 2[16-31]. The demographic characteristics for all included studies are provided in Table 3, and the rates of spinal fusion for all experimental groups are summarized in Table 4. Detailed information of each study is provided in Supplemental Table 1. All the included studies were preclinical animal studies (level of evidence of V).
Figure 1.
Consolidated standards of reporting trials diagram for article selection. GF: Growth factor.
Table 2.
Normal biological activity of each included growth factor
| Growth factors | Normal biologic activity[16-31] |
| AB204 | Chimera of activin A and BMP-2 - which are both members of the transforming growth factor-beta (TGF-beta) superfamily |
| Ang-1 | Pro-angiogenic growth factor that mediates reciprocal interactions between the endothelium and surrounding matrix, inhibits endothelial permeability, and contributes to blood vessel maturation and stability |
| Calcitonin | Secreted by the parafollicular (C cells) of the thyroid gland and is a direct inhibitor of osteoclasts |
| EPO | Produced in the kidney in response to hypoxia and is a well-known growth factor essential for hematopoiesis |
| bFGF | Broad mitogenic and angiogenic functions and is important for limb and nervous system development, wound healing, and tumor growth |
| GDF-5 | Structurally similar to BMP-2 and BMP-7, GDF-5 is a secreted member of the TGF-beta superfamily of proteins involved in the development of various tissues and cell types, as well as the growth of neuronal axons and dendrites |
| IGF-1/TGF-beta | IGF-1 is a protein with similar structure and function to insulin involved in mediating growth and development. TGF-beta is a secreted ligand capable of binding to various TGF-beta receptors involved in embryogenesis and cell differentiation and may play a role in wound healing |
| Insulin | A product of post-translational modification of proinsulin, involved in intracellular glucose uptake |
| NELL-1 | Secreted protein containing epidermal growth factor-like repeats. It binds to the cell surface heterodimer integrin α3β1, resulting in intracellular changes that induce osteoblastogenic programming. Its overexpression is associated with craniosynostosis |
| Noggin | Secreted polypeptide which binds and inactivates members of the transforming growth factor-beta superfamily of proteins. Noggin is important for developmental process such neural tube closure and joint formation, and when mutated can lead to proximal symphalangism and multiple synostoses syndrome |
| P-15 | Synthetic 15-amino acid peptide with an identical sequence to the cell-binding domain found on the α1(I) chain of Type-I collagen. It is combined with an anorganic bovine-derived hydroxyapatite matrix (ABM) to produce an osteoinductive and osteoconductive bone graft alternative |
| Peptide B2A | Synthetic, receptor-targeted peptide that cooperatively enhances biologic BMP-2 response |
| SPP24 | Secreted bone matrix protein that belongs to the cystatin superfamily and binds proteins in the transforming growth factor-beta family of cytokines |
BMP: Bone morphogenetic protein; GDF: Growth differentiation factor; TGF: Transforming growth factor; IGF: Insulin-like growth factor.
Table 3.
Demographic characteristics for all included studies
| Animal model | Studies (n) |
| Dog | 1[32] |
| Rat | 8[33,36,38,47-49,51,56] |
| Rabbit | 7[34,35,37,40,42,52,54] |
| Macaque | 1[20] |
| Sheep | 7[41,43-45,50,53,55] |
| Baboon | 1[39] |
| Goat | 1[46] |
| Spinal levels fused | |
| C3 – C4 | 4[43-46] |
| L1 – L2 | 1[32] |
| L2 – L3 | 1[55] |
| L3 – L4 | 3[20,50,56] |
| L4 – L5 | 12[32,35,36,38,39,41,47-49,51,54,55] |
| L3 – L5 | 2[33,53] |
| L5 – L6 | 7[20,34,37,40,42,50,52] |
| L6 – L7 | 1[35] |
| Spinal region | |
| Cervical | 4[43-46] |
| Lumbar | 22[20,32-42,47-56] |
| Biomechanical location | |
| Mobile segments | 11[20,32,33,43-46,50,53,55,56] |
| Junctional segments | 21[20,32-42,47-55] |
| Number of levels fused | |
| 1-Level fusion | 19[34,36-49,51,52,54,56] |
| Two separate 1-level fusions | 5[20,32,35,50,55] |
| 2-Level fusion | 2[33,53] |
| Surgical approach | |
| Anterior | 8[20,43-46,50,53,55] |
| Posterior | 18[32-42,47-49,51,52,54,56] |
Table 4.
Experimental models and rates of bony fusion for each included study
| Growth factor | Animal (n) | Levels (a/p)1 | Experimental groups | Fusion rate |
| AB204 | ||||
| Zheng et al[32], 2017 | Dog (n = 56) | L1-L2, L4-L5 (p) | (1) BCP; (2) BCP + rhBMP-2; (3) BCP + AB204 | (1) 6.3%; (2) 15%; (3) 90%2 |
| COMP-Ang-1 | ||||
| Park et al[33], 2011 | Rat (n = 56) | L3-L5 (p) | (1) ICBG; (2) ICBG + bovine serum albumin-impregnated collagen sponge; (3) ICBG + COMP-Ang-1-impregnated collagen sponge | (2) 38.9%; (2) 42.1%; (3) 89.5%2 |
| Calcitonin | ||||
| Babat et al[34], 2005 | Rabbit (n = 56) | L5-L6 (p) | (1) ICBG; (2) ICBG + calcitonin; (3) ICBG + pamidronate | (1) 56%; (2) 68%; (3) 37% |
| Liu et al[35], 2012 | Rabbit (n = 32) | L4-L5, L6-L7 (p) | (1) ICBG; (2) ICBG + calcitonin; (3) ICBG + interspinous fixation (steel wire); (4) ICBG + interspinous fixation (steel wire) + calcitonin | (1) 75%; (2) 100%; (3) 75% 100% |
| Liu et al[36], 2015 | Rat (n = 50) | L4-L5 (p) | (1) Sham surgery + saline vehicle; (2) Ovariectomy + saline vehicle; (3) Spinal fusion (ICBG) + saline vehicle; (4) Ovariectomy + Spinal fusion (ICBG); (5) Ovariectomy + Spinal fusion (ICBG) + calcitonin | Not reported |
| EPO | ||||
| Rolfing et al[37], 2011 | Rabbit (n = 28) | L5-L6 (p) | (1) ICBG + Epoetin beta subcutaneous injection; (2) ICBG + Saline subcutaneous injection | (1) 86%; (2) 71% |
| bFGF | ||||
| Inoue et al[38], 2017 | Rat (n = 20) | L4-L5 (p) | (1) Allograft; (2) Allograft + bFGF | Not reported |
| GDF-5 | ||||
| Spiro et al[39], 2000 | Baboon (n = 36) | L4-L5 (p) | (1) ICBG; (2) Collagen matrix strips; (3) Collagen matrix strips + rhGDF-5 (500μg/cm3); (4) Collagen matrix strips + rhGDF-5 (1500μg/cm3) | (1) 22%; (2) 0%; (3) 44%; (4) 11% |
| Spiro et al[40], 2001 | Rabbit (n = 31) | L5-L6 (p) | (1) ICBG; (2) Hydroxyapatite-mineralized collagen matrix (Matrix); (3) Matrix + bone marrow; (4) Healos strips + rhGDF-5 (0.1 mg/cc); (5) Healos strips + rhGDF-5 (1.0 mg/cc); (6) Non-crosslinked collagen strips + rhGDF-5 (0.1 mg/cc); (7) Non-crosslinked collagen strips + rhGDF-5 (1.0 mg/cc); (8) Collagen fiber slurry + rhGDF-5 (0.1 mg/cc); (9) Collagen fiber slurry + rhGDF-5 (1.0 mg/cc) | (1) 33%; (2) 0%; (3) 0%; (4) 0%; (5) 67%; (6) 75%; (7) 80%; (8) 25%; (9) 0% |
| Jahng et al[41], 2004 | Sheep (n = 8) | L4-L5 (p) | (1) ICBG; (2) Healos + GDF-5 | (1)100%; (2) 100% |
| Magit et al[42], 2006 | Rabbit (n = 65) | L5-L6 (p) | (1) ICBG; (2) Healos; (3) Healos + rhGDF-5 (0.5 mg/cc); (4) Healos + rhGDF-5 (1 mg/cc); (5) Healos + rhGDF-5 (1.5 mg/cc) | (1) 38%; (2) 0%; (3) 100%2; (4) 100%2; (5) 100%2 |
| IGF-1/TGF-β | ||||
| Kandziora et al[43], 2002 | Sheep (n = 32) | C3-C4 (a) | (1) Titanium cage (Cage); (2) Cage + ICBG; (3) Cage + PDLLA + BMP-2; (4) Cage + PDLLA + rh-IGF-1/TGF-β | (1) 0%; (2) 13%; (3) 13%; (4) 13% |
| Kandziora et al[44], 2002 | Sheep (n = 32) | C3-C4 (a) | (1) ICBG; (2) Titanium cage (Cage); (3) Cage + PDLLA; (4) Cage + PDLLA + rh-IGF-1/TGF-β | (1) 0%; (2) 0%; (3) 0%; (4) 12.5% |
| Kandziora et al[45], 2003 | Sheep (n = 32) | C3-C4 (a) | (1) Titanium cage + PDLLA (Cage); (2) Cage + rh-IGF-1/TGF-β (2.5/0.5%); (3) Cage + rh-IGF-1/TGF-β (5/1%); (4) Cage + rh-IGF-1/TGF-β (10/2%) | Not reported |
| Gu et al[46], 2006 | Goat (n = 32) | C3-C4 (a) | (1) ICBG; (2) Titanium cage (Cage); (3) Cage + hydroxyapatite; (4) Cage + IGF-1/TGF-β (5/1%) | (1) 0%; (2) 25%; (3) 38%; (4) 63% |
| Insulin | ||||
| Koerner et al[47], 2013 | Rat (n = 19) | L4-L5 (p) | (1) ICBG + 100% palmitic acid; (2) ICBG + Linplant (95% palmitic acid and 5% bovine insulin) | (1) 11%; (2) 60%2 |
| NELL-1 | ||||
| Lee et al[48], 2009 | Rat (n = 10) | L4-L5 (p) | (1) Demineralized bone putty (Putty) + PBS; (2) Putty + rh-NELL-1 | (1) 0%; (2) 60% |
| Li et al[49], 2010 | Rat (n = 24) | L4-L5 (p) | (1) DBX + PBS; (2) DBX + NELL-1 (2.5 g); (3) DBX + NELL-1 (5 μg) | (1) 25%; (2) 75%; (3) 88% |
| Siu et al[50], 2011 | Sheep (n = 32) | L3-L4 L5-L6 (a) | (1) Cage + DBM; (2) Cage + inactivated DBM; (3) Cage + DBM + NELL-1 (0.3 mg/mL) Cage + DBM + NELL-1 (0.6 mg/mL); (4) Cage + inactivated; (5) DBM + NELL-1 (0.3 mg/mL); (6) Cage + inactivated DBM + NELL-1 (0.3 mg/mL) | (1) 50%; (2) 50%; (3) 87.5%2; (4) 100%2; (5) 100%2; (6) 100%2 |
| Yuan et al[51], 2013 | Rat (n = 26) | L4-L5 (p) | (1) DBX + PBS; (2) DBX + NELL-1 (10μg); (3) DBX + NELL-1 (50μg); (4) Acellular collagen sponge (ACS) + PBS; (5) ACS + BMP-2; (6) ICBG | (1) 20%; (2) 100%; (3) 100%; (4) 0%; (5) 100%; (6) 0% |
| James et al[20], 2017 | Macaque (n = 12) | L3-L4 L5-L6 (a) | (1) Cage + aTCP; (2) Cage + DBX + rh-NELL-1-loaded aTCP (1mg/mL); (3) Cage + DBX + rh-NELL-1-loaded aTCP (1.7mg/mL) | (1) 25%; (2) 25%; (3) 100% |
| Noggin | ||||
| Klineberg et al[52], 2014 | Rabbit (n = 25) | L5-L6 (p) | (1) ICBG + Noggin scrambled siRNA bilateral; (2) ICBG + scrambled siRNA one side + siRNA other side; (3) ICBG + functional siRNA bilateral | (1) N/A (2) N/A (3) 50% |
| P-15 | ||||
| Sherman et al[53], 2010 | Sheep (n = 12) | L3-L5 (a) | (1) PEEK + ICBG; (2) PEEK + anorganic bovine-derived matrix/P-15 | (1) 83%; (2) 100% |
| Peptide B2A | ||||
| Smucker et al[54], 2008 | Rabbit (n = 45) | L4-L5 (p) | (1) ICBG; (2) ICBG + TCP (1:1); (3) ICBG + B2A (50 μg); (4) ICBG + B2A (100 μg); (5) ICBG + B2A (300 μg) | (1) 25%; (2) 22%; (3) 56%; (4) 78%2 (vs 1 and 2); (5) 40% |
| Cunningham et al[55], 2009 | Sheep (n = 40) | L2-L3 L4-L5 (a) | (1) PEEK + ICBG; (2) PEEK + ICBG + B2A (50 μg); (3) PEEK + ICBG + B2A (100 μg); (4) PEEK + ICBG + B2A (300 μg); (5) PEEK + ICBG + B2A (600 μg) | (1) 63%; (2) 88%; (3) 88%; (4) 88%; (5) 75% |
| SPP24 | ||||
| Sintuu et al[56], 2011 | Rat | L4-L5 (p) | (1) Collagen sponge (Sponge); (2) Sponge + rhBMP-2 (10 µg); (3) Sponge + rhBMP-2 (1 μg) + SPP24 (100 μg); (4) Sponge + rhBMP-2 (1 μg) + SPP24 (500 μg); (5) Sponge + rhBMP-2 (1 μg) + SPP24 (1 mg); (6) Sponge + rhBMP-2 (1 μg) + SPP24 (2.5 mg); (7) Sponge + rhBMP-2 (1 μg) + SPP18 (100 μg); (8) Sponge + rhBMP-2 (1 μg) + SPP18 (500 μg); (9) Sponge + rhBMP-2 (1 μg) + SPP18 (1 mg); (10) Sponge + rhBMP-2 (1 μg) + SPP18 (2.5 mg); (11) Sponge + rhBMP-2 (10 μg) + SPP24 (100 μg); (12) Sponge + rhBMP-2 (10 μg) + SPP24 (500 μg); (13) Sponge + rhBMP-2 (10 μg) + SPP24 (1 mg); (14) Sponge + rhBMP-2 (10 μg) + SPP24 (2.5 mg); (15) Sponge + rhBMP-2 (10 μg) + SPP18 (100 μg); (16) Sponge + rhBMP-2 (10 μg) + SPP18 (500 μg); (17) Sponge + rhBMP-2 (10 μg) + SPP18 (1 mg); (18) Sponge + rhBMP-2 (10 μg) + SPP18 (2.5 mg) | (1) 0%; (2) 100%2; (3) 0%; (4) 0%; (5) 0%; (6) 0%; (7) 0%; (8) 0%; (9) 0%; (10) 0%; (11) 0%; (12) 0%; (13) 0%; (14) 0%; (15) 0%; (16) 0%; (17) 0%; (18) 0% |
(a/p): anterior/posterolateral surgical approach; N2: Statistically significant compared to all other experimental groups. ICBG: Iliac crest bone graft. BCP: Biphasic calcium phosphate; rhBMP-2: Recombinant human bone morphogenetic protein 2; bFGF: Basic fibroblast growth factor; GDF-5: Growth differentiation factor 5; PDLLA: Poly-(D,L-lactide); TGF: Transforming growth factor; IGF: Insulin-like growth factor.
AB204
Zheng et al[32] compared the fusion rates between AB204 and rhBMP-2 in a beagle posterolateral lumbar (L1-L2 and L4-L5) model. Investigating biphasic calcium phosphate (BCP), rhBMP-2 + BCP, and AB204 + BCP, they reported that the AB204 group showed a significantly higher fusion rate (90%) compared to the rhBMP-2 group (15%) and the BCP-only group (6.3%) as assessed by manual palpation at 8 wk postoperatively.
Angiopoietin 1
Park et al[33] investigated the effects of COMP-Ang-1 on spinal fusion in a Sprague-Dawley rat posterolateral lumbar (L3-L5) model. Investigating iliac bone allograft (Allo), bovine serum albumin (BSA)-impregnated absorbable collagen sponge + Allo, and COMP-Ang-1-impregnated absorbable collagen sponge + Allo, they reported that the COMP-Ang-1 group showed a significantly higher fusion rate (89.5%) compared to the BSA group (42.1%) and the Allo-only group (38.9%) as assessed by manual palpation at 6 wk postoperatively.
Calcitonin
Babat et al[34] investigated the effects of calcitonin (postoperatively) and pamidronate (pre- and postoperatively) on spinal fusion in a New Zealand White rabbit posterolateral lumbar (L5-L6) model. Fusion rates were determined for each treatment group: autologous iliac crest bone graft (autograft) alone (56%), autograft + calcitonin (68%), and autograft + pamidronate (37%). Fisher exact test showed no significant differences between groups at 5 wk postoperatively.
In addition, Liu et al[35] investigated the effect of daily post-operative calcitonin administration on spinal fusion in a New Zealand White rabbit posterolateral lumbar (L4-5, without wire fixation of the spinous processes; and L6-L7, with wire fixation of the spinous processes) model. With both fixation and without fixation, the bone grafts receiving calcitonin had a higher fusion rate (100% vs 75%), higher histological score, and increased expression of pro-osteogenic and pro-angiogenic genes [i.e., Col I and BMP-2, IGF-1 and vascular endothelial growth factor (VEGF)] at 8 wk postoperatively.
Additionally, Liu et al[36] investigated the effects of calcitonin (postoperatively) on spinal fusion in an ovariectomized/normal Sprague–Dawley posterolateral lumbar (L4-5, with wire fixation of spinous processes) model. They found significantly enhanced fusion mass, bone mineral density, and microstructural parameters in calcitonin-treated ovariectomized animals at 12 wk postoperatively compared to non-treated ovariectomized animals as assessed via radiographs, micro-CT, and histologic analysis. Fusion rate was not reported.
EPO
Rolfing et al[37] investigated the effects of EPO (daily subcutaneous injection) on spinal fusion in a New Zealand White rabbit posterolateral lumbar (L5-L6) fusion model. At 6 wk postoperatively, the fusion rate was 86% in the EPO treated group + autograft and 71% in the autograft-alone group as assessed via manual palpation. Additionally, the bone fusion volume (micro-CT) and angiogenesis (actin stained blood vessels) were both significantly greater in the EPO treatment group.
bFGF
Inoue et al[38] investigated the effects of an engineered bFGF on spinal fusion in a Sprague-Dawley rat posterolateral lumbar (L4-L5) model. Two fusion groups were studied: femoral freeze-dried bone allograft incubated with an engineered bFGF or with phosphate buffered saline. They found that the bFGF group had a significantly higher mean grafted bone volume (radiography) as well as significantly greater new bone formation on the surface of the laminae and spinous processes (micro-CT) compared to the control group 14 d postoperatively. Fusion rate was not reported.
GDF-5
Spiro et al[39] investigated the effects of rh-GDF-5 on spinal fusion in a female baboon posterolateral lumbar (L4-L5) model. They investigated four groups: Healos® with or without rh-GDF-5 or iliac crest autograft. The fusion rate, as assessed via radiographic/CT analysis, was 44% for the 500 micrograms rh-GDF-5/cm3 Healos® group, 11% for the 1500 micrograms rh-GDF-5/cm3 Healos® group, 22% for the autograft group, and 0% for the Healos® alone group at 20 wk postoperatively.
In addition, Spiro et al[40] investigated the effects of rh-GDF-5 in several different formulations with collagen matrices in a New Zealand White rabbit posterolateral lumbar (L5-L6) model. They found that rh-GDF-5 added to non-crosslinked mineralized Type I bovine collagen strips resulted in a fusion rate, as assessed histologically, of 75% at a concentration of 0.1 mg growth factor/cm3 collagen and 80% at a concentration of 1.0 mg growth factor/cm3 collagen at 12 wk postoperatively. This compared to 33% for iliac crest autograft, 0% for the collagen strips alone, and 0% for the collagen strips with bone marrow aspirate from the iliac crest.
Additionally, Jahng et al[41] investigated the effects of Healos® with rh-GDF-5 in a sheep endoscopic instrumented (pedicle screws and plate) posterolateral lumbar (L4-L5) model. They found that at 4 and 6 mo 100% fusion was observed in both autograft and bone graft substitute groups, and no significant differences were observed between the groups via histological assessment, including the formation of vascular elements.
Magit et al[42] investigated the effects of rh-GDF-5 in New Zealand White rabbit posterolateral lumbar (L5-L6) model. The authors found that fusion rates, as assessed via manual palpation, were 38% in the autograft group, 0% in the Healos® alone group, and 100% in each of the experimental Healos®/rhGDF-5 groups at 8 wk of follow-up. Further, via micro-CT analysis, bone formation in the experimental rhGDF-5 groups were observed to be significantly greater than in the other study groups.
IGF-1/TGF- β
Kandziora et al[43] investigated the effects of combined IGF-1/TGF-beta-1 on spinal fusion in a sheep anterolateral cervical (C3/4) interbody model. They investigated using a titanium cage alone, titanium cage filled with autologous iliac crest bone graft, titanium cage coated with a biodegradable poly-(D,L-lactide) (PDLLA) carrier including rh-BMP-2, and titanium cage coated with a biodegradable PDLLA carrier including rh-IGF-1 (5% w/w) and rh-TGF-beta-1 (1% w/w). As assessed via CT, the BMP-2 and IGF-1/TGF-beta-1 groups led to intervertebral masses with a maximum intervertebral gap in the craniocaudal direction of less than 5 mm or complete fusion in 75% of animals, compared to 50% for the autograft group and 25% for the cage-alone group at 12-wk postoperatively.
In addition, Kandziora et al[44] investigated the effects of IGF-1/TGF-beta-1 in a sheep anterolateral cervical (C3-C4) interbody model using autologous tricortical iliac crest bone graft, a titanium cage alone, titanium cage with a PDLLA carrier, and a titanium cage with a PDLLA carrier including IGF-F and TGF-beta-1. The authors observed a fusion rate of 0% in the autograft, cage-alone, and cage plus PDLLA groups and 12.5% in the IGF-1/TGF-beta-1 group at 12 weeks postoperatively.
Additionally, Kandziora et al[45] investigated the effects of IGF-1/TGF-beta-1 in a sheep anterolateral (C3-C4) interbody model using a titanium cage coated with a PDLLA carrier including no growth factors, as well as with different concentrations of IGF-1 and TGF-beta-1. The authors concluded that the application of IGF-1 and TGF-beta-1 by a PDLLA-coated cage significantly improves interbody bone formation in a dose dependent manner, as assessed via micro-CT and histomorphometrical analysis, at 12 wk postoperatively. Fusion rate was not reported.
Further, Gu et al[46] investigated the effects of IGF-1 and TGF-beta-1 in a goat cervical (C3-C4) interbody model. Four groups were studied: autologous iliac crest bone graft, a hat-shaped titanium cage, a hat-shaped cage coated with hydroxyapatite, and a hat-shaped cage coated with hydroxyapatite plus IGF-1 and TGF-beta-1. As assessed via histomorphologic examination, the IGF-1 and TGF-beta-1 group led to fusion in 63% of animals, compared to 38% for the hydroxyapatite group, 25% for the cage alone group, and 0% for the autograft group at 12-wk postoperatively.
Insulin
Koerner et al[47] investigated the effects of time-released insulin on spinal fusion in a Sprague-Dawley posterolateral lumbar (L4-L5) model. The authors used iliac crest autograft plus either Linplant (95% micro-recrystallized palmitic acid and 5% bovine insulin) or a sham implant (100% palmitic acid). At 8 wk postoperatively, the fusion rate was 60% in the Linplant group compared to 11% in the control group, as assessed by manual palpation. In addition, half the animals in each group were euthanized on postoperative day 4 and analyzed for growth factors: IGF-I (but not TGF-beta-1, PDGF-AB, or VEGF) was significantly higher in the Linplant group.
Neural EGFL Like 1 (NELL-1)
Lee et al[48] investigated the effects of NELL-1 on spinal fusion in a male athymic rat posterolateral lumbar (L4-L5) model. They found that rh-NELL-1 lyophilized onto apatite-coated alginate/chitosan microparticles and mixed with demineralized bone matrix (DBM) led to a fusion rate of 60% at 4 wk postoperatively, compared to a fusion rate of 0% using PBS instead of rh-NELL-1.
Similarly, Li et al[49] investigated the effects of NELL-1 on spinal fusion in an athymic rat posterolateral lumbar (L4-5) model. The authors found that NELL-1 lyophilized onto β-tricalcium phosphate (TCP) microparticles and mixed with DBX (a type of DBM) led to a fusion rate of 75% (2.5 micrograms NELL-1) and 88% (5 micrograms NELL-1) at 4 wk postoperatively, compared to a fusion rate of 25% using PBS instead of NELL-1.
Siu et al[50] investigated the effects of NELL-1 on spinal fusion in a skeletally mature Rambouillet × Columbian ewe posterolateral lumbar (L3-L4 and L5-L6) model. Six groups with different implant compositions were studied: DBM alone or mixed with NELL-1 (0.3 or 0.6 mg/mL) and heat-inactivated DBM (inDBM) alone or mixed with NELL-1 (0.3 or 0.6 mg/mL). At 3 mo postoperatively, the fusion rates were 88% for DBM + 0.3 mg/mL NELL-1, 100% for DBM + 0.6 mg/mL NELL-1, and 50% for DBM alone. At 4 mo postoperatively, the fusion rates were 100% for inDBM + 0.3 mg/mL NELL-1, 100% for inDBM + 0.6 mg/mL NELL-1, and 50% for inDBM alone.
Yuan et al[51] investigated the effects of NELL-1 in a male athymic rat posterolateral lumbar (L4-5) model. They found that DBX mixed with NELL-1 (10 or 50 microgram) led to a 100% fusion rate at 4 wk postoperatively, compared to fusion rates of 20% using PBS instead of NELL-1, 100% using an acellular collagen sponge and BMP-2 (90 micrograms), and 0% using iliac crest autograft.
James et al[20] investigated the effects of NELL-1 on spinal fusion in a 5- to 7-year-old Rhesus macaque posterolateral lumbar (L3-L4 and L5-L6) model. Three groups were studied: intervertebral cage plus DBX mixed with saline- or rh-NELL-1-(1.0 or 1.7 mg/mL) loaded apatite-coated β-tricalcium phosphate (aTCP) particles. At 4 mo postoperatively, fusion rates as assessed via CT were 100% for the higher dose of rh-NELL-1, 25% for the lower dose of rh-NELL-1, and 25% for the saline control. Additionally, immunofluorescence staining showed increased Sca-1+CD31–CD45– stromal cells in the rh-NELL-1 treated groups compared to the saline control.
Noggin
Klineberg et al[52] investigated the effects of noggin on spinal fusion in a skeletally mature New Zealand White rabbit posterolateral lumbar (L5-L6). Noggin siRNA was injected into the paraspinal muscles to interrupt the negative feedback loop on endogenous BMP. Autologous iliac crest bone graft with paraspinal injections of either scrambled (non-functional) siRNA bilaterally, scrambled siRNA on one side of the spine and functional noggin siRNA on the other, or functional noggin siRNA bilaterally were studied. As assessed via manual palpation, the fusion rate of the bilateral functional siRNA group was 50%, which was not significantly different compared to historical autograft-only controls from the group, despite the fact that noggin protein was successfully knocked down in vivo for the initial 7 days before returning to normal levels by 6 wk.
P-15
Sherman et al[53] investigated the effects of an organic bovine-derived hydroxyapatite matrix combined with a synthetic 15 amino acid residue (ABM/P-15) on spinal fusion in a skeletally mature ewe anterolateral lumbar (L3-L5) model. Sheep were treated with polyetheretherketone (PEEK) interbody rings filled with autologous iliac crest bone graft at one level and AMB/P-15 formulated in a carboxymethylcellulose hydrogel matrix at the other. At 6 mo postoperatively, 100% of fusion sites in both groups achieved successful bony arthrodesis as assessed via CT, and histomorphometric analysis showed no statistically significant differences in the fusion masses between these groups.
Peptide B2A
Smucker et al[54] investigated the effects of B2A on spinal fusion in a skeletally mature New Zealand White rabbit posterolateral lumbar (L4-L5) model. The authors investigated iliac crest autograft alone and 1:1 mixtures of autograft and B2A-coated ceramic granules (CG) (0, 50, 100, and 300 μg B2A/mL CG). As assessed via manual palpation at 6 wk postoperatively, the fusion rates were 25% for the autograft alone group and 22%, 56%, 78%, and 40% for the 0, 50, 100, and 300 μg B2A/mL CG groups, respectively. The newly formed bone in the B2A-treated groups appeared morphologically normal without hyperplasia.
Cunningham et al[55] investigated the effects of B2A on spinal fusion in a 3- to 6-year-old crossbred Suffolk sheep anterolateral lumbar (L2-L3 and L4-L5) model. The sheep were treated with a PEEK interbody cage packed with 1:1 mixtures of autograft and B2A-coated ceramic granules (CG) (0, 50, 300, or 600 μg B2A/mL CG). As assessed via CT at 4 mo postoperatively, the fusion rates were 63%, 88%, 88%, and 75% for the 0, 50, 300, and 600 μg B2A/mL CG groups, respectively. In biomechanical testing, no statistically significant differences were observed between any of the groups.
SPP24
Sintuu et al[56] investigated the effects of SPP24 on spinal fusion in a 6- to 8-wk-old male Lewis rat posterolateral lumbar (L3-L4) model. Bilaterally placed implant materials consisted of collagen sponges soaked in high or low dose rh-BMP-2 (1 or 10 micrograms), plus treatment: 0, 0.1, 0.5, 1.0, or 2.5 mg of either full-length spp24 or truncated spp18 (solely the BMP-binding region of the full peptide). As assessed via manual palpation at 8 wk postoperatively, the fusion rate was 0% in all specimens treated with rh-BMP-2 and spp18 or spp24 (any concentration), compared to a fusion rate of 100% in specimens treated with rh-BMP-2 (10 micrograms) alone. Further, spp24 showed a greater inhibitory impact compared to spp18.
DISCUSSION
Pseudoarthrosis following spinal surgery can lead to significant patient morbidity and diminished quality of life, with unpredictable clinical outcomes following revision surgery. Optimizing the rate of spinal fusion relies on enhanced surgical technique, effective biologics (e.g., growth factors), instrumentation, and a greater appreciation of the local physiology[57]. Following FDA approval in 2002 for use in the anterior lumbar spine, rhBMP-2 revolutionized the role for growth factor adjuncts in spinal fusion[58,59], drastically increasing in use from 5.5% of all fusion cases in 2003 to 28.1% of all fusion cases in 2008 in the United States[60]. However, rhBMP-2 is associated with significant complications[6,7], which has fueled the investigation of different growth factors for spinal fusion. Despite significant research interest in this area, there are no published systematic reviews summarizing the state of the art in experimental growth factors for spinal fusion.
The present systematic review, across 4 databases, resulted in the inclusion of 26 spinal fusion animal studies comprising 14 investigational growth factors. The fusion rates of the current gold standard treatment (autologous iliac crest bone graft, ICBG) and the leading clinically used growth factor (BMP-2) ranged widely in the included studies, from 0-100% for ICBG[44,46,51] and from 13%-100% for BMP-2[43,51,56]. This variation reflects the unpredictable clinical outcomes following spinal fusion surgery and supports the need for efficacious materials that promote strong and reliable spinal fusion. Among the experimental growth factors, four resulted in fusion rates of 100% in some cases (Table 4): calcitonin[35], GDF-5[41,42], NELL-1[20,50,51], and P-15[53]. In addition, six growth factors resulted in significantly enhanced fusion rates compared to ICBG, BMP-2, or other internal control in some studies (Table 4): AB204 vs BMP-2[32], COMP-Ang-1 vs ICBG[33], GDF-5 vs ICBG[42], insulin (as Linplant) vs internal control (ICBG plus sham implant)[47], NELL-1 vs internal control (DBM)[50], and Peptide B2A vs ICBG[54]. The majority of other identified growth factors resulted in fusion rates similar to ICBG (Table 4); only SPP24 was shown to significantly decrease the rate of spinal fusion[56]. Directly comparing different growth factors herein is difficult given the extensive heterogeneity in animal species, fusion method, and experimental groups and timepoints across the studies (Tables 3 and 4). Further, it is known that the scaffolds themselves affect bone formation[61,62], possibly confounding the effects of the growth factors across studies.
In addition, similar effects on spinal fusion were generally, though not always, observed when multiple studies investigated the same growth factor. For example, several groups investigated the effects of NELL-1 on spinal fusion[20,48-51]. In all these experiments, NELL-1 was shown to enhance fusion rates. In contrast, for GDF-5, Magit et al[42] observed a significantly enhanced fusion rate compared to ICBG, while Jahng et al[41] observed a 100% fusion rate for both GDF-5 and ICBG groups, and Spiro et al[39] observed a non-significant decrease in fusion rate using GDF-5 (1500 micrograms/cm3) compared to ICBG. The differences in animal species (rabbit, sheep, and baboon) and surgical method (endoscopic with instrumentation and posterolateral without instrumentation) may help to account for these variations.
The successful clinical translation of any factor intended to enhance spinal fusion will depend not only on its capacity to promote strong and reliable spinal fusion in humans, but also on its safety profile (i.e., the associated local and systemic complications). At present, the growth factors AB204, COMP-Ang-1, GDF-5, NELL-1, P-15, insulin, and Peptide B2A represent some of the most promising investigational growth factors for promoting spinal fusion, with each demonstrating fusion efficacy in preclinical studies. However, the safety profiles of these growth factors in the setting of spinal fusion are largely unknown. In our review, none of the included studies reported complications directly related to the growth factors, though this absence of evidence obviously does not mean the absence of complications, any of which could hinder or halt clinical translation. Future work investigating the efficacy and safety of these growth factors not only in larger numbers of animals but also in higher-order species will be important for informing their potential clinical translation.
Interestingly, this systematic review found that, within the inclusion/exclusion criteria of our study, relatively few (i.e., fourteen) unique growth factors have been investigated in preclinical animal models of spinal fusion. This reveals that a relatively select group of growth factors in the overall setting of bone tissue engineering has been investigated in spinal fusion. For example, growth factors like stromal-derived growth factor 1 (SDF-1) and platelet derived growth factor (PDGF), both of which have been studied in the setting of regenerating critical sized bone defects[63,64], are notably absent in the preclinical spine fusion literature. While tissue engineering for spinal fusion is unique from other areas of bone tissue engineering in that the fusion site may be in motion during the fusion process, our review suggests potential new research strategies regarding the investigation of currently unexplored growth factors (e.g., SDF-1 and PDGF) for spinal fusion. Lastly, it is notable that relatively few studies involved combinations of growth factors[43-46]. We believe that the simultaneous or sequential delivery of multiple different growth factors may result in a synergistic enhancement in spinal fusion. We encourage future work in these areas, as well as in continued advancements in growth factor delivery methods and scaffold materials, towards the development of efficacious and safe, clinically translatable materials for spinal fusion.
Schimandle et al[65] in 1994, Sandhu et al[66] in 2002, and Drespe et al[67] in 2005 previously published reviews of animal models for spinal fusion. These reviews focused on the different species utilized, technical methodology, and representative outcomes. To the best of our knowledge, the present study is the first to systematically review investigational growth factors utilized in animal models of spinal fusion. Despite the novelty of this review, there are several limitations, including those inherent to systematic reviews. Additionally, many of the animal models differ with regard to the methodology and data collected (Tables 3 and 4). As a result of this heterogeneity, directly comparing end points (i.e., rates of fusion) across multiple studies is not possible. Further, three studies did not report fusion rates[36,38,45], limiting the interpretability of those studies. In addition, our review excludes growth factors that have been studied clinically in spinal fusion.
In conclusion, this is the first study to systematically review all the published investigational growth factors utilized in preclinical animal models for spinal fusion. Future studies aimed at directly comparing the most promising experimental growth factors identified herein - e.g., AB204, COMP-Ang-1, GDF-5, NELL-1, P-15, insulin, Peptide B2A, and others (Table 4) - in preclinical models may inform the development of efficacious, clinically translatable materials for spinal fusion. Further, future work involving the safety and cost of production of these growth factors, in comparison to BMP-2, may support the replacement of BMP-2 for safer and more cost-effective growth factors for spinal fusion.
ARTICLE HIGHLIGHTS
Research background
Over 400000 Americans undergo spinal fusion surgeries each year, with the number increasing yearly alongside a growing and aging population. However, pseudoarthrosis, or failed fusion, rates are reported to be as high as 40% in primary spinal fusion surgery and up to 60% in revision cases, even when the "gold standard" treatment of grafting bone from the patient’s own iliac crest is used.
Research motivation
To date, no study has systematically reviewed the experimental growth factors investigated for spinal fusion in preclinical animal models. Considering the efficacy and widespread use of recombinant growth factors (i.e., rhBMP-2 and rhPTH) to optimize spinal fusion, a broad assessment of experimental growth factors is essential to inform future work and clinical potential in this area.
Research objectives
Systematically review all published translational animal models assessing investigational growth factors for spinal fusion and identify promising agents for translation.
Research methods
A systematic review of the literature using PubMed, Embase, Cochrane Library, and Web of Science databases was performed. Inclusion criteria were original studies involving the implantation/administration of one or more identifiable, quantifiable, experimental growth factors (i.e., not BMPs or PTH) in an animal model of spinal fusion in the English language. Exclusion criteria were studies that involved the implantation of (1) scaffolds without growth factors; (2) BMPs or PTH, (3) non-peptide-based agents, and (4) cells, platelet-rich plasma, or other processed blood products that could confound effects of the growth factors. PRISMA guidelines were followed for this systematic review.
Research results
The literature search identified 4806 total articles, from which 26 articles met the inclusion/exclusion criteria and were included in this review. Among the included studies, 14 experimental growth factors were identified: AB204 (n = 1); angiopoietin 1 (n = 1); calcitonin (n = 3); erythropoietin (n = 1); basic fibroblast growth factor (n = 1); growth differentiation factor 5 (n = 4), combined insulin-like growth factor 1 + transforming growth factor beta (n = 4); insulin (n = 1); NELL-1 (n = 5); noggin (n = 1); P-15 (n = 1); peptide B2A (n = 2); and secreted phosphoprotein 24 (n = 1). Among the identified growth factors, calcitonin, GDF-5, NELL-1, and P-15 resulted in fusion rates of 100% in some cases. In addition, six growth factors - AB204, angiopoietin 1, GDF-5, insulin, NELL-1, and peptide B2A - resulted in significantly enhanced fusion rates compared to ICBG, BMP-2, or other internal control in some studies. Large heterogeneity in animal species, fusion method, and experimental groups and time points was observed across the included studies, limiting the direct comparison of the growth factors identified herein.
Research conclusions
This is the first study to systematically review all the published investigational growth factors utilized in preclinical animal models of spinal fusion. Future studies aimed at directly comparing the most promising investigational growth factors identified herein - e.g., AB204, COMP-Ang-1, GDF-5, NELL-1, P-15, insulin, Peptide B2A, and others - in preclinical models may inform the development of efficacious and safe, clinically translatable materials for spinal fusion.
Research perspectives
The successful clinical translation of any factor intended to enhance spinal fusion will depend not only on its capacity to promote strong and reliable spinal fusion in humans, but also on its safety profile. Our study reveals that relatively few growth factors and delivery strategies in the overall setting of bone tissue engineering have been investigated in spinal fusion. We encourage future investigation of currently unexplored growth factors for spinal fusion, as well as continued advancements in growth factor delivery methods and scaffold materials, towards the development of efficacious and safe, clinically translatable materials for spinal fusion.
ACKNOWLEDGEMENTS
We would like to thank Ms. Carrie Price, MLS, for technical assistance with the literature search.
Footnotes
Conflict-of-interest statement: Goodwin CR: Grants from NIH/NINDS K12 Physician Scientist Award, North Carolina Spine Society, and Burroughs Wellcome Funds; all outside the submitted work. Lo SF: Research support from Chordoma Foundation, Grant from AO Spine; all outside the submitted work. Sciubba DM: Consulting from Baxter, DePuy Synthes, Globus, K2M, Medical Device Business Services, Medtronic, NuVasive, and Stryker; Speaking/Teaching from Globus Medical, Medtronic, and DePuy Synthes; all outside the submitted work. Theodore N: Royalties from Globus Medical, Depuy Synthes; Stock in Globus Medical; Consulting from Globus Medical; Scientific Advisory Board from Globus Medical; Fellowship Support from AO North America; all outside the submitted work. Witham TF: Grants from Eli Lilly Company and the Gordon and Marilyn Macklin Foundation.
PRISMA Checklist: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Manuscript source: Unsolicited manuscript
Peer-review started: October 23, 2018
First decision: November 15, 2018
Article in press: January 26, 2019
Specialty type: Orthopedics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Pavone V; Paschos NK; Guerado E S-Editor: Dou Y L-Editor: A E-Editor:Wu YXJ
Contributor Information
Ethan Cottrill, Department of Neurosurgery, The Johns Hopkins Hospital, Baltimore, MD 21287, United States. ecottri1@jhmi.edu.
A Karim Ahmed, Department of Neurosurgery, The Johns Hopkins Hospital, Baltimore, MD 21287, United States.
Noah Lessing, Department of Neurosurgery, The Johns Hopkins Hospital, Baltimore, MD 21287, United States.
Zachary Pennington, Department of Neurosurgery, The Johns Hopkins Hospital, Baltimore, MD 21287, United States.
Wataru Ishida, Department of Neurosurgery, The Johns Hopkins Hospital, Baltimore, MD 21287, United States.
Alexander Perdomo-Pantoja, Department of Neurosurgery, The Johns Hopkins Hospital, Baltimore, MD 21287, United States.
Sheng-fu Lo, Department of Neurosurgery, The Johns Hopkins Hospital, Baltimore, MD 21287, United States.
Elizabeth Howell, Department of Neurosurgery, Duke University Medical Center, Durham, NC 27710, United States.
Christina Holmes, Department of Neurosurgery, The Johns Hopkins Hospital, Baltimore, MD 21287, United States.
C Rory Goodwin, Department of Neurosurgery, Duke University Medical Center, Durham, NC 27710, United States.
Nicholas Theodore, Department of Neurosurgery, The Johns Hopkins Hospital, Baltimore, MD 21287, United States.
Daniel M Sciubba, Department of Neurosurgery, The Johns Hopkins Hospital, Baltimore, MD 21287, United States.
Timothy F Witham, Department of Neurosurgery, The Johns Hopkins Hospital, Baltimore, MD 21287, United States.
References
- 1.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976) 2012;37:67–76. doi: 10.1097/BRS.0b013e31820cccfb. [DOI] [PubMed] [Google Scholar]
- 3.Buchbinder R, van Tulder M, Öberg B, Costa LM, Woolf A, Schoene M, Croft P Lancet Low Back Pain Series Working Group. Low back pain: a call for action. Lancet. 2018;391:2384–2388. doi: 10.1016/S0140-6736(18)30488-4. [DOI] [PubMed] [Google Scholar]
- 4.Albert TJ, Pinto M, Denis F. Management of symptomatic lumbar pseudarthrosis with anteroposterior fusion. A functional and radiographic outcome study. Spine (Phila Pa 1976) 2000;25:123–9; discussion 130. doi: 10.1097/00007632-200001010-00021. [DOI] [PubMed] [Google Scholar]
- 5.Cheh G, Bridwell KH, Lenke LG, Buchowski JM, Daubs MD, Kim Y, Baldus C. Adjacent segment disease followinglumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine (Phila Pa 1976) 2007;32:2253–2257. doi: 10.1097/BRS.0b013e31814b2d8e. [DOI] [PubMed] [Google Scholar]
- 6.James AW, LaChaud G, Shen J, Asatrian G, Nguyen V, Zhang X, Ting K, Soo C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng Part B Rev. 2016;22:284–297. doi: 10.1089/ten.teb.2015.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hustedt JW, Blizzard DJ. The controversy surrounding bone morphogenetic proteins in the spine: a review of current research. Yale J Biol Med. 2014;87:549–561. [PMC free article] [PubMed] [Google Scholar]
- 8.El Bialy I, Jiskoot W, Reza Nejadnik M. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm Res. 2017;34:1152–1170. doi: 10.1007/s11095-017-2147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebata S, Takahashi J, Hasegawa T, Mukaiyama K, Isogai Y, Ohba T, Shibata Y, Ojima T, Yamagata Z, Matsuyama Y, Haro H. Role of Weekly Teriparatide Administration in Osseous Union Enhancement within Six Months After Posterior or Transforaminal Lumbar Interbody Fusion for Osteoporosis-Associated Lumbar Degenerative Disorders: A Multicenter, Prospective Randomized Study. J Bone Joint Surg Am. 2017;99:365–372. doi: 10.2106/JBJS.16.00230. [DOI] [PubMed] [Google Scholar]
- 10.Ohtori S, Orita S, Yamauchi K, Eguchi Y, Ochiai N, Kuniyoshi K, Aoki Y, Nakamura J, Miyagi M, Suzuki M, Kubota G, Inage K, Sainoh T, Sato J, Shiga Y, Abe K, Fujimoto K, Kanamoto H, Inoue G, Takahashi K. More than 6 Months of Teriparatide Treatment Was More Effective for Bone Union than Shorter Treatment Following Lumbar Posterolateral Fusion Surgery. Asian Spine J. 2015;9:573–580. doi: 10.4184/asj.2015.9.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye F, Zeng Z, Wang J, Liu H, Wang H, Zheng Z. Comparison of the use of rhBMP-7 versus iliac crest autograft in single-level lumbar fusion: a meta-analysis of randomized controlled trials. J Bone Miner Metab. 2018;36:119–127. doi: 10.1007/s00774-017-0821-z. [DOI] [PubMed] [Google Scholar]
- 12.Malham GM, Parker RM, Ellis NJ, Blecher CM, Chow FY, Claydon MH. Anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2: a prospective study of complications. J Neurosurg Spine. 2014;21:851–860. doi: 10.3171/2014.8.SPINE13524. [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bomback DA, Grauer JN, Lugo R, Troiano N, Patel TCh, Friedlaender GE. Comparison of posterolateral lumbar fusion rates of Grafton Putty and OP-1 Putty in an athymic rat model. Spine (Phila Pa 1976) 2004;29:1612–1617. doi: 10.1097/01.brs.0000132512.53305.a1. [DOI] [PubMed] [Google Scholar]
- 15.Riordan AM, Rangarajan R, Balts JW, Hsu WK, Anderson PA. Reliability of the rabbit postero-lateral spinal fusion model: A meta-analysis. J Orthop Res. 2013;31:1261–1269. doi: 10.1002/jor.22359. [DOI] [PubMed] [Google Scholar]
- 16.Yoon BH, Esquivies L, Ahn C, Gray PC, Ye SK, Kwiatkowski W, Choe S. An activin A/BMP2 chimera, AB204, displays bone-healing properties superior to those of BMP2. J Bone Miner Res. 2014;29:1950–1959. doi: 10.1002/jbmr.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mofarrahi M, McClung JM, Kontos CD, Davis EC, Tappuni B, Moroz N, Pickett AE, Huck L, Harel S, Danialou G, Hussain SN. Angiopoietin-1 enhances skeletal muscle regeneration in mice. Am J Physiol Regul Integr Comp Physiol. 2015;308:R576–R589. doi: 10.1152/ajpregu.00267.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalb S, Mahan MA, Elhadi AM, Dru A, Eales J, Lemos M, Theodore N. Pharmacophysiology of bone and spinal fusion. Spine J. 2013;13:1359–1369. doi: 10.1016/j.spinee.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Gianoncelli A, Bonini SA, Bertuzzi M, Guarienti M, Vezzoli S, Kumar R, Delbarba A, Mastinu A, Sigala S, Spano P, Pani L, Pecorelli S, Memo M. An Integrated Approach for a Structural and Functional Evaluation of Biosimilars: Implications for Erythropoietin. BioDrugs. 2015;29:285–300. doi: 10.1007/s40259-015-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James AW, Shen J, Tsuei R, Nguyen A, Khadarian K, Meyers CA, Pan HC, Li W, Kwak JH, Asatrian G, Culiat CT, Lee M, Ting K, Zhang X, Soo C. NELL-1 induces Sca-1+ mesenchymal progenitor cell expansion in models of bone maintenance and repair. JCI Insight. 2017:2. doi: 10.1172/jci.insight.92573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin X, Zamora PO, Albright S, Glass JD, Peña LA. Multidomain synthetic peptide B2A2 synergistically enhances BMP-2 in vitro. J Bone Miner Res. 2005;20:693–703. doi: 10.1359/JBMR.041104. [DOI] [PubMed] [Google Scholar]
- 22.Hu B, Coulson L, Moyer B, Price PA. Isolation and molecular cloning of a novel bone phosphoprotein related in sequence to the cystatin family of thiol protease inhibitors. J Biol Chem. 1995;270:431–436. doi: 10.1074/jbc.270.1.431. [DOI] [PubMed] [Google Scholar]
- 23.Ornitz DM, Itoh N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin L, Li X. Growth differentiation factor 5 regulation in bone regeneration. Curr Pharm Des. 2013;19:3364–3373. doi: 10.2174/1381612811319190003. [DOI] [PubMed] [Google Scholar]
- 25.Laron Z. Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol Pathol. 2001;54:311–316. doi: 10.1136/mp.54.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Camillo B, Carlon A, Eduati F, Toffolo GM. A rule-based model of insulin signalling pathway. BMC Syst Biol. 2016;10:38. doi: 10.1186/s12918-016-0281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pakvasa M, Alverdy A, Mostafa S, Wang E, Fu L, Li A, Oliveira L, Athiviraham A, Lee MJ, Wolf JM, He TC, Ameer GA, Reid RR. Neural EGF-like protein 1 (NELL-1): Signaling crosstalk in mesenchymal stem cells and applications in regenerative medicine. Genes Dis. 2017;4:127–137. doi: 10.1016/j.gendis.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krause C, Guzman A, Knaus P. Noggin. Int J Biochem Cell Biol. 2011;43:478–481. doi: 10.1016/j.biocel.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Murray SS, Wang JC, Duarte ME, Zhao KW, Tian H, Francis T, Brochmann Murray EJ. The bone matrix protein secreted phosphoprotein 24 kD (Spp24): bone metabolism regulator and starting material for biotherapeutic materials. Histol Histopathol. 2015;30:531–537. doi: 10.14670/HH-30.531. [DOI] [PubMed] [Google Scholar]
- 32.Zheng GB, Yoon BH, Lee JH. Comparison of the osteogenesis and fusion rates between activin A/BMP-2 chimera (AB204) and rhBMP-2 in a beagle's posterolateral lumbar spine model. Spine J. 2017;17:1529–1536. doi: 10.1016/j.spinee.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Park BH, Song KJ, Yoon SJ, Park HS, Jang KY, Zhou L, Lee SY, Lee KB, Kim JR. Acceleration of spinal fusion using COMP-angiopoietin 1 with allografting in a rat model. Bone. 2011;49:447–454. doi: 10.1016/j.bone.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Babat LB, McLain R, Milks R, Ferrara L, Sohn MJ. The effects of the antiresorptive agents calcitonin and pamidronate on spine fusion in a rabbit model. Spine J. 2005;5:542–547. doi: 10.1016/j.spinee.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Fan Y, Cao D, Zhang J, Wu Z, Qiu G. Calcitonin enhanced lumbar spinal fusion in a New Zealand rabbit model: a study with morphologic and molecular analysis. Spine (Phila Pa 1976) 2012;37:E139–E146. doi: 10.1097/BRS.0b013e31822ba535. [DOI] [PubMed] [Google Scholar]
- 36.Liu CC, Tian FM, Zhou Z, Wang P, Gou Y, Zhang H, Wang WY, Shen Y, Zhang YZ, Zhang L. Protective effect of calcitonin on lumbar fusion-induced adjacent-segment disc degeneration in ovariectomized rat. BMC Musculoskelet Disord. 2015;16:342. doi: 10.1186/s12891-015-0788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rölfing JH, Bendtsen M, Jensen J, Stiehler M, Foldager CB, Hellfritzsch MB, Bünger C. Erythropoietin augments bone formation in a rabbit posterolateral spinal fusion model. J Orthop Res. 2012;30:1083–1088. doi: 10.1002/jor.22027. [DOI] [PubMed] [Google Scholar]
- 38.Inoue G, Uchida K, Matsushita O, Fujimaki H, Saito W, Miyagi M, Sekiguchi H, Nishi N, Ohtori S, Yogoro M, Takaso M. Effect of Freeze-Dried Allograft Bone With Human Basic Fibroblast Growth Factor Containing a Collagen-Binding Domain From Clostridium histolyticum Collagenase on Bone Formation After Lumbar Posterolateral Fusion Surgery in Rats. Spine (Phila Pa 1976) 2017;42:E995–E1001. doi: 10.1097/BRS.0000000000002074. [DOI] [PubMed] [Google Scholar]
- 39.Spiro RC, Liu L, Heidaran MA, Thompson AY, Ng CK, Pohl J, Poser JW. Inductive activity of recombinant human growth and differentiation factor-5. Biochem Soc Trans. 2000;28:362–368. [PubMed] [Google Scholar]
- 40.Spiro RC, Thompson AY, Poser JW. Spinal fusion with recombinant human growth and differentiation factor-5 combined with a mineralized collagen matrix. Anat Rec. 2001;263:388–395. doi: 10.1002/ar.1119. [DOI] [PubMed] [Google Scholar]
- 41.Jahng TA, Fu TS, Cunningham BW, Dmitriev AE, Kim DH. Endoscopic instrumented posterolateral lumbar fusion with Healos and recombinant human growth/differentiation factor-5. Neurosurgery. 2004;54:171–80; discussion 180-1. doi: 10.1227/01.neu.0000097516.00961.eb. [DOI] [PubMed] [Google Scholar]
- 42.Magit DP, Maak T, Trioano N, Raphael B, Hamouria Q, Polzhofer G, Drespe I, Albert TJ, Grauer JN. Healos/recombinant human growth and differentiation factor-5 induces posterolateral lumbar fusion in a New Zealand white rabbit model. Spine (Phila Pa 1976) 2006;31:2180–2188. doi: 10.1097/01.brs.0000232823.82106.0a. [DOI] [PubMed] [Google Scholar]
- 43.Kandziora F, Pflugmacher R, Scholz M, Knispel C, Hiller T, Schollmeier G, Bail H, Schmidmaier G, Duda G, Raschke M, Haas NP. Comparison of BMP-2 and combined IGF-I/TGF-ss1 application in a sheep cervical spine fusion model. Eur Spine J. 2002;11:482–493. doi: 10.1007/s00586-001-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kandziora F, Schmidmaier G, Schollmeier G, Bail H, Pflugmacher R, Görke T, Wagner M, Raschke M, Mittlmeier T, Haas NP. IGF-I and TGF-beta1 application by a poly-(D,L-lactide)-coated cage promotes intervertebral bone matrix formation in the sheep cervical spine. Spine (Phila Pa 1976) 2002;27:1710–1723. doi: 10.1097/00007632-200208150-00006. [DOI] [PubMed] [Google Scholar]
- 45.Kandziora F, Pflugmacher R, Scholz M, Schäfer J, Schollmeier G, Schmidmaier G, Duda G, Raschke M, Haas NP. Dose-dependent effects of combined IGF-I and TGF-beta1 application in a sheep cervical spine fusion model. Eur Spine J. 2003;12:464–473. doi: 10.1007/s00586-002-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu Y, Zhang F, Lineaweaver WC, Zhang J, Jia L, Qi J, Wang J, Zhen X. In Vivo Study of Hydroxyapatite-coated Hat Type Cervical Intervertebral Fusion Cage Combined With IGF-I and TGF-β1 in the Goat Model. Clin Spine Surg. 2016;29:E267–E275. doi: 10.1097/BSD.0b013e3182781d52. [DOI] [PubMed] [Google Scholar]
- 47.Koerner JD, Yalamanchili P, Munoz W, Uko L, Chaudhary SB, Lin SS, Vives MJ. The effects of local insulin application to lumbar spinal fusions in a rat model. Spine J. 2013;13:22–31. doi: 10.1016/j.spinee.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 48.Lee M, Li W, Siu RK, Whang J, Zhang X, Soo C, Ting K, Wu BM. Biomimetic apatite-coated alginate/chitosan microparticles as osteogenic protein carriers. Biomaterials. 2009;30:6094–6101. doi: 10.1016/j.biomaterials.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li W, Lee M, Whang J, Siu RK, Zhang X, Liu C, Wu BM, Wang JC, Ting K, Soo C. Delivery of lyophilized Nell-1 in a rat spinal fusion model. Tissue Eng Part A. 2010;16:2861–2870. doi: 10.1089/ten.tea.2009.0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siu RK, Lu SS, Li W, Whang J, McNeill G, Zhang X, Wu BM, Turner AS, Seim HB, 3rd, Hoang P, Wang JC, Gertzman AA, Ting K, Soo C. Nell-1 protein promotes bone formation in a sheep spinal fusion model. Tissue Eng Part A. 2011;17:1123–1135. doi: 10.1089/ten.tea.2010.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan W, James AW, Asatrian G, Shen J, Zara JN, Tian HJ, Siu RK, Zhang X, Wang JC, Dong J. NELL-1 based demineralized bone graft promotes rat spine fusion as compared to commercially available BMP-2 product. J Orthop Sci. 2013;18:646–657. doi: 10.1007/s00776-013-0390-5. [DOI] [PubMed] [Google Scholar]
- 52.Klineberg E, Haudenschild DR, Snow KD, Garitty S, Christiansen BA, Acharya C, Maitra S, Gupta MC. The effect of noggin interference in a rabbit posterolateral spinal fusion model. Eur Spine J. 2014;23:2385–2392. doi: 10.1007/s00586-014-3252-8. [DOI] [PubMed] [Google Scholar]
- 53.Sherman BP, Lindley EM, Turner AS, Seim HB, 3rd, Benedict J, Burger EL, Patel VV. Evaluation of ABM/P-15 versus autogenous bone in an ovine lumbar interbody fusion model. Eur Spine J. 2010;19:2156–2163. doi: 10.1007/s00586-010-1546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smucker JD, Bobst JA, Petersen EB, Nepola JV, Fredericks DC. B2A peptide on ceramic granules enhance posterolateral spinal fusion in rabbits compared with autograft. Spine (Phila Pa 1976) 2008;33:1324–1329. doi: 10.1097/BRS.0b013e3181732a74. [DOI] [PubMed] [Google Scholar]
- 55.Cunningham BW, Atkinson BL, Hu N, Kikkawa J, Jenis L, Bryant J, Zamora PO, McAfee PC. Ceramic granules enhanced with B2A peptide for lumbar interbody spine fusion: an experimental study using an instrumented model in sheep. J Neurosurg Spine. 2009;10:300–307. doi: 10.3171/2009.1.SPINE08565. [DOI] [PubMed] [Google Scholar]
- 56.Sintuu C, Simon RJ, Miyazaki M, Morishita Y, Hymanson HJ, Taghavi C, Brochmann EJ, Murray SS, Wang JC. Full-length spp24, but not its 18.5-kDa proteolytic fragment, inhibits bone-healing in a rodent model of spine fusion. J Bone Joint Surg Am. 2011;93:1022–1032. doi: 10.2106/JBJS.J.00081. [DOI] [PubMed] [Google Scholar]
- 57.Chun DS, Baker KC, Hsu WK. Lumbar pseudarthrosis: a review of current diagnosis and treatment. Neurosurg Focus. 2015;39:E10. doi: 10.3171/2015.7.FOCUS15292. [DOI] [PubMed] [Google Scholar]
- 58.Hsu WK, Nickoli MS, Wang JC, Lieberman JR, An HS, Yoon ST, Youssef JA, Brodke DS, McCullough CM. Improving the clinical evidence of bone graft substitute technology in lumbar spine surgery. Global Spine J. 2012;2:239–248. doi: 10.1055/s-0032-1315454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 60.Deyo RA, Ching A, Matsen L, Martin BI, Kreuter W, Jarvik JG, Angier H, Mirza SK. Use of bone morphogenetic proteins in spinal fusion surgery for older adults with lumbar stenosis: trends, complications, repeat surgery, and charges. Spine (Phila Pa 1976) 2012;37:222–230. doi: 10.1097/BRS.0b013e31821bfa3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calabrese G, Giuffrida R, Forte S, Salvatorelli L, Fabbi C, Figallo E, Gulisano M, Parenti R, Magro G, Colarossi C, Memeo L, Gulino R. Bone augmentation after ectopic implantation of a cell-free collagen-hydroxyapatite scaffold in the mouse. Sci Rep. 2016;6:36399. doi: 10.1038/srep36399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ripamonti U, Roden LC, Ferretti C, Klar RM. Biomimetic matrices self-initiating the induction of bone formation. J Craniofac Surg. 2011;22:1859–1870. doi: 10.1097/SCS.0b013e31822e83fe. [DOI] [PubMed] [Google Scholar]
- 63.Hwang HD, Lee JT, Koh JT, Jung HM, Lee HJ, Kwon TG. Sequential Treatment with SDF-1 and BMP-2 Potentiates Bone Formation in Calvarial Defects. Tissue Eng Part A. 2015;21:2125–2135. doi: 10.1089/ten.TEA.2014.0571. [DOI] [PubMed] [Google Scholar]
- 64.Badwelan M, Alkindi M, Ramalingam S, Nooh N, Al Hezaimi K. The Efficacy of Recombinant Platelet-Derived Growth Factor on Beta-Tricalcium Phosphate to Regenerate Femoral Critical Sized Segmental Defects: Longitudinal In Vivo Micro-CT Study in a Rat Model. J Invest Surg. 2018:1–13. doi: 10.1080/08941939.2018.1519048. [DOI] [PubMed] [Google Scholar]
- 65.Schimandle JH, Boden SD. Spine update. The use of animal models to study spinal fusion. Spine (Phila Pa 1976) 1994;19:1998–2006. doi: 10.1097/00007632-199409000-00023. [DOI] [PubMed] [Google Scholar]
- 66.Sandhu HS, Khan SN. Animal models for preclinical assessment of bone morphogenetic proteins in the spine. Spine (Phila Pa 1976) 2002;27:S32–S38. doi: 10.1097/00007632-200208151-00008. [DOI] [PubMed] [Google Scholar]
- 67.Drespe IH, Polzhofer GK, Turner AS, Grauer JN. Animal models for spinal fusion. Spine J. 2005;5:209S–216S. doi: 10.1016/j.spinee.2005.02.013. [DOI] [PubMed] [Google Scholar]