Abstract
Background:
Variability in glucagon-like peptide (GLP)-1 and GLP-2 plasma concentrations has been suggested in Celiac disease (CD), with inconclusive results. We assessed the association between serum levels of GLP-1 and GLP-2 and their duodenal receptor expression in children with and without CD.
Methods:
This was a two-part, cross-sectional and prospective cohort study. Group assignment, performed after duodenal samples for mRNA expression of GLP-1 receptor (GLP1R) and GLP-2 receptor (GLP2R), were taken during esophagogastroduodenoscopy. The control group consisted of patients with normal endoscopy and negative serology. The CD group consisted of patients with positive serology and endoscopy suggestive of CD. All had an oral glucose-tolerance test (OGTT). CD patients underwent a second OGTT after 6 months of a gluten-free diet (GFD).
Results:
The CD group included 12 patients; 7 males with mean age 9.2 ± 2.5 years. The control group included 10 patients; 5 males with mean age 12 ± 4 years, (p = 0.14). No differences were detected in basal or peak levels of GLP-1 or GLP-2 between control, naïve CD (before GFD) and treated CD (after GFD) groups. Expression of GLP1R and GLP2R mRNA was similar. Significant positive correlations between glucose and C-peptide secretion (r = 0.9, p < 0.01) and GLP-1 and GLP-2 (r = 0.8, p = 0.01) were detected in the control group. Significant negative correlations were found in the naïve CD group between GLP2R expression and glucose secretion (r = −0.68, p = 0.015) and GLP1R expression and serum GLP-1 (r = −0.7, p = 0.016).
Conclusions:
Although no significant differences were detected in secretion patterns or gut receptor expression of GLP-1 and GLP-2 in healthy versus CD pediatric patients, the detected discrepancy between the ligand levels and their tissue receptors requires additional study.
Keywords: cohort study, cross-sectional, C-peptide, oral glucose-tolerance test, duodenal receptor
Introduction
Celiac disease (CD) is an autoimmune disorder, induced in genetically predisposed individuals by gluten ingestion.1 Typical histological characteristics include: subtotal/total villous atrophy, crypt hypertrophy and chronic inflammatory infiltrate of the small intestine lamina propria.2 Altered profiles of gut endocrine cell peptide secretion have been demonstrated in animal studies, with several reports in CD patients. However, these reports describe mostly adults, and present inconclusive or contradictory results.3–5 L cells are specific enteroendocrine cells located in the small intestinal mucosa at increasing density, from a few cells in the duodenum to peak amounts in the ileum and colon.6 L cells have apical processes, with direct access to ingested nutrients, thus enabling the secretion of glucagon-like peptide (GLP)-1, GLP-2 and other hormones in response to nutrient ingestion.7 GLP-1 has several mechanisms of action: stimulation of nutrient-induced insulin release, reduction of glucagon secretion, and reduction of gastric emptying and secretions.8 GLP-2 has marked intestinotrophic properties, achieved through intestinal epithelial proliferation, crypt cell hyperplasia and inhibition of apoptosis.9,10 Because villous atrophy with crypt hypertrophy are typical histological features of CD, it has been suggested that GLP-1 and GLP-2 may affect, or be affected by CD pathology, but this hypothesis is still unproven. To clarify this, we performed a cohort study, including a cross-sectional part and a prospective part, in a pediatric population undergoing esophagogastroduodenoscopy (EGD) for clinical care due to gastrointestinal tract (GIT) symptoms to determine the correlation between serum and tissue parameters between those with and without CD, and after GFD in: (1) duodenal expression of GLP-1 receptor (GLP1R) and GLP2R mRNA among naïve CD patients and non-CD patients; and (2) basal and peak plasma concentrations, after carbohydrate stimulation, of GLP-1 and GLP-2 among the same patient groups prior to dietary intervention, and among the same CD patients after 6 months on a gluten-free diet (GFD).
Materials and methods
Study population
All parents of children aged 5–18 years, whose clinical complaints justified EGD with duodenal biopsies, were asked to participate. The patients were classified into two groups according to European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) criteria for the diagnosis of CD:11 a naïve CD group diagnosed with positive CD serology and at least Marsh 3A classification; a control group with negative serology for CD, and no pathology in small bowel biopsies. The patients in the naïve CD group were also prospectively studied after 6 months of GFD (treated CD group). Exclusion criteria included: negative serology and pathological biopsy or positive serology with atypical duodenal biopsy, signs of positive inflammatory markers, abnormal liver or renal function tests, use of oral medications for comorbidities, diabetes mellitus, prediabetes, and refusal to perform all study procedures.
Study design and ethical procedures
This two-part preliminary cross-sectional and prospective cohort study was performed between 2012 and 2016 at the Gastroenterology Institute and Research Unit of Assaf Harofeh Medical Center, Israel. The investigations were carried out in accordance with the principles of the Declaration of Helsinki as revised in 2008. All study phases and procedures were approved by the Assaf Harofeh Medical Center Institutional Medical Ethics Committee approval no.60/11, and by the National Ministry of Health Ethics Committee, no.920110232. Written informed consent was obtained from parents and oral assent was obtained from participants prior to EGD performance and enrollment (all participants were younger than 18 years of age).
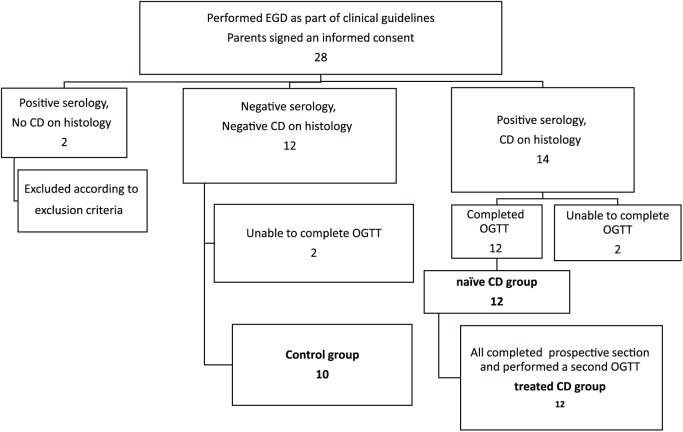
This was a preliminary cohort study in order to evaluate the association between several tissue parameters (specific receptors) and several serum hormones (ligands) in health, inflamed and treated CD. Previous reports include only a few studies, only one in the pediatric population, all with less than 14 CD patients, and none yet performed a simultaneous analysis of both hormones and their tissue receptors. All participants who were included in study in the two comparative groups according to EGD results (following fulfillment of inclusion criteria, as shown in Figure 1), underwent an oral glucose-tolerance test (OGTT) within 1 month of the EGD. The prospective part of the study was a second OGTT that was performed on the treated CD group after 6 months of GFD. Serum levels of glucose, insulin, C-peptide, GLP-1 and GLP-2 were assessed during the OGTT. To compare the GLP1R and GLP2R (mRNA expression) ratios between naïve CD patients and controls, two duodenal specimens were taken during the EGD and frozen at −70oC until analysis. Study participants flow is presented in Figure 1.
Figure 1.
Study and participants flow.
CD, celiac disease; EGD, esophagogastroduodenoscopy; OGTT, oral glucose-tolerance test.
Sample size
In order to evaluate a correlation coefficient of 0.7 or more (difference from zero), a study population of 14 patients was needed. The sample size was calculated using power of 80% and significance level of 5% in two-sided test. Sample size was calculated using Winpepi software, version 11.62, March 2016.12
Outcome measures
The primary outcome measures were: correlations of secretion patterns and duodenal receptor expression of GLP-1 and GLP-2 after carbohydrate stimulation between the same CD patients before and after GFD, and non-CD patients.
The secondary outcome measures were: associations between tissue and serum GLP-1 and GLP-2 parameters, according to C-peptide.
Clinical data collection
All participants were interviewed for clinical parameters, growth pattern, and family history of autoimmune diseases and diabetes mellitus. Physical examination included anthropometric measures and pubertal assessments. Height and body mass index (BMI) were reported as height standard deviation score (SDS) and BMI SDS.13 Tanner score was used to characterize sexual maturity. The prepubertal state was defined as Tanner stage 1 (testicular volume ⩽3 ml in boys and absence of breast tissue in girls).14,15
Laboratory assessments
OGTT was performed after ingestion of a 50% glucose solution at 1.75 g/kg per dose, maximum 75 g. Blood was drawn for glucose, C-peptide, insulin, GLP-1 and GLP-2 at 0, 15, 30, 60, 90 and 120 min from ingestion. Serum concentrations were expressed as the area under the curve (AUC), and baseline and peak concentrations. Serum C-peptide levels were determined using the Immulite 2000 analyzer. Inter- and intra-assay median coefficient of variation was 20%. Glycated hemoglobin (HbA1C) levels were determined in whole-blood samples from time 0 using the Cobas Integra 400 system by turbidometric inhibition immunoassay.
Serum GLP-1 and GLP-2 levels
Serum GLP-1 and GLP-2 levels were analyzed by enzyme-linked immunosorbent assay (ELISA) (EZGLP1T-36K and EZGLP2-37K, respectively, EMD Millipore, USA) according to the manufacturer’s protocols. Serum was kept at −70°C until use. All incubations were performed at room temperature on an orbital shaker at 400 rpm, and samples were tested in duplicate (50 μl/well). Standards, blanks, quality controls and sera were distributed in the precoated wells and incubated for 1.5 h for GLP-1 and 2 h for GLP-2 at room temperature. Plates were rewashed and incubated with primary antibody for 1 h (GLP-1) or 30 min (GLP-2). After washing, the plates were incubated with horseradish peroxidase-conjugated secondary antibody for 30 min (for both GLP-1 and GLP-2). After washing, plates were incubated with tetramethylbenzidine for 10–15 min in the dark for color development, followed by addition of 1 M H2SO4 to stop the reaction. Plates were read by a Multiskan-EX ELISA plate reader (Thermo, USA) within 5 min. The absorbance was measured at wavelengths of 450 nm and 590 nm. Total GLP-1 was measured in pmol/l, GLP-2 in ng/ml, and both were calculated according to the manufacturer’s protocol and resulting standard curve.
Analysis of GLP1R and GLP2R in duodenal tissue
GLP1R and GLP2R were analyzed by assessing their mRNA in tissue biopsies. Their expression was reported as the ratio of these receptors’ mRNA to glyceraldehyde 3-phosphate (GAPDH) (parameter of all normal cells). Total RNA was extracted using MagNA Pure Compact RNA Isolation kit (Roche Applied Science, Germany) and quantified by spectrophotometric assay. Reverse transcription was performed on individual samples of total RNA (3 μg) using a Transcriptor First Strand cDNA Synthesis kit (Roche Applied Science, Germany). Both procedures were according to the manufacturer’s protocols.
The primers used to amplify the GLP1R gene and GAPDH were the same as those described previously by Broide and colleagues16 and were synthesized by Integrated DNA Technologies, USA. The sequence for human GLP1R was (fwd) 5’-CTA CGC ACT CTC CTT CTC TGC T-3’ and (rev) 5’-CGG ACA ATG CTC GCA GGA TGA A-3’. The sequence for human GLP2R was (fwd) 5’-AGC TCC TTT CAT GGG TTC CT-3’ and (rev) 5’-TCC TAG GAA CCG GAA GTC CT-3’. The sequence for human GAPDH was (fwd) 5’-TCG GAG TCA ACG GAT TT-3’ and (rev) 5’-CCA CGA CGT ACT CAG C-3’. Quantitative real-time polymerase chain reaction (PCR) analysis was performed using a Light Cycler 480 II Instrument (Roche Diagnostics, Germany) and DNA Master SYBR Green I kit (Roche Diagnostics). PCR amplification was applied to 2 µl cDNA with 18 µl PCR Master Mix, and 1 µl of 10 µM primers. The samples were denatured at 95°C for 10 min and subjected to 45 amplification cycles with denaturation at 95°C for 10 s, annealing for 15 s at temperatures between 63°C and 55°C (touchdown PCR conditions were used for the initial annealing temperature of 63°C for 5 s with a decreasing temperature step of 0.5°C in each cycle), and extension at 72°C for 15 s.
The ‘E method’ was applied to analyze GLP1R and GLP2R mRNA expression levels in the different samples, and these were normalized to the GAPDH mRNA level and expressed as relative units.
Statistical analysis
Continuous variables were described as median and interquartile range (IQR). Categorical variables were described as frequency and percent. Fisher’s exact test was used to evaluate differences in categorical variables between groups. A Mann–Whitney test was used to observe differences in continuous variables between groups. AUCs of glucose, insulin, C-peptide, GLP-1 and GLP-2 levels during the OGTT were calculated by summing partial AUCs using the trapezoidal rule. The individual partial AUC segments (AUCi) were calculated as: AUCi = [(C0 + C1) × Time1–0]/2, where Time1–0 is the time elapsed between blood sample measurements.
Associations between continuous variables were evaluated using Spearman’s rank correlation coefficient. Wilcoxon signed-rank test was used to observe differences over time. Analysis was controlled for sex and BMI SDS. We used the generalized estimating equations model to evaluate the differences between control, naïve CD and CD after GFD (treated CD) groups, including controlling for sex and age. A two-tailed p < 0.05 was considered a statically significant result. Data analysis was performed with IBM SPSS Statistics for Windows, version 23.0 (released in 2015).
Results
Written informed consent was obtained from 28 parents of children prior to undergoing EGD, oral assent was obtained from the children. Overall, two of them were excluded due to serological or histological incompatibility and four patients failed to complete the OGTT. Ultimately, the CD group included 12 patients, and the control group included 10 participants. All satisfied the inclusion and exclusion criteria and completed the study requirements and conditions (Figure 1).
Clinical characteristics
All participants in the CD group had histological findings compatible with at least Marsh 3A according to the ESPGHAN criteria:11 two had Marsh 3A, nine had Marsh 3B and one had Marsh 3C. The clinical and demographic characteristics of the study population are presented in Table 1. No statistically significant differences were detected between groups with respect to GIT symptoms, BMI SDS or height SDS.
Table 1.
Clinical characteristics of the study population.
| Control group | CD group | p value | |
|---|---|---|---|
| Number | 10 | 12 | |
| Age (y)# | 13.6 (7.96, 15.73) | 9.0 (6.98, 10.64) | 0.14 |
| Sex (m:f) | 5:5 | 7:5 | 1 |
| Abdominal pain | 80% | 75% | 1 |
| Diarrhea | 0 | 8.3% | 1 |
| Vomiting | 10% | 0 | 0.46 |
| Short stature | 10% | 25% | 0.59 |
| Family history of T1DM | 10% | 16.7% | 1 |
| Height SDS# | −0.22 (−1.27, 0.05) | −0.27 (−1.27, 0.42) | 0.77 |
| BMI SDS# | 0.18 (−1.10, 1.12) | −0.55 (−2.10, 0.28) | 0.23 |
| HbA1c (%)# | 5.05 (5.00, 5.33) | 5.35 (5.15, 5.50) | 0.06 |
| HbA1c after GFD (%)# | NA | 5.4 (5.32, 5.5) | NA |
| HbA1c after GFD (%)# | NA | 5.4 (5.32, 5.5) | NA |
Data presented as median and IQR.
Fisher’s exact test was used to evaluate differences in categorical variables between groups. Mann–Whitney test was used to observe differences in continuous variables between groups.
BMI, body mass index; CD, celiac disease; GFD, gluten-free diet; HbA1c, glycated hemoglobin; IQR, interquartile range; NA, not applicable; SDS, standard deviation score; T1DM, type 1 diabetes mellitus;
Glycemic parameter profile
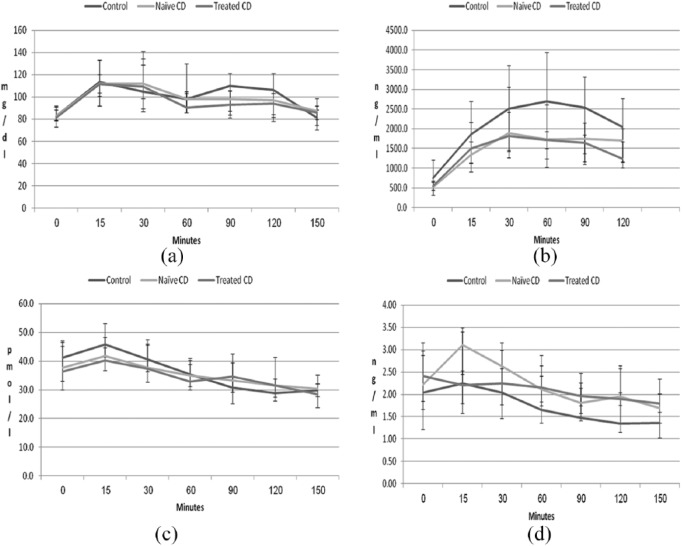
Glucose secretion peaked after 15 min, and C-peptide after 30 min in all groups. No differences were detected in the secretion patterns, basal levels, peak levels or AUCs of glucose or C-peptide among the three groups: control, naïve CD patients and treated CD patients [Table 2 and Figure 2(a and b)]. Similarly, no differences were detected in HbA1c.
Table 2.
Glucose, C-peptide, GLP-1 and GLP-2 concentrations and relative mRNA ratio of tissue receptors (GLP1R, GLP2R ) by groups.
| Control group N = 10 |
Naïve CD group (pre-GFD) N = 12 |
p value | Treated CD (post-GFD) N = 12 |
p value# | p value* | |
|---|---|---|---|---|---|---|
| Basal glucose (mg/dl) | 82.5 (73.0, 92.0) |
83 (78.5, 90.75) |
0.84 | 81.0 (79, 88.5) |
0.33 | 0.72 |
| Basal C-peptide (ng/ml) | 751.45 (446.48, 1209.75) | 511.95 (318.05, 675.45) | 0.16 | 547.9 (313.73, 653.45) |
0.7 | 0.06 |
| Basal GLP-1 (pmol/l) | 45.89 (41.35, 53.21) | 41.67 (34.18, 44.73) |
0.33 | 36.32 (30.02, 45.2) |
0.79 | 0.6 |
| Basal GLP-2 (ng/ml) | 2.04 (1.56, 2.87) |
2.22 (1.85, 3.16) |
0.50 | 2.42 (1.22, 2.87) |
0.28 | 0.7 |
| AUC glucose (mg/dl) | 207.19 (183.47, 238.34) | 206.13 (189.50, 214.13) |
0.5 | 199.8 (190.38, 224.69) |
0.66 | 0.5 |
| AUC C-peptide (ng/ml) | 4485.14 (2828.67, 6969.62) | 2964.86 (2458.8, 4138.18) | 0.14 | 3127.33 (2643.53, 4464.93) |
0.58 | 0.18 |
| AUC GLP-1 (pmol/l) | 72.53 (67.38, 80.44) | 70.45 (59.99, 77.34) | 0.5 | 70.73 (62.58, 87.86) |
0.58 | 0.92 |
| AUC GLP-2 (ng/ml) | 3.45 (2.47, 5.61) |
4.37 (3.80, 5.72) |
0.3 | 4.34 (3.54, 5.52) |
0.75 | 0.54 |
| Peak glucose (mg/dl) | 113.50 (92, 133.5) |
112 (104, 120) |
0.9 | 111.50 (100.75, 132.75) |
0.9 | 0.12 |
| Peak C-peptide (ng/ml) | 2705 (1026.45, 3937) |
1894 (1264, 2422) |
0.4 | 1820.0 (1451.5, 3065.5) |
0.72 | 0.7 |
| Peak GLP-1 (pmol/l) | 45.89 (41.35, 53.21) | 41.67 (34.18, 44.73) | 0.08 | 40.26 (36.79, 48.32) |
0.27 | 0.82 |
| Peak GLP-2 (ng/ml) | 2.04 (1.37, 2.70) |
2.63 (2.05, 3.16) |
0.3 | 2.42 (1.22, 2.87) |
0.28 | 0.42 |
| GLP1R/GAPDH mRNA | 0.006 (0.002, 0.008) | 0.009 (0.003, 0.25) | 0.3 | NA | NA | NA |
| GLP2R/GAPDH mRNA | 0.002 (0.0006, 0.003) | 0.002 (0.0008, 0.012) | 0.3 | NA | NA | NA |
Mann–Whitney test was used to observe differences in continuous variables between groups. Data presented as median and IQR.
AUC, area under the curve; CD, celiac disease; GAPDH, glyceraldehyde 3-phosphate; GFD, gluten-free diet; GLP, glucagon-like peptide; IQR, interquartile range; NA, not applicable.
Treated CD compared with naïve CD.
Treated CD compared with control.
Figure 2.
Concentrations of glucose (a), C-peptide (b), GLP-1 (c) and GLP-2 (d) during OGTT according to time during the test, in minutes. Secretion patterns and time of peak concentrations were similar, and only nonsignificant differences were detected among the control, naïve CD and treated CD groups.
CD, celiac disease; GLP, glucagon-like peptide; OGTT, oral glucose-tolerance test.
GLP-1 and GLP-2 secretion profiles
Similar secretion patterns for GLP-1 and GLP-2 were detected in all groups, with peak levels 15 min after oral ingestion of carbohydrate. No differences were detected in basal levels, peak levels or AUCs of GLP-1 or GLP-2 among the control, naïve CD and treated CD groups [Table 2 and Figure 2(c and d)].
Tissue expression of GLP1R and GLP2R
Expression of GLP1R and GLP2R in the duodenal tissue, as reflected by the mRNA ratio of those receptors to GAPDH, was similar between the control group and naïve CD patients (Table 2).
Associations between incretin secretion, glycemic parameters and receptor expression within and between groups
A significant positive correlation between the AUCs of glucose and C-peptide was only detected in the control group (r = 0.9, p < 0.01). A significant positive correlation was also found between the AUCs of GLP-1 and GLP-2 in the control group (r = 0.8, p = 0.01). These correlations were not reported as significant among CD patients (naïve or treated), as presented in Table 3.
Table 3.
Significant correlations between incretin secretion and tissue parameters compared among groups.
| Parameters assessed in correlation analysis | In control group | In naïve CD group | In treated CD group | p values for differences between groups | |
|---|---|---|---|---|---|
| AUC glucose | AUC C-peptide |
r = 0.9 p < 0.001 |
r = 0.36 p = 0.25 |
r = 0.03 p = 0.93 |
0.04# |
| 0.04* | |||||
| AUC GLP-2 | AUC GLP-1 |
r = 0.8 p = 0.01 |
r = 0.25 p = 0.43 |
r = 0.34 p = 0.29 |
0.11# |
| 0.02* | |||||
| AUC glucose | GLP2R ratio |
r = −0.52 p = 0.68 |
r = −0.68
p = 0.02 |
NA | 0.20# |
| NA* | |||||
| AUC GLP-1 | GLP1R ratio |
r = 0.37 p = 0.33 |
r = −0.7 p = 0.02 |
NA | 0.02# |
| NA* | |||||
| AUC C-peptide (naïve) | AUC C-peptide (GFD) | NA | NA | NA |
r = 0.72 p = 0.008 |
Demonstrated difference between control and naïve CD patients.
Demonstrated difference between control and treated CD patient.
AUC, area under the curve; CD, celiac disease; GFD, gluten-free diet; GLP, glucagon-like peptide; NA, not applicable.
A significant negative correlation was found in the naïve CD group between GLP2R expression in duodenal specimens and glucose AUC (r = −0.68, p = 0.015).
In the same naïve CD group, GLP1R expression in the duodenal tissue was negatively correlated to the AUC of serum GLP-1 (r = −0.7, p = 0.016). These correlations were not detected in the control group (Table 3). The AUCs of C-peptide in naïve and treated CD patients were positively correlated (r = 0.72, p = 0.008).
Discussion
This is the first report on plasma levels of GLP-1 and GLP-2 and expression of their duodenal tissue receptors in a pediatric population with GIT symptoms, including children with CD before and after GFD treatment. We demonstrate similar secretion patterns of these hormones in response to carbohydrate load, with similar glucose and C-peptide increments, and similar duodenal tissue expression of GLP1R and GLP2R mRNA.
The hypothesis that variations in gut-hormone profiles may influence, and be influenced by the pathophysiology of digestive diseases such as CD is based on several parameters: (1) the location of the L cells, and their probable damage due to mucosal atrophy; and (2) published, albeit inconsistent data regarding serum levels of glucose-dependent insulinotropic peptide (GIP), enteroglucagon, oxyntomodulin-like, GLP-1 and GLP-2 from studies performed mostly in adults,3,4,11,17–20 with only two of them addressing the pediatric population with and without CD.5,21
GLP-1 is released in response to glucose load and stimulates insulin secretion.22,23 We and others have hypothesized that abnormal or defective GLP-1 secretion will affect C-peptide response to glucose load (OGTT), with a difference before and after GFD. Interestingly, in our study, no differences in its secretion were detected in response to oral carbohydrate load. Similarly, Caddy and colleagues4 found no difference in GLP-1 plasma concentrations between naïve CD patients and healthy controls in an adult population that was similar in size to ours. In contrast, Papastamataki and colleagues5 demonstrated lower GLP-1 concentrations in a fasting state in naïve as well as GFD-treated CD patients compared with healthy controls. The reason for these discrepancies is unclear. However, they may be explained, at least in part, by the differences of the reports’ study designs and populations, such as age and type of incretin stimulation. Our study is unique and brings additive value since we used measurable carbohydrate-only stimulation and in addition to basal and peak incretin levels, we added measurements of the whole stimulation period by calculating the AUC during OGTT.
GLP-2 is secreted in a manner similar to GLP-1, from the same cells, but has more trophic local actions at the intestinal level, which are mainly evident in the setting of injured intestines.23 Caddy and colleagues,4 who studied both GLP-1 and GLP-2 levels in adults, demonstrated significantly higher basal and peak GLP-2 plasma concentrations after a carbohydrate meal in naïve CD patients compared with healthy individuals. These findings are surprising because both hormones (GLP-1 and GLP-2) are secreted from the same cells and therefore, one would expect similar secretion patterns: instead, they demonstrated different response patterns for GLP-1 and GLP-2. Furthermore, the basal GLP-2 level measured in their healthy group (seven adults) was significantly lower than the levels found in the comparable populations in our research and in that of Xiao and colleagues.10 Indeed, we demonstrated similar secretion of GLP-1 and GLP-2 after specific carbohydrate stimulation, as expected, released from the same cells.
We found no differences in secretion pattern of either GLP-1 or GLP-2 between CD and non-CD patients. This might be due in part to the fact that L cells are predominantly located in the distal small intestine,24 whereas in CD, mucosal atrophy is more pronounced in the proximal small intestine.
In this study, we demonstrated a significant positive correlation between glucose and C-peptide AUCs, and between GLP-1 and GLP-2 AUCs in the control group. This was not surprising, as we expected the specific carbohydrate oral stimulus to cause a correlated increase in glucose and C-peptide secretion, supporting the reliability of the stimulus used in this study. In response to a carbohydrate meal, glucose-dependent GLP-1 is released from gut endocrine cells into the circulation and interacts with their cognate G-protein coupled receptor (GLP1R ). Receptor activation results in tissue-selective pleiotropic responses that include augmentation of glucose-induced insulin secretion from pancreatic beta cells.25 The similar correlated responses of GLP-1 and GLP-2 were also expected because as already noted, both are secreted from the same L cells, and both are known to respond to glucose stimulation. Interestingly, those correlations were not detected as significant among CD patients, i.e., naïve and post-GFD. Furthermore, significant negative correlations between GLP2R expression in duodenal specimens and serum glucose AUC, and between GLP1R expression in duodenal tissue and serum GLP-1 AUC, were only found in the naïve CD group. This may indicate negative feedback at the receptor level. These correlations were not detected in the control non-CD population, which suggests possible dysregulation of glucose metabolism, as has been suggested in CD.3–5,11,17–21
Unfortunately, the data are not sufficient to imply any clinical relevance, but they do warrant further research. Previous studies have reported that multiple processes, such as N-glycosylation and receptor oligomerization, may regulate the exit of functional GLP1Rs from the endoplasmic reticulum and their maintenance in the plasma membrane.25 Alterations of the vesicular trafficking and relative receptors have been described previously in CD with the exposure to Gliadin.26,27 Our findings suggest that although the GLP-1 and GLP-2 levels are similar between CD and non-CD pediatric patients, different relationships between the tissue receptor and hormone in the CD population may indicate dysregulation of cell surface expression. This should be further analyzed for possible significance of expression modulation and clinical relevance.
Our study has several strengths. First, it includes comparable populations with similar clinical symptoms and normal body composition, eliminating selection bias. It is known that both may affect incretin secretion and incretin receptor stimulation.28
Second, our study design included L-cell stimulation by an accurately measurable oral solution in order to reach a maximally accurate similar effect in all tests. Previous reports used a meal stimulus, which may vary by a few grams between patients, and have different combinations of protein and fat, which might change the speed of glucose absorption and cause biases in interpreting the results. Third, to elucidate GLP-1 and GLP-2 secretion patterns, it is imperative to examine basal and peak levels as well as AUCs, as previously described in short bowel syndrome.29 Fourth, we added data regarding possible tissue changes related to dysregulation of hormonal and receptor expression. Assessment of the expression and modulation of GLP1R and GLP2R suggests possible up- or downregulation associated with the inconclusive reports. Finally, our study is the first to consider all of these parameters in a pediatric CD population.
The major limitation of the study is its small population, albeit no smaller than those used in previous studies on the topic. The study was limited in number because recruitment is difficult for pediatric population studies that require invasive procedures and recurrent blood tests. According to our preliminary results, a larger multicenter study, is needed in order to overcome the recruitment difficulty. Secondly, L cells are predominantly located in the distal small intestine,24 and tissue samples were taken only from the proximal small intestine, where mucosal atrophy is more pronounced in CD. For ethical reasons, the distal small bowel and the lower GIT were not assessed.
To our knowledge, this is the first study to simultaneously analyze carbohydrate-mediated gut hormones and their tissue receptors’ expression in naïve CD patients compared with non-CD patients with similar body composition presenting with similar clinical symptoms. Our results do not indicate alterations in serum GLP-1 or GLP-2 levels in CD patients and currently, these cannot be used clinically in the diagnosis of CD. However, additional studies are required to elucidate the significance of our findings with respect to the possible negative feedback in the receptor–ligand relationship of GLP-1 and GLP-2 and their duodenal receptors in patients with CD, with additional analysis of ileum incretin receptors.
Acknowledgments
We thank Dr Tomer Ziv for the statistical analysis, and Camille Vainstein for the scientific editing. Dr Marianna Rachmiel thanks The Israeli Society for Clinical Pediatrics for the research grant.
Footnotes
Funding: The study was supported in part by a research grant from The Israeli Society for Clinical Pediatrics (awarded to MR).
Conflict of interest statement: None of the authors have anything to declare, and all take complete responsibility for the integrity of the data and the accuracy of the data analyses.
ORCID iD: Marianna Rachmiel  https://orcid.org/0000-0002-0852-0831
https://orcid.org/0000-0002-0852-0831
Contributor Information
Marianna Rachmiel, Pediatric Endocrinology Unit, Assaf Harofeh Medical Center, Zerifin 70300, Israel.
Gilad Ben-Yehudah, Gastroenterology Unit, Assaf Harofeh Medical Center, Israel.
Haim Shirin, Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel; Gastroenterology Unit, Assaf Harofeh Medical Center, Israel.
Efrat Broide, Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel; Gastroenterology Unit, Assaf Harofeh Medical Center, Israel.
References
- 1. Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology 2009; 137: 1912–1933. [DOI] [PubMed] [Google Scholar]
- 2. Dickson BC, Streutker CJ, Chetty R. Coeliac disease: an update for pathologists. J Clin Pathol 2006; 59: 1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pietroletti R, Bishop AE, Carlei F, et al. Gut endocrine cell population in coeliac disease estimated by immunocytochemistry using a monoclonal antibody to chromogranin. Gut 1986; 27: 838–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caddy GR, Ardill JE, Fillmore D, et al. Plasma concentrations of glucagon-like peptide-2 in adult patients with treated and untreated coeliac disease. Eur J Gastroenterol Hepatol 2006; 18: 195–202. [DOI] [PubMed] [Google Scholar]
- 5. Papastamataki M, Papassotiriou I, Bartzeliotou A, et al. Incretins, amylin and other gut-brain axis hormones in children with coeliac disease. Eur J Clin Invest 2014; 44: 74–82. [DOI] [PubMed] [Google Scholar]
- 6. Parker HE, Gribble FM, Reimann F. The role of gut endocrine cells in control of metabolism and appetite. Exp Physiol 2014; 99: 1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sandoval DA, D’Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev 2015; 95: 513–548. [DOI] [PubMed] [Google Scholar]
- 8. Edholm T, Degerblad M, Gryback P, et al. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroenterol Motil 2010; 22: 1191–1200, e315. [DOI] [PubMed] [Google Scholar]
- 9. Drucker DJ. Glucagon-like Peptide 2. Trends Endocrinol Metab 1999; 10: 153–156. [DOI] [PubMed] [Google Scholar]
- 10. Xiao Q, Boushey RP, Drucker DJ, et al. Secretion of the intestinotropic hormone glucagon-like peptide 2 is differentially regulated by nutrients in humans. Gastroenterology 1999; 117: 99–105. [DOI] [PubMed] [Google Scholar]
- 11. Husby S, Koletzko S, Korponay-Szabo IR, et al. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2011; 54: 136–160. [DOI] [PubMed] [Google Scholar]
- 12. Abramson J. WINPEPI updated: computer programs for epidemiologist, and their teaching potential. Epidemiol Perspect Innov 2011; 8: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data 2000: 1–27. [PubMed] [Google Scholar]
- 14. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969; 44: 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970; 45: 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Broide E, Bloch O, Ben-Yehudah G, et al. GLP-1 receptor is expressed in human stomach mucosa: analysis of its cellular association and distribution within gastric glands. J Histochem Cytochem 2013; 61: 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dawson J, Bryant MG, Bloom SR, et al. Gastrointestinal regulatory peptide storage granule abnormalities in jejunal mucosal diseases. Gut 1984; 25: 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le Quellec A, Clapie M, Callamand P, et al. Circulating oxyntomodulin-like immunoreactivity in healthy children and children with celiac disease. J Pediatr Gastroenterol Nutr 1998; 27: 513–518. [DOI] [PubMed] [Google Scholar]
- 19. Besterman HS, Bloom SR, Sarson DL, et al. Gut-hormone profile in coeliac disease. Lancet 1978; 1: 785–788. [DOI] [PubMed] [Google Scholar]
- 20. Lauritsen KB, Lauritzen JB, Christensen KC. Gastric inhibitory polypeptide and insulin release in response to oral and intravenous glucose in coeliac disease. Scand J Gastroenterol 1982; 17: 241–245. [DOI] [PubMed] [Google Scholar]
- 21. Kilander AF, Stenhammar L, Lindstedt G, et al. Determination of enteroglucagon in plasma for detection of celiac disease in children. Clin Chem 1984; 30: 77–80. [PubMed] [Google Scholar]
- 22. Creutzfeldt W. The incretin concept today. Diabetologia 1979; 16: 75–85. [DOI] [PubMed] [Google Scholar]
- 23. Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology 2004; 145: 2653–2659. [DOI] [PubMed] [Google Scholar]
- 24. Green DW, Gomez G, Greeley GH., Jr Gastrointestinal peptides. Gastroenterol Clin North Am 1989; 18: 695–733. [PubMed] [Google Scholar]
- 25. Whitaker GM, Lynn FC, McIntosh CH, et al. Regulation of GIP and GLP1 receptor cell surface expression by N-glycosylation and receptor heteromerization. PLoS One 2012; 7: e32675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barone MV, Nanayakkara M, Paolella G, et al. Gliadin peptide P31–43 localises to endocytic vesicles and interferes with their maturation. PLoS One 2010; 5: e12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barone MV, Zimmer KP. Endocytosis and transcytosis of gliadin peptides. Mol Cell Pediatr 2016; 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith U, Kahn BB. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J Intern Med 2016; 280: 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jeppesen PB, Hartmann B, Thulesen J, et al. Elevated plasma glucagon-like peptide 1 and 2 concentrations in ileum resected short bowel patients with a preserved colon. Gut 2000; 47: 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]