Abstract
Introduction: Doxorubicin (DOX) is a widely used chemotherapeutic agent with known cardiotoxic properties, while calorie restriction (CR) and exercise have well-documented cardioprotective effects. No studies have investigated the effects of CR alone or the combined effects of CR and exercise on DOX cardiotoxicity. Methods: Rats were divided into 4 groups based on their food intake (ad libitum or CR) and activity (sedentary or voluntary wheel running [WR]). After completing a 16-week treatment, animals received either DOX (15 mg/kg) or saline (SAL) and cardiac function was measured 5 days after treatment. Chromatography was used to quantify left ventricular DOX accumulation. Results: Left ventricular developed pressure (LVDP), end systolic pressure (ESP), and left ventricular maximal rate of pressure development (dP/dtmax) were significantly higher in the CR + DOX group when compared with DOX. Fractional shortening, LVDP, ESP, dP/dtmax, and dP/dtmin were significantly higher in the CR + WR + DOX group compared with the DOX group. In addition, the CR + WR + DOX group showed significantly higher LVDP and ESP compared with the WR + DOX group. DOX accumulation in the heart was 5-fold lower (P < .05) in the CR + WR + DOX group compared with the DOX group. Conclusion: This is the first study to demonstrate that CR can reduce cardiac DOX accumulation, and confirms the protective role of CR against DOX-induced cardiac dysfunction. Our data also show that combining a known cardioprotective intervention, exercise training, with CR results in additive benefits in the protection against DOX cardiotoxicity.
Keywords: doxorubicin, cardiotoxicity, exercise, calorie restriction
Introduction
Doxorubicin (DOX) is a chemotherapeutic agent approved by the Food and Drug Administration for the treatment of a variety of cancers including non-Hodgkin’s lymphoma, acute leukemia, multiple myeloma, and cancers of the breast, adrenal cortex, endometrium, lung, and ovary. DOX enters cancerous cells and inhibits the replication of DNA, thereby inhibiting cellular proliferation. Clinical use of DOX is limited due to a dose-dependent cardiotoxicity, which can lead to acute cardiac dysfunction and may eventually result in heart failure long after the completion of treatment.1-3 The acute form of cardiotoxicity often develops within minutes, hours, days, or weeks following DOX treatment.4,5 Even though early signs of cardiac dysfunction are reversible, acute toxicities associated with DOX are highly predictive of chronic toxicities.6,7 Several molecular mechanisms for DOX-induced cardiotoxicity have been hypothesized8,9 and the exact mechanisms of DOX-mediated cardiotoxicity are unknown. However, oxidative stress10-13 and apoptosis8,14,15 appear to be primary contributors.
Calorie restriction (CR) is the only proven nongenetic means to lifespan extension. In the 1930s, McCay and colleagues16 reported that animals fed a specific amount of food each day lived longer than animals that were given free access to food. CR is defined as a decrease in calorie intake without becoming malnourished. Theories explaining the life span extension include the slowing of growth, decreased body fat, decreased body temperature, and increased physical activity due to CR.17-19 Lifespan extension by CR may also be related to its cardioprotective effects. Multiple studies have shown that CR lowers resting heart rate, lowers blood pressure, improves cardiac ischemic tolerance, and lowers the risk of cardiovascular disease.20,21 While there is limited evidence as to the role CR may play in protecting against DOX cardiotoxicity, Dutta et al22 reported that caloric restriction lowered the cardiac apoptotic index and attenuated cardiac enzyme release following DOX treatment. However, no studies have investigated the effects of CR on DOX-induced cardiac dysfunction.
Exercise has been shown to protect the heart against a wide range of insults including sepsis,23 ischemia-reperfusion injury,24 as well as cancer-induced cardiac cachexia.25 Studies have consistently shown that both endurance and resistance exercise training attenuate cardiac dysfunction in animals treated with DOX.26-29 This has been associated with a reduction in oxidative stress, attenuated apoptosis, and a preservation of myosin heavy chain expression.30,31 Although several mechanisms may explain these observations, studies have suggested that it may be related to the degree of DOX accumulation in the heart. Animals engaged in exercise training show a reduction in cardiac DOX accumulation,27,32 which may in turn preserve cardiomyocyte integrity and function. It is unclear whether CR protects against the cardiac dysfunction associated with DOX exposure, and it is unclear whether CR in combination with exercise training can provide enhanced protection against DOX-induced cardiotoxicity. Therefore, the purpose of this study was to determine if CR protects against DOX-induced cardiotoxicity and whether exercise training can provide additive or synergistic protective effects.
Methods
Animal Subjects, Voluntary Wheel Running, and Feeding Regimens
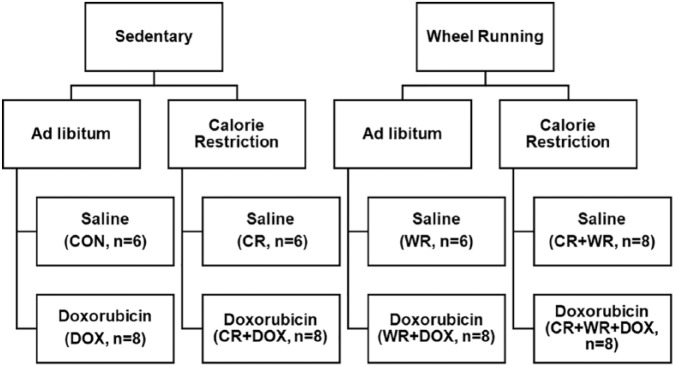
All experimental procedures were approved by the University of Northern Colorado Institutional Animal Care and Use Committee. Animals were initially housed 2 per cage in a temperature-controlled facility, provided standard rat chow and water ad libitum, and were adapted to a 12:12-hour light-dark cycle. Female Sprague-Dawley rats (175-200 g) obtained from Harlan (Indianapolis, IN) were used in this study. Animals (N = 58) were randomly assigned to 1 of 2 primary groups: sedentary or voluntary wheel running (WR). All animals were housed individually to control dietary intake. Animals in the WR groups were housed in cages equipped with commercially available running wheels (MiniMitter, Bend, OR) and given 24 h/day access to wheels throughout the exercise treatment. Running distances were recorded using a VitalView data acquisition system (MiniMitter, Bend, OR). Following a 1 week acclimation period, animals were randomly assigned to either ad libitum or CR groups. Weekly food intake of the ad libitum group was used to determine the daily intake of the CR group. The CR groups consumed 60% of the calories consumed by the ad libitum groups. Following the 4-month activity/feeding period, animals were randomly assigned to receive either 15 mg/kg DOX or saline bolus via an intraperitoneal injection. Following DOX/saline administration, all running wheels were removed from the animal cages. Cardiac function and cardiac DOX accumulation were then assessed 5 days post-DOX treatment. A total of 8 groups were used for these studies and included the following: control (n = 6), CR (n = 6), WR (n = 6), CR + WR (n = 8), DOX (n = 8), WR + DOX (n = 8), CR + DOX (n = 8), and CR + WR + DOX (n = 8; Figure 1). Control animals were fed ad libitum, remained sedentary, and received saline injections. CR animals underwent CR, remained sedentary, and received saline injections. WR animals were fed ad libitum, voluntarily exercised, and received saline injections. CR + WR animals underwent CR, voluntarily exercised, and received saline injections. DOX animals were fed ad libitum, remained sedentary, and received DOX injections. WR + DOX animals were fed ad libitum, voluntarily exercised, and received DOX injections. CR + DOX animals underwent CR, remained sedentary, and received DOX injections. CR + WR + DOX animals underwent CR, voluntarily exercised, and received DOX injections.
Figure 1.
Experimental design.
Echocardiography
In vivo cardiac function was assessed using echocardiography on sedated rats using a commercially available echocardiographic system (Toshiba Nemio 30; 10 MHz transducer). Animals were sedated with ketamine (40 mg/kg, ip), and echocardiography was completed within 10 minutes after the administration of the sedative. From the short-axis view, M-mode images of the left ventricle (LV) were obtained for measures of LV end systolic diameter (LVDs) and LV end diastolic diameter (LVDd). Fractional shortening (FS) was calculated as (LVDd − LVDs)/LVDd.
Isolated Working Heart
Ex vivo cardiac function was analyzed using an isolated working heart model (ADInstruments, Colorado Springs, CO). After the completion of echocardiography experiments, animals were anesthetized with heparinized sodium pentobarbital (50 mg/kg, ip). Anesthesia was confirmed by the absence of a tail pinch reflex, at which time the heart was rapidly excised and immersed in ice-cold Krebs Henseleit buffer (120 mM NaCl, 5.9 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl, 25 mM NaHCO3, 17 mM glucose, and 0.5 mM EDTA [ethylenediaminetetraacetic acid]). The aorta was cannulated within 1 minute and the heart was subjected to retrograde perfusion with the Krebs-Henseleit buffer saturated with 95% O2 and 5% CO2, and temperature was maintained in a water-jacketed column at 37°C for the duration of the experiment. The pulmonary vein was then cannulated to evaluate cardiac function using the working heart method and flow was redirected to enter through the left atrium and exit through the aorta. The heart was then given 15 minutes to equilibrate before data were collected. Preload and afterload were set at 10 cm H2O and 100 cm H2O above the cannula, respectively.
The heart was paced at 240 bpm using an electrode clipped to each cannula. A microtip catheter pressure transducer (Millar Instruments Inc, Houston, TX) was inserted directly into the left ventricular cavity through the apex of the heart to assess left ventricular function. The heart was allowed to stabilize before obtaining measures of LV end systolic pressure (ESP), developed pressure (LVDP), and the maximal (dP/dtmax) and minimal (dP/dtmin) rates of pressure development using a PowerLab data acquisition system (ADInstruments, Colorado Springs, CO).
High-Performance Liquid Chromatography
Cardiac DOX accumulation was measured via high-performance liquid chromatography (HPLC) using previously described techniques.32 Briefly, the LV was isolated, flash frozen in liquid nitrogen, and stored at −80°C. All chromatography experiments were conducted within 24 hours of obtaining the sample. Tissue samples were then subjected to a drug extraction procedure taken from previously validated methods.33 Approximately 50 mg of LV tissue was diluted with a 0.067 M phosphate buffer (pH 7.4) and homogenized at 8000 revolutions per minutes for 20 seconds using a Virtishear homogenizer (Virtis, Gardner, NJ). The concentration of heart tissue was approximately 25 mg/mL. Homogenates were then subjected to protein precipitation by adding 200 mL of a 50:50 (v/v) mixture of HPLC grade methanol and 40% of ZnSO4 to 150 mL of homogenized heart tissue. Fifty microliters of daunorubicin (Sigma, St Louis, MO) at an initial concentration of 500 ng/mL was added to the sample as an internal standard. The sample was vigorously vortexed for 1 minute before centrifugation at 1500g for 10 minutes. The supernatant was filtered through a 0.2-mM syringe filter and injected directly onto the column to initiate the analytical method.
The HPLC system consisted of 2 LC-10AT LC pumps for high-pressure gradient elution (Shimadzu Co, Kyoto, Japan). A reverse-phase Zorbax Rx-C8 4.6 mm·15 cm column (Agilent Technologies, Santa Clara, CA) was used for separation and operated at 40°C. The photo diode-array detector absorbance wavelength range was set to 328 to 342 nm. The fluorescence detector SPD-10 Avp UV (Shimadzu Co) used for excitation/emission wavelengths was maintained at 470/550 nm and the gain was raised to 16 nm. The detector lamps were turned on a minimum of 1 hour before analysis to allow for proper stabilization. Data analysis was performed using Shimadzu CLASS-VP 5.0 data analysis software (Shimadzu Co). Quantification of DOX in LV samples was determined from the peak area of each component relative to the calibration standard curve.
Statistical Analysis
All results are expressed as mean ± SEM. A multivariate analysis of variance (MANOVA) was used to identify model significance. If a significant F-value was observed, a 1-way analysis of variance (ANOVA) was used to identify which variables contained significant findings. A Tukey post hoc test was then performed to identify significant group differences. T tests were performed to determine significance in animal characteristics. P values ≤.05 were considered statistically significant.
Results
General Observations
Animals in this study underwent 4 months of feeding and activity before data were collected. Five days prior to sacrifice, animals were injected with 15 mg/kg of DOX, or an equivalent amount of saline. At sacrifice, body mass was significantly lower for those animals in the CR groups compared to the ad libitum groups (t-test, P < .05; Table 1). Other studies have shown that animals undergoing CR increase voluntary running behavior34-36 and this was confirmed in the present study with animals in the CR groups running significantly more than those in the ad libitum group (AL = 4900 ± 1370 m/day; CR = 7420 ± 2670 m/day; t-test, P < .05). After the CR/exercise intervention, animals were removed from wheel running cages and randomly assigned to drug treatment groups (SAL or DOX). For clarity, data from saline-treated groups are presented in Figure 2 and data from DOX-treated groups are presented in Figures 3 to 5. During the experimental period a total of 5 animals died (1 CR + WR, 2 WR + DOX, and 2 CR + DOX) and the final number of animals per group is reflected in Table 1.
Table 1.
Animal Characteristicsa.
| n | Body Mass (g) | Heart–Body Ratio (kg:g) | |
|---|---|---|---|
| CON | 6 | 292 (41) | 4.1 (0.9) |
| CR | 6 | 234 (10)* | 5.2 (0.4) |
| WR | 6 | 264 (38) | 4.9 (1.1) |
| CR + WR | 7 | 207 (12)*+ | 4.8 (0.5) |
| DOX | 8 | 242 (34)* | 4.3 (1.0) |
| WR + DOX | 6 | 220 (30)*+ | 4.9 (1.1) |
| CR + DOX | 6 | 221 (11)*+ | 4.1 (0.4) |
| CR + WR + DOX | 8 | 199 (10)*#+ | 4.6 (0.5) |
Abbreviation: CON, control; CR, calorie restriction; WR, wheel running; DOX, doxorubicin.
Data are mean (SD).
P < .05 versus CON. #P < .05 versus DOX. +P < .05 versus WR.
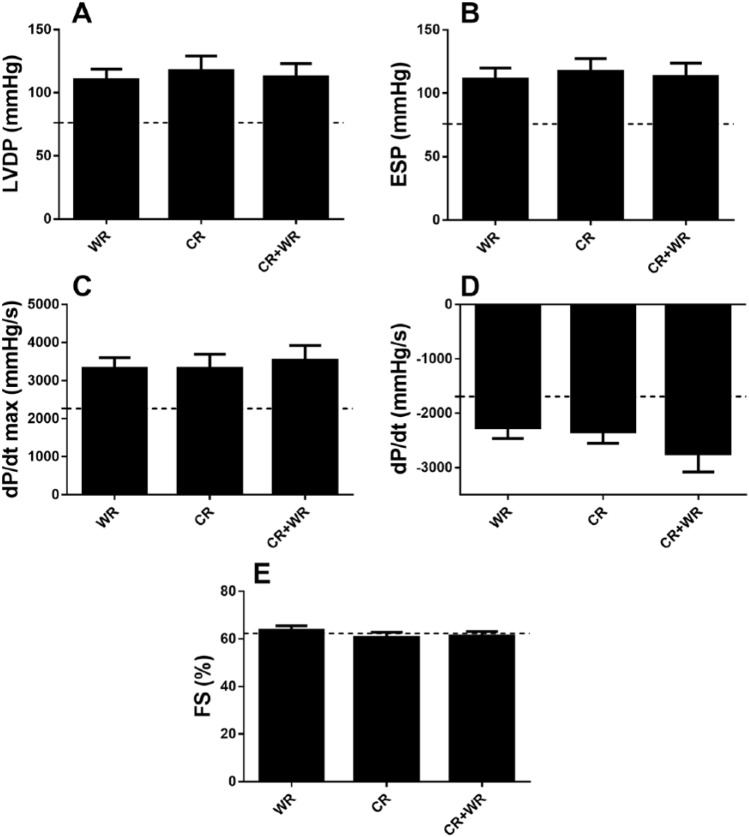
Figure 2.
Cardiac function data for saline groups. No significant differences were observed between saline-treated groups for left ventricular developed pressure (A), end systolic pressure (B), maximal rate of pressure development (C), minimal rate of pressure development (D), or fractional shortening (E). Dashed line represents Control group data.
Figure 3.
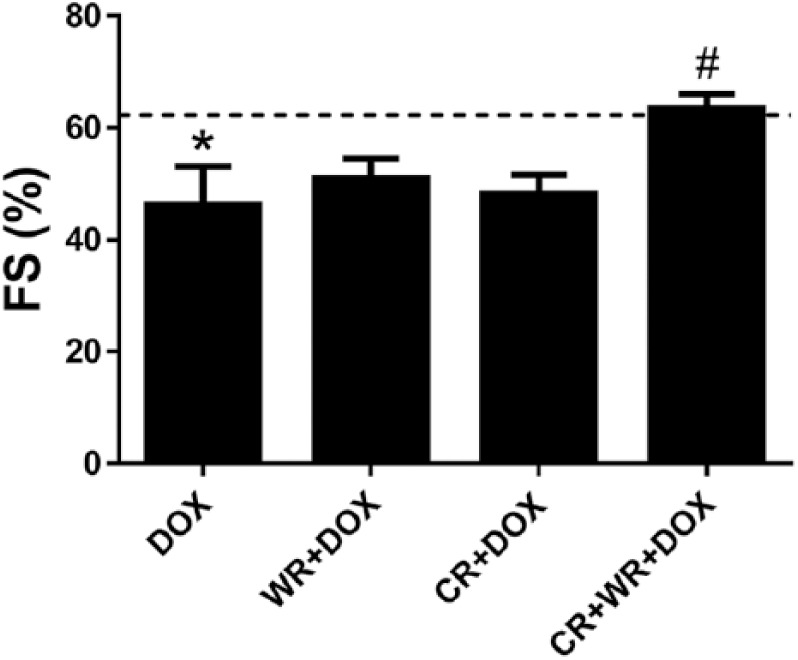
Fractional shortening. DOX significantly decreased FS, while the combination of CR and WR increased fractional shortening significantly. Dashed line represents Control group data. Data are mean ± SEM. *P < .05 versus CON. #P < .05 versus DOX.
Figure 5.
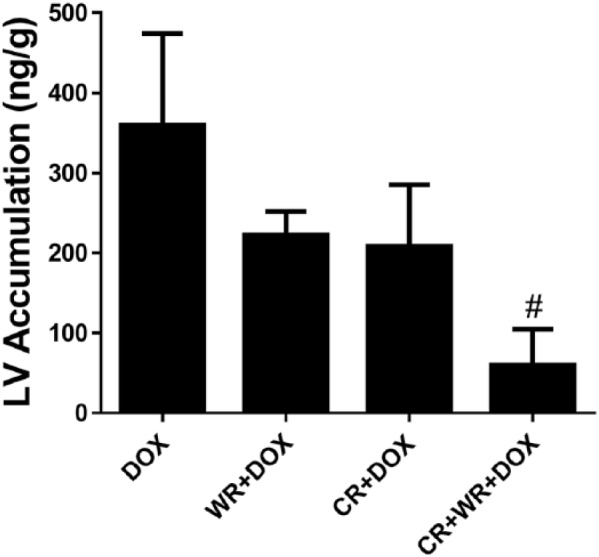
Cardiac doxorubicin accumulation. Animals in the CR + WR + DOX group had significantly less DOX accumulation in the left ventricle compared with animals the DOX group. Data are mean ± SEM. #P < .05 versus DOX.
In Vivo Cardiac Function Analysis
Following assigned treatments, animals were sedated and in vivo cardiac function was measured using echocardiography. DOX treatment resulted in a significant reduction in FS when compared with CON animals (CON 62 ± 8% vs DOX 46 ± 15%, P < .05; Figure 3). In contrast, no significant differences in FS were observed when comparing CR + DOX or WR + DOX with CON. When combining CR and WR there were further protective effects. CR + WR + DOX normalized FS to near control values and was significantly (P < .05) higher than DOX.
Ex Vivo Cardiac Function Analysis
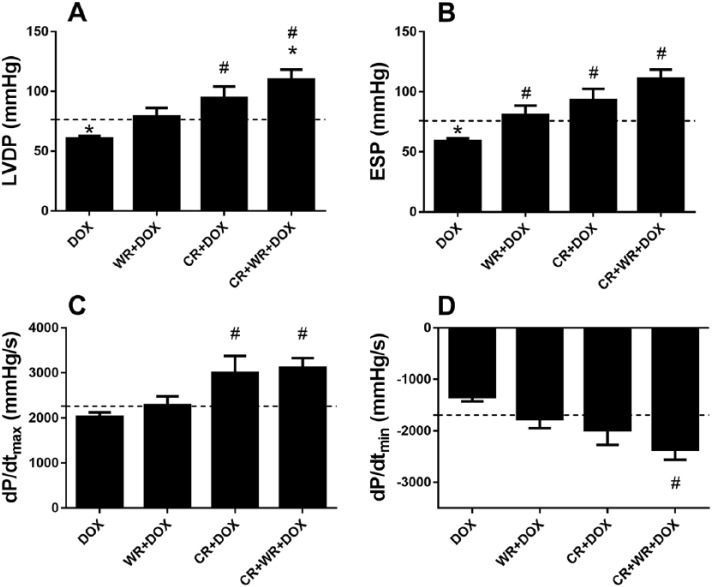
Left ventricular function was analyzed using an isolated working heart apparatus. This model allows for ex vivo LV function analysis without extrinsic influence from the nervous and endocrine systems. Acute DOX-mediated cardiotoxicity was evident as indicated by impaired ex vivo cardiac function 5 days post-DOX exposure. CR alone protected against DOX-induced impairments in LVDP, ESP, and the rate of pressure development (Figure 4A-D). LVDP (+56%, P < .05), ESP (+57%, P < .05) and dP/dtmax (+48%, P < .05) were significantly higher in the CR + DOX group when compared with DOX. Exercise alone also protected against DOX cardiac dysfunction, however, to a lesser degree, with LVDP (+31%, NS), ESP (+37%, P < .05), and dP/dtmax (+12%, NS) values significantly higher in WR + DOX when compared with DOX. Combination treatment was again protective, with significant increases in LVDP (+82%, P < .001), ESP (+87%, P < .001), dP/dtmax (+54%, P < .01), and dP/dtmin (+76%, P < .01) observed in the CR + WR + DOX group compared with DOX.
Figure 4.
Cardiac function in the isolated perfused working heart. CR + DOX and CR + WR + DOX had significantly increased LVDP (A) and ESP (B) compared with DOX. The maximal rate of left ventricle pressure development (C) was significantly greater in CR + DOX and CR + WR + DOX compared with DOX, while the minimum rate of left ventricle pressure development (D) was significantly decreased in CR + WR + DOX compared with DOX. Dashed line represents CON group data. Data are mean ± SEM. *P < .05 versus CON. #P < .05 versus DOX.
Doxorubicin Accumulation
DOX was undetectable in left ventricle samples obtained from saline-treated animals, while DOX animals averaged 360 ng of DOX per gram of left ventricle tissue. Animals that were CR averaged 208 ng/g (−42%, NS; Figure 5) and WR animals had a DOX accumulation of 222 ng/g (−38%, NS). However, combination treatment (CR + WR + DOX) resulted in a statistically significant reduction with animals in this group averaging 60 ng/g (−83%, P < .05).
Discussion
Cardiotoxicity associated with the use of anthracyclines such as DOX is a major clinical dilemma, and it is the primary reason for terminating their use during the course of cancer treatment. Continued use of these chemotherapeutic agents may be considered if there are effective strategies to mitigate cardiotoxic effects, which for some patients may be the best treatment option. As a result, there is a need to investigate interventions with the potential to provide cardioprotection against DOX toxicity. The present study shows that limiting caloric intake (ie, CR) can protect against the cardiotoxic effects of DOX. Furthermore, we found that while both CR alone and WR alone could independently protect, the combination of these 2 interventions resulted in an additive effect in the preservation of cardiac function.
DOX-induced cardiac dysfunction may be due in large part to an increase in reactive oxygen species. The quinone structure of DOX makes it prone to a one electron reduction forming a semiquinone,9 which is catalyzed by the exogenous NADH dehydrogenase in the cardiac mitochondria.37 The semiquinone then donates an electron to oxygen, forming a superoxide anion38 that can either trigger lipid peroxidation or be converted to hydrogen peroxide.38 Free radicals can be formed indiscriminately throughout the cell depending on the cell type, cellular conditions, and the abundance of oxidoreductase systems. Free radicals can also be formed from the interaction of DOX and iron.39 The interaction passes electrons between iron, DOX, and oxygen with a final product of one iron (III)-DOX (aldehyde) and 2 superoxide anions.38 The DOX-associated oxidative stress initiates voltage-dependent anion channel release of cytochrome c, resulting in the activation of caspase-3 and apoptosis.40
Previous work from our laboratory and others has consistently revealed a protective effect of exercise against DOX-induced cardiotoxicity. This protection has been documented following resistance exercise training26 an acute bout of endurance exercise41,42 and after long-term chronic exercise training.43 Several of these studies have demonstrated that the protective effects of exercise are associated with a reduction in cardiac oxidative stress. It is well documented that DOX administration results in increased formation of reactive oxygen species and oxidative damage to cardiac tissue,31,41,44,45 while exercise has been shown to mitigate those effects by upregulating antioxidants and providing protection against DOX-induced oxidative damage.42,43,46 Such adaptations with exercise may also provide protection from oxidative stress-induced apoptosis in cardiac myocytes by muting upstream oxidative signaling pathways. In addition, DOX has been shown to interact with DNA, leading to strand breaks and subsequent cardiac apoptosis. Exercise, however, has been shown to decrease activity of apoptotic pathways and promote cell survival.41,43
The interventions implemented in this study reduced cardiac DOX accumulation and one potential mechanism to explain this is an upregulation of multidrug resistance proteins. Multidrug resistant protein 1 (MRP1) is a member of the ATP-binding cassette transporter protein superfamily, subfamily C.47 MRP1 is ubiquitously expressed in several tissues, including heart,48 where it localizes in sarcolemma.49 Its localization in the plasma membrane positions it to efflux substrates, such as DOX, from the cell thus serving a protective role against DOX-induced cardiotoxicity. Previous work in our laboratory has shown that exercise significantly decreases the accumulation of DOX in left ventricular tissue.32 In a follow-up study, Parry and Hayward27 found that decreased cardiac DOX accumulation was associated with an increase in MRP1 and MRP2 following exercise training. It was also reported that while exercise decreased cardiac DOX accumulation, DOX accumulation in tumors was unchanged. Thus, it is possible that an upregulation of cardiac MRPs results in the extrusion of DOX from the heart thereby averting exposure to a host of deleterious DOX-induced cardiotoxic effects such as calcium dysregulation, inhibition of mitochondrial respiration, metabolic enzyme dysfunction (PFK, CK, and AMPK), alterations in cardiac contractile proteins (MHC, a-actin, and troponin), formation of mitochondrial permeability transition pores, and stimulation of cellular apoptosis.
There appears to be a wide array of potential mechanisms involved with the cardioprotective effects of CR. CR has been shown to reduce infarct size, improve systolic and diastolic function, enhance inotropic reserve, and attenuate deleterious cardiac remodeling.50-54 These functional and morphological benefits of CR have been associated with increased adiponectin production,52,54 normalized cardiac β-adrenergic receptor levels,51 and the modulation of genes involved with oxidative stress, apoptosis, metabolism, and angiogenesis.53 Other studies suggest that calorie restriction protects against the development of cardiac and aortic fibrosis and it has the potential to increase cardiomyocyte density.50 Considering the well-documented cardiac oxidative stress associated with DOX exposure, protection via mechanisms that reduce oxidative stress or enhance antioxidant protections could play an important role. Mitra et al52 reported that a 35% dietary restriction decreased cardiac lipid peroxidation, attenuated superoxide production, and increased antioxidant levels, which was accompanied by a downregulation of mitochondrial uncoupling proteins. They also demonstrated favorable improvements in ATP levels and ATP/ADP ratios, and activation of the pro-survival cardioprotective JAK/STAT3 pathway. Studies in humans suggest that the protective effects of calorie restriction may be linked to insulin signaling, inflammation, autophagy, and mitochondrial biogenesis.55,56
Cardiac mitochondria are highly vulnerable to DOX-mediated toxicity and mechanisms related to mitochondrial biogenesis may be another important mechanism to alleviate DOX toxicity. CR activates sirtuin (SIRT1) and AMP-activated protein kinase (AMPK), which are known regulators of peroxisome-proliferator-activated receptor γ co-activator-1α (PGC-1α). PGC-1α is a key regulator of mitochondrial biogenesis and an upregulation of PGC-1α by calorie restriction may serve to replace or repair cardiac mitochondria damaged by DOX exposure. Maayan et al57 recently demonstrated that the cardioprotective effects of CR against oxidative stress involved increased expression of SIRT1 and PGC-1α. They showed that pharmacological inhibition of SIRT1 decreased PGC-1α expression, increased the production of reactive oxygen species, and abolished the cardioprotective effects of calorie restriction.
Conclusions
This is the first study to demonstrate that CR can reduce cardiac DOX accumulation, and confirms the protective role of CR against DOX-induced cardiac dysfunction. Our data also show that combining a known cardioprotective intervention, exercise training, with CR results in additive benefits in the protection against DOX cardiotoxicity. Based on the fact that these interventions reduce cardiac DOX accumulation, it is possible that cardioprotection is mediated through the attenuation of multiple DOX-induced damaging pathways. In addition, a number of studies have demonstrated that CR is a potent and reproducible intervention for cancer prevention. Clinical studies58 are indeed underway to assess the protective effects of aerobic exercise and CR against DOX-induced cardiotoxicity, and the data presented here support further clinical studies into these cardioprotective interventions for cancer survivors.
Footnotes
Authors’ note: Ashley J. Smuder is now affiliated to University of Florida, Gainesville, FL, USA.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Reid Hayward  https://orcid.org/0000-0002-6324-5190
https://orcid.org/0000-0002-6324-5190
References
- 1. Larussi D, Pisacane C, Indolfi P, Casale F, Martino V, Di Tullio MT. Evaluation of left ventricular function in long-term survivors of childhood Hodgkin disease. Pediatr Blood Cancer. 2005;45:700-705. [DOI] [PubMed] [Google Scholar]
- 2. Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629-2636. [DOI] [PubMed] [Google Scholar]
- 3. Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem. 2000;207:77-86. [DOI] [PubMed] [Google Scholar]
- 4. Tokarska-Schlattner M, Zaugg M, Zuppinger C, Wallimann T, Schlattner U. New insights into doxorubicin-induced cardiotoxicity: the critical role of cellular energetics. J Mol Cell Cardiol. 2006;41:389-405. [DOI] [PubMed] [Google Scholar]
- 5. Ferrans VJ, Clark JR, Zhang J, Yu ZX, Herman EH. Pathogenesis and prevention of doxorubicin cardiomyopathy. Tsitologiia. 1997;39:928-937. [PubMed] [Google Scholar]
- 6. Cardinale D, Sandri MT, Martinoni A, et al. Myocardial injury revealed by plasma troponin I in breast cancer treated with high-dose chemotherapy. Ann Oncol. 2002;13:710-715. [DOI] [PubMed] [Google Scholar]
- 7. Nousiainen T, Jantunen E, Vanninen E, Hartikainen J. Early decline in left ventricular ejection fraction predicts doxorubicin cardiotoxicity in lymphoma patients. Br J Cancer. 2002;86:1697-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2014;56:185-229. [DOI] [PubMed] [Google Scholar]
- 9. Zucchi R, Danesi R. Cardiac toxicity of antineoplastic anthracyclines. Curr Med Chem Anticancer Agents. 2003;3:151-171. [DOI] [PubMed] [Google Scholar]
- 10. Andreadou I, Sigala F, Iliodromitis EK, et al. Acute doxorubicin cardiotoxicity is successfully treated with the phytochemical oleuropein through suppression of oxidative and nitrosative stress. J Mol Cell Cardiol. 2007;42:549-558. [DOI] [PubMed] [Google Scholar]
- 11. Krause MS, Oliveira LP, Jr, Silveira EM, et al. MRP1/GS-X pump ATPase expression: is this the explanation for the cytoprotection of the heart against oxidative stress-induced redox imbalance in comparison to skeletal muscle cells? Cell Biochem Funct. 2007;25:23-32. [DOI] [PubMed] [Google Scholar]
- 12. Schimmel KJ, Richel DJ, van den Brink RB, Guchelaar HJ. Cardiotoxicity of cytotoxic drugs. Cancer Treat Rev. 2004;30:181-191. [DOI] [PubMed] [Google Scholar]
- 13. DeAtley SM, Aksenov MY, Aksenova MV, et al. Antioxidants protect against reactive oxygen species associated with adriamycin-treated cardiomyocytes. Cancer Lett. 1999;136:41-46. [DOI] [PubMed] [Google Scholar]
- 14. Ascensão A, Magalhães J, Soares JM, et al. Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am J Physiol Heart Circ Physiol. 2005;289:H722-H731. [DOI] [PubMed] [Google Scholar]
- 15. Wang S, Konorev EA, Kotamraju S, Joseph J, Kalivendi S, Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. Intermediacy of H2O2- and p53-dependent pathways. J Biol Chem. 2004;279:25535-25543. [DOI] [PubMed] [Google Scholar]
- 16. McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63-79. [PubMed] [Google Scholar]
- 17. Giani JF, Bonkowski MS, Munoz MC, et al. Insulin signaling cascade in the hearts of long-lived growth hormone receptor knockout mice: effects of calorie restriction. J Gerontol. 2008;63:788-797. [DOI] [PubMed] [Google Scholar]
- 18. Faulks SC, Turner N, Else PL, Hulbert AJ. Calorie restriction in mice: effects on body composition, daily activity, metabolic rate, mitochondrial reactive oxygen species production, and membrane fatty acid composition. J Gerontol A Biol Sci Med Sci. 2006;61:781-794. [DOI] [PubMed] [Google Scholar]
- 19. Lopez-Lluch G, Hunt N, Jones B, et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A. 2006;103:1768-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weiss EP, Albert SG, Reeds DN, et al. Effects of matched weight loss from calorie restriction, exercise, or both on cardiovascular disease risk factors: a randomized intervention trial. Am J Clin Nutr. 2016;104:576-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Melo DS, Costa-Pereira LV, Santos CS, et al. Severe calorie restriction reduces cardiometabolic risk factors and protects rat hearts from ischemia/reperfusion injury. Front Physiol. 2016;7:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dutta D, Xu J, Dirain ML, Leeuwenburgh C. Calorie restriction combined with resveratrol induces autophagy and protects 26-month-old rat hearts from doxorubicin-induced toxicity. Free Radic Biol Med. 2014;74:252-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tyml K, Swarbreck S, Pape C, et al. Voluntary running exercise protects against sepsis-induced early inflammatory and pro-coagulant responses in aged mice. Crit Care. 2017;21:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Powers SK, Quindry JC, Kavazis AN. Exercise-induced cardioprotection against myocardial ischemia–reperfusion injury. Free Radic Biol Med. 2008;44:193-201. [DOI] [PubMed] [Google Scholar]
- 25. Parry TL, Hayward R. Exercise protects against cancer-induced cardiac cachexia. Med Sci Sports Exerc. 2018;50:1169-1176. [DOI] [PubMed] [Google Scholar]
- 26. Pfannenstiel K, Hayward R. Effects of resistance exercise training on doxorubicin-induced cardiotoxicity. J Cardiovasc Pharmacol. 2018;71:332-339. [DOI] [PubMed] [Google Scholar]
- 27. Parry TL, Hayward R. Exercise training does not affect anthracycline antitumor efficacy while attenuating cardiac dysfunction. Am J Physiol Regul Integr Comp Physiol. 2015;309:R675-R683. [DOI] [PubMed] [Google Scholar]
- 28. Lien CY, Jensen BT, Hydock DS, Hayward R. Short-term exercise training attenuates acute doxorubicin cardiotoxicity. J Physiol Biochem. 2015;71:669-678. [DOI] [PubMed] [Google Scholar]
- 29. Hayward R, Lien CY. Echocardiographic evaluation of cardiac structure and function during exercise training in the developing Sprague-Dawley rat. J Am Assoc Lab Anim Sci. 2011;50:454-461. [PMC free article] [PubMed] [Google Scholar]
- 30. Campos EC, Souza FR, Freitas AC, Ramos SG, Resende ES. Effects of physical exercise and thyroid hormone on myosin heavy chain expression in doxorubicin-induced cardiotoxicity in rats. FASEB J. 2016;30(1 suppl):1178-1183. [Google Scholar]
- 31. Min K, Kwon OS, Smuder AJ, et al. Increased mitochondrial emission of reactive oxygen species and calpain activation are required for doxorubicin-induced cardiac and skeletal muscle myopathy. J Physiol. 2015;593:2017-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jensen BT, Lien CY, Hydock DS, Schneider CM, Hayward R. Exercise mitigates cardiac doxorubicin accumulation and preserves function in the rat. J Cardiovasc Pharmacol. 2013;62:263-269. [DOI] [PubMed] [Google Scholar]
- 33. Alvarez-Cedron L, Sayalero ML, Lanao JM. High-performance liquid chromatographic validated assay of doxorubicin in rat plasma and tissues. J Chromatogr B Biomed Sci Appl. 1999;721:271-278. [DOI] [PubMed] [Google Scholar]
- 34. Ruegsegger GN, Speichinger KR, Manier JB, Younger KM, Childs TE, Booth FW. Hypothalamic Npy mRNA is correlated with increased wheel running and decreased body fat in calorie-restricted rats. Neurosci Lett. 2016;618:83-88. [DOI] [PubMed] [Google Scholar]
- 35. Overton JM, Williams TD. Behavioral and physiologic responses to caloric restriction in mice. Physiol Behav. 2004;81:749-754. [DOI] [PubMed] [Google Scholar]
- 36. Holloszy JO. Mortality rate and longevity of food-restricted exercising male rats: a reevaluation. J Appl Physiol (1985). 1997;82:399-403. [DOI] [PubMed] [Google Scholar]
- 37. Nohl H, Gille L, Staniek K. The exogenous NADH dehydrogenase of heart mitochondria is the key enzyme responsible for selective cardiotoxicity of anthracyclines. Z Naturforsch C. 1998;53:279-285. [DOI] [PubMed] [Google Scholar]
- 38. Olson RD, Mushlin PS. Doxorubicin cardiotoxicity: analysis of prevailing hypotheses. FASEB J. 1990;4:3076-3086. [PubMed] [Google Scholar]
- 39. Minotti G, Cairo G, Monti E. Role of iron in anthracycline cardiotoxicity: new tunes for an old song? FASEB J. 1999;13:199-212. [PubMed] [Google Scholar]
- 40. Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2: Bax ratio. Cancer Res. 2002;62:4592-4598. [PubMed] [Google Scholar]
- 41. Wonders KY, Hydock DS, Schneider CM, Hayward R. Acute exercise protects against doxorubicin cardiotoxicity. Integr Cancer Ther. 2008;7:147-154. [DOI] [PubMed] [Google Scholar]
- 42. Chicco AJ, Schneider CM, Hayward R. Exercise training attenuates acute doxorubicin-induced cardiac dysfunction. J Cardiovasc Pharmacol. 2006;47:182-189. [DOI] [PubMed] [Google Scholar]
- 43. Chicco AJ, Hydock DS, Schneider CM, Hayward R. Low-intensity exercise training during doxorubicin treatment protects against cardiotoxicity. J Appl Physiol. 2006;100:519-527. [DOI] [PubMed] [Google Scholar]
- 44. Kavazis AN, Morton AB, Hall SE, Smuder AJ. Effects of doxorubicin on cardiac muscle subsarcolemmal and intermyofibrillar mitochondria. Mitochondrion. 2017;34:9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Myers CE, McGuire WP, Liss RH, Ifrim I, Grotzinger K, Young RC. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1997;197:165-167. [DOI] [PubMed] [Google Scholar]
- 46. Kavazis AN, Smuder AJ, Min K, Tümer N, Powers SK. Short-term exercise training protects against doxorubicin-induced cardiac mitochondrial damage independent of HSP72. Am J Physiol Heart Circ Physiol. 2010;299:H1515-H1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cole SPC, Bhardwaj G, Gerlach JH, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650-1654. [DOI] [PubMed] [Google Scholar]
- 48. Flens MJ, Zaman GJ, van der Valk P, et al. Tissue distribution of the multidrug resistance protein. Am J Pathol. 1996;148:1237-1247. [PMC free article] [PubMed] [Google Scholar]
- 49. Jungsuwadee P, Nithipongvanitch R, Chen Y, et al. Mrp1 localization and function in cardiac mitochondria after doxorubicin. Mol Pharmacol. 2009;75:1117-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ahmet I, Tae HJ, de Cabo R, et al. Effects of calorie restriction on cardioprotection and cardiovascular health. J Mol Cell Cardiol. 2011;51:263-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Lucia C, Gambino G, Petraglia L, et al. Long-term caloric restriction improves cardiac function, remodeling, adrenergic responsiveness, and sympathetic innervation in a model of postischemic heart failure. Circ Heart Fail. 2018;11:e004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mitra MS, Donthamsetty S, White B, et al. Mechanism of protection of moderately diet restricted rats against doxorubicin-induced acute cardiotoxicity. Toxicol Appl Pharamacol. 2007;225:90-101. [DOI] [PubMed] [Google Scholar]
- 53. Noyan H, El-Mounayri O, Isserlin R, et al. Cardioprotective signature of short-term caloric restriction. PLoS One. 2015;10:e0130658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809-2017. [DOI] [PubMed] [Google Scholar]
- 55. Mercken EM, Crosby SD, Lamming DW, et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013;12:645-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang L, Licastro D, Cava E, et al. Long-term calorie restriction enhances cellular quality-control processes in human skeletal muscle. Cell Rep. 2016;14:422-428. [DOI] [PubMed] [Google Scholar]
- 57. Maayan W, Keren C, Dor Y, et al. Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving “SIRT1 and PGC-1α.” Cardiovas Diabetol. 2018;17:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kitkham AA, Paterson DI, Prado CM, et al. Rationale and design of the Calorie Restriction and Exercise protection from Anathracycline Toxic Effects (CREATE) study: a 3-arm parallel group phase II randomized controlled trial in early breast cancer. BMC Cancer. 2018;18:864. [DOI] [PMC free article] [PubMed] [Google Scholar]