Abstract
Around 75% to 90% of people who experience a traumatic brain injury (TBI) are classified as having a mild TBI (mTBI). The term mTBI is synonymous with concussion or mild head injury (MHI) and is characterized by symptoms of headache, nausea, dizziness, and blurred vision. Problems in cognitive abilities such as deficits in memory, processing speed, executive functioning, and attention are also considered symptoms of mTBI. Since these symptoms are subtle in nature and may not appear immediately following the injury, mTBI is often undetected on conventional neuropsychological tests. Current neuroimaging techniques may not be sensitive enough in identifying the array of microscopic neuroanatomical and subtle neurophysiological changes following mTBI. To this end, electrophysiological tests, such as auditory evoked potentials (AEPs), can be used as sensitive tools in tracking physiological changes underlying physical and cognitive symptoms associated with mTBI. The purpose of this review article is to examine the body of literature describing the application of AEPs in the assessment of mTBI and to explore various parameters of AEPs which may hold diagnostic value in predicting positive rehabilitative outcomes for people with mTBI.
Keywords: mild traumatic brain injury, auditory brainstem response, auditory evoked potentials, auditory middle latency response, auditory event-related potentials, amplitude, latency
Introduction
Traumatic brain injury (TBI) is an acquired injury to the brain that can occur following sudden trauma to the head (National Institute of Neurological Disorders and Stroke, 2017). The extent of brain injury is typically determined during the acute phase following neurotrauma. Based on the clinical indices of the Glasgow Coma Scale (GCS) and the post-traumatic amnesia (PTA) duration, severity of TBI is categorized as mild, moderate, or severe. The GCS is recorded as a composite score consisting of three individual components: eye-opening, verbal response, and motor response. A GCS score in the range of 13 to 15 is interpreted as mild TBI (mTBI), 9 to 12 as moderate TBI, and 3 to 8 as severe TBI (Teasdale & Jennette, 1974). The duration of PTA refers to the time interval between the injury and when the person is oriented and able to form and recall new memories. A PTA of less than 24 h is categorized as mTBI, 1 to 7 days as moderate TBI, and more than 7 days as severe TBI (Friedland, 2013; Nakase-Richardson et al., 2011). mTBI is used interchangeably with other terms, such as mild closed head injury, MHI, or concussion, all of which represent the same level of brain dysfunction (Nuwer, Hovda, Schrader, & Vespa, 2005). The Mild Traumatic Brain Injury Committee of the American Congress of Rehabilitation Medicine (1993) defines mTBI as:
A condition in which a person has sustained a traumatically induced physiological disruption of brain function, as manifested by at least one of the following: 1) any period of loss of consciousness; 2) any loss of memory for events immediately before or after the accident; 3) any alteration in mental state at the time of accident; and 4) focal neurological deficit(s) that may or may not be transient. (p. 86)
The public health impact of mTBI is significant. Around 1.6 to 3.8 million sports and recreation-related concussions are reported every year (Langlois, Rutland-Brown, & Wald, 2006; UPMC Sports Medicine, 2019). According to a report from the Defense and Veterans Brain Injury Center (2017), mTBI accounted for about 312,000 of the total 380,000 TBIs recorded in combat operations during a 17-year time frame between 2000 and 2017. It is estimated that around 75% to 90% of all TBIs are classified as mild, thus making mTBI the most common type of brain injury overall, yet around 50% to 90% of these cases go undetected (Centers for Disease Control and Prevention, 2017; National Center for Injury Prevention and Control, 2003; Prince & Bruhns, 2017).
People with mTBI often experience postconcussion syndrome, characterized by a complex set of symptoms that may appear and persist for days, weeks, and even months following brain injury. These secondary symptoms mainly include nausea, confusion, dizziness, blurred vision, headaches, agitation, and mood changes (Centers for Disease Control and Prevention, 2017; National Institute of Neurological Disorders and Stroke, 2018). In addition, people with mTBI may also experience problems in cognitive abilities including memory, attention, executive functioning, and processing speed (Gaetz & Bernstein, 2001; Mathias, Beall, & Bigler, 2004; Mathias & Wheaton, 2007; Tulsky et al., 2017). The availability of a wide spectrum of neuropsychological instruments for assessing these cognitive domains has made it possible to identify and differentiate cognitive impairments associated with moderate and severe TBI (Benedict, 1997; Delis, Kaplan, & Kramer, 2001; Gronwall, 1977; Heaton, Chelune, Talley, Kay, & Curtiss, 1993; Shallice, 1982; Wechsler, Coalson, & Raiford, 2008). However, detecting cognitive profiles associated with mTBI still remains a challenge. There are two potential reasons for this limitation. First, mTBI symptoms may be transient and not appear immediately following the injury, making it difficult to obtain a reliable cognitive profile of the individual with suspected mTBI. Second, cognitive impairments associated with mTBI often show significant variability within an individual and across groups of individuals with a similar mTBI profile, as determined by premorbid cognitive functioning levels, heterogeneity in the etiology of mTBI (e.g., motor vehicle accident vs. a blast exposure during combat), and diffuse neuronal damage. This variability poses limitations to standardizing neuropsychological tests for use in mTBI assessment protocols (Iverson, Holdnack, & Lange, 2013; Prince & Bruhns, 2017; Tulsky et al., 2017).
To address some of the limitations related to existing neuropsychological tests, the National Institutes of Health Toolbox for the Assessment of Neurological and Behavioral Functioning Cognition Battery (NIHTB-CB; Gershon et al., 2013) was tested in a group of 182 individuals with TBI (Tulsky et al., 2017). The authors identified that a small assortment of neuropsychological tests may provide a robust estimate of cognitive functioning in people with TBI, regardless of the severity. These tests included a set of composite scores indexed across multiple measures of various cognitive domains, including memory, attention, and executive functioning. The aim of the study was to establish a construct validity and clinical utility of using NIHTB-CB in people with mTBI. However, three main challenges remain in adapting the toolbox in the evaluation of people with mTBI. First, participants were tested for a total of 8 h over a period of 2 days. This type of test duration poses a serious concern regarding the ecological validity of using this battery of tests in people with mTBI, given that extensive testing may lead to declines in cognitive performance, which may be incorrectly attributed to cognitive impairments. Second, the authors testing NIHTB-CB suspect that the assessment may underestimate cognitive impairment in some domains such as processing speed. Third, the assortment of tests within NIHTB-CB fails to account for previous exposure to similar neuropsychological tests that might have influenced test performance in current testing sessions.
Conventional neuroimaging techniques are designed to identify structural and functional brain changes. Neuroimaging findings of a person with suspected TBI are often linked with neuropsychological data for the purposes of obtaining concurrent validity (Eierud et al., 2014). Some of the common neuroimaging techniques used in the evaluation of moderate to severe TBI are computerized tomography (CT), functional magnetic resonance imaging (fMRI), single photon emission computed tomography, magnetoencephalography, and positron emission tomography. However, these tools may not be sensitive enough to detect the array of microscopic neuroanatomical and subtle neurophysiological changes that occur at the micron and nanometer cellular level in mTBI.
Neuroanatomical changes in mTBI are attributed to the acceleration or deceleration forces that cause diffuse neuronal injury—an important factor that presents additional challenges for injury detection using neuroimaging techniques. While the CT scan has been recommended as the neuroimaging procedure of choice in TBI assessment, repeated exposure to harmful ionizing radiation and difficulties in assessing children and young adults pose additional limitations to wider use of the procedure. Conventional fMRIs in clinical setups such as T-2 and T-1 weighted structural scans index both structural and functional data of neuronal changes following mTBI and pose no health risk to people undergoing the procedures. However, fMRI scans are time-consuming, expensive, and insufficient in providing supplemental information beyond findings obtained from CT scans. Taken together, limitations concerning the lack of sensitivity and specificity at the individual patient level and concerns with ecological validity have presented important challenges to developing mTBI diagnostic tools based on neuroimaging techniques (Bigler, 2004; Eierud et al., 2014; Holmes, Goodacre, Stevenson, Pandor, & Pickering, 2012).
Electrophysiological tests, such as auditory evoked potentials (AEPs), offer hope as potential sensitive assessment tools in identifying the fine-grained neurophysiological changes and diffuse neuroanatomical aberrations accompanying mTBI deficits. The purpose of this review article is to provide an overview of the application of AEPs in the assessment of mTBI and to explore various parameters of AEPs which may have diagnostic value in the assessment of mTBI. To this end, the review covers important AEPs which have shown promise in testing people with mTBI. They include auditory brainstem response (ABR), complex ABR, auditory middle latency response (AMLR), and auditory late latency response (ALLR) subsuming N2 and P300. The review excludes AEPs that were not tested in mTBI, such as mismatch negativity, N-400, P-600, and auditory steady-state response.
AEPs in mTBI Assessment
The most common neurocognitive problems observed in individuals with mTBI are associated with memory, attention, and processing speed (Gaetz & Bernstein, 2001; Mathias et al., 2004; Mathias & Wheaton, 2007). The neural correlates corresponding to these cognitive domains are innately intertwined to the auditory pathway, and efficient auditory processing is contingent upon fast and well-coordinated integration of several neural systems. AEPs have been established as sensitive tools for detecting neural aberrations in the auditory pathway. They offer a precise recording of the synchronicity of neural firing events throughout the auditory nerve, from the cochlea to cortex (Kraus & White-Schwoch, 2015). This level of sensitivity is crucial as it has been shown that the physiological functioning may still be impaired despite normal performance on traditional cognitive assessments in mTBI (Segalowitz, Bernstein, & Lawson, 2001). Thus, we infer that AEPs have the potential to be used as assessment tools, in conjunction with other neuropsychological and neuroimaging tests in obtaining a sophisticated mechanistic picture of neurophysiological and neurocognitive deficits in mTBI. We provide a brief review of the commonly used AEPs and their potential role as assessment tools in mTBI evaluation.
AEPs can be classified into early, middle, and late latency types. Early AEPs include the ABR, elicited using simple and complex auditory stimuli; middle AEPs include the AMLR, and late AEPs include the ALLR or auditory event-related potentials. Early AEPs are used to evaluate the integrity of the cochlea, auditory nerve, and brainstem auditory pathways, while middle AEPs are used to measure auditory cortical functions. Late AEPs are used to evaluate functional brain activity and record changes in information processing due to diffuse axonal injury (Gaetz & Bernstein, 2001).
Early AEPs
Noseworthy, Miller, Murray, and Regan (1981) conducted one of the earlier ABR studies in people with TBI. Participants included 11 people with postconcussion status and 12 matched controls. A significantly delayed latency of Waveform III was recorded in people with postconcussion. These results indicated the involvement of higher neural centers, such as the superior olivary complex (a generating site for Waveform III) in a concussion. In another study, Munjal, Panda, and Pathak (2010) recorded binaural ABRs from 290 participants with TBI (150 with mTBI, 100 with moderate TBI, and 40 with severe TBI) and 50 matched participants with no hearing or neurocognitive problems. ABRs were recorded in both ears separately. GCS was used to classify participants into mild, moderate, and severe TBI groups. The results showed positive associations between the severity of TBI and prolonged Waveform V and I–V interwave latencies in the right ear. The side of impact on the head was not documented in the study, making it difficult to explain differences between the right and left ears in terms of absolute and interwave latencies. In summary, the findings of this study indicate the involvement of the brainstem as a direct consequence of the severity of TBI.
Gallun et al. (2012) studied the efficacy of ABRs in detecting deficits in the cochlear structures and central auditory pathway in 19 military personnel with a history of mTBI following blast exposure and 29 control participants with no history of mTBI. The authors reported no significant differences in the latencies and amplitudes of Waveforms I, III, and V between the blast-exposed and the control group. In some studies, researchers have suggested that ABRs offer no significant prognostic information in the assessment of individuals with mTBI or even with those with severe TBI (Cusumano et al., 1992; Gaetz & Weinberg, 2000; Haglund & Persson, 2009; Keren, Sazbon, Groswasser, & Shmuel, 1994; Nölle, Todt, Seidl, & Ernst, 2004; Werner & Vanderzant, 1991). It should be noted here that such inconsistencies in results across the studies may stem from the variability in time intervals between the brain injury and ABR assessment, as well as the variability in the site of lesion among participants with mTBI (e.g., brainstem vs. cortical lesions; Hall, Speilman, & Gennarelli, 1982; Musiek, Baran, & Shein, 2004).
Another important parameter in ABR acquisition is the rate of stimulus presentation. A high rate ABR protocol is typically used in diagnosing retro-cochlear pathology. Thus, it can be expected that high rate ABRs may have better sensitivity in detecting changes in neural synchrony due to a lesion or injury following mTBI. Podoshin, Ben-David, Fradis, and Pratt (1990) examined ABR recordings in 15 people with minor head trauma and 35 people without a history of brain injury. ABRs were elicited at two different stimulus rates (10/s and 55/s). Follow-up ABR recordings were performed on the same individuals with minor head trauma after 2 months. It was observed that there were no significant differences in interwave latencies V-I, III-I, and V-III at a stimulus rate of 10/s. However, there was a significant delay in all three interwave latencies at a stimulus rate of 55/s, specifically recorded in the first testing session. It can be noted here that an ABR with an increased stimulus rate is sensitive to changes in synaptic efficiency secondary to ischemic changes, while an ABR with a low stimulus rate (e.g., 10/s) is more sensitive to white matter lesions in TBI (Podoshin et al., 1990; Rosahl, Schuhmann, Thomas, Brinker, & Samii, 1998).
Frequency Following Response
ABR recordings typically involve using transient and simple stimuli. However, brainstem responses can also be evoked using complex stimuli with long durations (e.g., speech sounds, music, and amplitude-modulated sounds). Such brainstem responses are also known as complex auditory brainstem responses (cABRs). A cABR waveform consists of an onset response, a frequency following response (FFR), and an offset response. Given that FFR is a crucial component of cABRs, these terms have been used interchangeably as well (Anderson & Kraus, 2013; Skoe & Kraus, 2010). The neural generators of FFRs change with the modulation frequency. The auditory cortex is the primary generator of FFRs at modulation rates ∼40 Hz. FFRs obtained with 70 to 150 Hz modulation frequency reflects sustained phase-locked activity to the individual cycles of the stimulus waveform or the envelope of periodic stimuli in the brainstem (Holmes & Herrmann, 2017; Moushegian, Rupert, & Stillman, 1973; Smith, Marsh, & Brown, 1975). Recently, FFRs have also been used to elucidate deficits in sound processing with extreme granularity (Banai, Nicol, Zecker, & Kraus, 2005; Kraus et al., 2016).
Kraus et al. (2016) showed that FFRs have the potential to be used as an auditory biological marker of concussion in children. The study included 20 children with a history of concussion and 20 children without concussion history. Inclusion criteria for both groups entailed that participants had no history of hearing problems, neurological diseases, or severe TBI. FFRs were recorded from the right ear using a 40 ms sound/d/ delivered through insert earphones at 80.4 dB SPL. The Post-Concussion Symptom Scale was administered to all participants from the concussion group to assess the symptom load. The concept of symptom load refers to the sum total of the intensity of symptoms subsuming neurocognitive, emotional, and somatic domains. The scale included a total of 19 symptoms, with higher scores indicating greater symptom loads. The FFRs of children with concussion exhibited poorer pitch coding and delayed smaller neural responses than children without a history of concussion. The results of the study suggested that neural processing of the auditory stimuli correctly identifies 90% of the concussion and clears 95% of the control population. Thus, it can be construed that FFRs hold the potential to be a reliable biological marker of concussion. However, further studies are required to confirm the validity of FFR markers in different populations with mTBI.
In summary, we infer that early AEPs show potential diagnostic utility in mTBI. Specifically, ABRs with a higher stimulus repetition rate will further augment AEP recordings by indexing synchronicity of neural firing events at a finer level during cognitive performance. The main challenge will be to attenuate the influence of noise during data acquisition resulting from higher stimulus repetition rates (more than 100 stimuli per second). Algorithm-based novel techniques such as continuous loop averaging deconvolution and maximum length sequencing have been used to suppress noise and recover transient AEPs from the data (Peng, Yuan, Chen, Wang, & Ding, 2017). Lastly, investigating the potential influence of different types of auditory stimuli like clicks and tone bursts (frequency specific stimuli) at various presentation rates and levels will offer new insights into understanding deficits in central and peripheral auditory pathways of people with mTBI.
Middle AEPs
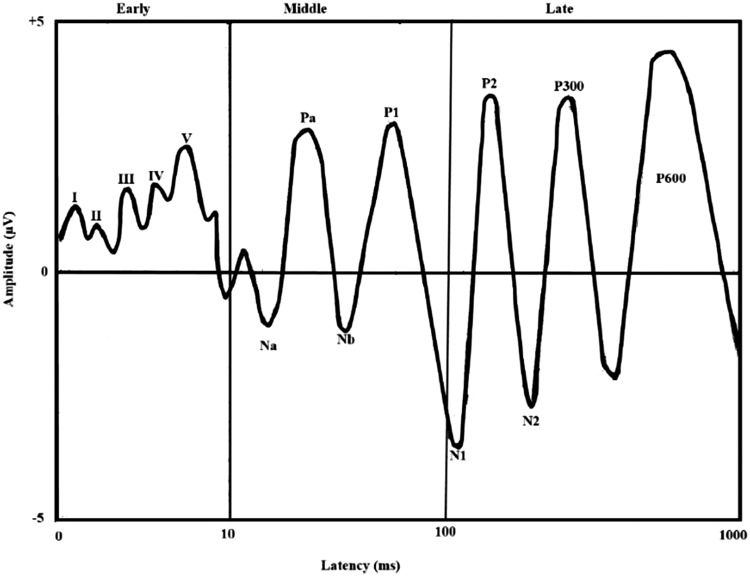
AMLR is a middle AEP mainly used for evaluating auditory cortical functions. It is engendered from the medial geniculate body of the thalamus, inferior colliculus, primary auditory cortex, thalamocortical tracts, and lateral supratemporal gyrus (Kraus & McGee, 1990; Musiek, Geurkink, Weider, & Donnelly, 1984). Typically, an AMLR waveform comprises a total of four constituent waveforms: Two negative waveforms labeled Na and Nb and two positive waveforms labeled Pa and Pb/P50. The Pb/P50 waveform of AMLR is the same waveform as P1 of the ALLR. Waveforms of individual AEPs are shown in Figure 1.
Figure 1.
Schematic representation of early, middle, and late auditory evoked potentials.
There is evidence to show that AMLR generator regions in the brain may be affected in mTBI, highlighting the diagnostic value of using AMLRs in the evaluation of people with mTBI (Drake, Weate, & Newell, 1996; Soustiel, Hafner, Chistyakov, Barzilai, & Feinsod, 1995). Soustiel et al. (1995) tested 40 participants with mTBI using brainstem AEPs and AMLR. Participants were examined in two sessions: first, 2 days following hospital admission and second, at 3 months following injury. Results showed significantly prolonged Na and Pa waveforms of AMLRs in 15 out of 40 participants. The authors suggested the involvement of diencephalic and paraventricular structures (indexed by abnormal AMLR waveforms) as a plausible pathological basis for some of the postconcussion symptoms, such as memory problems observed in people with mTBI. Drake et al. (1996) studied AMLRs in 20 people with a history of concussion and 20 people without any history of brain trauma. AMLR recordings in people with concussion showed reduced amplitudes of Na and Pa AMLR waveforms and significantly longer Pa latency compared with participants without brain injuries. The authors suggested that amplitude reduction of Na and Pa waveforms may indicate posttraumatic disturbances in subcortical AMLR generators or in frontotemporal cortical structures that modulate them. Further, there is evidence to show that the phenomenon of amplitude reduction of Na and Pa waveforms of AMLRs may be associated with increasing severity of TBI, thus underlining the potential role of AMLRs as one of the main evaluation systems in establishing both the severity of TBI and the associated auditory dysfunction in mTBI assessments (Munjal et al., 2010).
In addition to the Na and Pa waveforms, the AMLR P50 waveform, which is generated from Heschl's gyrus of the temporal lobe, may be crucial in recording neurophysiological events accompanying mTBI (Korzyukov et al., 2007). The P50 waveform has been used as a sensitive measure to evaluate auditory sensory gating—a phenomenon that refers to the inherent ability of the auditory system in preventing incoming irrelevant auditory information from reaching the auditory cortex, thus ensuring relevant auditory processing. It is suspected that persistent attention and memory impairments accompanying TBI may lead to an impaired auditory sensory gating regardless of severity. Therefore, the P50 waveform can be used in obtaining objective and relative measures of auditory sensory gating and record deficits in attention and memory accompanying mTBI (Adler et al., 1998; Boutros, Overall, & Zouridakis, 1991; Judd, McAdams, Budnick, & Braff, 1992). Arciniegas et al. (2000) assessed auditory sensory gating using AMLR on a group of 20 participants with TBI: five with mTBI, six with moderate TBI, and nine with severe TBI. The severity of TBI among participants was defined using the duration of PTA. The AMLR findings of this group were compared with the findings of the nonbrain-injured control group (n = 20). The findings of this study revealed reduced sensory gating in the TBI group when compared with the control group. Interestingly, the mild, moderate, and severe subgroups of this study revealed similar degrees of impaired auditory gating.
In contrast to the earlier findings, two studies have shown no evidence of significant AMLR changes in people with head trauma (Gaetz & Weinberg, 2000; Musiek et al., 2004). We argue that such inconsistencies with respect to AMLR findings among individuals with and without mTBI may be attributed to the differences in the site of lesion (e.g., peripheral vs. central), test protocols, and the time interval between the brain injury and AMLR assessments (Hall et al., 1982; Musiek et al., 2004). Future studies in this line of research will need to address the above confounds when considering AMLRs as a potential tool for assessment of individuals with TBI. At present, the Pb waveform of AMLR holds promise in capturing auditory sensory gating data, which may have imminent application in the assessment and rehabilitation of people with mTBI.
Late AEPs
ALLRs or auditory event-related potentials are a type of late AEPs. The major waveforms of the ALLR range from about 100 to 600 ms following stimuli presentation. The major waveforms of the ALLR include N1, P2, N2, and P300 (also known as P3), although some researchers consider P1 to be the part of the ALLR (Nelson, Hall, & Jacobson, 1997; Polich, Aung, & Dalessio, 1988; Purdy, Kelly, & Davies, 2002). The amplitudes and latencies of these waveforms have been used as reliable indices of auditory stimulus processing. Higher amplitudes of ALLRs reflect the allocation of greater attentional resources in stimulus processing, while delayed latency of ALLRs represents slowed sensory and cognitive processing (Duncan, Kosmidis, & Mirsky, 2005; Mazzini, 2004; Pratap-Chand, Sinniah, & Salem, 1988). ALLRs have also been used to test cognitive deficits associated with TBI including auditory memory, attention, and processing speed (Clark, Ohanlon, Wright, & Geffen, 1992; Mazzini, 2004; Potter & Barrett, 1999; Pratap-Chand et al., 1988; Solbakk, Reinvang, Nielsen, & Sundet, 1999). The generators of ALLR waveforms are described in Table 1.
Table 1.
Generators of Auditory Late Latency Response.
| Components | Latency | Neural generators |
|---|---|---|
| N1 | 75–140 ms | Auditory cortex |
| P2 | 150–230 ms | Auditory cortex |
| N2 | 150–250 ms | Auditory cortex, frontal cortex |
| P300 | 250–350 ms | Reticulothalamus, frontal cortex, and medial septal area |
ALLRs are most commonly elicited by auditory two-tone and three-tone oddball tasks. In terms of P3 waveforms, the auditory two-tone oddball task evokes either P3a or P3b, and the auditory three-tone oddball task simultaneously elicits both P3a and P3b waveforms. While P3a is typically elicited by an infrequent and uninstructed novel stimulus, P3b is indexed by an instructed and infrequently presented target stimulus (Polich, 2004, 2007). There is evidence to show that P3a is associated with the efficient allocation of attentional resources to new auditory stimuli (Kopp, Tabeling, Moschner, & Wessel, 2006). On the other hand, P3b is associated with stimulus evaluation and allocation of attentional resources while updating working memory (Broglio, Moore, & Hillman, 2011).
Studies using ALLRs have been at the forefront of electrophysiological research for almost 30 years. Pratap-Chand et al. (1988) compared P3b between 20 participants with mTBI and 20 matched control participants with no history of brain injury. They observed a significant delay in the P3b latency and reduction in P3b amplitude in the postconcussion period in people with mTBI. However, these delays and reductions in latency and amplitude of waveform P3b diminished completely on repeat testing, indicating that P3b is sensitive to cerebral dysfunction in mTBI and the potential recovery phase that may ensue over time. In a similar study, Solbakk et al. (1999) studied information processing and sustained selective auditory attention in participants with MHI, verified frontal lobe damage, and matched controls. They defined MHI as any blow to the head forcing one to stop whatever one was doing. A dichotic listening task was used to index cortical AEPs. The results of this study revealed significantly reduced N2 and P3 amplitudes in participants with MHI compared with participants with frontal lobe damage and nonbrain-damaged controls, reconfirming the notion that people with mTBI have deficits in focused sustained auditory attention and auditory information processing.
Segalowitz et al. (2001) examined the attentional capacity of university students with a history of mTBI (n = 10) and no self-reported neurocognitive impairment (n = 12). The average time postinjury in the mTBI group was 6.4 years. Experimental protocol included administration of four auditory three-tone oddball tasks and neuropsychological tests, such as Wechler Adult Intelligence Scale-Revised (WAIS-R; Wechsler, 1981) and the Woodcock Johnson Psycho-Educational Battery: Tests of Cognitive Ability (Woodcock & Johnson, 1977). In addition, three self-reported questionnaires assessing memory and attention were administered. It was observed that people with mTBI showed significantly reduced amplitudes of P3a and P3b compared with matched controls, while no significant difference was observed in cognitive performance on neuropsychological tests. The results of the study are important for two reasons. First, cognitive problems, especially related to attentional performance, may continue to persist in people with mTBI several years following injury. Second, ALLRs are a sensitive tool that can index cognitive declines that may be missed during routine neuropsychological tests. Similar results of delayed P3 responses have been observed in military veterans with a history of blast exposure, reconfirming earlier evidence on blast-induced mTBI affecting high level auditory and cognitive processing (Eskridge et al., 2012; Folmer et al., 2014; Gallun et al., 2012; McCrea et al., 2009; Papesh, Billing, Folmer, & Gallun, 2016).
In addition to people with experience serving in the military, a relatively higher prevalence of mTBI is observed among athletes, who are vulnerable to sports-related injuries. A range of postconcussion symptoms is commonly found in this population, including slower information processing, impaired focused attention, problems on tasks involving divided attention, and overall inconsistency in cognitive performance (Gosselin, Thériault, Leclerc, Montplaisir, & Lassonde, 2006; Thériault, De Beaumont, Gosselin, Filipinni, & Lassonde, 2009). In a study conducted by Gosselin et al. (2006), ALLR waveforms were examined in 30 athletes that included 20 symptomatic and asymptomatic athletes with a history of concussion and 10 athletes without concussion (controls). The results of the study revealed significantly reduced the amplitude of N1, P2, and P3 waveforms and delayed latency of P3 in both symptomatic and asymptomatic concussed athletes compared with athletes without a history of concussions. In a similar study, Thériault et al. (2009) compared P3a and P3b waveforms in 30 participants, who were recruited from various college sports teams (athletes from football, basketball, and volleyball teams) and divided into three groups: a nonbrain-damaged control group, a recently concussed group (examined between 5 and 12 months after the last concussion), and a late concussed group (examined between 22 and 60 months after the last concussion). A three-tone auditory oddball paradigm was used to record event-related potentials (i.e., P3a and P3b). A neuropsychological battery was also administered to all three groups, which comprised of a symbol digit modality test, a controlled oral word association test, a verbal learning test, a brief visuospatial memory test, a test for visual search and inhibition, and an orientation test. The findings showed a significant amplitude reduction of the P3a and P3b waveforms in the recently concussed group when compared with the nonbrain-damaged control athletes. On the other hand, P3a and P3b amplitudes in the late concussed group were equivalent to the nonbrain-damaged control group. None of the neuropsychological tests showed a significant difference among the three groups. The results indicate that despite functioning within a normal range on neuropsychological tests and sports activities, concussed athletes may still exhibit subtle deficits in auditory information processing within 12 months of head injury, which is identified by sensitive electrophysiological tests like the ALLR.
ALLR studies discussed earlier highlight the potential sensitivity of P3 as a marker of auditory attention and auditory memory deficits in participants with mTBI. The conflicting results of the studies that did not show a significant difference between individuals with mTBI and controls in terms of P3a and P3b amplitude and latency (Potter & Barrett, 1999; Potter, Bassett, Jory, & Barrett, 2001) can be attributed to multiple differences in experimental protocol and different study populations. Taken together, findings of AEP studies involving athletes with mTBI are critical for three main reasons. First, athletes with a history of concussion and exhibiting no symptoms may continue to have cognitive deficits related to early and late stages of auditory processing. Second, the absence of symptoms or the performance on neuropsychological tests may not be valid indicators for determining return to play. Third, AEPs are sensitive to indexing potential cognitive changes accompanying mTBI, even in the absence of reported symptoms and following a longer time interval (few months to several years) postinjury (Elting, Naalt, Weerden, Keyser, & Maurits 2005; Segalowitz et al., 2001; Solbakk et al., 1999). An overview of important research studies indexing AEPs in mTBI is provided in Table 2.
Table 2.
An Overview of Studies Indexing Auditory Evoked Potentials in Mild Traumatic Brain Injury.
| Authors | Study sample | Methods | AEP parameters | Major findings |
|---|---|---|---|---|
| Arciniegas et al. (2000) | Twenty adult participants with TBI (mild TBI = 5, moderate TBI = 6, severe TBI = 9) and 20 control participants. | Pulse-evoked AMLR. | Auditory pulse stimuli of 0.04 ms were presented in pairs, with an intrapair interval of 0.5 s, and an interstimulus interval of 10 s at 35–40 dB above hearing threshold. Three sets of average responses were collected. | Significant differences in P50 amplitude and P50 ratio between mTBI and control groups were observed. |
| Drake et al. (1996) | Twenty participants with mTBI and 20 control participants. | Alternating polarity clicks presented monaurally at 70 dB SL at a rate 4/s with 40 dB contralateral masking. | Prolonged latency of Pa and reduced amplitude of Pa and Na waveforms of AMLR were observed in participants with mTBI. | |
| Gallun et al. (2012) | Nineteen blast-exposed participants with mTBI and 29 without mTBI | Click-evoked ABR. | Rarefaction clicks presented at 70 dB nHL, with a rate of 17/s. | No significant differences were observed in the latencies and amplitude of waveforms I, III, and V between the groups. |
| Gosselin et al. (2006) | Twenty symptomatic and asymptomatic collegiate athletes and 20 control participants. | AERPs evoked using auditory oddball task. | Dichotic sequence of standard (70 ms; 1000 Hz-tone) and deviant (70 ms; 1100 Hz-tone) stimuli were presented at 80 dB SPL. The interstimulus interval was 2 s. A total of 400 stimuli were presented. | Smaller P2 amplitudes were observed in symptomatic participants; Prolonged P3 latencies and reduced amplitude of N1, P2, and P3 waveforms were observed in the concussed groups. |
| Kraus et al. (2016) | Twenty children with concussion and 20 control participants. | FFR of complex ABR. | FFRs elicited from the right ear by a 40 ms /da/ sound, presented with alternating polarities at 80.4dB SPL, with a rate of 10.9/s. | Reduced and slower responses to fundamental frequency (F0) and poor pitch coding were observed in children with concussion. |
| Munjal, Panda, and Pathak (2010) | Two hundred and ninety participants with TBI (mild TBI = 100, moderate TBI = 150, severe TBI = 40) and 50 control participants. | Click-evoked ABR and AMLR. | ABRs were recorded from each ear at 70 and 90 dB nHL using click stimuli presented at a rate of 19.3/s. AMLRs were measured for each ear using click stimuli at a rate of 5.1/s. The AMLR recordings were obtained at 70 dB nHL. | Wave V latency and I-V interpeak latency of ABR increased with severity of TBI. The amplitude of AMLR's Na and Pa component decreased with increasing severity of mTBI. |
| Noseworthy et al. (1981) | Eleven adults with a history of concussion and 12 control participants. | Click-evoked ABR. | Alternating condensation and rarefaction clicks of 80 dB SL were presented at a stimulus rate of 10/s. | Concussed participants showed a delayed wave III latency compared with control participants. |
| Podoshin et al. (1990) | Fifteen participants with minor head trauma and 35 control participants. | Click-evoked ABR. | Click stimuli presented at 70 dB nHL at two rates; 10 and 55/s for each ear. | Reduced interpeak latency difference at high stimulation rates was observed in participants with minor head trauma. |
| Pratap-Chand et al. (1988) | Twenty participants with mTBI and 20 matched controls. | AERPs evoked using auditory oddball task. | Tone bursts of 2 kHz presented randomly in a sequence of frequent (nontarget) 750 Hz tone bursts at 90 dB. | Decrease P3b amplitude with increased latency observed in people with mTBI. |
| Segalowitz et al. (2001) | Ten collegiate students with MHI and 12 control participants. | AERPs evoked using auditory oddball task. | Four auditory oddball tasks were presented with varying levels of difficulty. | Reduced amplitudes of P3a and P3b were observed in participants with MHI. |
| Solbakk et al. (1999) | Fifteen participants with mTBI and 13 control participants. | AERPs evoked auditory oddball task. | Two tone pips of 1000 Hz: standard (25 ms) and deviant (75 ms) were presented at 80 dB SPL in a random order at a fixed 1 s interstimulus interval and event-related potentials were recorded. | Reduced N2 and P3 amplitudes were observed in participants with mTBI. |
| Soustiel et al. (1995) | Forty participants with mTBI and 23 control participants. | Click-evoked AMLR. | Alternating polarity clicks presented at 75 dB nHL at a rate 4/s. | Prolonged Na and Pa waveforms of AMLRs were observed in 15 participants with mTBI. |
| Thériault et al. (2009) | High school athletes: three groups: Recent concussion (n = 10); late concussion (n = 10); control participants (n = 10). | AERPs evoked using auditory oddball task. | Event-related potentials were recorded using a three-tone auditory oddball paradigm. The frequent tone (1700 Hz, 80 dB) was presented in 80% of the trials. The rare target (2000 Hz, 80 dB) and the rare deviant (4000 Hz, 90 dB) tones were each presented in 10% of trials. | Smaller P3a and P3b amplitudes were observed in recently concussed athletes compared with control participants. The late concussion group also showed smaller P3a and P3b amplitude compared with controls. Larger P3b amplitude in the late concussed group was observed compared with the recent concussed group. |
AEP = auditory evoked potential; AMLR = auditory middle latency response; TBI = traumatic brain injury; mTBI = mild traumatic brain injury; ABR = auditory brainstem response; AERPs = auditory event-related potentials; FFR = frequency following response; MHI = mild head injury.
Future Implications
Neurocognitive deficits accompanying mTBI may be transient, subtle, and complex, involving problems with attention, working memory, episodic memory, processing speed, fluid reasoning, and executive functioning (Tulsky et al., 2017). Current limitations of neuropsychological and neuroimaging assessment tools may result in an underdiagnosis of mTBI, leading to challenges in acute management, long-term recovery, and rehabilitation for people with mTBI. These limitations can be addressed to some extent by including AEPs in the standard test battery of assessment tests in mTBI. The inclusion of AEPs in a standard test battery is suggested because AEPs are not an independent diagnostic tool for mTBI and have certain limitations such as targeting only auditory sense modality and providing less spatial or localizing information than neuroimaging tests. In addition, the clinical usefulness of AEPs in the assessment of mTBI is limited by the lack of standardized experimental paradigms. Nevertheless, various waveforms of AEPs offer a promising assessment protocol as a simultaneous or supplemental tool in recording accompanying neural events in conjunction with other neuroimaging tests in mTBI. The range of neurocognitive deficits observed in people with mTBI may also be a result of problems in the processing of auditory information itself (since cognitive and auditory domains are interrelated in tasks of information processing, as in auditory comprehension). However, it is likely that deficits resulting from mTBI may encompass both neurocognitive and auditory domains, depending on the site and the extent of neurotrauma. AEPs offer a highly sensitive index to measure potential auditory deficits in mTBI, thus providing enhanced information for making clinical decisions. The high rate ABR is sensitive to changes in synaptic efficiency, secondary to ischemic changes, and may identify subtle synaptic impairment associated with mTBI (Podoshin et al., 1990). The sensitivity of high rate ABRs could be enhanced by manipulating stimulus parameters and incorporating newer techniques like maximum length sequencing and continuous loop averaging deconvolution. Future studies are warranted to identify the influence of these techniques in the detection of deficits in patients with mTBI. The attention and memory deficits in mTBI patients could be associated with impaired sensory gating. The P50 waveform of the AMLR is a sensitive parameter to identify sensory gating deficits in participants with mTBI (Arciniegas et al., 2000). The clinical potential of P50 needs to be explored in future studies. A recent study examined the processing of fundamental frequency using FFRs in children with concussions and suggested that FFRs could be used as a biological marker for sports-related concussions (Kraus et al., 2016). There is a need to validate FFRs in different types of mTBI populations. The P3 waveform and its subtypes, P3a and P3b, are viable tools for investigating the integrity of sensory pathways, including their efficiency for conducting auditory inputs and the covert aspects of information processing, auditory memory, and auditory attention (Eierud et al., 2014). Despite the efficacy of P3, it is not used widely or routinely in general clinical neurology. One of the major barriers in clinical utility of all these promising AEP waveforms in mTBI assessment is a lack of standard testing paradigms. The development of standard testing paradigms for high rate ABR, FFR, P50, and P3 waveforms is the next step in considering AEPs as a clinical tool in mTBI assessment protocols.
Conclusions
Structural and functional brain changes following mTBI entail both acute and long-term neurocognitive implications, regardless of the etiopathology and the extent of the neuronal injury. Conventional neuropsychological tests and neuroimaging techniques may not be sensitive or specific enough in delineating varied cognitive deficits and neural correlates underlying these deficits. AEPs are cost-effective, easily accessible at most clinical care centers, and sensitive to indexing neural events accompanying performance on cognitive tasks. Including AEPs as part of the standard assessment tools will provide concurrent validity by offering triangulation of data from neuroimaging and neuropsychological tests, resulting in better diagnostic outcomes for people with mTBI. This step will be crucial in performing comprehensive evaluations of neurocognitive profiles of people with mTBI for meeting acute management and rehabilitation goals as well as identifying predictors for mTBI recovery.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Adler L. E., Olincy A., Waldo M., Harris J. G., Griffith J., Stevens K., Freedman R. (1998) Schizophrenia, sensory gating, and nicotinic receptors. Schizophrenia Bulletin 24(2): 189–202. doi:10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Anderson, S., & Kraus, N. (2013). The Potential Role of the cABR in Assessment and Management of Hearing Impairment. International Journal of Otolaryngology, 2013, 1–10. doi:10.1155/2013/604729. [DOI] [PMC free article] [PubMed]
- Arciniegas D., Olincy A., Topkoff J., Mcrae K., Cawthra E., Filley C. M., Adler L. E. (2000) Impaired auditory gating and p50 nonsuppression following traumatic brain injury. The Journal of Neuropsychiatry and Clinical Neurosciences 12(1): 77–85. doi:10.1176/jnp.12.1.77. [DOI] [PubMed] [Google Scholar]
- Banai K., Nicol T., Zecker S. G., Kraus N. (2005) Brainstem timing: Implications for cortical processing and literacy. Journal of Neuroscience 25(43): 9850–9857. doi:10.1523/jneurosci.2373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict R. H. B. (1997) Brief visuospatial memory test – Revised, Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Bigler E. D. (2004) Neuropsychological results and neuropathological findings at autopsy in a case of mild traumatic brain injury. Journal of the International Neuropsychological Society 10(05): 794–806. doi:10.1017/s1355617704105146. [DOI] [PubMed] [Google Scholar]
- Boutros N. N., Overall J., Zouridakis G. (1991) Test-retest reliability of the P50 mid-latency auditory evoked response. Psychiatry Research 39(2): 181–192. doi:10.1016/0165-1781(91)90086-5. [DOI] [PubMed] [Google Scholar]
- Broglio S. P., Moore R. D., Hillman C. H. (2011) A history of sport-related concussion on event-related brain potential correlates of cognition. International Journal of Psychophysiology 82(1): 16–23. doi:10.1016/j.ijpsycho.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2017). What are the signs and symptoms of concussion? Retrieved from https://www.cdc.gov/traumaticbraininjury/symptoms.html.
- Clark C., Ohanlon A., Wright M., Geffen G. (1992) Event-related potential measurement of deficits in information processing following moderate to severe closed head injury. Brain Injury 6(6): 509–520. doi:10.3109/02699059209008148. [DOI] [PubMed] [Google Scholar]
- Cusumano S., Paolin A., Paola F. D., Boccaletto F., Simini G., Palermo F., Carteri A. (1992) Assessing brain function in post-traumatic coma by means of bit-mapped SEPs, BAEPs, CT, SPET and clinical scores. Prognostic implications. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section 84(6): 499–514. doi:10.1016/0168-5597(92)90039-e. [DOI] [PubMed] [Google Scholar]
- Defense and Veterans Brain Injury Center. (2017). DoD worldwide TBI totals for 2017 Q4. Retrieved from https://dvbic.dcoe.mil/files/tbi-numbers/worldwide-totals-2000-2017_feb-14-2018_v1.0_2018-03-08.pdf.
- Delis D. C., Kaplan E., Kramer J. (2001) Delis Kaplan Executive Function System, San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Drake M. E., Weate S. J., Newell S. A. (1996) Auditory evoked potentials in postconcussive syndrome. Electromyography and Clinical Neurophysiology 36(8): 457–462. . [PubMed] [Google Scholar]
- Duncan C. C., Kosmidis M. H., Mirsky A. F. (2005) Closed head injury-related information processing deficits: An event-related potential analysis. International Journal of Psychophysiology 58(2–3): 133–157. doi:10.1016/j.ijpsycho.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson C. C., Donchin E. (1982) The P300 component of the event-related brain potential as an index of information processing. Biological Psychology 14(1–2): 1–52. doi:10.1016/0301-0511(82)90016-3. [DOI] [PubMed] [Google Scholar]
- Eierud C., Craddock R. C., Fletcher S., Aulakh M., King-Casas B., Kuehl D., LaConte S. M. (2014) Neuroimaging after mild traumatic brain injury: Review and meta-analysis. NeuroImage: Clinical 4: 283–294. doi:10.1016/j.nicl.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elting J., Naalt J., Weerden T., Keyser J., Maurits N. (2005) P300 after head injury: Pseudodelay caused by reduced P3A amplitude. Clinical Neurophysiology 116(11): 2606–2612. doi:10.1016/j.clinph.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Eskridge S. L., Macera C. A., Galarneau M. R., Holbrook T. L., Woodruff S. I., Macgregor A. J., Shaffer R. A. (2012) Injuries from combat explosions in Iraq: Injury type, location, and severity. Injury 43(10): 1678–1682. doi:10.1016/j.injury.2012.05.027. [DOI] [PubMed] [Google Scholar]
- Folmer, R. L., Hutter, M., Belding, H., Papesh, M., Elinger, R., Leek, M., & Gallun, F. J. (2014, June). Electrophysiological evidence of auditory and cognitive dysfunction in veterans exposed to high-intensity blasts. Poster session presented at 32nd Annual National Neurotrauma Symposium, San Francisco, CA.
- Friedland D. P. (2013) Improving the classification of traumatic brain injury: The Mayo classification system for traumatic brain injury severity. Journal of Spine S4: 5. [Google Scholar]
- Gaetz M., Bernstein D. M. (2001) The current status of electrophysiologic procedures for the assessment of mild traumatic brain injury. Journal of Head Trauma Rehabilitation 16(4): 386–405. doi:10.1097/00001199-200108000-00008. [DOI] [PubMed] [Google Scholar]
- Gaetz M., Weinberg H. (2000) Electrophysiological indices of persistent post-concussion symptoms. Brain Injury 14(9): 815–832. doi:10.1080/026990500421921. [DOI] [PubMed] [Google Scholar]
- Gallun F. J., Diedesch A. C., Kubli L. R., Walden T. C., Folmer R. L., Lewis M. S., Leek M. R. (2012) Performance on tests of central auditory processing by individuals exposed to high-intensity blasts. Journal of Rehabilitation Research and Development 49(7): 1005–1025. doi:10.1682/jrrd.2012.03.0038. [DOI] [PubMed] [Google Scholar]
- Gavett, B. E. (2011). Neuropsychological assessment battery. In J. S. Kreutzer, J. DeLuca, & B. Caplan (Eds.), Encyclopedia of clinical neuropsychology (pp. 2427–2431). New York, NY: Springer..
- Gershon R. C., Wagster M. V., Hendrie H. C., Fox N. A., Cook K. F., Nowinski C. J. (2013) NIH toolbox for assessment of neurological and behavioral function. Neurology 80(3): S2–S6. doi:10.1212/WNL.0b013e3182872e5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin N., Thériault M., Leclerc S., Montplaisir J., Lassonde M. (2006) Neurophysiological anomalies in symptomatic and asymptomatic concussed athletes. Neurosurgery 58(6): 1151–1161. doi:10.1227/01.neu.0000215953.44097.fa. [DOI] [PubMed] [Google Scholar]
- Gronwall D. M. (1977) Paced auditory serial-addition task: A measure of recovery from concussion. Perceptual & Motor Skills 44(2): 367–373. [DOI] [PubMed] [Google Scholar]
- Haglund Y., Persson H. E. (2009) Does Swedish amateur boxing lead to chronic brain damage? 3. A retrospective clinical neurophysiological study. Acta Neurologica Scandinavica 82(6): 353–360. doi:10.1111/j.1600-0404.1990.tb03316.x. [DOI] [PubMed] [Google Scholar]
- Hall J. W., Speilman G., Gennarelli T. A. (1982) Auditory evoked responses in acute severe head injury. Journal of Neuroscience Nursing 14(5): 225–231. doi:10.1097/01376517-198210000-00004. [DOI] [PubMed] [Google Scholar]
- Heaton S. K., Chelune G. J., Talley J. L., Kay G. G., Curtiss G. (1993) Wisconsin card sorting test manual: Revised and expanded, Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Holmes, E., & Herrmann, B. (2017, May 24). Revisiting the contribution of auditory cortex to frequency-following responses. Retrieved from http://www.jneurosci.org/content/37/21/5218. [DOI] [PMC free article] [PubMed]
- Holmes M. W., Goodacre S., Stevenson M. D., Pandor A., Pickering A. (2012) The cost-effectiveness of diagnostic management strategies for adults with minor head injury. Injury 43(9): 1423–1431. [DOI] [PubMed] [Google Scholar]
- Iverson G. L., Holdnack J., Lange R. T. (2013) Using the WAIS-IV/WMS-IV/ACS following moderate-severe traumatic brain injury. In: Holdnack J., Drozdick L., Weiss L. G., Iverson G. L. (eds) WAIS-IV/WMS-IV/ACS: Advanced clinical interpretation, San Diego, CA: Elsevier Science, pp. 485–544. [Google Scholar]
- Judd L. L., McAdams L., Budnick B., Braff D. L. (1992) Sensory gating deficits in schizophrenia: New results. American Journal of Psychiatry 149(4): 488–493. doi:10.1176/ajp.149.4.488. [DOI] [PubMed] [Google Scholar]
- Keren O., Sazbon L., Groswasser Z., Shmuel M. (1994) Follow-up studies of somatosensory evoked potentials and auditory brainstem evoked potentials in patients with post-coma unawareness (PCU) of traumatic brain injury. Brain Injury 8(3): 239–247. doi:10.3109/02699059409150976. [DOI] [PubMed] [Google Scholar]
- Kopp B., Tabeling S., Moschner C., Wessel K. (2006) Fractionating the neural mechanisms of cognitive control. Journal of Cognitive Neuroscience 18(6): 949–965. doi:10.1162/jocn.2006.18.6.949. [DOI] [PubMed] [Google Scholar]
- Korzyukov O., Pflieger M. E., Wagner M., Bowyer S. M., Rosburg T., Sundaresan K., Boutros N. N. (2007) Generators of the intracranial P50 response in auditory sensory gating. NeuroImage 35(2): 814–826. doi:10.1016/j.neuroimage.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus, N., & McGee, T. (1990). Clinical applications of the middle latency response. Journal of the American Academy of Audiology, 1(3), 130–133. [PubMed]
- Kraus N., Thompson E. C., Krizman J., Cook K., White-Schwoch T., Labella C. R. (2016) Auditory biological marker of concussion in children. Scientific Reports 6(1): 39009 doi:10.1038/srep39009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus N., White-Schwoch T. (2015) Unraveling the biology of auditory learning: A cognitive-sensorimotor-reward framework. Trends in Cognitive Sciences 19(11): 642–654. doi:10.1016/j.tics.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois J. A., Rutland-Brown W., Wald M. M. (2006) The epidemiology and impact of traumatic brain injury. Journal of Head Trauma Rehabilitation 21(5): 375–378. [DOI] [PubMed] [Google Scholar]
- Mathias J. L., Beall J. A., Bigler E. D. (2004) Neuropsychological and information processing deficits following mild traumatic brain injury. Journal of the International Neuropsychological Society 10(2): 286–297. doi:10.1017/s1355617704102117. [DOI] [PubMed] [Google Scholar]
- Mathias J. L., Wheaton P. (2007) Changes in attention and information-processing speed following severe traumatic brain injury: A meta-analytic review. Neuropsychology 21(2): 212–223. doi:10.1037/0894-4105.21.2.212. [DOI] [PubMed] [Google Scholar]
- Mazzini L. (2004) Clinical applications of event-related potentials in brain injury. Physical Medicine and Rehabilitation Clinics of North America 15(1): 163–175. doi:10.1016/s1047-9651(03)00101-3. [DOI] [PubMed] [Google Scholar]
- Mccrea M., Iverson G. L., Mcallister T. W., Hammeke T. A., Powell M. R., Barr W. B., Kelly J. P. (2009) An integrated review of recovery after mild traumatic brain injury (MTBI): Implications for clinical management. The Clinical Neuropsychologist 23(8): 1368–1390. doi:10.1080/13854040903074652. [DOI] [PubMed] [Google Scholar]
- Mild Traumatic Brain Injury Committee of the American Congress of Rehabilitation Medicine (1993) Definition of mild traumatic brain injury. Journal of Head Trauma Rehabilitation 8(3): 86–87. doi:10.1097/00001199-199309000-00010. [Google Scholar]
- Moushegian G., Rupert A. L., Stillman R. D. (1973) Scalp-recorded early responses in man to frequencies in the speech range. Electroencephalography and Clinical Neurophysiology 35(6): 665–667. doi:10.1016/0013-4694(73)90223-x. [DOI] [PubMed] [Google Scholar]
- Munjal S. K., Panda N. K., Pathak A. (2010) Relationship between severity of traumatic brain injury (TBI) and extent of auditory dysfunction. Brain Injury 24(3): 525–532. doi:10.3109/02699050903516872. [DOI] [PubMed] [Google Scholar]
- Musiek F. E., Baran J. A., Shinn J. (2004) Assessment and remediation of an auditory processing disorder associated with head trauma. Journal of the American Academy of Audiology 15(2): 117–132. doi:10.3766/jaaa.15.2.3. [DOI] [PubMed] [Google Scholar]
- Musiek F. E., Geurkink N. A., Weider D. J., Donnelly K. (1984) Past, present, and future applications of the auditory middle latency response. The Laryngoscope 94(12): 1545 doi:10.1288/00005537-198412000-00002. [DOI] [PubMed] [Google Scholar]
- Nakase-Richardson R., Sherer M., Seel R. T., Hart T., Hanks R., Arango-Lasprilla J. C., Hammond F. (2011) Utility of post-traumatic amnesia in predicting 1-year productivity following traumatic brain injury: Comparison of the Russell and Mississippi PTA classification intervals. Journal of Neurology Neurosurgery Psychiatry 82(5): 494–499. [DOI] [PubMed] [Google Scholar]
- National Center for Injury Prevention and Control. (2003). Report to congress on mild traumatic brain injury in the united states: Steps to prevent a serious public health problem. Retrieved from https://www.cdc.gov/traumaticbraininjury/pdf/mtbireport-a.pdf.
- National Institute of Neurological Disorders and Stroke. (2018). Traumatic brain injury information page. Retrieved from https://www.ninds.nih.gov/disorders/all-disorders/traumatic-brain-injury-information-page.
- Nelson M. D., Hall J. W., Jacobson G. P. (1997) Factors affecting the recordability of auditory evoked response component Pb (P1). Journal of American Academy of Audiology 8(2): 89–99. [PubMed] [Google Scholar]
- Nölle C., Todt I., Seidl R. O., Ernst A. (2004) Pathophysiological changes of the central auditory pathway after blunt trauma of the head. Journal of Neurotrauma 21(3): 251–258. doi:10.1089/089771504322972040. [DOI] [PubMed] [Google Scholar]
- Noseworthy J. H., Miller J., Murray T. J., Regan D. (1981) Auditory brainstem responses in postconcussion syndrome. Archives of Neurology 38(5): 275–278. doi:10.1001/archneur.1981.00510050041004. [DOI] [PubMed] [Google Scholar]
- Nuwer M. R., Hovda D. A., Schrader L. M., Vespa P. M. (2005) Routine and quantitative EEG in mild traumatic brain injury. Clinical Neurophysiology 116(9): 2001–2025. doi:10.1016/j.clinph.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Papesh, M. A., Billings, C., Folmer, R. L., & Gallun, F. J. (2016, February). Late auditory evoked potentials in blast-exposed veterans. Poster session presented at Association for Research in Otolaryngology MidWinter Meeting, San Diego, CA.
- Peng X., Yuan H., Chen W., Wang T., Ding L. (2017) New metric for optimizing Continuous Loop Averaging Deconvolution (CLAD) sequences under the 1/f noise model. Plos One 12(4): e0175354 doi:10.1371/journal.pone.0175354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podoshin L., Ben-David J., Fradis M., Pratt H. (1990) Brainstem auditory evoked potentials with increased stimulus rate in minor head trauma. The Journal of Laryngology and Otology 49(6): 287–293. doi:10.1017/S0022215100112241. [DOI] [PubMed] [Google Scholar]
- Polich J. (2004) Clinical application of the P300 event-related brain potential. Physical Medicine and Rehabilitation Clinics of North America 15(1): 133–161. doi:10.1016/s1047-9651(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Polich J. (2007) Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology 118(10): 2128–2148. doi:10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J., Aung M., Dalessioo D. (1988) Long latency auditory evoked potentials: Intensity, inter-stimulus interval, and habituation. The Pavlovian Journal of Biological Science 23(1): 35–40. [PubMed] [Google Scholar]
- Potter D., Barrett K. (1999) Assessment of mild head injury with ERPs and neuropsychological tasks. Journal of Psychophysiology 13(3): 173–189. doi:10.1027//0269-8803.13.3.173. [Google Scholar]
- Potter D. D., Bassett M. R., Jory S. H., Barrett K. (2001) Changes in event-related potentials in a three-stimulus auditory oddball task after mild head injury. Neuropsychologia 39(13): 1464–1472. doi:10.1016/s0028-3932(01)00057-4. [DOI] [PubMed] [Google Scholar]
- Pratap-Chand R., Sinniah M., Salem F. A. (1988) Cognitive evoked potential (P300): A metric for cerebral concussion. Acta Neurologica Scandinavica 78(3): 185–189. doi:10.1111/j.1600-0404.1988.tb03643.x. [DOI] [PubMed] [Google Scholar]
- Prince C., Bruhns M. (2017) Evaluation and treatment of mild traumatic brain injury: The role of neuropsychology. Brain Sciences 7(12): 105 doi:10.3390/brainsci7080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy S., Kelly A., Davies M. (2002) Auditory brainstem response, middle latency response, and late cortical evoked potentials in children with learning disabilities. Journal of American Academy of Audiology 13(7): 367–382. [PubMed] [Google Scholar]
- Rosahl S. K., Schuhmann M. U., Thomas S., Brinker T., Samii M. (1998) Brain-stem auditory evoked potential monitoring in experimental diffuse brain injury. Intracranial Pressure and Neuromonitoring in Brain Injury 71: 88–90. doi:10.1007/978-3-7091-6475-4_27. [DOI] [PubMed] [Google Scholar]
- Segalowitz S. J., Bernstein D. M., Lawson S. (2001) P300 event-related potential decrements in well-functioning university students with mild head injury. Brain and Cognition 45(3): 342–356. doi:10.1006/brcg.2000.1263. [DOI] [PubMed] [Google Scholar]
- Shallice T. (1982) Specific impairments of planning. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences 298: 199–209. doi:10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Skoe, E., & Kraus, N. (2010). Auditory Brain Stem Response to Complex Sounds: A Tutorial. Ear and Hearing, 31(3), 302–324. doi:10.1097/aud.0b013e3181cdb272. [DOI] [PMC free article] [PubMed]
- Smith J. C., Marsh J. T., Brown W. S. (1975) Far-field recorded frequency-following responses: Evidence for the locus of brainstem sources. Electroencephalography and Clinical Neurophysiology 39(5): 465–472. doi:10.1016/0013-4694(75)90047-4. [DOI] [PubMed] [Google Scholar]
- Solbakk A., Reinvang I., Nielsen C., Sundet K. (1999) ERP indicators of disturbed attention in mild closed head injury: A frontal lobe syndrome? Psychophysiology 36(6): 802–817. doi:10.1111/1469-8986.3660802. [PubMed] [Google Scholar]
- Soustiel J. F., Hafner H., Chistyakov A. V., Barzilai A., Feinsod M. (1995) Trigeminal and auditory evoked responses in minor head injuries and post-concussion syndrome. Brain Injury 9(8): 805–813. doi:10.3109/02699059509008236. [DOI] [PubMed] [Google Scholar]
- Teasdale G., Jennett B. (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet 2(7872): 81–84. [DOI] [PubMed] [Google Scholar]
- Thériault M., Beaumont L. D., Gosselin N., Filipinni M., Lassonde M. (2009) Electrophysiological abnormalities in well-functioning multiple concussed athletes. Brain Injury 23(11): 899–906. doi:10.1080/02699050903283189. [DOI] [PubMed] [Google Scholar]
- Tulsky D. S., Carlozzi N. E., Holdnack J., Heaton R. K., Wong A., Goldsmith A., Heinemann A. W. (2017) Using the NIH Toolbox Cognition Battery (NIHTB-CB) in individuals with traumatic brain injury. Rehabilitation Psychology 4(62): 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UPMC Sports Medicine. (2019). Concussions facts and statistics. Retrieved from https://www.upmc.com/services/sports-medicine/services/concussion/facts-statistics.
- Wechsler D. (1981) Wechsler Adult Intelligence Scale—Revised, San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D., Coalson D. L., Raiford S. E. (2008) WAIS-IV technical and interpretive manual, San Antonio, TX: Pearson. [Google Scholar]
- Werner R. A., Vanderzant C. W. (1991) Multimodality evoked potential testing in acute mild closed head injury. Archives of Physical Medicine and Rehabilitation 72(1): 31–34. [PubMed] [Google Scholar]
- Woodcock R. W., Johnson M. B. (1977) Woodcock–Johnson psycho-educational battery: Tests of cognitive ability, Hingham, MA: Teaching Resources Corporation. [Google Scholar]