Abstract
The polyphenol plant extracts have previously been demonstrated to act as chemopreventive and anticancer agents. Ficus carica is a rich source of polyphenols, yet its antioxidant and anticancer activities remain poorly characterized. This study aimed to determine the anticancer activity of F carica leaf and fruit extracts by investigating their impact on proliferation, apoptosis, and Huh7it cell necrosis. Leaves and fruits were extracted using methanol, and the phytochemical contents were analyzed using Fourier-transform infrared spectroscopy. The antioxidant activity was measured using the 2,2-diphenyl-1-picrylhydrazyl method. Anticancer activities were examined through MTT (3-(4,5-dimethylthiazol-2yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay on Huh7it liver cancer cells. The apoptosis and necrosis conditions were examined using Annexin biomarkers V-PI and later analyzed in flow cytometry. F carica leaves and fruit examined were found to have strong antioxidant activities with IC50 values of 7.9875 µg/mL and 13.402 µg/mL, respectively. MTT assay results indicated F carica leaves and fruit had IC50 values >653 μg/mL and >2000 μg/mL, respectively. The flow cytometry analysis indicated a higher percentage of Huh7it apoptosis and necrosis in leaf extracts compared with fruit extracts. The difference in anticancer activity was attributed to differing compounds present in each extract.
Keywords: anticancer, F carica, antioxidant, leaves, fruits, methanol extract
Introduction
Liver cancer is the sixth most common human disease and the third most common cause of death after lung and colon cancer. In 2012, there were 14 067 894 new cases of cancer and 8 201 575 deaths from cancer worldwide.1 In 2013, there were over 700 000 deaths from malignancies in liver cancer, the lead causes of which were hepatitis A, hepatitis B, and alcohol abuse. Of all the cases that occur, 75% of them are in men with an average age of 63 to 69 years.2
Liver cancer is also called hepatocellular carcinoma (HCC). This disease is highly associated with chronic liver disease, especially hepatitis B virus (HBV) and hepatitis C virus (HCV) infection. About 52.3% of patients with liver cancer have had chronic HBV infection and 20% have had HCV infection.3 Other causes of HCC are a nonalcoholic fatty liver disease (NAFLD), aflatoxin, and alcoholic liver disease. The risk of liver cancer from cirrhosis patient ranges from 1% to 6% per year. Cirrhosis, regardless of its etiology, has a risk of liver cancer 3 to 4 times higher than chronic hepatitis. Increased hepatocellular proliferation can lead to the activation of tumor suppressor gene mutations. This change will later initiate hepatocarcinogenesis. Hepatocarcinogenesis can develop into HCC.3 Currently, the most common HCC treatment is a surgical therapy. However, success rates are low and often patients do not qualify. Less than 30% of HCC patients can be treated with liver resection or transplantation as the majority of HCC cases have an advanced phase liver condition. Other options include percutaneous ethanol injection, radiofrequency ablation, and transarterial chemoembolization.4
Many efforts have been made to create effective chemotherapy drugs, but there are still problems in terms of toxicity and selectivity. The toxicity of modern chemotherapy and the resistance of cancer cells to anticancer agents have induced investigations for treatment alternatives and methods of prevention. Natural plant products may offer a solution. The use of plants as an anticancer agent has previously been investigated by Apriyanto et al5 which found that methanol extract of Annona muricata leaves could inhibit the growth of Huh7it cells with IC50 < 0.3 µg/mL. Other plants have also been able to suppress the growth of HCC in vitro, such as Ailanthus altissima, Tabernaemontana elegans, Asplenium ramlowii Hieron, Toona sureni, Melicope latifolia, Melanolepis multiglandulosa S., and Ficus fistulosa.6–8
Ficus is one of the largest genus of medicinal plants with around 750 species of woody plants, trees, and shrubs found in subtropical and tropical regions throughout the world. F carica Linn, commonly called “Tin” in Indonesia and globally known as F carica, has previously been studied for health-promoting properties. These effects were mediated through phenolic acids, chlorogenic acids, flavones, and flavonols present in F carica.9,10 Quercetin compounds are the main phenolic compounds found in F carica. Quercetin has the ability to stimulate the apoptosis of Caco-2 and HT-29 colon cancer and HL-60 leukemia cancer cells by stimulating the release of cytochrome c from mitochondria.10 This compound also showed a synergistic effect with cisplatin (chemotherapeutic drug) in vitro and in vivo through the inhibition of protein kinase C (PKC).10 F carica also contains fiber, vitamin A, vitamin C, calcium, magnesium, and potassium which are needed by the body. Other bioactive compounds of F carica are arabinose, β-amirin, β-carotene, glycosides, β-sitosterol, and xanthol, which are antioxidant compounds.11
F carica may also have anticancer activities. F carica has previously been demonstrated to inhibit the growth of HeLa cancer cells and MDA-megabyte (MB)-231 breast cancer cells.11,12 However, F carica extracts have never been tested on Huh7it liver cancer cells. In this research, F carica’s potential as anticancer agent was explored by testing the methanol extract of F carica leaves and fruit on Huh7it cell cultures in vitro.
Material and Methods
Plant material and chemicals
F carica fruits and leaves were obtained from Tulangan district, Sidoarjo city, East Java, Indonesia, in August 2018. The other materials used were absolute Methanol (Sigma-Aldrich, St. Louis, MO, USA), ultraviolet-visible (UV-vis) spectrophotometers, Quvetes Glass, 2,2-diphenyl-1-picrylhydrazyl (DPPH), MTT (3-(4,5-dimethylthiazol-2yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) solution (Sigma-Aldrich, St. Louis, MO, USA), dimethyl sulfoxide (DMSO), GloMax-Multi Microplate Multimode Reader (Promega Corp., WI, USA), Huh7it cell line, phosphate-buffered saline (PBS), Annexin-PI, BD Biosciences FACS CaliburTM flow cytometry, DMEM (Gibco BRL, Grand Island, NY, USA), t-EDTA, Dulbecco PBS (D-PBS, Gibco BRL, Grand Island, NY, USA), nonessential amino acid, fetal bovine serum (FBS, Gibco BRL, Grand Island, NY, USA), Dulbecco’s modified Eagle’s Medium (Invitrogen, Carlsbad, CA, USA), kanamycin (Sigma-Aldrich, St. Louis, MO, USA), and nonessential amino acids (Invitrogen).
F carica fruit and leaf extract preparation
Hundred grams of the fruit and leaves of F carica were cleaned with water, cut into pieces and macerated with 400 mL of methanol, and allowed to stand overnight 3 times. Precipitates were collected and remaining methanol was evaporated with a rotary evaporator at 50°C. The extract was then lyophilized to form a powder. The stock solution was made by solubilizing each powder (5 mg) with DMSO (50 µL).12
Antioxidant DPPH assay
The leaf and fruit extracts were tested for antioxidant activity using the DPPH method.13 The stock solution was made by adding various concentrations of fruit and leaf extracts and dissolving in 5 mL absolute methanol. A control solution containing 3 mL of methanol and 1 mL of DPPH 100 ppm solution was also prepared. One milliliter of sample or control solution, 1 mL DPPH, and 2 mL methanol were prepared and incubated for 30 minutes at 27°C until there was a change in color from DPPH activity. Color change was quantified using absorbance at 517 nm using a UV-vis spectrophotometer. Free radical antidote activities were calculated as a percentage of DPPH color reduction. Antioxidant capacity (%) to inhibit free radicals was determined by the following equation:
A curve was made between the percent of free radical antidote activities to the concentration of the test solution. The results were measured using this following linear equation: Y = aX + b
where Y = % inhibition; a = gradient; X = concentration (μg/mL); b = constant.
The resulting linear equation was used to obtain the IC50 value. IC50 value is the concentration obtained when the % inhibition is 50 or 50 = aX + b. All measurements were duplicated.
Identification of extracts functional group
The functional group identification test was carried out using Fourier-transform infrared spectroscopy (FTIR). This instrument is used to predict active organic compounds by identifying functional groups through infrared ray absorption. One milligram of dried F carica leaf and fruit were mixed with 200 mg potassium bromide. IR spectra were recorded in the mid-IR area at frequency 400-4000 cm−1. Wavelength measurements were carried out by FTIR spectrometer.
Huh7it cell culture
Huh7it cells were cultured in Petri dishes on DMEM (Invitrogen, Carlsbad, CA, USA) with 10% addition of FBS (Biowest, Nuaille, France), kanamycin 150 μg / mL (Sigma-Aldrich), and nonessential amino acids (Invitrogen).
Cancer cell proliferation analysis
Cancer cell proliferation test was carried out using an MTT assay (Sigma-Aldrich). Huh7it cells were cultured on 96-well plates with a density of 2.4 × 104 per well and incubated at 37°C for 24 hours. After incubation, 100 µL of F carica leaf and fruit extracts were added in each well and diluted with DMSO with concentrations of 2000, 1000, 800, 400, 200, 100, 50, 25, 12, 5, 6, and 3 μg/mL and incubated at temperature 37°C for 48 hours. The positive control group was given doxorubicin (10 μg/mL), based on IC50 value from previous research.14 Medium was discarded after incubation and 150 μL of new medium containing 0.5 mg/mL MTT solution concentration in DMEM were added to each well. Then, the plate was incubated at 37°C for 4 hours until a formazan crystal formed. Cells were observed using an inverted microscope, if formazan was clearly formed, the medium was discarded and 100 μL DMSO was added to dissolve the precipitate formed from the MTT reaction. Absorbance was measured at a wavelength of 560 nm and 750 nm using GloMax-Multi Microplate Multimode Reader (Promega). The measurement results were then compared with controls.
Apoptosis and necrosis analysis
Huh7it cells 60 × 104 were placed on 6-well plates and incubated at 37°C overnight. F carica leaf and fruit extracts were added at concentrations of 400, 600, 800, 1000 μg/mL. The plate was then incubated for 48 hours at 37°C. Cells were collected by adding trypsin EDTA. All aspirates were placed on the microtube and centrifuged 13 000 rpm for 5 minutes at 4°C and washed with cold PBS. The cells were harvested and then centrifuged at speeds of 2500 rpm, 10°C for 5 minutes. The supernatant was removed and the pellet was washed with PBS 1 mL and centrifuged at a speed of 2500 rpm, 10°C for 5 minutes. Pellets were added with Annexin-PI in PBS (50 μL) and incubated for 30 minutes. Flow cytometry analysis was carried out at the end of incubation by adding 300 μL of PBS containing incubated Huh7it cells directly to the flow cytometer cuvette.
Results
Phytochemical content and antioxidant activity
Phytochemical test results indicated that F carica leaf extract contained active compounds such as phenol, tannin, flavonoid, and saponin, while F carica fruit extract contained phenol, flavonoid, and saponin. These compounds have the potential to act as an antioxidant because their structure contains a hydroxyl (-OH) group that can donate protons to free radicals. F carica leaf was found to have more active compounds than fruit, resulting in differing antioxidant activity. The antioxidant activity in F carica leaves is greater than the fruit, based on the IC50 values. However, both extracts have very good potential to tackle free radicals. The results of comparing the concentration with the % value of antioxidant activity from each sample are shown in Table 1.
Table 1.
IC50 value of F carica fruit and leaf extracts.
| Extract sample | Equation | R 2 | Y value | X value (IC50) |
|---|---|---|---|---|
| F carica fruits | y = 3.6868x + 0.5895 | 0.8213 | 50 | 13.402 |
| F carica leaves | y = 7.2556x − 7.1555 | 0.8179 | 50 | 7.9875 |
The linear regression curves represent the concentration of samples added DPPH on X-axis and the percent inhibition of antioxidants on Y-axis. The equation for F carica leaf extract is y = 7.2556x − 7.1555; R2 = 0.8179, while the fruit extract is y = 3.6868x + 0.5895; R2 = 0.8213. The y coefficient in this equation is 50% deterrence of free radicals, while the x coefficient in this equation is the IC50 value. The IC50 value of F carica leaf and fruit extracts was found to be 7.9875 µg / mL and 13.402 µg / mL, respectively. It has previously reported that compounds with an IC50 value < 10 µg / mL have very strong anti-free radical properties, strong if the IC50 value ranges between 10 and 50 µg / mL, moderate if the IC50 value ranges from 50 to 100 µg / mL, weak if the IC50 value ranges from 100 to 250 µg / mL, and not active if the IC50 value is above 250 µg / mL.15 Therefore, F carica leaf extract has been proven to contain a very strong antioxidant activity because its IC50 value is less than 10 µg / mL, while F carica fruit extract has a strong antioxidant activity because its IC50 value ranges from 10 to 50 µg / mL.
FTIR characterization
FTIR analysis was used to determine the functional groups of active compounds contained in F carica leaf and fruit extracts. The wavelength of light absorbed is salient feature of the chemical bond as can be seen in the annotated spectrum. By interpreting the infrared absorption spectrum, the chemical bonds in a compound can be determined. The FTIR spectra of F carica leaves and fruits both contained wave peaks characteristic of hydroxyl, alkane, alkene groups that could indicate the presence of phenolic and saponin compounds in the extract. Ether groups were also present in both but only aliphatic spectra were found in the leaf samples. Therefore, it is predicted that F carica leaf and fruit extracts contain phenolic active compounds and their derivatives (flavonoids and tannins) as well as saponin compounds.
The peaks contained in FTIR spectra of F carica leaf extract are shown in Table 2. The peak at frequency of 2936.9, 2100.2, 1635.9, 1416.8, and 1058.3 were strong. The peaks contained in FTIR spectra of F carica fruit extract are shown in Table 3. The peak at frequency of 2926.6, 2149.16, 1633.9, and 1048.7 were strong.
Table 2.
FTIR peak spectra of F carica leaf extract.
Table 3.
FTIR peak spectra of F carica fruit extract.
| No. | Functional group | Absorption frequency (cm−1) | Intensity |
|---|---|---|---|
| 1 | Alcohol (-OH) | 3377.4 | Broad |
| 2 | Alkane (sp3 C-H) | 2926.6 | Strong |
| 2 | Alkene (-C = C-) | 2149.16 | Strong |
| 3 | Carbonyl (-C = O) | 1633.9 | Strong |
| 4 | C-O | 1048.7 | Strong |
Antiproliferation using MTT assay
Anticancer activity was evaluated using an MTT in vitro cell proliferation assay, which is a general anticancer activity evaluation test. Based on the MTT assay absorbance, the anticancer activity of F carica leaves was higher than F carica fruit (Figure 1). The concentration of 1000 μg/mL F carica leaf extract was found to inhibit 82.78% of cell growth, whereas the same concentration of F caricas fruits showed 11.84% cell growth inhibition. The data of cell growth inhibition is provided in Table 4. The 50% cell growth inhibition activity, which was based on a probit log base 10 regression, showed that F carica leaf extract had IC50 values >653 μg/mL and F carica fruit extract had IC50 values >2000 μg/mL. Therefore, it can be concluded that F carica leaf extract could inhibit the growth of liver cancer cells more than the fruit. The difference in the content of compounds found in leaf and fruit extracts may be the cause of differences in liver anticancer activity in between the extracts.
Figure 1.
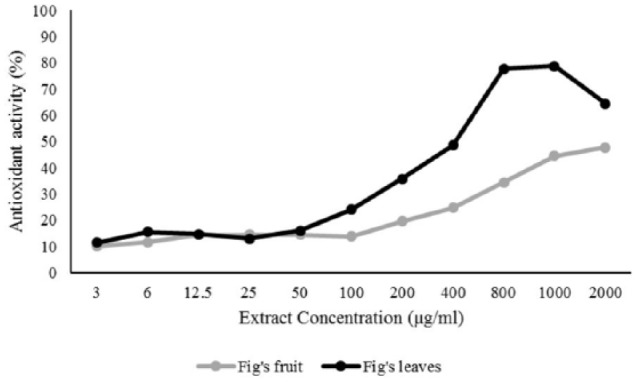
Antioxidant activity of F carica leaf and fruit extracts at various concentrations.
Table 4.
Antioxidant activity of F carica extracts and its effect on Huh7it cells growth inhibition.
| Concentration (µg/mL) | % Growth inhibition |
% Antioxidant activity |
||
|---|---|---|---|---|
| Leaves | Fruits | Leaves | Fruits | |
| 3 | 0.161 | 9.83 | 11.38 | 10.04 |
| 6 | 2.769 | 4.25 | 15.53 | 11.56 |
| 12.5 | 0.361 | 2.77 | 14.68 | 14.52 |
| 25 | 2.97 | 2.41 | 13.03 | 14.47 |
| 50 | 1.485 | 3.01 | 16.1 | 14.5 |
| 100 | 3.652 | 10.19 | 24.1 | 13.76 |
| 200 | 14.687 | 2.73 | 35.76 | 19.54 |
| 400 | 13.604 | 6.98 | 48.62 | 24.7 |
| 800 | 35.032 | 9.67 | 77.68 | 34.43 |
| 1000 | 82.785 | 11.84 | 78.82 | 44.5 |
| 2000 | 98.676 | 10.43 | 64.41 | 47.75 |
Apoptosis and necrosis analysis using flow cytometry
Apoptosis and necrosis analysis of liver cancer cells were performed using flow cytometry. Cells undergoing apoptosis secrete phosphatidylserine on the extracellular surface so that it can be characterized by Annexin V-labeled fluorescence. Phosphatidylserine is normally present in the cytosolic surface of the plasma membrane but is redistributed to the extracellular surface during apoptosis by a hypothetical protein known as scramblase.
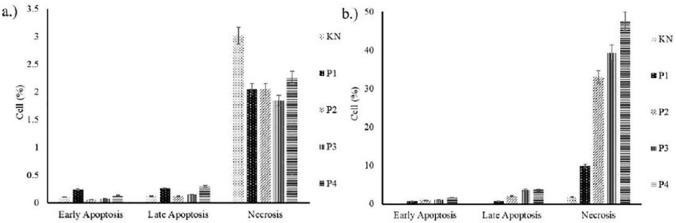
Huh7it cells were treated with F carica leaf and fruit extracts and analyzed using Annexin-V and PI antibodies. Cells expressing Annexin – PI + were experiencing necrosis, those expressing Annexin + PI + were experiencing late apoptosis, and Annexin – PI – were experiencing early apoptosis.The percentage of Huh7it cells found to be experiencing necrosis and cell apoptosis at respective dose levels are shown in Table 5. The sum of early and late apoptosis percentage was defined as the total apoptosis. The total apoptosis of the negative and positive (given doxorubicin 10 ppm) control groups was 0.23% and 65.68%, respectively. About 0.44% of Huh7it cells experienced total apoptosis at the highest F carica fruit extract dose, whereas 5.37% of Huh7it cells experienced total apoptosis at the highest F carica leaf dose.
Table 5.
The percentage of Huh7it cell apoptosis and necrosis in various treatments.
| Group | Early apoptosis (%) |
Late apoptosis (%) |
Total apoptosis (%) |
Necrosis (%) |
||||
|---|---|---|---|---|---|---|---|---|
| Leaves | Fruits | Leaves | Fruits | Leaves | Fruits | Leaves | Fruits | |
| Normal control | 0.06 | 0.11 | 0.08 | 0.12 | 0.14 | 0.23 | 1.87 | 3.02 |
| Positive control | 0.01 | 0.00 | 65.29 | 65.68 | 65.30 | 65.68 | 34.39 | 34.10 |
| 400 µg/mL extracts | 0.67 | 0.24 | 0.69 | 0.26 | 1.36 | 0.50 | 9.94 | 2.05 |
| 600 µg/mL extracts | 0.90 | 0.06 | 2.06 | 0.12 | 2.96 | 0.18 | 33.06 | 2.05 |
| 800 µg/mL extracts | 1.10 | 0.08 | 3.63 | 0.15 | 4.73 | 0.23 | 39.37 | 1.85 |
| 1000 µg/mL extracts | 1.69 | 0.14 | 3.68 | 0.30 | 5.37 | 0.44 | 47.66 | 2.26 |
Discussion
F carica is estimated to contain a total phenol of 17.8 to 815 mg/100 g in leaves, and 3241.2 to 3447.36 mg/100 g in fruits.16–19 Similarly, the results of this indicate that F carica leaf and fruit extracts contained several antioxidants, such as flavonoid and tannin. The presence of flavonoid, tannin, and other polyphenolic compounds can counteract free radicals by donating protons to free radicals and therefore terminate potentially damaging chain reactions. Saponin content in F carica leaf and fruit extracts can also perform antioxidant functions through the reduction of superoxide through the formation of hydroperoxide intermediates.20
In our study, the antioxidant activity of F carica leaf and fruit extracts was shown by the IC50 value, the ability to reduce free radicals by 50%. The smaller the IC50 value, the higher the free radical reduction activity. The IC50 value of F carica leaf extract was 7.9875 µg/mL while the IC50 value of F carica fruit extract was 13.402 µg/mL. The IC50 value of cancer cell growth was found to be >653 µg/mL leaf extract and >2000 µg/mL in fruit extract. The smaller IC50 value shows the higher inhibitory activity of extracts against cancer cells. Therefore, F carica leaf extract was more active in inhibiting Huh7it cancer cell growth compared with the fruit extract (Figure 2). Polyphenols have antioxidants properties and are known to play an important role in preventing cancer.21 One of the polyphenol derivatives contained in F carica leaves and fruits was found to be flavonoids. Multiple mechanisms involving flavonoids inhibiting cancer cells can occur, such as releasing hydrogen atoms and electrons, inducing the release of protective conjugate enzymes, increasing apoptosis, inhibiting lipid peroxidation, inhibiting angiogenesis, and inhibiting DNA oxidation.22
Figure 2.
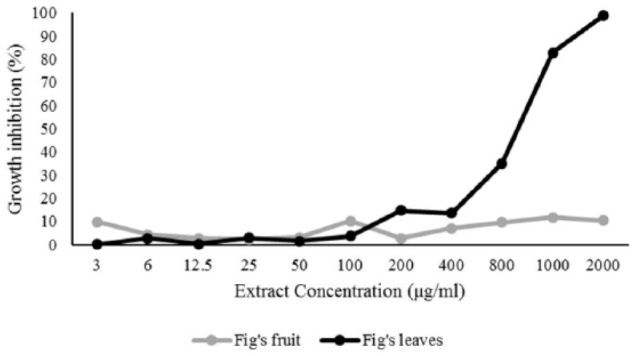
Growth inhibition of F carica leaf and fruit extracts against Huh7it liver cancer cells.
Flow cytometry analysis indicated that 0.5% Huh7it cells exposed to a low dose of F carica leaf extract experience total apoptosis, whereas 5.37% exposed to a high dose experience total apoptosis. The higher the dose resulted in more cancer cells undergoing apoptosis and a lower percentage of cell growth (Figure 3). Effects were therefore dose dependent.
Figure 3.
The effect of F carica extract on Huh7it cells apoptosis and necrosis: (A) F carica fruit extract; (B) F carica leaf extract. KN = normal control (Huh7it without treatment), P1 = Huh7it + 400 µg/mL extracts, P2 = Huh7it + 600 µg/mL extracts, P3 = Huh7it + 800 µg/mL extracts, P4 = Huh7it + 1000 µg/mL extracts.
The ability of F carica leaf and fruit extracts to cause cell apoptosis is through its ability to induce cell cycle arrest and increase the activity of apoptotic regulating genes including p53 and p21. It has been demonstrated that luteolin, a type of flavonoids, can induce apoptosis of Hep3B liver cancer cells and cause cell cycle arrest in G1 phase of HepG2 liver cancer cells.23 This was caused by the increase of p53 gene activity, which in turn activates p21 transcription factors. Activation of p21 triggers cyclin dependent kinase 2 (CDK2) to bind with cyclin E, causing the cell cycle to stop. The increase of p53 activation in the cytosol also triggers Bax activity and suppresses Bcl-2 activity. This in turn changes the mitochondrial membrane permeability and causes cytochrome c to exit the cytosol. Cytochrome c which reacts with APAF-1 can activate a cascade and caspase reaction and thus triggers DNA-se activation. The DNA-se enters the nucleus and causes fragmentation of the DNA, cleavages of PARP (poly ADP-ribose polymerase), and apoptosis.24
Quercetin has been shown to trigger many cellular events such as the activation of p53, cell cycle arrest in the G0/G1 phase of leukemia, cell cycle arrest in the S phase of colorectal carcinoma, and cell cycle arrest in the G2 / M phase of breast cancer, leukemia, and esophageal cancer cells.25–29 A recent study by Srivastava et al30 showed that administration of quercetin in breast cancer cell cultures causes a reaction in the DNA of cancer cells. The accumulation of this reaction can lead to apoptosis. This apoptotic process was also related to role of p53 and p21. Quercetin stimulates the expression of p21 and suppresses cyclin D1 expression which cause cell cycle cessation. Quercetin also increases the ratio of Bax/Bcl-2 and stabilizes p53 which lead to apoptosis in HepG2 liver cancer cells.31
This study is the first attempt on analyzing F carica effects toward liver cancer cells growth, apoptosis, and necrosis. However, further molecular analysis of the protein pathway and how F carica active ingredients affect it needs to be carried out.
Conclusion
Based on the results of antioxidant activity, anti-cell proliferation, and Huh7it cell apoptosis and necrosis, this study demonstrates that F carica leaf extract had a higher anticancer activity compared with its fruit extracts. This was due to their different antioxidant activity performed by different numbers of active compounds contained in each extract.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by Universitas Airlangga Mandat Research Grant, fiscal year 2018, No. 004/SP2 H/LT/ DRPM/IV/2017, April 8, 2018.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: RP, AAP, SH performed the analysis and prepared the manuscript. EA performed the FITR and DPPH analysis. DW and WD designed and supervised the project, and reviewed the manuscript. All authors read and approved the final version of the manuscript.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Hamilton JP, Gurakar A, Koteish A, Li ZP, Mezey E. Liver cancer. Paper presented at: 39th Annual Topics in Gastroenterology and Hepato-Biliary Update Conference; November 5–9, 2013; Baltimore, MD. [Google Scholar]
- 3. Fong ZV, Tanabe KK. The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence-based comparison and review. Cancer. 2014;120:2824–2838. [DOI] [PubMed] [Google Scholar]
- 4. Teo EK, Fock KM. Hepatocellular carcinoma: an Asian perspective. Dig Dis. 2001;19:263–268. [DOI] [PubMed] [Google Scholar]
- 5. Apriyanto DR, Hartati S, Dewi BE, Aoki-Utsubo C, Hotta H. Aktivitas sitotoksisitas ekstrak metanol daun sirsak (Annona muricata L.) terhadap karsinoma hepatoseluler strain Huh7it-1 cell line. J Kedokteran dan Kesehatan. 2018;4:1–4. [Google Scholar]
- 6. Bandgar BP, Gawande SS, Bodade RG, Totre JV, Khobragade CN. Synthesis and biological evaluation of simple methoxylated chalcones as anticancer, anti-inflammatory and antioxidant agents. Bioorg Med Chem. 2010;18:1364–1370. [DOI] [PubMed] [Google Scholar]
- 7. Ammirante M, Di Giacomo R, De Martino L, et al. 1-methoxy- canthin-6-one induces c-Jun NH2-terminal kinase-dependent apoptosis and synergizes with tumor necrosis factor-related apoptosis-inducing ligand activity in human neoplastic cells of hematopoietic or endodermal origin. Cancer Res. 2006;66: 4385–4393. [DOI] [PubMed] [Google Scholar]
- 8. Mansoor TA, Ramalho RM, Mulhovo S, Rodrigues CMP, Ferreir MJU. Induction of apoptosis in Huh-7 cancer cells by monoterpene and b-carboline indole alkaloids isolated from the leaves of Tabernaemontana elegans. Bioorg Med Chem Lett. 2009;19:4255–4258. [DOI] [PubMed] [Google Scholar]
- 9. Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23:519–534. [DOI] [PubMed] [Google Scholar]
- 10. Mawa S, Husain K, Jantan I. Ficus carica L. (Moraceae): phytochemistry, traditional uses and biological activities. Evid Based Complement Alternat Med. 2013;2013:974256. doi: 10.1155/2013/974256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joseph B, Raj JS. Pharmacognostic and phytochemical properties of Ficus carica Linn—an overview. Int J PharmTech Res. 2011;3:8–12. [Google Scholar]
- 12. Harborne JB. Metode fitokimia: penuntun cara modern menganalisis tumbuhan. Bandung, Indonesia: ITB Press; 1987:234–245. [Google Scholar]
- 13. Jami’ah SR, Ifaya M, Pusmarani J, Nurhikma E. Uji aktivitas antioksidan ekstrak metanol kulit pisang raja (Musa paradisiaca sapientum) dengan metode DPPH (2,2-difenil-1-pikrilhidrazil). J Mandala Pharm Indonesia. 2018;4:33–38. [Google Scholar]
- 14. Noor R, Astuti I, Mustofa Cytotoxicity of a-terpineol in Hela cell line and its effects to apoptosis and cell cycle. J Med Sci. 2014;46:1–9. [Google Scholar]
- 15. Phongpaichit S, Nikom J, Rungjindamai N, et al. Biological activities of extracts from endophytic fungi isolated from Garcinia plants. FEMS Immunol Med Microbiol. 2007;51:517–525. [DOI] [PubMed] [Google Scholar]
- 16. Bey MB, Louaileche H, Zemouri S. Optimization of phenolic compound recovery and antioxidant activity of light and dark dried fig (Ficus carica L.) varieties. Food Sci Biotechnol. 2013;22:1613–1619. [Google Scholar]
- 17. Chawla A, Kaur R, Sharma AK. Ficus carica Linn: a review on its pharmacognostic, phytochemical and pharmacological aspect. Int J Pharm Phytopharm Res. 2012;1:215–232. [Google Scholar]
- 18. Slatnar A, Klancar U, Stampar F, Veberic R. Effect drying of figs (Ficus carica L.) on the contents of sugars, organic acids and phenolic compounds. J Agr Food Chem. 2011;59:11696–11702. [DOI] [PubMed] [Google Scholar]
- 19. Vallejo F, Marin JG, Fransisco A, Tomás-Barberán FA. Phenolic compound content of fresh and dried figs (Ficus carica L.). Food Chem. 2012;130:485–492. [Google Scholar]
- 20. Zhang Y, Wan Y, Huo B, Li B, Jin Y, Hu X. Extracts and components of Ficus carica leaves suppress survival, cell cycle, and migration of triple-negative breast cancer MDA-MB-231 cells. Onco Targets Ther. 2018;11:4377–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laandrault N, Pouchert P, Ravel P, Gase F, Cros G, Teissedro PL. Antioxidant activities and phenolic level of French wines from different varieties and vintages. J Agric Food Chem. 2001;49: 3341–3343. [DOI] [PubMed] [Google Scholar]
- 22. Chahar MK, Sharma N, Dobhal MP. Flavonoids: a versatile source of anticancer drugs. Pharmacogn Rev. 2011;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yee SB, Hye JC, Sang WC, et al. Growth inhibition of luteolin on HepG2 cells is induced via p53 and Fas/Fas-ligand besides the TGF-β pathway. Int J Oncol. 2015;47:747–754. [DOI] [PubMed] [Google Scholar]
- 24. Wang IK, Lin-Shiau SY, Lin JK. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. Eur J Cancer. 1999;35:1517–1525. [PubMed] [Google Scholar]
- 25. Yuan Z, Long C, Junming T, Qihuan L, Youshun Z, Chan Z. Quercetin-induced apoptosis of HL-60 cells by reducing PI3k/Akt. Mol Biol Rep. 2012;39:7785–7793. [DOI] [PubMed] [Google Scholar]
- 26. Richter M, Ebermann R, Marian B. Quercetin-induced apoptosis in colorectal tumor cells: possible role of EGF receptor signaling. Nutr Cancer. 1999;34:88–99. [DOI] [PubMed] [Google Scholar]
- 27. Choi JA, Kim JY, Lee JY, et al. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int J Oncol. 2001;19:837–844. [DOI] [PubMed] [Google Scholar]
- 28. Lee TJ, Kim OH, Kim YH, et al. Quercetin arrests G2/M phase and induces caspase-dependent cell death in U937 cells. Cancer Lett. 2006;240:234–242. [DOI] [PubMed] [Google Scholar]
- 29. Zhang Q, Zhao XH, Wang ZJ. Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem Toxicol. 2008;46:2042–2053. [DOI] [PubMed] [Google Scholar]
- 30. Srivastava S, Somasagara RR, Hegde M, et al. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci Rep. 2016;6:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tanigawa S, Fujii M, Hou DX. Stabilization of p53 is involved in quercetin-induced cell cycle arrest and apoptosis in HepG2 cells. Biosci Biotechnol Biochem. 2008;72:797–804. [DOI] [PubMed] [Google Scholar]