Abstract
The study aimed at determining nutritional, antioxidant and blood glucose lowering potentials of improved plantain-based dough meals enriched with defatted soybean and tigernut flour. The constituted dough meals [PSB (plantain 64.46, defatted soybean 35.54%), TNS (tigernut 59.83, defatted soybean 40.17%); PTS (plantain 51.07, tigernut, 11.50, defatted soybean, 37.43%); TNT (100% tigernuts); PLT (100% plantain) and CNT (a commercial flour)] were evaluated for nutritional, antioxidant and blood glucose concentration in streptozotocin-induced diabetics rats. The improved dough meals contained appreciable amount of protein, energy value, and high in antioxidative activity than PLT. Blood glucose reducing potential of improved plantain-based dough meals (60.5–71.9%) in streptozotocin-induced diabetic rats was higher than PLT, but comparable to acarbose (anti-diabetic drug) (69%). The present study established that improved traditional plantain-based dough meals (particularly PTS) was high in essential nutrients, antioxidative activities, and blood glucose reducing potentials. Hence, the dough-meals may be suitable for diabetes management.
Keywords: Food science, Nutrition
1. Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by increased blood glucose levels (Ozougwu et al., 2013), due to either lack of insulin production or inefficient in the activity of insulin (Maitra and Abbas, 2005). Diabetes Mellitus is one of five leading causes of deaths and debilitating disease in the world (WHO, 2010; International diabetes foundation, 2012). The number of people with type 2 diabetes mellitus is increasing in every country with 80% of people with diabetes mellitus living in low- and middle-income countries (Mufunda et al., 2006; Levitt, 2008). In Nigeria, epidemiological studies have reported that diabetes mellitus is one of the commonest causes of admission and death in tertiary health institutions (Osuafor and Ele, 2004; Odenigbo and Oguejiofor, 2009). Several strategies such as regular use of different antidiabetic medications, dietary modifications and change in lifestyle have been adopted to manage diabetes (Bantle et al., 2008; Hawley and Gibala, 2012). These strategies, particularly using synthetic antidiabetic agents are very costly, and also have some side effects (Prasad et al., 2014). In view of this, efforts have focused on the development of non-toxic food-based antidiabetic agents, that is, functional foods (Inzucchi et al., 2012; Agius, 2014).
One of the current issue in nutrition and health is to consume food products, that is, functional foods that have the potential to provide adequate nutrient requirements, and also to promote good health status on regular ingestion (Juárez-García et al., 2006; Academy of Nutrition and Dietetics, 2017). Functional food is an emerging field in food science due to its increasing popularity among health conscious consumers. The increasing interest in therapeutic foods reflects the fact that a specific diet or component of a diet is associated with a lower risk of certain diseases. For instance, Odom et al. (2013) and Famakin et al. (2016) formulated plantain-based foods fortified with legumes for the management of diabetes. Plant-based functional foods are now getting more attention than ever before, because they have the potential of myriad benefit to the society or indeed to the entire mankind especially in the line of nutrition, medicine and pharmacology. The medicinal values of these plant-based foods lies in bioactive compounds like phytochemicals and proteins that produce definite physiological action on the human body (Edeoga and Gomina, 2000; Igwe et al., 2012).
Plantain is widely grown in the southern parts of Nigeria and other African countries. In many parts of African countries, plantain is consumed as a cheap source of energy, and also, medically recommended for diabetic patient, due to its low glycaemic index properties (Akubor and Ishiwu, 2013; Eleazu and Okafor, 2015). Plantain, particularly unripe, is a good source of energy, dietary fiber, irons, potassium, and vitamins (Randy et al., 2007; Tribess et al., 2009). Apart from being a good source of calories and other nutrients, plantain is considered nutritionally poor, because it is deficient in fat and protein (Odenigbo et al., 2013). Therefore, supplementation of plantain flour with inexpensive staples, such as legumes, cereals and pulses, helps to improve the nutritional quality of plantain products (Famakin et al., 2016). Plantain flour has been successfully added to cereals to produce bread (Juárez-García et al., 2006), spaghetti (Mastromatteo et al., 2014) and other food products, demonstrating that its addition results in higher protein and resistant starch content and a lower starch digestion rate.
Tigernut (Cyperus esculentus Lativum) is a perennial grass-like plant, and underutilised tubers that produces sweet nut-like taste (Coşkuner et al., 2002), widely grown in tropical and Mediterranean regions (Adejuyitan, 2011). The tuber serves as a potentially valuable food for both human and animal in Southern Europe and many parts of developing countries (Pascual et al., 2000). The tubers contain appreciable amount of essential phytonutrients and phytochemicals, which are effective in the treatment and prevention of many diseases like coronary heart diseases, obesity, diabetes, and gastrointestinal diseases (Sabiu et al., 2017).
Soybean (Glycine max (L)Merrill) belongs to the family of leguminoisae and sub-family papilionnideae. Soy protein is a major component of the food for animals and is increasingly important in the human diet (Usman et al., 2016). Soy protein is deficient in methionine, but high in lysine. Hence, there is a needs to complement soybean based food products with cereals, which is high in lysine, but low in methionine (Coulibaly et al., 2012). In Nigeria, Soybean is used in various food products in different homes such as soymilk, soyogi, soy-daddawa, soy-akara, soy-gari, soy-soup, and hot soy-drink (Coulibaly et al., 2012; Samson, 2014; Usman et al., 2016).
This study aimed at formulating and to evaluate nutritional composition, glycaemic index and antidiabetics potentials of dough meals from locally available food materials.
2. Materials and methods
2.1. Source of food materials
Tigernuts (Cyperus esculentus Lativum) yellow variety and matured, unripe fruits of plantain were purchased from Erekesan market, Akure and Owena market, Ondo, Nigeria, respectively.
2.2. Processing of food materials into flour
2.2.1. Tigernut tuber flour processing
Tigernut tuber was processed into flour using the method of Oladele and Aina (2017) with slight modification. Yellow tigernut tubers were sorted to remove unwanted materials like stones, pebbles and other foreign seeds, washed with double distilled water and drained. The tubers were oven dried at 60 °C for 20 h using a hot-air oven (Plus11 Sanyo Gallenkamp PLC, Loughborough, Leicestershire, UK), milled with a laboratory blender (Model KM 901D; Kenwood Electronic, Hertfordshire, UK) and passed through a 60 mm mesh sieve (British Standard) to obtain tigernut tuber flour. The flour was packed in a plastic container, sealed and stored at room temperature (∼27 °C) until analysis.
2.2.2. Plantain flour processing
The plantain flour was processed using method described by Mepba et al. (2007) with slight modification. The plantain heads were cut into separate bunches which were subsequently de-fingered. The fingers were washed to remove adhering soil particles, peeled, cut into thin slices of about 2-cm thick, blanched in 1.25% NaHS03 solution at 80 °C for 5 min and drained. The blanched plantain slices were oven dried at 60 °C for 20 h using a hot-air oven (Plus11 Sanyo Gallenkamp PLC, Loughborough, Leicestershire, UK), milled with a laboratory blender (Model KM 901D; Kenwood Electronic, Hertfordshire, UK) and passed through a 60 mm mesh sieve (British Standard) to obtain plantain flour. The flour was packed in a plastic container, sealed and stored at room temperature (∼27 °C) until analysis.
2.2.3. Processing of defatted soybean flour
The defatted soybean flour was processed using method described by Ijarotimi and Owoeye (2017). The defatted soybean was oven dried at 60 °C for 20 h using a hot-air oven (Plus11 Sanyo Gallenkamp PLC, Loughborough, Leicestershire, UK), milled with a laboratory blender (Model KM 901D; Kenwood Electronic, Hertfordshire, UK) and passed through a 60 mm mesh sieve (British Standard) to obtain defatted soybean flour. The flour was packed in a plastic container, sealed and stored at room temperature (∼27 °C) until analysis.
2.3. Formulations of food samples
The plantain, tigernut tubers and defatted soybean flour were blended with reference to 50% recommended daily intakes (RDI) of protein (56 g/day) and fat (20 g/day) for diabetes patient's adult using material balance equations. This was done in order to compensate for carbohydrate reduction, and also to maintain the energy value of the product for the target population. The following food combinations were thereafter obtained, that is, PSB (plantain flour, 64.5% & defatted soybean flour, 35.5%), TNS (tiger nut flour, 59.8% & defatted soybean flour 40.2%), PTS (plantain flour 51.1%, tigernut flour 11.5% & defatted soybean flour 37.4%), PLT (100% plantain flour), TNT (100% tigernut flour) and a commercial dough meal flour (control) (CNT).
2.4. Chemical analyses
2.4.1. Determination of proximate composition flour blends
Proximate compositions, that is, moisture content, ash, crude fiber, crude fat and crude protein content of experimental food samples were determined using the standard methods (AOAC, 2012). Carbohydrate content was determined by difference as follow:
The gross energy values of the samples were determined (MJ/kg) by using Gallenkamp Adiabatic bomb calorimeter (Model CBB-330-01041; UK).
2.4.2. Determination of antioxidative potential of flour blends
The scavenging effect of improved plantain-based dough meal samples on 2, 2- Diphenyl-1-picryhydrazyl (DPPH) was measured according to the method of Hwang et al. (2006), metal chelating activity was determined using a modified method of Decker and Welch (1990), nitric assay was carried out as described by scientific methods (Kumar et al., 2008; Gupta et al., 2011), and ferric reducing power was determined according to the method of Mau et al. (2002).
2.4.3. Glycaemic index and nutritional evaluation of dough meals from plantain, tigernut and defatted soybean composite flour
2.4.3.1. Experimental animals
Forty white albino rats (Rattus novergicus) of both sex weighing between 120 and 200 g was obtained from the Animal House Unit of the Department of Biochemistry, Federal University of Technology, Akure. The rats were divided into eight groups (5 rats per group) and were kept in clean plastic cages and maintained under standard laboratory conditions (temperature, 22 ± 3 °C; photo period, 12 h natural light and 12 h dark; humidity, 40–45%) (Lawal et al., 2015). The animals were maintained on standard animal feeds and water ad libitum.
2.4.4. Statement of animal rights
The study protocol was approved by the Ethical Committee for Laboratory Animals of School of Agriculture and Agricultural Technology, Akure, Nigeria (FUTA/SAAT/2017/033). The experiments on animals were conducted in accordance with the force laws and regulations as regards animal use and care as contained in the Canadian Council on Animal Care Guidelines and Protocol Review (CCAC, 1993).
2.4.5. Determination of glycaemic index and glycaemic load of flour blends
2.4.5.1. Experimental animals
Twenty-five Wistar Albino rats of body weights between 140-150g were divided into 5 groups (5 rats/group), and the rats were housed individually in metabolic cages in a climate-controlled environment with free access to feed and water. The rats were allowed to acclimatize to the new environment for 7 days. After the adaptation period, the animals were reweighed and fasted for 12 h (overnight fasting). The blood glucose of the animals were taken at zero time from the tail vein before feeding them with the test products and glucose (a control) in a portion size that was calculated to contain 2.0 g of available carbohydrate, which were consumed within 25 min. After the consumption, the serum glucose levels of the animals were measured using an automatic glucose analyzer (‘Accu-chek Active’ Diabetes monitoring kit; Roche Diagnostic, Indianapolis, USA) at 0, 30, 60, 90 and 120 min intervals. The glycaemic response was determined as the Incremental Area under the Blood Glucose Curve (IAUC) measured geometrically from the blood glucose concentration-time graph ignoring area beneath the fasting level (Wolever et al., 1991).
2.4.5.2. Measurement of blood glucose response
Blood glucose curves were constructed from blood glucose values of animals at time 0, after 15, 30, 45, 60, 90 and 120 min intervals after consumption of the glucose (control) and experimental food samples of each group. The Incremental Area Under the Curve (IAUC) was calculated for reference food (glucose) by the trapezoidal rule in every rats in each group separately as the sum of the surface of trapezoids between the blood glucose curve and horizontal baseline going parallel to x-axis from the beginning of blood glucose curve at time 0 to the point at time 120 min to reflect the total rise in blood glucose concentration after eating the reference food (glucose). The Incremental Area Under the Curve (IAUC) from the animals fed with the formulated food samples were similarly obtained. The glycaemic Index (GI) for each diet was calculated by ratio of Incremental Area Under two hours of blood glucose response or Curve (IAUC) for each diet to the IAUC for glucose solution standard according to the method of Wolever et al. (1991) using the equation below, and classified as follows: Low-GI = < 55%, Medium-GI = 56–69%, and High-GI = >70% (Dona et al., 2010).
2.4.5.3. Calculation of glycaemic load (GL)
Glycaemic Load (GL) for each food sample was determined by the method of Salmerón et al. (1997). In each individual glycaemic load was calculated by taking the percentage of the food's carbohydrate content in a typical serving food and multiplying it by its glycaemic index (GI) value. The following formula was used:
Net Carbs = Total Carbohydrates in the food sample served. The GL was classified as follows: Low-GL = < 10, Medium-GL = 11–19 and High-GL = >20 (Dona et al., 2010).
2.4.6. Growth performance and protein digestibility in rats of the dough meals
Rats in each group were fed on experimental food samples and water ad libitum for 28 days. All diets were served to the rats in ground form after proper mixing. Records were kept on the weight and length changes, and total food intake. The total faeces and urine voided for the last 7 days of the trial were collected continuously for 24 h. The faeces were oven dried at 60 °C, while urine was preserved in 10 mL of 10% sulfuric acid to eliminate microbial activities and prevent nitrogen losses by evaporation of ammonia, and the samples were stored in a deep freezer (-20 °C) prior to nitrogen determination. The feed efficiency, protein efficiency ratio, biological value and feed conversion ratio were computed (Agbede and Aletor, 2003).
2.4.7. Determination of haematological and biochemical properties of albino rats fed on dough meals from plantain, tigernut and defatted soybean composite flour
2.4.7.1. Collection of blood sample
At the end of experimental period, that is, 28 days, the Albino rats were fasted overnight with access to water ad libitum and sacrificed underchloroform anaesthesia. The blood samples were collected via cardiac puncture with syringe and poured into heparinised and non-heparinised tubes. The non-heparinised tubes were allowed to clot and were centrifuged at 3000 xg for 25 min to obtain the sera, and the blood samples were stored in a deep freezer (-20 °C) prior to haematological and biochemical analyses at the Medical Laboratory Unit of University Health Centre, Federal University of Technology, Akure (Agbede and Aletor, 2003; Shittu et al., 2013). The rats' organs, that is, whole liver, two kidney and heart were excised, freed of fat, blotted with clean tissue paper and then weighed.
2.4.7.2. Haematological and biochemical determination
The Automated Haematologic Analyzer (Sysmex,KX-21, Systmex Corporation, Kobe, Japan) was used to analyze the haematological parameters, that is, red blood cells (RBC), pack cell volume (PCV), haemoglobin concentration (Hbc), white blood cells (WBC), neutrophil (NEU) and lymphocytes (LYM) using methods described by Dacie and Lewis (2002). Mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentrated (MCHC) and mean corpuscular volume (MCV) were calculated from values obtained from RBC, PCV and Haemoglobin (Hbc) content (Dacie and Lewis, 2002).
The biochemical parameters were analysed using methods described by Jasper et al. (2012). The blood sample was first centrifuged at 1,500 x g for 10 min at ambient temperature. The serum was then separated and used for liver function assessment employing measurements of the enzymes aspartate aminotransferase (AST), alanine aminotransferase (ALT) and Alkaline Phosphate (ALP). Renal function was evaluated using serum concentrations of urea and creatinine. These tests were performed using disposable kits obtained from LabtestDiagnostica S.A. (Lagoa Santa, Minas Gerais, Brazil).
2.4.7.3. Evaluation of antidiabetic potential of formulated dough meals from plantain, tigernut and defatted soybean composite flour in streptozocin-induced diabetic rats
The anti-diabetic potential of dough meals from plantain, tigernut and defatted soybean composite flour was determined. The baseline blood glucose levels of animals were measured before being induced with streptozocin. Forty Wistar albino rats were induced by single intraperitoneal injection of freshly prepared solution of streptozocin (150 mg·kg-1 body weight) dissolved in physiological saline in overnight fasted rats (Abu et al., 2010). The rats were allowed to drink 5% glucose solution to avoid hypoglycaemic effects of the drug. Blood glucose levels in animals were measured 72 h after streptozocin administration through tail tipping using glucometer (Accu-Chek, Active, Roche Diagnostic's, Indianapolis, IN, Lot No 115764) The diabetic induced rats with serum glucose ≥250 mg·dL-1 level were used (Ramdas and Balakrishnan, 2012; Parks et al., 2013), and randomly divided into 8 groups containing 5 rats per group. Six of the groups were fed on experimental dough meals and control sample (a commercial dough meal flour). The remaining two groups were fed on basal diet with and without Acabose (a synthetic antidiabetic agent). The animals were fed with the diets for 28 days and blood glucose levels measured in the morning at regular intervals by drawing blood from each rat through tail tipping and blood glucose level measured using Accu check® Glucometer kit (Meiton, 2006).
2.5. Statistical analysis
Data were subjected to analysis of variance using SPSS (IBM version. 20.0, SPSS Inc., Quarry Bay, Hong Kong) and presented as means (±SEM). Comparisons between different groups was done using Analysis of Variance (ANOVA) and Duncan's Multiple Range Test (DMRT). Values of p < 0.05 were considered as statistically significant as described by Yalta and Talha (2008).
3. Results and discussion
3.1. Nutrient composition and energy values of dough meals from plantain, tigernut and defatted soybean composite flour and control samples
The proximate composition of improved plantain-based dough meals enriched with tigernut and defatted soybean and control sample is presented in Table 1. The range values of crude protein and energy of the food samples were 6.20 ± 0.03 g/100g in PLT to 25.82 ± 0.01 g/100g in PTS and 357.6 ± 3.11 kcal/100g in PTS to 422.1 ± 2.02 kcal/100g in TNT, respectively. Whereas, the crude fibre content ranged between 1.52 ± 0.01 g/100g in PLT and 9.17 ± 0.10 g/100g in TNT. Comparatively, the protein content of enriched plantain-based dough meals were higher than traditional plantain-based dough (PLT, a 100% plantain flour), commercial dough meal flour (CNT) (13.50 ± 0.07 g/100g), and value obtained for plantain-tigernut composite flour reported by Adegunwa et al. (2017). This observation agreed with the reports that plantain is a good source of calories, however, it is deficient in protein (Odenigbo et al., 2013), hence, there is a need to complement plantain flour with inexpensive staples, such as legumes, to further improve the nutritional quality of the plantain-based products (Famakin et al., 2016). The fibre content of food samples, particularly TNT and TNS, was significantly higher than PLT and CNT, this observation further establishedthe nutritional qualities of the present study food samples. Recently, epidemiological studies reported on health benefits that associated with dietary fibre intakes such as inhibiting the absorption of glucose and lipids in small intestine, slows gastric emptying, maintaining levels of satiety and contributing towards less weight gain (James et al., 2003; Lunn and Buttriss, 2007). Soluble fibre and resistant starch molecules are additionally fermented by bacteria in the large intestine, producing short chain fatty acids, which help to reduce circulating cholesterol levels (Slavin et al., 1999). For instance, consumption of different types of dietary fibre (DF) hasbeen demonstrated to increase satiety, lower energy density, delay of gastric emptying, and/or modulation of gastrointestinal hormones (Smith and Tucker, 2011). Hence, dietary fibre is used to manage obesity and diabetes (Roberfroid et al., 2010; Sánchez et al., 2012).
Table 1.
Proximate composition (g/100 g) and energy values (kcal/100g) of dough meals from plantain, tigernut and defatted soybean and control samples.
| Samples | PLT | TNT | TNS | PSB | PTS | CNT |
|---|---|---|---|---|---|---|
| Moisture | 16.95a ± 0.06 | 9.01c ± 0.19 | 7.53e ± 0.11 | 8.75d ± 0.09 | 9.07c ± 0.21 | 10.46b ± 0.20 |
| Ash | 1.59e ± 0.03 | 2.31c ± 0.01 | 3.73a ± 0.07 | 3.35b ± 0.01 | 2.34c ± 0.06 | 1.79d ± 0.01 |
| Fat | 9.37c ± 0.11 | 20.81a ± 0.42 | 17.32b ± 0.40 | 2.50f ± 0.23 | 2.67e ± 0.06 | 4.07d ± 0.03 |
| Fibre | 1.52c ± 0.01 | 9.17a ± 0.07 | 7.984b ± 0.03 | 1.53c ± 0.04 | 2.52c ± 0.01 | 0.76d ± 0.01 |
| Protein | 6.20f ± 0.03 | 7.29e ± 0.10 | 23.93b ± 0.10 | 15.55c ± 0.22 | 25.82a ± 0.02 | 13.50d ± 0.07 |
| Carbohydrate | 64.37c ± 0.04 | 51.41e ± 0.33 | 39.51f ± 0.22 | 68.32b ± 0.19 | 57.58d ± 0.26 | 69.39a ± 0.30 |
| Energy | 366.61c ± 0.81 | 422.1a ± 2.02 | 409.6b ± 2.89 | 357.9d ± 3.11 | 357.6d ± 4.34 | 368.2c ± 2.03 |
Means (±SEM) with different alphabetical superscripts in the same row are significantly different at P < 0.05.
Key: PLT: 100% Plantain; TNT: 100% Tigernut; CNT: 100% Commercial dough meal; TNS: Tigernut: Defatted soybean (59.83:40.17%); PSB: Plantain: Defatted soybean (64.46:35.54%); PTS: Plantain: Tigernut: Defatted soybean (51.07:11.50:37.43%).
3.2. Antioxidant properties of dough meal from plantain, tigernut and defatted soybean composite flour and control samples
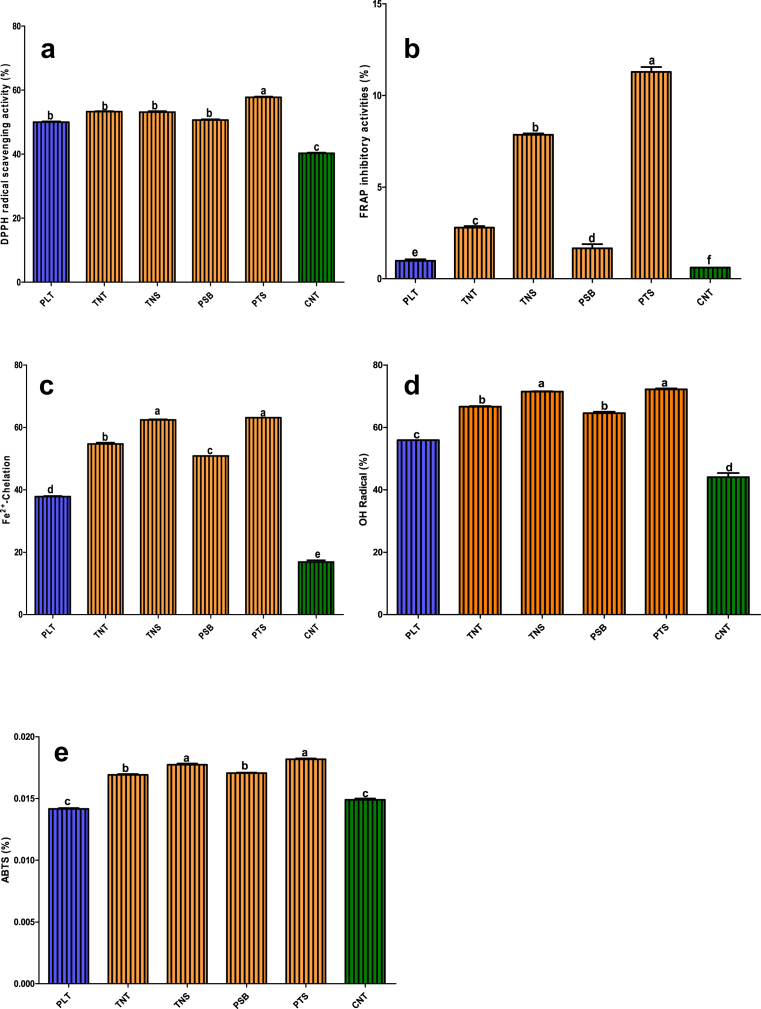
The results of antioxidant properties of dough meals made from plantain, tigernut and defatted soybean composite flour and control samples are presented Fig 1a–e. The ability of improved plantain-based composite flour and control flour samples to scavenge free radical against 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay ranged from 50.64% in PSB to 57.76% in PTS, and the values were significantly (p < 0.05) higher than PLT (50.00%) and CNT (40%). The ability of improved plantain-based composite flour samples to scavenge free radical against 2, 2-Azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) ranged between 0.0172 and 0.0182 mg TEAC/g for PBS and PTS, respectively; and free radical scavenging potentials was higher in plantain-based composite flour samples than CNT (0.0149 mg TEAC/g) and PLT (42 mg TEAC/g). The ferric reducing antioxidant power (FRAP) of the improved composite flour samples ranged from 1.66 mg AAE/g in PSB to 11.29 mg AAE/g in PTS, while that of CNT and PLT were 0.98 mg AAE/g and 0.60 mg AAE/g, respectively. The Fe2+chelation antioxidant power of improved flour samples ranged between 50.86 mg/mL in PSB and 63.43 mg/mL in PTS, while PLT sample was 37.86 mg/mL and CNT was 16.86 mg/mL. The ability of composite flour samples to prevent free hydroxyl radicals ranged from 64.57% in PSB and 72.22% in PTS, and the values were higher in composite flour than PLT (44.08%) and CNT (55.92%).
Fig. 1.
(a–e). Antioxidative activities of composite flour from plantain, tigernut tuber and defatted soybean flour [PLT: 100% Plantain; TNT: 100% Tigernut; CNT: 100% Commercial dough meal; NS: Tigernut: Defatted Soybean (59.83:40.17%); PSB: Plantain: Defatted soybean (64.46:35.54%); PTS: Plantain: Tigernut: Defatted soybean (51.07:11.50:37.43%)].
The antioxidant activities and free radical scavenging abilities of the composite flour samples were comparatively higher than control sample (CNT). This finding could be attributed to variations in food composition and bioactive components like phytochemicals, fibres and bioactive proteins, which were significantly presents in these experimental food samples than control sample. In the last decades, several studies have demonstrated the significant of antioxidant in diseases prevention and managements (Valabhji et al., 2001; Polidori et al., 2001). For instance, studies have reported on the contributions of antioxidants in diabetes and its complications (Pickup, 2004; Chertow, 2004).
3.3. Glycaemic index (GI) and glycaemic load (GL) of formulated dough meal and control samples
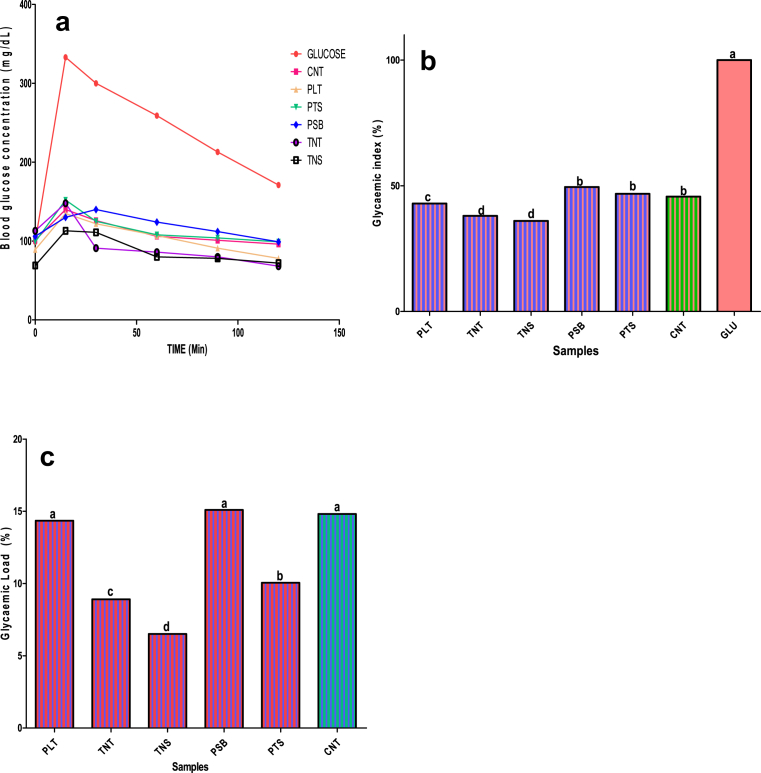
The in-vivo blood glucose concentrations, glycaemic index and glycaemic load of Albino Wistar rat fed on improved plantain-based dough meals, control sample and glucose are presented in Fig. 2 (a–c). The result showed that blood glucose concentration of experimental rat fed on improved plantain-dough meals and control sample ranged as follows: 69–113 mg/dL in TNS, 106–130 mg/dL in PSB, 99–152 mg/dL in PTS, 89–135 mg/dL in PLT and 101–140 mg/dL in CNT, while those rats fed on glucose ranged between (99–333 mg/dL) after food samples were administered (Fig. 2a).
Fig. 2.
(a–c). Blood Glucose concentration, Glycaemic index (%) and Glycaemic load of Albino rats fed on formulated dough meals and control samples [PLT: 100% Plantain; TNT: 100% Tigernut; CNT: 100% Commercial dough meal; NS: Tigernut: Defatted Soybean (59.83:40.17%); PSB: Plantain: Defatted soybean (64.46:35.54%); PTS: Plantain: Tigernut: Defatted soybean (51.07:11.50:37.43%)].
Dietary glycaemic index (GI) is an indicator of carbohydrate quality that reflects the effect on blood glucose, foods and usually classified into three, that is, high (>70%), medium (56–69%) and low GI (<55) depending on the rate at which blood sugar level rises, whereas glycaemic load is an indicator of both carbohydrate quality and quantity in food (Wolever et al., 1991; Salmerón et al., 1997). The glycaemic load of experimental food samples ranged from 6.51% in TNS to 15.10 in PSB, while that of CNT was 14.82%. For glycaemic index, the values ranged from 36. 05% in TNS to 42.95% in PLT, while CNT (a commercial dough meal flour) was 45.67%. This finding showed that the GI and GL of improved plantain-based dough meals enriched with tigernut and defatted soybean were lower than 55% and 20%, hence, these dough meals could be classified as low glycaemic index and load foods, hence, the food samples may be suitable for diabetic patients. Findings have reported on the benefits inherent in low GI and GL food intakes. For instance, it is evident that low-GI foods usually reduce the risk of diabetes (Salmerón et al., 1997), increase insulin sensitivity (Rizkalla et al., 2004), reduce food intake and body weight, and may reduce serum cholesterol (Warren et al., 2003; McMillan-Price et al., 2006). Whereas, the higher the glycaemic load (GL) value, the greater the elevation in blood glucose and in the insulinogenic effect of the food. It is evident that long-term consumption of a diet with a relatively high GI and GL is associated with an increased risk of type 2 diabetes and coronary heart disease (Foster-Powell et al., 2002). Hence, the low GI and GL values that were observed in the present study food samples may not elevate the blood glucose of their consumers. This observation agreed with other scientific studies which established that consumption of unripe plantain did not elevate the blood glucose of its consumers (Menezes et al., 2010; Dan, 2011).
3.4. Nutritional evaluation of dough meals prepared from plantain, tigernut and defatted soybean composite flour in Wistar Albino Rat
3.4.1. Growth patterns of Albino Wistar rat fed on dough meal and control sample
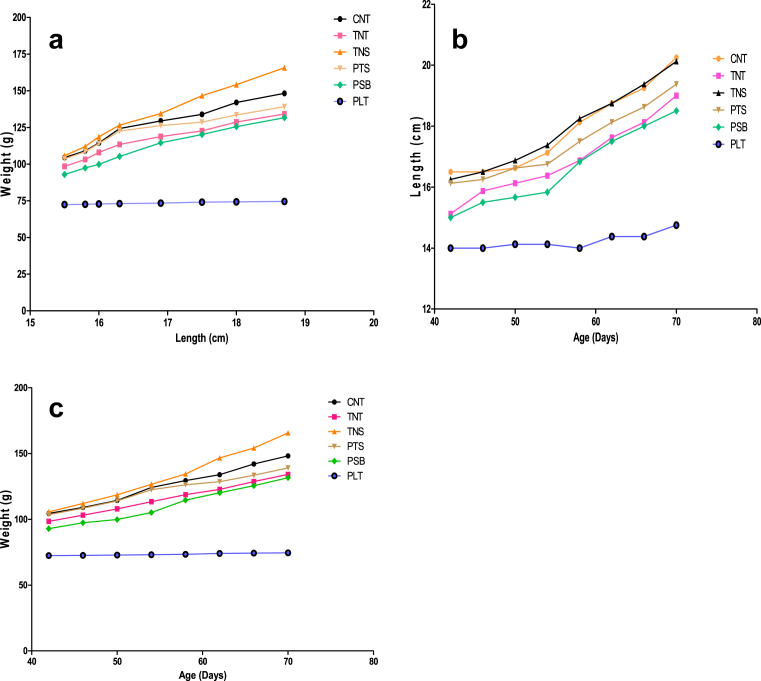
The growth pattern of Wistar Albino rat fed on the improved plantain-based dough meals and control sample are presented in Fig. 3 a–c. The nutritional status of the experimental rats showed that the rats fed on improved plantain-based dough meals had better growth performance when compared with those rats fed on 100% plantain, but were comparable to those rats fed on CNT. However, the rats fed on TNS had the highest growth performance when compared to other formulated dough meals in terms of weight-for-age (underweight, a measure of combination of chronic and acute malnutrition), length-for-age (LFA) (stunting, a measure of past nutritional status) and weight-for-length (WFL) (Wasting, a measure of short-term fluctuation in nutritional status) indices. Nutritionally, from this present study, it could be deduced that these improved plantain-based food samples, particular TNS, may be suitable as a functional food to prevent malnutrition among the young children and adults. Studies have reported on the increase of protein-energy malnutrition (PEM) in developing countries due to low nutritional qualities of traditional foods (Ikujenlola and Adurotoye, 2014; Adepoju and Etukumoh, 2014). Hence, a low cost food that is high in protein and energy-density such as TNS may be a desirable substitute for expensive imported foods and low qualities local foods (Ijarotimi and Olopade, 2009; Ijarotimi and Keshinro, 2012).
Fig. 3.
a–c. Weight-For-Length (wasting), Length-For-Age (stunting) and Weight-For-Age (underweight) of Albino rats fed on formulated dough meals and control samples. Key: [PLT: 100% Plantain; TNT: 100% Tigernut; CNT: 100% Commercial dough meal; NS: Tigernut: Defatted Soybean (59.83:40.17%); PSB: Plantain: Defatted soybean (64.46:35.54%); PTS: Plantain: Tigernut: Defatted soybean (51.07:11.50:37.43%)].
3.4.2. Protein quality and relative organ weight of experimental rat fed on formulated dough and control samples
The protein quality and relative organ weight of experimental rats fed on formulated and control dough meals are presented in Table 2. The range values of biological values (BV), net protein utilization (NPU) and protein efficiency ratio (PER) of improved dough meal samples were 81.07–93.03%, 42.38–70.80% and 0.22–3.36, respectively. Comparatively, the biological value (BV), Net protein utilization (NPU) and Protein efficiency ratio (PER) of the improved plantain dough meal samples were higher than 100% plantain (PLT) (BV = 41.46%; NPU = 12.43% and PER = 2.39). However, these values agreed with the values obtained for the control sample (CNT) (BV = 86.25%; NPU = 50.12% and PER = 3.06) and far above FAO/WHO (1989) recommendations (BV, 70%; PER, 2.7). This observation indicates that the protein content of the formulated dough meals may be able to support children and adults physiological needs. The relative weight organs of the experimental rats, that is, kidney, liver and heart, fed on plantain-based dough meals ranged as follows: 0.70%–1.08%, 2.56–4.93% and 0.38%–0.68%, respectively, and these values were comparatively higher than PLT (kidney = 0.67%; liver = 3.38% and heart = 0.39%) and CNT (a commercial market sample) (kidney = 0.70%; liver = 2.96 and heart = 0.35%). The bio-efficacy of these experimental dough meals in rats further indicate the nutritional quality of these food samples, and also their suitability as functional food to support growth and body maintenance. This finding agreed with other works that reported on the nutritional qualities of foods that were formulated from the combinations of two or more plant-based food materials (Okpala and Okoli, 2011; Ijarotimi and Keshinro, 2012).
Table 2.
Nutritional quality and relative organ weight of rat feed with formulated dough meals and control samples.
| Parameters | PLT | TNT | TNS | PSB | PTS | CNT |
|---|---|---|---|---|---|---|
| Weight gained (g) | 2.13 | 35.75 | 60.03 | 38.73 | 35.58 | 43.75 |
| Food intake (g) | 298.50 | 400.25 | 465.45 | 383.15 | 484.00 | 465.45 |
| Feed Efficiency Ratio | 0.13 | 0.09 | 0.13 | 0.01 | 0.07 | 0.09 |
| Nitrogen Retention (%) | 0.96 | 2.89 | 13.46 | 2.88 | 16.30 | 5.33 |
| Biological Value (%) | 41.46 | 81.07 | 89.14 | 83.96 | 93.03 | 86.25 |
| Net Protein Utilization (%) | 12.43 | 42.38 | 68.76 | 49.68 | 70.80 | 50.12 |
| Protein Efficiency Ratio | 2.39 | 3.36 | 2.28 | 0.22 | 1.20 | 3.06 |
| Relative organs weight (%) | ||||||
| Kidney | 0.67 | 0.77 | 0.70 | 1.08 | 0.71 | 0.70 |
| Liver | 3.38 | 2.65 | 2.56 | 4.93 | 3.44 | 2.96 |
| Heart | 0.39 | 0.42 | 0.38 | 0.68 | 0.39 | 0.35 |
Key: PLT: 100% Plantain; TNT: 100% Tigernut; CNT: 100% Commercial dough meal; TNS: Tigernut: Defatted soybean (59.83:40.17%); PSB: Plantain: Defatted soybean (64.46:35.54%); PTS: Plantain: Tigernut: Defatted soybean (51.07:11.50:37.43%).
3.4.3. Haematological and biochemical properties of Albino Wistar rat fed on formulated dough meal and control samples
Haematological properties of Albino Wistar rat fed on plantain-based dough meals and control sample are presented in Table 3. The range values of packed cell volume (PCV), haemoglobin concentration (Hbc) and white blood cells (Wbc) were 33.50–42.00%, 11.20–14.10 g/dL and 2.86–6.10 × 103mm3, respectively. While that of red blood cells concentrations (Rbc) and Lymphocytes were 3.67–4.65 × 103mm3 and 46.0–64.0%, respectively. The high concentration of PCV, Hbc, RBC and lymphocytes of rats fed on experimental dough meals further established nutritional quality of these products. This finding agreed with the report of Roberts et al. (2000) who established that diets containing quality protein and iron usually enhance production of haemoglobin and immunity in animals). In contrary, low PCV and Hbc that were observed in rats fed on 100% plantain dough meal could be attributed to low protein quality of the food sample, which may lead to poor production of haemoglobin, hence, anaemia (Osundahunsi and Aworh, 2002; Ijarotimi and Keshinro, 2012). The range values of mean cell haemoglobin concentration (MCHC), mean cell haemoglobin (MCH) and mean cell volume (MCV) of rats fed on the experimental foods were 33.26 ± 0.06–33.50 ± 0.01 g/dL, 29.23 ± 0.23–30.70 ± 0.11 pg and 89.40 ± 0.01–91.40 ± 0.17 fL, respectively, while that of CNT were 33.25 ± 0.08 g/dL, 31.55 ± 0.89 pg and 90.55 ± 0.49 fL, respectively. For the neutrophils, the values ranged between 36.00 ± 0.57 and 50.33 ± 0.33% for the experimental foods, while that of CNT was 54.66 ± 1.48%. The MCHC, MCH and MCV are a useful indices of the average Hb concentration of the red blood cells, and low concentration of these haematological parameters in animals is indicative of haemolytic anaemia, while their increased also indicate massive intravascular haemolysis (Sridhar Prasad et al., 2018).
Table 3.
Haematological and Biochemical properties of Albino rats fed on formulated dough meals made from plantain, tigernut and defatted soybean flour using rats.
| Parameters | PLT | TNT | TNS | PSB | PTS | CNT | *NR |
|---|---|---|---|---|---|---|---|
| PVC% | 34.50 ± 0.86bc | 39.50 ± 1.44ab | 33.50 ± 0.86c | 34.33 ± 0.33bc | 42.00 ± 1.73a | 38.00 ± 3.46abc | 37.6–50.6 |
| Hb (g/dl) | 11.50 ± 0.28b | 13.20 ± 0.51ab | 11.20 ± 0.28b | 11.30 ± 0.11b | 14.10 ± 0.57a | 12.65 ± 1.18ab | 11.5–16.1 |
| WBC (x103mm3) | 4.65 ± 0.37ab | 5.45 ± 0.37ab | 4.00 ± 0.23bc | 2.86 ± 0.03c | 6.10 ± 0.23a | 4.75 ± 1.18ab | 6.6–12.6 |
| RBC (x103mm3) | 3.77 ± 0.10b | 4.35 ± 0.14ab | 3.67 ± 0.10b | 3.70 ± 0.15b | 4.65 ± 0.20a | 4.20 ± 0.40b | 6.76–9.75 |
| MCHC (g/dL) | 33.3 ± 0.00b | 33.35 ± 0.08ab | 33.40 ± 0.00ab | 33.26 ± 0.06b | 33.50 ± 0.00a | 33.25 ± 0.08b | 28.2–34.1 |
| MCH (pg) | 30.45 ± 0.08ab | 30.30 ± 0.17ab | 30.70 ± 0.11a | 29.23 ± 0.23b | 30.35 ± 0.08ab | 31.55 ± 0.89a | 16.0–23.1 |
| MCV (fl) | 91.40 ± 0.17a | 90.75 ± 0.31ab | 91.15 ± 0.14ab | 89.40 ± 0.00c | 90.30 ± 0.17b | 90.55 ± 0.49ab | 50.0–77.8 |
| Neutrophils (%) | 48.67 ± 0.33a | 60.00 ± 1.15bc | 48.00 ± 0.00a | 50.33 ± 0.33a | 36.00 ± 0.57c | 54.66 ± 5.48ab | 5.3–38.1 |
| Lymphocytes (%) | 50.00 ± 0.00cd | 60.00 ± 1.15ab | 50.00 ± 1.15cd | 46.00 ± 0.57d | 64.00 ± 0.57a | 54.67 ± 5.47bc | 56.7–93.1 |
| Monocytes (%) | 0.00 ± 0.00b | 0.00 ± 0.00b | 2.00 ± 1.00a | 3.00 ± 0.00a | 0.00 ± 0.00b | 2.00 ± 1.00a | 0.00–7.7 |
| Eosinophils (%) | 1.67 ± 0.33a | 0.00 ± 0.00a | 0.00 ± 0.00b | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00b | 0.0–3.4 |
| Basophils (%) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.0–0.4 |
| Creatinine (mg/dL) | 44.36 ± 1.77b | 57.66 ± 6.56a | 30.16 ± 0.87c | 43.15 ± 4.24b | 14.44 ± 0.84d | 28.63 ± 3.33c | 0.2–0.8 |
| Urea (mg/dL) | 4.18 ± 0.11b | 7.61 ± 1.91a | 2.22 ± 0.64bc | 4.10 ± 0.27b | 0.28 ± 0.09c | 3.17 ± 0.21b | 7–20 |
| Total protein (g/dL) | 2.79 ± 0.05b | 3.18 ± 0.05ab | 3.15 ± 0.15ab | 3.38 ± 0.07a | 1.28 ± 0.13c | 2.96 ± 0.32ab | 5.6–7.6 |
| Albumin (g/dL) | 4.24 ± 0.17d | 6.46 ± 0.17b | 3.81 ± 0.26d | 5.70 ± 0.27c | 4.31 ± 0.10d | 8.33 ± 0.20a | 3.8–4.8 |
| Globulin (g/dL) | 1.45 ± 0.12d | 3.71 ± 0.13b | 1.21 ± 0.91d | 2.32 ± 0.19c | 1.05 ± 0.02d | 5.87 ± 0.24a | - |
| AST (μ/L) | 34.16 ± 0.83d | 68.88 ± 0.62b | 77.83 ± 1.02a | 44.78 ± 1.33c | 48.50 ± 1.95c | 25.22 ± 1.75e | 45.7–80.8 |
| ALT (μ/L) | 136.34 ± 18.26a | 122.14 ± 2.65b | 117.97 ± 0.55c | 110.95 ± 1.31d | 115.67 ± 1.83c | 116.56 ± 2.52c | 17.5–30.2 |
| ALP (μ/L) | 131.81 ± 3.61c | 214.27 ± 0.96a | 123.37 ± 1.75d | 16.45 ± 1.09e | 121.95 ± 3.39d | 195.75 ± 1.95b | 56.8–128 |
| AST/ALT ratio | 0.25d ± 0.00 | 0.56b ± 0.01 | 0.66a ± 0.01 | 0.40c ± 0.00 | 0.42c ± 0.01 | 0.22e ± 0.00 | <1.0 |
Means (±SEM) with different alphabetical superscripts in the same row are significantly different at P < 0.05.PLT: 100% Plantain; TNT: 100% Tigernut; CNT: 100% Commercial dough meal; TNS: Tigernut: Defatted soybean (59.83:40.17%); PSB: Plantain: Defatted soybean (64.46:35.54%); PTS: Plantain: Tigernut: Defatted soybean (51.07:11.50:37.43%). *Normal range (NR): Giannini et al. (1999); Diana (2007).
The total blood proteins (1.28–3.38 g/dL) and serum albumin concentrations (3.81–5.70 g/dL) of rats fed on formulated dough meals were comparatively lower than normal range values for serum protein (6–8 g/dL) and albumin (3.5–5.0 g/dL) (Giannini et al., 1999; Diana, 2007), this could be as a result of the nature of protein source, that is, from plant. It well known that the bioavailability of plant proteins is usually lower compare to animal-based proteins, and that the concentration of plasma proteins, especially albumin, depends on the amount of protein intakes and its quality (Fujita et al., 1978).
The creatinine value of rat fed on the formulated dough meal ranged from 14.44 mg/dl in PTS to 57.66 mg/dL in TNT, while that of CNT sample was 28.63 mg/dL and PLT was 44.36 mg/dL. The Urea concentration of rats fed on experimental dough meals ranged from 0.28 mg/dL in PTS to 7.61 mg/dL in PSB group, and were comparable to those rats fed on CNT (3.17 mg/dL). Comparatively, the creatinine level of the rat fed on the formulated dough meal and control samples were within the normal ranged of Kunitoshi et al. (1997), which implies that both the formulated dough meal and the control samples had no negative side effect on the kidney functionality. Healthy kidneys remove creatinine and urea nitrogen from blood, but the level of creatinine and urea in blood rises with kidney failure and function, that is, the higher the creatinine and urea value the less effective the kidney function (RusulArif and Haider, 2014).
Alanine aminotransferase (ALT), Aspartate aminotransferase (AST) and Alkaline Phosphatase (ALP) are all enzyme markers used to determine the functionality of the liver. The AST values of the formulated dough meal ranged from 44.78 μ/L in PSB to 77.83 μ/L in TNS, and were significantly (p < 0.05) higher than 100% plantain (PLT) (25.22 μ/L) and control sample (CNT) (34.16 μ/L). However, the AST values for experimental food samples were within the normal range values (45.70–80.50 μ/L) Giannini et al. (1999); Diana (2007). For ALT, the values ranged from 110.95 μ/L in PSB to 122.14 μ/L in TNT, while that of CNT was 116.56 μ/L and PLT was 136.34 μ/L. Statistically, the ALT value of rat fed on 100% plantain flour dough meals (PLT) was significantly (p < 0.05) higher than those rats fed on formulated dough meal and control sample (CNT). The ALP values ranged from 106.45 μ/L in PSB to 123 μ/L in TNS, while that of PLT and CNT were 131.81 μ/L and 195.75 μ/L, respectively. The AST/ALT ratios of experimental food samples ranged between 0.25 and 0.66 for PLT and TNS, respectively, and were lower than that of CNT (commercial dough meal flour) (0.22) and normal value (<1.0). This observation implies that the formulated diets are suitable for consumption, and that consumption of these food samples would not damage liver cells. Scientific study has reported that high concentration of AST or ALT in the blood is an indication of liver function and damage (Al-Mamary et al., 2002; Aniagu et al., 2005; Aliyu et al., 2007).
3.4.4. Trend of blood glucose concentration of streptozotocin-induced diabetic rats fed on dough meals and control samples
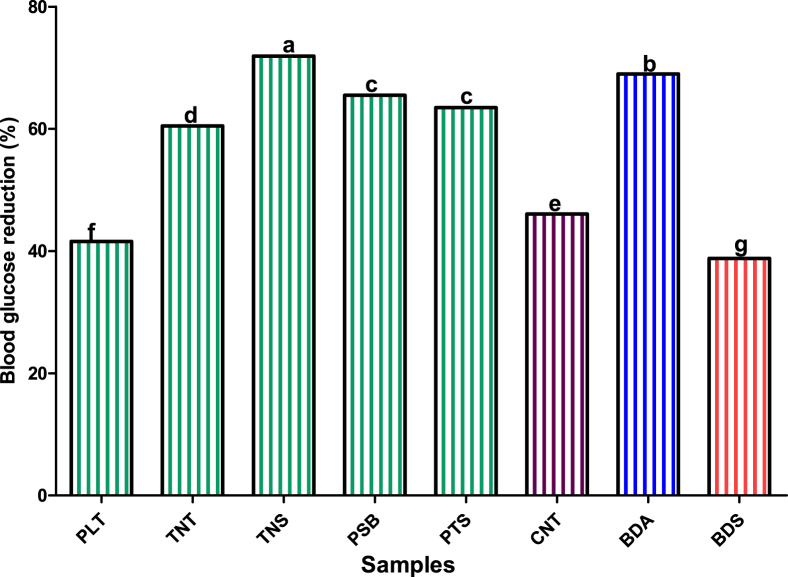
The percentage of blood glucose concentration reduction of streptozotocin-induced diabetic rat fed on formulated dough meals and control sample is presented in Fig. 4. The percentage of blood glucose reduction in streptozotocin-induced diabetic rats fed on experimental food samples were in this order TNS > PSB > PTS > TNT, that is, TNS (71.9%) had the highest blood glucose reduction; while TNT (60.5%) had the lowest percentage reduction. In comparison, this study showed that the experimental dough meals (TNS > PSB > PTS > TNT) had higher potentials to lower blood glucose in streptozotocin-induced diabetic rats than 100% plantain (41.6%) and CNT, a commercial dough meals (46.1%), but comparable to that of BDA (an acarbose, synthetic anti-diabetic agent). This observation could be due to the activities of bioactive compounds such as dietary fibres, phytochemicals and peptides that are presents in these experimental dough meals, which mighty have inhibited the activities of apha-amylase and apha-glucosidase, delaying the gastric emptying rate and reducing active transport of glucose across intestinal brush border membrane (Kobayashi et al., 2000; Heilbronn et al., 2004). Also, several studies have equally reported on antidiabetic potentials of plantain-based food products (Shodehinde and Oboh, 2013; Iroaganachi et al., 2015; Eleazu and Okafor, 2015).
Fig. 4.
Percentage reduction of blood glucose (%) in streptozocin-induced diabetic rats fed on formulated dough meals and control samples [PLT: 100% Plantain; TNT: 100% Tigernut; CNT: 100% Commercial dough meal; NS: Tigernut: Defatted Soybean (59.83:40.17%); PSB: Plantain: Defatted soybean (64.46:35.54%); PTS: Plantain: Tigernut: Defatted soybean (51.07:11.50:37.43%); BDS: Normal Rodent Basal Diet Pellet; BDA: Normal Rodent Basal Diet Pellet administered with Acarbose (2 mg/day)].
4. Conclusion
The study established that the formulated dough meals made from plantain, tigernut and defatted soybean contained appreciable amount of protein and energy values. The dough meals also exhibited good free radical scavenging abilities, better nutritional qualities in terms of growth performances, high biological values (>70%) and low glycaemic index (33–50%). These experimental dough meals, especially TNS and PTS showed significant reduction in blood glucose of diabetic-induced rats, when compared with 100% plantain flour, control (a commercial dough meal flour) and acarbose (anti-diabetic drug). However, out of the formulated dough meals, the PTS (plantain, 51.07%; tigernut, 11.5% & defatted soybeans flour, 37.43%) was rated best in terms of nutritional composition, antioxidant properties and blood glucose reducing potential, hence, this food sample may be suitable as functional food in managing diabetic mellitus.
Declarations
Author contribution statement
Oluwajuyitan Timilehin: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Oluwole Ijarotimi: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abu H., Zulfiker F.A.R., Mahbubur R., Obayed M.U., Kaiser H., Mahbubur R.K., Sohel R. Antidiabetic and antioxidant effect of scopariadulcis in alloxan induced albino mice. Int. J. Pharm. Tech. Res. 2010;2(4):2527–2534. [Google Scholar]
- Academy of Nutrition and Dietetics . 2017. Functional Foods Reviewed by Taylor Wolfram, MS, RDN, LDN.www.eatright.org/food/nutrition/healthy-eating/functional-foods [Google Scholar]
- Adegunwa M.O., Adelekan E.O., Adebowale A.A., Bakare H.A., Alamu E.O. Evaluation of nutritional and functional properties of plantain (Musa paradisiaca L.) and tigernut (Cyperus esculentus L.) flour blends for food Formulations. Cogent Chemistry. 2017;3:2–15. [Google Scholar]
- Adejuyitan J.A. Tigernut processing: its food uses and health benefit. Am. J. Food Technol. 2011;6(3):197–201. [Google Scholar]
- Adepoju O.T., Etukumoh A.U. Nutrient composition and suitability of flour commonly used local complementary foods in Akwa Ibom state, Nigeria. Afr. J. Food Nutr. Sci. 2014;14(7):9544–9560. [Google Scholar]
- Agbede J.O., Aletor V.A. Evaluation of fishmeal replaced with leaf protein concentrate in diets of broiler chicks. Effects on performance, muscle growth, haematology and serum metabolism. Int. J. Poult. Sci. 2003;2:14–19. [Google Scholar]
- Agius L. Lessons from glucokinase activitors: the problem of declining efficacy. Expert Opin. Ther. Pat. 2014;24(11):1155–1159. doi: 10.1517/13543776.2014.965680. [DOI] [PubMed] [Google Scholar]
- Akubor P.I., Ishiwu C. Chemical composition, physical and sensory properties of cakes supplemented with plantain peel flour. Int. Agric. Pol. Res. 2013;1:87–92. [Google Scholar]
- Aliyu R., Adebayo A.H., Gasting D., Garba I.H. The effects of ethanolic leaf extract of Commiphora Africana (Burseraceae) on rat liver and kidney functions. J. Pharmacol. Toxicol. 2007;2:373–379. [Google Scholar]
- Al-Mamary A.M., Al-Habori M., Al-Meeri Ali. Antioxidant activities and total phenolic of different types of honey. Nutr. Res. 2002;22(9):1041–1047. [Google Scholar]
- Aniagu S., Nwinyi C.F., Akumka D.D., Gamaniel K. Toxicity studies in rats fed nature cure bitters. Afr. J. Biotechnol. 2005;4(1):72–78. [Google Scholar]
- AOAC . Official Methods of Analysis of the Analytical Chemist International. eighteenth ed. 2012. Association of official analytical chemist. Gathersburg, MD USA. [Google Scholar]
- Bantle J.P., Wylie-Rosett J., Albright A.L., Apovian C.M., Clark N.G., Franz M.J., Hoogwerf B.J., Lichtenstein A.H., Mayer-Davis E., Mooradian A.D., Wheeler M.L. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(1):S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- CCAC [Canadian Council on Animal Care] In: second ed. Olfert E.D., Cross B.M., McWilliam A.A., editors. Vol. 1. CCAC; Ontario: 1993. (Guide to the Care and Use of Experimental Animals). [Google Scholar]
- Chertow B. Advances in diabetes for the millennium. Vitamins and oxidative stress in diabetes and its complications. Medsc. Gen. Med. 2004;6(4):15647709. [PMC free article] [PubMed] [Google Scholar]
- Coşkuner Y., Ercan R., Karababa E., Nazlican A.N. Physical and chemical properties of chufa (Cyperus esculentus L) tubers grown in the climate region of Turkey. J. Sci. Food Agric. 2002;82:625–631. [Google Scholar]
- Coulibaly A.Y., Konate K., Youl E.N.H., Sombie P.A.E.D., Kiendrebeogo M., Meda Nâg-Tiéro. R., Lamien A., Zeba B., Nacoulma O.G. Anti-proliferative effect of Scoparia dulcis L. against bacterial and fungal strains. Int. J. Brain Cogn. Sci. 2012;6(6):3055–3063. [Google Scholar]
- Dacie J.V., Lewis S.M. eleventh ed. Elsevier Science Ltd; 2002. Practical Haematology; pp. 380–382. [Google Scholar]
- Dan M.C.T. Universidade de São Paulo; São Paulo, Brasil: 2011. Avalição da potencialidade da farinha de banana verdecomo ingredient functional: Estudo in vivo e in vitro. Doctoral thesis. [Google Scholar]
- Decker E.A., Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990;38:674–677. [Google Scholar]
- Diana Nicoll C. Appendix: therapeutic drug monitoring and laboratory reference ranges. In: Stephen J.M., Maxine A.P., editors. Current Medical Diagnosis and Treatment. 46th edition. McGraw hill; 2007. pp. 1767–1775. [Google Scholar]
- Dona A.C., Guilhem P., Robert G.G., Philip W.K. Digestion of starch: in vivo and in vitro kinetic models used to characterize oligosaccharide or glucose release. Carbohydr. Polym. 2010;80:599–617. [Google Scholar]
- Edeoga H.O., Gomina A. Nutritional values of some non-conventional leafy vegetables of Nigeria. J. Econ. Taxon. Bot. 2000;24:7–13. [Google Scholar]
- Eleazu C.O., Okafor P.N. Antioxidant effect of unripe plantain (Musa paradisiacae) on oxidative stress in Alloxan-induced diabetic rabbits. Int. J. Med. Biomed. Res. 2015;1(3):232–241. [Google Scholar]
- Famakin O., Fatoyinbo A., Ijarotimi O.S., Badejo A.A., Fagbemi T.N. Assessment of nutritional quality, glycaemic index, antidiabetic and sensory properties of plantain (Musa paradisiaca)-based functional dough meals. J. Food Sci. Technol. 2016;53(11):3865–3875. doi: 10.1007/s13197-016-2357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO . 1989. Protein Quality Evaluation. Report of the joint FAO/WHO Expert consultation Bethesda, MD., USA. [Google Scholar]
- Foster-Powell K., Holt H.A.S., Brand-Miller J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Yoshimura Y., Inoue G. Effect of low-protein diets on free amino acids in plasma of young men: effect of protein quality with maintenance or excess energy intake. J. Nutr. Sci. Vitaminol. 1978;24(3):297–309. doi: 10.3177/jnsv.24.297. [DOI] [PubMed] [Google Scholar]
- Giannini E., Botta F., Fasoli A., Ceppa P., Risso D., Lantieri P.B., Celle G., Testa R. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig. Dis. Sci. 1999;44(6):1249–1253. doi: 10.1023/a:1026609231094. [DOI] [PubMed] [Google Scholar]
- Gupta D., Bleakley B., Gupta R.K. Phytochemical analysis and antioxidant activity of herbal plant Doronicumhookeri Hook f. (Asteraceae) J. Med. Plants Res. 2011;5(13):2736–2742. [Google Scholar]
- Hawley J.A., Gibala M.J. Exercise intensity and insulin sensitivity: how low can you go? Diabetologia. 2012;52(9):1709–1713. doi: 10.1007/s00125-009-1425-5. [DOI] [PubMed] [Google Scholar]
- Heilbronn L., Smith S.S., Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and Type II diabetes mellitus. Obes. Rev. 2004;28:S12–S21. doi: 10.1038/sj.ijo.0802853. [DOI] [PubMed] [Google Scholar]
- Hwang I.G., Woo G.S., Kim T.M., Kim D.J., Yang M.H., Jeong H.S. Change of physicochemical characteristics of Korean pear (Pyruspyri folia Nakai) juice with heat treatment conditions. Korean J. Food Sci. Technol. 2006;38:342–347. [Google Scholar]
- Igwe C.U., Ojiako A.O., Emejulu A.A., Iweke A.V. Phytochemical Analysis of plants traditionally used in malaria treatment in southeastern Nigeria. J. Res. Biochem. 2012;1:015–022. [Google Scholar]
- Ijarotimi O.S., Keshinro O.O. Protein quality, hematological properties and nutritional status of albino rats fed complementary foods with fermented popcorn, African locust bean, and Bambara groundnut flour blends. Nutr. Res. Pract. 2012;6(5):381–388. doi: 10.4162/nrp.2012.6.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijarotimi O.S., Olopade A.J. Determination of amino acid content and protein quality of complementary food produced from locally available food materials in Ondo state, Nigeria. Malays. J. Nutr. 2009;15(1):87–95. [PubMed] [Google Scholar]
- Ijarotimi O.S., Owoeye O.R. Study on energy-nutrient density, functional and organoleptic properties of complementary foods from indigenous plant Based food materials. J. Adv. Food Sci. Technol. 2017;4(2):73–83. [Google Scholar]
- Ikujenlola A.V., Adurotoye E.A. Evaluation of quality characteristics of high nutrient dense complementary food from mixtures of malted quality protein maize (Zea mays L.) and Steamed cowpea (Vigna unguiculata) J. Food Process. Technol. 2014;5:291. [Google Scholar]
- International Diabetes Foundation . International Diabetes Federation; 2012. Diabetes Atlas.http://www.idf.org/diabetesatlas/. 8 Available from: [Google Scholar]
- Inzucchi S.E., Bergenstal R.M., Buse J.B., Diamant M., Ferrannini E., Nauck M., Peters A.L., Tsapas A., Wender R., Matthews D.R. Management of hyperglycemia in type 2 diabetes: a patient-centered approach position statement of the American diabetes association (ADA) and the European association for the study of diabetes (EASD) Diabetes Care. 2012;35(6):1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iroaganachi M., Eleazu C.O., Okafor P.N., Nwaohu N. Effect of unripe plantain (Musa paradisiaca) and ginger (Zingiber officinale) on blood glucose, body weight and feed intake of streptozotocin-induced diabetic rats. Open Biochem. J. 2015;9:1–6. doi: 10.2174/1874091X01509010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S.L., Muir J.G., Curtis S.L., Gibson P.R. Dietary fibre: a roughage guide. Int. Med. J. 2003;33:291–296. doi: 10.1046/j.1445-5994.2003.00404.x. [DOI] [PubMed] [Google Scholar]
- Jasper R., Locatelli O.G., Pilati C., Locatelli C. Evaluation of biochemical, haematological and oxidative parameters in mice exposed to the herbicide glyphosate-Roundup. Interdiscip. Toxicol. 2012;5(3):133–140. doi: 10.2478/v10102-012-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez-García E., Agama-Acevedo E., Sáyago-Ayerdi S.G., Rodríguez-Ambriz S.L., Bello-Pérez L.A. Composition, digestibility and application in breadmaking of banana flour. Plant Foods Hum. Nutr. 2006;61(3):131–137. doi: 10.1007/s11130-006-0020-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Saito Y., Nakazawa I., Yoshizaki F. Screening of crude drugs for influence on amylase activity and postprandial blood glucose in mouse plasma. Biol. Pharm. Bull. 2000;23:1250–1253. doi: 10.1248/bpb.23.1250. [DOI] [PubMed] [Google Scholar]
- Kumar R.V., Venkatrajireddy G., Bikshapathi T., Reddy M.K. Antioxidant-the maximum expressed activity among 63 medicinal plants. J. Phyto Pharmacol. 2008;1(5):1–13. [Google Scholar]
- Kunitoshi I., Yoshiharu I., Koshiro F. Risk factors of end stage renal disease and serum creatinine in a community based mass screening. Kidney Int. 1997;51:850–854. doi: 10.1038/ki.1997.119. [DOI] [PubMed] [Google Scholar]
- Lawal B., Shittu O.K., Abubakar A.N., Haruna G.M., Sani S., Ossai P.C. Haematopoetic effect of methanol extract of Nigerian honey bee (Apismellifera) propolis in mice. J. Coastal Life Med. 2015;3(8):648–651. [Google Scholar]
- Levitt S.D. Evidence that seat belts are as effective as child safety seats in preventing death for children aged two and up. Rev. Econ. Stat. 2008;90(1):158–163. [Google Scholar]
- Lunn J., Buttriss J.L. Carbohydrates and dietary fibre. Nutr. Bull. 2007;32:21–64. [Google Scholar]
- Maitra A., Abbas A.K. Endocrine system. In: Kumar V., Fausto N., Abbas A.K., editors. Robbins and Cotran Pathologic Basis of Disease. seventh ed. Saunders; Philadelphia: 2005. pp. 1156–1226. 2005. [Google Scholar]
- Mastromatteo M., Danza A., Lecce L., Spinelli S., Lampignano V., Laverse J., Contò F., Nobile M.A. Effect of durum wheat varieties on bread quality. Int. J. Food Sci. Technol. 2014;49:72–81. [Google Scholar]
- Mau J.L., Lin H.C., Song S.F. Antioxidant properties of several specialty mushrooms. Food Res. Int. 2002;35:519–526. [Google Scholar]
- McMillan-Price J., Petocz P., Atkinson F. Comparison of 4 diets of varying glycaemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults. Arch. Int. Med. Assoc. 2006;166:1466–1475. doi: 10.1001/archinte.166.14.1466. [DOI] [PubMed] [Google Scholar]
- Meiton D.A. Reversal of Type -1 diabetes in mice. N. Engl. J. Med. 2006;355:89–90. doi: 10.1056/NEJMcibr062559. [DOI] [PubMed] [Google Scholar]
- Menezes E.W., Dan M.C., Cardenette G.H., Goñi I., Bello-Pérez L.A., Lajolo F.M. In vitro colonic fermentation and glycemic response of different kinds of unripe banana flour. Plant Foods Hum. Nutr. 2010;65(4):379–385. doi: 10.1007/s11130-010-0190-4. [DOI] [PubMed] [Google Scholar]
- Mepba H.D., Eboh L., Nwajigwa S.U. Chemical composition, functional and baking properties of wheat-plantain composite flours. Afr. J. Food Nutr. Sci. 2007;7(1):1–22. [Google Scholar]
- Mufunda J., Chatora R., Ndambakuwa Y., Nyarango P., Kosia A., Chifamba J., Filipe A., Usman A., Sparks V.H. Emerging non-communicable disease epidemic in Africa: preventive measures from the WHO Regional office for Africa. Ethn. Dis. 2006;16(2):521–526. [PubMed] [Google Scholar]
- Odenigbo A.M., Asumugha V.U., Ubbor S., Nwauzor C., Otuonye A.C., Offia-Olua B.I., Princewill-Ogbunna I.L., Nzeagwu O.C., HenryUneze H.N., Anyika J.U., Ukaegbu P., Umeh A.S., Anozie G.O. Proximate composition and consumption pattern of plantain and cooking-banana. Br. J. Appl. Sci. Technol. 2013;3:1035–1043. [Google Scholar]
- Odenigbo C.U., Oguejiofor O.C. Pattern of medical admissions at the Federal Medical Centre, Asaba-a two year review. Niger. J. Clin. Pract. 2009;12:395–397. [PubMed] [Google Scholar]
- Odom T.C., Udensi E.A., Ogbuji C.A. Evaluation of hypoglycaemic properties of Mucuna cochichinensis, unripe Carica papaya and unripe Musa paradisiaca flour blends. Eur. J. Biol. Med. Sci. Res. 2013;1(1):15–22. [Google Scholar]
- Okpala L.C., Okoli E.C. Nutritional evaluation of cookies produced from pigeon pea, cocoyam and sorghum flour blends. Afr. J. Biotechnol. 2011;10(3):433–438. [Google Scholar]
- Oladele A.K., Aina J.O. Chemical composition and functional properties of flour produced from two varieties of tigernut (Cyperus esculentus) Afr. J. Biotechnol. 2017;6:2473–2476. [Google Scholar]
- Osuafor T.O., Ele P.U. The pattern of admissions in the medical wards of Nnamdi azikiwe University Teaching hospital Nnewi. Orient J. Med. 2004;16:11–15. [Google Scholar]
- Osundahunsi O.F., Aworh A.C. A preliminary study on the use of Tempe-based formula-plant. Foods Hum. Nutr. 2002;57(3-4):365–376. doi: 10.1023/a:1021805117084. [DOI] [PubMed] [Google Scholar]
- Ozougwu J.C., Obimba K.C., Belonwu C.D., Unakalamba C.B. The pathogenesis and pathophysiology of Type 1 and Type 2 diabetes mellitus. J. Physiol. Pathophysiol. 2013;4(4):46–57. [Google Scholar]
- Parks B.W., Nam E., Org E., Kostem E., Norheim F., Hui S.T., Pan C., Civelek M., Rau C.D., Bennett B.J., Mehrabian M., Ursell L.K., He A., Castellani L.W., Zinker B., Kirby M., Drake T.A., Drevon C.A., Knight R., Gargalovic P., Kirchgessner T., Eskin E., Lusis A.J. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metabol. 2013;17(1):141–152. doi: 10.1016/j.cmet.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual J., Martinez-Yamout M., Dyson H.J., Wright P.E. Structure of the PHD zinc finger from human Williams-Beuren syndrome transcription factor. J. Mol. Biol. 2000;304(5):723–729. doi: 10.1006/jmbi.2000.4308. [DOI] [PubMed] [Google Scholar]
- Pickup J.C. Inflammation and activated innate immunity in the pathogenesis of Type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- Polidori M.C., Stahl W., Eichler O., Niestroj I., Sies H. Profile of antioxidants in human plasma. Free Radic. Biol. Med. 2001;30(5):456–462. doi: 10.1016/s0891-5849(00)00345-2. [DOI] [PubMed] [Google Scholar]
- Prasad S., Krishnadas M., McConkey K., Datta A. The tangled causes of population decline in two harvested species: a comment on Ticktin. J. Appl. Ecol. 2014;51(3):555–559. [Google Scholar]
- Ramdas P., Balakrishnan S. Extract of Adenanthera pavonina L. seed reduces development of diabetic nephropathy in streptozotocin-induced diabetic rats. Avicenna J. Phytomed. 2012;2(4):233–242. [PMC free article] [PubMed] [Google Scholar]
- Randy C.P., Kepler A.K., Daniells J., Nelson S.C. 2007. Banana and Plantain- an Overview with Emphasis on the pacific Island Cultivars. Species Profile for pacific Island Agro-Forestry Island Cultivars. Species Profile for pacific Island Agroforestry.www.traditionaltree.org Retrieved 5/7/2018. [Google Scholar]
- Rizkalla S.W., Taghrid L., Laromiguiere M. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycaemic index diet in Type 2 diabetic men: a randomized trial. Diabetes Care. 2004;27:1866–18872. doi: 10.2337/diacare.27.8.1866. [DOI] [PubMed] [Google Scholar]
- Roberfroid M., Gibson G.R., Hoyles L., McCartney A.L., Rastall R., Rowland I., Wolvers D., Watzl B., Szajewska H., Stahl B., Guarner F., Respondek F., Whelan K., Coxam V., Davicco M.J., Léotoing L., Wittrant Y., Delzenne N.M., Cani P.D., Neyrinck A.M., Meheust A. Prebiotics effects: metabolic and health benefits. Br. J. Nutr. 2010;104:S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- Roberts K.M., Daryl K.G., Peter A.M., Victor W.R. 25th edition. Vol. 25. McGraw Hill; New York: 2000. pp. 763–765. (Mayer’s Biochemistry). [Google Scholar]
- RusulArif A.A., Haider S. A study of some biochemical changes in patients with chronic renal failure undergoing hemodialysis. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:581–586. [Google Scholar]
- Sabiu S., Ajani O.E., Sunmonu O.T., Ashafa T.O.A. Kinetics of modulatory role of Cyperusesculentus L. on the specific activity of key carbohydrate metabolism enzymes. Afr. J. Tradit. Complement. Altern. Med. 2017;14(4):46–53. doi: 10.21010/ajtcam.v14i4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmerón J., Manson J.E., Stampfer M.J., Colditz G.A., Wing A.L., Willett W.C. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. Joint Am. Med. Assoc. 1997;277:472–477. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- Samson I.I. Formulation of infant weaning foods from vegetable proteins and cereal. Am. J. Food Technol. 2014;9:104–110. [Google Scholar]
- Sánchez A., Sharma S., Rozenzhak S., Roguev A., Krogan N.J., Chabes A., Russell P. Replication fork collapse and genome instability in a deoxycytidylatedeaminase mutant. MCB (Mol. Cell. Biol.) 2012;32(21):4445–4454. doi: 10.1128/MCB.01062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shittu O.K., Musa F., Gbadamosi D.F. Trypanocidal activity and haematological changes in T. brucei infected rats treated with methanolic leaf extract of thymus vulgaris. Int. J. Appl. Biol. Res. 2013;5(1 and 2):109–114. 2013. [Google Scholar]
- Shodehinde S.A., Oboh G. Antioxidant properties of aqueous extracts of unripe Musa paradisiaca on sodium nitroprusside induced lipid peroxidation in rat pancreas in vitro. Asian Pac. J. Trop. Biomed. 2013;3(6):449–457. doi: 10.1016/S2221-1691(13)60095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin J.L., Martini M.C., Jacobs D.R., Jr., Marquart L. Plausible mechanisms for the protectiveness of whole grains. Am. J. Clin. Nutr. 1999;70(3) doi: 10.1093/ajcn/70.3.459s. 459-63S. [DOI] [PubMed] [Google Scholar]
- Smith C.E., Tucker K. Health benefits of cereal fibre: a review of clinical trials. Nutr. Res. Rev. 2011;24(1):118–131. doi: 10.1017/S0954422411000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar Prasad Y.P., Padmaja H., Shajina M., Mirshad P.V., Fasalu Rahiman O.M. Hematinic and antioxidant potential of aqueous extract of Sesamum indicum seeds against phenylhydrazine-induced hemolytic anemia in albino rats. Natl. J. Physiol. Pharm. Pharmacol. 2018;8(8):1092–1096. [Google Scholar]
- Tribess T., Hernandez-Uribe P.J., Mendez-Montealvo G., Tadini C.C. Thermal properties and resistant starch content of green banana flour (Musa cavendishii) produced at different drying conditions. LWT Food Sci. Technol. 2009;42(5):1022–1025. [Google Scholar]
- Usman M.N., Ibrahim F.D., Tanko L. Perception and adaptation of crop farmers to climate change to in Niger state, Nigeria. Niger. J. Agric. Food Environ. 2016;12(4):186–193. [Google Scholar]
- Valabhji J., McColi A.J., Richmond W., Schachter M., Rubens M.B., Elkeles R.S. Antioxidant status and coronary artery calcification in Type 1 diabetes. Diabetes Care. 2001;24(9):1608–1613. doi: 10.2337/diacare.24.9.1608. [DOI] [PubMed] [Google Scholar]
- Warren I.J., Burnette M., South C.S., Patten V.I. Psychopathy in women: structural modeling and comorbidity. Int. J. Law Psychiatry. 2003;26(3):223–243. doi: 10.1016/S0160-2527(03)00034-7. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2010. Global Recommendations on Physical Activity for Health. 2010. [PubMed] [Google Scholar]
- Wolever T.M.S., Jenkins D.J.A., Jenkins A.L., Josse R.G. The glycemic index: methodology and clinical implications. Am. J. Clin. Nutr. 1991;54:846–854. doi: 10.1093/ajcn/54.5.846. [DOI] [PubMed] [Google Scholar]
- Yalta A., Talha The accuracy of statistical distributions in Microsoft Excel 2007. Comput. Stat. Data Anal. 2008;52:4579–4586. [Google Scholar]