Abstract
BACKGROUND
Studies implicate that angiotensin 1–7 (Ang1-7) imparts protective effects in the kidney. However, its relevance in hypertensive kidney disease is not fully understood. The purpose of this study was to explore the role of Ang1-7 on renal damage/remodeling during hypertension and its potential underlying molecular–cellular mechanisms.
METHODS
Hypertension was induced in adult Sprague–Dawley rats by infusion of aldosterone (ALDO; 0.75 μg/hour) for 4 weeks with or without co-treatment of Ang1-7 (1 mg/kg/day). Untreated rats served as controls. Systolic blood pressure was monitored by tail-cuff technique. Renal fibrosis was evaluated by picrosirius red staining and renal collagen volume fraction was quantitated using imaging analyzing system. The expression of profibrotic factors [transforming growth factor-β1 (TGF-β1), platelet-derived growth factor-D (PDGF-D), fibroblast growth factor-1 (FGF-1), vascular endothelial growth factor-D (VEGF-D), and tissue inhibitors of metalloproteinases (TIMPs)] and free radical producing enzymes (inducible nitric oxide synthase and nicotinamide adenine dinucleotide phosphate [NADPH] oxidase) in the kidney were examined by reverse transcription–polymerase chain reaction and western blot. Renal oxidative stress was assessed by malondialdehyde (MDA) measurement.
RESULTS
Chronic ALDO infusion caused hypertension and hypertensive renal disease represented as glomerular damage/sclerosis. Ang1-7 co-treatment did not affect blood pressure in ALDO-treated rats, but significantly attenuated the glomerular damage/fibrosis. ALDO treatment significantly elevated renal expression of profibrogenic factors, including TGF-β1, TIMP-1/TIMP-2, FGF-1, PDGF-D, and VEGF-D, whereas Ang1-7 co-treatment significantly reduced renal TGF-β1, TIMP-1/TIMP-2, and FGF-1, but not PDGF-D and VEGF-D. Furthermore, ALDO infusion elevated NADPH oxidase (gp91phox) and MDA in the kidney, which was attenuated by Ang1-7 co-treatment.
CONCLUSIONS
Ang1-7 plays a protective role in the hypertensive kidney disease independent of blood pressure. The beneficial effects of Ang1-7 are likely mediated via suppressing TGF-β/FGF-1 pathways and oxidative stress.
Keywords: angiotensin 1–7, blood pressure, fibrotic mediators, hypertension, hypertensive kidney disease, oxidative stress
Angiotensin-converting enzyme/angiotensin II/type 1 angiotensin receptors (ACE/AngII/AT1) axis of the rennin–angiotensin system plays an integral role in maintaining vascular tone, salt and water homeostasis, as well as cardiac function in humans. Chronic activation of AngII/AT1 causes hypertension accompanied by cardiac, renal, and vascular injury with remodeling that eventually leads to end-organ damage, such as renal failure, heart failure, and coronary artery disease.1
During the last decade, classical view of the rennin–angiotensin system has been challenged by the discovery of ACE2. This membrane-bound enzyme has been identified in rodents and humans, and is found mainly in the heart and kidney.2 The major role of ACE2 is to cleave AngII to generate Ang1-7. The biological effects of Ang1-7 in the kidney are primarily mediated by interaction with the G-protein-coupled receptor Mas. However, other complex effects have been described that may involve receptor–receptor interactions with AT1 or type 2 angiotensin receptors (AT2) receptors, as well as nuclear receptor binding.3 The ACE2/Ang1-7/Mas axis counterbalances the role of ACE/AngII/AT1 axis, thus imparting broader influences on the cardiovascular system, including vasodilatation, myocardial protection, antiarrhythmic effects, and inhibition of pathological cardiac remodeling.4 Furthermore, a number of experimental findings suggest that Ang1-7 also plays a protective role in the kidney.5,6
Hypertensive kidney disease is a clinical condition that causes damage to the kidney because of chronic high blood pressure. The renal pathological changes are gradual and progressive, characterized by glomerular damage, followed by glomerular sclerosis and interstitial fibrosis.7 The intensity of fibrosis correlates with the degree of glomerular filtration deficit. Multiple factors, including Ang1-7, have been shown to contribute to the renal pathological changes. Ang1-7 is reported to attenuate fibrosis in liver, skeletal muscle, and heart.8 A growing body of evidence supports a role for endogenous or exogenous Ang1-7 serving as a protectant against nephron injury in certain experimental conditions.3 Using a well-established hypertensive rat model, the first objective of this study was to determine whether Ang1-7 exerts an effect on hypertensive kidney disease.
The development of tissue fibrosis, including hypertensive kidney disease, is a complex process with tight regulation of extracellular matrix synthesis and degradation. Transforming growth factor-β (TGF-β) is the most well-characterized profibrotic mediator for the fibrous tissue formation.9 Other growth factors, including platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), and connective tissue growth factor, also promote fibrosis in the damaged tissue.10,11 These fibrotic mediators initiate signal transduction pathways that ultimately lead to the proliferation/activation of fibroblasts and extracellular matrix deposition. Ang1-7 has been reported to reduce collagen deposition in the infarcted heart through suppressing TGF-β.12 Moreover, Ang1-7 is shown to play a protective role in the kidneys of diabetes and liver fibrosis through suppressing oxidative stress-mediated signaling pathway.13,14 Further studies are needed to elucidate the regulatory mechanisms of Ang1-7 in hypertensive kidney disease. The second objective of the study was to determine the potential molecular–cellular mechanisms responsible for the role of Ang1-7 in renal fibrosis of hypertensive kidney disease.
METHODS
Animal experiment
Eight-week-old male Sprague–Dawley rats were used in the study. Three animal groups (n = 6/group) were studied: (i) untreated and unoperated rats, which served as controls; (ii) uninephrectomized rats receiving aldosterone (ALDO; 0.75 μg/hour), administered subcutaneously via implanted minipump, together with 1% sodium chloride in drinking water, causing hypertension15; and (iii) ALDO-treated rats receiving Ang1-7 (1mg/kg/day) given subcutaneously by implanted minipump.16 At the end of 4 weeks of treatment, kidneys were harvested, frozen in isopentane with dry ice, and kept at −80 °C for the following studies. This study protocol was approved by the University of Tennessee Health Science Center Animal Care and Use Committee.
Systolic blood pressure
Systolic blood pressure was monitored at the end of animal experiment by tail-cuff technique as we reported previously.15 Briefly, rats were placed in plastic restrainers. A cuff with a pneumatic pulse sensor was attached to the tail. Rats were allowed to accustom to this procedure for a week before blood pressure were recorded and were averaged from 8–10 consecutive readings from each rat.
Morphology
Cryostat sections (6 μm) of frozen kidney were prepared to evaluate renal morphology by hematoxylin/eosin and the fibrillar collagen accumulation (fibrosis) by collagen-specific picrosirius red staining as previously reported.17 Collagen volume fraction (CVF) in the kidney was quantitated using a computer image analysis system (NIH image, 1.60) and was calculated as the sum of connective tissue areas, divided by the sum of connective tissue area and nonconnective tissue area in all fields of the kidney section (3 sections/kidney).
Reverse transcription–polymerase chain reaction
The total RNA was extracted from the kidney. Gene expression of renal type 1 collagen, TGF-β1, tissue inhibitors of metalloproteinase-1 (TIMP-1), TIMP-2, PDGF-D, PDGF receptor- β (PDGFR-β), FGF-1, FGF receptor-1 (FGFR-1), VEGF-D, VEGF receptor-3 (VEGFR-3), gp91phox (a subunit of nicotinamide adenine dinucleotide phosphate [NADPH] oxidase), and inducible nitric oxide synthase (iNOS) was deduced by reverse transcription–polymerase chain reaction as previously reported.18 Fold changes were used to compare the difference among groups.
Western blotting
Growth factor protein levels in the kidney were measured by western blot. Briefly, the renal tissue was homogenized and the supernatant was collected and separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. After electrophoresis, samples were transferred to polyvinylidene difluoride membranes and incubated with antibody against TGF-β1, TIMP-1, FGF-1, and gp91phox (Invitrogen, Carlsbad, CA; Sigma, St. Louis, MO). Blots were subsequently incubated with peroxidase-conjugated secondary antibody (Sigma). After washing, the blots were developed with an enhanced chemoluminescence method. The amount of protein detected was assessed by means of quantitative densitometry analysis with a computer image analyzing system. Fold changes were used to compare the statistical difference among groups.18
Measurement of MDA
Malondialdehyde (MDA) results from lipid peroxidation and polyunsaturated fatty acids that serve as a marker of oxidative stress. Renal tissue was homogenized (1:10 w:v) in ice-cold phosphate-buffered saline (pH 7.4) containing 5 mM butylated hydroxytoluene, sonicated, and centrifuged at 3000×g for 10 minutes. MDA concentration in the supernatant was determined colorimetrically using a commercially available kit (Oxis Research, Portland, OR). Briefly, duplicate aliquots of supernatant were incubated at 45 °C with 7.7 mM N-methyl-2-phenylindole in acetonitrile/methanol (75:25 v:v) and 15.4 mM methanesulfonic acid. After clarification by centrifugation at 15000×g for 15 minutes, absorbance was measured at 586 nm using 1,1,3,3-tetramethoxypropane as a standard. The MDA content of the renal tissue was expressed as nanomol per milligram protein.19
Statistical analysis
Statistical analyses of systolic blood pressure, renal CVF, reverse transcription–polymerase chain reaction, western blot, and MDA data were performed using analysis of variance. The values were expressed as mean ± SEM, with P < 0.05 considered significant. Multiple group comparisons among the controls and each group were made by the Scheffe F test.
RESULTS
Systolic blood pressure
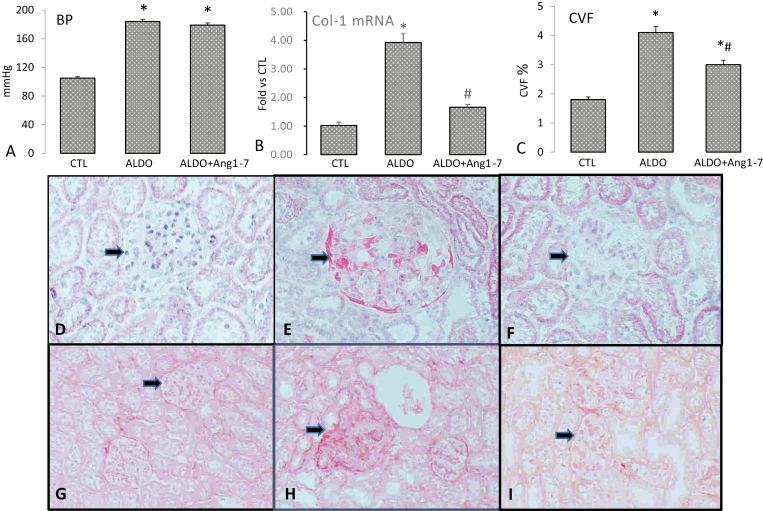
In this study, uninephrectomized rats receiving ALDO/salt treatment significantly increased systolic blood pressure compared to untreated control rats. However, co-treatment with Ang1-7 did not alter the blood pressure in rats with ALDO infusion (Figure 1a).
Figure 1.
Blood pressure, renal collagen synthesis and morphology in rats received aldosterone (ALDO) with/without angiotensin 1–7 (Ang1-7) co-treatment. ALDO infusion significantly elevated blood pressure in rats, which was not affected by Ang1-7 co-treatment (a). Type 1 collagen (Col-1) mRNA (b) and CVF (c) in the kidney were significantly increased in ALDO-treated rats, which was significantly suppressed by Ang1-7. Compared to the control kidney (d and g), glomerular damage/necrosis (arrow, e) and sclerosis (h) appeared in ALDO-treated rats. Ang1-7 co-treatment improved glomerular injury () and fibrosis (i). Panels a–c: *P < 0.05 compared to control group (CTL); #P < 0.05 compared to ALDO group (n = 6/group); Panels d–f: ×400.
Collagen synthesis in the kidney
Collagen is the main structural protein in the extracellular space of the connective and fibrous tissue. Type 1 collagen is the major component of fibrous tissue. As assessed by reverse transcription–polymerase chain reaction, renal type 1 collagen was significantly elevated in rats receiving ALDO treatment. However, Ang1-7 co-treatment significantly suppressed renal type 1 collagen mRNA in rats receiving ALDO (Figure 1b).
Detected by collagen-specific picrosirius red staining and image analyzing system, we observed very low levels of renal CVF in the normal rat kidney. Renal CVF was significantly increased in ALDO-treated rats compared to controls, whereas Ang1-7 co-treatment significantly suppressed renal CVF in ALDO-treated rats (Figure 1c).
Kidney morphology
Chronic hypertension leads to hypertensive kidney disease, characterized as glomerular damage, followed by glomerular sclerosis. Renal damage was evident in rats receiving chronic ALDO infusion. As detected by hematoxylin/eosin and picrosirius red staining, glomeruli exhibited fibroid necrosis (Figure 1e) and sclerosis (Figure 1h) compared to the normal kidney (Figure 1d, g), whereas ALDO-induced renal injury and fibrosis were markedly reduced by Ang1-7 co-treatment (Figure 1f, i).
Gene expression of renal TGF-β and TIMPs
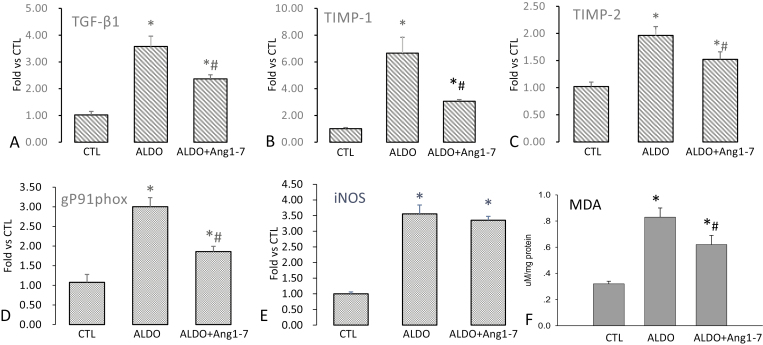
Tissue collagen synthesis is primarily regulated by profibrogenic cytokines and growth factors. TGF-β is a multifunctional cytokine belonging to the TGF superfamily. TGF-β1 is a key modulator of tissue fibrosis by stimulating fibroblast proliferation and collagen synthesis. Renal TGF-β1 expression has been shown to be upregulated in the hypertensive kidney disease.20 Our data revealed that renal TGF-β1 mRNA levels were significantly increased in ALDO-treated rats, whereas Ang1-7 co-treatment significantly attenuated the pathogenic upregulation (Figure 2a).
Figure 2.
Transforming growth factor-β1 (TGF-β1), tissue inhibitors of metalloproteinase-1/-2 (TIMP-1/-2), gp91phox and inducible nitric oxide synthase (iNOS) mRNA, and malondialdehyde (MDA) levels in the kidney. Compared to controls, TGF-β1 (a), TIMP-1 (b) and TIMP-2 (c), and gp91phox (d) mRNA and MDA levels (f) were significantly elevated in aldosterone (ALDO)-treated rats, which was significantly attenuated by angiotensin 1–7 (Ang1-7) co-treatment. However, renal iNOS mRNA level was significantly increased in rats receiving ALDO, which was not altered by Ang1-7 co-treatment (e). *P < 0.05 compared to control group (CTL); #P < 0.05 compared to ALDO group (n = 6/group).
Collagen synthesis and degradation coexist in the remodeling tissue and their temporal equilibrium determines the collagen volume in tissue. Elevated collagen synthesis and/or suppressed collagen degradation lead to excessive accumulation of collagen in tissue undergoing remodeling. Matrix metalloproteinases are responsible for collagen degradation and matrix metalloproteinase activity is intrinsically regulated by TIMPs. Our study revealed that the expressions of renal TIMP-1 and TIMP-2 were significantly elevated in ALDO-treated rats, which was significantly attenuated in those co-treated with Ang1-7 (Figure 2b, c).
Gene expression of gp91phox and iNOS in the kidney
Oxidative stress is involved in the pathogenesis of various diseases and plays a role in the development of tissue fibrosis.21 Specifically, NADPH oxidase is a multiprotein complex and is a major contributor of reactive oxygen species.22 In this study, we observed markedly increased expression of gp91phox, a subunit of NADPH oxidase, in the kidney of ALDO-treated rats, which was significantly attenuated by Ang1-7 co-treatment (Figure 2d).
Nitric oxide is a free radical, primarily generated by nitric oxide synthase. Induction of the activated iNOS state usually occurs in an oxidative environment, and thus higher levels of nitric oxide have the greater propensity to react with superoxides leading to formation of peroxynitrite, causing cellular toxicity. It has been suggested that pathologic generation of nitric oxide through increased iNOS production may stimulate tissue fibrosis.23 Our study revealed that iNOS expression in the kidney was significantly increased in ALDO-treated rats compared to their controls, which, however, was not altered by Ang1-7 co-treatment (Figure 2e).
MDA levels in the kidney
MDA is a marker of oxidative stress. Our study showed that compared to the control rats, renal MDA levels were elevated in ALDO-treated rats, which was significantly suppressed by Ang1-7 co-treatment (Figure 2f).
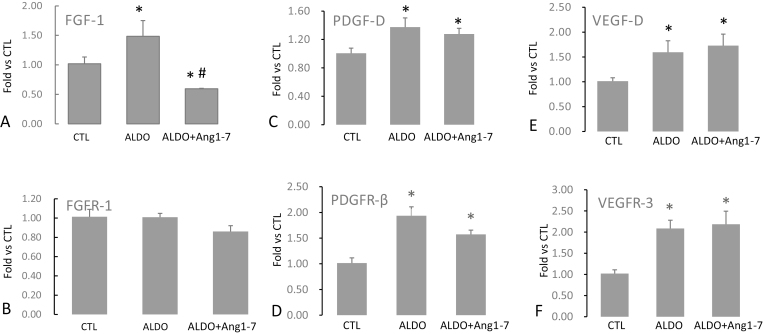
Gene expression of fibrotic mediators in the kidney
It has been known that multiple growth factors, including FGF, PDGF, and VEGF, contribute to the development of tissue fibrosis. FGF-1 is a member of the FGF family, which possesses broad mitogenic activity, and is involved in a variety of biological processes, including tissue repair and fibrosis. We observed markedly increased renal expression of FGF-1 in ALDO-treated rats, which was significantly suppressed by Ang1-7 co-treatment (Figure 3a). Cellular actions of FGF-1 are mediated by FGFR. However, our study showed that renal FGFR-1 expression was not altered by ALDO infusion with or without Ang1-7 co-treatment (Figure 3b).
Figure 3.
Gene expression of fibroblast growth factor-1/FGF receptor-1 (FGF-1/FGFR-1), platelet-derived growth factor-D/PDGF receptor-β (PDGF-D/PDGFR-β), and vascular endothelial growth factor-D/VEGF receptor-3 (VEGF-D/VEGFR-3) in the kidney. Aldosterone (ALDO) treatment significantly increased expression of FGF-1 in the kidney, which was significantly suppressed by angiotensin 1–7 (Ang1-7) (a). Renal FGFR-1 was not affected by ALDO and Ang1-7 (b). Renal expression of PDGF-D (c), PDGFR-β (d), VEGF-D (e), and VEGFR-3 (f) were significantly increased in ALDO-treated rats, but was not altered by Ang1-7 co-treatment. *P < 0.05 compared to control group (CTL) #P < 0.05 compared to ALDO group (n = 6/group).
PDGF-D is a crucial mediator of fibrous tissue formation and its cellular actions are mediated by PDGFR-β. Our study revealed that renal expression of PDGF-D and PDGFR-β was significantly increased in ALDO-treated rats. However, the elevated expressions of PDGF-D and PDGFR-β in the hypertensive kidney were not affected by Ang1-7 co-treatment (Figure 3c, d).
VEGF is a subfamily of growth factors consisted of its 5 members: VEGF-A, VEGF-B, VEGF-C, VEGF-D, and the placental growth factor. VEGFs are important signaling proteins involved in angiogenesis. VEGF-C and VEGF-D also serve as profibrogenic mediators that promote tissue fibrosis.24,25 In this study, we observed significantly increased expression of VEGF-D (Figure 3e) and VEGFR-3 (Figure 3f) in the kidney of ALDO-treated rats. However, Ang1-7 co-treatment did not modify renal gene expression of VEGF-D and VEGFR-3 in ALDO-treated rats.
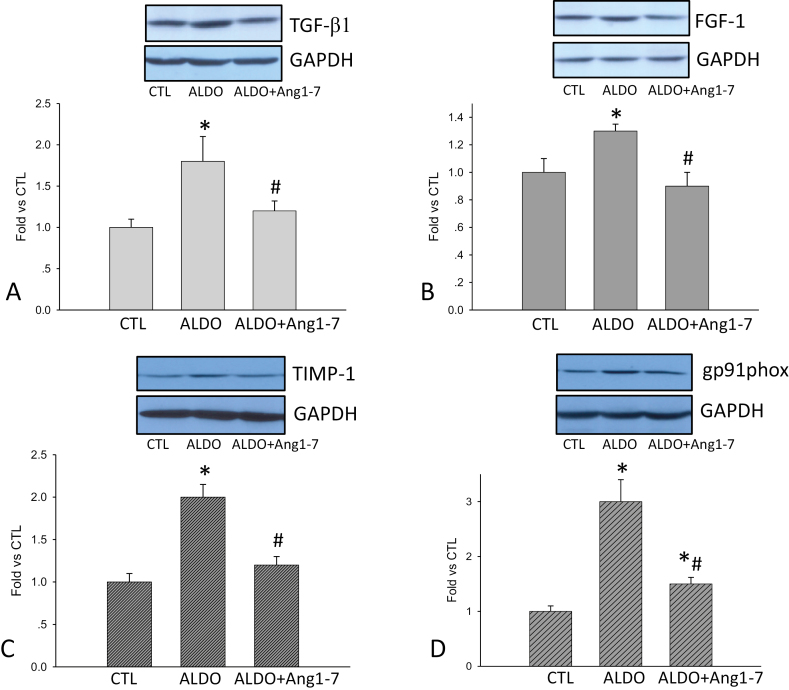
Protein levels of profibrogenic factors in the kidney
Detected by western blot, we observed elevated renal TGF-β1, FGF-1, TIMP-1, and gp91phoxlevels in ALDO-treated rats compared to control rats, which were significantly reduced by Ang1-7 co-treatment (Figure 4).
Figure 4.
Transforming growth factor-β1 (TGF-β1), fibroblast growth factor-1 (FGF-1), tissue inhibitors of metalloproteinase-1 (TIMP-1), and gp91phox levels in the kidney. Compared to controls, renal TGF-β1 (a), FGF-1 (b), TIMP-1 (c), and gp91phox levels (d) were significantly increased in aldosterone (ALDO) group, which were all significantly suppressed by angiotensin 1–7 (Ang1-7) co-treatment. *P < 0.05 compared to control group (CTL); #P < 0.05 compared to ALDO group (n = 6/group).
DISCUSSION
This study explored the role of Ang1-7 on hypertensive kidney disease and its underlying molecular and cellular mechanisms. Our results demonstrated that Ang1-7 plays a protective role in the hypertensive kidney via multiple pathways.
The kidney is one of the major sources of Ang1-7 production.26 ACE2 protein is increased in kidneys from diabetic mice.27 By contrast, other studies reported that ACE2 expression in the kidney was significantly decreased in hypertensive, diabetic, and pregnant rats.28 Our previous study showed that renal ACE2 and Mas levels remained unchanged in ALDO-induced hypertensive rats.15 Although the reported difference in ACE2 expression in kidney diseases remains hitherto unidentified, it is likely related to the varied stages of renal damage and repair.
Experimental findings on the effects of Ang1-7 on blood pressure have been controversial. It is reported that systemic infusion of Ang1-7 to hypertensive rats lowered the blood pressure,29 whereas others indicated it had no effect.30 This study also revealed that Ang1-7 did not alter blood pressure in hypertensive rats induced by ALDO infusion. In humans, Ang1-7 infusion induced noticeable vasodilatory effects in individuals with normal blood pressure and hypertension.31 Furthermore, Ang1-7 infusion leads to renal vasodilation in hypertensive individuals.32 However, another study reported that Ang1-7 treatment did not change the blood pressure in subjects with normal blood pressure.33 Thus, the therapeutic relevance of Ang1-7 on blood pressure remains to be confirmed.
Chronic hypertension leads to multiple-organ injury, including kidneys. High blood pressure can damage blood vessels by scaring and weakening the vessel wall. In rats receiving chronic ALDO infusion, we observed glomerular damage and sclerosis, which were improved by co-treatment with Ang1-7. Quantitative studies further indicated that Ang1-7 significantly attenuated type 1 collagen mRNA and collagen volume in the hypertensive kidney. A protective role for Ang1-7 in hypertension-induced renal injury has been strongly supported by other studies. In rats with hypertension because of renal wrap, chronic treatment with Ang1-7 prevented glomerulosclerosis and tubulointerstitial fibrosis without lowering blood pressure.34 However, in diabetic spontaneous hypertensive rats with Ang1-7 improved renal function without lowering blood pressure.35 Taken together, these findings suggest that Ang1-7 plays a protective role in hypertensive kidney disease independent of blood pressure.
We further investigated the potential mechanisms responsible for the protective role of Ang1-7 on hypertensive kidneys. Fibrosis is initiated when macrophages release profibrogenic cytokines and growth factors that stimulate fibroblasts for laying down extracellular matrix at the sites of injury. The most well-characterized profibrotic mediator is TGF-β. This study shows that TGF-β1 expression was upregulated in the hypertensive kidney, which was significantly suppressed by Ang1-7 co-treatment. Other studies have also evaluated the role of Ang1-7 in modifying the expression of TGF-β and key components of the TGF-β pathway. Ang1-7 was reported to decrease TGF-β1 mRNA levels in cultured cardiac fibroblasts36 and improved vascular remodeling through downregulation of TGF-β and inhibition of the Smad2 pathway.37 Ang1-7, therefore, participates in renal fibrosis by regulating TGF-β synthesis.
Other growth factors that stimulate tissue fibrosis include FGF, PDGF, and VEGF. These profibrotic mediators initiate signal transduction pathways that ultimately lead to the proliferation and activation of fibroblasts, which, in turn, deposit extracellular matrix. This study revealed upregulation of FGF-1/FGFR1, PDGF-D/PDGFR-β, and VEGF-D/VEGFR-3 in the hypertensive kidney. Our study further demonstrated that Ang1-7 treatment significantly attenuated FGF-1, but not PDGF-D and VEGF-D and their respective receptors, suggesting that in addition to TGF-β, the antifibrotic role of Ang1-7 in the hypertensive kidney is also mediated through FGF-1 pathway.
Collagen synthesis and degradation coexist in the repairing tissue and its equilibrium dictates tissue collagen volume. Enhanced collagen production and reduced collagen degradation facilitate tissue collagen deposition. The function of TIMP is to inhibit metalloproteinase activity and promote collagen accumulation. This study showed that TIMP-1 and TIMP-2 are upregulated in the hypertensive kidney, which was significantly attenuated in Ang1-7-treated rats. These findings implicate that Ang1-7 can promote collagen degradation by suppressing TIMPs, thus attenuating fibrosis in the hypertensive kidney.
Oxidative stress often occurs in the injured tissue, which plays an important role in inflammatory and fibrogenic responses.38 In this study, we observed elevated expression of NADPH oxidase subunit, gp91phox, and iNOS in the hypertensive kidney, which contributes to the activation of local oxidative stress. In addition, Ang1-7 significantly suppressed renal gp91phox expression. Other study has reported that recombinant human ACE2 significantly blunts AngII infusion–induced NADPH oxidase and reactive oxygen species production in the heart.39 These results suggest that Ang1-7 confers antioxidant property in the hypertensive kidney.
Although our work has achieved its primary objectives, there were some unavoidable limitations. First, blood pressure was measured by tail-cuff method in the study, which may be influenced by stress. The direct method using catheter technique can overcome the problem. However, the technique requires general anesthesia, which can significantly increase or drop blood pressure. Therefore, it is not an ideal method to measure blood pressure. Tail-cuff method is currently the best way to monitor the blood pressure when rats are well trained to reduce the stress level before measurement. Second, circulating Ang1-7 levels following Ang1-7 treatment were not measured in the study, because the previous study revealed that Ang1-7 infusion increased plasma Ang1-7 level.40 Third, Ang1-7 has been shown to reduce plasma renin and subsequent ALDO endogenous release. This effect of Ang1-7 was not addressed in this study, because when exogenous ALDO was introduced by a minipump, circulating renin and AngII were significantly suppressed and the potential effect of Ang1-7 on renin and ALDO could be considered negligible.
In summary, this study demonstrated the protective effect of Ang1-7 in the hypertensive kidney disease. However, the beneficial role of Ang1-7 on the hypertensive kidney is independent of blood pressure but is mediated via multiple mechanisms. Ang1-7 improves renal injury and fibrosis by suppressing the expression of TGF, FGF, and TIMP as well as reactive oxygen species production.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENT
This work was supported by the NIH National Heart, Lung, and Blood Institute (1R01HL128350, Y.S.)
REFERENCES
- 1. Zhuo JL, Li XC. New insights and perspectives on intrarenal renin–angiotensin system: focus on intracrine/intracellular angiotensin II. Peptides 2011; 32:1551–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burrell LM, Johnston CI, Tikellis C, Cooper ME. ACE2, a new regulator of the renin–angiotensin system. Trends Endocrinol Metab 2004; 15:166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zimmerman D, Burns KD. Angiotensin-(1-7) in kidney disease: a review of the controversies. Clin Sci (Lond) 2012; 123:333–346. [DOI] [PubMed] [Google Scholar]
- 4. Mercure C, Yogi A, Callera GE, Aranha AB, Bader M, Ferreira AJ, Santos RA, Walther T, Touyz RM, Reudelhuber TL. Angiotensin(1-7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res 2008; 103:1319–1326. [DOI] [PubMed] [Google Scholar]
- 5. Dilauro M, Burns KD. Angiotensin-(1-7) and its effects in the kidney. ScientificWorldJournal 2009; 9:522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Igase M, Yokoyama H, Ferrario CM. Attenuation of hypertension-mediated glomerulosclerosis in conjunction with increased angiotensin (1-7). Ther Adv Cardiovasc Dis 2011; 5:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension 2004; 44:595–601. [DOI] [PubMed] [Google Scholar]
- 8. Chappell MC, Al Zayadneh EM. Angiotensin-(1-7) and the regulation of anti-fibrotic signaling pathways. J Cell Signal 2017; 2:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee SY, Kim SI, Choi ME. Therapeutic targets for treating fibrotic kidney diseases. Transl Res 2015; 165:512–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 2004; 15:255–273. [DOI] [PubMed] [Google Scholar]
- 11. Lee AS, Lee JE, Jung YJ, Kim DH, Kang KP, Lee S, Park SK, Lee SY, Kang MJ, Moon WS, Kim HJ, Jeong YB, Sung MJ, Kim W. Vascular endothelial growth factor-C and -D are involved in lymphangiogenesis in mouse unilateral ureteral obstruction. Kidney Int 2013; 83:50–62. [DOI] [PubMed] [Google Scholar]
- 12. Zhao W, Zhao T, Chen Y, Sun Y. Angiotensin 1-7 promotes cardiac angiogenesis following infarction. Curr Vasc Pharmacol 2015; 13:37–42. [DOI] [PubMed] [Google Scholar]
- 13. Dhaunsi GS, Yousif MH, Akhtar S, Chappell MC, Diz DI, Benter IF. Angiotensin-(1-7) prevents diabetes-induced attenuation in PPAR-gamma and catalase activities. Eur J Pharmacol 2010; 638:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mak KY, Chin R, Cunningham SC, Habib MR, Torresi J, Sharland AF, Alexander IE, Angus PW, Herath CB. ACE2 therapy using adeno-associated viral vector inhibits liver fibrosis in mice. Mol Ther 2015; 23:1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meng W, Zhao W, Zhao T, Liu C, Chen Y, Liu H, Sun Y. Autocrine and paracrine function of angiotensin 1-7 in tissue repair during hypertension. Am J Hypertens 2014; 27:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nematbakhsh M, Mansouri A. Renal vascular response to angiotensin 1-7 in rats: the role of Mas receptor. Res Pharm Sci 2018; 13:177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol 2002; 161:1773–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao T, Zhao W, Chen Y, Ahokas RA, Sun Y. Acidic and basic fibroblast growth factors involved in cardiac angiogenesis following infarction. Int J Cardiol 2011; 152:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamalov G, Deshmukh PA, Baburyan NY, Gandhi MS, Johnson PL, Ahokas RA, Bhattacharya SK, Sun Y, Gerling IC, Weber KT. Coupled calcium and zinc dyshomeostasis and oxidative stress in cardiac myocytes and mitochondria of rats with chronic aldosteronism. J Cardiovasc Pharmacol 2009; 53:414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saito K, Ishizaka N, Aizawa T, Sata M, Iso-O N, Noiri E, Ohno M, Nagai R. Role of aberrant iron homeostasis in the upregulation of transforming growth factor-beta1 in the kidney of angiotensin II-induced hypertensive rats. Hypertens Res 2004; 27:599–607. [DOI] [PubMed] [Google Scholar]
- 21. Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med 2000; 21:49–98. [DOI] [PubMed] [Google Scholar]
- 22. Meitzler JL, Antony S, Wu Y, Juhasz A, Liu H, Jiang G, Lu J, Roy K, Doroshow JH. NADPH oxidases: a perspective on reactive oxygen species production in tumor biology. Antioxid Redox Signal 2014; 20:2873–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iwakiri Y. Nitric oxide in liver fibrosis: the role of inducible nitric oxide synthase. Clin Mol Hepatol 2015; 21:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang L, Kwon J, Popov Y, Gajdos GB, Ordog T, Brekken RA, Mukhopadhyay D, Schuppan D, Bi Y, Simonetto D, Shah VH. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology 2014; 146:1339–50.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao T, Zhao W, Meng W, Liu C, Chen Y, Bhattacharya SK, Sun Y. Vascular endothelial growth factor-D mediates fibrogenic response in myofibroblasts. Mol Cell Biochem 2016; 413:127–135. [DOI] [PubMed] [Google Scholar]
- 26. Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol 2005; 289:H2281–H2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ye M, Wysocki J, Naaz P, Salabat MR, LaPointe MS, Batlle D. Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: a renoprotective combination? Hypertension 2004; 43:1120–1125. [DOI] [PubMed] [Google Scholar]
- 28. Danilczyk U, Penninger JM. Angiotensin-converting enzyme II in the heart and the kidney. Circ Res 2006; 98:463–471. [DOI] [PubMed] [Google Scholar]
- 29. Garcia-Espinosa MA, Shaltout HA, Gallagher PE, Chappell MC, Diz DI. In vivo expression of angiotensin-(1-7) lowers blood pressure and improves baroreflex function in transgenic (mRen2)27 rats. J Cardiovasc Pharmacol 2012; 60:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nautiyal M, Shaltout HA, de Lima DC, do Nascimento K, Chappell MC, Diz DI. Central angiotensin-(1-7) improves vagal function independent of blood pressure in hypertensive (mRen2)27 rats. Hypertension 2012; 60:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sasaki S, Higashi Y, Nakagawa K, Matsuura H, Kajiyama G, Oshima T. Effects of angiotensin-(1-7) on forearm circulation in normotensive subjects and patients with essential hypertension. Hypertension 2001; 38:90–94. [DOI] [PubMed] [Google Scholar]
- 32. van Twist DJ, Houben AJ, de Haan MW, Mostard GJ, Kroon AA, de Leeuw PW. Angiotensin-(1-7)-induced renal vasodilation in hypertensive humans is attenuated by low sodium intake and angiotensin II co-infusion. Hypertension 2013; 62:789–793. [DOI] [PubMed] [Google Scholar]
- 33. Plovsing RR, Wamberg C, Sandgaard NC, Simonsen JA, Holstein-Rathlou NH, Hoilund-Carlsen PF, Bie P. Effects of truncated angiotensins in humans after double blockade of the renin system. Am J Physiol Regul Integr Comp Physiol 2003; 285:R981–R991. [DOI] [PubMed] [Google Scholar]
- 34. Ji H, Menini S, Zheng W, Pesce C, Wu X, Sandberg K. Role of angiotensin-converting enzyme 2 and angiotensin(1-7) in 17beta-oestradiol regulation of renal pathology in renal wrap hypertension in rats. Exp Physiol 2008; 93:648–657. [DOI] [PubMed] [Google Scholar]
- 35. Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1-7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol 2008; 28:25–33. [DOI] [PubMed] [Google Scholar]
- 36. Ruiz-Ortega M, Esteban V, Egido J. The regulation of the inflammatory response through nuclear factor-kappab pathway by angiotensin IV extends the role of the renin angiotensin system in cardiovascular diseases. Trends Cardiovasc Med 2007; 17:19–25. [DOI] [PubMed] [Google Scholar]
- 37. Zeng W, Chen W, Leng X, He JG, Ma H. Chronic angiotensin-(1-7) administration improves vascular remodeling after angioplasty through the regulation of the TGF-beta/Smad signaling pathway in rabbits. Biochem Biophys Res Commun 2009; 389:138–144. [DOI] [PubMed] [Google Scholar]
- 38. Agarwal R, Campbell RC, Warnock DG. Oxidative stress in hypertension and chronic kidney disease: role of angiotensin II. Semin Nephrol 2004; 24:101–114. [DOI] [PubMed] [Google Scholar]
- 39. Lo J, Patel VB, Wang Z, Levasseur J, Kaufman S, Penninger JM, Oudit GY. Angiotensin-converting enzyme 2 antagonizes angiotensin II-induced pressor response and NADPH oxidase activation in Wistar-Kyoto rats and spontaneously hypertensive rats. Exp Physiol 2013; 98:109–122. [DOI] [PubMed] [Google Scholar]
- 40. Velkoska E, Dean RG, Griggs K, Burchill L, Burrell LM. Angiotensin-(1-7) infusion is associated with increased blood pressure and adverse cardiac remodelling in rats with subtotal nephrectomy. Clin Sci (Lond) 2011; 120:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]