Abstract
BACKGROUND
Although numerous recent studies have shown a strong link between inflammation and hypertension, the underlying mechanisms by which inflammatory cytokines induce hypertension remain to be fully elucidated. Hypertensive disorders are also associated with elevated pressor sensitivity. Tissue transglutaminase (TG2), a potent cross-linking enzyme, is known to be transcriptionally activated by inflammatory cytokines and stabilize angiotensin II (Ang II) receptor AT1 (AT1R) via ubiquitination-preventing posttranslational modification. Here we sought to investigate the TG2-mediated AT1R stabilization in inflammation-induced hypertension and its functional consequences with a focus on receptor abundance and Ang II responsiveness.
METHODS AND RESULTS
Using an experimental model of inflammation-induced hypertension established by introducing the pro-inflammatory tumor necrosis factor cytokine LIGHT, we provide pharmacologic and genetic evidence that TG2 is required for LIGHT-induced hypertension (systolic pressure on day 6: LIGHT = 152.3 ± 7.4 vs. LIGHT+ERW1041E [TG2 inhibitor] = 105.8 ± 13.1 or LIGHT+TG2−/− = 114.3 ± 4.3 mm Hg, P < 0.05, n = 4–5) and renal compromise (urine albumin/creatinine: LIGHT = 0.17 ± 0.05 vs. LIGHT+ERW1041E = 0.03 ± 0.01 or LIGHT+TG2−/− = 0.06 ± 0.01 mg/mg; plasma creatinine: LIGHT = 1.11 ± 0.04 vs. LIGHT+ERW1041E = 0.94 ± 0.04 or LIGHT+TG2−/− = 0.88 ± 0.09 mg/dl; urine volume: LIGHT = 0.23 ± 0.1 vs. LIGHT+ERW1041E = 0.84 ± 0.13 or LIGHT+TG2−/− = 1.02 ± 0.09 ml/24 hour on day 14, P < 0.05, n = 4–5). Our mechanistic studies showed that the TG2-mediated AT1R modification and accumulation (relative renal AT1R level: phosphate-buffered saline [PBS] = 1.23 ± 0.22, LIGHT = 3.49 ± 0.37, and LIGHT+ERW1041E = 1.77 ± 0.46, P < 0.05, n = 3; LIGHT+TG2+/+ = 85.28 ± 36.11 vs. LIGHT+TG2−/− = 7.01 ± 5.68, P < 0.05, n = 3) induced by LIGHT is associated with abrogated β-arrestin binding (AT1R/associated β-arrestin ratio: PBS = 2.62 ± 1.07, LIGHT = 38.60 ± 13.91, and LIGHT+ERW1041E = 6.97 ± 2.91, P < 0.05, n = 3; LIGHT+TG2+/+ = 66.43 ± 44.81 vs. LIGHT+TG2−/− = 2.45 ± 1.78, P < 0.01, n = 3) and could be found in renal medulla tubules of kidneys (relative tubular AT1R level: PBS = 5.91 ± 2.93, LIGHT = 92.82 ± 19.54, LIGHT+ERW1041E = 28.49 ± 11.65, and LIGHT+TG2−/− = 0.14 ± 0.10, P < 0.01, n = 5) and the blood vasculature (relative vascular AT1R level: PBS = 0.70 ± 0.30, LIGHT = 13.75 ± 2.49, and LIGHT+ERW1041E = 3.28 ± 0.87, P < 0.01, n = 3), 2 of the tissues highly related to the genesis of hypertension. Our in vitro cellular assays showed that LIGHT stimulation triggered a rapid TG2-dependent increase in the abundance of AT1Rs (relative AT1R level after 2-hour LIGHT treatment: AT1R (WT)+TG2 = 2.21 ± 0.23, AT1R (Q315A)+TG2 = 0.18 ± 0.23, P < 0.05 vs. starting point = 1, n = 2) and downstream calcium signaling (fold increase in NFAT-driven luciferase activity: Saline = 0.02 ± 0.03, Ang II = 0.17 ± 0.08, LIGHT = 0.05 ± 0.04, LIGHT+Ang II = 0.90 ± 0.04 (P < 0.01 vs. Ang II), and LIGHT+Ang II+ERW1041E = 0.15 ± 0.15 (P < 0.01 vs. LIGHT+Ang II), n = 3).
CONCLUSIONS
Our data indicate an essential and systemic role for TG2 in bridging inflammation to hypertension via its posttranslational modifications stabilizing AT1 receptor and sensitizing Ang II. Our findings also suggest that TG2 inhibitors could be used as a novel group of cardiovascular agents.
Keywords: ACE–angiotensin receptors–renin angiotensin system, blood pressure, GPCR, hypertension, inflammation, tissue transglutaminase
Hypertension is a major risk factor for cardiovascular disorders in millions of individuals worldwide.1–3 Research has traditionally focused on the renin–angiotensin–aldosterone system, renal function, endothelial cells, sympathetic nervous system, and genetics4 to understand the multitude of factors contributing to hypertension. More recently, the contribution of pro-inflammatory cytokines that are significantly elevated in multiple hypertensive conditions has been recognized. These cytokines include interleukin-1β,5 tumor necrosis factors,6,7 interleukin-6,8 and interleukin-17.9 A causal role for these cytokines in hypertension is supported by experiments with rodents showing that introduction of these cytokines results in increased blood pressure.7,10–14 The essential and intrinsic role of inflammation in hypertension has been summarized and highlighted in several recent reviews.15–19 Despite considerable evidence that inflammatory cytokines cause hypertension, the molecular mechanisms underlying cytokine-induced hypertension remain to be fully elucidated.
To understand how cytokines cause hypertension, we developed an experimental model of hypertension established by the introduction of the pro-inflammatory cytokine LIGHT, a member of the tumor necrosis factor superfamily (TNFSF14).7 LIGHT triggers inflammatory responses by activating 2 widely distributed receptors, the herpes virus entry mediator20 and the lymphotoxin β receptor,21,22 upstream of the NFκB signaling pathway.23,24 Using this experimental model of inflammation-induced hypertension, we discovered that transglutaminase is a critical link in this process.25 Transglutaminases are a family of cross-linking enzymes that catalyze the formation of inter- or intramolecular ε-(γ-glutamyl)-lysine isopeptide bonds or the incorporation of primary amines on peptide-bound glutamine residues in a calcium-dependent manner.26–29 Among the transglutaminase enzyme family, tissue transglutaminase (TG2) is the most ubiquitous member and present in most cell types.28 The potent enzyme is involved in multiple cardiovascular disorders including atherosclerosis,30–33 cardiac hypertrophy,34,35 and chronic kidney disease/renal fibrosis36–38 by cross-linking extracellular matrix proteins.39–42 Our previous study also found TG2 plays an essential role in an autoantibody-induced model of preeclampsia (pregnancy-induced hypertension) in mice and stabilizes angiotensin II (Ang II) receptor AT1 (AT1R) via ubiquitination-preventing posttranslational isopeptide modification.43 Of note, β-arrestin, the key adaptor protein in the desensitization of G-protein-coupled receptors (GPCRs), has long been shown to participate in the receptor ubiquitination process.44 These facts drove us to hypothesize that in LIGHT-induced hypertension, the TG2-mediated posttranslational modification (PTM) of AT1Rs may impair the receptor’s desensitization process, finally contributing to increased Ang II sensitivity, a phenomenon consistently observed in multiple hypertensive patient groups and animal models.45–49 In this study, we present evidence that TG2 is an essential and systemic contributor to the inflammatory cytokine LIGHT-induced hypertension via PTMs that stabilize AT1 receptors and result in increased Ang II sensitivity.
METHODS
Please see Supplementary Data.
RESULTS
TG2 is required for LIGHT-induced hypertension and renal impairment
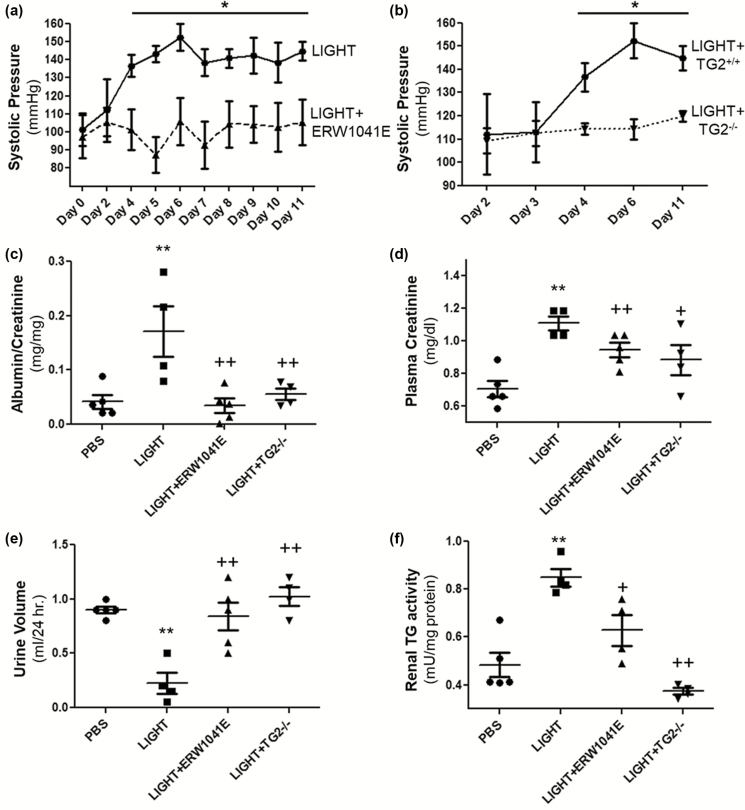
To specifically address the role of TG2 in LIGHT-induced hypertension, we inhibited its enzyme activity with the specific inhibitor ERW1041E or abrogated its expression with genetic ablation in the animal model. LIGHT-induced increase in blood pressure was significantly ameliorated in mice co-treated with ERW1041E, as measured by tail-cuff plethysmography (Figure 1a). Consistent with the finding from the Ang II model,50 TG2-deficient mice are also resistant to the LIGHT-induced increase in blood pressure (Figure 1b). These results indicate an essential role for TG2 in LIGHT-induced hypertension.
Figure 1.
TG2 is required for LIGHT-induced hypertension and renal dysfunction. TG2 inhibitor ERW1041E treatment (a) or genetic ablation (b) significantly attenuated LIGHT-induced increase in blood pressure. Systolic blood pressure of mice injected with LIGHT (4 ng/day) in the presence or absence of the TG2 inhibitor ERW1041E (0.125 mg/day) or genetic ablation was determined on the days indicated by tail cuff plethysmography. (*P < 0.05 vs. LIGHT+ERW1041E or LIGHT+ TG2−/−; n = 4 or 5 mice per group). TG2 inhibitor ERW1041E treatment or genetic ablation also significantly ameliorated LIGHT-induced proteinuria (c), plasma creatinine accumulation (d), urine retention (e), and renal TGase activation (f) (**P < 0.01 vs. PBS; +P < 0.05, ++P < 0.01 vs. LIGHT). All mice in panels c–f were treated for 14 consecutive days prior to measurements (n = 4 or 5 mice per group). Abbreviations: PBS, phosphate-buffered saline; TG2, tissue transglutaminase.
Because renal impairment is associated with the progression of hypertensive disorders, we next looked into renal functions in the treated mice. LIGHT treatment resulted in severe albuminuria that was evident at the end of the 14-day treatment compared with the control mice treated with phosphate-buffered saline vehicle. In contrast, the ERW1041E treatment or TG2 ablation significantly attenuated the LIGHT-induced albuminuria (Figure 1c). In addition to proteinuria, a decline in renal function is usually associated with elevated plasma creatinine and reduced urine volume.51 Consistent with the proteinuria data, the LIGHT-induced plasma creatinine accumulation and reduction in urine volume were significantly ameliorated in the ERW1041E-treated mice and TG2-deficient mice (Figure 1d and e). Altogether, these results indicate that TG2 is required for LIGHT-induced renal impairment.
To further address the involvement of renal TG2 in these processes, we then assessed the effect of TG2 inhibition or genetic ablation on LIGHT-induced increase in renal TGase activity. A significant repression in LIGHT-induced renal TGase activity was observed in ERW1041E-treated mice or TG2-deficient mice compared to controls treated with LIGHT only (Figure 1f) as measured by in vitro TGase assay, indicating TG2 as a major source or requirement for the LIGHT-induced increase in kidney TGase activation. Altogether, the pharmacologic and genetic data presented in Figure 1 suggest an essential role of TG2 in LIGHT-induced hypertension and renal compromise.
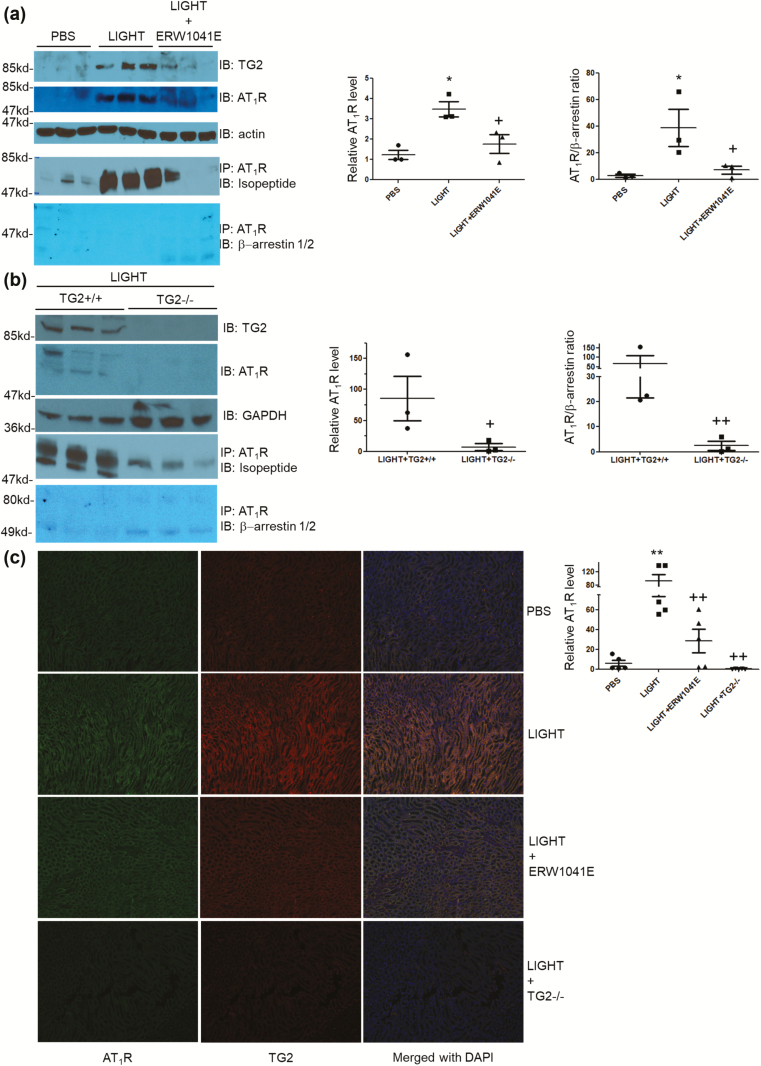
LIGHT induces TG2-dependent accumulation of AT1 receptor dissociated with β-arrestin in kidneys
To determine if the elevated TG2 in kidneys of LIGHT-treated mice25 resulted in the TG2-mediated accumulation of AT1Rs,43 we measured the relative receptor abundance in the kidneys of LIGHT-treated mice with or without ERW1041E treatment or TG2 ablation. Western blot analysis showed that there was a significant increase in renal AT1Rs in LIGHT-treated animals compared to phosphate-buffered saline controls (Figure 2a). Treatment with TG2 inhibitor ERW1041E or genetic ablation of TG2 abrogated the LIGHT-induced AT1R accumulation in kidneys (Figure 2a and b). To determine if the elevated AT1Rs were modified by TG2 and resistant to β-arrestin-mediated desensitization, we immunoprecipitated AT1Rs from the renal lysates of the LIGHT-treated mice (Figure 2a and b). From the pull-down products, we found an increased level of AT1Rs with TGase-mediated isopeptide modification and abrogated β-arrestin association in LIGHT-treated animals that was repressed by either ERW1041E treatment or TG2 ablation (Figure 2a and b), suggesting that TG2 played a crucial role in LIGHT-induced AT1 receptor accumulation and sensitization in kidneys. Using immunofluorescent staining, we co-localized increased TG2 and AT1Rs in the renal medulla tubules of LIGHT-treated animals but not in mice co-treated with ERW1041E or in mice with genetic ablation of TG2 (Figure 2c). Taken together, data presented in Figure 2 indicate that activation/elevation of TG2 in the renal medulla leads to accumulation of AT1Rs with TG2-mediated isopeptide modification.
Figure 2.
TG2-mediated LIGHT-induced AT1 receptor accumulation and dissociation with β-arrestin in kidneys. (a) LIGHT treatment resulted in a significant increase in renal AT1 receptors with TG2 modification, and dissociated from β-arrestin that was attenuated by TG2 inhibitor ERW1041E. (b) LIGHT-induced increase in AT1 receptor abundance, isopeptide modification, and dissociation with β-arrestin was abrogated in TG2−/− mice. (c) Increased TG2 and AT1Rs were co-localized in renal medulla tubules of LIGHT-treated animals, but not those co-treated with ERW1041E or with TG2 genetic ablation (n = 5 in each group). (*P < 0.05, **P < 0.01 vs. PBS; +P < 0.05, ++P < 0.01 vs. LIGHT). Abbreviations: PBS, phosphate-buffered saline; TG2, tissue transglutaminase.
Besides renal dysfunction, vascular perturbation has long been recognized as another major contributor to the genesis of hypertension. Thus in this study, we also examined the TG2-mediated AT1R accumulation in blood vessels using immunofluorescent staining. As shown in Supplementary Figure 1, LIGHT treatment resulted in TG2-dependent AT1R elevation in pulmonary vasculature. Interestingly, we also observed a TG2-dependent AT1R accumulation in the smooth muscle cell layer of the bronchial airways of LIGHT-treated animals, indicating the event’s ubiquitous presence in cells with AT1R and TG2 expression.
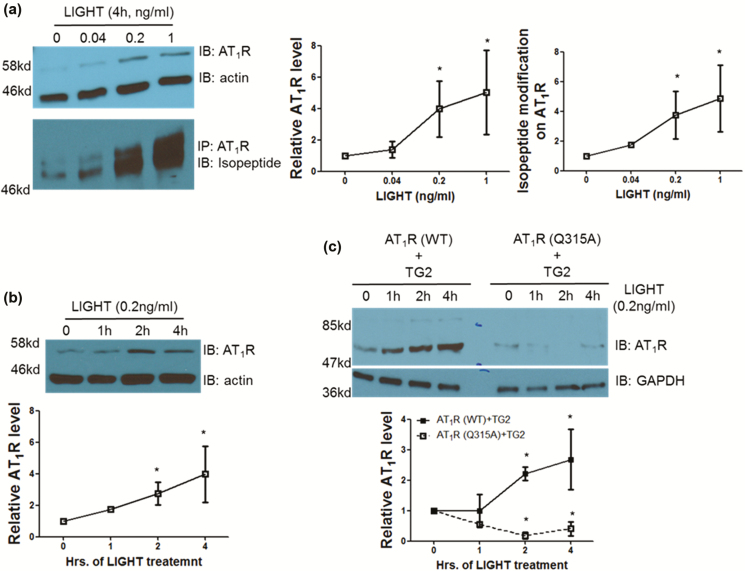
LIGHT stimulation causes TG2-dependent AT1 receptor accumulation and Ang II sensitization
To investigate the kinetics of the TG2-dependent AT1R accumulation induced by LIGHT, we carried out studies with the human trophoblast cell line, HTR-8/SVneo, which is characterized by high expression levels of both AT1Rs and TG243 mimicking their upregulation in the renal tubular or vascular cells exposed to inflammation and/or hypoxia. And these cells also express membrane receptors for LIGHT, the herpes virus entry mediator and the lymphotoxin β receptor.7 Our results showed a dose-dependent LIGHT-induced accumulation of AT1Rs that started to peak at 0.2 ng/ml LIGHT after a 4-hour treatment and was accompanied with an increase in the level of ε-(γ-glutamyl)-lysine isopeptide modification of the receptor (Figure 3a). Using the peak dose of 0.2 ng/ml LIGHT, we next observed an increase in the abundance of AT1Rs after 2 hours in a time-course treatment (Figure 3b). To determine if the LIGHT-induced accumulation of AT1Rs depended on TG2 modification, we provided similar LIGHT stimulation to Chinese hamster ovary cells overexpressing TG2 and either wild-type or the Q315A mutant AT1R in which the glutamine residue in the cytoplasmic tail of the receptor for TG2 modification is replaced with an alanine.43,52 LIGHT stimulation resulted in a time-dependent accumulation (Figure 3c) of AT1Rs in cells overexpressing wild-type receptors but not the Q315A mutant where exacerbated ubiquitination-dependent receptor degradation could be observed with the help of proteasome inhibitor MG132 (data not shown). Taken together, these results show that LIGHT stimulation causes a TG2-mediated increase in the abundance of AT1Rs by interfering with their ubiquitination-dependent degradation.
Figure 3.
LIGHT stimulation stabilizes AT1 receptor via TG2-mediated isopeptide modification at Q315. (a) LIGHT stimulation stabilized AT1 receptor with ε-(γ-glutamyl)-lysine isopeptide modification in a dose-dependent fashion in HTR cells (*P < 0.05 vs. 0; n = 2–3). (b) LIGHT-induced AT1 receptor stabilization peaked within 2-hour treatment in HTR cells (*P < 0.05 vs. 0; n = 2–3). (c) LIGHT-induced AT1R accumulation was abolished in Chinese hamster ovary cells overexpressing TG2 and AT1R Q315A mutant but not those overexpressing TG2 and wild-type AT1R (*P < 0.05 vs. 0, n = 2). Abbreviations: PBS, phosphate-buffered saline; TG2, tissue transglutaminase.
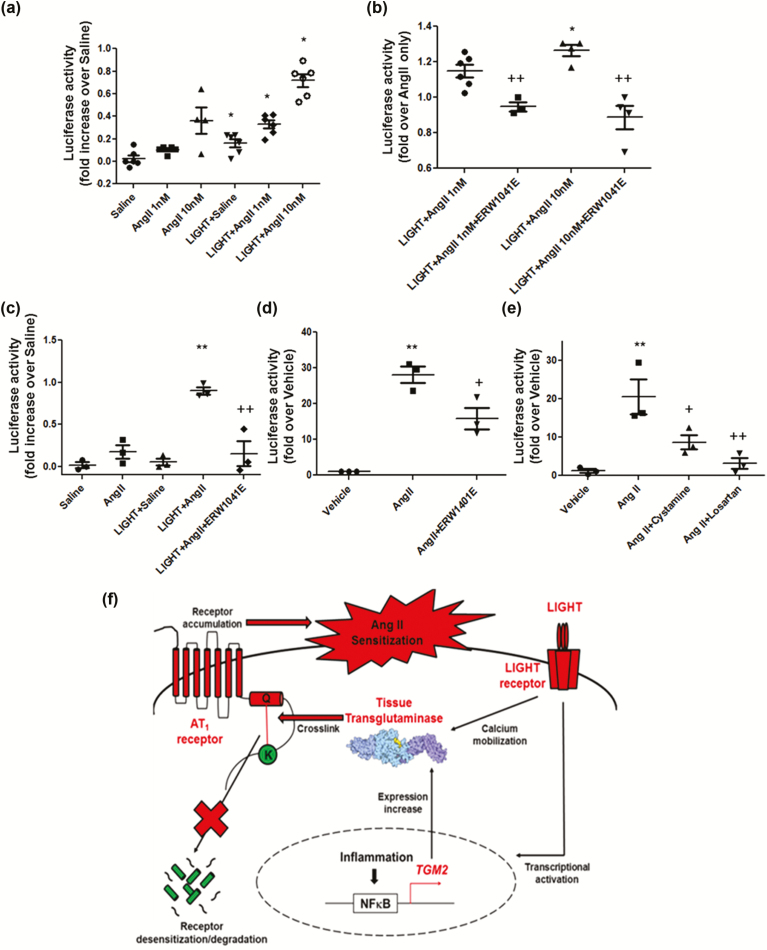
To explore the functional consequences of LIGHT-induced TG2 modifications on AT1R signaling, we assessed the Ang II-induced downstream calcium response by measuring luciferase activity in AT1R-NFAT-luciferase reporter cells.53 Initially, the reporter cells were incubated with or without LIGHT (200 pg/ml) for 2 hours followed by overnight incubation with Ang II (1 or 10 nM). The luciferase assay results (Figure 4a) indicate that preincubation with LIGHT resulted in a significantly higher calcium response following Ang II stimulation. TG2 inhibitor ERW1041E co-pretreatment was able to prevent the increase in the follow-up Ang II responsiveness (Figure 4a). Prolonged incubation with LIGHT at a lower dose (50 pg/ml, overnight) resulted in a more pronounced enhancement of the low-dose (1 nM) Ang II-induced luciferase expression (Figure 4b). Importantly, this LIGHT-induced hypersensitivity to Ang II was prevented by the TG2 inhibitor ERW1041E (Figure 4c), indicating the crucial role of LIGHT-induced TG2 modifications in this process. To further assess the endogenous role of TG2 in AT1R signaling, we treated the reporter cells with a high-dose Ang II (100 nM) in the presence or absence of TG2 inhibitor ERW1041E or the pan-TGase inhibitor cystamine. The results (Figure 4d and e) show that Ang-II-induced calcium signaling was significantly inhibited by ERW1401E or cystamine, suggesting that TG2 may function as a downstream enhancer in the endogenous Ang II–AT1R signaling pathway.
Figure 4.
TG2 contributes to LIGHT-induced Ang II sensitization. (a) Ang II-induced calcium response was significantly pronounced in AT1R-NFAT-luciferase reporter cells pretreated with LIGHT (200 pg/ml) for 2 hours (n = 4–6; *P < 0.05 vs. treatment w/o LIGHT pretreatment; duration of Ang II incubation is overnight at the indicated dose). (b) TG2 inhibitor ERW1041E inhibited the enhanced Ang II response triggered by 2-hour LIGHT (200 pg/ml) pretreatment (n = 3–6; *P < 0.05 vs. LIGHT+AngII 1nM; ++P < 0.01 vs. w/o ERW1041E (10 μM) co-pretreatment; duration of Ang II incubation is overnight at the indicated dose). (c) Low-dose Ang II (1 nM)-induced calcium response was enhanced by low-dose LIGHT (50 pg/ml) but not low-dose LIGHT (50 pg/ml) plus TG2 inhibitor ERW1041E (10 μM) co-incubation (n = 3, **P < 0.01 vs. Ang II; ++P < 0.01 vs. LIGHT+Ang II; duration of all the indicated treatments is overnight). TG2 inhibitor ERW1041E (200 μM) (d) or TGase inhibitor cystamine (500 μM) (e) significantly attenuated high-dose Ang II (100 nM)-induced calcium signaling in AT1R-NFAT-luciferase reporter cells (n = 3, **P < 0.01 vs. Vehicle; +P < 0.05, ++P < 0.01 vs. Ang II; duration of all the indicated treatments is overnight; losartan = 2 μM). (f) Mechanistic model for a role of TG2 in LIGHT-induced cardio-renal syndrome: By activating its membrane receptors, the TNF cytokine LIGHT transcriptionally increases TGM2 gene expression and/or directly activates TG2 via calcium mobilization. TG2-mediated modification of AT1Rs at Q315 impairs receptor desensitization/proteasomal degradation, contributing to Ang II sensitization. Abbreviations: Ang II, angiotensin II; TG2, tissue transglutaminase; TNF, tumor necrosis factor.
DISCUSSION
In this report, we provide pharmacologic and genetic evidence that TG2 is required for pro-inflammatory cytokine LIGHT-induced hypertension. Our mechanistic studies indicated that the TG2-mediated AT1R modification and accumulation induced by LIGHT is associated with abrogated β-arrestin binding and could be found in renal medulla tubules of kidneys and the blood vasculature. In vitro cellular assays showed that the TG2-dependent accumulation of AT1Rs contributes to Ang II sensitization. Taken together, our findings reveal a previously unrecognized role of the inflammatory signals in elevating pressor sensitivity via TG2-mediated PTMs. Our results may also suggest a systemic mechanism of TG2-mediated enhancement of AT1R signaling in hypertensive disorders linked with inflammation.
Kidney is one of the central organs in the regulation of blood pressure, and its malfunction results in hypertension.54–56 Our previous results indicate that renal TGase activation is associated with hypertension and renal impairment induced by LIGHT. We report here that LIGHT treatment caused upregulation of TG2 and increased modification of AT1Rs in the renal medulla (Figure 2) where a hypoxic environment may be maintained or even pronounced.57,58 The presumably hypoxic and inflammatory environment of renal medulla of LIGHT-treated animals stimulates the activation of TG2 that in turn resulted in AT1R accumulation. Of note, over-activation of renal renin–angiotensin–aldosterone machinery is considered as a prominent contributing factor of essential hypertension,59–61 and perturbation in medullary tubules has also been recognized as the driving force of salt and water retention. In this way, enhanced Ang II signaling caused by TG2-mediated AT1R accumulation in medullary tubules may contribute to the downstream outputs favoring hypertension. Consistently, LIGHT-induced accumulation of TG2-modified AT1Rs in kidneys was associated with impaired renal function as evident from increased urine retention, albuminuria, and elevated plasma creatinine, and the renal impairment was prevented in TG2-deficient mice or in mice treated with the TG2 inhibitor ERW1041E. Besides kidney, previous studies from us and others25,50,62 suggest the possible involvement of vascular TG2 in hypertension, and the TG2-dependent AT1R accumulation was also found in endothelial and smooth muscle cells of the vasculature (Supplementary Figure 1). Given the ubiquitous presence of Ang II–AT1R signaling axis among related organ/tissues including kidney, vasculature,63 T lymphocytes,64 and central nervous system,65 our findings may suggest a TG2-mediated general mechanism downstream of inflammation for the genesis of hypertension. Mice with cell-type-specific deletions will be especially important to determine the cellular source of TG2 that is required for LIGHT-induced pathophysiology.
Elevated pressor sensitivity is consistently observed among multiple hypertensive patient groups and animal models,45–49 and the contributing role of inflammatory cytokines in this process has been noticed.66,67 Also, people have long been intrigued by the accumulation of pressor receptors including alpha-adrenergic receptor68–71 and AT1R72 in hypertensive animals. Together with the studies50 addressing the role of TG2 in vascular remodeling and endothelial function, our studies might provide a mechanistic insight into the intrinsic link among these phenomena. We found that the inflammatory cytokine LIGHT treatment leads to a TG2-dependent elevation of AT1R with abrogated β-arrestin association in kidneys. To explore its functional consequences, we used overexpression cell lines to mimic the cell types with high levels of AT1R and TG2. With them, we showed that the rapid TG2-dependent accumulation of AT1Rs induced by LIGHT is associated with elevation of Ang II sensitivity. As a 7 transmembrane receptor, AT1R is regulated by GPCR kinases (GRKs) and β-arrestin-mediated desensitization.73 The increased ligand sensitivity and abrogated arrestin association suggest the inhibitory effect of TG2-mediated receptor modification on the desensitization process. The TG2-dependent AT1R accumulation further supports this concept if β-arrestin-mediated receptor ubiquitination could be considered as a long-term outcome of the desensitization.44 Consistently, transglutaminase inhibitors dampened Ang II-induced signaling response. Taken together, these results are suggesting an intrinsic role of TG2 in the maintenance of endogenous GPCR signaling.
We have recently shown that LIGHT-induced TG2 triggers the production of AT1R activating autoantibodies (AT1-AA) that contribute to hypertension and renal impairment. These autoantibodies were originally observed in women with preeclampsia and subsequently observed in other hypertensive conditions. These pathogenic autoantibodies (in humans and mice) recognize a common epitope, AFHYESQ, present on the second extracellular loop of the AT1R. Thus, one possible mechanism by which LIGHT-induced TG2 contributes to hypertension is by PTM of the epitope glutamine (Q187) thereby creating a neoantigen that contributes to AT1-AA production via the adaptive immune response. However, this process requires activation of the adaptive immune system, and is expected to take longer time. Another, more rapid mechanism by which TG2 may contribute to LIGHT-induced hypertension is by PTM of AT1Rs at Q315. Modification of this glutamine by TG2 prevents ubiquitin-dependent proteasomal degradation, thereby allowing AT1Rs to accumulate quickly (~2 hours). Modification at this site also blocks β-arrestin binding, resulting in prolonged receptor activation. Increased AT1R abundance and reduced β-arrestin binding (due to TG2-mediated PTM at Q315) occur more rapidly than the extended time required to activate the adaptive immune system to produce AT1-AA (due to TG2-mediated PTM at Q187). It is also noteworthy that the TG2-mediated receptor modification at Q315 may enhance the autoimmune response because of increased receptor abundance. Moreover, our recent studies43 also suggest that AT1-AA is able to generate biased downstream signaling favoring receptor accumulation. Taken together, our data suggest a pathogenic vicious cycle is formed between the receptor accumulation and autoantibody generation under inflammatory conditions.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by National Institutes of Health (HL137990), UTHealth Pulmonary Center of Excellence Discovery Award and National Natural Science Foundation of China fund (81629003) to Y.X. and the Bob and Hazel Casey Endowed Chair in Biochemistry to R.E.K.
REFERENCES
- 1. Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med 1993; 153:598–615. [DOI] [PubMed] [Google Scholar]
- 2. Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001; 345:1291–1297. [DOI] [PubMed] [Google Scholar]
- 3. Wang W, Lee ET, Fabsitz RR, Devereux R, Best L, Welty TK, Howard BV. A longitudinal study of hypertension risk factors and their relation to cardiovascular disease: the Strong Heart Study. Hypertension 2006; 47:403–409. [DOI] [PubMed] [Google Scholar]
- 4. Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J, Hall ME. Hypertension: physiology and pathophysiology. Compr Physiol 2012; 2:2393–2442. [DOI] [PubMed] [Google Scholar]
- 5. Dalekos GN, Elisaf MS, Papagalanis N, Tzallas C, Siamopoulos KC. Elevated interleukin-1 beta in the circulation of patients with essential hypertension before any drug therapy: a pilot study. Eur J Clin Invest 1996; 26:936–939. [DOI] [PubMed] [Google Scholar]
- 6. Xu W, Yang Q, Chen H. [Tumor necrosis factor in pregnancies associated with pregnancy induced hypertension]. Zhonghua Fu Chan Ke Za Zhi 1997; 32:9–11. [PubMed] [Google Scholar]
- 7. Wang W, Parchim NF, Iriyama T, Luo R, Zhao C, Liu C, Irani RA, Zhang W, Ning C, Zhang Y, Blackwell SC, Chen L, Tao L, Hicks MJ, Kellems RE, Xia Y. Excess LIGHT contributes to placental impairment, increased secretion of vasoactive factors, hypertension, and proteinuria in preeclampsia. Hypertension 2014; 63:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension 2001; 38:399–403. [DOI] [PubMed] [Google Scholar]
- 9. Yao W, Sun Y, Wang X, Niu K. Elevated serum level of interleukin 17 in a population with prehypertension. J Clin Hypertens (Greenwich) 2015; 17:770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takahashi H, Nishimura M, Sakamoto M, Ikegaki I, Nakanishi T, Yoshimura M. Effects of interleukin-1 beta on blood pressure, sympathetic nerve activity, and pituitary endocrine functions in anesthetized rats. Am J Hypertens 1992; 5:224–229. [DOI] [PubMed] [Google Scholar]
- 11. Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens 2002; 15:170–175. [DOI] [PubMed] [Google Scholar]
- 12. Orshal JM, Khalil RA. Reduced endothelial NO-cGMP-mediated vascular relaxation and hypertension in IL-6-infused pregnant rats. Hypertension 2004; 43:434–444. [DOI] [PubMed] [Google Scholar]
- 13. LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension 2005; 46:1022–1025. [DOI] [PubMed] [Google Scholar]
- 14. Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res 2013; 97:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caillon A, Schiffrin EL. Role of inflammation and immunity in hypertension: recent epidemiological, laboratory, and clinical evidence. Curr Hypertens Rep 2016; 18:21. [DOI] [PubMed] [Google Scholar]
- 16. McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res 2015; 116:1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 2014; 126:267–274. [DOI] [PubMed] [Google Scholar]
- 18. Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension 2011; 57:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu C, Kellems RE, Xia Y. Inflammation, autoimmunity, and hypertension: the essential role of tissue transglutaminase. Am J Hypertens 2017; 30:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kwon BS, Tan KB, Ni J, Oh KO, Lee ZH, Kim KK, Kim YJ, Wang S, Gentz R, Yu GL, Harrop J, Lyn SD, Silverman C, Porter TG, Truneh A, Young PR. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem 1997; 272:14272–14276. [DOI] [PubMed] [Google Scholar]
- 21. Browning JL, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow EP, Hession C, O’Brine-Greco B, Foley SF, Ware CF. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell 1993; 72:847–856. [DOI] [PubMed] [Google Scholar]
- 22. Crowe PD, VanArsdale TL, Walter BN, Ware CF, Hession C, Ehrenfels B, Browning JL, Din WS, Goodwin RG, Smith CA. A lymphotoxin-beta-specific receptor. Science 1994; 264:707–710. [DOI] [PubMed] [Google Scholar]
- 23. Mackay F, Majeau GR, Hochman PS, Browning JL. Lymphotoxin beta receptor triggering induces activation of the nuclear factor kappaB transcription factor in some cell types. J Biol Chem 1996; 271:24934–24938. [DOI] [PubMed] [Google Scholar]
- 24. Marsters SA, Ayres TM, Skubatch M, Gray CL, Rothe M, Ashkenazi A. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem 1997; 272:14029–14032. [DOI] [PubMed] [Google Scholar]
- 25. Luo R, Liu C, Elliott SE, Wang W, Parchim N, Iriyama T, Daugherty PS, Tao L, Eltzschig HK, Blackwell SC, Sibai BM, Kellems RE, Xia Y. Transglutaminase is a critical link between inflammation and hypertension. J Am Heart Assoc 2016; 5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 2003; 4:140–156. [DOI] [PubMed] [Google Scholar]
- 27. Király R, Demény M, Fésüs L. Protein transamidation by transglutaminase 2 in cells: a disputed Ca2+-dependent action of a multifunctional protein. FEBS J 2011; 278:4717–4739. [DOI] [PubMed] [Google Scholar]
- 28. Gundemir S, Colak G, Tucholski J, Johnson GV. Transglutaminase 2: a molecular Swiss Army knife. Biochim Biophys Acta 2012; 1823:406–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eckert RL, Kaartinen MT, Nurminskaya M, Belkin AM, Colak G, Johnson GV, Mehta K. Transglutaminase regulation of cell function. Physiol Rev 2014; 94:383–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bowness JM, Venditti M, Tarr AH, Taylor JR. Increase in epsilon(gamma-glutamyl)lysine crosslinks in atherosclerotic aortas. Atherosclerosis 1994; 111:247–253. [DOI] [PubMed] [Google Scholar]
- 31. Haroon ZA, Wannenburg T, Gupta M, Greenberg CS, Wallin R, Sane DC. Localization of tissue transglutaminase in human carotid and coronary artery atherosclerosis: implications for plaque stability and progression. Lab Invest 2001; 81:83–93. [DOI] [PubMed] [Google Scholar]
- 32. Sumi Y, Inoue N, Azumi H, Seno T, Okuda M, Hirata K, Kawashima S, Hayashi Y, Itoh H, Yokoyama M. Expression of tissue transglutaminase and elafin in human coronary artery: implication for plaque instability. Atherosclerosis 2002; 160:31–39. [DOI] [PubMed] [Google Scholar]
- 33. Cho BR, Kim MK, Suh DH, Hahn JH, Lee BG, Choi YC, Kwon TJ, Kim SY, Kim DJ. Increased tissue transglutaminase expression in human atherosclerotic coronary arteries. Coron Artery Dis 2008; 19:459–468. [DOI] [PubMed] [Google Scholar]
- 34. Small K, Feng JF, Lorenz J, Donnelly ET, Yu A, Im MJ, Dorn GW II, Liggett SB. Cardiac specific overexpression of transglutaminase II (G(h)) results in a unique hypertrophy phenotype independent of phospholipase C activation. J Biol Chem 1999; 274:21291–21296. [DOI] [PubMed] [Google Scholar]
- 35. Shinde AV, Su Y, Palanski BA, Fujikura K, Garcia MJ, Frangogiannis NG. Pharmacologic inhibition of the enzymatic effects of tissue transglutaminase reduces cardiac fibrosis and attenuates cardiomyocyte hypertrophy following pressure overload. J Mol Cell Cardiol 2018; 117:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson TS, Fisher M, Haylor JL, Hau Z, Skill NJ, Jones R, Saint R, Coutts I, Vickers ME, El Nahas AM, Griffin M. Transglutaminase inhibition reduces fibrosis and preserves function in experimental chronic kidney disease. J Am Soc Nephrol 2007; 18:3078–3088. [DOI] [PubMed] [Google Scholar]
- 37. Shweke N, Boulos N, Jouanneau C, Vandermeersch S, Melino G, Dussaule JC, Chatziantoniou C, Ronco P, Boffa JJ. Tissue transglutaminase contributes to interstitial renal fibrosis by favoring accumulation of fibrillar collagen through TGF-beta activation and cell infiltration. Am J Pathol 2008; 173:631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin CH, Chen J, Zhang Z, Johnson GV, Cooper AJ, Feola J, Bank A, Shein J, Ruotsalainen HJ, Pihlajaniemi TA, Goligorsky MS. Endostatin and transglutaminase 2 are involved in fibrosis of the aging kidney. Kidney Int 2016; 89:1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bakker EN, Buus CL, Spaan JA, Perree J, Ganga A, Rolf TM, Sorop O, Bramsen LH, Mulvany MJ, Vanbavel E. Small artery remodeling depends on tissue-type transglutaminase. Circ Res 2005; 96:119–126. [DOI] [PubMed] [Google Scholar]
- 40. Eftekhari A, Rahman A, Schaebel LH, Chen H, Rasmussen CV, Aalkjaer C, Buus CL, Mulvany MJ. Chronic cystamine treatment inhibits small artery remodelling in rats. J Vasc Res 2007; 44:471–482. [DOI] [PubMed] [Google Scholar]
- 41. Santhanam L, Tuday EC, Webb AK, Dowzicky P, Kim JH, Oh YJ, Sikka G, Kuo M, Halushka MK, Macgregor AM, Dunn J, Gutbrod S, Yin D, Shoukas A, Nyhan D, Flavahan NA, Belkin AM, Berkowitz DE. Decreased S-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circ Res 2010; 107:117–125. [DOI] [PubMed] [Google Scholar]
- 42. Foote CA, Castorena-Gonzalez JA, Staiculescu MC, Clifford PS, Hill MA, Meininger GA, Martinez-Lemus LA. Brief serotonin exposure initiates arteriolar inward remodeling processes in vivo that involve transglutaminase activation and actin cytoskeleton reorganization. Am J Physiol Heart Circ Physiol 2016; 310:H188–H198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu C, Wang W, Parchim N, Irani RA, Blackwell SC, Sibai B, Jin J, Kellems RE, Xia Y. Tissue transglutaminase contributes to the pathogenesis of preeclampsia and stabilizes placental angiotensin receptor type 1 by ubiquitination-preventing isopeptide modification. Hypertension 2014; 63:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 2001; 294:1307–1313. [DOI] [PubMed] [Google Scholar]
- 45. Wambach G, Meiners U, Bönner G, Konrads A, Helber A. Cardiovascular and adrenal sensitivity to angiotensin II in essential hypertension. Klin Wochenschr 1984; 62:1097–1101. [DOI] [PubMed] [Google Scholar]
- 46. Wisgerhof M, Brown RD. Increased adrenal sensitivity to angiotensin II in low-renin essential hypertension. J Clin Invest 1978; 61:1456–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest 1973; 52:2682–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Webb RC, Johnson JC, Vander AJ, Henry JP. Increased vascular sensitivity to angiotensin ii in psychosocial hypertensive mice. Hypertension 1983; 5:I165–I169. [DOI] [PubMed] [Google Scholar]
- 49. Campese VM, Karubian F, Chervu I, Parise M, Sarkies N, Bigazzi R. Pressor reactivity to norepinephrine and angiotensin in salt-sensitive hypertensive patients. Hypertension 1993; 21:301–307. [DOI] [PubMed] [Google Scholar]
- 50. Savoia C, Arrabito E, Sada L, Michelini S, Pucci L, Briani M, Nicoletti C, Candi E, Schiffrin EL, Volpe M. Reduced vascular remodeling and improved endothelial function in transglutaminase 2 knock-out mice treated with angiotensin II. Hypertension (Dallas, Tex: 1979) 2013; 62:A45. [Google Scholar]
- 51. Clark WF, Sontrop JM, Macnab JJ, Suri RS, Moist L, Salvadori M, Garg AX. Urine volume and change in estimated GFR in a community-based cohort study. Clin J Am Soc Nephrol 2011; 6:2634–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. AbdAlla S, Lother H, Langer A, el Faramawy Y, Quitterer U. Factor XIIIA transglutaminase crosslinks AT1 receptor dimers of monocytes at the onset of atherosclerosis. Cell 2004; 119:343–354. [DOI] [PubMed] [Google Scholar]
- 53. Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension 2010; 55:386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guyton AC. Blood pressure control—special role of the kidneys and body fluids. Science 1991; 252:1813–1816. [DOI] [PubMed] [Google Scholar]
- 55. Navar LG. The role of the kidneys in hypertension. J Clin Hypertens (Greenwich) 2005; 7:542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Coffman TM, Crowley SD. Kidney in hypertension: guyton redux. Hypertension 2008; 51:811–816. [DOI] [PubMed] [Google Scholar]
- 57. Brezis M, Rosen S. Hypoxia of the renal medulla—its implications for disease. N Engl J Med 1995; 332:647–655. [DOI] [PubMed] [Google Scholar]
- 58. Haase VH. Mechanisms of hypoxia responses in renal tissue. J Am Soc Nephrol 2013; 24:537–541. [DOI] [PubMed] [Google Scholar]
- 59. Hall JE. Control of sodium excretion by angiotensin II: intrarenal mechanisms and blood pressure regulation. Am J Physiol 1986; 250:R960–R972. [DOI] [PubMed] [Google Scholar]
- 60. Granger JP, Schnackenberg CG. Renal mechanisms of angiotensin II-induced hypertension. Semin Nephrol 2000; 20:417–425. [PubMed] [Google Scholar]
- 61. Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension 2011; 57:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rautureau Y, Paradis P, Schiffrin EL. Transglutaminase 2 is a regulator of angiotensin II-induced ERK1/2 activation in vascular smooth muscle cells. Hypertension (Dallas, Tex: 1979) 2012; 60:A485. [Google Scholar]
- 63. Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 2002; 90:1205–1213. [DOI] [PubMed] [Google Scholar]
- 64. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 2007; 204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wei SG, Yu Y, Zhang ZH, Felder RB. Angiotensin II upregulates hypothalamic AT1 receptor expression in rats via the mitogen-activated protein kinase pathway. Am J Physiol Heart Circ Physiol 2009; 296:H1425–H1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zera T, Ufnal M, Szczepanska-Sadowska E. Central TNF-alpha elevates blood pressure and sensitizes to central pressor action of angiotensin II in the infarcted rats. J Physiol Pharmacol 2008; 59 (Suppl 8):117–121. [PubMed] [Google Scholar]
- 67. Xue B, Thunhorst RL, Yu Y, Guo F, Beltz TG, Felder RB, Johnson AK. Central renin-angiotensin system activation and inflammation induced by high-fat diet sensitize angiotensin II-elicited hypertension. Hypertension 2016; 67:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. U’Prichard DC, Greenberg DA, Snyder SH. CNS alpha-adrenergic receptor binding, studies with normotensive and spontaneously hypertensive rats. In Meyer P, Schmitt H (eds), Nervous System and Hypertension. Wiley: New York, 1979, pp. 38. [Google Scholar]
- 69. Graham RM, Pettinger WA, Sagalowsky A, Brabson J, Gandler T. Renal alpha-adrenergic receptor abnormality in the spontaneously hypertensive rat. Hypertension 1982; 4:881–887. [DOI] [PubMed] [Google Scholar]
- 70. Pettinger WA, Sanchez A, Saavedra J, Haywood JR, Gandler T, Rodes T. Altered renal alpha 2-adrenergic receptor regulation in genetically hypertensive rats. Hypertension 1982; 4:188–192. [PubMed] [Google Scholar]
- 71. Lin CI, Lu HH, Lin KY. Adrenergic receptors and increased reactivity of aortic smooth muscle in renal hypertensive rats. J Auton Nerv Syst 1982; 5:253–264. [DOI] [PubMed] [Google Scholar]
- 72. Zhuo J, Ohishi M, Mendelsohn FA. Roles of AT1 and AT2 receptors in the hypertensive Ren-2 gene transgenic rat kidney. Hypertension 1999; 33:347–353. [DOI] [PubMed] [Google Scholar]
- 73. Kohout TA, Lefkowitz RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol 2003; 63:9–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.