Abstract
BACKGROUND:
Mechanisms for persistent atrial fibrillation (AF) are unclear. We hypothesized that putative AF drivers and disorganized zones may interact dynamically over short time scales. We studied this interaction over prolonged durations, focusing on regions where ablation terminates persistent AF using 2 mapping methods.
METHODS:
We recruited 55 patients with persistent AF in whom ablation terminated AF prior to pulmonary vein isolation from a multicenter registry. AF was mapped globally using electrograms for 360±45 cycles using (1) a published phase method and (2) a commercial activation/phase method.
RESULTS:
Patients were 62.2±9.7 years, 76% male. Sites of AF termination showed rotational/focal patterns by methods 1 and 2 (51/55 vs 55/55; P=0.13) in spatially conserved regions, yet fluctuated over time. Time points with no AF driver showed competing drivers elsewhere or disordered waves. Organized regions were detected for 61.6±23.9% and 70.6±20.6% of 1 minute per method (P=nonsignificant), confirmed by automatic phase tracking (P<0.05). To detect AF drivers with >90% sensitivity, 8 to 32 s of AF recordings were required depending on driver definition.
CONCLUSIONS:
Sites at which persistent AF terminated by ablation show organized activation that fluctuate over time, because of collision from concurrent organized zones or fibrillatory waves, yet recur in conserved spatial regions. Results were similar by 2 mapping methods. This network of competing mechanisms should be reconciled with existing disorganized or driver mechanisms for AF, to improve clinical mapping and ablation of persistent AF.
CLINICAL TRIAL REGISTRATION:
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02997254.
Keywords: atrial fibrillation, mapping, network, patients, pulmonary vein, registries, tachycardia
Incomplete understanding of the mechanisms for persistent atrial fibrillation (AF) impacts therapy.1 Pulmonary vein isolation (PVI) remains the foundation of AF ablation, yet is not uniformly successful at eliminating AF and success may not be improved by ablation of complex electrograms or linear lesions.2 Organized AF drivers have been proposed as ablation targets, yet it is currently unclear how/why drivers fluctuate and if this reflects interaction with disordered waves.
We hypothesized that AF may exhibit rapid short-term interactions between regions of organized AF activity and disorganized waves that may explain why some studies show AF drivers, whereas others show disorder. Optical mapping in human AF4 and mapping studies at ablation5–8 provide evidence for drivers of human persistent AF, of which some are stable over time,6,7,9 whereas others are unstable5 and could be interpreted as disordered waves. Clinically, ablation at organized regions was promising in a recent metaanalysis,10,11 yet other studies have been negative.12–14
We set out to define spatiotemporal interactions between potential AF drivers and disorganized activity in human persistent AF in an international registry.15 We used global mapping of the atria, to reduce the possibility of missing dynamics in either atrium. To quantify trends in AF even given potential short-term fluctuations, we mapped for prolonged durations. Moreover, because differences in reported AF mechanisms may reflect mapping method,1 in each patient, we compared 2 methods reported to show disordered AF waves16 and drivers,17 respectively. Finally, as a clinical reference, we studied only patients in whom ablation at a documented site terminated persistent AF before PVI.
METHODS
Patient Inclusions
We identified 55 consecutive patients with persistent AF as part of the international COMPARE-AF registry (Comparison of Algorithms for Rotational Evaluation in Atrial Fibrillation) at 5 centers (Stanford University Hospital, Palo Alto, CA; Veterans Affairs Medical Center, San Diego, CA; University of Colorado, Denver, CO; Indiana University, Indianapolis, IN; and Klinikum Coburg, Coburg, Germany), in whom ablation in a documented region terminated persistent AF to sinus rhythm or atrial tachycardia (AT) before PVI. Persistent AF was defined by guidelines,2 and AF was refractory to ≥1 antiarrhythmic medication. We excluded patients in whom AF terminated to sinus rhythm or AT during PVI, linear lesions, or ablation of complex fractionated electrograms. The study was approved by each local Institutional Review Body. Online data, videos, and software code used in this project are available to other researchers for the purposes of reproducing the results or replicating the procedure on http://narayanlab.stanford.edu and as Data Supplement for this article.
Electrophysiological Study and Ablation
Patients were studied in the postabsorptive state. Classes I and III antiarrhythmic medications were discontinued for >5 half-lives (>30 days for amiodarone). Catheters were advanced to the right atrium, coronary sinus, and transseptally to left atrium (LA). Contact basket catheters (64 poles, FIRMap; Abbott) were positioned in right atrium, then LA for AF mapping, based on 3-dimensional electroanatomic imaging (NavX, St Jude Medical, Sylvar, CA; or Carto, Biosense Webster, Diamond Bar, CA).
Radiofrequency energy was delivered via an irrigated catheter (Thermocool, Biosense Webster; or Sapphire-Blue, St Jude Medical) at 25 to 35 W. All patients had prospective ablation at regions of interest identified by a commercial system (RhythmView; Abbott, Inc). This approach may reveal rotational or focal sites during AF and was compared in this study to an independent mapping algorithm.16,18,19 Lesions were applied for 15 to 30 s at each site, successively, to cover areas of 2 to 3 cm2 as described by Miller et al.9 PVI was then performed, comprising circumferential ablation of left and right PV pairs with verification of PV isolation using dedicated circular mapping catheters.
Each case in this series had termination of persistent AF, at sites marked prospectively on the shell relative to electrodes, for example, AB23 referencing an ablation site bracketed by electrodes A2, A3, B2, and B3. Examples are shown in Figures 1A and 2B.
Figure 1. Rotational activity at atrial fibrillation (AF) termination site, revealed by 2 mapping systems for most AF cycles over a minute.
In a 67-year-old woman with persistent AF, (A) atrial shell, where ablation at site A, (B) terminated persistent AF via atrial tachycardia (AT) to sinus rhythm (SR). C, Counterclockwise rotation at site A by both methods. Another site B showed clockwise rotation that was also continuous but did not perturb site A. Randomly selected control site C showed no rotations. D, Prevalence of rotations at site A for successive 4-s windows over 1 min of AF by both methods. At arrowed time points; Movie I in the Data Supplement shows continuous rotations at site A by both methods, and Movie II in the Data Supplement shows interrupted rotations by method 1 but continuous rotations by method 2. FIRM indicates focal impulse and rotor modulation; LAA, left atrial appendage; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; and RSPV, right superior pulmonary vein.
Figure 2. At atrial fibrillation (AF) termination site, rotational activity without competing organized sites.
In a 78-year-old man with persistent AF, (A) atrial shell on which ablation at site A, (B) terminated persistent AF. C, Clockwise rotation at site A by both mapping methods. No rotations are seen at control site C. D, Prevalence of rotations at site A for successive 4-s windows over 1 minute of AF by both methods. At arrowed time points, Movie III in the Data Supplement shows continuous rotations at site A by both methods; Movie IV in the Data Supplement shows interrupted rotations by method 1 but continuous rotations by method 2. FIRM indicates focal impulse and rotor modulation; LAA, left atrial appendage; LIPV, left inferior pulmonary vein; RIPV, right inferior pulmonary vein; and RSPV, right superior pulmonary vein.
Data Acquisition for Analysis
Unipolar electrograms were recorded at 0.05 to 500 Hz bandpass, at 1 kHz sampling with electroanatomic location turned off to reduce electromagnetic interference. Analysis used 1 minute of data initially focusing on the 4 s used clinically to guide ablation that terminated persistent AF. Raw electrograms comprising 64 basket and other intracardiac channels (eg, coronary sinus) and 12-lead ECG were exported from Bard (LabSystem Pro), Prucka (GE Cardiolab), or Siemens recorders for analysis. Raw basket recordings of AF are available online at http://narayanlab.stanford.edu.
AF Mapping Methods
Two AF mapping methods were applied to the same electrographic data: (1) an independent mapping approach,16,18,19 which was compared with (2) the clinical approach focal impulse and rotor modulation (FIRM)7,17 used prospectively.
Mapping Method 1
Mapping method 1 is based on a published phase approach for AF,16,18,19 selected because it avoids proprietary algorithms, does not use activation maps (the basis of clinical method number 2), and uses phase analysis yet minimizes false rotations20 (reporting <1% rotational sites in clinical studies with 1–2 cm2 epicardial plaques and in sheep AF16,18,19).
We coded the Kuklik/Schotten algorithm16,18,19 by first computing an average QRS complex for each electrogram channel that we subtracted from its parent channel. Next, we applied a 1.5 to 25 Hz fourth-order Butterworth band-pass filter and computed the dominant cycle length for each channel from the Welch Power Spectrum Density estimate (window 2000 ms, overlap 1000 ms, and cycle length cutoff 130–280 ms). The recomposed signal was constructed as a sum of single-period sinusoidal waves. The Hilbert transform was used to compute phase maps. This software is available online at https://narayanlab.stanford.edu (download tab).
Mapping Method 2
Mapping method 2 was used clinically (FIRM, Abbott) to guide AF termination in each case and has been described.7,17 This approach is distinct from method 1 as it displays activation maps of AF (after filtering electrograms using repolarization) as grayscale movies and uses phase as a secondary analysis to indicate regions of interest in red (rotational activity profiles). AF electrograms often show multiple deflections, and classical rules for marking activation may inadvertently tag far-field signals.21 FIRM attempts to reduce far-field tagging by analyzing onsets between each electrode and its neighbors, filtered to exclude activation times shorter than rate-adjusted human action potential duration (repolarization).22,23 If no signal is detected, that region remains black on maps. Reconstructing signals of normalized amplitude,22,23 phase is used to quantify rotations. In preliminary comparisons, FIRM identified microreentrant AF drivers detected on optical mapping of human atria.24
Quantification of AF Drivers and Fibrillatory Conduction: Clinical
In each case, blinded reading of AF maps prospectively identified sites where ablation may terminate AF, and 1 control site at randomly assigned grid coordinates remote from the termination site. Rotational activation was defined as reentry/spiral wave, that is, activation >75% of a full rotation (ie, 270°–360°) as in prior studies. A focal impulse was defined as an electrical origin of centrifugal activation, either uniform or anisotropic, during AF.
Rotational or focal patterns were identified in each 4-s mapping window if present for ≥3 cycles25 by 3 reviewers (F.S., S.H., C.A.B.K.). To allow for limited spatial meander, patterns repeating within ≤1 electrode were considered to represent precession (wobble)26 of the same organized AF pattern. Temporal prevalence of each organized AF region was calculated as the percentage of time for which it was present in a 4-s window (Figure 1D).
Quantification of AF Drivers and Fibrillatory Conduction: Computational
We automatically tracked phase singularities in clinical AF maps using software written in Matlab (Release 2015b; The MathWorks, Inc, Natick, MA).27,28 This nonsupervised analysis used signals from methods 1 and 2, applied the Hilbert transform then tracked AF singularity points spatially over time to define their domain of meander (Figure 3A). We quantified overlap in rotational activation at the AF termination site for 1 minute between methods 1 and 2, whose barycenter (center of precession over time) was ≤1 electrode from the termination site. The area covered by drivers was defined as all pixels inside this domain (Figure 3B). Proportional overlap was the area of overlap divided by the area from method 1 (Figure 3C).
Figure 3. Phase singularities in similar locations on automatic tracking.
A, Automatic phase singularity tracking along its atrial path for methods 1 (red) and 2 (blue). B, Area enclosed by the meandering path of both methods showed a proportional overlap of 65.7% between methods. C, Area overlap of phase singularities between both methods for the entire cohort was 79.6±23.1%. Four patients did not show organized activity by method 1.
Analyzing Fluctuations in AF Activation for 1 Minute
AF was analyzed for 1 minute, repeating all 4 s analyses >15× for ≈19 800 AF cycles in 55 patients. Analysis was performed by 3 observers (F.S., V.J., C.A.B.K.) and discrepancies resolved by consensus. Fluctuation analysis focused on sites of AF termination. We defined substantial fluctuations as >20% variations of a rotational or focal AF pattern, that is, twice our expected SD for an AF driver, between consecutive 4-s windows.
Statistical Analysis
Continuous data are represented as mean±SD or median and interquartile range as appropriate. Normality was evaluated using the Kolmogorov–Smirnov test. Comparisons between 2 groups were made with Student t tests and summarized with means and SD for independent samples if normally distributed (eg, age and LA diameter), or if not normally distributed, with the Mann–Whitney U test and summarized with medians and quartiles. Nominal values were expressed as n (%) and compared with χ2 tests (eg, hypertension, AF type, diabetes mellitus, and obstructive sleep apnea), the Fisher exact test when expected cell frequency was <5 (eg, sex). The McNemar test was used to test paired dichotomous comparisons, and the paired t test was used for paired comparisons with continuous variables. Pearson and point biserial correlation was applied for clinical, demographic correlations. A probability of <0.05 was considered statistically significant.
RESULTS
Table 1 shows demographics. Patients in whom AF terminated to AT (n=23) had higher rates of hypertension (P<0.01) and coronary disease (P<0.01), and lower left ventricular ejection fraction (P=0.04) than those terminating to sinus rhythm. No conversions to AT or sinus rhythm occurred before ablation.
Table 1.
Clinical Demographics
| N=55 | Entire Cohort | Terminations to Sinus Rhythm (n=32) | Terminations to Atrial Tachycardia (n=23) | P Value |
|---|---|---|---|---|
| Age, y | 62.2±9.7 | 61.8±9.8 | 62.8±9.4 | 0.71 |
| Male, n (%) | 42 (76) | 23 (72) | 19 (83) | 0.52 |
| Persistent AF, n (%) | 55 (100) | 32 (100) | 23 (100) | |
| Prior AF ablation, n (%) | 27 (49) | 17 (53) | 10 (43) | 0.59 |
| AF history, y | 3.94 (2.2–8.8) | 3.83 (2.2–8.8) | 3.94 (2.2–8.2) | 0.86 |
| Hypertension, n (%) | 43 (78.1) | 20 (62.5) | 23 (100) | <0.01 |
| Left atrial diameter, mm | 47.8±7.6 | 48.8±9.3 | 46.4±5.3 | 0.37 |
| LV ejection fraction, % | 52.1±12.3 | 56.4±10.0 | 46.2±15.4 | 0.04 |
| CHADSVASc score | 2 (1–3) | 2 (1–4) | 3 (2–3) | 0.42 |
| Coronary disease, n (%) | 14 (25.5) | 3 (21.9) | 11 (47.8) | <0.01 |
AF indicates atrial fibrillation; and LV, left ventricular.
AF Mapping at Sites of Termination
AF was disordered yet with regions of spatial organization in each patient. Using mapping method 1, each patient had 4.2±2.0 organized sites. By comparison, method 2 revealed 4.3±2.3 organized sites (P=non significant versus method 1).
Ablation terminated persistent AF before PVI in each patient (n=55). Sites at which ablation terminated AF were predominantly rotational by both methods (51 versus 55; P=0.13 methods 1 versus 2; Table 2) and showed significantly more organized rotational/focal activity than control sites for method 1 (55 versus 4; P<0.001) and method 2 (55 versus 5; P<0.001). Table I in the Data Supplement shows patient-level comparison of disordered, rotational, or focal activation patterns between mapping methods at sites of AF termination.
Table 2.
Atrial Fibrillation Mapping at Sites of Termination
| Characteristic | Entire Cohort | Terminations to Sinus Rhythm (n=32) | Terminations to Atrial Tachycardia (n=23) | P Value |
|---|---|---|---|---|
| Site of AF termination | ||||
| Left atrial/right atrial | 48/7 | |||
| Technique 1 (phase); no. of cycles/index map | N=55; 11.1±4.3 | |||
| No. sources (LA/RA) | (45/6) | (29/3) | (16/3) | 0.66 |
| Activation at AF term site | ||||
| Rotational | 48 | 30 | 18 | 0.16 |
| Focal | 3 | 2 | 1 | |
| Disordered (ie, no source) | 4 | 0 | 4 | |
| Technique 2 (activation/phase); no. of cycle/index map | N=55; 12.7±3.7 | |||
| No. sources (LA, RA) | (48/7) | (29/3) | (19/4) | 0.43 |
| Activation at AF term site | ||||
| Rotational | 52 | 30 | 22 | 0.96 |
| Focal | 3 | 2 | 1 | |
| Disordered (ie, no source) | 0 | 0 | 0 | |
AF indicates atrial fibrillation; LA, left atrium; and RA, right atrium.
Figure 1 illustrates persistent AF in a 67-year-old woman in whom ablation on the anterior LA roof (Figure 1A) terminated persistent AF to sinus rhythm before PVI (Figure 1B). Mapping method 1 (phase) identified counterclockwise rotational activation localized to the AF termination site, surrounded by disordered waves (Figure 1C, left). Mapping method 2 (activation+phase) also revealed rotational activity (Figure 1C, right). Movies I and II in the Data Supplement (from mapping methods 1 and 2) show rotational activation at the termination site for many cycles with surrounding fibrillatory conduction.
AF Patterns at Sites of Termination Can be Stable Over Time
The rotational AF pattern in Figure 1 was stable over time. Figure 1D quantifies its prevalence for 1 minute, which for method 1 (green) was 88.1±9.3% (15 consecutive 4-s windows). Driver stability was slightly higher but similar for method 2 (blue).
Notably, Figure 1C and Movies I and II in the Data Supplement reveal a second site of concurrent rotational activity in AF maps (site B) by both methods. Activity at site B was continuous (not plotted), yet dominated by rotations from site A (terminating site). The patient was AF and AT free at 1-year post ablation with ablation at organized AF sites and PVI.
Figure 2 (Movies III and IV in the Data Supplement) shows another patient with persistent AF in whom the site of AF termination showed rotational activation for the majority of cycles over 1 minute by both mapping methods.
Quantitative Comparison in AF Drivers by Both Mapping Methods
Both mapping methods identified qualitatively similar organized patterns at sites of AF termination as shown in Figures 1 and 2 (Table I in the Data Supplement shows per patient data).
Organized patterns at sites of AF termination occupied slightly shorter percentages of the mapped window by method 1 than 2 (Table 2; 11.1±4.3 versus 12.7±3.7 cycles for each method, respectively; P=0.04).
Spatial overlap in AF drivers is shown in Figure 3A, indicating automatic tracking of phase singularity for the case in Figure 1 for methods 1 (red) and 2 (blue). Area overlap of AF drivers for this patient was 65.7% (Figure 3B). On patient-by-patient analysis, Figure 3C illustrates area overlap for the entire cohort of 79.6±23.1% (in 4 patients, method 1 did not show drivers so overlap could not be computed).
AF Patterns at Sites of Termination May Fluctuate Because of Competing Organized Sites
Figure 4 illustrates persistent AF in a 49-year-old woman at first ablation. Figure 4A shows site A near the left superior pulmonary vein (Figure 4B) where targeted ablation terminated AF to sinus rhythm before commencing PVI. Ablation was guided to maps of rotational activation (Figure 4C).
Figure 4. Fluctuating rotational activation at atrial fibrillation (AF) termination site, because of intermittent reciprocal activity from competing organized site.
In a 49-year-old woman with persistent AF, (A) Atrial shell on which ablation at Site A, (B) terminated persistent AF. C, Clockwise rotational activation at site A by method 1 (phase). D, Inspection of separate time windows revealed clear focal activity emerging from site B. Randomly selected control site C showed no rotational activation. E, Prevalence of rotational activation at sites A (blue) and B (orange) for successive 4-s windows for 1 min of AF. Sites A/B were reciprocal, high prevalence at site A at times of low prevalence at site B and vice versa. Movie V in the Data Supplement shows a transition from rotations at site A to focal activation at site B. Abl indicates ablation; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; PVI, pulmonary vein isolation; RIPV, right inferior pulmonary vein; and RSPV, right superior pulmonary vein.
Rotational activity at the AF termination site in this case (site A) was not continuous over 1 minute. Figure 4E shows that rotations at site A fluctuated for successive 4-s time windows in 1 minute. Close inspection revealed a second site of focal activation with centrifugal emanation (Figure 4D) arising from site B on the opposite side of the LA (inferior; Figure 4A), and Figure 4E quantifies that appearance/disappearance of rotations at site B (orange) reciprocated with disappearance/appearance at site A (blue).
Movie V in the Data Supplement shows reciprocal dominance of sites A and B by both mapping methods. In Movie V in the Data Supplement, times 00:00 to 00:05 show rotations at site A and the focus at site B is not seen. This is followed by a transition toward site B dominance. From 00:20 to 00:30, focal activity from site B is seen, but rotations at site A are not. Clinically, site A was ablated first which terminated AF. Site B was then ablated in sinus rhythm, all before PVI. The patient is free of AF and AT off medications at 2 years.
In n=8 patients, the site of termination was the first ablation target. Overall, termination occurred at the 1.9±1.3th ablated site out of 2.8±1.4 in LA (3.3±1.8th overall).
Quantitative Analysis of Temporal Fluctuations at Spatial Sites of AF Termination
Figure 5 shows prevalence of organized AF activity (rotational/focal) at AF-terminating sites for successive 4-s windows over 1 minute (360±45 AF cycles) for each patient. Median prevalence ranged from 22.2% (interquartile range, 13.9%–83.3%) in patient number 1 to 100% (interquartile range, 100%–100%) in patient numbers 48 to 55. Forty-seven of 55 patients showed drivers for >50% of all mapped intervals.
Figure 5. Patient-level prevalence of organized activity patterns over 1 min for the entire cohort.
Columns indicate median presence for all 4-s windows during the minute. Error bars represent interquartile range. Forty-seven of 55 patients showed organized activity for >50% of 1 min at the site of atrial fibrillation (AF) termination by ablation.
There was no difference in organized site prevalence in patients whose AF terminated to sinus rhythm or AT by method 1 (37.1±10.5 versus 36.8±15.5 s/min; P=0.95) or method 2 (41.7±13.0 versus 43.3±9.0 s/min; P=0.61).
Impact of AF Fluctuations on Clinical Recording Duration to Detect Drivers
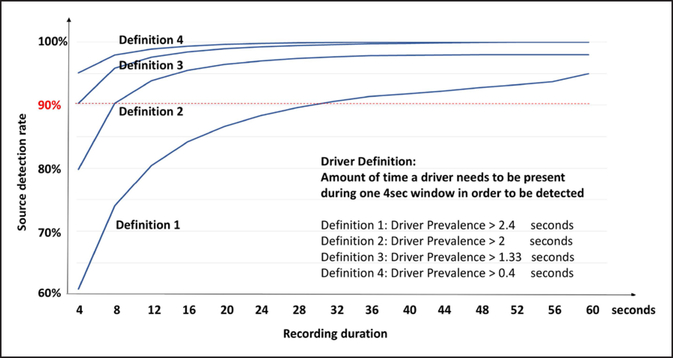
We hypothesized that fluctuations in organized AF sites may impact the duration of AF required to detect AF drivers. Figure 6 shows the probability of detecting an organized pattern at the site of AF termination for cumulative random 4-s time windows. We plotted 4 definitions of an AF driver: (1) ≥66% prevalence over time; (2) ≥50% prevalence over time; (3) ≥33% prevalence over time; and (4) ≥10% prevalence over time. For definition 1 (most stringent), 32 s of mapping would detect 90% of organized patterns at AF-terminating sites; for definitions 2 to 4 (less stringent), 4 to 8 s would be sufficient.
Figure 6. Recording duration to detect potential atrial fibrillation (AF) drivers at sites of termination varies with definition.
Ordinate shows detection rate of organized AF patterns, abscissa shows increasing cumulative durations of recording time as randomly selected (nonduplicated) 4-s windows for up to 1 min. Distributions are shown for 4 definitions of potential AF drivers. For definition 1 (≥66% prevalence), 32 s of AF achieves a 90% probability of detecting a potential AF driver. For definition 2 (≥50% prevalence), definition 3 (≥33% prevalence), and definition 4 (≥10% prevalence), 4 to 8 s of recording is sufficient
Clinical Correlations
Correlations were observed between clinical parameters and fluctuations in organized AF patterns. The number of time windows in which sources were identified correlated weakly with history of AF (r=0.31; P=0.02), prior ablation (r=0.46; P<0.01), and age (r=0.25; P=0.07). The number of organized sites was nonsignificantly and inversely associated with LA diameter (r=−0.26; P=0.06) and female sex (r=−0.16; P=0.24).
DISCUSSION
We reveal a potential pathophysiological network in AF exhibiting spatiotemporal interplay between organized patterns at sites of AF termination by ablation and surrounding disorganized activity in patients with persistent AF. Organized AF patterns were mostly rotational, with similarity and topographical overlap by 2 separate mapping methods. However, such organized AF patterns waxed and waned over 360±45 AF cycles, because of competition from other organized AF sites or fibrillatory waves, before reappearing in similar spatial locations. Results were similar for patients undergoing first or repeat ablation. Future studies should further define this network of competing mechanisms, in terms of mechanisms of fluctuation at organized AF sites, whether interplay with fibrillatory conduction explains differences in reports on potential AF drivers, and whether knowledge of such interactions may improve the mapping and ablation of persistent AF.
Stability of AF Sources in Prior Literature
Several studies report organized regions in human AF. Support for AF drivers comes from optical mapping of human AF,4 acute and long-term impact of AF driver ablation in multicenter, nonrandomized trials,5,7,9,29–31 other experimental,26 and computational32 studies.
A major debate in studies showing AF drivers is whether they are spatially or temporally stable. Our study shows spatially stable AF drivers with temporal variability using 2 mapping methods, including one which reported few organized sites in animal models. These results agree with optical mapping in human AF performed by Li et al,33 who reported reentrant AF drivers stabilized spatially by atrial structure which fluctuated because of competing drivers. That study showed 74.0±21.7% prevalence over time (81.4±26.2% for rotational circuits), similar to our data. Those authors have shown4 that ablation at spatially stable temporally fluctuating drivers can terminate AF. Lin et al34 used a roving multipolar catheter and a novel wave similarity index to identify potential AF drivers for ablation, also supporting spatially stability rotational (high similarity index and rotor identification index) and focal (high similarity index but low rotor identification index) sites.
Conversely, other studies show AF driver instability,5,35 which may preclude successful ablation.35 If instability reflects meander between border-zones of fibrosis,36,37 it is unclear if precession loci may indicate critical fibrotic regions that could be ablation targets. It has been suggested that spatial meander may be exaggerated by projecting cardiac signals to the body surface27 or by studying short 1- to 2-s periods between QRS complexes.5
Impact of Different Mapping Methods
Traditional activation mapping shows disordered waves38 with short-lived organized patterns. These studies were typically for shorter continuous time periods than our study and using small mapping plaques which could miss interacting regions of organization. It is not clear if the discrepant results reflect these factors, different mechanisms in surgical patients typically with advanced AF undergoing valve replacement,38 challenges of marking AF electrogram deflections to plot contours,39 or other factors.
Few prior studies compared different AF mapping methods in the same data sets. In preliminary studies, FIRM-detected rotational drivers of AF were detected simultaneously by optical mapping of the human atria.24 The present study shows similarity between activation and phase (FIRM) and a phase mapping approach previously reported to show few rotations in animal models of AF.16 Next steps in AF mapping include combining different approaches, and in theory, each patient may have tailored treatment based on a fusion between methods.
Clinical and Mechanistic Implications
Depending on different definitions/cutoff for potential AF driver, 8 to 32 s of mapping would detect 90% of AF-terminating drivers. Given AF fluctuations, a search for competing AF mechanisms is essential and may also require recording from 2 to 3 basket positions to map most of the atria.40 Patients in whom driver ablation terminated AF to AT rather than sinus rhythm showed worse indices of substrate (Table 1), and it remains unclear if this promoted AT. Finally, it should be confirmed if different mapping methods may yield similar results if used on the same data, as in this study, with differences being quantitative (eg, sensitivity and specificity for organized sites) rather than qualitative.41
Limitations
This study design used true positives—cases in whom ablation terminated AF. Other studies are examining fluctuations at non–AF-terminating sites. In theory, observed terminations could be spontaneous, although AF did not terminate before ablation and is less likely in persistent AF before PVI. It is possible that cumulative ablation led to termination, although most drivers in this study were the first or second to be ablated in the LA. Delayed termination of AF after ablation of a driver is an emerging concept but may not alter our conclusions that ablation before PVI terminated AF at sites showing organized activity on 2 mapping methods.
Inclusion of AT termination events may be a limitation, yet transition to AT indicates termination of the fibrillatory process and was included in most prior studies of termination.42 In this study, AT was sustained after AF terminated and did not transition back to AF.
Subjects in this study were enrolled on different protocols, including patients with prior ablation, yet AF termination before PVI was common to each. Long-term outcomes on each protocol are being collected.
The method 2 algorithm is commercial and may not be fully disclosed, although it has been described scientifically.17,23,43 However, this limitation may be mitigated by the fact that its results were similar to method 1 (for which code is downloadable).
The basket has resolution limits, may be sufficient to record organized rotational/focal activity,44 likely improves on prior designs40 yet may produce false positives. The resolution required for AF mapping may follow an inverse principle: global mapping with existing catheters allows detection of macroscopic events (eg, multiple or reciprocating sources in Figures 1 and 4) but may miss fine detail for which higher resolution is needed. Conversely, small mapping plaques may see high-resolution detail yet miss precessing AF organized activity (Figure 3) or reciprocal sites outside the mapping field (Figure 4). Future improvements in recordings catheters may provide additional mechanistic insights and clinical improvements.
Finally, we did not have simultaneous epicardial or body surface mapping. We recognize that because of limitations in mapping resolution, for instance, focal sources could represent the exit point of interatrial fibers.
Conclusions
Sites at which persistent AF is terminated by ablation showed organized, mostly rotational activity, that fluctuated over time yet recurred in spatially conserved regions. Fluctuations resulted from collision/fusion from identified competing organized sites or fibrillatory waves. Depending on definition of an AF driver, 8 to 32 s of recording may detect >90% of organized activity patterns at sites of AF termination. Our findings were supported by 2 independent mapping methods. These findings reveal a network of interacting mechanisms in AF, which may help interpret differing reports between AF drivers and disorganized sites, and may help to improve clinical mapping and ablation of persistent AF.
Supplementary Material
WHAT IS KNOWN?
In persistent atrial fibrillation (AF), organized drivers interact with complex fibrillatory activity.
Localized ablation which terminates persistent AF may indicate a localized driver of fibrillatory conduction.
WHAT THE STUDY ADDS?
Drivers of human persistent AF wax and wane as they compete with other potential driver sites or regions of disordered activation.
These interacting mechanisms may represent a dynamic pathophysiological AF network.
A minimum of up to 32 s of global AF recordings is required to capture this dynamic interplay.
Acknowledgments
The present study was performed in (partial) fulfillment of the requirements for obtaining the degree “Dr Med” (Doctor Medicinae).
Sources of Funding
J.A.B. Zaman acknowledges funding from a Fulbright-British Heart Foundation fellowship. Dr Baykaner acknowledges funding from a Josephson-Wellens Heart Rhythm Society Fellowship grant. Dr Peters acknowledges funding from the British Heart Foundation (RG/16/3/32 175 and Centre of Research Excellence), Rosetrees Trust, and National Institute for Health Research UK Biomedical Research Centre. Dr Krummen acknowledges funding from the University of California San Diego Clinical Translational Research Institute and National Institute for Health (NIH; HL83359). Dr Narayan reports research grants from NIH (HL103800, HL83359).
Footnotes
Disclosures
Dr Brachmann reports modest honoraria from Medtronic, Bayer, and Biotronik. Dr Miller reports consulting fees from Biosense Webster and Abbott Electrophysiology (both modest); honoraria from Biosense Webster, Medtronic Inc, and Boston Scientific (all modest); fellowship support from Biosense Webster, Medtronic Inc, and Boston Scientific (all significant). Dr Krummen reports consulting for Abbott Laboratories (modest) and fellowship support from Abbott, Biosense Webster, Biotronik, Boston Scientific, and Medtronic. Dr Narayan reports consulting compensation from Up to Date, Abbott Laboratories, American College of Cardiology Foundation (all modest); speaking/consulting fees from Medtronic, Inc (modest), and St. Jude Medical (modest); equity interests from Topera Medical (significant); and intellectual property rights from University of California Regents (significant). Dr Wang reports honoraria/consultant from Janssen, St. Jude Medical, Amgen, and Medtronic; (all modest) fellowship support from Biosense Webster (moderate), Boston Scientific (moderate), Medtronic, and St. Jude Medical (all modest); clinical studies from Medtronic, Siemens, Cardiofocus, and ARCA Biopharma (all modest); and stock options from VytronUS (modest). The other authors report no conflicts.
The Data Supplement is available at http://circep.ahajournals.org/lookup/suppl/doi:10.1161/CIRCEP.117.005846/-/DC1.
Contributor Information
Christopher A.B. Kowalewski, Department of Medicine, Stanford University, CA Department of Cardiology, Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany.
Fatemah Shenasa, Department of Medicine, Stanford University, CA.
Miguel Rodrigo, Department of Medicine, Stanford University, CA.
Paul Clopton, Department of Medicine, Stanford University, CA.
Gabriela Meckler, Department of Medicine, Stanford University, CA.
Mahmood I. Alhusseini, Department of Medicine, Stanford University, CA.
Mark A. Swerdlow, Department of Medicine, Stanford University, CA.
Vijay Joshi, Department of Medicine, Stanford University, CA.
Samir Hossainy, Department of Engineering, University of California, Berkeley.
Junaid A.B. Zaman, Department of Medicine, Stanford University, CA; ElectroCardioMaths Programme, Imperial College, London, United Kingdom.
Tina Baykaner, Department of Medicine, Stanford University, CA.
Albert J. Rogers, Department of Medicine, Stanford University, CA.
Johannes Brachmann, Klinikum Coburg, Germany.
John M. Miller, Department of Medicine, Indiana University, Indianapolis.
David E. Krummen, Department of Medicine, University of California San Diego.
William H. Sauer, Department of Medicine, University of Colorado, Denver.
Nicholas S. Peters, ElectroCardioMaths Programme, Imperial College, London, United Kingdom.
Paul J. Wang, Department of Medicine, Stanford University, CA.
Sanjiv M. Narayan, Department of Medicine, Stanford University, CA.
REFERENCES
- 1.Nattel S, Dobrev D. Controversies about atrial fibrillation mechanisms: aiming for order in chaos and whether it matters. Circ Res. 2017;120:1396–1398. doi: 10.1161/CIRCRESAHA.116.310489. [DOI] [PubMed] [Google Scholar]
- 2.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen PS, Chen SA, Chung MK, Nielsen JC, Curtis AB, Davies DW, Day JD, d’Avila A, de Groot NMSN, Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao HM, Verma A, Wilber DJ, Yamane T. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nattel S, Xiong F, Aguilar M. Demystifying rotors and their place in clinical translation of atrial fibrillation mechanisms. Nat Rev Cardiol. 2017;14:509–520. doi: 10.1038/nrcardio.2017.37. [DOI] [PubMed] [Google Scholar]
- 4.Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, Kalyanasundaram A, Lim P, Bratasz A, Powell KA, Simonetti OP, Higgins RS, Kilic A, Mohler PJ, Janssen PM, Weiss R, Hummel JD, Fedorov VV. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J. 2015;36:2390–2401. doi: 10.1093/eurheartj/ehv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, Daly M, Amraoui S, Zellerhoff S, Picat MQ, Quotb A, Jesel L, Lim H, Ploux S, Bordachar P, Attuel G, Meillet V, Ritter P, Derval N, Sacher F, Bernus O, Cochet H, Jais P, Dubois R. Driver domains in persistent atrial fibrillation. Circulation. 2014;130:530–538. doi: 10.1161/CIRCULATIONAHA.113.005421. [DOI] [PubMed] [Google Scholar]
- 6.Lin Y-J, Lo M-T, Chang S-L, Lo L-W, Hu Y-F, Chao T-F, Chung F-P, Liao J-N, Lin C-Y, Kuo H-Y, Lin Y-CC, Tuan T-C, Young H-WV, Suenari K, Do VBD, Raharjo SB, Huang NE, Chen S-A. Benefits of atrial substrate modification guided by electrogram similarity and phase mapping techniques to eliminate rotors and focal sources versus conventional defragmentation in persistent atrial fibrillation. JACC: Clin Electrophysiol. 2016;2:667–678. [DOI] [PubMed] [Google Scholar]
- 7.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seitz J, Bars C, Théodore G, Beurtheret S, Lellouche N, Bremondy M, Ferracci A, Faure J, Penaranda G, Yamazaki M, Avula UM, Curel L, Siame S, Berenfeld O, Pisapia A, Kalifa J. AF Ablation guided by spatiotemporal electrogram dispersion without pulmonary vein isolation: a wholly patient-tailored approach. J Am Coll Cardiol. 2017;69:303–321. doi: 10.1016/j.jacc.2016.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller JM, Kalra V, Das MK, Jain R, Garlie JB, Brewster JA, Dandamudi G. Clinical Benefit of Ablating Localized Sources for Human Atrial Fibrillation: the Indiana University FIRM Registry. J Am Coll Cardiol. 2017;69:1247–1256. doi: 10.1016/j.jacc.2016.11.079. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez FD, Birnie DH, Nair GM, Szczotka A, Redpath CJ, Sadek MM, Nery PB. Efficacy and safety of driver-guided catheter ablation for atrial fibrillation: a systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2017;28:1371–1378. doi: 10.1111/jce.13313. [DOI] [PubMed] [Google Scholar]
- 11.Baykaner T, Rogers AJ, Meckler GL, Zaman J, Navara R, Rodrigo M, Alhusseini M, Kowalewski CAB, Viswanathan MN, Narayan SM, Clopton P, Wang PJ, Heidenreich PA. Clinical implications of ablation of drivers for atrial fibrillation: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2018;11:e006119. doi: 10.1161/CIRCEP.117.006119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buch E, Share M, Tung R, Benharash P, Sharma P, Koneru J, Mandapati R, Ellenbogen KA, Shivkumar K. Long-term clinical outcomes of focal impulse and rotor modulation for treatment of atrial fibrillation: a multicenter experience. Heart Rhythm. 2016;13:636–641. doi: 10.1016/j.hrthm.2015.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Natale A, Mohanty S, Gianni C, Philipp Halbfass M, Mohanty P, Metz T, Trivedi C, Deneke T, Tomassoni GF, Bai R, Al-Ahmad A, Bailey S, Burkhardt JD, Gallinghouse GJ, Horton RP, Hranitzky P, Sanchez JE, Biase LD. Late-breaking clinical trials II: Impact of Rotor Ablation in Non-Paroxysmal AF Patients (OASIS) (Abstract). Heart Rhythm. 2016;13: [Google Scholar]
- 14.Steinberg JS, Shah Y, Bhatt A, Sichrovsky T, Arshad A, Hansinger E, Musat D. Focal impulse and rotor modulation: acute procedural observations and extended clinical follow-up. Heart Rhythm. 2017;14:192–197. doi: 10.1016/j.hrthm.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Alhusseini M, Vidmar D, Meckler GL, Kowalewski CA, Shenasa F, Wang PJ, Narayan SM, Rappel WJ. Two independent mapping techniques identify rotational activity patterns at sites of local termination during persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2017;28:615–622. doi: 10.1111/jce.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuklik P, Zeemering S, Maesen B, Maessen J, Crijns HJ, Verheule S, Ganesan AN, Schotten U. Reconstruction of instantaneous phase of unipolar atrial contact electrogram using a concept of sinusoidal recomposition and Hilbert transform. IEEE Trans Biomed Eng. 2015;62:296–302. doi: 10.1109/TBME.2014.2350029. [DOI] [PubMed] [Google Scholar]
- 17.Narayan SM, Krummen DE, Enyeart MW, Rappel W. Computational mapping approach identifies stable and long-lived electrical rotors and focal sources in human atrial fibrillation. PLos One. 2012;7:e46034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuklik P, Lau DH, Ganesan AN, Brooks AG, Sanders P. High-density mapping of atrial fibrillation in a chronic substrate: evidence for distinct modes of repetitive wavefront propagation. Int J Cardiol. 2015;199:407–414. doi: 10.1016/j.ijcard.2015.07.057. [DOI] [PubMed] [Google Scholar]
- 19.Kuklik P, Zeemering S, van Hunnik A, Maesen B, Pison L, Lau DH, Maessen J, Podziemski P, Meyer C, Schaffer B, Crijns H, Willems S, Schotten U. Identification of rotors during human atrial fibrillation using contact mapping and phase singularity detection: technical considerations. IEEE Trans Biomed Eng. 2017;64:310–318. doi: 10.1109/TBME.2016.2554660. [DOI] [PubMed] [Google Scholar]
- 20.Vijayakumar R, Vasireddi SK, Cuculich PS, Faddis MN, Rudy Y. Methodology considerations in phase mapping of human cardiac arrhythmias. Circ Arrhythm Electrophysiol. 2016;9:e004409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narayan SM, Wright M, Derval N, Jadidi A, Forclaz A, Nault I, Miyazaki S, Sacher F, Bordachar P, Clémenty J, Jaïs P, Haïssaguerre M, Hocini M. Classifying fractionated electrograms in human atrial fibrillation using monophasic action potentials and activation mapping: evidence for localized drivers, rate acceleration, and nonlocal signal etiologies. Heart Rhythm. 2011;8:244–253. doi: 10.1016/j.hrthm.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narayan SM, Kazi D, Krummen DE, Rappel WJ. Repolarization and activation restitution near human pulmonary veins and atrial fibrillation initiation: a mechanism for the initiation of atrial fibrillation by premature beats. J Am Coll Cardiol. 2008;52:1222–1230. doi: 10.1016/j.jacc.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation. 2011;123:2922–2930. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen BJ, Briggs C, Moore BT, Csepe TA, Li N, Zhao J, Garikipati NV, Janssen PM, Mohler PJ, Hummel JD, Fedorov VV. Human atrial fibrillation drivers seen simultaneously by focal impulse and rotor mapping and high-resolution optical mapping [abstract]. Circulation. 2015;132:A18402. [Google Scholar]
- 25.Haissaguerre M, Shah AJ, Cochet H, Hocini M, Dubois R, Efimov I, Vigmond E, Bernus O, Trayanova N. Intermittent drivers anchoring to structural heterogeneities as a major pathophysiological mechanism of human persistent atrial fibrillation. J Physiol. 2016;594:2387–2398. doi: 10.1113/JP270617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandit SV, Jalife J. Rotors and the dynamics of cardiac fibrillation. Circ Res. 2013;112:849–862. doi: 10.1161/CIRCRESAHA.111.300158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigo M, Guillem MS, Climent AM, Pedrón-Torrecilla J, Liberos A, Millet J, Fernández-Avilés F, Atienza F, Berenfeld O. Body surface localization of left and right atrial high-frequency rotors in atrial fibrillation patients: a clinical-computational study. Heart Rhythm. 2014;11:1584–1591. doi: 10.1016/j.hrthm.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodrigo M, Climent AM, Liberos A, Fernández-Avilés F, Berenfeld O, Atienza F, Guillem MS. Highest dominant frequency and rotor positions are robust markers of driver location during noninvasive mapping of atrial fibrillation: a computational study. Heart Rhythm. 2017;14:1224–1233. doi: 10.1016/j.hrthm.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller JM, Kowal RC, Swarup V, Daubert JP, Daoud EG, Day JD, Ellenbogen KA, Hummel JD, Baykaner T, Krummen DE, Narayan SM, Reddy VY, Shivkumar K, Steinberg JS, Wheelan KR. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol. 2014;25:921–929. doi: 10.1111/jce.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rashid H, Sweeney A. Approaches for focal impulse and rotor mapping in complex patients: a US private practice perspective. J Innovations in Cardiac Rhythm Management. 2015;6:2193–2198. [Google Scholar]
- 31.Gianni C, Di Biase L, Deneke T, Tami Metz T, Halbfass P, Müller P, Schade A, Mohanty S, Trivedi C, Bai R, Al-Ahmad A, Burkhardt JD, Gallinghouse GJ, Horton RP, Hranitzky PM, Sanchez JE, Tomassoni GF, Natale A. Acute and short-term outcomes in persistent and long-standing persistent patients undergoing rotors only ablation (abstract). Heart Rhythm. 2015;12:PO01–PO58. [Google Scholar]
- 32.Rappel WJ, Zaman JA, Narayan SM. Mechanisms for the termination of atrial fibrillation by localized ablation: computational and clinical studies. Circ Arrhythm Electrophysiol. 2015;8:1325–1333. doi: 10.1161/CIRCEP.115.002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li N, Csepe TA, Hansen BJ, Sul LV, Kalyanasundaram A, Zakharkin SO, Zhao J, Guha A, Van Wagoner DR, Kilic A, Mohler PJ, Janssen PM, Biesiadecki BJ, Hummel JD, Weiss R, Fedorov VV. Adenosine-induced atrial fibrillation: localized reentrant drivers in lateral right atria due to heterogeneous expression of adenosine a1 receptors and girk4 subunits in the human heart. Circulation. 2016;134:486–498. doi: 10.1161/CIRCULATIONAHA.115.021165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin YJ, Lo MT, Chang SL, Lo LW, Hu YF, Chao TF, Chung FP, Liao JN, Lin CY, Kuo HY, Chang YC, Lin C, Tuan TC, Vincent Young HW, Suenari K, Dan Do VB, Raharjo SB, Huang NE, Chen SA. Benefits of atrial substrate modification guided by electrogram similarity and phase mapping techniques to eliminate rotors and focal sources versus conventional defragmentation in persistent atrial fibrillation. JACC: Clin Electrophysiol. 2016;2:667–678. [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Sahadevan J, Khrestian CM, Cakulev I, Markowitz A, Waldo AL. Simultaneous biatrial high-density (510–512 electrodes) epicardial mapping of persistent and long-standing persistent atrial fibrillation in patients: new insights into the mechanism of its maintenance. Circulation. 2015;132:2108–2117. doi: 10.1161/CIRCULATIONAHA.115.017007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P, Brachmann J. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 37.Kottkamp H Human atrial fibrillation substrate: towards a specific fibrotic atrial cardiomyopathy. Eur Heart J. 2013;34:2731–2738. doi: 10.1093/eurheartj/eht194. [DOI] [PubMed] [Google Scholar]
- 38.Allessie MA, de Groot NM, Houben RP, Schotten U, Boersma E, Smeets JL, Crijns HJ. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease: longitudinal dissociation. Circ Arrhythm Electrophysiol. 2010;3:606–615. doi: 10.1161/CIRCEP.109.910125. [DOI] [PubMed] [Google Scholar]
- 39.Sahli Costabal F, Zaman JAB, Kuhl E, Narayan SM. Interpreting activation mapping of atrial fibrillation: a hybrid computational/physiological study. Ann Biomed Eng. 2018;46:257–269. doi: 10.1007/s10439-017-1969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honarbakhsh S, Schilling RJ, Providência R, Dhillon G, Sawhney V, Martin CA, Keating E, Finlay M, Ahsan S, Chow A, Earley MJ, Hunter RJ. Panoramic atrial mapping with basket catheters: a quantitative analysis to optimize practice, patient selection, and catheter choice. J Cardiovasc Electrophysiol. 2017;28:1423–1432. doi: 10.1111/jce.13331. [DOI] [PubMed] [Google Scholar]
- 41.Zaman JAB, Rogers AJ, Narayan SM. Rotational drivers in atrial fibrillation: are multiple techniques circling similar mechanisms? Circ Arrhythm Electrophysiol. 2017;10:e006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haïssaguerre M, Hocini M, Sanders P, Sacher F, Rotter M, Takahashi Y, Rostock T, Hsu LF, Bordachar P, Reuter S, Roudaut R, Clémenty J, Jaïs P. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol. 2005;16:1138–1147. doi: 10.1111/j.1540-8167.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 43.Lalani GG, Schricker A, Gibson M, Rostamian A, Krummen DE, Narayan SM. Atrial conduction slows immediately before the onset of human atrial fibrillation: a bi-atrial contact mapping study of transitions to atrial fibrillation. J Am Coll Cardiol. 2012;59:595–606. doi: 10.1016/j.jacc.2011.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roney CH, Cantwell CD, Bayer JD, Qureshi NA, Lim PB, Tweedy JH, Kanagaratnam P, Peters NS, Vigmond EJ, Ng FS. Spatial resolution requirements for accurate identification of drivers of atrial fibrillation. Circ Arrhythm Electrophysiol. 2017;10:e004899. doi: 10.1161/CIRCEP.116.004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.