Abstract
Aims
To evaluate the effects of (-)-epicatechin (Epi) in the progression of kidney damage.
Material and methods
We assessed the effects of Epi [0.01–20 mg/kg of body weight/day] during 14 days, in a 5/6 nephrectomy model in mice.
Key findings
Nephrectomy-induced systolic arterial hypertension was significantly reduced in a dose dependent manner with Epi treatment. Increased serum creatinine and urea were reduced almost to normal values. The concentration of tetrahydrobiopterin (BH4), used as subrogate of endothelial dysfunction, decreased in nephrectomyzed animals, Epi treatment increased BH4 levels almost reaching normal values. The expression of angiotensin II receptor (AT1-R) and NADPH oxidase-4 (NOX-4) and 3-nitrotyrosine levels increased with nephrectomy and were reduced with Epi treatment. Renal tissue morphology in the remaining tissue was conserved with Epi treatment in a dose dependent manner.
Significance.
Chronic kidney disease (CKD) is an independent cardiovascular risk factor associated with a mortality rate 10 to 20 times higher than that of the general population. High blood pressure, endothelial dysfunction and oxidative stress are important factors determining kidney damage progression. Findings of this study indicate that Epi is able to counteract the deleterious effects of subtotal nephrectomy and the structural and functional changes in the remnant kidney tissue, decreasing the progression of CKD. These results warrant the possibility of implement clinical trials to limit the progression of CKD in humans.
Keywords: Cell biology, Pathology
1. Introduction
Chronic kidney disease (CKD), a condition in which there is a gradual impairment of renal function, it is an independent risk marker of cardiovascular events and death [1]. Its diagnosis involves; a decrease of glomerular flow rate (GFR) and the occurrence of one or more, for at least three months, of markers of kidney structural damage; albuminuria, abnormalities of the urinary sediment, electrolyte and acid-basic anomalies, histology evidence of renal damage, imaging demonstration of structural abnormalities [2]. Progression of kidney disease is a complex process in which several factors are intertwined: traditional risk factors, as dyslipidemia, hypertension and diabetes, as well as, the classical triad of vascular damage: low grade inflammation, nitroxidative stress and endothelial dysfunction [3, 4], plus other factors as vascular calcinosis, anemia and genetic influence [5]. Natural history of CKD is characterized by a torpid evolution. When GFR drops to <15 ml/min/1.73 m2 there is an established renal failure and patients would require dialytic procedures or renal transplant to survive. Then, it is imperative to prolong as much as possible the useful life of the kidney, in order not to reach the end-stage of renal failure, with all his ominous and costly consequences. Common etiologies of CKD are diabetes mellitus, high blood pressure and ageing, a general strategy to slow down the decreasing glomerular flow consist in normalize glycaemia, as well as, blood pressure levels and lipid disorders, in addition to dietary adjustments and the adoption of a healthy lifestyle.
Overexpression of the renin angiotensin aldosterone system (RAAS) particularly in patients with diabetes mellitus, obesity or both, is involved in another phenomenon that determines the progression of kidney damage: the nitroxidative stress, defined as a state in which a redox imbalance surges, with an increase in the formation of reactive oxygen species (ROS). In this process, angiotensin II (Ang II) increases the activity of the renal NADPH oxidases (NOX-4 and NOX-2 isoforms mainly) increasing the production of superoxide anion (O2•-) [6], which in turn oxidizes tetrahydrobiopterin (BH4), indispensable co-factor of endothelial nitric oxide synthase (eNOS), which conditions the decoupling of the enzyme and the deviation of its activity towards the production of a greater quantity of O2•-.
The loss of balance between oxidant and antioxidant mechanisms is one of the main features of endothelial dysfunction and the loss of vasodilator power of the endothelium, favoring vasoconstriction and increasing blood pressure [7]. In this regard, the effects of (-)-epicatechin (Epi), the most abundant flavanol of cacao, can have potential beneficial uses in CKD treatment since it has positive effects on carbohydrate and lipid metabolism, blood pressure, inflammation, endothelial function [8], decreasing NOX-4 activity [9] and restoration of striated muscle function and mitochondrial biogenesis including an antioxidant effect acting as a positive regulator of mitochondrial structure/function endpoints and redox balance control systems and in a lesser manner acting as a radical scavenger [10]. Thus, the aim the present study was to analyze the effect of Epi, in a model of CKD induced by a 5/6 nephrectomy in mice, that resemble CKD changes in blood pressure, urea and creatinine serum concentrations (as a measure of renal function), overexpression of the RAAS and NOX-4.
2. Methods
The study was carried out on 88 male mice of the C57BL6 strain (25–30 g, 4–5 weeks of age), in accordance with the guidelines for the care and use of laboratory animals, issued by the Section of Graduate Studies and Research of the Superior School of Medicine (ESM-CICUAL-05/4-12-2017), of the National Polytechnic Institute and the Official Mexican Norm. Animals were kept in a 12/12 light/obscurity cycle with food and water provided at libitum.
Mice were divided in eight groups (n = 11) as follows; 1) Sham (S); 2) Sham + vehicle (Sham + V); 3–8) The remaining groups were composed by mice in which a 5/6 nephrectomy, as an experimental model for reducing most of the renal mass (Nf) was performed, and treated during 14 days with vehicle or Epi at the doses of 0 (vehicle), 0.01, 0.1, 1, 10 and 20 mg/kg of body weight/day by gavage respectively.
2.1. Nephrectomy
Surgical 5/6 nephrectomy is a well-known animal model to reduce the mass of nephrons, leaving the remaining renal tissue unharmed. Mice were anesthetized with intraperitoneal sodium pentobarbital at the dose of 30 mg/kg of body weight. Through an incision in the right paravertebral dorsal region, right kidney was exposed and then extirpated. A similar incision was performed in the left side of the same region. After dissecting the left renal hilum, two out of three renal arteries were ligated; leaving untouched only the inferior pole artery. This procedure leaves unscathed just one fifth of the entire renal mass. In animals pertaining to the sham group, the incision just reached the peritoneum, but no nephrectomy or arterial ligation was done.
Once recovered from the surgical procedures mice were randomly allocated into treatment groups. Afterwards, animals were sacrificed with an intraperitoneal injection of 200 mg/kg of body weight of sodium pentobarbital.
2.2. Blood pressure measurements
Previous to the sacrifice and after fourteen days of treatment, systolic blood pressure (SBP) was measured with a method utilizing an inflatable rat's tail-cuff using a computerized system (MRBP Mouse and Rat Tail Cuff Method Blood Pressure Systems, IITC Life Science, USA). Conscious mice were placed inside an immobilizing container trap for the non-invasively measurement of blood pressure. Following a known standard procedure, the pressure in the cuff (whose dimensions were appropriated according to the corpulence of the animal) was automatically controlled and systolic pressure was detected by a proper transducer and displayed in the monitor. Blood pressure was measured three times, at one-minute intervals, and the mean value was estimated.
2.3. Estimation of markers of renal function
Before sacrifice and under anesthesia, blood samples were extracted by direct heart puncture. After centrifugation of samples, urea and creatinine concentrations were determined using colorimetric commercial kits (Sandoz, co., Mexico) following manufacturer instructions.
2.4. Measurement of BH4 as a marker of endothelial function
50 μL of serum were employed to measure the concentration of serum tetrahydrobiopterin (BH4) by capillary electrophoresis, with fluorescence emission created with laser excitation (Capillary Electrophoresis Systems P/ACETM MDQ, Beckman Coulter Inc., USA.)
2.5. Renal histology
Once blood samples were obtained, mice were perfused through the left ventricle with phosphate-saline buffer solution (PBS, pH 7.4; 0.1 M), just before the extraction of the remnant of left kidney. After a thorough cleansing of the tissue, it was deposited in a 4% formaldehyde solution. Afterwards, the conventionally dehydrated tissues were fixed in paraffin and sectioned in slices 5 μm thick, which then were stained with hematoxylin and eosin (H&E). Tissue was examined using light field optical microscopy by two blinded to treatments experts. Analysis was performed in at least 10, randomly chosen optical fields where at least 50 glomeruli were analyzed.
2.6. Western blot
For determining the expression of angiotensin II receptor (AT1-R) and NADPH oxidase 4 (NOX-4) proteins a Western blot technique was employed. For that purpose, 100 mg of kidney tissue were homogenized with lysis buffer supplemented with complete™ Mini Protease Inhibitor Cocktail, Roche Diagnostics, Germany). Then, homogenates were sonicated during 2 minutes, at 4 °C, and were centrifuged at 10,956 g during 10 minutes, at 4 °C. Protein concentration in the supernatant was determined with Bradford Protein Assay Kit (Bio- RAD, USA). 50 μg of protein were separated by dodecyl sulfate polyacrylamide gel electrophoresis, SDS-PAGE, transferred to a PVDF Blotting Membrane (GE Healthcare Life Science, Germany) in a semi-dry system. Membranes were blocked with Blotting-Grade Blocker (Bio-RAD, USA), for 60 min at room temperature. Incubation was done with primary antibodies against AT1-R and NOX-4 (PA5-20812 Thermo Fisher, USA and GTX121929, GeneTex Inc, USA) overnight at 4 °C. Then a secondary antibody mouse anti-rabbit IgG-HRP was incubated during 90 min at room temperature. Finally Western blots were developed by chemiluminescence using Western Blotting Luminol Reagent (sc-2048, Santa Cruz Biotechnology, USA) and analyzed with C-Digit Chemiluminescence Western Blot Scanner (LI-COR Biosciences, USA). β2-Tubulin was used as control.
2.7. Nitroxidative stress marker (3-nitrotyrosine)
For the measurement of 3-nitrotyrosine, as a marker of nitroxidative stress, the dot blot technique was utilized [11]. Aliquots with 20 μg of total protein in a volume of 10 μL were prepared and were placed in a PVDF membrane. After drying, membranes were exposed to the blocking reagent and incubated with the antibody against nitrotyrosine (sc-32757, Santa Cruz Biotechnology, Inc., USA) overnight, at 4 °C, followed by incubation with the secondary antibody antimouse (sc-516102, Santa Cruz Biotechnology, Inc., USA) and developed by Chemiluminescence.
2.8. Statistical analysis
In the comparison among multiple groups a variance analysis was performed (ANOVA) and the Tukey post-hoc test. Also, the Student test was used when it was applicable. The significance level was set at p < 0.05.
3. Results
In the sham (S) and sham plus vehicle (ethanol/water, 1:10 solution) (Sham + V) groups, the observed ciphers of systolic blood pressure (SBP) were 96 ± 2 mm Hg and 90 ± 2 mm Hg respectively, without significant differences between them, so from now on we only report data from the group of mice with sham operations that received vehicle.
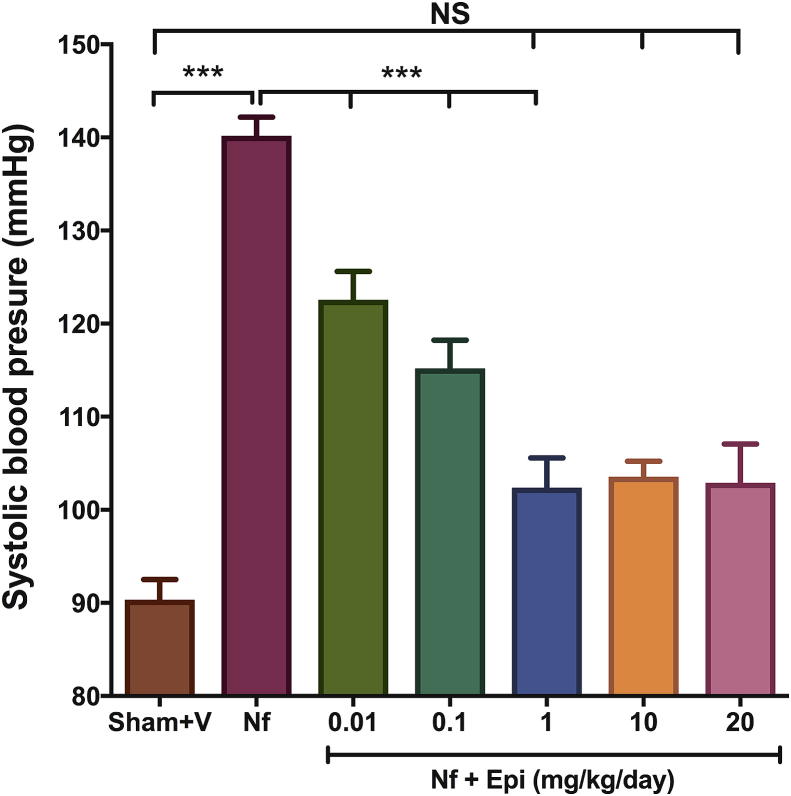
In the partially nephrectomized animals (Nf), SBP rose significantly to 140 ± 2 mm Hg (p < 0.01 when compared with the Sham + V group). In contrast, in the Nf groups treated with Epi al the doses of 0.01, 0.1, 1, 10 y 20 mg/kg of body weight/day, the levels of SBP were 123 ± 3 mm Hg, 115 ± 3 mm Hg, 102 ± 3 mm Hg, 104 ± 2 mm Hg, y 103 ± 4 mm Hg, respectively. Results show that that the administration of Epi decreased SBP 18, 25, 38, 37 y 38 mm Hg as compared to the Nf group (Fig. 1). Notice that there is a dose-response curve up to the dose of 1 mg/kg of body weight, with higher doses (10 and 20 mg/kg of body weight/day) a plateau was reached. Interestingly, SBP at Epi doses of 1, 10, and 20 mg/kg of body weight/day is similar to SBP in the Sham + V group suggesting that Epi blocks the Nf-induced increases in SBP.
Fig. 1.
Systolic blood pressure (SBP) at 14 days post-surgery. There is an increase in SBP in the group with nephrectomy 5/6 (Nf) vs Sham. A dose dependent decrease in SBP was found in the Nf treated with Epi (Nf + Epi) vs (Nf) without treatment. Data are presented as mean ± sem (n = 11). Analysis performed with one-way ANOVA with Post-Hoc test Tukey. ***p < 0.001, NS: not significant.
For this reason, from now on only results in groups of 0.01, 0.1 and 1 mg/kg of body weight/day will be reported.
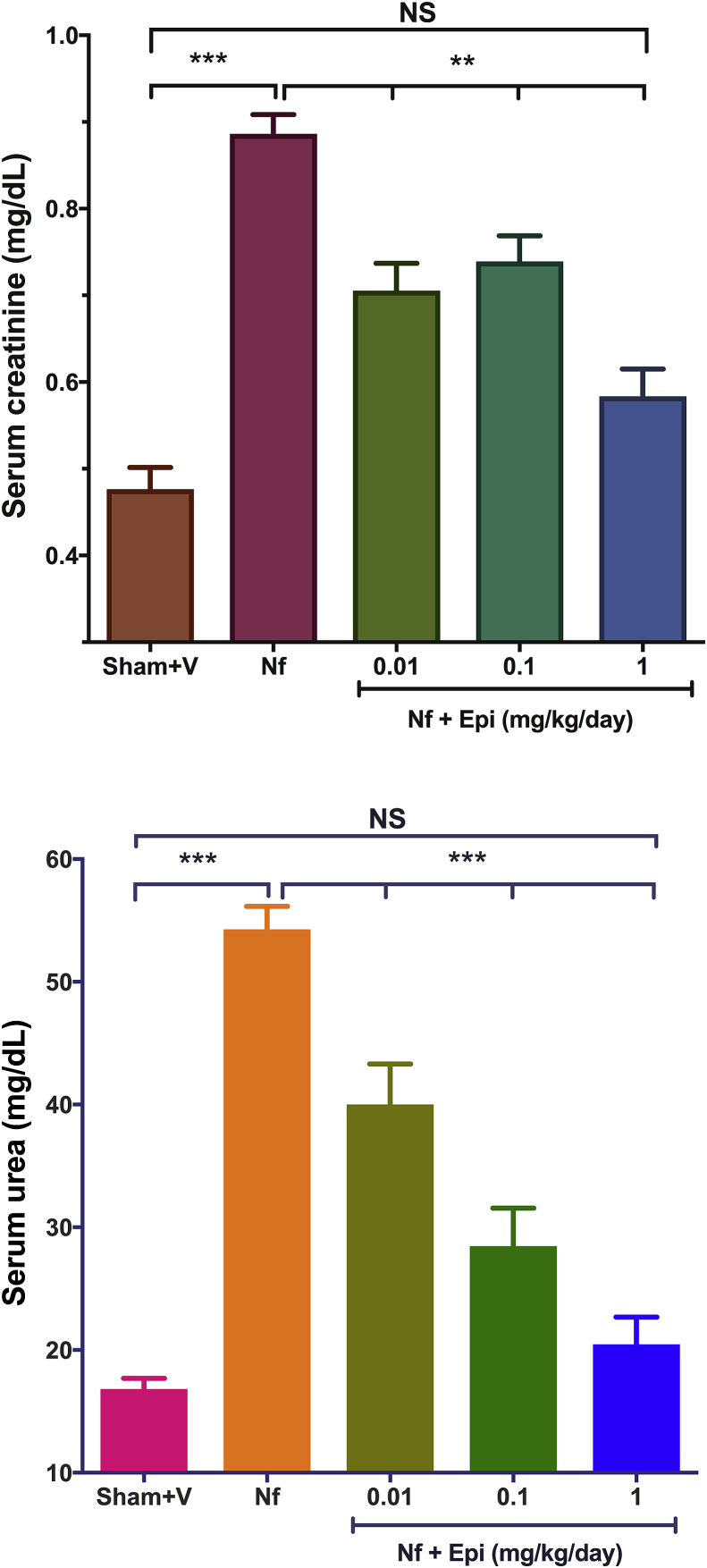
In the group Sham + V the concentrations of creatinine and urea were 0.47 ± 0.03 mg/dL and 16.81 ± 0.88 mg/dL, respectively, while in the NF group, without treatment, creatinine and urea concentrations rose to 0.88 ± 0.02 mg/dL and 54.27 ± 1.9 mg/dL respectively, representing an increment of 1.87 and 3.2 times as compared to control (Fig. 2A & B) these differences were highly significant (p < 0.01).
Fig. 2.
Determination of the concentration of serum creatinine and urea at 14 days post-surgery. A) There is an increase in creatinine in the group with nephrectomy 5/6 (Nf) vs Sham. A dose dependent decrease in creatinine was found in the Nf treated with Epi (Nf + Epi) [0.01 to 1 mg/kg of body weight/day] vs (Nf) without treatment. B) There is an increase in Urea in the group with nephrectomy 5/6 (Nf) vs Sham. A dose dependent decrease in urea was found in the Nf treated with Epi (Nf + Epi) [0.01 to 1 mg/kg of body weight/day] vs (Nf) without treatment. Data are presented as mean ± sem (n = 11). Analysis performed with one-way ANOVA with Post-Hoc test: Tukey. **p < 0.01, ***p < 0.001, NS: not significant.
In the groups treated with Epi at doses of 0.01, 0.1 and 1 mg/kg of body weight/day (Fig. 2A y B), concentrations of serum creatinine were 0.70 ± 0.03 mg/dL, 0.73 ± 0.03 mg/dL and 0.58 ± 0.03 mg/dL, while those of serum urea were 40 ± 3.3 mg/dL, 28.5 ± 3 mg/dL and 20.5 ± 2.2 mg/dL respectively. At the most effective doses of 1 mg/kg of body weight/day, creatinine was reduced 34% and urea 62% (p < 0.01 vs Nf group), reaching values of mice in the control Sham + V group.
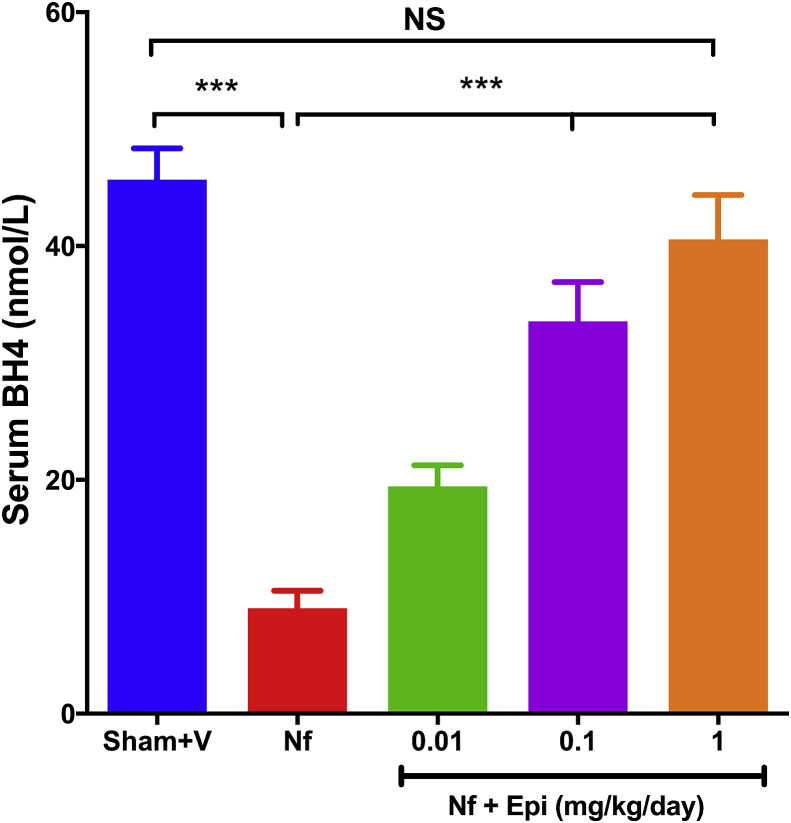
Serum concentration of tetrahydrobiopterin (BH4) in Sham + V group was 46 ± 2.7 nmol/L. In the Nf group, this value decrease to 9 ± 1.5 nmol/L (p < 0.01). On the contrary, in Nf groups treated with Epi [0.01, 0.1 y 1 mg/kg of body weight/day] concentrations of BH4 raised 18 ± 1.8, 35 ± 3.4 and 44 ± 3.8 nmol/L respectively (Fig. 3, p < 0.01). At a 1 mg/kg of body weight/day dose, the serum value of BH4 was similar to the concentrations in Sham + V group.
Fig. 3.
Serum concentration of tetrahydrobiopterin (BH4) at 14 days post-surgery. There is a decrease of BH4 concentration in the nephrectomy 5/6 (Nf) without treatment group vs Sham. In contrast there is an increase in BH4 concentration in the Nf treated with Epi (Nf + Epi) groups [0.01 to 1 mg/kg of body weight/day]. Data are presented as mean ± sem (n = 11). Analysis performed with one-way ANOVA with Post-Hoc test: Tukey. ***p < 0.001, NS: not significant.
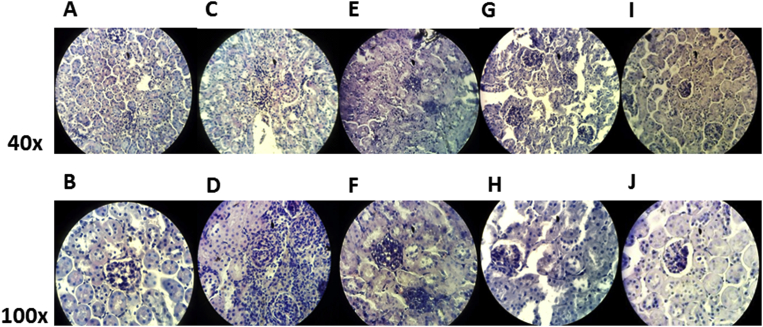
In the control group (Sham + V) (Fig. 4A (40x) and B (100x)) renal tubules had normal appearance, with glomeruli capsule components well conserved, with parietal and visceral membrane and Bowman's space without alterations. Meanwhile, in mice with 5/6 nephrectomy, an inflammatory infiltration, as well as, loss of the integrity of the components of the glomerular capsule, especially the Bowman space (Fig. 4C (40x) and D (100x)) was found. In contrast, in the Nf groups of mice treated with Epi an attenuation of tissue damage was observed. In mice that received Epi dose of 0.01 mg/kg of body weight/day, tubule-interstitial increase cellularity with conserved glomeruli were observed (Fig. 4E (40x) and F (100x)). Whereas, in mice treated with Epi at the dose of 0.1 mg/kg of body weight/day a greater preservation of the integrity of glomerular capsules and less damage of both visceral and parietal membranes and Bowman space was evident (Fig. 4G (40x) and H (100x)). Finally with the dose of 1 mg/kg of body weight/day, renal normal morphology was kept (Fig. 4I (40x) and J(100x)).
Fig. 4.
Representative images of Hematoxylin & Eosin staining of histological sections of kidney (8μm), obtained from sham surgery groups treated with vehicle (Sham + V) (Fig. 4A [40x] and B [100x]), with nephrectomy 5/6 (Nf) without treatment (Fig. 4C [40x] and D [100x]) and with Nf treated with Epi (Nf + Epi) at the doses of 0.01 mg/kg of body weight/day (Fig. 4E [40x] and F [100x]), 0.1 mg/kg of body weight/day, (Fig. 4G [40x] and H [100x]) and 1 mg/kg of body weight/day (Fig. 4I [40x] and J [100x]).
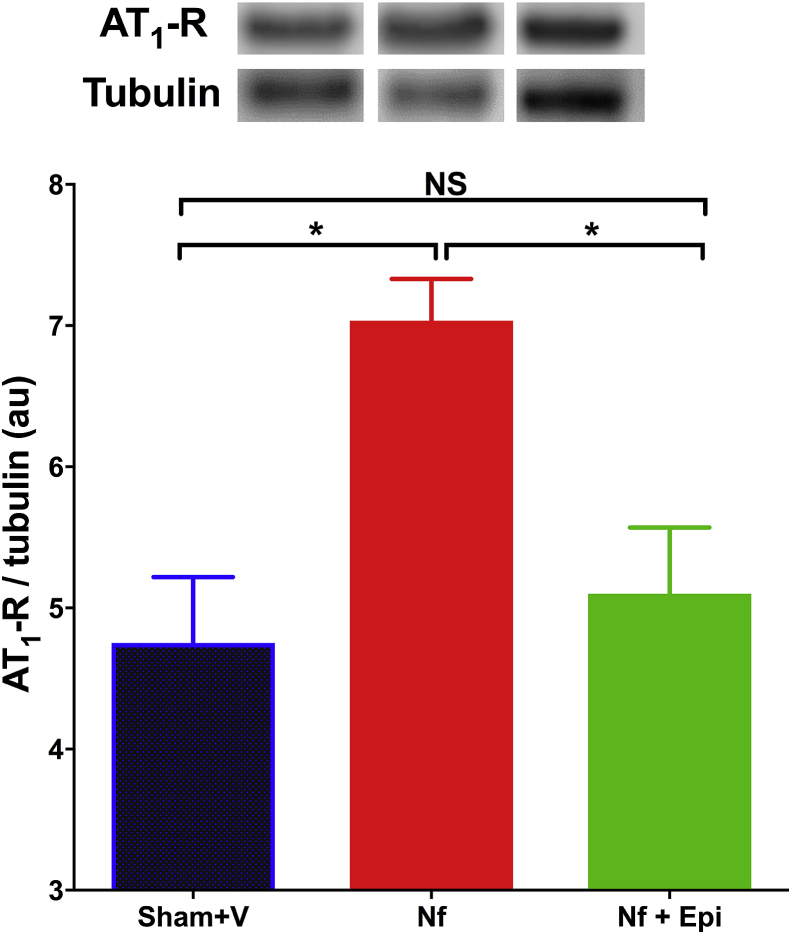
Since the maximal effects of Epi were observed at the dose of 1 mg/kg/day, we only report the effects of Epi on the expression of AT1-R at this dose.
In mice with Nf a significant increment of the relative expression of the receptor was observed in comparison with animals in the Sham + V group (p < 0.05), in contrast, in mice treated with Epi, no change of the expression was observed (Fig. 5).
Fig. 5.
Analysis of the expression of angiotensin II type 1 receptor (AT1-R) in renal tissue analyzed by western blot, at 14 days post-surgery. The AT1-R/tubulin index is reported in arbitrary units (A.U.). Significant increase in the expression of ATR-1 in the group with the nephrectomy 5/6 (Nf) vs Sham. There is no change in the group treated with Epi 1 mg/kg of body weight/day as compared with Sham group. Data are presented as mean ± sem. Analysis performed with one-way ANOVA with Post-Hoc test: Tukey. *p < 0.05, NS: not significant.
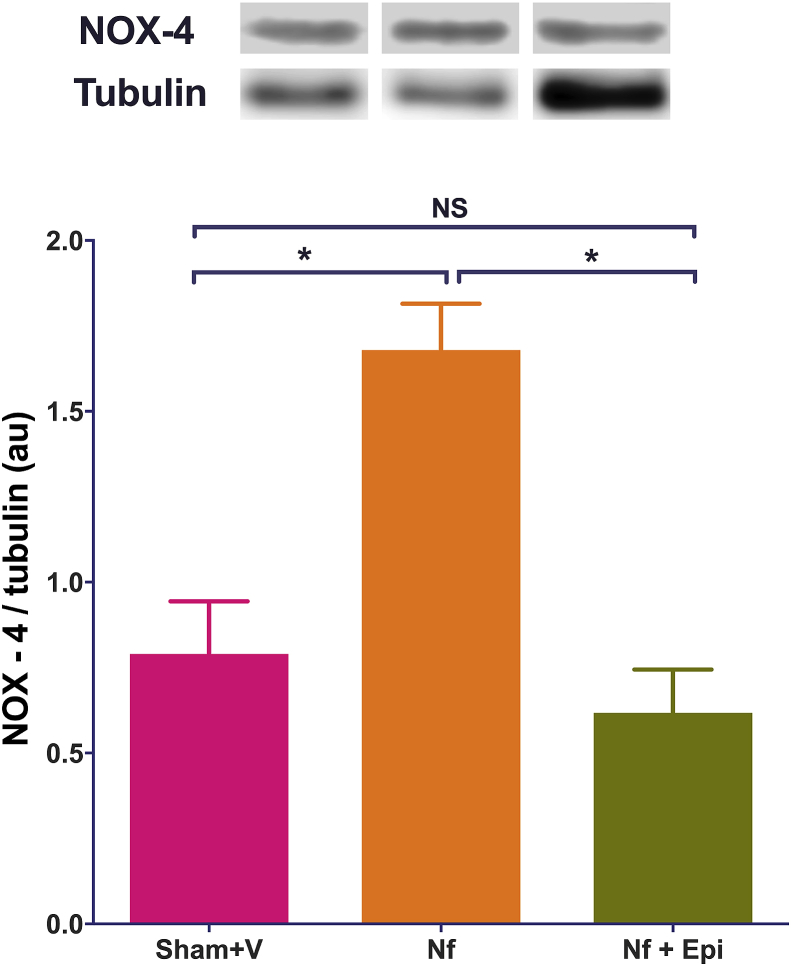
The expression of NOX-4 increased significantly (p < 0.05) in Nf mice as compared with values in Sham + V group. Whilst, Epi treatment blocked the Nf-induced increase in the expression to NOX-4, keeping the values comparable to Sham + V groups (Fig. 6).
Fig. 6.
Analysis of NOX-4 expression in renal tissue analyzed by western blot, at 14 days post-surgery. The index NOX-4/tubulin is reported in arbitrary units (A.U.). The expression of NOX-4 was significantly increased in the group with the nephrectomy 5/6 (Nf). In contrast, in the Nf group treated with Epi (Nf + Epi) at 1mg/kg of body weight/day NOX-4 the expression remained without significant difference vs Sham. Data are presented as mean ± sem. Analysis performed with one-way ANOVA with Post-hoc test: Tukey. *p < 0.05, NS: not significant.
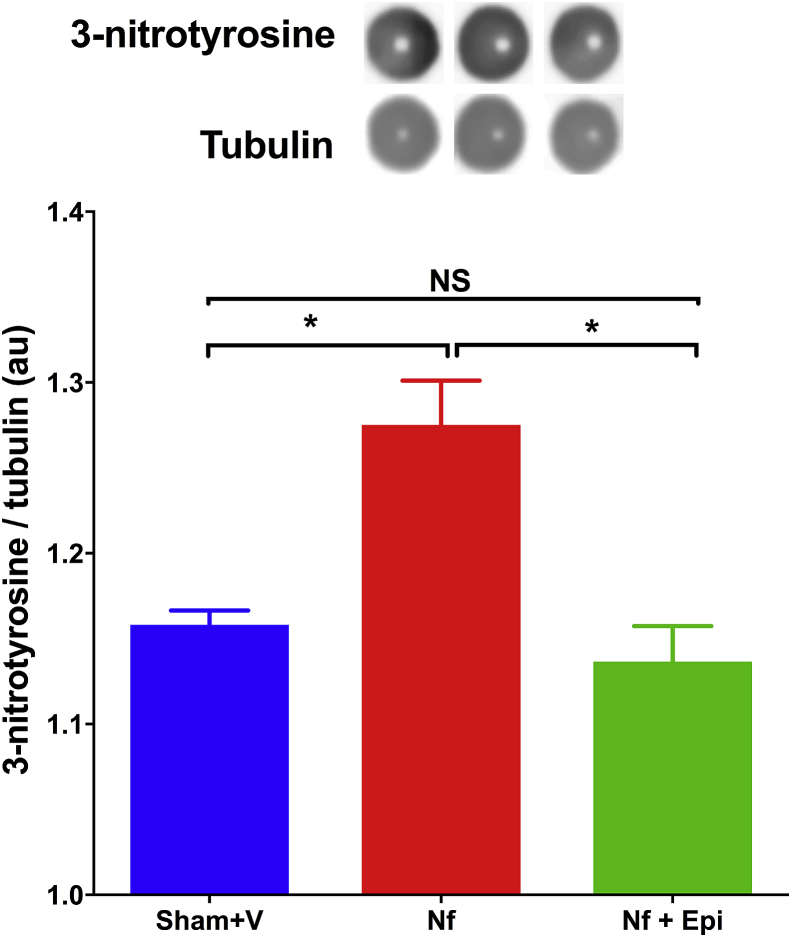
Relative expression of 3-nitrotyrosine in the remnant renal tissue increased in Nf mice in comparison with the Sham + V control group (p < 0.01). Instead, this increment was attenuated in mice treated with Epi, whose values were indistinguishable from that of the control mice (Fig. 7).
Fig. 7.
Dot blot analysis of the formation of 3-nitrotyrosine in the renal tissue at 14 days post-surgery. 3-nitrotyrosine/tubulin index was reported in arbitrary units (A.U.). A significant increase in the formation of 3-nitrotyrosine in the group with Nf vs Sham group was noted. The group with Nf treated with Epi at 1 mg/kg of body weight/day showed a decrease in 3-nitrotyrosine without significant differences vs Sham group. Data are presented as mean ± sem. Analysis performed with one-way ANOVA with Post-Hoc test: Tukey. *p < 0.05, NS: not significant.
4. Discussion
The main findings of this study were: the remarkable Epi-induced dose-dependent positive effects on blood pressure levels, BH4 concentration, AT1-R, NOX-4 and 3-nitrotyrosine expressions and in the progression of renal damage induced by removal of 5/6 of renal tissue. The microscopic findings signal that Epi, has the capacity to preserve renal structure, in concordance with the salutary effects on the serum concentration of waste nitrogen organic compounds, as indices of renal function and also on substances that reflect the activity of the angiotensin-renin system, nitroxidative stress and endothelial dysfunction.
This study employed a model of chronic renal damage, via the ablation of 5/6 parts of renal tissue in C-57BL6 mice [12], which mimics the conditions of progressive renal failure associated to extensive kidney mass loss. The residual kidney tissue goes through structural remodeling and over compensatory function, which lead to glomerular damage, focal fibrosis, proteinuria and a fall of glomerular flow rate, mimicking the natural history of many human cases of CKD [13].
It has been reported that in this model, glomerular hypertrophy and mesangial expansion occur, animals exhibit glomerular sclerosis, interstitial fibrosis and tubule-interstitial atrophy, alongside with the development of high blood pressure, massive proteinuria and progressive renal failure [14], similarly to kidney damage in human patients in classes 4 and 5 of chronic renal disease, according with KDIGO classification [14].
Epi in this model of renal damage produces a substantial improvement on all these variables. Many of the abnormalities caused by the reduction of renal mass were corrected to the point that reached the level of the control animals. Our study, however, did not explore the probable mechanisms by which the flavonoid exerts its beneficial actions. On this regard, it has been established that Epi is able to increase the production of endothelial nitric oxide (NO) [15]. Reestablishing endothelial function in the remaining portion of kidney tissue, the clearance renal capacity improves; serum nitrogenous wasting substances descend, as well as, the blood pressure levels. In this sense, it is important to consider the results observed in the concentration of BH4, an essential cofactor in the synthesis of NO [16]. Partial nephrectomy reduced BH4 serum concentration, which signals endothelial dysfunction. The restoration of such concentration up to the levels of control mice suggests the normalization of endothelial function. Equally striking is the significant effect of Epi on blood pressure, the vasodilatation effect of a restored endothelium at least in part, can explain this salutary effect.
The results reported in this work agree with those reported by Yang et al [17] using quercetin in a rat model of adenine-induced chronic kidney disease and with those from Cao et al [18] and Galisteo et al [19], using catechin an Epi epimer that in our hand is only a partial agonist and may be an antagonist of Epi-induced effects. Our results also agree with those of Gomez-Guzman et al showing that Epi prevents the increase in blood pressure and proteinuria in DOCA-salt rat model with reduction in oxidative stress and endothelin-1 [20], and also with the results of Prince et al, showing that in fructose-fed rats the increases in oxidative stress, inflammation, proteinuria were ameliorated by dietary supplementation with Epi [21]. The potential Epi beneficial effects protecting kidney have shown also in an acute model of LPS-induced nephrotoxicity where Epi prevented TLR4 upregulation and NF-kB activation [22] and in cisplatin-induced nephrotoxicity where Epi prevent the reduction of mitochondrial mass, oxidative phosphorylation complexes and superoxide dismutase levels [23].
In fact, great attention to the usefulness of flavonoids and Epi in particular, as possible agents for the treatment of chronic kidney pathologies has been recently developed [24].
It must be taken in consideration that in humans the progression of renal lesion in is rather complex and multifactorial, intertwining the level of intra-glomerular pressure. Angiotensin II in the first place, but also renin and aldosterone stimulate the expression of TGF-β1 [25]. A common mechanism of renal harm is glomerular hyperfiltration, generally secondary to vasodilation of afferent arteriole, or constriction of the efferent vessel [26]. The intra-glomerular hydraulic changes are partially explained by the fact that there are more AT1-R in the efferent that in the afferent artery [27]. In clinical conditions as diabetes mellitus, obesity or hypertension, in which there is an overexpression of the renin-angiotensin system occur an efferent arteriole constriction that elevates the intra-glomerular filtration pressure [28]. Glomerular lesion involves podocyte harm, allowing an increased transit of macromolecules to the mesangial space, where they prompt inflammatory and fibrotic responses [29]. The deregulation of the renin-angiotensin system is one of the processes that leads to an inflammatory state, to nitroxidative stress and endothelial dysfunction and finally to the progressive and terminal damage of the kidney [28, 30]. Our results show clearly that after the tissue is damaged, an increase in AT1-R and NOX-4 expression/activity take place increasing 3-nitrotyrosine levels, Epi decrease the expression of these markers of kidney damage.
For the aforementioned reasons, markers and variables assessed in this study reflect not only the state of renal function and structure, but moreover exhibit their more important determinants: oxidation, inflammation, endothelial damage and arterial hypertension. The findings in our study indicate that Epi is able to counteract the deleterious effects of subtotal nephrectomy and the structural and functional changes in the remnant kidney tissue. Taken into account the absence of known secondary effects of this flavanol, is pertinent now to explore the possibility of its use in human patients, especially in the first stages of CKD, with the purpose of preserve as much as possible and as long as possible renal functionality and structure.
Declarations
Author contribution statement
Jorge Montes-Rivera, Mónica Arellano-Mendoza, Leonardo Del Valle-Mondragón, Javier Perez Duran, Francisco Villarreal, Ivan Rubio-Gayosso, Eduardo Meaney: Performed the experiments; Analyzed and interpreted the data.
Nayelli Nájera: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Guillermo Ceballos: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Jorge Montes-Rivera was supported by CONACYT, Francisco Villarreal was supported by NIH (DK98717) and Guillermo Ceballos by Conacyt (253769 grant).
Competing interest statement
The authors declare the following conflict of interests: Francisco Villarreal and Guillermo Ceballos are stockholders of Cardero Therapeutics, Co.
Additional information
No additional information is available for this paper.
References
- 1.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Webster A., Nagler E., Morton R., Masson P. Chronic kidney disease. Lancet. 2017;389:1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 3.Szu-Chia C., Jiun-Chi H Ho-Ming S., Yi-Wen C., Jer-Ming C., Shang-Jyh H., Hung-Chun C. Prognostic cardiovascular markers in chronic kidney disease. Kidney Blood Press. Res. 2018;43:1388–1407. doi: 10.1159/000492953. [DOI] [PubMed] [Google Scholar]
- 4.Vidi S. Role of hypertension in progression of chronic kidney disease in children. Curr. Opin. Pediatr. 2018;30:247–251. doi: 10.1097/MOP.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 5.Staples A., Wong C. Risk factors for progression of chronic kidney disease. Curr. Opin. Pediatr. 2010;22:161–169. doi: 10.1097/MOP.0b013e328336ebb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sedeek M., Nasrallah R., Touyz R.M., Hébert R.L. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J. Am. Soc. Nephrol. 2013;24:1512–1518. doi: 10.1681/ASN.2012111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jun M., Venkataraman V., Razavian M., Cooper B., Zoungas S., Ninomiya T., Webster A.C., Perkovic V. Antioxidants for chronic kidney disease. Cochrane Database Syst. Rev. 2012;10:CD008176. doi: 10.1002/14651858.CD008176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutiérrez-Salmeán G., Ortiz-Vilchis P., Vacaseydel C.M., Rubio-Gayosso I., Meaney E., Villarreal F., Ramírez-Sánchez I., Ceballos G. Acute effects of an oral supplement of (-)-epicatechin on postprandial fat and carbohydrate metabolism in normal and overweight subjects. Food Func. 2014;5:521–527. doi: 10.1039/c3fo60416k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prince P., Rodriguez C., Fraga C., Galleano M. Dietary (-)-epicatechin affects NF-kB activation and NADPH oxidases in the kidney cortex of high-fructose-fed rats. Food & Function. 2019;10:26–32. doi: 10.1039/c8fo02230e. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez-Sanchez I., De los Santos S., Gonzalez-Basurto S., Canto P., Mendoza-Lorenzo P., Palma-Flores C., Ceballos-Reyes G., Villarreal F., Zentella-Dehesa A., Coral-Vazquez R. (–)-Epicatechin improves mitochondrial-related protein levels and ameliorates oxidative stress in dystrophic d-sarcoglycan null mouse striated muscle. FEBS J. 2014;281:5567–5580. doi: 10.1111/febs.13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinicke E., Kumar U., Munoz D.G. Quantitative dot-blot assay for proteins using enhanced chemiluminescence. J. Immunol. Methods. 1992;152:227–236. doi: 10.1016/0022-1759(92)90144-i. [DOI] [PubMed] [Google Scholar]
- 12.Ma L.J., Fogo A.B. Model of robust induction of glomerulosclerosis in mice: importance of genetic background. Kidney Int. 2003;64:350–355. doi: 10.1046/j.1523-1755.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 13.Yang H.-C., Zuo Y., Fogo A.B. Models of chronic kidney disease. Drug Discov. Today Dis. Model. 2010;7:13–19. doi: 10.1016/j.ddmod.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey A.S., de Jong P.E., Coresh J., El Nahas M., Astor B.C., Matsushita K., Gansevoort R.T., Kasiske B.L., Eckardt K.U. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies. Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez-Sanchez I., Maya L., Ceballos G., Villarreal F. −)-Epicatechin activation of endothelial cell eNOS, NO and related signaling pathways. Hypertension. 2010;55:1398–1405. doi: 10.1161/HYPERTENSIONAHA.109.147892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan J.C., Pollock J.S. Coupled and uncoupled NOS: separate but equal? Uncoupled NOS in endothelial cells is a critical pathway for intracellular signaling. Circ. Res. 2006;98:717–719. doi: 10.1161/01.RES.0000217594.97174.c2. [DOI] [PubMed] [Google Scholar]
- 17.Hu Yang, Song Yan, Liang Ya-nan, Rong Li. Quercetin treatment improves renal function and protects the kidney in a rat model of adenine-induced chronic kidney disease. Med. Sci. Monit. 2018;24:4760–4766. doi: 10.12659/MSM.909259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao Y., He X.J., Xiang W., Yi Z.W. Protective effect of catechin on renal microvessels in 5/6 nephrectomized rats and its mechanism. Zhong Xi Yi Jie He Xue Bao. 2007;7(6):557–562. doi: 10.3736/jcim20090612. [DOI] [PubMed] [Google Scholar]
- 19.Galisteo M., Garcia-Saura M.F., Jimenez R., Villar I.C., Zarzuelo A., Vargas F. Effects of chronic quercetin treatment on antioxidant defence system and oxidative status of deoxycorticosterone acetate-salt-hypertensive rats. Mol. Cell. Biochem. 2004;259:91–99. doi: 10.1023/b:mcbi.0000021360.89867.64. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Guzman M., Jimenez R., Sanchez M., Zarzuelo M.J., Galindo P., Quintela A.M., López-Sepúlveda R., Romero M., Tamargo J., Vargas F., Pérez-Vizcaíno F., Duarte J. Epicatechin lowers blood pressure, restores endothelial function and decreases oxidative stress, endothelin-1, NADPH oxidase activity in DOCA-salt hypertension. Free Radic. Biol. Med. 2012;52:70–79. doi: 10.1016/j.freeradbiomed.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Prince P.D., Lanzi C.R., Toblli J.E., Elesgaray R., Oteiza P.I., Fraga C.G., Galleano M. Dietary (-)-epicatechin mitigates oxidative stress, NO metabolism alterations, and inflammation in renal cortex from fructose-fed rats. Free Radic. Biol. Med. 2016;90:35–46. doi: 10.1016/j.freeradbiomed.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Prince P., Fischerman L., Toblli J., Fraga C., Galleano M. LPS-induced renal inflammation is prevented by (−)-epicatechin in rats. Redox Biol. 2017;11:342–349. doi: 10.1016/j.redox.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanabe K., Tamura Y., Lanaspa M., Miyazaki M., Suzuki N., Sato W., Maeshima Y., Schreiner G., Villarreal F., Johnson R., Nakagawa T. Epicatechin limits renal injury by mitochondrial protection in cisplatin nephropathy. Am. J. Physiol. Renal. Physiol. 2012;303:F1264–F1274. doi: 10.1152/ajprenal.00227.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vargas F., Romecín P., García-Guillén A.I., Wangesteen R., Vargas-Tendero P., Paredes M., Atucha N., García-Estañol J. Flavonoids in kidney health and disease. Front. Physiol. 2018;9:394. doi: 10.3389/fphys.2018.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf G. Renal injury due to renin-angiotensin-aldosterone system activation of the transforming growth factor-beta pathway. Kidney Int. 2006;70:1914–1929. doi: 10.1038/sj.ki.5001846. [DOI] [PubMed] [Google Scholar]
- 26.Helal I., Fick-Brosnahan G.M., Reed-Gitomer B., Schrier R.W. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 2012;8:293–300. doi: 10.1038/nrneph.2012.19. [DOI] [PubMed] [Google Scholar]
- 27.Razga Z., Nyengaard J.R. Up- and down-regulation of angiotensin II AT1-A and AT1-B receptors in afferent and efferent rat kidney arterioles. J. Renin Angiotensin Aldosterone Syst. 2008;9:196–201. doi: 10.1177/1470320308098592. [DOI] [PubMed] [Google Scholar]
- 28.Siragy H.M., Carey R.M. Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am. J. Nephrol. 2010;31:541–550. doi: 10.1159/000313363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavenstädt H. Roles of the podocyte in glomerular function. Am. J. Physiol. Renal. Physiol. 2000;278:F173–F179. doi: 10.1152/ajprenal.2000.278.2.F173. [DOI] [PubMed] [Google Scholar]
- 30.deQueiroz T., Monteiro M., Braga V. Angiotensin-II-derived reactive oxygen species on baroreflex sensitivity during hypertension: new perspectives. Front. Physiol. 2013;4:105. doi: 10.3389/fphys.2013.00105. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]