Abstract
Incorporation of natural ingredients antioxidants in edible fats can profitably affect their oxidative stability during production and storage. The purposes of the current work were to assess the antioxidant and antimicrobial effect of walnut kernel septum membranes hydroalcohol extract (WHE) in traditional butter (TB). Antioxidant characterization of the extract was screened through methods of DPPH, reducing power and total phenolic assays. After preparation of traditional butter from yogurt, WHE was incorporated into TB at three different concentrations; 0.05%, 0.1% and 0.5% and compared with a control, BHT and tocopherol treated samples (200 mg of BHT and tocopherol/kg). Microbiological studies (Staphylococcus aureus, Coliforms, Psychrotrophic bacteria, yeasts and molds) were done during 90 days of storage time. Changes in Anisidine value (AV), acid value, peroxide value (PV) free fatty acids (FFA), Schaal and Totox value were monitored at 45-day intervals. Sensory evaluation was done using 10 semi-trained panelists based on the 5-point hedonic scale.
It was found that the total phenolic content of WHE was 368.86 mg GAE/g. The BHT had higher antioxidant activity than WHE inhibiting 92.3% of the DPPH radical at 600 μg/mL. Peroxide value of TB treated with tocopherol, BHT and WHE 0.5% was 0.29 ± 0.07, 0.39 ± 0.07 and 0.52 ± 0.04 respectively. Furthermore, the WHE incorporated butter has shown low levels of free fatty acids, Schaal and Totox value when compared to control treatment. The WHE 0.5% incorporated sample had the most antimicrobial activity and it inhibited the growth of all the microorganisms (except Staphylococcus aureus) used in the study. Among the treated TB, the samples treated with the control and WHE 0.05% had the highest sensory attributes score. The study showed that WHE could be an excellent natural origin of antimicrobial and antioxidant agents which can be used in butter.
Keyword: Food science
1. Introduction
Butter with 80–83% lipid contents is an important edible fat in most countries of the world (Mofid et al., 2018). This dairy product is one of the main complexes of every dietary fats, containing further 400 different fatty acids (Papadopoulou and Roussis, 2008). In Iran, the traditional butter (TB) locally is obtained after milk fermentation by churning and in this way the fat content concentrates about 20 times. Since butter is kept for a lengthy time, sensory attributes can transform owing to oxidative rancidity as a result of oxygen catalyzed by lipases from microorganisms or food. At the first step of oxidation, the reaction involving unsaturated fatty acids leads to the configuration of peroxides with a resultant reduction in the safety caused by the formation of secondary toxic compounds. Oxidation eventually leads to formation of a rancid flavor and decreases the economic and nutritional value of fats (Ozkan et al., 2007).
Lipid oxidation may be expeditiously retarded when antioxidants are present. Synthetic antioxidants for instance butylated hydroxyanisole (BHA) as well as butylated hydroxytoluene (BHT) at concentrations ranging from 50-500 ppm in butter are appropriate in the protection of fats, thus, are applied as suppressors of oxidative reaction (Nadeem et al., 2015). However, their usage have been asked because of their toxicity, hence today the quest for developing natural antioxidants has increased. Using natural antioxidants is considered as anti-food borne bacteria and a great opportunity for the oxidative stabilization of milk products (Ozkan et al., 2007).
Among the different sources of natural antioxidants, walnuts (Juglans regia L. family Juglandaceae) are rich in compounds with potent antioxidant and antibacterial activity such as tocopherols and polyphenols (gallic acid and ellagic acid), tannins and melatonin (Tapsell, 2010). Production of walnuts is almost 1,500,000 Tons and the United States, China, Iran, Turkey and Ukraine are the main producers of walnut. Different parts of it such as kernel, leaves and septum have a significant antioxidant effects. It has been recognized that about 50 g of walnuts contains further entirety of phenolics than about 240 ml of apple juice or even a milk chocolate (43 g) (Miraliakbari and Shahidi, 2008). Polyphenols have been recognized to be capable of disturbing the activities of enzymes concerned in the generation of reactive oxygen species, chelating transition metals (Pereira et al., 2008). Besides antioxidant activity, numerous researches confirmed the antimicrobial activity of phenolic extracts such as walnut extract (Sousa et al., 2006), making them a good option to synthetic preservatives. Ajaiyeoba and Fadare (2006) reported that African walnut has antibacterial and antifungal activities linked to phenolic compounds against Staphyloccocus aureus (S. aureus), coliforms and molds. Ajaiyeoba, Onocha, Nwozo, and Sama (2003) showed that the chloroform, methanol, hexane and ethyl acetate fractions of the kernel extract of walnut displayed good antimicrobial activities which were concentration-dependent. Generally, little information in the literature is connected to the augmented shelf life of butter using natural or synthetic antioxidants. Li et al. (2006) have confirmed the antimicrobial and antioxidant activity of walnut products, but information about the kernel septum membranes is almost inexistent.
Despite this interest, no study has been done so far to investigate the WHE as a natural antioxidant for the stabilization of traditional butter. For the reason, this research aimed to examine the effect of treating WHE compared with other antioxidants, namely Butylated hydroxytoluene (BHT), tocopherol on oxidation, chemical, microbial against (S. aureus, coliforms, psychrotrophic bacteria and yeast/mold) and sensory properties of TB during refrigerated storage.
2. Materials and methods
2.1. Preparation of walnut kernel septum membranes extract
Walnut kernel septum membranes fruits (Juglans regia L.) were purchased from local market. An amount of 700 ml Ethyl alcohol (Merck Millipore) and 300 ml of distilled water was mixed with 250 g of walnut kernel septum membranes powder. The mixture was shaken for 24 hr and incubated for one hr at 40 °C and 160 rpm. After cooling the extract was filtered also the ethanol was detached by using a rotary evaporator (Heidolph, Germany). The extract was transferred into the plate and the solvents were removed by using an oven at 45 °C, and then was stored in a glass tube with aluminum foil and kept at 4 °C for antimicrobial and antioxidant activities assays.
2.2. Treatments
Ripened, unsalted butter was purchased from farmers (it was manufactured according to the traditional method) for use in preliminary experiments. To make traditional butter (TB), firstly, cow milk separated to two high in fat and almost no fat phase with hand-operated centrifuges called the “milk wheel”, then yogurt starter added to high fat phase. After preparation of yogurt, it was transferred to goatskin and churning process for fat separation from buttermilk was done. The TB samples were primarily melted at 70 °C then tempered incessantly and moved into 25 °C water bath. WHE was incorporated into TB at three different concentrations; 500 (0.05%), 1000 (0.1%), and 5000 (0.5%) ppm and compared with a control (without WHE, BHT and tocopherol), BHT and tocopherol treated samples (200 mg of BHT and tocopherol/kg). Each treatment was replicated three times, kept at refrigerator temperature and studied at 0, 45 and 90 days.
2.3. Antioxidant characterization of extract
2.3.1. DPPH, reducing power and total phenolic assays
The methodology as modified by Lee et al. (1998) in order to investigate the capability of extracts inhibited the decomposition activity of the DPPH radical.
The reducing power and total phenol of WHE were determined based on the method of Oyaizu (1986) and Siripatrawan and Harte (2010), respectively.
2.4. Physicochemical analysis
2.4.1. Moisture
Five grams of TB was weighed into a dish which is contained 10 g of pumice. The plate was dried at 102 ± 2 °C for 2 h. This drying process was repeated until constant weight was obtained. The moisture content was measured by the weight loss corrected for a blank.
2.4.2. Solids-non-fat (SNF)
Five grams of butter was measured as well as the plate was placed in an oven at 102 ± 2 °C for almost 15 h to eliminate the water. Petroleum ether was added later and then cooled in order to dissolve the fat and SNF. The SNF content was measured from the increase in the mixed weight of the plate, rod and crucible (Evers et al., 1999).
2.4.3. Fat and sodium chloride content
The crude fat content was analyzed according to the fat in butter direct method (938.06B) and sodium chloride content were determined by the Mohr methods (AOAC, 2000., Pearson, 1976).
2.4.4. Free fatty acid and acid value
Fatty acid analysis was measured based on the method of Bannon et al. (1982).
In order to obtain the acid value, 5 g of melted TB was dissolved in 50 ml of alcohol and then titrated with KOH (0.1 N) (Farag et al., 1989).
2.4.5. Peroxide value (PV)
The primary products from oxidation were determined by the International Dairy Federation (IDF) and standard method 74a was applied (IDF, 1991). Approximately 0.01–0.3 g of butter was combined with 9.8 mL chloroform 70%-methanol 30%. Afterward ammonium thiocyanate solution was added. Following this, 50 μL iron solution was added and stored for 5 minutes at ambient temperature. Finally the solution absorbance was read at 500 nm.
2.4.6. Anisidine test (AV)
The oil samples were allowed to react with p-anisidine to produce a colored compound, and the absorbance values were read at 350 nm using a spectrometer (LKB Novaspec II; Pharmacia, Sweden) AV was measured based on the method of Wu et al. (2009). Isooctane is the fat solvent in this test. The investigative reagent is 1 ml 0.25% anisidine in glacial acetic acid.
2.4.7. Schaal test
The oxidation of 50 g butter in 125 ml enclosed glass cups was controlled via evaluating the formation of hydroperoxides at 63 °C. The level of hydroperoxide was evaluated as PV with regard to the procedure of the American Oil Chemists' Society (AOCS, 1986).
| CPV (%) = 100(PV2-PV1)/PV1 |
Where PV1 is peroxide value at the start of the experiment and PV2 is peroxide value at the last part of the test.
2.4.8. Totox value
The Totox (total oxidation) value was calculated as Totox V = (2 × PV) + AV (Pearson, 1976). Where AV is anisidine value and PV is peroxide value.
2.5. Microbiological analysis
Ten grams of TB was mixed in 90 mL of 0.85% (w/v) sterilized NaCl solution and kept in a water bath at 45 °C until the butter melted. Thereafter, serial dilutions were provided. Coliforms were determined using violet red bile glucose agar (VRBGA). The plates were kept at 37 °C for 24 h. Staphylococcus aureus were counted on Baird Parker agar (BPA, Merck) including potassium tellurite and egg yolk (Merck), and lastly incubation at 37 °C for 48 h. Yeasts and molds were counted on Sabouraud Dextrose Agar (SDA, Merck, Germany) with Chloramphenicol and kept at 25 °C for 3–5 days. Psychrotrophic bacteria were counted by Plate Count Agar (PCA, Merck, Germany), following the incubation for ten days at 6.5 °C.
2.6. Sensory evaluation
Sensory investigation of oils was performed using quantitative descriptive analysis. For this purpose a group of ten semi-trained panelists as of the academic personnel of Department of Food Hygiene and Quality Control of the Urmia University, Iran (4 women and 6 men, ages 23–55 yr) were recruited. Butter samples in terms of texture, color, flavor and odor and overall acceptability were determined using five point hedonic level. Sensory panel members were needed so as to score the samples from 1 to 5. (1 = Very bad; 2 = bad; 3 = good; 4 = very good; 5 = excellent) (Dimić et al., 2012; Janiaski et al., 2016).
2.7. Statistical analysis
All data were analyzed by means of the SPSS statistical package (version 21.0; IBM Corp, Armonk, USA). The experimental design used in this study was a completely randomized design. All tests were done in triplicate and the means were evaluated using Tukey's test (P < 0.05). Microbiological counts were reported with log CFU/g.
3. Results and discussion
3.1. Antioxidant characterization of extract
3.1.1. DPPH
There are many methods to evaluate the antioxidant properties that are so useful but many researches had emphasized the necessity to apply more than one kind of antioxidant activity analysis to evaluate the antioxidant action (Mojaddar Langroodi et al., 2018; Nadeem et al., 2015). The WHE, at 37.5 μg/mL, indicated a scavenging effect of 56.40% so as to augment to 91.73% at 600 μg/mL (Fig. 1). The BHT had higher antioxidant activity than WHE inhibiting 92.3% of the DPPH radical at 600 μg/mL. On the contrary, by decreasing the sample concentrations, the WHE showed more antioxidant ability than BHT. The scavenging capabilities of WHE exhibited concentration dependant effects, the higher the concentration of WHE with the stronger antioxidant activity. Espı'n et al. demonstrated that the radical scavenging capacity of the lipid fraction of walnut oil was dependent on the amount of tocopherols Espín et al. (2000). As reported by Bakkalbasi et al. (2013), methanolic walnut extract with 38.8–85.2% inhibition had been authenticated as the strongest free radical scavengers among other nuts. Proestos, Chorianopoulos, Nychas, and Komaitis (2005) showed that the higher level of phenolic substances is correlated to the content of free radical scavenging activity. The tocopherols accompanied by 76–98% to the total antioxidant activity of every nut oils and α-tocopherol alone contributed 65–96% in the DPPH assay (Ferreira et al., 2007).
Fig. 1.
2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity of walnut kernel septum membranes hydroalcoholic extract (WHE) and butylated hydroxytoluene (BHT). Values are given as mean ± SD. Different letters indicate a statistically significant difference (p < 0.05).
3.1.2. Reducing power
This assay is often used to evaluate the ability of an antioxidant to donate electrons. This method is founded on the decrease of the ferricyanide complex to the ferrous ion. In the present study, at all concentrations especially at 0.25 mg/ml, WHE has more reducing capacity than BHT. As it showed at 0.25 and 2 mg/ml concentrations the lowest (1.5 and 1.76 mg/ml in BHT and WHE, respectively) and highest (2.08 and 2.16 mg/ml in BHT and WHE, respectively) reducing power respectively (Fig. 2). In Pereira et al. (2007) study walnut leaves indicated high reducing powers at very low concentrations (<1 mg/ml), being even more powerful than BHA (3.6 mg/ml). Furthermore, Meir et al. (1995) showed that walnut extracts have a high potent of reducing power. The results of DPPH and reducing power test was demonstrated high antioxidant capability of WHE in compare to BHT.
Fig. 2.
Reducing power of walnut kernel septum membranes hydroalcoholic extract (WHE) and BHT. Values are given as mean ± SD. Different letters indicate a statistically significant difference (p < 0.05).
3.1.3. Total phenol
The total phenolic content of WHE was 368.86 mg GAE/g. However, other researchers registered a large extent of lower levels in walnuts: between 58.9 and 95.1 mg GAE/g (Slatna et al., 2015). The result of phenolic content revealed that there is a significant correlation between phenolic compounds and antioxidant properties. Miraliakbari and Shahidi (2008) indicated that the amount of total phenol in walnuts is detectably more than in other nuts. Although, Stampar et al. (2006) investigated low concentrations of the total phenols (0.32 mg/g oil) in walnut extract. The quantity of total phenol can vary due to plant age, harvesting season, geographical sources, climate, and extraction method (Miraliakbari and Shahidi, 2008).
3.2. Physicochemical results
3.2.1. The chemical composition of butter
Fat, salt, SNF, and moisture of the butter used in this study were 79.7 ± 0.8%, 0.3 ± 0.04%, 5.2 ± 0.6% and 15.1 ± 0.2% respectively. In this study, butter was prepared using traditional method. In this method, because of the extraction of butter from yogurt, some buttermilk containing protein, phosphorus and calcium also enters the final product, so the amount of SNF is higher than commercial butter. Other results were consistent with the standards.
3.2.2. Peroxide value
Evaluation of PV has been extensively practical to butter with mixed results about the sensory realization of off-flavors. This value is considered to be the one of the most efficient methods for determining the initial stages of oxidative rancidity. Changes in PV of samples for 90 days are presented in Fig. 3. In this study, which is in agreement with Savage et al. (2001) research, the mean of walnut PV at day 0 was 0.2 meq O2/kg oil. The Control group had detected more PV compared with the treated samples during storage time (p < 0.05). With increasing storage time, the PV tends to increase more rapidly in WHE 0.05% treated sample, in comparison with WHE 0.5%, WHE 0.1, BHT and tocopherol samples. The PV of all groups was higher at the last day of storage time compared to the 0 and 45 days of storage. The current outcomes are in accordance with Simsek (2011), who showed that the least PV of butter was at the beginning stages of storage time. Jebson, McDowell, and Russell (1974) concluded an increase in the PV of butter treatments kept which were at 4 °C after 120 and 240 days. At the end of storage period, control sample revealed the highest PV of 1.42 as compared to 0.29 (meq/kg), in tocopherol treated sample which are lower than the threshold (5 meq O2/kg) which shows the commencement of the lipid peroxidation (FAO, 1992). The antioxidant activities of WHE increased with increasing amount of extract. The sample treated with tocopherol was found to be the least advancement of peroxides than other samples of the peroxides. The variation in peroxides development in tocopherol and BHT samples could be caused by the existence of various antioxidant compounds (Christopoulos and Tsantili, 2015).
Fig. 3.
The changes in PV (meq O2 per kg fat) of butter in different treatment groups during storage at at different time points. Values are given as mean ± SD.
3.2.3. Anisidine value, Totox value, Schaal oven test
AV test is the most broadly used method for the purpose of secondary and tertiary steps of autoxidation of fats, and oils. Acording to the results (Fig. 4) the control had the highest AV, thus showed a higher level of oxidation that might be due to the presence of prooxidants, which speeded the autoxidation process. BHT and WHE 0.05% treated samples were found to be the most effective in reducing the oxidation of butter in this test. Addition of BHT and WHE 0.05% was more competent in the retardation of oxidation products and significantly lower AV values of the experimental samples could be attributed to the phenolic antioxidants naturally present in WHE. In this study, AV of sample treated with tocopherol was increased gradually until 45 days (1.94 ± 0.18 on 45 days) of storage then decreased to (1.08 ± 0.07 on 90 days). The decrease in AV after 45 days may be due to the dimerization of two aldehydes to form stable malonaldehydes (Fedele and Bergamo, 2001).
Fig. 4.
The changes in anisidine value of butter in different treatment groups during storage at different time points. Values are given as mean ± SD.
TotoxV is thought to combine evidence about the past history of the oil (AV) with the present state of the oil (PV). TotoxV and the Schaal oven changes in samples are shown in Table 1. TotoxV of control sample generally was the highest value between treatments. By increasing the amount of the WHE, TotoxV values were decreased which can be correlated to the presence of phenolic agents, which offered resistance against the PV in the accelerated oxidation reaction. Tocopherol treated sample with 1.66 ± 0.29 and control sample with 7.51 ± 1.2 had the lowest and highest TotoxV, respectively. Jahurul et al. (2014) concluded that tocopherols the same as antioxidants can stabilize oil. No significant differences were found in the WHE 0.1% and WHE 0.05% incorporated samples.
Table 1.
Effect of different treatments on lipid oxidation in schaal and Totox tests. Values are given as mean ± SD. Different letters in each column indicate a statistically significant difference (P < 0.05).
| Treatment/test | Totox | Schaal (%) |
|---|---|---|
| Control | 7.51 ± 1.2a | 12.1 ± 1.82a |
| WHE 0.5% | 3.74 ± 0.22b | 2.75 ± 0.37d |
| WHE 0.1% | 4.12 ± 0.31c | 11 ± 1.91a |
| WHE 0.05% | 3.8 ± 0.14b | 8.1 ± 0.77b |
| BHT | 1.96 ± 0.30d | 3.76 ± 0.42c |
| Tocopherol | 1.66 ± 0.29d | 11.3 ± 1.05a |
Ordinarily, Schaal oven method is applied for evaluation of oxidation of oils but it is also applied in dairy industry. The control sample showed the highest Schaal test value followed by tocopherol sample. There are no significant differences among the control, WHE 0.1% and tocopherol butter samples in Schaal test. The addition of WHE to TB decreased the oxidation of treated samples in comparison with the control group. In the present study WHE 0.5% and BHT showed more antioxidant ability than other samples. By increasing the level of phenolic substances, the number of hydroxyl groups present in the reaction increased. Therefore, the possibility of hydrogen conveyed to free radicals will increase (Chen et al., 2014). Walnut phenolics supposedly demonstrate strong antioxidant and free radical-scavenging capacities (Colaric et al., 2005).
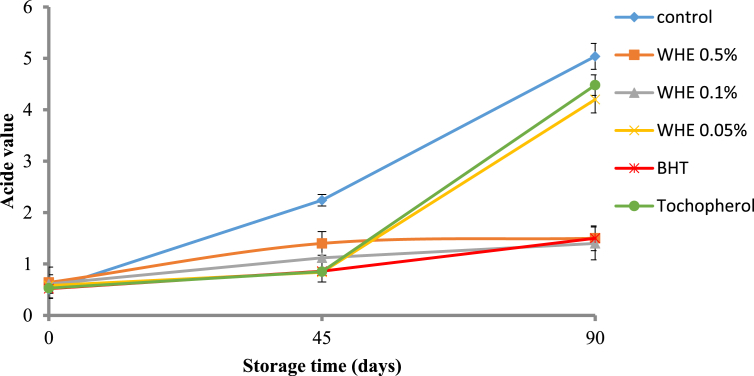
3.2.4. Titratable acidity
In the present study the titratable acidity value of all butter samples increased during the storage period (see Fig. 5). This increase has a detected effect on the quality of the product during storage time, mainly regarding sensorial characteristics such as color, texture and odor, which are negatively affected (Nadeem et al., 2015). Among all samples the WHE 0.1% had the lowest titratable acidity value, 1.4 at 90 day, followed by BHT.
Fig. 5.
The changes in acid value butter in different treatment groups during storage at different time points. Values are given as mean ± SD.
3.2.5. Free fatty acids
The formation of free fatty acids are due to hydrolysis as well as by cleavage and oxidation of lipids double bonds. The increase in free fatty acid contents of all the treatments and control during the storage of 90 days contributed to this phenomenon (Nadeem et al., 2013). On the other hand, least increase in FFA was in sample treated with WHE 0.1% and BHT throughout storage period which could be due to the presence of more antioxidant compounds like phenolic compounds in WHE which is in agreement with the results reported by Shende et al. (2014). A free fatty acid value of fresh butter which is between 0.14 to 39% is generally assumed to be passable for butter fat; a higher content of free fatty acid is related to poor storage condition (Nadeem et al., 2013; Erkaya and Sengul, 2015). In the present study all treated samples were in this level on 0 day (0.25, 0.32, 0.29, 0.3, 0.26 and 0.26 in the control, WHE 0.5%, WHE 0.1%, WHE 0.5%, BHT and tocopherol, respectively). Statistical analysis showed no significant differences in free fatty acid between the WHE 0.5%, WHE 0.1% treated samples and BHT on 90 days (see Fig. 6).
Fig. 6.
The changes in free fatty acid of butter in different treatment groups during storage at different time points. Values are given as mean ± SD.
3.3. Microbiological analysis
Presence of substances such as serum and water affect storage time of butter because they are a suitable medium for spoilage microorganisms. The growth of proteolytic organisms such as lactobacillus, yeasts and Pseudomonas spp in butter is a major cause of its deterioration (Ayar et al., 2007). Microbiological results are presented in Table 2. Results indicate that the extension of storage time increased all bacteria count in samples. In the present study, WHE 0.5% treatment was the most effective in control Coliforms and Psychrotrophic bacteria. Increase in total psychrotrophic bacteria is more pronounced when the dairy products are produced in poor hygiene situations. The primary PTC (day 0) of samples which was between 1.08 log CFU/g, in BHT samples, to 2.24 log CFU/g in the controls was studied. After 90 days of storage, PTC reached 9.06, 7.11, 7.39 and 7.88 log/CFU, in control, WHE 0.5%, BHT and tocopherol treated samples respectively. It is evident that WHE treatment can inhibit the PTC in TB. The present outcomes could be credited to the inhibitory effect of the WHE controlling Gram-negative bacteria population. The mechanism of action of WHE is normally considered to be the derangement of the cytoplasmic membrane, active transport, electron flow and coagulation of cell ingredients. The bacteria behavior was alike (except S. aureus), but different percentages of bacteria growth inhibition were observed, this can be attributed mostly to the concentration of WHE and chemical composition of treatments containing compounds such as polyphenol and flavones which cause rupture of membrane (Altuntas and Ozkan, 2008). Initial S. aureus count was 1.19 log CFU/g, increasing during storage to reach final count of 8.33, 8.69, 8.77 and 8.81 log CFU/g, in the control, WHE 0.5%, WHE 0.1% and WHE 0.05% samples, respectively, which indicates that the extract does not have effect on gram-positive bacteria. Contrary to the present study, Pereira et al. (2007) showed that Gram positive bacteria such as S. aureus which were most sensitive were inhibited by walnut extracts. Alkhawajah (1997) reported that the walnut inhibited the growth of several species of microorganisms, showing both E. coli and pathogenic yeast.
Table 2.
The changes in Psychrotrophic bacteria, Staphylococcus aureus, Coliforms and yeasts–molds counts of butter during storage at different time points. Values are given as mean ± SD. Means within the same row and the same column with different letters are significantly different (P < 0.05).
| Treatment | Days | Psychrotrophic bacteria | Yeasts–molds | Coliforms | Staphylococcus aureus |
|---|---|---|---|---|---|
| Control | 1 | 2.24 ± 0.82aA | 1.3 ± 0.74aA | 0aA | 1.19 ± 0.16aA |
| 45 | 8.54 ± 0.14bA | 8.67 ± 0.77bA | 3.7 ± 0.43bA | 6.83 ± 0.61bA | |
| 90 | 9.06 ± 0.74cA | 8.77 ± 0.6bA | 4.01 ± 0.24bA | 8.33 ± 0.97cA | |
| WHE 0.5% | 1 | 1.19 ± 0.19aB | 1.01 ± 0.34aB | 0aA | <10aA |
| 45 | 5.07 ± 0.25bB | 5.01 ± 0.85bB | 0aB | 4.14 ± 0.51bB | |
| 90 | 7.11 ± 0.64cB | 7.39 ± 0.36cB | 2.17 ± 0.14bB | 8.69 ± 0.34cA | |
| WHE 0.1% | 1 | 1.49 ± 0.98aB | 1.26 ± 0.23aA | 0aA | 1.11 ± 0.24aA |
| 45 | 6.03 ± 0.74bC | 5.11 ± 0.43bB | 2.35 ± 0.09bC | 4.6 ± 0.32bB | |
| 90 | 9.14 ± 0.33cA | 8.51 ± 0.27cA | 2.17 ± 0.34bC | 8.77 ± 0.17cA | |
| WHE 0.05% | 1 | 2.09 ± 1.12aA | 1.5 ± 0.32aA | 0aA | 1.62 ± 0.23aA |
| 45 | 8.36 ± 0.44bA | 7.21 ± 0.71bA | 4. 1 ± 0.68bD | 4.65 ± 0.46bB | |
| 90 | 9.81 ± 0.61cA | 9.02 ± 0.18cC | 2.14 ± 0.71cC | 8.81 ± 0.57cA | |
| BHT | 1 | 1.08 ± 0.24aB | <10aB | 0aA | <10aA |
| 45 | 5.94 ± 0.33bC | 4.69 ± 0.29bB | 4.15 ± 0.39bD | 4.01 ± 0.71bB | |
| 90 | 7.39 ± 0.91cB | 7.22 ± 0.72cB | 3.49 ± 0.16cB | 3.81 ± 0.62bB | |
| Tocopherol | 1 | 2.16 ± 0.17aA | 1.74 ± 0.11aA | 0aA | 1.77 ± 0.19aC |
| 45 | 6.04 ± 0.19bC | 4.11 ± 1.24bB | 3.69 ± 0.14bD | 3.9 ± 0.29bB | |
| 90 | 7.88 ± 0.15cB | 7.17 ± 0.57cB | 3.11 ± 0.18cB | 6.71 ± 0.23cC |
According to yeasts/molds species recognized to be concerned in the spoilage of TB, all of the antimicrobial treatments evaluated in this study lead to significantly lower counts for yeasts/molds in WHE 0.5%, WHE 0.1%, WHE 0.05%, BHT and tocopherol TB as compared to the control groups during storage time. In Wei et al. (2017) study, it was reported that yeasts/molds are associated with traditional dairy products such as butter (Wei et al., 2017). In agreement with this study, Erkaya and Sengul (2015) showed that the mold-yeast counts of TB detectably differed depending on the storage time. In the current study the yeasts/molds count increased eventually in all samples during storage. There were statistically (P > 0.05) different outcomes among TB treatments throughout the storage. Few studies have been conducted on the antimicrobial effects of plant extracts in butter. Ayar et al. (2007) study, showed that oregano extracts had highest inhibition effect on yeasts and moulds, proteolytic, lipolytic microorganisms and coliform in butter, and sage was effective on lactobacilli and total bacteria.
Gharibzahedi et al. (2014) concluded that walnut leaves and hazelnuts extracts had good antimicrobial activities. The phenolic combinations reduced the microbial population in treated samples with WHE, through enzyme inhibition or protein binding. For example, DNA gyrase enzyme of polyphenols showed antimicrobial activity (Cushnie and Lamb, 2005).
3.4. Sensory evaluation
Colors and flavors are the most important factors for acceptance of butter by consumers. Furthermore, the shelf-life of butter is affected by a number of chemical alterations through oxidation, and consequently, development of inappropriate changes in sensory characteristics (Mert and Demirkesen, 2016). Mean values of sensory properties of treatment groups during storage time are presented in Fig. 7. It was observed that the color scores of the TB sample treated with WHE 0. 5% were not in the acceptable range. Among the treated TB, the samples treated with the control and WHE 0.05% had the highest sensory attributes score. There was no difference in flavor and texture of the TB samples treated with WHE 0.5% and WHE 0.05% was considered. The flavor and overall acceptability scores of WHE 0.05% and WHE 0.1% were not different together. The scores of sensorial evaluation of the treated samples were decreased by WHE. This is due to the unpleasant effect of herbal extract and essential oils. The total amount of PV and free fatty acids may be the cause of the expansion of negative flavor changes in butter throughout the storage (Blake and Marangoni, 2015). According to the International Dairy Federation the standard value for milk fat is 0.2 meq oxygen/kg fat. In this study, treatments containing tocopherol and 0.5% extract were below this limit until the 45th day.
Fig. 7.
The changes in sensory attributes of butter in different treatment groups.
4. Conclusions
The present results indicate that the utilization of WHE as natural origin agent is safe in the 0.05%, 0.1% and 0.5% concentrations and has benefits for oxidative stability and inhibited the microbial growth of butter stored under suitable conditions. Butter treated with WHE 0.5% was found to be better for inhibiting the growth of bacteria and resistance against the development of peroxides than the TB incorporated with tocopherol. In addition, it was proved that WHE has a powerful antioxidant activity and inhibit the population of different gram-negative bacteria, which can lead to health problems. Antioxidant activity of samples treated with WHE 0.5% and WHE 0.05% in Schaal and AV tests, respectively was more than TB treated with tocopherol. Eventually it was found that WHE 0.05% was the most appropriate WHE concentration for sensory properties of TB.
In addition to examining the effect of the WHE on the fatty acid profiles of the butter by instrumental methods, further work needs to be done in order to elucidate the mechanism of action of the active compounds contained in the WHE and extraction of these compounds for use in food as antioxidant.
Declarations
Author contribution statement
Tooraj Mehdizadeh: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Neda Mohammadipour: Performed the experiments.
Ali Mojaddar Langroodi: Analyzed and interpreted the data; Wrote the paper.
Mojtaba Raeisi: Analyzed and interpreted the data.
Funding statement
This work was supported by the Faculty of Veterinary Medicine, and Biotechnology Research Center of Urmia University.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Ajaiyeoba E.O., Fadare D.A. Antimicrobial potential of extracts and fractions of the African walnut – Tetracarpidium conophorum. Afr. J. Biotechnol. 2006;5(22):2322–2325. [Google Scholar]
- Ajaiyeoba E.O., Onocha P.A., Nwozo S.O., Sama W. Antimicrobial and cytotoxicity evaluation of Buchholzia coriacea stem bark. Fitoterapia. 2003;74(7-8):706–709. doi: 10.1016/s0367-326x(03)00142-4. [DOI] [PubMed] [Google Scholar]
- Alkhawajah A.M. Studies on the antimicrobial activity of Juglans regia. Am. J. Chin. Med. 1997;25(2):175–180. doi: 10.1142/S0192415X97000202. [DOI] [PubMed] [Google Scholar]
- Altuntas E., Ozkan Y. Physical and mechanical properties of some walnut (Juglans regia L.) cultivars. Int. J. Food Eng. 2008;4(4):1–14. [Google Scholar]
- AOAC . seventeenth ed. AOAC; Gaithersburg, Md: 2000. Official Analytical Chemists Intl. Official Methods of Analysis. [Google Scholar]
- AOCS . In: Official Methods and Recommended Practices. third ed. Walker R., editor. American Oil Chemists' Society; Champaign, IL, USA: 1986. [Google Scholar]
- Ayar A., Özcan M., Akgul A. Effect of oregano and sage extracts on microbiological quality of molten butter. Food Sci. Technol. Res. 2007;10(2):111–113. [Google Scholar]
- Bakkalbasi E., Yilmaz O.M., Yemis O., Artik N. Changes in the phenolic content and free radical-scavenging activity of vacuum packed walnut kernels during storage. Food Sci. Technol. Res. 2013;19(1):105–112. [Google Scholar]
- Bannon C.D., Craske J.D., Breen G.J., Hai N.T., Harper N.L., Kerry L., Orourke L. Analysis of fatty acid methyl esters with high accuracy and reliability. J. Chromatogr. 1982;247(1):63–69. doi: 10.1016/s0021-9673(01)92621-4. [DOI] [PubMed] [Google Scholar]
- Blake A.I., Marangoni A.G. Factors affecting the rheological properties of a structured cellular solid used as a fat mimetic. Food Res. Int. 2015;74:284–293. doi: 10.1016/j.foodres.2015.04.045. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhang Y., Zu Y., Yang L., Lu Q., Wang W. Antioxidant effects of rosemary extracts on sunflower oil compared with synthetic antioxidants. Int. J. Food Sci. Technol. 2014;49(2):385–391. [Google Scholar]
- Christopoulos M.V., Tsantili E. Participation of phenylalanine ammonialyase (PAL) in increased phenolic compounds in fresh cold stressed walnut (Juglans regia L.) kernels. Postharvest Biol. Technol. 2015;104:17–25. [Google Scholar]
- Colaric M., Veberic R., Solar A., Hudina M., Stampar F. Phenolic acids, syringaldehyde, and juglone in fruits of different cultivars of Juglans regia L. J. Agric. Food Chem. 2005;53(16):6390–6396. doi: 10.1021/jf050721n. [DOI] [PubMed] [Google Scholar]
- Cushnie T.P.T., Lamb A.J. Antimicrobial activity of flavanoids. Int. J. Antimicrob. Agents. 2005;26(5):343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimić E., PrEmović T., Takač&i A. Effects of the contents of impurities and seed hulls on the quality of cold-pressed sunflower oil. Czech J. Food Sci. 2012;30(4):343–350. [Google Scholar]
- Erkaya T., Sengul M. Bioactivity of water soluble extracts and some characteristics of white cheese during the ripening period as effected by packaging type and probiotic adjunct cultures. J. Dairy Res. 2015;82(1):47–55. doi: 10.1017/S0022029914000703. [DOI] [PubMed] [Google Scholar]
- Espín J.C., Soler-Rivas C., Wichers H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000;48(3):648–656. doi: 10.1021/jf9908188. [DOI] [PubMed] [Google Scholar]
- Evers J.M., Crawford R.A., Wightman L.M., Gijsbert J., Beutick G.,J., Contarini G., Farrington D.S. An accurate and rapid method for the direct determination of fat in butter, butter-margarine blends and milk fat spreads. Int. Dairy J. 1999;9(10):675–682. [Google Scholar]
- FAO . Programme mixte FAO/OMS sur les normes alimentaires; Rome: 1992. Codex Alimentarius. [Google Scholar]
- Farag R.S., Daw Z.Y., Abo-Raya S.H. Influence of some spice essential oils on Aspergillus parasiticus growth and production of aflatoxins in a synthetic medium. J. Food Sci. 1989;54(1):74–76. [Google Scholar]
- Fedele E., Bergamo P. Protein and lipid oxidative stresses during cheese manufacture. J. Food Sci. 2001;66(7):932–935. [Google Scholar]
- Ferreira I.C.F.R., Barros L., Soares M.E., Bastos M.L., Pereira J.A. Antioxidant activity and total phenolic contents of Olea europaea L. leaves sprayed with different copper formulations. Food Chem. 2007;103(1):188–195. [Google Scholar]
- Gharibzahedi S.M.T., Mousavi S.M., Hamedi M., Khodaiyan F. Determination and characterization of kernel biochemical composition and functional compounds of Persian walnut oil. J. Food Sci. Technol. 2014;51(1):34–42. doi: 10.1007/s13197-011-0481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Dairy Federation (IDF) IDF; Brussels, Belgium: 1991. Anhydrous milk fat: determination of peroxide value. IDF standard 74A. [Google Scholar]
- Jahurul M.H.A., Zaidul I.S.M., Norulaini N.A.N., Sahena F., Abedin M.Z., Ghafoor K., Mohd Omar A.K. Characterization of crystallization and melting profiles of blends of mango seed fat with palm oil mid-fraction as cocoa butter replacers using differential scanning calorimetry and pulse nuclear magnetic resonance. Food Res. Int. 2014;55:103–109. [Google Scholar]
- Janiaski D.R., Pimentel T.C., Cruz A.G., Prudencio S.H. Strawberry-flavored yogurts and whey beverages: what is the sensory profile of the ideal product? J. Dairy Sci. 2016;99:5273–5283. doi: 10.3168/jds.2015-10097. [DOI] [PubMed] [Google Scholar]
- Jebson R., McDowell A., Russell R.W. The effect of storage temperature on the quality of salted sweet cream butter. N. Z. J. Dairy Sci. 1974;9:163–165. [Google Scholar]
- Langroodi A.M., Tajik H., Mehdizadeh T., Moradi M., Moghaddas Kia E., Mahmoudian A. Effects of sumac extract dipping and chitosan coating enriched with Zataria multiflora Boiss oil on the shelf-life of meat in modified atmosphere packaging. LWT Food Sci. Technol. 2018;98:372–380. [Google Scholar]
- Lee S.K., Mbwambo Z.H., Chung H.S., Luyengi L., Games E.J.C., Mehta R.G. Evaluation of the antioxidant potential of natural products. Comb. Chem. High Throughput Screen. 1998;1:35–46. [PubMed] [Google Scholar]
- Li L., Tsao R., Yang R., Liu C.M., Zhu H.H., Young J.C. Polyphenolic profiles and antioxidant activities of heartnut (Juglans ailanthifolia var. cordiformis) and Persian walnut (Juglans regia L.) J. Agric. Food Chem. 2006;54(21):8033–8040. doi: 10.1021/jf0612171. [DOI] [PubMed] [Google Scholar]
- Meir S., Kanner J., Akiri B., Philosoph-Hadas S. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J. Agric. Food Chem. 1995;43(7):1813–1819. [Google Scholar]
- Mert B., Demirkesen I. Reducing saturated fat with oleogel/shortening blends in a baked product. Food Chem. 2016;199:809–816. doi: 10.1016/j.foodchem.2015.12.087. [DOI] [PubMed] [Google Scholar]
- Miraliakbari H., Shahidi F. Oxidative stability of tree nut oils. J. Agric. Food Chem. 2008;56(12):4751–4759. doi: 10.1021/jf8000982. [DOI] [PubMed] [Google Scholar]
- Mofid V., Mousavi M., Emam-Djomeh Z., Razavi S.H., Gharibzahedi S.M.T., Jahanbakhsh F. Rheological characterization of functional walnut oil-enriched butters stabilized by the various polysaccharides. J. Dispers. Sci. Technol. 2018;39(4):469–477. [Google Scholar]
- Nadeem M., Chen Situ C., Mahmud A., Khalique A., Imran M., Fazal Rahman F., Sabir Khan S. Antioxidant activity of sesame (Sesamum indicum L.) cake extract for the stabilization of olein based butter. J. Am. Oil Chem. Soc. 2013;91(6):967–977. [Google Scholar]
- Nadeem M., Mahud A., Imran M., Khalique A. Enhancement of the oxidative stability of whey butter through Almond (Prunus dulcis) peel extract. J. Food Process. Preserv. 2015;39(6):591–598. [Google Scholar]
- Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986;44:307–315. [Google Scholar]
- Ozkan G., Simsek B., Kuleasan H. Antioxidant activities of Satureja cilicica essential oil in butter and in vitro. J. Food Eng. 2007;79(4):1391–1396. [Google Scholar]
- Papadopoulou D., Roussis I.G. Inhibition of butter oxidation by N-acetyl-cysteine and glutathione. Eur. Food Res. Technol. 2008;227:905–910. [Google Scholar]
- Pearson D. seventh ed. Churchill Livingstone; Edinburgh: 1976. The Chemical Analysis of Food. [Google Scholar]
- Pereir J.A., Oliveira I., Sousa A., Valentão P., Andrade P.B., Ferreira I.C.F.R., Ferreres F., Albino Bent A., Seabra R., Estevinho L. Walnut (Juglans regia L.) leaves: phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007;45(11):2287–2295. doi: 10.1016/j.fct.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Pereira J.A., Oliveira I., Sousa A., Ferreira I.C.F.R., Bento A., Estevinho L. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem. Toxicol. 2008;46(6):2103–2111. doi: 10.1016/j.fct.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Proestos C., Chorianopoulos N., Nychas G.-J.E., Komaitis M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005;53(4):1190–1195. doi: 10.1021/jf040083t. [DOI] [PubMed] [Google Scholar]
- Savage G.P., McNeil D., Ö sterberg K. Oxidative stability of walnuts during long term storage. 4th International Walnut Symposium. Acta Hortic. 2001;544:591–597. [Google Scholar]
- Shende S., Patel S., Arora S., Sharma V. Oxidative stability of ghee incorporated with clove extracts and BHA at elevated temperatures. Int. J. Food Prop. 2014;17(7):1599–1611. [Google Scholar]
- Simsek B. Studies on the storage stability of yayik butter. J. Verbrauch. Lebensm. 2011;6(2):175–181. [Google Scholar]
- Siripatrawan U., Harte B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocolloid. 2010;24(8):770–775. [Google Scholar]
- Slatna A., Mikulic-Petkovsek M., Stampar F., Veberic R., Solar A. Identification and quantification of phenolic compounds in kernels, oil and bagasse pellets of common walnut (Juglans regia L.) Food Res. Int. 2015;67:255–263. doi: 10.1016/j.foodres.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Sousa A., Ferreira I.C.F.R., Calhelha R., Andrade P.B., Valentao P., Seabra R., Estevinho L., Bento A., Pereira J.A. Phenolics and antimicrobial activity of traditional stoned table olives. Bioorg. Med. Chem. 2006;14(24):8533–8538. doi: 10.1016/j.bmc.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Stampar F., Solar A., Hudina M., Veberic R., Colaric M. Traditional walnut liqueur – cocktail of phenolics. Food Chem. 2006;95(4):627–631. [Google Scholar]
- Tapsell L.C. Health benefits of walnut consumption. Acta Horticult. 2010;86:409–416. [Google Scholar]
- Wei Y., Siewers V., Nielsen J. Cocoa butter-like lipid production ability of non-oleaginous and oleaginous yeasts under nitrogen-limited culture conditions. Biotechnol. Prod. Proc. Eng. 2017;101(9):3577–3585. doi: 10.1007/s00253-017-8126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Zhang C.M., Kong X.Z., Hua Y.F. Oxidative modification of soy protein by peroxyl radicals. Food Chem. 2009;116(1):295–301. [Google Scholar]