Abstract
Purple-fleshed sweetpotatoes (PFSPs) are considered to be a healthy food and there are many methods to process in family. This study aimed to investigate the effects of various domestic cooking on the anthocyanin variation and antioxidant activity of a newly bred purple-fleshed cultivar Guangzishu 9 (GZ9) with anthocyanin content up to 1,500 mg/100g dry weight. As a result, total 15 individual anthocyanins were separated and identified by using UPLC-QTOF-MS. Top three anthocyanins were cyanidin 3-dicaffeoyl sophoroside-5-glucoside, cyanidin 3-caffeoyl- p-coumaryl sophoroside-5-glucoside and peonidin 3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside, which accounting for 57.27% of the total anthocyanin content. Acylated anthocyanins were the major constituents in GZ9, and the type of anthocyanins was dominated by cyanidin. Boiling, steaming and mircrowaving had no significant effect on the total anthocyanin content. But baking, frying, air-frying and stir-frying reduced 11–45% of total anthocyanin content. Through ABTS+ radical scavenging capacity and reducing power, antioxidant variations were also observed during different family cooking, and the variation had a strong correlation with total anthocyanin content.
Keywords: Food science, Chemistry, Agriculture
1. Introduction
Sweetpotato (Ipomoea batatas) is known as a highly nutritious vegetable with rich vitamin Bs, minerals, dietary fiber, and dietary carbohydrates [1, 2]. Besides of these, purple-fleshed sweetpotatoes (PFSPs) contain significant amount of anthocyanins. Anthocyanins are a class of flavonoid compounds responsible for the attractive blue, purple and red colours of most fruits and vegetables. In nature, hundreds of anthocyanins have been identified. Anthocyanins are glycosylated and/or acylated derivatives of anthocyanidins. Among of anthocyanidins, the six common ones are pelargonidin, cyanidin, peonidin, delphinidin, petunidin and malvidin. At present, four anthocyanidins and about 40 anthocyanins have been identified in sweetpotato storage roots, leaf and cell lines [3, 4]. The main anthocyanins in PFSPs are 3, 5-diglucoside derivatives of cyanidin or peonidin with acylated p-hydroxybenzoic acid, ferulic acid, or caffeic acid, which contribute more than ninety percent of the total anthocyanin content in purple-fleshed sweetpotatoes [5, 6, 7].
It is reported that anthocyanins of PFSPs could provide a wide range of health benefits, such as antioxidative activity [8, 9], anti-inflammatory activity [10, 11], antimutagenic activity [12, 13], ultraviolet light protection and reduction in memory impairment effects [14]. The new results reported that the peonidin-based components in purple sweetpotato anthocyanins could induce the proliferation of four kinds of probiotics, like Bifidobacterium bifidum, and they inhibited the growth of harmful bacteria, Staphylococcus aureus and Salmonella typhimurium in vitro. [15] Moreover, a research of anthocyanins from blood plasma or urine in animal models, including humans, who consumed raw PFSP or beverage, found that only 0.01%–0.03% of the anthocyanins remained upon excretion. This demonstrated that the anthocyanins were not only selectively well absorbed, but were also well maintained in their original form [16, 17, 18].
In the past few years, PFSPs were developed in many countries to meet a growing demand because of its high nutrition value [14, 19]. In some countries, like Japan, PFSP such as Yamagawa-murasaki and Ayamurasaki are utilized as natural food colorants, juices, bread, noodles, jams, sweetpotato chips, confectionary, and alcoholic beverages [10, 20, 21]. The reason why PFSPs have so many applications is that the anthocyanins have been found to be stable after heat and light treatment. As cooking does not lose their functional activity, particular care is not needed in processing and storage [20]. In family, PFSPs are usually cooked in different originally ways, like boiling, steaming and baking [5, 22]. Some of them have been analyzed. However, the comprehensive information of the effects of different cooking methods on anthocyanin content and the antioxidant activity are insufficient and few researches focus on individual anthocyanin variations during domestic cooking of Chinese cultivars. Especially, air-frying was evaluated for the first time in PFSPs home processing. Thus, in this present study, the purpose is to characterize anthocyanin content and composition, the antioxidant capacity variations through different domestic cooking methods.
2. Materials and methods
2.1. Chemicals and materials
The HPLC-grade acetonitrile and formic acid was obtained from Merck (Germany). Standard of cyanidin 3-O-glucoside chloride, phosphate buffered saline (PBS), potassium ferricyanide, trichloroacetic acid and ferric chloride were purchased from MACKLIN (Shanghai, China). Hydrochloric acid, 95% ethanol, and hexane were obtained from Sinophorm Chemical Reagent Co., Ltd (Shanghai China). Six-hydroxy-2, 5, 7, 8-tetramethylchromane-2-carboxylic acid (Trolox), 2, 2 - azino-bis 3-ethylbenzothiazoline-6-sulfonic acid diammonium salt (ABTS) and 1,1-Diphenyl-2-picrylhydrazyl radical 2,2-Diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl (DPPH) were purchased from Sigma-Aldrich. Potassium persulfate was obtained from Dingguo, China.
2.2. Sample preparation and cooking methods
GZ9 is a newly bred, high anthocyanin-containing cultivar and grown in the field at the experimental station of Guangdong Academy of Agricultural Sciences (GDAAS). Storage roots were collected at 140 days after transplanting. The root of GZ9 were washed with tap-water and air-dried at room temperature before their exposure to different cooking methods. Peanut oil was purchased from a local supermarket (Guangzhou, China). For boiling, steaming, baking and microwaving, whole roots (unpeeled) were used, and a stainless-steel probe was inserted in the roots to ensure they were cooked thoroughly (Table 1) [23]. For normal frying and air-frying, roots were washed, peeled and cut into strips (8 × 7 × 60 mm) manually, whereas for stir-frying, the strips were 2 × 3 × 60 mm (Table 1). After cooking, the raw and cooked samples were freeze-dried (-80 °C) and powdered using a commercial grinder. An additional de-fatting procedure was performed on fried, air-fried and stir-fried samples according to the method of Xu et al. [24].
Table 1.
Cooking methods used in present study.
| Cooking methods | Pretreatment | Cooking condition | Cooking equipment |
|---|---|---|---|
| Boiling | Whole, unpeeled | 100 °C, 30 min | Steel worm |
| Steaming | Whole, unpeeled | 30 min | Steel worm |
| Baking | Whole, unpeeled | 210 °C, 45 min | Media MT10NE-AA |
| Microwaving | Whole, unpeeled | 800 W, 10 min | Galanz G80F23CN3L Ⅶ-C2K(R8) |
| Frying | 8*7*60mm | 160 °C, 4 min; 300g/1000 mL | Steel worm |
| Air-frying | 8*7*60mm | 180 °C, 12 min; 300g/8 mL | Joyoung KL-26J601 |
| Stir-frying | 2*3*60mm | 220 °C, 10 min; 200g/15 mL | ASD WG8332QB |
2.3. Anthocyanin extraction
One gram of powder sample in a conical tube (50 mL) was extracted with 15 mL of 1.5% concentrated HCL in 95% ethanol in an ultrasonic cleaner (UC-900R, QILI, China) at 25 °C, 720 W for 30 min and centrifuged (5000 rpm, 5 min, 4 °C) with a centrifuge (ST16R, Thermo Scientific, America). The extraction was repeated twice and the supernatants were pooled. Finally, the supernatants were brought to 50 mL with the extraction solvent. Five mL extractions were filtered with 0.22 μm organic membrane, and then used for further analysis.
2.4. HPLC and UPLC-QTOF-MS for quantitation and characterization of anthocyanins
HPLC analysis was performed on a HPLC (LC-20A, Shimadzu, Japan) equipped with a Kinetex C18 reversed phase column (150 × 4.6 mm, 2.6 μm). Elution was performed with mobile phase A (4.5% formic acid in ultrapure water) and mobile phase B (acetonitrile) according to previous report [25]. The column temperature was 35 °C and the injection volume of the extract was 5 μL. The gradient started with 5% B for 10 min, and then increased to 10% B between 10 and 30 min, reached 15 % B at 30 min and stayed for 10 min, and then increased to 25 % B at 50 min, 10 min later, reached 30 % B and maintained at this level for 5 min. The flow rate was set to 0.43 mL/min with a re-equilibration time of 20 min. Quantitation of individual anthocyanin in PFSPs was obtained at 520 nm and cyanidin 3-glucoside was used as standard, which were then expressed as mg/100 g dry weight sweetpotato. MS analysis was performed on a UPLC-QTOF-MS (UPLC 1290-6540B Q-TOF, Agilent, USA) using an Eclipse plus C18 chromatographic column (100 × 2.1 mm, 1.8 μm, Agilent) run in positive ionization mode using an electrospray ionisation (ESI) source, performing a scanning interval 105–1700 m/z. The MS conditions were set as follows: capillary voltage 4.0 kV, gas temperature 300 °C, drying gas flow rate 8.0 L/min and nebulizer gas pressure 1.5 bar.
2.5. Antioxidant activity
The antioxidant capacity of anthocyanin extract was evaluated synthetically through ABTS+ radical scavenging activity, DPPH radical scavenging activity and Ferric Reducing Antioxidant Power (FRAP). ABTS+ radical scavenging activity was determined according to the method of Re et al [26] with some modifications. ABTS.+ was produced by reacting 5 mL of 7 mM ABTS solution with 88 μL of 140 mM potassium persulfate solution and allowing the mixture to stand in dark at room temperature for 12–16 h before use. For the assay, the ABTS.+ solution was diluted in absolute ethanol to an absorbance of 0.7 (±0.02) at 734 nm. A 4 μL aliquot of the extraction was added to 36 μL of absolute ethanol in a 96-well flat-bottomed microplate. After 200 μL of ABTS radical solution was added, the plates were stored in the dark for 6 min. The absorbance was measured using a plate reader (Multiskan Spectrum) at 734 nm. A control containing 40 μL of absolute ethanol (no extraction solution) was also included in each plate. The ABTS radical scavenging activity was calculated using equation with a Trolox (0, 20, 40, 60, 80, 100 and 120 μM) and was expressed as μM Trolox equivalent (TE) per gram dry weight (DW). ABTS radical scavenging activity (%) = %. In the formula, A1 and Ai meant absorbance of solvent control and samples. A0 and Aj represented absorbance of blank control and blank sample.
DPPH radical scavenging activity and FRAP were investigated using the previously reported methods [25, 27]. Similarly, Trolox was chosen to be the reference substance and the result of antioxidant activity was expressed as μM Trolox equivalent (TE) per gram dry weight (DW).
2.6. Statistical analysis
The anthocyanins and antioxidant activity variations were analyzed by one-way ANOVA where domestic cooking methods were main factors. Duncan test was used to assess the multiple differences of individual anthocyanin and antioxidant activity. The correlation between anthocyanins and antioxidant activity was evaluated by pearson coefficient of association. All of the contents are expressed as the means ± standard deviations (SD) of triplicate determinations and a probability of P < 0.05 was considered significant. Statistical procedures were done by SPSS 19.
3. Results and discussion
3.1. Anthocyanin separation and composition identification
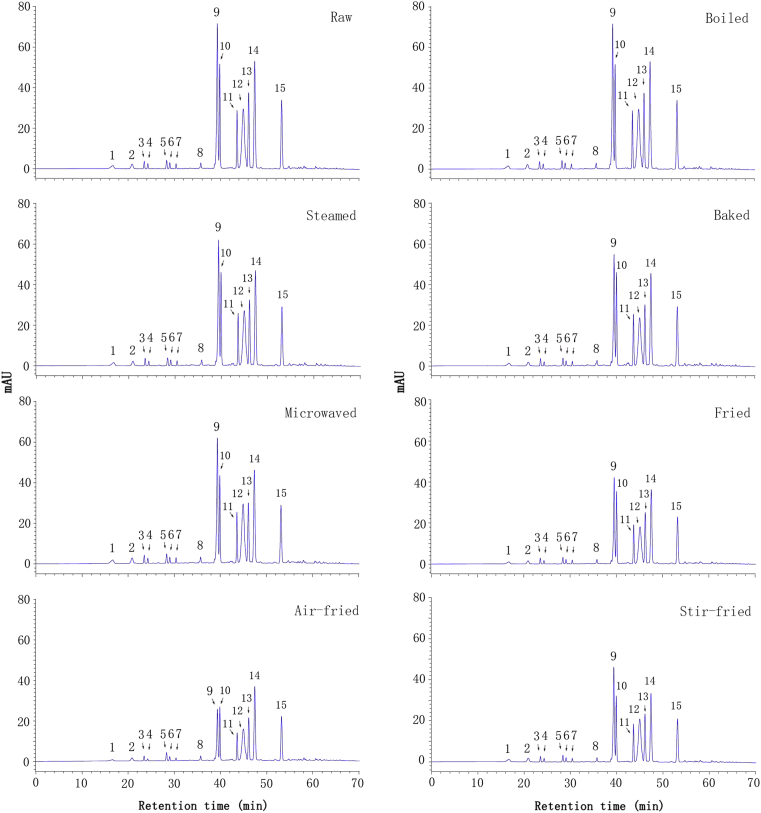
As shown in Fig. 1, anthocyanins were separated under the elution conditions from water extraction. A total of 15 major compounds were isolated in this cultivar. Peak 9, 10, 11, 12, 13, 14, 15 represented the major anthocyanins and they contributed to more than 90% of the total anthocyanin content. Peak number, retention time, and % of total areas were summarized in Table 2.
Fig. 1.
HPLC chromatograms of anthocyanins in raw and different cooking for cultivar GZ9.
Table 2.
Identification of anthocyanin in purple-fleshed sweetpotato using UPLC-QTOF-MS.
| Peak | TR (min) | Mass (m/z) | Fragment ions (m/z) | Formula | Identification | Content (%) |
|---|---|---|---|---|---|---|
| 1 | 16.90 | 773.2133 | 611,449,287 | C33H41O21 | Cy 3-soph-5-glc | 1.07 |
| 2 | 21.00 | 787.2295 | 625,463,301 | C34H43O21 | Peo 3-soph-5-glc | 1.15 |
| 3 | 23.66 | 893.2351 | 731,449,287 | C40H45O23 | Cy 3-p-hydroxybenzoyl soph-5-glc | 0.81 |
| 4 | 24.26 | 935.2445 | 773,449,287 | C42H47O24 | Cy 3-(6‴-caffeoyl soph)-5-glc | 0.55 |
| 5 | 28.50 | 907.2505 | 745,463,301 | C41H47O23 | Peo 3-p-hydroxybenzoyl soph-5-glc | 1.00 |
| 6 | 29.00 | 949.2606 | 787,463,301 | C43H49O24 | Peo 3-(6‴-caffeoyl soph)-5-glc | 0.70 |
| 7 | 30.51 | 919.2487 | 757,449,287 | C42H47O23 | Cy 3-p-coumaryl soph-5-glc | 0.52 |
| 8 | 35.81 | 963.2757 | 801,463,301 | C42H51O24 | Peo 3-feruloyl soph-5-glc | 0.71 |
| 9 | 39.59 | 1097.2769 | 935,449,287 | C51H53O27 | Cy 3-dicaffeoyl soph-5-glc | 20.58 |
| 10 | 40.11 | 1055.2686 | 893,449,287 | C49H51O26 | Cy 3-caffeoyl-p-hydroxybenzoyl soph-5-glc | 10.00 |
| 11 | 43.74 | 1111.2924 | 949,449,287 | C52H53O27 | Cy 3-caffeoyl-feruloyl soph-5-glc | 6.00 |
| 12 | 45.12 | 1081.2818 | 919,449,287 | C51H53O26 | Cy 3-caffeoyl- p-coumaryl soph-5-glc | 19.99 |
| 13 | 46.25 | 1111.2931 | 949,463,301 | C52H55O27 | Peo 3-dicaffeoyl soph-5-glc | 10.12 |
| 14 | 47.49 | 1069.2816 | 907,463,301 | C50H53O26 | Peo 3-caffeoyl-p-hydroxybenzoyl soph-5-glc | 16.70 |
| 15 | 53.14 | 1125.3083 | 963,463,301 | C53H57O27 | Peo 3-caffeoyl-feruloyl soph-5-glc | 10.10 |
Cy:cyanidin, Peo:peonidin, soph:sophorside, glc:glucoside.
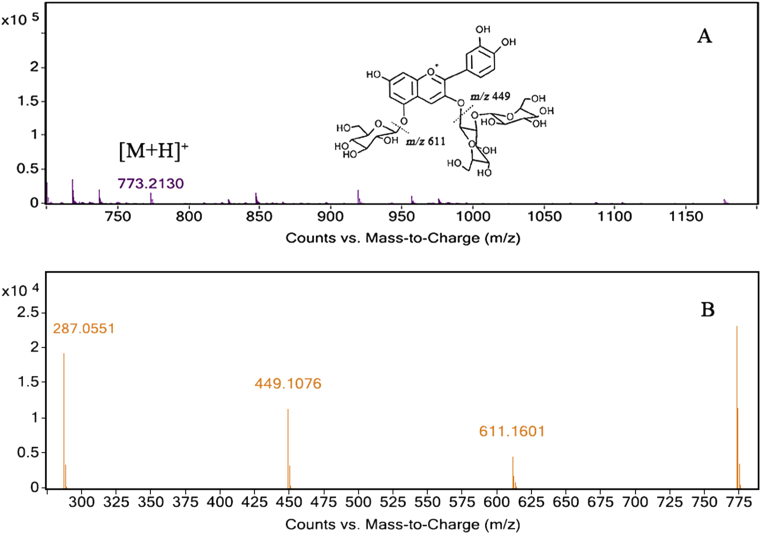
The m/z ratio of each intact anthocyanin with fragment ions was captured using LC-QTOF-MS. With referencing to the LC-MS library of purple fleshed sweetpotato anthocyanins, a total of 15 compounds were identified. The chemical structures of the individual anthocyanins could be determined by HPLC retention time, absorbance spectra, and MS fragment database according to previous researches, in which the phenolic acids such as p-hydroxybenzoic acid (m/z 120), caffeic acid (m/z 162) and ferulic acid (m/z 176) were disconnected from their structures with glucoside (m/z 162) or sophoroside (m/z 324) [4, 5, 7, 28, 29]. For example, peak 1 represented Cyanidin 3- sophoroside-5-glucoside. As shown in Fig. 2, a molecular ion ([M + H]+) of m/z 773 was broken up into three fragments of m/z 611, 449 and 287. The fragment m/z 611 represented that a glucose moiety (m/z 162) was disconnected from the molecular. And the fragment m/z 449 meant that the sophoroside moiety (m/z 324) was lost from the molecular. The fragment m/z 287 represented the loss of both the glucoside and sophoroside moieties from the molecular. As a result, structure could be determined by referencing to the previous researches of PFSP anthocyanins with the addition of the retention time. Another example, peak 14, Peonidin 3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside, a di-acylated peonidin-type anthocyanin, a molecular ion ([M + H]+) of m/z 1069 produced three fragments of m/z 907, 463 and 301. The fragment m/z 907 indicated that a glucoside moiety (m/z 162) was disconnected from the molecular. The fragment m/z 463 represented the loss of caffeic acid, p-hydroxybenzoic acid and sophoroside moieties (total m/z 606) from the molecular. The fragment m/z 301 meant that glucoside moiety (m/z 162), as well as caffeic acid, p-hydroxybenzoic acid and sophoroside moieties (total m/z 606) were both disconnected from the molecular. Similarly, it could be inferred through referencing to the LC-MS library of PFSP anthocyanins with the retention time additionally. Identification of the remaining anthocyanins was carried out in a similar way. All the MS fragment patterns and contents of individual anthocyanins were summarized in Table 2.
Fig. 2.
A:Structrue and MS of Cyanidin 3- sophoroside-5-glucoside; B: MS fragment of Cyanidin 3- sophoroside-5-glucoside.
In GZ9, 60% of anthocyanins were cyanidin derivatives, which indicating cyaniding--type anthocyanins were dominated in this cultivar. Acylated anthocyanins were the major constituents in this cultivar, which was in good agreement with the previous researches [5, 6, 7]. Di-acylated anthocyanins showed a remarkably 42 times higher level than that of non-acylated anthocyanins, and 21 times higher level than that of mono-acylated anthocyanins. The top three major anthocyanins of GZ9 were represented by peak 9 (cyanidin 3-dicaffeoyl sophoroside-5-glucoside), peak 12 (cyanidin 3-caffeoyl- p-coumaryl sophoroside-5-glucoside) and peak 14 (peonidin 3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside), which in total contributed for 57.27% of the total anthocyanin content.
3.2. Anthocyanin variations during cooking
The effects of several representative domestic cooking methods on anthocyanin content and composition were investigated. The HPLC chromatograms of anthocyanins in raw plant material and after cooking were presented in Fig. 1. The variations in anthocyanin content and composition during cooking were presented in Table 3. Air-fried, fried and stir-fried cooking induced the greatest losses in total content of anthocyanin (44.87%, 31.97% and 31.38%, respectively), followed by baking (11.01%), whereas steaming and microwaving caused no significant difference (P > 0.05). Surprisingly, 6.55% increase of the total anthocyanin content was observed after boiling. Application of different cooking methods induced method-specific changes in the content of individual anthocyanin. For cyanidin 3-dicaffeoyl sophoroside-5-glucoside (peak 9), the top one major component in GZ9, a reduction of 7.32%–63.43% was measured after the cooking methods except boiling, which increased 8.83%. The top two major peak 12 (cyanidin 3-caffeoyl- p-coumaryl sophoroside-5-glucoside) had a greatest reduction (46.09%) after air-fried, followed by fried and stir-fried (36.25%, 27.56%, respectively), while boiled, steamed and microwaved methods induced no significant difference (P > 0.05). Interestingly, nearly all mono-acylated anthocyanins (peak 3, 4, 5, 6 and 7) increased significantly after boiling and microwaving. On the other hand, total di-acylated anthocyanins decreased significantly after frying, air-frying and stir-frying. It suggested that boiling and microwaving lead to an increase of most mono-acylated anthocyanins, whereas frying, air-frying and stir-frying induced reduction of total di-acylated anthocyanins. Thermal treatments might release more anthocyanins from physical entrapment in other structures [30]. Furthermore, di-acylated anthocyanins might transform and strengthen mono-acylated anthocyanins [31]. For instance, peonidin 3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside (peak 14) would turn into peonidin 3-p-hydroxybenzoyl sophoroside-5-glucoside (peak 5) due to the loss of caffeic acid and it could be reduced to peonidin 3-caffeoyl-sophoroside-5-glucoside (peak 6) if losing p-hydroxybenzoic acid. Even more, it might be peonidin 3-sophoroside-5-glucoside (peak 2) when lost both of the phenolic acids. However, it was difficult to distinguish the content of the non- and mono-acylated compounds generated from the translation of di-acylated compounds. Taking into consideration of the tested variety, the retention rate of total anthocyanins was higher in boiled, steamed, baked and microwaved samples than in fried, air-fried and stir-fried. The result was similar to that of Kim et al [22] for the anthocyanin variations.
Table 3.
Effect of domestic cooking methods on the content of individual anthocyanins of GZ9 (mg/100g DW).
| Peak | Raw | Boiled | Steamed | Baked | Microwaved | Fried | Air-fried | Stir-fried |
|---|---|---|---|---|---|---|---|---|
| 1 | 16.03 ± 0.71d | 20.52 ± 0.57e | 15.88 ± 1.02d | 12.88 ± 0.78c | 19.90 ± 0.84e | 9.80 ± 0.84b | 6.22 ± 1.05a | 12.40 ± 0.32c |
| 2 | 17.15 ± 0.63c | 20.31 ± 0.15d | 17.31 ± 1.19c | 15.03 ± 0.53b | 21.69 ± 0.65e | 11.97 ± 0.19a | 12.12 ± 0.13a | 14.19 ± 0.48b |
| 3 | 12.04 ± 0.45c | 15.88 ± 0.42e | 12.53 ± 0.75c | 12.23 ± 0.63c | 14.12 ± 0.50d | 9.87 ± 0.39b | 8.31 ± 0.09a | 9.28 ± 0.27b |
| 4 | 8.26 ± 0.29d | 9.63 ± 0.23f | 7.91 ± 0.44d | 7.01 ± 0.29c | 8.85 ± 0.30e | 6.07 ± 0.18b | 4.23 ± 0.06a | 6.31 ± 0.28b |
| 5 | 14.96 ± 0.55bc | 18.67 ± 0.40d | 15.64 ± 0.95c | 14.46 ± 0.72b | 17.67 ± 0.67d | 12.23 ± 0.52a | 15.47 ± 0.09bc | 12.33 ± 0.41a |
| 6 | 10.39 ± 0.37d | 11.61 ± 0.23e | 10.57 ± 0.66d | 9.50 ± 0.60c | 11.29 ± 0.38e | 8.34 ± 0.31b | 7.54 ± 0.07a | 7.83 ± 0.20ab |
| 7 | 7.75 ± 0.31c | 9.73 ± 0.20e | 8.02 ± 0.41c | 7.54 ± 0.36c | 8.90 ± 0.32d | 6.19 ± 0.19b | 4.98 ± 0.08a | 6.10 ± 0.17b |
| 8 | 10.57 ± 0.27bc | 11.54 ± 0.40bcd | 11.94 ± 1.75cd | 10.33 ± 0.62b | 12.14 ± 0.71d | 8.44 ± 0.50a | 8.32 ± 0.32a | 8.18 ± 0.57a |
| 9 | 307.64 ± 11.41f | 334.79 ± 5.43g | 285.13 ± 16.97e | 251.94 ± 11.13d | 296.43 ± 9.02ef | 190.03 ± 5.09b | 112.49 ± 2.19a | 213.87 ± 5.99c |
| 10 | 149.52 ± 6.11e | 169.19 ± 3.28f | 143.85 ± 8.87de | 144.37 ± 7.24de | 137.04 ± 4.15d | 109.86 ± 4.17c | 78.98 ± 1.32a | 99.50 ± 3.23b |
| 11 | 89.68 ± 3.35d | 97.16 ± 1.82e | 85.24 ± 5.09cd | 83.70 ± 3.79c | 84.37 ± 2.86cd | 62.62 ± 1.79b | 43.19 ± 0.80a | 59.60 ± 1.73b |
| 12 | 298.76 ± 11.07ef | 311.29 ± 4.77f | 285.15 ± 16.39e | 255.83 ± 11.26d | 312.43 ± 8.70f | 190.47 ± 3.12b | 161.05 ± 1.96a | 216.42 ± 6.01c |
| 13 | 151.29 ± 5.95e | 144.56 ± 2.50de | 138.30 ± 8.42cd | 131.09 ± 6.08c | 129.77 ± 3.68c | 104.81 ± 2.08b | 85.98 ± 2.23a | 96.96 ± 3.59b |
| 14 | 249.61 ± 9.96c | 265.80 ± 4.77d | 232.41 ± 14.28b | 231.89 ± 11.44b | 234.23 ± 6.53b | 176.77 ± 4.48a | 174.73 ± 3.11a | 162.00 ± 5.45a |
| 15 | 150.94 ± 5.83cd | 151.79 ± 2.60d | 142.03 ± 8.50bc | 142.24 ± 6.59bc | 140.28 ± 4.47b | 109.36 ± 3.28a | 100.34 ± 1.89a | 100.60 ± 3.28a |
| Cy | 889.68 ± 33.70d | 968.19 ± 16.63e | 843.71 ± 49.87d | 775.50 ± 35.37c | 882.04 ± 26.38d | 584.91 ± 15.65b | 419.45 ± 6.67a | 623.48 ± 17.79b |
| Peo | 604.91 ± 23.53c | 624.29 ± 10.95c | 568.20 ± 35.57b | 554.55 ± 26.53b | 567.06 ± 16.26b | 431.92 ± 11.22a | 404.49 ± 7.50a | 402.10 ± 13.83a |
| NA | 33.18 ± 1.34d | 40.83 ± 0.72e | 33.19 ± 2.20d | 27.91 ± 1.27c | 41.59 ± 1.48e | 21.77 ± 0.95b | 18.34 ± 1.15a | 26.59 ± 0.63c |
| MA | 63.96 ± 2.24bc | 77.06 ± 1.81d | 66.62 ± 4.91c | 61.08 ± 3.21b | 72.97 ± 2.53d | 51.14 ± 2.01a | 48.84 ± 0.57a | 50.04 ± 1.73a |
| DA | 1397.45 ± 53.67ef | 1474.60 ± 25.16f | 1312.11 ± 78.25cd | 1241.07 ± 57.43c | 1334.55 ± 38.81de | 943.91 ± 23.89b | 756.76 ± 12.20a | 948.94 ± 29.24b |
| TA | 1494.59 ± 57.21d | 1592.49 ± 27.57e | 1411.92 ± 85.34cd | 1330.06 ± 61.88c | 1449.11 ± 42.54d | 1016.82 ± 26.85b | 823.94 ± 13.84a | 1025.57 ± 31.58b |
Cy: Cyanidin. Peo: Peonidin. NA: none-acylated. MA: mono-acylated. DA: di-acylated. TA: Total anthocyanin.
a–g Significantly different at p < 0.05.
3.3. Antioxidant activity variations during cooking
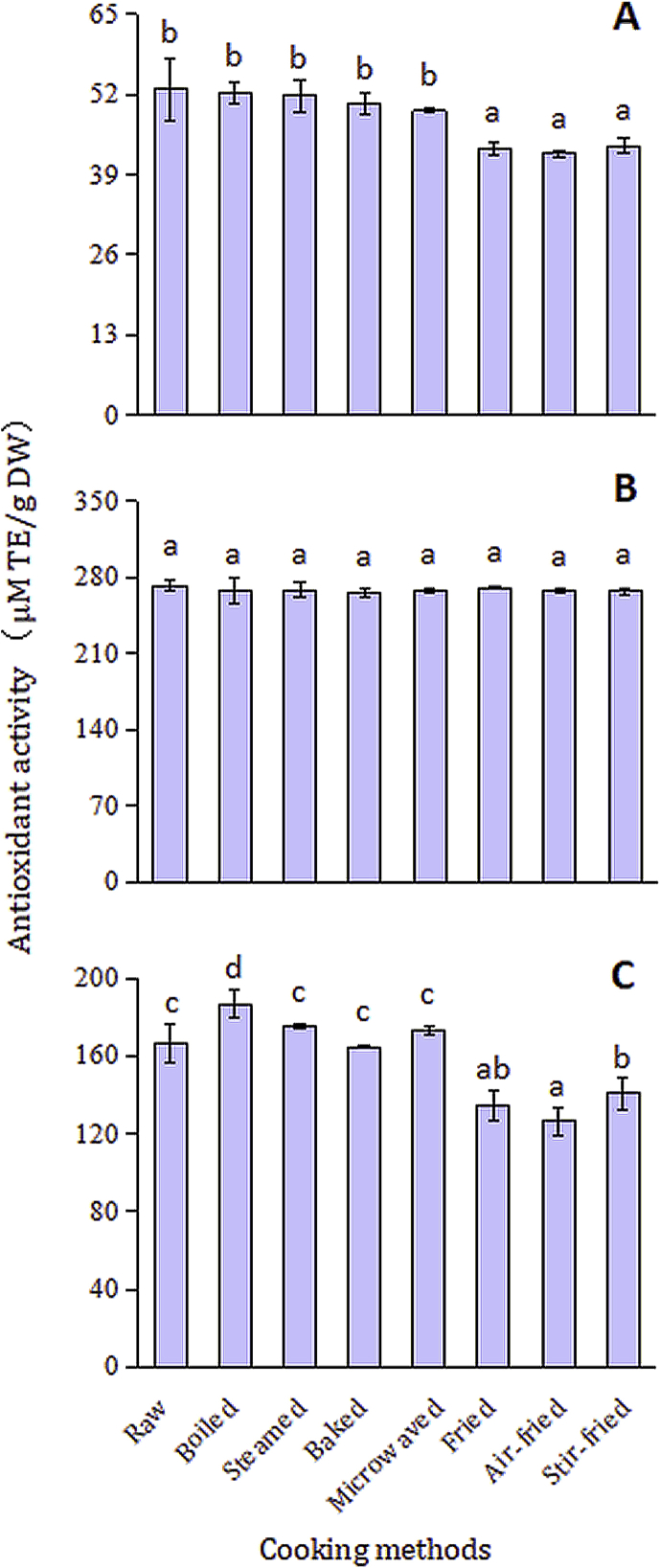
Antioxidant variations were observed during domestic cooking for GZ9 in the test of ABTS+ radical scavenging activity and FRAP, except DPPH radical scavenging activity (as shown in Fig. 3). Compared with the ABTS radical-scavenging activity of the no-processing raw anthocyanin extract, there was no significant modification caused by microwaving, boiling, steaming and baking (P > 0.05). On the contrary, air-frying, frying and stir-frying had a great effect on the ABTS radical-scavenging activity, making a reduction of 12.63%–15.20% (Fig. 3A). For the test of FRAP, differential performance of various domestic cooking tended to be more segmented. For example, air-frying had greater impact on the reduction of antioxidant activity than that of frying and stir-frying (P < 0.05), reaching 24.03% decrease comparing with the no-processing raw anthocyanin extract (Fig. 3C). But for DPPH activity, there was no significant differences among all the cooking methods (P > 0.05) (Fig. 3B). These results suggested that ABTS+ radical scavenging activity and FRAP were more suitable for antioxidant activity examination of PFSP than DPPH radical scavenging activity. Similar phenomenon was also found from previous report [25]. In addition, through measuring the DPPH and ORAC (Oxygen radical absorbance capacity) values among the raw and steamed samples for the whole roots, there was no significant difference in antioxidant activity either [19]. In order to further reveal the possible key influence factor on the antioxidant activity, the correlation between anthocyanins and antioxidant activity was analyzed. As shown in Table 4, some interesting information can be obtained. All the analyzed factors except peak 5 and the ratio of cyanidin/peonidin, had significant effect on its antioxidant activity in the test of ABTS+ radical scavenging activity (p < 0.05 or p < 0.01). Significant positive correlation with its antioxidant activity of all the analyzed factors was observed in the test of FRAP (p < 0.05 or p < 0.01). Interesting, like the situation in the test of ABTS+ radical scavenging activity, peak 5 and the ratio of cyanidin/peonidin had less effect on its antioxidant activity (p < 0.05) than other analyzed factors (p < 0.01) in the test of FRAP. Apparently, total anthocyanin content had a strong correlation with antioxidant activity (p < 0.01), and acylated anthocyanins played a more important role than none-acylated forms on the antioxidant activity for ABTS+ and FRAP testing.
Fig. 3.
Effect of domestic cooking on the antioxidant activities in GZ9. A: ABTS+ radical scavenging activity; B: DPPH radical scavenging activity; C: Ferric Reducing Antioxidant Power (FRAP).
Table 4.
Correlation between anthocyanins and antioxidant activity.
| Type |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
| ABTS | 0.80* | 0.75* | 0.83* | 0.85** | 0.6 | 0.89** | 0.85** | 0.87** | 0.91** | 0.95** | 0.95** | 0.92** | 0.98** |
| DPPH | 0.06 | -0.04 | -0.05 | 0.16 | -0.20 | 0.09 | 0.00 | -0.06 | 0.19 | 0.13 | 0.15 | 0.12 | 0.29 |
| FRAP | 0.93** | 0.89** | 0.96** | 0.95** | 0.72* | 0.96** | 0.97** | 0.94** | 0.95**∗ | 0.95**∗ | 0.96** | 0.96** | 0.92** |
|
| |||||||||||||
| Type |
14 |
15 |
TA |
Cy |
Peo |
NA |
MA |
DA |
Cy/Peo |
Hy |
Co |
Fe |
Ca |
| ABTS | 0.96** | 0.98** | 0.96** | 0.93** | 0.98** | 0.79* | 0.85** | 0.96** | 0.57 | 0.96** | 0.92** | 0.98** | 0.96** |
| DPPH | 0.12 | 0.15 | 0.15 | 0.16 | 0.15 | 0.02 | -0.03 | 0.18 | 0.14 | 0.11 | 0.00 | 0.14 | 0.17 |
| FRAP | 0.93** | 0.94** | 0.98** | 0.97** | 0.95** | 0.92** | 0.96** | 0.97** | 0.72* | 0.96** | 0.97** | 0.96** | 0.97** |
1-15: the peak number of HPLC; TA: Total anthocyanin, Cy: Cyanidin. Peo: Peonidin. NA: none-acylated. MA: mono-acylated. DA: di-acylated. Cy/Peo: cyanindin/peonidin, Hy: hydroxybenzoyl, Co: coumaryl, Fe: feruloyl, Ca: caffeoyl. *, **. Significantly different at p < 0.05 and p < 0.01.
In summary, the anthocyanin content and composition from different domestic cooking methods were profiled for the newly released purple-fleshed sweetpotato cultivar (GZ9). A total of fifteen individual anthocyanins were separated and identified. Cyanidin-type anthocyanins were dominated in this cultivar. Taking consideration of the different domestic cooking methods, the total content of anthocyanins was higher in boiled, steamed, baked and microwaved samples than in fried, air-fried and stir-fried. The total anthocyanin content had a strong correlation with its antioxidant activity. Additionally, this study also revealed that the acylated anthocyanins played a more important role than none-acylated forms on the antioxidant activity. Taken all together, these results should provide helpful information for selecting preferable method for cooking purple-flesh sweetpotato from a health-promotion point of view, may be an advantage to the development of functional products, and also will be great value to breeders for well-directed breeding.
Declarations
Author contribution statement
Minghuan Liao: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Bo Zou, Jingyi Chen, Zhufang Yao, Lifei Huang, Zhongxia Luo: Performed the experiments.
Zhangying Wang: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by key Project of Science and Technology of Guangdong Province (No. 2016B020233003), the grants from the Guangdong Modern Agro industry Technology Research System (No. 2017LM1073), the Project of Science and Technology of Guangdong Province (No. 2015A030302051). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Katayama K., Kitahara K., Sakai T., Kai Y., Yoshinaga M. Resistant and digestible starch contents in sweet potato cultivars and lines. J. Appl. Glycosci. 2011;58(2):53–59. [Google Scholar]
- 2.Yoshimoto M., Yamakawa O., Tanoue H. Potential chemopreventive properties and varietal difference of dietary fiber from sweetpotato (Ipomoea batatas L.) root. Japan Agric. Res. Q. Jarq. 2005;39(1):37–43. [Google Scholar]
- 3.Castañeda-Ovando A., de Pacheco-Hernández M.L., Páez-Hernández M.E., Rodríguez J.A., Galán-Vidal C.A. Chemical studies of anthocyanins: a review. Food Chem. 2009;113(4):859–871. [Google Scholar]
- 4.Tian Q., Konczak I., Schwartz S.J. Probing anthocyanin profiles in purple sweet potato cell line (Ipomoea batatas L. Cv. Ayamurasaki) by high-performance liquid chromatography and electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2005;53(16):6503–6509. doi: 10.1021/jf050671m. [DOI] [PubMed] [Google Scholar]
- 5.Kim H.W., Kim J.B., Cho S.M., Mi N.C., Lee Y.M., Chu S.M., Che J.H., Kim S.N., Kim S.Y., Cho Y.S. Anthocyanin changes in the Korean purple-fleshed sweet potato, shinzami, as affected by steaming and baking. Food Chem. 2012;130(4):966–972. [Google Scholar]
- 6.Li J., Li X.D., Zhang Y., Zheng Z.D., Qu Z.Y., Liu M., Zhu S.H., Liu S., Wang M., Qu L. Identification and thermal stability of purple-fleshed sweet potato anthocyanins in aqueous solutions with various PH values and fruit juices. Food Chem. 2013;136(3–4):1429–1434. doi: 10.1016/j.foodchem.2012.09.054. [DOI] [PubMed] [Google Scholar]
- 7.Truong V. Den, Deighton N., Thompson R.T., Mcfeeters R.F., Dean L.O., Pecota K.V., Yencho G.C. Characterization of anthocyanins and anthocyanidins in purple-fleshed sweetpotatoes by HPLC-DAD/ESI-MS/MS. J. Agric. Food Chem. 2010;58(1):404–410. doi: 10.1021/jf902799a. [DOI] [PubMed] [Google Scholar]
- 8.Kano M., Takayanagi T., Harada K., Makino K., Ishikawa F. Antioxidative activity of anthocyanins from purple sweet potato, ipomoera batatas cultivar Ayamurasaki. Biosci. Biotechnol. Biochem. 2005;69(5):979–988. doi: 10.1271/bbb.69.979. [DOI] [PubMed] [Google Scholar]
- 9.Oki T., Masuda M., Furuta S., Nishiba Y., Terahara N., Suda I. Involvement of anthocyanins and other phenolic compounds in radical-scavenging activity of purple-fleshed sweet potato cultivars. J. Food Sci. 2002;67(5):1752–1756. [Google Scholar]
- 10.Suda I., Ishikawa F., Hatakeyama M., Miyawaki M., Kudo T., Hirano K., Ito A., Yamakawa O., Horiuchi S. Intake of purple sweet potato beverage affects on serum hepatic biomarker levels of healthy adult men with borderline hepatitis. Eur. J. Clin. Nutr. 2008;62(1):60–67. doi: 10.1038/sj.ejcn.1602674. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z.F., Fan S.H., Zheng Y.L., Lu J., Wu D.M., Shan Q., Hu B. Purple sweet potato color attenuates oxidative stress and inflammatory response induced by D-galactose in mouse liver. Food Chem. Toxicol. 2009;47(2):496–501. doi: 10.1016/j.fct.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Lim S., Xu J., Kim J., Chen T.Y., Su X., Standard J., Carey E., Griffin J., Herndon B., Katz B. Role of anthocyanin-enriched purple-fleshed sweet potato P40 in colorectal cancer prevention. Mol. Nutr. Food Res. 2013;57(11):1908–1917. doi: 10.1002/mnfr.201300040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshimoto M., Okuno S., Yamaguchi M., Yamakawa O. Antimutagenicity of deacylated anthocyanins in purple-fleshed sweetpotato. J. Agric. Chem. Soc. Jpn. 2001;65(7):1652–1655. doi: 10.1271/bbb.65.1652. [DOI] [PubMed] [Google Scholar]
- 14.Wu D.M., Lu J., Zheng Y.L., Zhou Z., Shan Q., Ma D.F. Purple sweet potato color repairs D-galactose-induced spatial learning and memory impairment by regulating the expression of synaptic proteins. Neurobiol. Learn. Mem. 2008;90(1):19–27. doi: 10.1016/j.nlm.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Sun H., Zhang P., Zhu Y., Lou Q., He S. Antioxidant and prebiotic activity of five peonidin-based anthocyanins extracted from purple sweet potato (Ipomoea batatas (L.) lam.) Sci. Rep. 2018;8(1):1–12. doi: 10.1038/s41598-018-23397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suda I., Oki T., Masuda M., Nishiba Y., Furuta S., Matsugano K., Sugita K., Terahara N. Direct absorption of acylated anthocyanin in purple-fleshed sweet potato into rats. J. Agric. Food Chem. 2002;50(6):1672–1676. doi: 10.1021/jf011162x. [DOI] [PubMed] [Google Scholar]
- 17.Harada K., Kano M., Takayanagi T., Yamakawa O., Ishikawa F. Absorption of acylated anthocyanins in rats and humans after ingesting an extract of Ipomoea batatas purple sweet potato tuber. Biosci. Biotechnol. Biochem. 2004;68(7):1500–1507. doi: 10.1271/bbb.68.1500. [DOI] [PubMed] [Google Scholar]
- 18.Oki T., Suda I., Terahara N., Sato M., Hatakeyama M. Determination of acylated anthocyanin in human urine after ingesting a purple-fleshed sweet potato beverage with various contents of anthocyanin by LC-ESI-MS/MS. Biosci. Biotechnol. Biochem. 2006;70(10):2540–2543. doi: 10.1271/bbb.60187. [DOI] [PubMed] [Google Scholar]
- 19.Steed L.E., Truong V.D. Anthocyanin content, antioxidant activity, and selected physical properties of flowable purple-fleshed sweetpotato purees. J. Food Sci. 2008;73(5):215–221. doi: 10.1111/j.1750-3841.2008.00774.x. [DOI] [PubMed] [Google Scholar]
- 20.Yamakawa O., Yoshimoto M. Sweetpotato as food material with physiological functions. Acta Hort. 2002;583:179–185. [Google Scholar]
- 21.Suda I., Oki T., Masuda M., Kobayashi M., Nishiba Y., Furuta S. Physiological functionality of purple-fleshed sweet potatoes containing anthocyanins and their utilization in foods. Japan Agric. Res. Q. 2003;37(3):167–173. [Google Scholar]
- 22.Kim H.J., Park W.S., Bae J.Y., Kang S.Y., Yang M.H., Lee S., Lee H.S., Kwak S.S., Ahn M.J. Variations in the carotenoid and anthocyanin contents of Korean cultural varieties and home-processed sweet potatoes. J. Food Compos. Anal. 2015;41:188–193. [Google Scholar]
- 23.Tian J., Chen J., Lv F., Chen S., Chen J., Liu D., Ye X. Domestic cooking methods affect the phytochemical composition and antioxidant activity of purple-fleshed potatoes. 2016;197:1264–1270. doi: 10.1016/j.foodchem.2015.11.049. [DOI] [PubMed] [Google Scholar]
- 24.Xu J., Su X., Lim S., Griffin J., Carey E., Katz B., Tomich J., Smith J.S., Wang W. Characterisation and stability of anthocyanins in purple-fleshed sweet potato P40. Food Chem. 2015;186:90–96. doi: 10.1016/j.foodchem.2014.08.123. [DOI] [PubMed] [Google Scholar]
- 25.Zou B., Zeng D., Wu J., Yu Y., Xiao G., Xu Y. Antioxidant capacity and anthocyanins of purple-fleshed sweet potato cultivars. Food Sci. 2018;39(02):38–44. [Google Scholar]
- 26.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Riceevans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 27.Feng-wei Y.A.N., Zu-you L.U.O., Ji-qin W.U., Mou-cheng W.U. Study on the anti-oxidative effect and mechanism of polysaccharide from rapeseed ( RSPS ) Sci. Agric. Sin. 2005;38(1):157–162. http://www.chinaagrisci.com/CN/Y2005/V38/I01/157 [Google Scholar]
- 28.Giusti M.M., Rodríguez-Saona L.E., Griffin D., Wrolstad R.E. Electrospray and tandem mass spectroscopy as tools for anthocyanin characterization. J. Agric. Food Chem. 1999;47(11):4657–4664. doi: 10.1021/jf981242+. [DOI] [PubMed] [Google Scholar]
- 29.Tian Q., Giusti M.M., Stoner G.D., Schwartz S.J. Screening for anthocyanins using high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry with precursor-ion analysis, product-ion analysis, common-neutral-loss analysis, and selected reaction monitoring. J. Chromatogr. A. 2005;1091(1–2):72–82. doi: 10.1016/j.chroma.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 30.Xu B., Chang S.K.C. Total phenolics, phenolic acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. J. Agric. Food Chem. 2008;56(16):7165–7175. doi: 10.1021/jf8012234. [DOI] [PubMed] [Google Scholar]
- 31.Xu J. Vol. 40. 2013. Identification and stability of acylated anthocyanins in purple-fleshed sweet potato P40. [Google Scholar]