Abstract
Restrained shrinkage cracking that appears on concrete surfaces during construction, or while concrete in the curing process adversely affect the long-term durability and other performance attributes of concrete-based-infrastructures. This study investigates the effects of early-age surface cracking on restrained shrinkage concrete made of alkali activated cement. Concrete produced using this class of cement differs from ordinary Portland cement concrete in terms of surface qualities and some other properties. Bleeding which could be a main cause of plastic shrinkage cracking was measured and compared with that of Portland cement. Rheological tests were performed to gain further insight into factors that may influence the early-age performance of the cement paste. Portland cement was used as control for comparative assessment of the alkali activated cement performance. Experimental test showed that alkali activated cement improved the characteristics of the newly produced concrete. The concrete prepared using alkali activated cement has low tendency to bleeding and higher resistance to plastic shrinkage, also, the viscosity and yield stress of the alkali activated cement paste were relatively higher when compared to those of ordinary Portland cement paste. Test data collected from rheological and bleeding tests on the alkali activated cement concrete were used to explain its desired resistance to plastic shrinkage cracking.
Keyword: Civil engineering
1. Introduction
Cracking of concrete reduces its barrier qualities and accelerates weathering attack, resulting corrosion of embedded reinforcing steel bars. Cracking of concrete also negatively affects the structural performance of the concrete-based infrastructure. The shrinkage of concrete when it is still in semi-fluid (plastic) state is referred to as plastic shrinkage [1]. Plastic shrinkage cracking generally occurs on the exposed concrete surfaces which, due to more rapid drying, undergo greater plastic shrinkage movements when compared with the body of concrete. Internal restraint of the surface plastic shrinkage can thus cause cracking of concrete surfaces between the time of placement and the final setting of concrete [2]. Exposure of concrete surfaces to windy, warm and dry conditions accelerates plastic shrinkage cracking.
Research on plastic shrinkage mechanism in concrete have generally concluded that capillary stresses near the exposed concrete surfaces which usually caused by the imbalance between the rates of bleeding and water evaporation, are the primary drivers of plastic shrinkage movements [3, 4]. Plastic settlements have also been found to influence plastic shrinkage of concrete [5]. The mechanisms of plastic shrinkage are mainly physical [4, 6]; whereas chemical phenomena have minimal effects on the early-age (plastic) shrinkage of concrete.
Control of plastic shrinkage cracking of concrete has been a subject of several investigations. Plastic shrinkage reducing admixtures have been used to lower the moisture evaporation rate, leading to lower settlement [7, 8], thereby reducing the buildup of capillary pressure.
The concrete potential for cracking due to plastic shrinkage was reduced by using either superabsorbent polymers [9] or cellulose-based stabilizers [10]. Fibrous materials such as fibrillated polypropylene fibers were also used to minimize the cracking resulted from plastic shrinkage [11, 12]. Precautions such as moistening concrete surfaces with water or curing agents [13], or by applying cover sheets to minimize evaporation during construction should be taken to prevent plastic shrinkage [14]. Cracking of concrete due to plastic shrinkage is still a major concern especially in areas of large exposed surfaces such as slabs on grade, repairs on thin surfaces, tunnel linings, patching, etc. [15, 16]. In such applications, the ratio of the concrete surface area to its total volume is relatively high, and the original concrete subgrade surface also works as a high degree restraint.
Efforts to develop new classes of hydraulic cements should address the concerns with plastic shrinkage cracking and the related material properties of concrete. In this work, concrete was prepared using a new cement chemistry (alkali activated cement) and tested for plastic shrinkage cracking.
The hydraulic cement used in this work relies on alkali activation of aluminosilicate for production of hardened binder. Alkali activated cements have been recently used as a sustainable replacement to Portland cement binder [17]. Alkali activated binders can be synthesized using several aluminosilicate precursors with different availability and reactivity levels (such as slag, coal fly ash, clay, volcanic tuffs, etc.) [18, 19]. The concrete material prepared using alkali activated binders have shown promising results regarding resistance to severe conditions such as acids and sulfates, fire and corrosion resistance [20, 21]. Even though alkali activated aluminosilicate binders have been used in many investigations, the plastic shrinkage and bleeding characteristics have not been studied yet.
The work reported herein investigated the plastic shrinkage cracking resistance of alkali activated cement concrete. The bleeding and rheological attributes of alkali activated cement pastes were also measured. Portland cement paste, and concrete were tested as control materials. The distinctions between the alkali activated and Portland cement were identified and explained based on the experimental results.
2. Materials and methods
2.1. Materials
The alkali activated cement used in this investigation was developed using mechanochemical processing via ball milling described in the previous work of the same authors [22]. The proportions of the raw materials used for production of the alkali activated cement were coal fly ash at 45 wt.%, slag at 25 wt.%, albites at 15 wt.%, sodium silicate 7 wt.%, sodium hydroxide at 3 wt.% and borax at 1 wt.%. Portland cement (Type I per ASTM C150 acquired from Lafarge-Holcim) was used as control. Chemical compositions and Blaine fineness for the Portland cement and the alkali activated cement used in this investigation are presented in Table 1. The initial set time for the alkali activated cement and Portland cement used in this investigation are 53 and 132 minutes, respectively [22].
Table 1.
Chemical compositions (wt.%) and Blaine finesses of the Portland cement and the alkali activated cement used in this investigation.
| SiO2 | CaO | Al2O3 | Fe2O3 | MgO | K2O | Na2O | SO3 | Blaine Fineness, cm2/g | |
|---|---|---|---|---|---|---|---|---|---|
| Alkali activated cement | 35.2 | 28.1 | 13.6 | 4.03 | 3.73 | 1.14 | 8.89 | 0.53 | 3960 |
| Portland cement | 20.1 | 64.2 | 5.31 | 2.86 | 2.65 | 0.10 | 0.02 | 2.14 | 3870 |
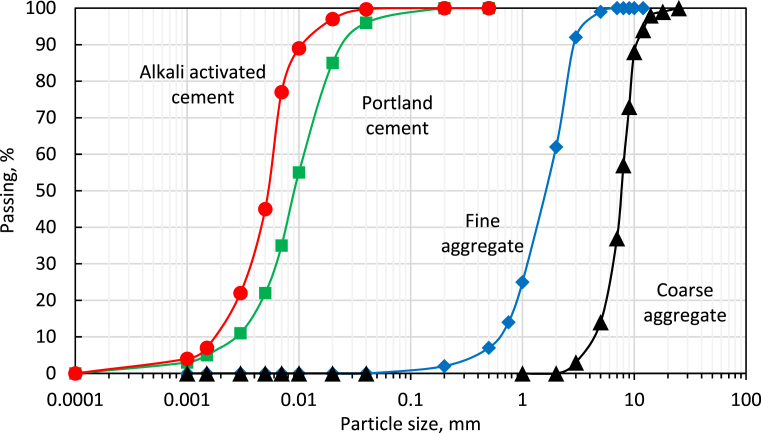
The particle size distributions of the Portland cement, the alkali activated cement and coarse and fine aggregate are presented in Fig. 1. The median particle sizes were 9.8 and 7.4 μm for Portland cement and the alkali activated cement, respectively. Based on previous investigations, the cement particle size distribution has a significant effect on the plastic shrinkage cracking; the higher the cement surface area, the more vulnerable the concrete would be to plastic shrinkage cracking [23]. Adjustment of cement particle size distribution through addition of finer powder (i.e. silica fume) raised the concrete potential for plastic shrinkage cracking [24]. In this investigation, this effect has not been considered since the two cements are very close in their particle size distributions and Blaine fineness. Natural sand with maximum particle size of 4.75 mm, was used as fine aggregate. Crushed limestone with a maximum particle size of 19 mm was used as coarse aggregate.
Fig. 1.
Particle size distributions of the Portland cement, the alkali activated cement and the coarse and fine aggregates used in this investigation.
2.2. Methods
Plastic shrinkage crack resistance of concrete was measured per ASTM C1579. A 19 L planetary mixer (Hobart A-200, Lombard) was used to prepare the concrete mixture with the mix proportions presented in Table 2.
Table 2.
Mix design for the concrete used in this investigation.
| Material | Quantity, kg/m3 |
|---|---|
| Cement | 400 |
| Fine aggregate | 910 |
| Coarse aggregate | 1100 |
| Water-to-cement ratio∗ | 0.45 for alkali activated cement concrete 0.55 for Portland cement concrete |
water cement ratio was adjusted so that concrete produce similar fresh mix workability (slump of 60 ± 10 mm).
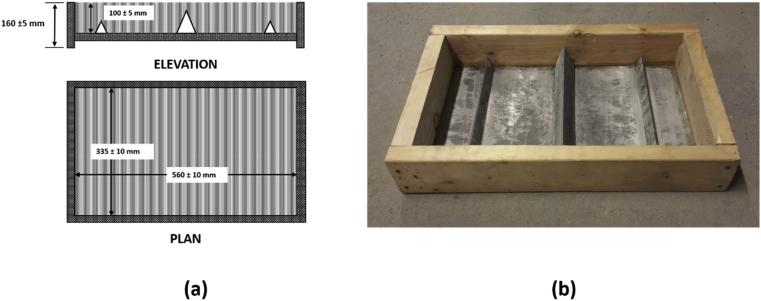
The hydraulic cement was added first to the operating mixer followed by water, and mixing was continued for 2 minutes. Fine and coarse aggregates were then added, and mixing was continued for another three minutes until a homogeneous fresh concrete mixture was produced. The fresh concrete was placed in the plastic shrinkage mold (Fig. 2), consolidated using rods, and finished with a trowel.
Fig. 2.
Schematics of the plastic shrinkage mold with stress risers (a), and picture of the mold with stress risers used in this investigation (b).
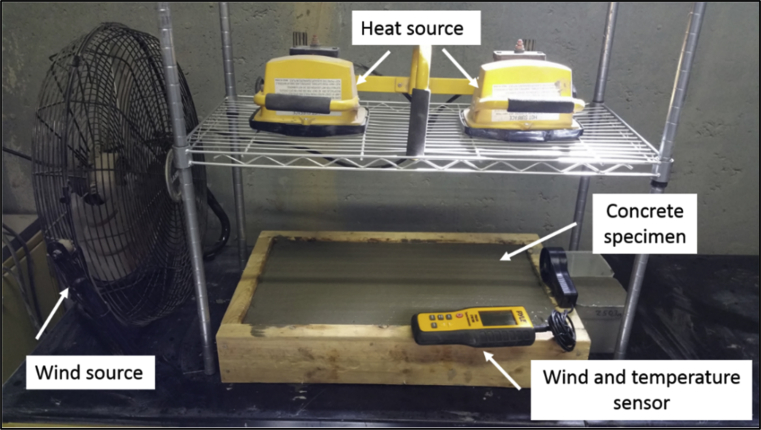
Twenty-five minutes after the addition of water to cement, the specimens were placed in an environmental chamber with a temperature of 36 ± 3 °C, relative humidity of 30 ± 2%, and wind velocity of 24 ± 2 km/h (Fig. 3). Pictures of the concrete surfaces were taken after 2, 4 and 6 hours. The fans were turned off after 6 hours, and the specimens were exposed to the chamber temperature and humidity for an additional 18 hours during which surface cracking was monitored at 1-hour time intervals. The specimens were removed from the environmental chamber after 24 ± 2 hours, and inspected visually in order to evaluate plastic shrinkage cracking and surface condition. Water loss with exposure time was also monitored during the first 6 hours of testing. Two specimens were tested for each condition, and the average value was recorded.
Fig. 3.
Plastic shrinkage test set-up.
Bleeding tests were performed on fresh concrete specimens per ASTM C232 (Method-B For a sample consolidated by vibration). Fresh concrete (mix design presented in Table 2) was filled into a metal container with 150 mm diameter and 150 mm height in two layers, with each layer vibrated for 10 seconds on a vibrating table at medium speed. The container was then placed on a level platform free from vibration and covered with a film to prevent the evaporation of water. A pipette was used to draw off the bleed water at 10-minute intervals during the first 70 minutes, and at 30-minute intervals thereafter until cessation of bleeding. The bleeding test set up is shown in Fig. 4.
Fig. 4.
Bleeding test set up.
The rheological features of fresh (Portland and alkali activated cement) pastes were measured using a digital rheometer (DV-III ULTRA) with stress control and data acquisition capabilities. The fresh mix was placed in a sample holder comprising an external sleeve and an internal rotator. The dimensions of the internal rotator and the external sleeve can be selected based on the rheological properties of the fresh mix. The stress controller controls the torque of the internal rotator. If the relationship between torque and rotational speed is linear, the linearity coefficients are preoperational to the Bingham constants of the fluid. The water-to-cement ratio considered here was 0.35 for both cement pastes.
3. Results and discussion
3.1. Plastic shrinkage
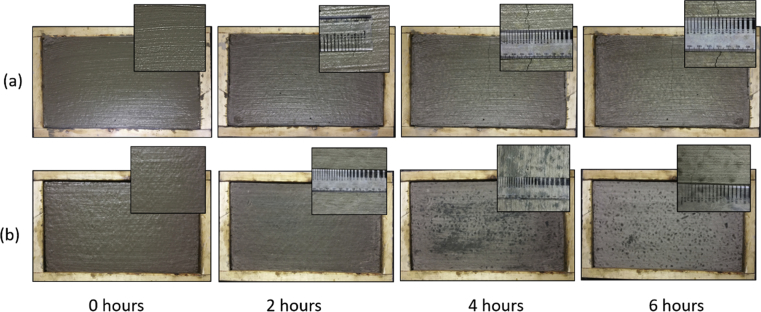
Fig. 5 shows the images taken from the top surfaces of concrete specimens (and zoomed images of any cracks) after 2, 4, and 6 hours of exposure; the measured values of plastic shrinkage crack areas are presented in Table 3.
Fig. 5.
Visual appearance of concrete specimen at different exposure time; (a) Portland cement concrete and (b) alkali activated cement concrete.
Table 3.
Total plastic shrinkage crack areas (mm2) versus time of exposure for Portland cement and alkali activated cement concrete materials.
| Exposure time, hours |
|||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Portland cement concrete | 0 | 8.2 | 28.1 | 92.7 | 138.4 | 177.8 | 190.2 |
| Alkali activated cement concrete | 0 | 0 | 0 | 1.2 | 4.8 | 8.8 | 9.6 |
Small cracks with total area of 8 mm2 started to appear on the Portland cement concrete surface after 1 hour. The cracks were more visible after 2 hours of exposure (as shown in Fig. 5) with total area of 28 mm2. Substantial increase in the crack area was observed after 3 and 4 hours of exposure with areas of 93 and 138 mm2, respectively. By the end of 6 hours, large cracks were observed with total crack area of 190 mm2. Alkali activated cement concrete, on the other hand, did not exhibit any signs of cracking after 2 hours of exposure. After 3 and 4 hours, microcracks were hardly detected with total area of less than 5 mm2. By the end of 6 hours, small cracks were noted on the top surface of alkali activated cement concrete with total area of 10 mm2. These results indicate that the alkali activated cement concrete provided significantly more resistance to plastic shrinkage cracking when compared with Portland cement concrete.
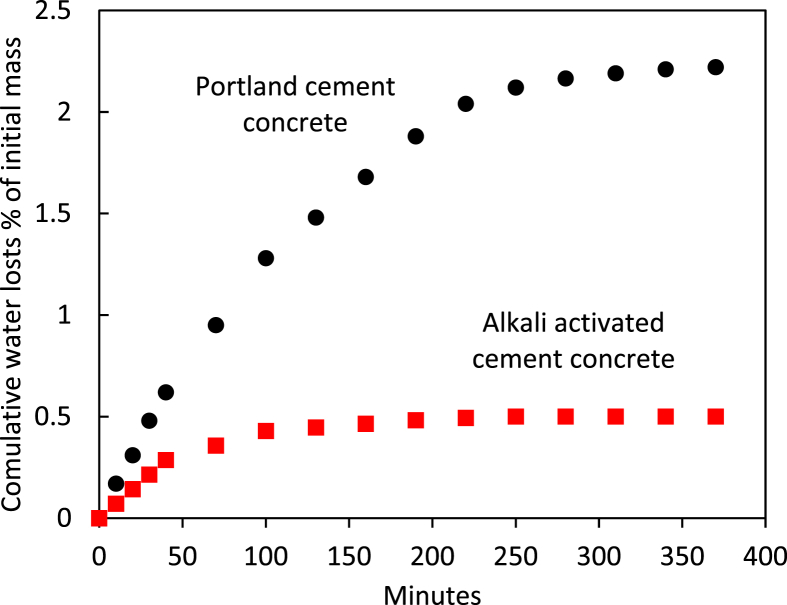
Fig. 6 presents the measured values of cumulative water loss versus time from the Portland cement and alkali activated cement concrete specimens under exposure to drying conditions over 6 hours. Portland cement concrete exhibited greater water loss with exposure time, which reached about 2% of the initial mass of the specimen by the end of 4 hours, after which the rate of moisture loss was smaller and reached 2.2% of the initial mass after 6 hours. The rate and extent of moisture loss from concrete specimens were significantly smaller for the alkali activated cement concrete. Moisture loss was 0.3% of the initial weight of the concrete specimen after 1 hour of exposure. After 6 hours, the total moisture loss was 0.5%. These minimal values of moisture loss point at minimal bleeding of the alkali activated cement concrete; rapid setting of the alkali activated cement concrete partly explains these observations.
Fig. 6.
Cumulative water loss of the alkali activated cement and Portland cement concrete materials versus time under exposure to drying conditions.
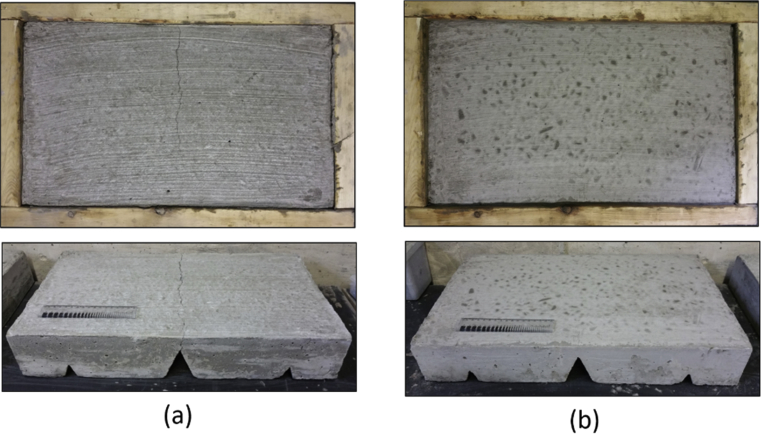
Fig. 7 presents top and side views of the alkali activated and Portland cement concrete surfaces after 24 hours. A continuous crack was observed on the Portland cement concrete surface whereas no notable cracks were observed on the alkali activated cement concrete surface. This observation could be explained partly by the thicker layer of cement paste formed on the Portland cement concrete surface, which exacerbates moisture loss and thus the plastic shrinkage and consequent cracking. While the coarse aggregates were not visible on the surface of Portland cement concrete, they could be observed on the surface of the alkali activated cement concrete. This observation pointed at the relatively small thickness of the cement paste formed on the alkali activated cement concrete surface. The heavy presence of coarse aggregates on the top surface of the alkali activated cement concrete lowers the plastic shrinkage values and raises the resistance to plastic shrinkage cracking. These effects seem to more than compensate for the adverse effects of the reduced bleeding of the alkali activated cement concrete versus Portland cement concrete (presented later).
Fig. 7.
Top and side views after 24 hours for Portland cement concrete specimen (a) and alkali activated cement concrete specimen (b).
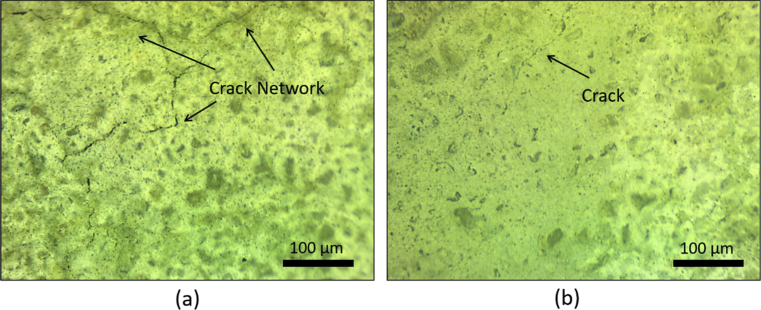
Fig. 8 compares the optic microscope images taken from concrete surfaces after 24 hours of drying. Portland cement concrete was noted to exhibit networked microcracking which covered most of its surface area. Only fine and discontinuous microcracks could be observed on the alkali activated concrete surface. The rapid moisture loss from the Portland cement concrete surface could reduce the degree of hydration at the surface, compromise the quality of aggregate-paste interfaces, and made the surfaces more vulnerable to plastic shrinkage cracking [25]. One could argue that the presence of calcium hydroxide (where calcium oxide was used as a raw material in the alkali activated cement) may reduce the shrinkage of the hardened binder throughout the formation of calcium hydroxide crystals [26].
Fig. 8.
Optic microscope images for the concrete specimens' surfaces exposed to drying conditions; (a) Portland cement concrete and (b) alkali activated cement concrete.
3.2. Bleeding
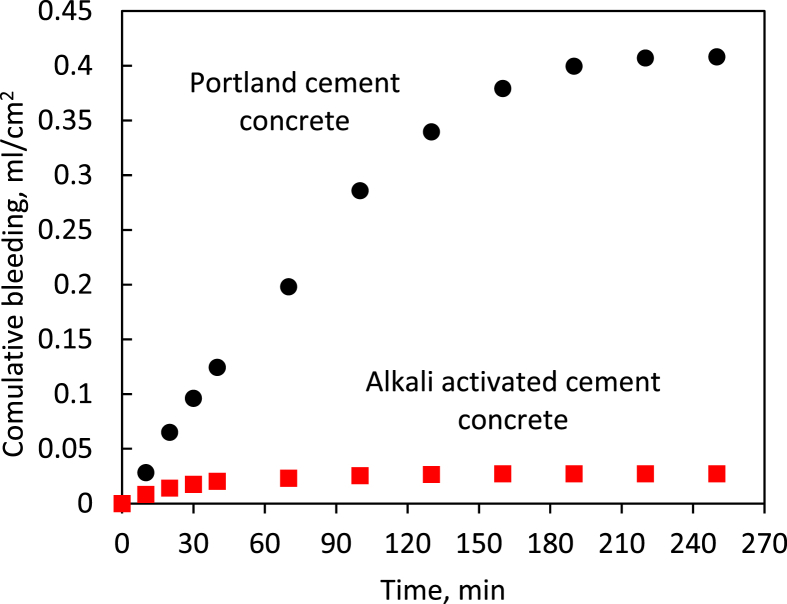
Bleeding has mixed effects on concrete properties; It could raise the water content of the exposed concrete surface that experienced strong weathering effects. At the same time, in conditions that accelerate moisture loss from the concrete surface, it could have beneficial effects towards mitigating plastic shrinkage cracking of the concrete surface by restoring the moisture lost to evaporation. Excess bleeding could also signal segregation tendencies of fresh concrete mixtures [27]. Fig. 9 compares the cumulative bleed water of Portland and alkali activated cement concrete materials. Bleeding of the alkali activated cement concrete is observed to be negligible when compared with that of Portland cement concrete. Alkali activated cements generally exhibit rapid setting and distinct rheological attributes (discussed later). These features could be used to explain the minimal bleeding of the alkali activated cement concrete measured in this investigation. Bleeding generally benefits the resistance of concrete to plastic shrinkage cracking. In spite of its low bleeding, the alkali activated cement concrete was observed to exhibit high resistance to plastic shrinkage cracking. As noted earlier, this could be because some favorable features of the alkali activated cement concrete, including its high aggregate content at the surface and reduced rates of moisture evaporation, more than compensate for its reduced bleeding as far as the effects on plastic shrinkage cracking are concerned.
Fig. 9.
Cumulative bleeding versus time for Portland and alkali activated cement concrete materials.
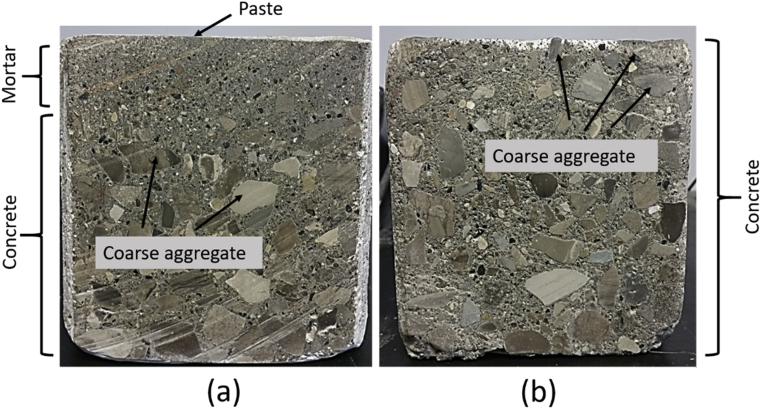
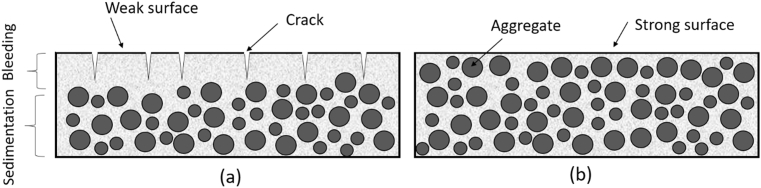
Fig. 10 shows cross sections of the Portland and alkali activated cement concrete specimens after performing the bleeding test. Three layers could be identified in Portland cement concrete (Fig. 10a): (i) a thin paste layer of cement paste with about 8 mm thickness on top; (ii) a mortar layer of about 4 cm thickness; and (iii) concrete. The alkali activated cement concrete (Fig. 10b) was homogeneous, and coarse aggregates could be detected on the surface. As schematically depicted in Fig. 11, the formation of the layered structure with the top cement paste (or mortar) layer that does not benefit from the stabilizing effect of aggregates exacerbates the potential for plastic shrinkage cracking. It seems that the distinctly high viscosity and the relatively high yield stress of the alkali activated cement concrete (when compared with Portland cement concrete), discussed in the next section, produced a segregation-resistant concrete mix which mitigated settlement of aggregates and bleeding of water to the surface. Finally, it should be noted that (barring notable evaporation of surface moisture) bleeding raises the moisture content of the concrete surface, and thus raises its water/cement ratio and lowers its quality [28]. Furthermore, the formation of a network of capillary pores due to the movement of bleeding water towards the surface also reduces the durability of concrete in this zone [29].
Fig. 10.
Cross sections of the bleed test specimens; (a) Portland cement concrete and (b) Alkali activated cement concrete.
Fig. 11.
Schematics of the surface composition and cracking in Portland cement concrete (a) and alkali activated cement concrete (b).
3.3. Rheological characteristics
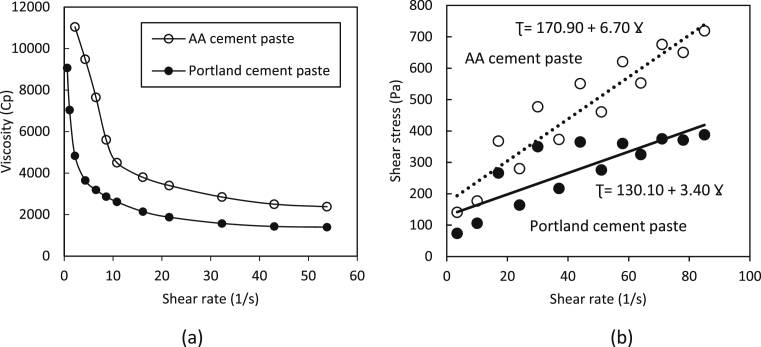
Fig. 12 shows the viscosity-shear rate and the shear stress-shear rate relationships for Portland cement and the alkali activated cement pastes. These test results suggest that the alkali activated cement paste has a higher viscosity when compared with the Portland cement paste (Fig. 12a). The test results on viscosity-shear rate relationships were analyzed in order to determine if the rheological behavior of the two pastes follow the Bingham model. The resulting shear stress-shear rate relationships presented Fig. 12b indicate that application of the Bingham model to these pastes is appropriate. Linear regression analyses of these relationships produced the yield stress and viscosity values summarized in Table 4. These results indicate that the yield stress of the alkali activated cement paste is about 30% higher than that of the Portland cement paste. The plastic viscosity of the alkali activated cement paste is two times that of the Portland cement paste.
Fig. 12.
Apparent Viscosity test results for Portland and alkali activated (AA) cement pastes (a), and the shear stress-shear rate relationships (b).
Table 4.
Yield stress and viscosity of Portland and alkali activated cement pastes.
| Yield stress, Pa | Viscosity, Pa.S | |
|---|---|---|
| Portland cement paste | 130.1 | 3.40 |
| Alkali activated cement paste | 170.9 | 6.70 |
The relatively higher viscosity and yield stress of alkali activated cement paste could also explain the lower bleeding of the alkali activated cement concrete when compared with Portland cement concrete. Knowing that both mixtures have same workability (slump 60 ± 10 mm). The higher attractive forces in the viscous paste could hinder settlement of particles which causes upward movement (bleeding) of water. This is actually the concept used in development of viscosity-enhancing admixtures which enhance the segregation resistance of fresh (e.g., self-consolidating) Portland cement concrete [30].
Previous studies have shown that several additives can be used to enhance the rheological characteristics of alkali activated binders. The addition of citric acid has altered the rheological characteristics of alkali activated binder; the use of 3 wt.% has produced alkali activated binder that has similar yield stress and viscosity that are comparable to those of Portland cement concrete [31].
4. Conclusions
Concrete specimens prepared using an alkali activated cement with predominantly alkali activated chemistry and also specimens prepared with Portland cement were tested for plastic shrinkage, bleeding and rheological characteristics. The following primary conclusions were derived based on the data generated in this experimental work.
-
•
The alkali activated cement concrete exhibited significantly higher resistance to plastic shrinkage cracking when compared with Portland cement concrete. Moisture evaporation from the alkali activated cement concrete was distinctly low when compared with Portland cement concrete.
-
•
Bleeding of the alkali activated cement concrete was negligible when compared with that of Portland cement concrete. Viscosity of the fresh alkali activated cement paste was, at comparable water/cement ratio, twice that of Portland cement paste. Yield stress of the fresh alkali activated cement paste was also relatively high when compared with Portland cement paste.
-
•
Cross-sectional comparisons of the alkali activated, and Portland cement concrete specimens indicated that the surface of Portland cement concrete exhibited indications of segregation where a surface layer that was rich in Portland cement paste formed, and the coarse aggregate content increased with depth. Alkali activated cement concrete did not exhibit such segregation; it did not have a notable compositional gradient with respect to depth, and coarse aggregates were present at the surface; they were visible on the top surface.
-
•
The relatively high viscosity and yield stress of the fresh alkali activated cement paste reduced the settlement of particles, and decreased the upward movement of water (bleeding). The reduced bleeding of the alkali activated cement concrete explains the reduction in water evaporation occurred when the concrete is exposed to drying conditions. Finally, the presence of aggregates near the surface of the alkali activated cement concrete has a stabilizing effect as far as plastic shrinkage movements and cracking potential are concerned. These effects seem to more than compensate for the reduced bleeding of alkali activated cement concrete which could delay drying, and thus improve the plastic shrinkage cracking of the alkali activated concrete surface.
-
•
Recommendation: The work presented in this manuscript investigate the plastic shrinkage cracking for alkali activated cement with specific formulation. In order to establish a general trend for plastic shrinkage behavior of alkali activated concrete, concrete mixtures with different parameters and under several testing conditions should be investigated.
Declarations
Author contribution statement
Faris Matalkah: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yaser Jaradat: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Parviz Soroushian: Conceived and designed the experiments.
Funding statement
This work was supported by the U.S. Department of Energy (Award No. DE-SC0015197).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Powers T.C. 1969. The Properties of Fresh concrete. [Google Scholar]
- 2.Holt E., Leivo M. Cracking risks associated with early age shrinkage. Cement Concr. Compos. 2004;26(5):521–530. [Google Scholar]
- 3.Ravina D., Shalon R. Plastic shrinkage cracking. J. Proc. 1968 [Google Scholar]
- 4.Wittmann F. On the action of capillary pressure in fresh concrete. Cement Concr. Res. 1976;6(1):49–56. [Google Scholar]
- 5.Weyers R., Conway J., Cady P. Photoelastic analysis of rigid inclusions in fresh concrete. Cement Concr. Res. 1982;12(4):475–484. [Google Scholar]
- 6.Radocea A. Chalmers University of Technology; 1992. A Study on the Mechanism of Plastic Shrinkage of Cement-Based Materials. [Google Scholar]
- 7.Mora-Ruacho J., Gettu R., Aguado A. Influence of shrinkage-reducing admixtures on the reduction of plastic shrinkage cracking in concrete. Cement Concr. Res. 2009;39(3):141–146. [Google Scholar]
- 8.Bentz D.P., Geiker M.R., Hansen K.K. Shrinkage-reducing admixtures and early-age desiccation in cement pastes and mortars. Cement Concr. Res. 2001;31(7):1075–1085. [Google Scholar]
- 9.Dudziak L., Mechtcherine V. Enhancing early-age resistance to cracking in high-strength cement-based materials by means of internal curing using super absorbent polymers. Additions improving properties of concrete. RILEM Proc. PRO. 2010;77:129–139. [Google Scholar]
- 10.Lin S.-T., Huang R. Effect of viscosity modifying agent on plastic shrinkage cracking of cementitious composites. Mater. Struct. 2010;43(5):651–664. [Google Scholar]
- 11.Soroushian P., Mirza F., Alhozajiny A. Plastic shrinkage cracking of polypropylene fiber reinforced concrete. Mater. J. 1993;92(5):553–560. [Google Scholar]
- 12.Banthia N., Gupta R. Influence of polypropylene fiber geometry on plastic shrinkage cracking in concrete. Cement Concr. Res. 2006;36(7):1263–1267. [Google Scholar]
- 13.Slowik V. Proceedings of the 8th International Conference on Creep, Shrinkage and Durability of Concrete and Concrete Structures-CONCREEP. 2008. Early age cracking and its influence on the durability of concrete structures. [Google Scholar]
- 14.Alsayed S., Amjad M. Effect of curing conditions on strength, porosity, absorptivity, ans shrinkage of concrete in hot and dry climate. Cement Concr. Res. 1994;24(7):1390–1398. [Google Scholar]
- 15.Shaeles C.A., Hover K.C. Influence of mix proportions and construction operations on plastic shrinkage cracking in thin slabs. Mater. J. 1988;85(6):495–504. [Google Scholar]
- 16.Uno P.J. Plastic shrinkage cracking and evaporation formulas. ACI Mater. J. 1998;95:365–375. [Google Scholar]
- 17.Pacheco-Torgal F., Castro-Gomes J., Jalali S. Alkali-activated binders: a review. Part 2. About materials and binders manufacture. Constr. Build. Mater. 2008;22(7):1315–1322. [Google Scholar]
- 18.Mwiti M.J., Thiong'o J.K., Muthengia W.J. Properties of activated blended cement containing high content of calcined clay. Heliyon. 2018;4(8) doi: 10.1016/j.heliyon.2018.e00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andini S. Coal fly ash as raw material for the manufacture of geopolymer-based products. Waste Manag. 2008;28(2):416–423. doi: 10.1016/j.wasman.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Cheng T., Chiu J. Fire-resistant geopolymer produced by granulated blast furnace slag. Miner. Eng. 2003;16(3):205–210. [Google Scholar]
- 21.Matalkah F., Salem T., Soroushian P. Acid resistance and corrosion protection potential of concrete prepared with alkali aluminosilicate cement. J. Build. Eng. 2018;20:705–711. [Google Scholar]
- 22.Matalkah F., Soroushian P. Synthesis and characterization of alkali aluminosilicate hydraulic cement that meets standard requirements for general use. Constr. Build. Mater. 2018;158:42–49. [Google Scholar]
- 23.Cohen M.D., Olek J., Dolch W.L. Mechanism of plastic shrinkage cracking in portland cement and portland cement-silica fume paste and mortar. Cement Concr. Res. 1990;20(1):103–119. [Google Scholar]
- 24.Al-Amoudi O.S.B., Maslehuddin M., Abiola T.O. Effect of type and dosage of silica fume on plastic shrinkage in concrete exposed to hot weather. Constr. Build. Mater. 2004;18(10):737–743. [Google Scholar]
- 25.Abel J., Hover K. Effect of water/cement ratio on the early age tensile strength of concrete. Transport. Res. Rec. J. Transp. Res. Board. 1998;(1610):33–38. [Google Scholar]
- 26.Yuan X.-h. Shrinkage compensation of alkali-activated slag concrete and microstructural analysis. Constr. Build. Mater. 2014;66:422–428. [Google Scholar]
- 27.Powers T.C. The bleeding of potland cement paste, mortar, and concrete. J. Proc. 1939 [Google Scholar]
- 28.Giaccio G., Giovambattista A. Bleeding: evaluation of its effects on concrete behaviour. Mater. Struct. 1986;19(4):265–271. [Google Scholar]
- 29.Deacon C., Dewar J. Concrete durability-specifying more simply and surely by strength. Concrete. 1982;16:19–21. [Google Scholar]
- 30.Khayat K.H. Viscosity-enhancing admixtures for cement-based materials—an overview. Cement Concr. Compos. 1998;20(2-3):171–188. [Google Scholar]
- 31.Xu L. Effects of citric acid on the rheology, hydration and strength development of alkali aluminosilicate cement. Adv. Cem. Res. 2017;30(2):75–82. [Google Scholar]