Abstract
Background:
Preoperative urinary tract infection (UTI) may be associated with surgical site infections secondary to hematogenous spread of bacteria. The association between preoperative UTI and postoperative complications has not been evaluated in general surgery populations.
Materials and methods:
Patients undergoing elective general surgery procedures from 2011 to 2013 were selected from the American College of Surgeons National Surgical Quality Improvement Program database. Patients with UTI present at the time of surgery (PATOS) were identified as cases. Patients without UTI PATOS were selected and matched 2:1 on age, American Society of Anesthesiologists class, and Current Procedural Terminology code with identified cases. Univariate and multivariate analyses compared postoperative outcomes between the two groups.
Results:
A total of 434,802 patients were identified for inclusion in the study, with an overall preoperative UTI rate of 0.1% (n = 363). On univariate analysis, the UTI group had a significantly higher incidence of overall complications, infectious complications, and noninfectious complications. Multivariate analysis confirmed that patients with UTI had a higher risk of postoperative complications compared with those without preoperative UTI (odds ratio [OR] 1.551, 95% confidence interval [CI] 1.071–2.247). This relationship persisted for both infectious (OR 1.515, 95% CI 1.000–2.296) and noninfectious (OR 1.683, 95% CI 1.012–2.799) complications.
Conclusions:
We demonstrated an increased rate of 30-d complications in elective general surgery patients with UTI PATOS. These findings suggest that diligent efforts to diagnose and treat UTI before surgery may result in improved outcomes. Furthermore, surgeons should consider postponing elective procedures to allow for the complete resolution of preoperative UTI.
Keywords: Urinary tract infection, Postoperative, Complication, UTI
Introduction
Over 4%−5% of patients undergoing colorectal surgeries and nearly 3% of patients undergoing elective vascular surgeries develop postoperative urinary tract infection (UTI).1–4 In the perioperative setting, UTI is associated with higher costs, longer hospital stay, and increased incidence of other postoperative complications.3–8 Although the implications of postoperative UTI are well known, the impact of preoperative UTI on postoperative outcomes remains controversial. The urinary tract is the most common source of bacteremia in the elderly, which highlights the potential role of urinary pathogens in systemic illness.9 In the orthopedic surgery literature, bacteria from the urinary tract have been implicated in the development of surgical site infection (SSI). Specifically, a mechanism of hematogenous spread of urinary pathogens and subsequent seeding at the site of orthopedic implants has been described.10–13 Conversely, others argue that patients with preoperative UTI have no increased risk of SSI, and therefore, this should not be a reason to delay surgery.14
Despite the well-described negative consequences of postoperative UTI, fewer studies have examined the relationship between UTI present at the time of surgery (PATOS) and postoperative complications. Diagnosis and treatment of UTI preoperatively may prove to be an easily modifiable factor that could have the potential to improve postoperative outcomes. The goal of this study was to evaluate the risk of postoperative complications in the setting of preoperative UTI in patients undergoing elective general surgery procedures. We hypothesized that the presence of UTI at the time of elective surgery is associated with increased postoperative complication rates compared with those without preoperative UTI.
Methods
Data source and patient selection
All patients in the multi-institutional American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) participant use files from 2011 to 2013 who underwent elective general surgery procedures were considered for inclusion in this study. Patients who had missing UTI PATOS information were excluded. Additional exclusion criteria included American Society of Anesthesiologists (ASA) class 5 or unknown, presence of preoperative sepsis or septic shock, and preoperative ventilator dependence. Patients with UTI PATOS were identified as cases. Patients without UTI PATOS were selected and matched 2:1 on age, ASA class, and Current Procedural Terminology (CPT) code with the previously identified cases (Fig. 1). The University of Wisconsin granted Institutional Review Board exemption for this study. Patient consent was not required for this study.
Fig. 1 –
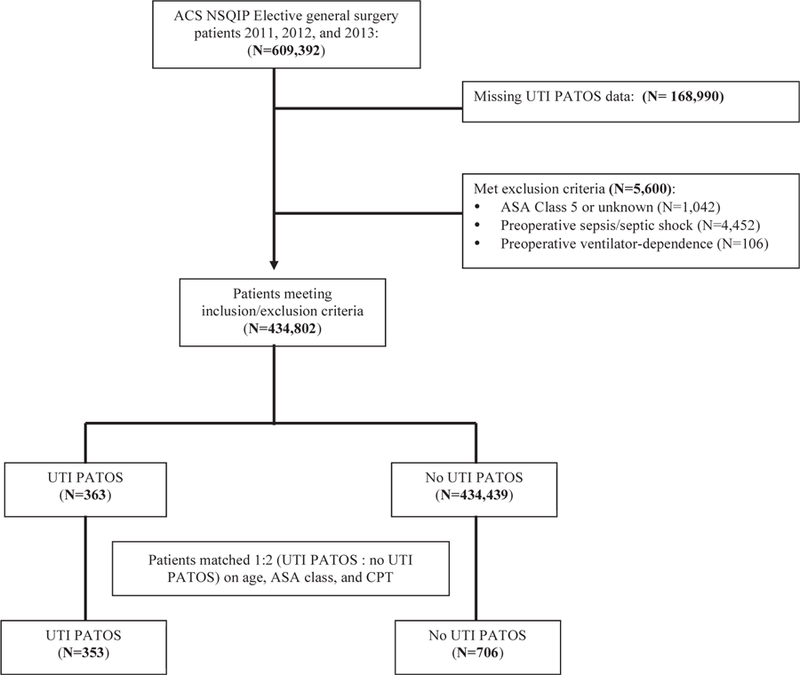
Flow diagram depicting construction of cohorts.
Variable definitions
“Elective surgery” is defined in NSQIP as surgery in which the patient presents to the facility for a scheduled surgery on the day that the procedure is to be performed and specifically excludes emergent and urgent cases, along with cases in which the patient is inpatient status before surgery, presents through the emergency department, or is transferred from a clinic. General surgery procedures were defined as those being performed by a general surgeon and included a variety of different procedures ranging from skin and soft-tissue surgery to solid organ resections (Table 1). Procedures were categorized by CPT code into seven major groups as summarized in Table 1.
Table 1 –
Categorical grouping of CPT codes based on procedure type and location.
| CPT category | CPT codes included |
|---|---|
| Skin/soft tissue/breast/thyroid/parathyroid | 10140, 11004, 11008, 11042, 11043, 11044, 15271, 15734, 15936, 19120, 19125, 19301, 19302, 19303, 19304, 19307, 38760, 49021, 60220, 60240, 60252, 60260, 60271, 60500 |
| Major vascular and lower extremity amputation | 27590, 27880, 35565, 35646, 35656 |
| Major thoracic surgery | 32663, 43112, 43122, 43130 |
| Major noncolorectal intra-abdominal surgery | 38100, 38120, 43280, 43281, 43282, 43332, 43621, 43634, 43644, 43645, 43775, 43999, 44005, 44120, 44125, 44130, 44180, 44187, 44625, 44310, 44314, 44640, 44799, 47120, 47130, 47562, 47563, 47600, 47605, 47741, 47780, 48140, 48150, 48153, 48548, 48999, 49000, 49999, 58150, 58542, 60650 |
| Colorectal surgery | 44140, 44141, 44143, 44144, 44145, 44147, 44150, 44155, 44158, 44160, 44188, 44204, 44205, 44206, 44207, 44208, 44210, 44320, 44340, 44345, 44346, 44620,, 44626, 44960, 44970, 45110, 45111, 45112, 45119, 45130, 45136, 45402, 45540, 45999 |
| Urologic | 44660, 44661, 45805, 50546, 50760, 51595, 52240 |
| Inguinal or ventral hernia | 49205, 49505, 49521, 49560, 49561, 49565, 49566, 49568, 49570, 49587, 49650, 49651, 49652, 49654, 49655 |
Preoperative UTI was defined according to the UTI PATOS variable, which was added to the NSQIP database in 2011. This variable captures UTIs that were likely or definitely present preoperatively, specifically in patients in whom UTI is diagnosed as a postoperative complication. If UTI is diagnosed within 30 d of surgery (Table 2), the UTI is considered to have been present before surgery if “there was any preoperative evidence of a symptomatic UTI (that had not started treatment or is currently undergoing treatment) or preoperative evidence was highly suggestive or suspicious of a UTI at the time of surgery.”15 Importantly, this includes only cases in which either treatment was not started preoperatively or treatment was started and remained underway at the time of the postoperative UTI diagnosis. This does not include patients who had been diagnosed with a UTI before the time of surgery and had completed treatment, without evidence of ongoing infection.
Table 2 –
NSQIP definition of urinary tract infection as a postoperative complication.
| A* | B |
|---|---|
| ONE of the following: | TWO of the following: |
| Fever (>38° C or 100.4° F) | Fever (>38° C or 100.4° F) |
| Urgency | Urgency |
| Frequency | Frequency |
| Dysuria | Dysuria |
| Suprapubic tenderness | Suprapubic tenderness |
| Costovertebral angle pain or tenderness | Costovertebral angle pain or tenderness |
| AND | AND |
| Urine culture with >100,000 colonies/mL urine with _two species of organisms | ONE of the following: |
| Dipstick test positive for leukocyte esterase and/or nitrate Pyuria | |
| Organisms seen on Gram stain of unspun urine | |
| Two urine cultures with repeated isolation of the same uropathogen with >100,000 colonies/mL urine in nonvoided specimen | |
| Urine culture with <100,000 colonies/mL urine of single uropathogen in patient being treated with appropriate antimicrobial therapy Physician’s diagnosis | |
| Physician institutes appropriate antimicrobial therapy |
Criteria listed in either A or B must be met for postoperative diagnosis of urinary tract infection.
Age was treated as a categorical variable with the following definitions: <40 y, 40–55 y, 56–70 y, and >70 y. Body mass index (BMI) was also divided into categories as nonobese (BMI < 30) and obese (BMI ≥ 30).
Outcomes
The primary outcome for this study was the occurrence of at least one postoperative complication within 30 d of surgery (“any complication”). Complications reported in NSQIP were grouped into two categories to more clearly understand the effect of UTI PATOS on postoperative morbidity. The composite outcome “infectious complication” included the presence of at least one of the following: superficial incisional SSI, deep incisional SSI, organ space SSI, and pneumonia. Similarly, “noninfectious complication” included the presence of at least one of: dehiscence, unplanned intubation, pulmonary embolism, ventilator use >48 h, progressive renal insufficiency, acute renal failure, cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, and deep vein thrombosis/thrombophlebitis. Additional secondary outcomes included the occurrence of each individual complication reported in the NSQIP database.
By definition in NSQIP, a condition can only be coded as PATOS if it has already been noted as a postoperative complication. For this reason, postoperative UTI was not included in any of the composite outcomes or as an individual postoperative outcome. Furthermore, if any condition was noted to be present at the time of surgery (e.g., pneumonia) in a particular patient, it was not considered a postoperative complication in that individual.
Statistical analysis
Univariate statistical analysis was performed using chi-square or Fisher’s exact tests to compare the UTI PATOS and no UTI PATOS groups with regard to patient demographics, comorbidities, and postoperative complication rates. Multivariate analysis was then performed using multiple logistic regression with no UTI PATOS as the reference category. Explanatory variables of interest for the multivariate model included patient demographics, comorbid conditions, procedure class, and presence or absence of UTI PATOS. Any preoperative variable found to be significantly associated with an outcome on univariate analysis at a significance level of 0.05 was included as a covariate in the multivariate models. Finally, two sensitivity analyses were undertaken in which the multivariate regression models were performed first excluding patients with a preoperative wound infection and subsequently excluding patients who received a preoperative blood transfusion. P < 0.05 was considered statistically significant for all analyses.
Results
A total of 434,802 patients were identified for inclusion in the study before matching. Of these patients, the overall rate of UTI at the time of surgery was 0.1% (n = 363). After matching cases with controls 1:2, there were 353 patients with UTI and 706 without UTI (Fig. 1). Table 3 summarizes and compares the baseline characteristics of the two cohorts. The two groups were similar with regard to age, BMI, and ASA class. There were more women and higher frequency of dependent functional status in the UTI group. Not surprisingly, the patients with UTI PATOS also had a higher incidence of other comorbid conditions, such as congestive heart failure (1.7% versus 0.6%, P = 0.093), wound infection (10.8% versus 3.4%, P ≤ 0.001), weight loss >10% (6.5% versus 2.8%, P = 0.004), and receipt of preoperative transfusion (2.0% versus 0.6%, P = 0.032; Table 3).
Table 3.
– Comparison of baseline demographics and comorbidities between the two cohorts.
| Characteristic, % (n) | UTI PATOS (n = 353) | No UTI PATOS (n = 706) | P value |
|---|---|---|---|
| Female | 73.1 (258) | 73.1 (258) | <0.001 |
| Race | 0.382 | ||
| White | 80.2 (283) | 76.8 (542) | |
| Black and/or African American | 9.9 (35) | 10.3 (73) | |
| Other | 3.4 (12) | 3.3 (23) | |
| Unknown | 6.5 (23) | 9.6 (68) | |
| Age, y | 0.988 | ||
| <40 | 10.8 (38) | 10.2 (72) | |
| 41–55 | 15.6 (55) | 16.1 (114) | |
| 56–70 | 34.6 (122) | 34.8 (246) | |
| 70+ | 39.1 (138) | 38.8 (274) | |
| BMI | 0.291 | ||
| Nonobese (BMI < 30) | 53.6 (187) | 57.0 (398) | |
| Obese (BMI _ 30) | 46.4 (162) | 43.0 (300) | |
| Outpatient classification | 20.4 (72) | 29.6 (209) | 0.001 |
| Diabetes | 0.275 | ||
| Noninsulin dependent | 13.9 (49) | 12.5 (88) | |
| Insulin dependent | 10.5 (37) | 7.9 (56) | |
| Smoker | 20.7 (73) | 17.0 (120) | 0.143 |
| Dyspnea | 7.4 (26) | 8.5 (60) | 0.525 |
| Dependent functional status | 10.5 (37) | 3.4 (24) | <0.001 |
| ASA classification | 0.999 | ||
| I | 2.8 (10) | 2.8 (20) | |
| II | 33.1 (117) | 33.1 (234) | |
| III | 53.3 (188) | 53.5 (378) | |
| IV | 10.8 (38) | 10.5 (74) | |
| Chronic obstructive pulmonary disease | 5.4 (19) | 5.7 (40) | 0.850 |
| Ascites | 0.3 (1) | 0.3 (2) | 1.000* |
| Congestive heart failure | 1.7 (6) | 0.6 (4) | 0.093* |
| Hypertension | 55.0 (194) | 56.5 (399) | 0.630 |
| Acute renal failure | 0.6 (2) | 0.3 (2) | 0.604* |
| Dialysis-dependent | 0.8 (3) | 2.0 (14) | 0.167 |
| Disseminated cancer | 5.7 (20) | 5.2 (37) | 0.773 |
| Wound infection | 10.8 (38) | 3.4 (24) | <0.001 |
| Steroid use | 7.9 (28) | 5.9 (42) | 0.221 |
| Weight loss | 6.5 (23) | 2.8 (20) | 0.004 |
| Bleeding disorder | 5.9 (21) | 4.4 (31) | 0.269 |
| Transfusion | 2.0 (7) | 0.6 (4) | 0.032 |
| CPT code groupings | 1.000 | ||
| Skin/soft tissue/breast/ thyroid/parathyroid | 11.6 (41) | 11.6 (82) | |
| Major vascular and lower extremity amputation | 2.0 (7) | 2.0 (14) | |
| Major thoracic surgery | 1.1 (4) | 1.1 (8) | |
| Major noncolorectal intra-abdominal surgery | 31.7 (112) | 31.7 (224) | |
| Colorectal surgery | 36.5 (129) | 36.5 (129) | |
| Urologic | 2.8 (10) | 2.8 (10) | |
| Inguinal or ventral hernia | 14.2 (50) | 14.2 (50) |
Fisher’s exact P value.
On univariate analysis, we found that patients with a UTI PATOS had a higher rate of any postoperative complication compared with controls (19.3% versus 11.9%, P = 0.001; Fig. 2). When the outcome measure was categorized by type of complication, preoperative UTI was associated with a significantly higher rate of both infectious complications (14.2% versus 9.1%, P = 0.012) and noninfectious complications (9.9% versus 5.2%, P = 0.004) when compared with those without UTI (Fig. 2). Finally, comparison of individual complications demonstrated that the proportion of patients with each postoperative complication was higher in the UTI PATOS cohort with the exception of cardiac arrest requiring CPR, which was equally incident between the two groups (Table 4). Specifically, the difference in complication rates between the two groups was most dramatic for respiratory complications, including unplanned intubation (3.4% versus 1.0%, P = 0.005) and on ventilator >48 h (3.4% versus 1.3%, P = 0.019; Table 4).
Fig. 2 –
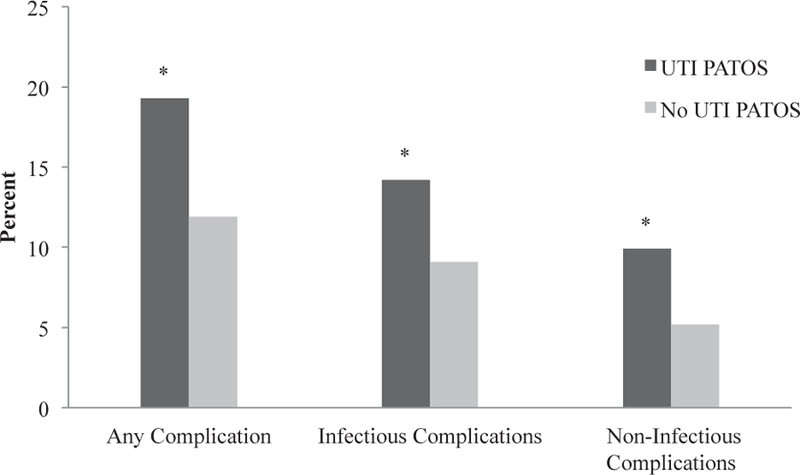
Rates of postoperative morbidity using composite end points in patients with and without UTI present at the time of elective general surgery procedures. *P < 0.05.
Table 4 –
Rates of specific postoperative complications in patients with and without UTI PATOS.
| Characteristic, % (n) | UTI PATOS (n = 353) | No UTI PATOS (n = 706) | P value |
|---|---|---|---|
| Any complication | 19.3 (68) | 11.9 (84) | 0.001 |
| Infectious complication | 14.2 (50) | 9.1 (64) | 0.012 |
| Superficial incisional surgical site infection | 4.8 (17) | 3.4 (24) | 0.260 |
| Deep incisional surgical site infection | 2.3 (8) | 1.1 (8) | 0.154 |
| Organ space surgical site infection | 3.1 (11) | 2.3 (16) | 0.408 |
| Pneumonia | 4.5 (16) | 2.5 (18) | 0.084 |
| Noninfectious complication | 9.9 (35) | 5.2 (37) | 0.004 |
| Wound dehiscence | 1.1 (4) | 0.7 (5) | 0.491* |
| Unplanned intubation | 3.4 (12) | 1.0 (7) | 0.005 |
| Pulmonary embolism | 0.8 (3) | 0.6 (4) | 0.692* |
| Ventilator > 48 h | 3.4 (12) | 1.3 (9) | 0.019 |
| Progressive renal insufficiency | 1.4 (5) | 0.1 (1) | 0.018* |
| Acute renal failure | 1.4 (5) | 0.4 (3) | 0.126* |
| Cardiac arrest requiring CPR | 0.3 (1) | 0.3 (2) | 1.000* |
| Myocardial infarction | 1.1 (4) | 0.8 (6) | 0.739* |
| DVT/thrombophlebitis | 3.4 (12) | 1.6 (11) | 0.053 |
CPR, cardiopulmonary resuscitation; DVT, deep vein thrombosis.
Fisher’s exact test P value.
Multivariate analysis controlling for baseline differences between the two groups confirmed a greater risk of postoperative complications in patients with UTI PATOS. We found that patients with UTI had over 50% increased risk of any postoperative complication compared with those without preoperative UTI (odds ratio [OR] 1.551, 95% confidence interval [CI] 1.071–2.247). This observed relationship again held true for both infectious complications (OR 1.515, 95% CI 1.000–2.296) and noninfectious complications (OR 1.683, 95% CI 1.012–2.799; Table 5). Other significant predictors of postoperative complications in the multivariate analysis were receipt of preoperative blood transfusion and inpatient case classification.
Table 5 –
Results of multivariate analyses for primary outcomes.
| Factor | Any complication |
Infectious complication |
Noninfectious complication |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| UTI PATOS | 1.551 (1.071–2.247) | 0.020 | 1.515 (1.00–2.296) | 0.050 | 1.683 (1.012–2.799) | 0.045 |
| Gender | ||||||
| Female | Ref | Ref | Ref | |||
| Male | 0.952 (0.656–1.380) | 0.794 | 0.906 (0.599–1.372) | 0.642 | 0.873 (0.524–1.454) | 0.602 |
| Case classification | ||||||
| Outpatient | Ref | Ref | Ref | |||
| Inpatient | 4.616 (2.507–8.502) | <0.001 | 3.458 (1.820–6.567) | <0.001 | 12.953 (3.145–53.354) | <0.001 |
| Functional status | ||||||
| Independent | Ref | Ref | Ref | |||
| Dependent | 1.259 (0.610–2.598) | 0.533 | 0.906 (0.376–2.181) | 0.826 | 2.199 (0.953–5.072) | 0.065 |
| Wound infection | 1.117 (0.526–2.373) | 0.773 | 0.960 (0.398–2.315) | 0.927 | 1.099 (0.378–2.690) | 0.968 |
| >10% weight loss in 6 mo before surgery | 1.096 (0.493–2.439) | 0.822 | 1.470 (0.643–3.361) | 0.361 | 1.187 (0.431–3.269) | 0.740 |
| Preoperative transfusion | 5.689 (1.651–19.607) | 0.006 | 3.615 (1.008–12.956) | 0.049 | 2.071 (0.411–10.441) | 0.378 |
Bold values indicate statistical significance.
The sensitivity analysis excluding patients with preoperative wound infection did not significantly change the association between UTI PATOS and each of the three primary outcomes (any complication OR 1.561, 95% CI 1.062–2.293; infectious complication OR 1.465, 95% CI 0.954–2.251; and noninfectious complication OR 1.698, 95% CI 1.000–2.882). Similarly, exclusion of patients receiving preoperative blood transfusion also did not alter the results (any complication OR 1.542, 95% CI 1.061–2.243; infectious complication OR 1.558, 95% CI 1.024–2.370; and noninfectious complication OR 1.617, 95% CI 0.966–2.706).
Discussion
In this study, we have demonstrated that patients undergoing elective, general surgery procedures who have an unrecognized or incompletely treated UTI at the time of surgery have increased rates of postoperative complications compared with those without a preoperative UTI. Importantly, this increased risk of postoperative complications is observed for both infectious and noninfectious complications. To our knowledge, this relationship has not been clearly demonstrated previously. Although it is evident that preoperative UTI is associated with many other markers of worse postoperative outcomes, UTI itself appears to be an independent predictor of postoperative complications in this analysis.
Our finding of increased postoperative complications in patients with UTI PATOS is consistent with those of previous studies and case reports that have highlighted the possibility of hematogenous spread and distant seeding of bacteria from the urinary tract. D’Ambrosia et al.12 have reported three fatal cases of postoperative infections with an identified hematogenous source. Similarly, Stinchfield et al.16 described hematogenous spread of bacteria responsible for infection in nine patients. Specific to the urinary tract, multiple authors have reported cases of hematogenous spread with evidence of the urinary tract as the source of infection.10,11,13
In evaluating the consequences of UTI after colorectal surgery, Sheka et al.3 studied 47,781 colorectal surgery patients in the NSQIP database and found that postoperative UTI negatively impacts patient outcomes and is associated with increased incidence of other complications. Specifically, they found that over half of patients with postoperative UTI developed at least one additional complication.3 Our findings suggest that preoperative UTI is associated with similarly poor outcomes, with nearly 20% of the UTI PATOS group experiencing at least one complication after elective general surgery. The higher incidence of complications observed by Sheka et al. is likely related to their study population, composed entirely of patients undergoing colorectal surgery, a population known to have higher average complication rates compared with other general surgery procedures.3,17
Although we have shown that preoperative UTI is an independent predictor of both infectious and noninfectious postoperative complications, whether asymptomatic bacteriuria would similarly increase risk of postoperative complications remains an area of controversy. Currently, screening and subsequent treatment of asymptomatic bacteriuria is only recommended in pregnant women, and those undergoing invasive genitourinary procedures where treatment has been shown to improve outcomes and decrease complications.18 Aside from these populations, it has been reported that treatment of asymptomatic bacteriuria provides no measurable improvement in morbidity and mortality, promotes higher rates of antibiotic resistant pathogens and secondary infections, and can even paradoxically increase rates of postoperative UTI and wound infections compared with untreated asymptomatic bacteriuria.19–22 Although the definition used by NSQIP to identify UTI PATOS is likely capturing predominantly patients who were symptomatic, it may also include asymptomatic patients who had other evidence of UTI. In addition, the UTI PATOS group in our study included patients in whom treatment may have been started preoperatively but complete resolution of UTI had not been achieved at the time of surgery. Therefore, it remains unclear from these data if a complete course of antimicrobials is necessary to mitigate the risks associated with active urinary infection. Prospective studies could help clarify issues such as timing of treatment in relation to surgery, duration of treatment, and extent to which these interventions can improve outcomes.
Although we have demonstrated a significantly increased risk of postoperative complications in the setting of preoperative UTI, our study has several major limitations. The primary limitation is the retrospective and nonrandomized design. There are clearly substantial baseline differences between patients with and without UTI PATOS. To address this issue, we selected controls by matching on age, ASA classification, and CPT code and additionally performed a multivariate analysis controlling for any variables that significantly differed between the two groups on univariate analysis. Although NSQIP provides many comorbidities and variables for risk adjustment, there are certainly unmeasured differences that remain between the two groups for which we were unable to control. Therefore, baseline differences in health and risk for poor outcomes may have been partly responsible for our observed increased complication rate in patients with UTI PATOS.
The second major limitation of this study is related to the definition of UTI PATOS. To satisfy the NSQIP definition of UTI PATOS, patients must have documented evidence or high suspicion of a preoperative UTI in the setting of a postoperative UTI diagnosis. Therefore, all patients in the UTI PATOS group, by definition, have already been diagnosed with a UTI as a complication. Given known negative consequences of UTI in the postoperative setting, this definition makes it difficult to discern to what extent poor outcomes are related to the UTI being present specifically at the time of surgery versus during the postoperative period. We also did not have specific information on what constituted “evidence of a symptomatic UTI” in any given patient. Furthermore, the definition of UTI PATOS suggests that patients may or may not have started treatment for UTI preoperatively. This significantly limits our ability to determine whether partial treatment of a UTI is adequate to mitigate the risk or if surgery should be delayed until complete resolution of UTI.
Finally, we were unable to fully explore the mechanism behind UTI PATOS and increased postoperative complications. Specifically, to address whether the urinary tract served as a direct source of bacteria for postoperative infections via hematogenous spread, confirmation through culture of the etiologic agent at both the urinary tract and source site and potential metastatic sites would be needed. Unfortunately, culture data are not available in the NSQIP database.
Despite these limitations, we have found a clear association between UTI PATOS and increased risk of complications. Future studies will be helpful in understanding how this information can be used not only as a marker of worse outcomes but also for risk reduction.
Conclusions
To our knowledge, this is the first study to comprehensively evaluate the impact of UTI PATOS on postoperative outcomes in patients undergoing elective general surgery procedures. As part of ongoing quality improvement efforts, it is crucial to identify any potentially modifiable risk factors for complications. It is clear from our study that UTI PATOS is associated with worse postoperative outcomes in general surgery patients. Therefore, surgeons should maintain a high index of suspicion and obtain a relevant urinary history to identify high-risk patients. Furthermore, surgeons may consider postponing elective procedures to allow for the complete resolution of preoperative UTI.
Acknowledgment
Salary support for CM Papageorge is provided by a T32 Surgical Oncology Training Grant (NIH NCI T32 CA090217–11).
Footnotes
Disclosures
The authors have no conflicts of interest or financial ties to disclose.
Contributor Information
Courtney J. Pokrzywa, Department of Surgery, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin.
Christina M. Papageorge, Department of Surgery, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin.
Gregory D. Kennedy, Department of Surgery, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin.
REFERENCES
- 1.Vogel TR, Dombrovskiy VY, Carson JL, Haser PB, Lowry SF, Graham AM. Infectious complications after elective vascular surgical procedures. J Vasc Surg 2010;51:122–130. [DOI] [PubMed] [Google Scholar]
- 2.Regenbogen SE, Read TE, Roberts PL, Marcello PW, Schoetz DJ, Ricciardi R. Urinary tract infection after colon and rectal resections: more common than predicted by risk-adjustment models. J Am Coll Surg 2011;213:784–792. [DOI] [PubMed] [Google Scholar]
- 3.Sheka AC, Tevis S, Kennedy GD. Urinary tract infection after surgery for colorectal malignancy: risk factors and complications. Am J Surg 2016;211:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang CY, Chaudhry OO, Halabi WJ, et al. Risk factors for postoperative urinary tract infection and urinary retention in patients undergoing surgery for colorectal cancer. Am Surg 2012;78:1100–1104. [PubMed] [Google Scholar]
- 5.Núñez-Pereira S, Rodrı´guez-Pardo D, Pellisé F, et al. Postoperative urinary tract infection and surgical site infection in instrumented spinal surgery: is there a link? Clin Microbiol Infect 2014;20:768–773. [DOI] [PubMed] [Google Scholar]
- 6.Yadla S, Ghobrial GM, Campbell PG, et al. Identification of complications that have a significant effect on length of stay after spine surgery and predictive value of 90-day readmission rate 2015;23(December):1–5. doi: 10.3171/2015.3.SPINE14318. [DOI] [PubMed] [Google Scholar]
- 7.Rogers MAM, Langa KM, Kim C, et al. Contribution of infection to increased mortality in women after cardiac surgery. Arch Intern Med 2006;166:437–443. [DOI] [PubMed] [Google Scholar]
- 8.Khuri SF, Henderson WG, Depalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg 2005;242:341–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mouton CP, Bazaldua OV, Pierce B, Espino DV. Common infections in older adults. Am Fam Physician 2001;63:257–268. [PubMed] [Google Scholar]
- 10.Donovan TL, Gordon RO, Nagel DA. Urinary infections in total hip arthroplasty: influences of prophylactic cephalosporins and catheterization. J Bone Joint Surg Am 1980;58A:2630–2634. [PubMed] [Google Scholar]
- 11.Hall AJ. Late infection about a total knee prosthesis: report of a case secondary to urinary tract infection. J Bone Joint Surg Am 1974;56B:144–147. [PubMed] [Google Scholar]
- 12.D’Ambrosia RD, Shoji H, Heater R. Secondarily infected total joint replacements by hematogenous spread. J Bone Joint Surg Am 1976;58:450–453. [PubMed] [Google Scholar]
- 13.Cruess R, Bickel W, VonKessler K. Infections in total hips secondary to a primary source elsewhere. Clin Orthop Relat Res 1975:99–101. [DOI] [PubMed] [Google Scholar]
- 14.Koulouvaris P, Sculco P, Finerty E, Sculco T, Sharrock NE. Relationship between perioperative urinary tract infection and deep infection after joint arthroplasty. Clin Orthop Relat Res 2009;467:1859–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American College of Surgeons National Surgical Quality Improvement Program. User Guide for the Participant UseData File https://www.facs.org/w/media/files/qualityprograms/nsqip/nsqip_puf_userguide_2014.ashx. Published 2013.
- 16.Stinchfield FE, Bigliani LU, Neu HC, Goss TP, Foster CR. Late hematogenous infection of total joint replacement. J Bone Joint Surg Am 1980;62:1345–1350. [PubMed] [Google Scholar]
- 17.Schilling PL, Dimick JB, Birkmeyer JD. Prioritizing quality improvement in general surgery. J Am Coll Surg 2008;207:698–704. [DOI] [PubMed] [Google Scholar]
- 18.Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005;40:643–654. [DOI] [PubMed] [Google Scholar]
- 19.Cai T, Nesi G, Mazzoli S, et al. Asymptomatic bacteriuria treatment is associated with a higher prevalence of antibiotic resistant strains in women with urinary tract infections. Clin Infect Dis 2015;61:1655–1661. [DOI] [PubMed] [Google Scholar]
- 20.Dull RB, Friedman SK, Risoldi ZM, Rice EC, Starlin RC, Destache CJ. Antimicrobial treatment of asymptomatic bacteriuria in noncatheterized adults: a systematic review. Pharmacotherapy 2014;34:941–960. [DOI] [PubMed] [Google Scholar]
- 21.Ferroni M, Taylor AK. Asymptomatic bacteriuria in noncatheterized adults. Urol Clin North Am 2015;42:537–545. [DOI] [PubMed] [Google Scholar]
- 22.Gonza E, Martı D. Prevalence of asymptomatic bacteriuria in knee arthroplasty patients and subsequent risk of prosthesis infection 2015. doi: 10.1007/s00590-015-1720-4. [DOI] [PubMed] [Google Scholar]


