Abstract
Background:
The number of patients with end-stage pulmonary disease awaiting lung transplantation is at an all-time high, while the supply of available organs remains stagnant. Utilizing donation after circulatory death (DCD) donors may help to address the supply-demand mismatch. The objective of this study is to determine the potential donor pool expansion with increased procurement of DCD organs from patients who die at hospitals.
Material and Methods:
The charts of all patients who died at a single, rural, quaternary-care institution between August 2014 and June 2015 were reviewed for lung transplant candidacy. Inclusion Criteria were age <65 years, absence of cancer and lung pathology, and cause of death other than respiratory or sepsis.
Results:
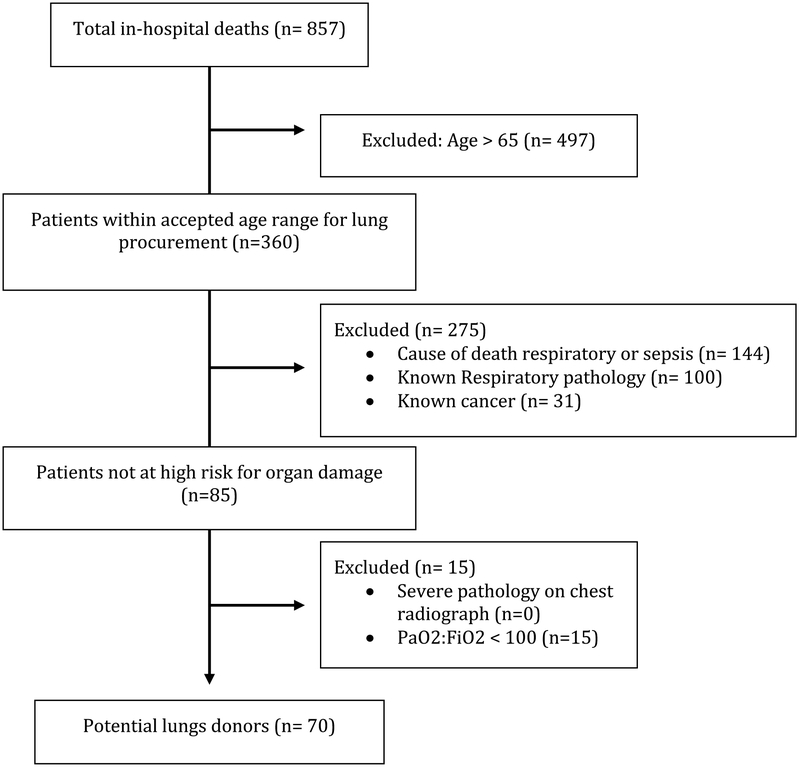
A total of 857 patients died within a one-year period and were stratified by age: Pediatric <15 years (n=32, 4%), Young 15–64 years (n=328, 38%), and Old >65 years (n=497, 58%). Those without cancer totaled 778 (90.8%) and 512 (59%) did not have lung pathology. This leaves 85 patients qualifying for DCD lung donation (Pediatric n=10, Young n=75, and Old n=0). Potential donors were significantly more likely to have clear chest X-rays (24.3% vs 10.0%, p<0.0001) and higher mean PaO2/FiO2 (342.1 vs 197.9, p<0.0001) compared to ineligible patients.
Conclusions:
A significant number of DCD lungs are available every year from patients that die within hospitals. We estimate the use of suitable DCD lungs could potentially result in a significant increase in the number of lungs available for transplantation.
Keywords: Lung transplantation, donation after circulatory death, high-risk donation, donor pool size
INTRODUCTION
Lung transplantation remains the standard of care for patients with end-stage pulmonary disease that is refractory to medical therapy. However, despite modest increases in donor authorization rates, the supply of donor lungs is not adequate to meet the growing demand.1 The number of patients waiting for a transplant at year’s end has increased dramatically over the last decade and waitlist mortality remains unacceptably high.2 According to data from the U.S. Department of Health and Human Services Organ Procurement and Transplantation Network, there were 2057 lung transplants performed in 2015, while more than 2400 patients were added to the waiting list during the previous year.3 Thus, the number of patients needing a life-saving or life-sustaining lung transplant is at an all-time high and there is a critical need to increase the supply of donor lungs.
The number of registered organ donors has increased steadily over the past several years due to efforts by governments, physician groups, and organ procurement agencies. Campaigns have increased public awareness, which has lead to small increases in total donor authorizations.4,5 However, the number of patients awaiting a lung transplant has risen even faster. With donor authorization rates now as high as 89% in some areas, there is clearly a need to find other mechanisms to increase the donor pool.6
In the past, when thoracic transplant surgeons faced critical shortages of available organs, they expanded donation criteria to include lungs that would have otherwise been declined. The addition of lungs from donors older than 55 years old, those from donors with a significant smoking history, and those with evidence of mild pulmonary disease helped to meet the growing demand for transplantable organs.7,8 Similarly, in light of the current donor shortage, one method to increase lung procurement rates is to increase the number of organs utilized from donation after circulatory death (DCD) donors. Several observational studies show no difference in survival or rates of primary graft dysfunction in lungs used from donors after circulatory death when compared to donors diagnosed with brain death (DBD).9,10 There is a growing body of experimental evidence that suggests utilizing DCD lungs, especially when ex vivo lung perfusion (EVLP) is utilized, is a safe method to increase the overall donor pool.11,12 The purpose of this study was to determine the potential for donor pool expansion with increased procurement of DCD organs from patients who die at hospitals.
MATERIALS AND METHODS
Participants
A retrospective chart review was performed to analyze the potential for lung donation after circulatory death in a rural referral hospital. All in hospital mortalities from Aug 2014 to June 2015 at our institution were identified. Due to the post-mortem nature of the review Institutional Review Board approval was not required for this study. The electronic medical records were evaluated for patient demographics, comorbid disease, cause of death, and clinical results. Demographic information including age, race, and smoking status were collected. All patients were classified as pediatric (less than 15 years of age), young (15–65 years), or old (>65 years) based on convention in lung transplantation. Comorbidities of interest included the presence of any cancer or significant lung pathology, including traumatic lung injury, acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, lung cancer, and pneumonia at the time of death. Additional clinical data abstracted included arterial blood gas data and chest radiograph interpretations. Patients were stratified for analysis by lung donor suitability. Donor exclusion criteria included: 1) Age greater than 65 years old, 2) Any known lung pathology, 3) Any recorded history of cancer, 4) Respiratory or septic cause of death, 5) PaO2/FiO2 <100, and 6) Severe pathology on chest radiograph.
Variable Definitions
Chest radiograph findings were classified as mild (absence of pathology, minimal atelectasis, or small pleural effusion), moderate (large pleural effusion, consolidation, or infiltrate), or severe pathology (traumatic injury or lung mass) based on radiographs taking within 7 days of death. Arterial blood gas data collected within 24 hours of death were compared between donor candidates and non-candidates using PaO2/FiO2.
Statistical Analysis
Continuous variables were presented as mean ± standard deviation while categorical variables as number and percentage. Patients were stratified by the primary outcome of lung donor potential for univariate analysis. Categorical variables were analyzed by Chi-Square test while continuous variables by independent T-test or Mann Whitney U-test based on normality of the distribution. All data analysis was performed using SAS version 9.4 (SAS Company, Cary NC), with an alpha less than 0.05 defining statistical significance.
RESULTS
There were 857 in-hospital mortalities over a one-year period. The median age was 64 years old (IQR 56–79). Potential donors were grouped by age into Pediatric (n=32, 4%), Young (n=328, 38%), and Old (n=497, 58%) age groups. Pediatric and Young patients were considered transplant candidates while Old patients (>65) were disqualified based on widely accepted donor criteria. The presence of any known lung pathology was recorded including lung trauma, pneumonia, COPD, ARDS, lung cancer, or pulmonary fibrosis. Causes of death were reported by organ systems or mechanism of injury where appropriate and are shown in Table 1. Candidates for lung donation were those less than 65 years old, free of any cancer, without lung pathology, and who did not die of respiratory causes or sepsis. Based on these criteria 85 patients were considered candidates for lung donation.
Table 1:
Demographic information and cause of death
| Age Criterion (years) | Pediatric (n = 32) |
Young (n = 328) |
Old (n = 497) |
Total (n = 857) |
|---|---|---|---|---|
| <15 | 15–65 | >65 | all | |
| Cause of Death | ||||
| Neuro | 2 (6.3%) | 27 (8.2%) | 69 (13.4%) | 98 (11.4%) |
| Trauma | 1 (3.1%) | 13 (4.0%) | 25 (5.0%) | 39 (4.6%) |
| Cancer | 0 | 40 (12.2%) | 39 (7.9%) | 79 (9.2%) |
| Renal | 1 (3.1%) | 0 | 2 (0.4%) | 3 (0.4%) |
| Liver | 0 | 27 (8.2%) | 20 (4.0%) | 77 (9.0%) |
| Respiratory | 6 (18.8%) | 27 (8.2%) | 59 (12.0%) | 92 (10.7%) |
| Sepsis | 11 (34.4%) | 100 (30.5%) | 144 (29.0%) | 255 (29.8%) |
| Cardiac | 9 (28.3%) | 90 (27.4%) | 132 (26.6%) | 231 (27.0%) |
| Lung Pathology | ||||
| Lung Trauma | 0 | 9 (2.7%) | 15 (3.02%) | 24 (2.8%) |
| COPD | 1 (3.1%) | 43 (13.1%) | 89 (17.9%) | 133 (15.5%) |
| PNA | 3 (9.4%) | 88 (26.8%) | 152 (30.6%) | 243 (28.4%) |
| ARDS | 3 (9.4%) | 36 (11.0%) | 27 (5.4%) | 66 (7.7%) |
| Lung Cancer | 0 | 30 (9.2%) | 30 (6.0%) | 60 (7.0%) |
| Fibrosis | 1 (3.1%) | 5 (1.5%) | 11 (2.2%) | 17 (2.0%) |
Of the 85 patients who met clinical criteria for lung donation, 70 (82%) had a PaO2/FiO2 greater than 100. The mean PaO2/FiO2 for candidates was 342±135 versus 198±126 for non-candidates (p<0.0001). Chest radiographs taken within 7 days of death were available for 61 of the 70 candidates. There was no pathology/mild pathology seen in 9 (13%), moderate pathology in 44 (63%), and no candidate had findings classified as severe. Of those who were not candidates, 78 (10%) had no pathology or mild pathology, 505 (64%) had moderate, 44 (6%) had severe, and 157 (20%) did not have a chest radiograph (Table 3).
Table 3:
Severity of Chest Radiograph findings, and Mean PaO2:FiO2 by candidacy for DCD lung donation
| Candidate | Non Candidate | p-value | |
|---|---|---|---|
| CXR | <0.0001 | ||
| No CXR | 9 (12.9%) | 157 (20.1%) | |
| No or Mild pathology | 17 (24.3%) | 78 (9.9%) | |
| Moderate pathology | 44 (62.9%) | 505 (64.4%) | |
| Severe pathology | 0 | 44 (5.6%) | |
| PaO2/FiO2 | 342 ± 135 | 197 ± 126 | <0.0001 |
DISCUSSION
Of the 857 deaths at our institution, 497 (58%) were older than 65 years, thus automatically disqualified based on traditional lung donation criteria. Of the 360 patients under 65 years old, we determined that 70 (19%) were suitable candidates for lung donation because they did not have any cancer or lung pathology, they did not die of respiratory causes or sepsis, and they had PaO2/FiO2 >100 and no severe chest radiograph findings. During the time of this study there were 16 DBD organ donors at our institution and 4 lungs were recovered for transplantation. There were no DCD organ donors at our hospital during the study period. Given that 70 patients in our study were deemed to be appropriate candidates for lung donation, we could potentially increase the total number of organ donors by more than 400% by evaluating all deaths at our institution. This prediction is in line with other projections including a population study by the Organ Procurement and Transplantation Network that reports the actual number of deceased donors is only one fourth of the number of estimated potential donors.13
It has been over 20 years since the initial report on the successful use of lungs procured after circulatory death for transplantation.14 Enthusiasm has grown slowly over that time and only recently have DCD lungs become more widely accepted. Still, last year they accounted for just 5% of the total lungs transplanted.1 Estimates of the potential impact of DCD lungs on the overall donor pool vary. The United Network for Organ Sharing has predicted a stable donor pool through the year 2020, with 75% of all potentially transplantable organs going unused. They identified donation after circulatory death as a major area for increasing the overall donor pool.13 Halpern et al. conservatively suggests a possible 50% increase in transplantable lungs with increased utilization of organs from controlled donation after circulatory death in ICUs.15
Despite the obvious benefits of a larger donor pool and the increasing body of evidence in favor of DCD organ utilization, the majority of US centers do not perform DCD lung transplantation.16 Ethical concerns regarding the consent process, performance metrics that incentivize conservatism among transplant centers, and changes in end-of-life care act as barriers to the routine transplantation of DCD organs. Multiple agencies, including the Institute of Medicine and the American Thoracic Society, have determined that donation after circulatory death is ethically sound and have worked to establish protocols for the use of DCD organs.17,18 Although favorable outcomes have been demonstrated using a variety of protocols for DCD lung procurement and transplantation, Australia has been particularly successful by implementing national consensus protocols. Nationwide, DCD accounts for 20% of Australia’s lung transplants, which is largely due to standardization of procurement practices.19,20
In order to utilize 100% of in-hospital deaths as potential organ donors, Maastricht I and II uncontrolled DCD (uDCD) organs would have to be considered. Internationally, uDCD lungs are transplanted, although less often than those from controlled DCD donors.22 In the US, Suzuki et al. recently published a case report of successful uDCD lung transplantation calling for reconsideration of this yet unused resource.23 At this time uDCD lung transplants have higher rates of primary graft dysfunction than those who received lungs from DBD donors.24 More research is warranted to determine the effect of prolonged warm ischemic time and secondary lung injury and whether those effects can be mitigated by existing technology. Further, the processes of notifying families and Organ Procurement Organizations, obtaining consent, and organ allocation without delaying post mortem interventions such as ventilation or topical cooling will prove challenging. Clinical trials are underway to assess the suitability of uDCD lungs for transplantation and investigators are thoughtfully and carefully overcoming these ethical and logistical challenges as they arise.25 Despite the apparent barriers to organ donation after uncontrolled circulatory death, we should remain committed to honoring the wishes of all organ donors and taking full advantage of the precious resources they provide.
Ex Vivo Lung Perfusion has been shown to improve outcomes when lungs are transplanted from high-risk or DCD donors. Using EVLP, physicians can evaluate and even rehabilitate lungs that may have previously been gone unused due to concerns about their quality.26 EVLP provides the opportunity to procure more lungs and gain valuable information before transplanting them into a critically ill patient. EVLP also allows treatment of infection, pulmonary edema, or atelectasis in donor lungs before transplantation.22 For all of these reasons, increased use of EVLP could facilitate the procurement and ultimate transplantation of uDCD lungs.
Our study has several limitations including those associated with all retrospective studies such as inclusion bias. Additionally, the single center nature limits generalizability. Categorization of patients was limited by lack of established criteria for lung donation, and therefore all deaths and results were reported. Some patients also would experience uncontrolled circulatory death, which is less widely accepted. Finally, consent for donation may be difficult to obtain in some patients. Therefore, our findings represent a high-end estimate that may be useful for extrapolation based on local consent rates.
Conclusion
Laboratory and clinical studies have shown non-inferiority of DCD lungs yet they remain dramatically underutilized. This study demonstrates that 8% of in-hospital deaths at our institution qualify as potential candidates for lung donation. This represents a significant potential increase in the total number of lung donors at our institution from the previous year. While not all donors would ultimately meet donor criteria and consent for donation, having protocols in place for evaluation could help increase the number of donations.
Figure 1:
Criteria for identifying potential lung donors
Table 2:
Cause of death by candidacy for lung donation
| Candidate | Non-candidate | |
|---|---|---|
| Neuro | 16 (22.9%) | 80 (10.2%) |
| Trauma | 5 (7.1%) | 34 (4.3%) |
| Cancer | 0 | 79 (10.1%) |
| Renal | 0 | 3 (0.4%) |
| Liver | 14 (20%) | 33 (4.2%) |
| Respiratory | 0 | 92(11.8%) |
| Sepsis | 0 | 255 (32.5%) |
| Cardiac | 35 (50%) | 194 (24.8%) |
DISCLOSURE
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article. This work was supported by the National Institutes of Health [grant numbers T32 HL007849 and UM1 HL088925]. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
This work was supported by the National Heart, Lung, and Blood Institute (grants UM1 HL088925 and T32 HL007849). The authors have no conflicts of interest to report.
Footnotes
Meeting: Oral Presentation at the 12th Annual Academic Surgical Congress, February 7–9, 2017 in Las Vegas, NV
REFERENCES
- 1.United Network for Organ Sharing.UNOS Data. http://www.unos.org/data. Accessed November 11, 2015.
- 2.Valapour M, Skeans MS, Smith JM, et al. Lung. Am J Transplant 2016; 16 Suppl 2: 141–68. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services. Organ Procurement and Transplantation Network National Data. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/. Accessed November 11, 2015. [Google Scholar]
- 4.Chatterjee P, Venkataramani AS, Vijayan A, Weelen JR, Martin EG. The effect of state policies on organ donation and transplantation in the United states. JAMA Intern Med. 2015; 175(8) 1323–9. [DOI] [PubMed] [Google Scholar]
- 5.Wynn J, Alexander C. Increasing organ donation and transplantation: the U.S. experience over the past decade. Transpl Int. 2011; 24(4):324–32. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg DS, French B, Abt PL, Gilroy RK. Increasing the number of organ transplants in the United States by optimizing donor authorization rates. Am J Transplant. 2015; 15(8): 2117–25. [DOI] [PubMed] [Google Scholar]
- 7.Kron IL, Tribble CG, Kern JA et al. Successful transplantation of marginally acceptable thoracic organs. Ann Surg. 1993; 217(5) 518–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhorade SM, Vigneswaran W, McCabe MA, Garrity ER. Liberalization of donor criteria may expand the donor pool without adverse consequences in lung transplantation. J Heart Lung Transplant. 2000; 19: 1200–04. [DOI] [PubMed] [Google Scholar]
- 9.Krutsinger D, Blevins A, et al. Lung transplantation from donation after cardiocirculatory death: a systematic review and meta-analysis. J Heart Lung Transplant, 2014; 34: 675–684. [DOI] [PubMed] [Google Scholar]
- 10.Cypel M, Levvey B, VanRaemdonck D, et al. Favorable outcomes of donation after cardiac death in lung transplantation: a multicenter study. J Heart Lung Transplant. 2013; 32:S15. [Google Scholar]
- 11.Wagner CE, Pope NH, Charles EJ, et al. Ex vivo lung perfusion with adenosine A2A receptor agonist allows prolonged cold preservation of lungs donated after cardiac death. J Thorac Cardiovasc Surg. 2016; 151:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charles EJ, Huerter ME, Wagner CE, et al. Donation after circulatory death lungs transplantable up to six hours after ex vivo lung perfusion. Ann Thorac Surg. 2016;102:1845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klassen DK, Edwards LB, Stewart DE, et al. The OPTN deceased donor potential study: implications for policy and practice. Am J Transplant. 2016; 16: 1707–1714. [DOI] [PubMed] [Google Scholar]
- 14.D’Alessandro AM, Hoffmann RM, Knechtle SJ, et al. Successful extrarenal transplantation from non- heart-beating donors. Transplantation. 1995; 59(7): 977–82. [DOI] [PubMed] [Google Scholar]
- 15.Halpern S, Hasz R, Abt P. Incidence and distribution of transplantable organs from donors after circulatory determination of death the U.S. intensive care units. Ann Am Thorac Soc. 2013; 10(2): 73–80. [DOI] [PubMed] [Google Scholar]
- 16.Dark JH, Egan TM . Lungs from the controlled donation after circulatory determination of death donor: perspectives from the United States and beyond. Am J Transplant. 2016; 16: 1047–1048. [DOI] [PubMed] [Google Scholar]
- 17.Gries CJ, White DB, Truog RD, et al. An Official American Thoracic Society/International Society for Heart and Lung Transplantation/Society of Critical Care Medicine/Association of Organ and Procurement Organizations/United Network of Organ Sharing Statement: Ethical and Policy Considerations in Organ Donation after Circulatory Determination of Death. Am J Respir Crit Care Med. 2013; 188(1): 103–9. [DOI] [PubMed] [Google Scholar]
- 18.Institute of Medicine, National Academy of Sciences. Non-heart- beating organ transplantation: Practice and protocols. Washington, DC: National Academy Press, 2000. [PubMed] [Google Scholar]
- 19.Wigfield C Donation after cardiac death for lung transplantation: a review of current clinical practice. Curr Opin Organ Transplant. 2014; 19:455–459. [DOI] [PubMed] [Google Scholar]
- 20.Levvey BJ, Harkess M, Hopkins P, et al. Excellent clinical outcomes from a national donation after determination of cardiac death lung transplant collaborative. Am J Transplant. 2012; 12: 2406–2413. [DOI] [PubMed] [Google Scholar]
- 21.Mooney JJ, Hedlin H, Mohabir R, et al. Lung quality and utilization in controlled donation after circulatory determination of death within the United Sates. Am J Tranplant. 2016; 16: 1207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeb J, Cypel M. Ex vivo lung perfusion. Clin Transplant. 2016; 30(3): 183–94. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki Y, Tiwari JL, Lee J, et al. Should we reconsider lung transplantation through uncontrolled donation after circulatory death? Am J Transplant. 2014; 14:966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez-de-Antonio D, Campo-Canaveral JL, Crowley S, et al. Clinical lung transplantation from uncontrolled non-heart-beating donors revisited. J Heart Lung Transplant. 2012;31(4):349–53. [DOI] [PubMed] [Google Scholar]
- 25.Egan TM, Requard JJ. Uncontrolled donation after circulatory determination of death donors (uDCD) as a source of lungs for transplant. Am J Transplant. 2015; 15: 2031–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011; 364:1431–1440. [DOI] [PubMed] [Google Scholar]