Abstract
Although clinical outcomes for patients with rheumatoid arthritis (RA) have greatly improved with the use of biologic and conventional DMARDs, approximately 40% of patients do not achieve primary clinical outcomes in randomized trials, and only a small proportion achieve lasting remission. Over the past decade, studies in murine models point to the critical role of the lymphatic system in the pathogenesis and therapy of inflammatory-erosive arthritis, presumably by the removal of catabolic factors, cytokines and inflammatory cells from the inflamed synovium. Murine studies demonstrate that lymphatic drainage increases at the onset of inflammatory-erosive arthritis but, as inflammation progresses to a more chronic phase, lymphatic clearance declines and both structural and cellular changes are observed in the draining lymph node. Specifically, chronic damage to the lymphatic vessel from persistent inflammation results in loss of lymphatic vessel contraction followed by lymph node collapse, reduced lymphatic drainage, and ultimately severe synovitis and joint erosion. Notably, clinical pilot studies in patients with RA report lymph node changes following treatment, and thus draining lymphatic vessels and nodes could represent a potential biomarker of arthritis activity and response to therapy. Most importantly, targeting lymphatics represents an innovative strategy for therapeutic intervention for RA.
Introduction
Rheumatoid arthritis (RA) is an inflammatory joint disorder that affects 0.5-1% of the population worldwide.1,2 Despite major advances in our understanding of RA pathogenesis, major unmet clinical needs remain for a large proportion of patients, whose disease is refractory to current treatments.3,4 Although autoantibodies have long been implicated in RA, it is now broadly accepted that environmental and epigenetic factors are also critical in the development and pathogenesis of joint disease.5 Moreover, the aetiology of seronegative RA is not well understood.6 An additional relatively under-recognized mechanism, altered lymphatic function, has the potential to modulate disease activity and clinical course in RA via multiple pathways. Herein, we provide an overview of the synovial lymphatic system in the context of joint homeostasis and inflammatory arthritis, review the experimental and clinical evidence that support the central importance of the lymphatic system in the progression and resolution of inflammatory arthritis, and introduce the concept that the lymphatic vasculature is a potential clinical diagnostic biomarker and novel therapeutic target in RA.
The lymphatic system
Structure and function
In general, the peripheral lymphatic system has two primary functions: immune cell surveillance and interstitial fluid balance.7 The lymphatic system maintains interstitial fluid balance by shifting fluid from the periphery through vessels that connect to a series of draining lymph nodes,which empty into final ducts (for example, the right lymphatic duct and thoracic duct) where the lymph is returned to the vascular supply via the subclavian veins.8
Interstitial fluid becomes lymph when it enters the initial lymphatics or lymphatic capillaries that are present in most tissues, including the synovium (Figure 1A). These vessels are lined by a single layer of lymphatic endothelial cells (LECs) with specialized button-like junctions that are highly permeable to solutes and macromolecules, which physically attach to the extracellular matrix by anchoring filaments (Figure 1B).9–14 LECs overlap, creating primary valves that prevent the backflow of fluid into the tissue (Figure 1C).12,13 Entry of fluid into the lymphatic vessels takes place when external compressive forces are higher than the intraluminal fluid pressure, thereby pushing fluid, macromolecules and cells into the initial lymphatics.15,16 These external forces are generated by skeletal muscle contractions, ambulation or other joint movement.
Figure 1. Structure and function of the synovial lymphatic system.
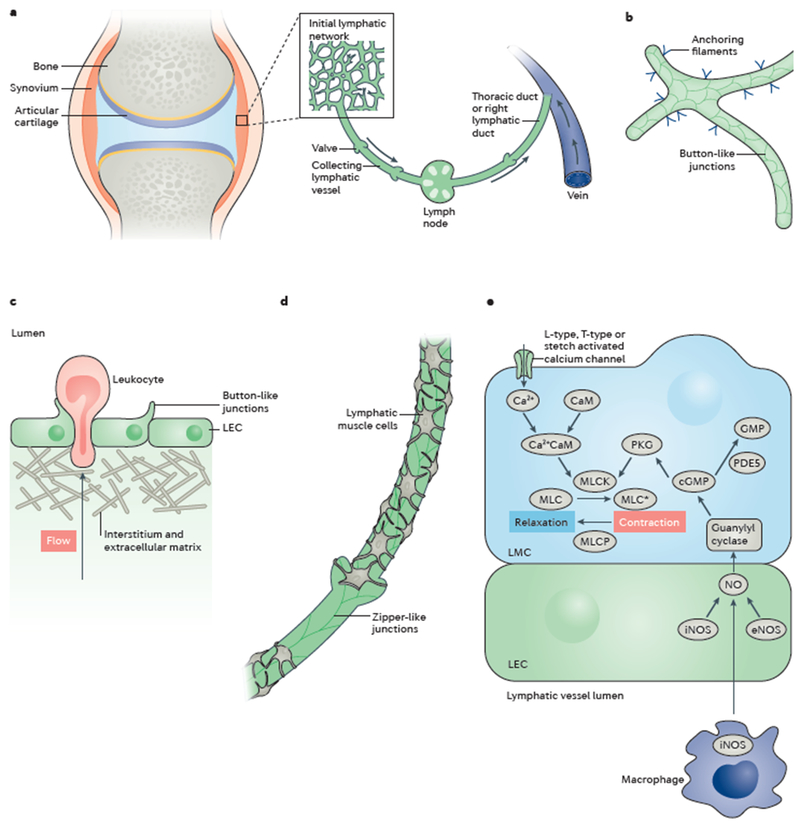
(A) The synovial lymphatic system of arthrodial joints includes initial lymphatics interspersed throughout the synovium, which join to form collecting lymphatics that lead to joint-draining lymph nodes and, eventually, to the thoracic or right lymphatic duct, which joins the venous supply. (B) Initial lymphatics are composed of a network of lymphatic endothelial cells (LECs) with anchoring filaments to the basement membrane. Overlapping portions of LEC membranes create button-like junctions that act as primary valves to enable macromolecules, cells and fluid to enter the initially lymphatic system. (C) Lymphocytes enter the initial lymphatics from the interstitial space. (D) Collecting lymphatic vessels comprise a single layer of LECs joined together by impermeable zipper-like junctions and one or more layers of continuous lymphatic muscle cells (LMCs). (E) Contractions in LMCs are triggered by calcium signalling, which is inhibited by nitric oxide (NO) signalling through soluble guanylyl cyclase (sGC). Under homeostatic conditions, NO produced by endothelial nitric oxide synthase (eNOS) in adjacent LECs inhibits LMC contractions to the lymphatic vessels to fill. In the setting of inflammation, the cycle of lymphatic vessel contractions can be disrupted by ‘squelching’ NO produced from inducible nitric oxide synthase (iNOS) expressed in adjacent LECs and/or immune cells, such as activated-adherent macrophages. CaM, calmodulin.
The initial lymphatics converge to form larger collecting lymphatic vessels. These vessels are less permeable than the initial lymphatics owing to their tight zipper-like junctions between adjacent LECs.14 In addition, collecting lymphatics contain secondary bicuspid valves, which help prevent backflow, and one or more layers of lymphatic muscle cells (LMCs), which allow for vessel contraction to move lymph against the force of gravity (Figure 1D).9,10,17–19 LMCs have a myosin and actin cytoskeletal architecture with characteristics of both smooth muscle (SMA and SMB myosin heavy chain isoforms) and striated muscle (β-myosin heavy chain).20 Thus, these cells possess characteristics of both vascular smooth muscle and cardiac striated muscle, facilitating the generation of tone as well as strong, phasic contractions.20–22 These contractile components respond to a tightly controlled cascade of signalling molecules that balances myosin light chain kinase, smooth muscle (MLCK) and myosin light chain phosphatase (a holoenzyme composed of 3 subunits, a catalytic subunit of type 1 phosphatase, PPlc; a targeting subunit, termed myosin phosphatase target subunit, MYPT; and a smaller subunit, M20, of unknown function23) activity to produce the tone and contractile events (reviewed elsewhere10). Briefly, contractions can be generated by stretch-mediated, L-type or T-type calcium channels that generate an influx of Ca2+ ions, which bind to calmodulin.24 Calmodulin, in turn, activates MLCK, phosphorylating myosin light chain and shortening its length. LMCs are relaxed by nitric oxide (NO), which is synthesized by LECs and derived from various sources, including inducible nitric oxide synthase (iNOS), produced either directly or indirectly from immune cells travelling along the lumen of the vessel, and endothelial nitric oxide synthase (eNOS) activated by shear stress.25 NO translocates to the LMCs, where it activates soluble guanylyl cyclase and downstream cyclic GMP-dependent protein kinase (cGK-1, also known as protein kinase G), which blocks MLCK, enabling dephosphorylation of myosin light chain by its phosphatase and subsequent vessel relaxation (Figure 1E).26
Fluid courses through the lymphatic vessels until it enters a series of lymph nodes, which are highly-organized organs containing immune cells, where adaptive immune responses are generated. Antigenic stimuli travel directly through the lymph, or are captured by antigen-presenting cells in the periphery and transported to the lymph node. Finally, lymph enters the sinuses via the afferent lymphatic vessel, exits in the efferent lymphatic vessel, and moves to the next lymph node, eventually returning to the circulation through the great veins. The pathways that lead to generation of an immune response are reviewed comprehensively elsewhere27–31 and are beyond the scope of this Review. Whereas much is known about antigen transport and immune interactions in the peripheral lymph nodes, understanding of the specific processes that lead to lymph generation and lymphatic contraction in human joints is extremely limited, and studies to elucidate the synovial lymphatic system are at the forefront of this field.
Effects of inflammation on lymphatics
The lymphatic system and immune function are closely connected because antigen-presenting cells enter lymphatic vessels to travel to the lymph nodes where they initiate adaptive immune responses. Dendritic cells (DCs), neutrophils and macrophages travel through these vessels.32–36 The importance of this lymphatic network is highlighted in mice that lack dermal lymphatics and show decreased humoral immunity.37 Interestingly, immune cells travelling adjacent to and within lymphatics can substantially alter the vessels by releasing factors that induce lymphangiogenesis and/or alter lymphatic vessel functions (such as contraction or permeability), and can also damage the vessel wall.38
Lymphangiogenesis, or the growth of lymphatic vessels, is a primary response during inflammation, and disruption of this process leads to a variety of diseases.39 Vascular endothelial growth factor C (VEGF-C), and its cognate receptor VEGF receptor 3 (VEGFR-3), expressed on LECs, are the master regulators of lymphangiogenesis.39 Macrophages are a well-known source of VEGF-C and are abundant in inflamed tissues.40,41 Importantly, activated macrophages and T cells cooperate to enhance expression of VEGF-C.42 During inflammation, VEGFR-3 also regulates LEC expression of CC-chemokine 21 (CCL21), a potent chemotactic signal for DCs.32,43 These complex interactions reveal the presence of a coordinated system controlling lymphangiogenesis and immune cell migration.
Immune cell regulation of lymphatic contraction is facilitated primarily through cytokine-mediated NO signalling, as demonstrated by the reduced lymphatic contraction in a model of sterile inflammation, which was not observed in iNOS−/− mice.44 The reduction in lymphatic contraction frequency and ejection fraction was shown to be dependent on iNOS-expressing CD11b+Gr1+ cells that surround collecting lymphatic vessels.44 Additionally, direct application of TNF and IL-1β in vivo can decrease lymphatic contraction frequency, which is mediated though iNOS.45 This finding was confirmed in ex vivo studies in which lymphatic contraction was inhibited by direct application of TNF to mesenteric lymphatic vessels, and restored by antagonizing iNOS and guanylate cyclase.46 The mechanism of this impairment is known to be partially dependent on arachidonic acid metabolites; the subject has been reviewed elsewhere.10,47–49
From ex vivo cannulation experiments, it is known that lymphatics respond to a variety of mechanical cues, including pressure, flow, and stretch.50–53 The increased fluid load traversing lymphatics during inflammation can increase all three of these factors. Contraction frequency increases in response to elevated transmural pressure, partially owing to increased shear that results in more NO production.50,52,54,55 However, it should be noted that there seems to be a Gaussian relationship between pressure and flow, as at high pressures, frequency reduces.52 When fluid load is lowered, and thus transmural pressure decreases, homeostatic contraction frequency returns. Therefore, whether a reduction in lymphatic contraction is mechanically and/or cytokine-mediated is difficult to determin from in vivo experiments.
Both initial and collecting lymphatics have an inherent permeability that is integral to their function. However, during inflammation, barrier integrity can be breached, leading to leakage of lymph into the interstitium. In the initial lymphatics, unidirectional flow facilitates the entry of fluid, cells, and macromolecules into the lymphatic system.9–14 During acute inflammation, permeability of the initial lymphatics increases, and tracer molecules flow back into the interstitium.56 True measurements of initial lymphatic permeability are rare, but many groups have reported increased leakiness of the initial lymphatics in the presence of inflammation, which is a prospective sign of increased permeability.57–59 Similarly, in a murine model of psoriasis, permeability increased in the initial dermal lymphatics of psoriatic plaques, but not in adjacent uninvolved skin.60 In the collecting lymphatics, their inherent permeability is benefical as it enables antigen sampling by macrophages and DCs in the surrounding adipose tissue, which facilitates downstream T cell responses.61 DCs regulate collecting lymphatic vessel permeability via CC-chemokine receptor 7 (CCR7)-dependent mechanisms.62 Diabetic mice, and mice after bacterial infection, also have increased collecting lymphatic permeability.63,64 Mechanistically, in vitro studies showed that TNF and IL-1β can both increase LEC monolayer permeability.65,66 Thus, in contrast to the blood vasculature, permeability of lymphatic vessels is a highly regulated and dynamic process that changes during normal physiology and pathologic conditions.
Lymphatic phenotypes in RA
Evidence from mouse models
Although several animal models of inflammatory erosive arthritis exist, none precisely recapitulates the pathophysiology of RA.67 As such, they are broadly divided into models of autoimmunity, which are characterized by sudden onset of disease, and models of general joint inflammation that display a more stochastic progression. K/B×N68 and TNF-transgenic (TNF-Tg)69 mice are representative examples of these different RA models, respectively, and have distinct aetiologies of inflammatory erosive arthritis. Because joint pathogenesis is a dynamic process, an approach to study arthritic changes has focused on longitudinal imaging studies using contrast enhanced MRI (CE-MRI), power Doppler ultrasonography (PDUS), near-infrared indocyanine green (NIR-ICG) imaging, and in vivo micro-CT (μCT).59,70–73 With the use of these longitudinal imaging modalities, we found that K/B×N mice demonstrated a marked increase in lymphatic vessel contraction frequency during the acute phase of synovitis, which in the chronic phase of arthritis returns to levels similar to those seen in wild-type mice.59 By contrast, the slow onset of inflammatory arthritis in TNF-Tg mice enabled us to identify that ankle and knee arthritis progresses by two distinct mechanisms, as illustrated in Figure 2. During the initial stages of arthritis and inflammation in the ankle joints of TNF-Tg mice, increased lymphangiogenesis maintains lymphatic drainage from the joint without a detectable increase in lymphatic vessel contractions compared with wild-type littermates (Supplemental Video 2).74 This phase of arthritic progression is termed the ‘expansion’ phase because the popliteal lymph node enlarges in size owing to increased lymphangiogenesis, increased pressure from afferent vessels, and infiltration of IgM+CD23+CD21hiCD1dhi B cells.59,75–81 Interestingly, this subset of B cells is distinct from marginal zone and follicular B cells in the lymph node, suggesting they are a unique population of B cells in inflamed lymph nodes, which we therefore named ‘Bin’ cells.79,82 The increase in lymphatic vessel contraction frequency in K/B×N mice, and lymphangiogenesis in both K/B×N and TNF-Tg mice, represent a compensatory response that enhances the removal of inflammatory cells and catabolic factors from the synovium. Functionally, this process limits joint inflammation and focal erosion in the expanding phase. In these models, however, this compensatory mechanism is incomplete because synovial inflammatory cells and catabolic factors directly damage LECs and LMCs when they pass through afferent lymphatic vessels and draining lymph nodes.83
Figure 2. Overview of lymphatic phenotypes in normal (-healthy), expanding and collapsed lymph nodes in mice.

(A) The distal lymphatic vessels drain the footpad to the popliteal lymph node (PLN), and the proximal lymphatic vessels drain lymph from the PLN and knee synovium to the iliac lymph node (ILN). Inset: In contracted and dilated lymphatic vessels, tight connections exist between lymphatic endothelial cells (LECs) and lymphatic muscle cells (LMCs) during transport of lymph. At homeostasis, these lymphatic vessels contract 0.5–2 times per minute. Near infrared-indocyanine green (NIR-ICG) image shows the lower limb of a wild-type mouse (Supplemental Video 1). (B) The initiation of inflammatory arthritis is followed by an ‘expansion’ phase, in which lymphangiogenesis in the synovium facilitates removal of inflammatory cells and lymph to the draining lymph nodes, which expand up to 10-fold in volume owing to lymphangiogenesis, excess lymphatic flow, and accumulation of B cells. Inset: CD11b+ macrophages are present in contracted and dilated lymph vessels and travel at great speed (~0.2 mm/s). In models of autoimmune arthritis, lymphatic vessel contractions increase to ~5 contractions/min at initiation of autoimmunity before stabilizing at ~1 contractions per min in the chronic phase of inflammatory arthritis (C) During chronic synovitis, ipsilateral PLNs and ILNs simultaneously collapse via poorly understood mechanisms. Lymphatic contractions are absent or rare in this ‘collapsed’ phase (Supplemental Video 3). PLNs and ILNs decrease in size owing to fluid loss, and B cells translocate from B cell follicles into the lymphatic sinuses effectively ‘clog’ the lymphatic vessels. A lack of lymphatic transport results in a sudden, severe exacerbation of synovitis. Anti-CD20 therapy removes B cells and restores passive lymphatic flow from the joint. Inset: Although the initiating event(s) of the collapsed phase are not known, several pathologic features of the degenerated lymphatic vessel suggest the involvement of intersecting mechanism.
Following the expansion phase in the TNF-Tg model, the popliteal lymph node reduces in volume and power Doppler signal decreases, indicating reduced blood flow, marking what has been termed the ‘collapsed’ phase of arthritic progression.70,72 This collapse coincides with several pathologic processes, including increased synovial hyperplasia, poor lymphatic clearance, lymphatic vessel damage with subequent increased leakiness and loss of contractions (Supplemental Video 3), leukocyte stasis within lymphatic vessels, translocation of Bin cells from the lymph node follicles to the lymph node sinuses, and reduction in draining lymph node volume with increased fluid pressure.70,75,79,81,84,85 Interestingly, the lymph node collapse is asymmetrical, and occurs along the ipsilateral axis of the lower limb.85 Although a direct cause and effect relationship between the altered lymphatic dynamics outlined above and arthritic processes is not firmly established, the sequence of events fits with a unifying model to explain the collapsed phase (Figure 2). In this model, increased inflammatory cells, cytokines/chemokines and catabolic factors generated during persistent inflammation pass through the afferent lymphatic vessels, and damage LECs and underlying LMCs. Concomitantly, these catabolic factors trigger LECs to express high levels of iNOS, resulting in ‘NO squelching’ of eNOS-mediated contractions. Thus, lymphatic vessel structural damage and NO production by LECs results in loss of lymphatic vessel contractions owing to constitutive activation of soluble guanylyl cyclase and downstream cGK-1-mediated inhibition of calcium signalling in LMCs (Figure 1E). At the same time, activated macrophages become static in the vessel lumen because of the loss of contractions. These cells also express iNOS, which furthers inhibition of lymphatic vessel contraction due to NO squelching. Additionally, activated macrophages in the draining lymph nodes express the potent B cell chemotactic factor CXC-chemokine 13 (CXCL13)86. A decrease or absence of lymphatic flow from afferent lymphatic vessels enables a CXCL13 gradient to form, increasing CXCL13 levels in the lymph node sinuses, which drives the migration of Bin cells from the follicles into the sinuses. As a result, these Bin cells accumulate and clog the lymphatic vessel. Both active and passive lymphatic transport from the inflamed joint is consequently markedly reduced or abolished, and is followed by progressive joint inflammation and destruction.
Lymph node collapse in RA
Although lymph node involvement and lymphadenopathy in patients with RA was first described in 1896 by Chauffard and Ramond,87,88 follow-up studies have been hampered by the absence of reliable clinical outcome measures and biomarkers to assess lymphatic function, and to monitor disease progression and response to therapy. Knowledge of human lymphatics has advanced considerably in the last 10 years, based on recent pioneering studies using cadaveric dissection, which mapped the lymphatic anatomy of the upper limb in detail.89,90 Moreover, clinical imaging strategies (CE-MRI, PDUS and NIR-ICG)91–98 have provided the first evidence that the lymphatics draining inflamed joints can serve as biomarkers of arthritis and response to therapy.95 Additionally, ex vivo histopathology and immunofluorescence analyses of popliteal lymph node tissues harvested from patients undergoing total knee replacement surgery or lower limb amputation have confirmed the presence of B follicular hyperplasia and histological alterations in lymph nodes, and the existence of Bin-like B cells, in tissue from patients with RA, findings not observed in tissues from patients with other (non-RA) severe lower limb diseases.80
The next critical question is whether the phases of lymph node changes identified in murine studies are relevant to lymph node changes in RA. To address this question, Manzo and colleagues designed a proof-of-concept pilot study to formally examine whether RA is associated with alterations in the joint-draining lymph nodes.98 Clinical and PDUS examination revealed hypertrophy of the lymph node cortex in the absence of clinical lymphadenopathy in 7 out of 10 patients with active disease, but there were no lymph node abberations in the 5 patients in clinical remission or 5 healthy controls. Moreover, PDUS signal was amplified in the cortical and hilar regions of lymph nodes at baseline but returned to normal levels after 3 months of anti-TNF and/or methotrexate therapy 98. In a follow-up study of 40 consecutive patients with RA refractory to treatment with DMARDs and 20 healthy individuals,97 PDUS demonstrated considerable alterations of RA lymph node morphology and volume following anti-TNF treatment, and a degree of concordance of these alterations with synovitis activity in peripheral joints. Interestingly, low PDUS signal in the lymph nodes (which has also been observed in collapsed murine lymph nodes)70,72 at baseline was strongly associated with a poor clinical response to anti-TNF therapy. These studies provide initial evidence that lymph node dynamics, including changes in volume and PDUS signal, a marker of blood flow, are associated with joint pathology and responsiveness to treatment, and thus are a biomarker of RA disease activity and response to therapy.
Advances in RA flare assessment
The murine models outlined above demonstrate a strong temporal association between altered lymphatic flow and progressive joint inflammation and damage. A major question that emerges from these studies is whether the lymphatic dynamics contribute to joint inflammation in RA. Joint flare, a prevalent but poorly understood process in inflammatory arthritis, provides an opportunity to examine a potential relationship between the lymphatics and arthritis exacerbation.
In spite of more aggressive treatment approaches based on combination therapies of conventional and biological DMARDs, one of the perplexing problems in RA care is the loss of response to therapies over time, and the well-recognized phenomenon of periods of episodic worsening of disease activity, often referred to as disease flare. Ongoing research has advanced our understanding of the descriptive characterization of flares from the perpsectives of patients and health care providers, yet we lack insight into the central pathophysiological correlates. Moreover, we lack the biomarkers of impending flare that are needed to facilitate early recognition and targeted intervention. As outlined in this Review, the discovery that altered lymph node dynamics and lymphatic flow precedes exacerbated arthritis in murine models provides critical clues to the existence of alternative pathways responsible for acute and chronic synovial inflammation, which might have relevance in patients with RA.
Inflammatory flares of RA are a well-recognized feature of the underlying disease. Some flares are self-limited, mild in nature, and resolve without intervention, whereas others persist and worsen, and ultimately represent failure of a previously effective therapy and necessitate a change in treatment. Work ongoing over a number of years by multiple groups has sought to better characterize flares in RA. To facilitate research in the area, the Outcome Measures in Rheumatology Clinical Trials (OMERACT) group proposed to define the concept of RA flare as “worsening of signs and symptoms of sufficient intensity and duration to lead to change in therapy”,99,100 and further identified events that characterized flares along their continuum of severity.101 This framework led to the development of a set of core domains to assess flare in RA, which included standard assessments of RA disease activity (swollen and tender joints, laboratory markers), patient and physician global assessments of disease activity, patient pain ratings, and assessment of physical function.102 Additional assessments including fatigue, stiffness, and participation in life activities were subsequently added and validated.103–105
Demonstrating that patient reports and clinical assessments actually reflect events in the synovial microenvironment are important for linking clinical phenomenology with pathobiology. Ultrasonography and MRI have been used for such evaluations.95 Ultrasonography is a powerful tool that provides point-of-care testing and objective evidence of active inflammation including the presence of joint effusions, synovial hypertrophy, and bone erosions.107,108 However, the utility of ultrasonography is limited by operator proficiency and the inability to visualize deeper anatomical structures.109,110 Compared with ultrasonography, MRI can image deep structures with high resolution, and therefore provide greater detail regarding disease activity, although its use is limited by cost, feasibility, and availability.110,111 Therefore, both imaging modalities are currently applied in a complementary fashion to evaluate arthritic disease.
Although limited in number, studies investigating the relationship between imaging outcomes and instruments assessing the OMERACT core domains have helped refine our understanding of the progression of RA flare. Applying the OMERACT definition of flare, Saleem and colleagues showed that some patients in clinical remission demonstrated power Doppler signals in the upper extremities (wrist and hand) that were predictive of subsequent flare.112 Furthermore, in a cohort of patients with RA in clinical remission (n=427), imaging of the hand, wrists and associated tendons by PDUS113–115 detected tenosynovitis in 22.7% (95% CI 0.19–0.27), and these patients had an increased likelihood of flare as determined by the self-reported Flare Assessment in Rheumatoid Arthritis (FLARE) questionnaire (OR 1.95; 95% CI 1.17–3.26). These studies suggest that in clinically asymptomatic patients, ultrasonography (PDUS in particular) can reveal unstable or fluctuating inflammation that develops into flares.
MRI has also been used to evaluate synovial inflammation and its ability to assess or predict flare.116–119 Gaffney et al. used DCE-MRI, a method to assess ‘wash in’ and ‘wash out’ of contrast dye, to compare changes in knee synovial contrast enhancement to histological synovial changes via synovial biopsies in 20 patients with RA.117 They found good correlation (r=0.63, P <0.01) between histology and imaging. However, they were unable to correlate MRI synovial enhancement with flare as determined by clinical findings and use of the Health Assessment Questionnaire (HAQ, global assessment), or by the modified Ritchie Articular Index (local assessment). In a study performed in a cohort of patients with juvenile idiopathic arthritic, those who subsequently experienced a disease flare (12 of 32 patients), as defined by no longer meeting Wallace criteria for remission, had significantly different maximal enhancement of synovium on DCE-MRI at baseline compared with patients in remission over the course of the study (P=0.05); a higher maximal enhancement was suggestive of flare potential.120 The applicability of these findings to adults with RA require confirmation, but this study suggests the potential usefulness of DCE-MRI for flare prediction.
Taken together, the efforts to define flare by use of questionnaires or imaging have provided valuable tools for diagnosis and possible intervention, yet the mechanisms that underlie this sudden alteration in joint inflammation remain enigmatic.
Lymphatic alterations in flare
The association between arthritic flare and lymph node dynamics is poorly understood. To explore this association, we performed a pilot clinical study to investigate the relationship between arthritis flare and popliteal lymph node volume by use of CE-MRI in 10 patients with RA, before and after anti-TNF therapy.92 Knee flare in patients with chronic arthritis (disease duration >20 years) demonstrated advanced synovitis adjacent to focal bone erosions and small popliteal lymph nodes. In keeping with the findings in our preclinical studies,75 all patients with detectable lymph nodes on CE-MRI showed decreased lymph node volume after 16 weeks of anti-TNF therapy (mean decrease 37%; P=0.002).92 Also, a significant improvement in knee pain was observed, as determined by the Rheumatoid and Arthritis Outcome Score,121 which inversely correlated with a decreased in popliteal lymph node volume (R2=0.94; P<0.05). In aggregate, these results suggest that patients with RA in whom draining lymph node volume is maintained during anti-TNF therapy experience the greatest amelioration of pain in flaring joints.
To formally establish quantitative biomarkers for draining lymphatics in RA, several human NIR-ICG imaging studies are ongoing. An example of this pioneering work comes from Sevick-Muraca and colleagues, who evaluated lymphatic vessels in patients with lymphoedema.122–124 This group was the first to demonstrate lymphatic vessel contraction rates in healthy people.125 An example of this experimental NIR-ICG imaging technology and its potential as a clinical diagnostic tool is presented in Box 1 (See also Supplematal Video 4, 5, and 6). Although no NIR-ICG imaging studies have been reported in patients with RA to date, open clinical protocols are actively enrolling participants126, and it is likely that data will be forthcoming in the next few years.
Box 1. Near-infrared imaging of lymphatic vessels in humans.
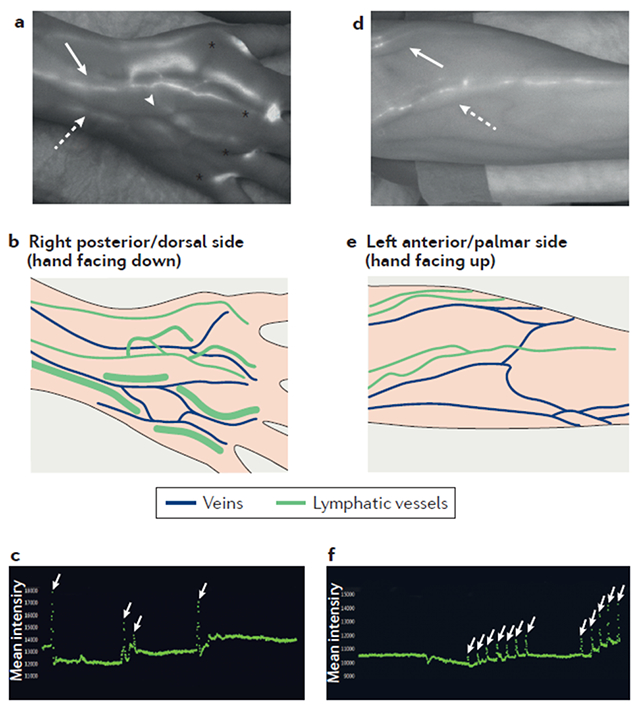
Visualization and quantification of lymphatic function with near-infrared (NIR) imaging provides a unique opportunity to examine the role of lymphatic contraction and flow in human disease.122,123 Following intradermal injections of a NIR dye, usually indocyanine green (ICG), images are captured by cameras sensitive to the NIR spectrum where ICG emits (peak of ~825 nm) to reveal lymphatic vessel anatomy and function. From cadaveric studies, we know that all areas of the dorsal hand drain through the major collecting vessels that initiate at the mid-dorsal aspect and cross the wrist, primarily running adjacent to the basilic or cephalic veins.89,90,173 It is therefore likely that the metacarpal joints are also drained by these collecting vessels. Experimental NIR-ICG imaging (as described by Rahimi et al.95) has been used for the analysis of human lymphatic flow in a study in which ICG was injected into the four web spaces of the hands of a healthy volunteer following informed consent126. In the NIR-ICG image on the left-hand side (a still image from Supplementary video 4), the major veins of the hand appear as dark vessels and lymphatic vessels (which fill with ICG) appear white. ICG signal is also apparent at the injection sites (*). The collecting lymphatic network is adjacent to the dorsal venous arch (#), and further joins together forming larger vessels adjacent to the basilic vein (red arrow), and cephalic vein (yellow arrow). Also shown is a schematic representation of these veins and lymphatic vessels. The graph (a still image from Supplementary video 5) quantifies lymphatic vessel contractions in the hand during a 10-minute imaging session, by plotting the signal intensity in a region of interest over time; spikes in signal intensity (white arrows) are scored as lymphatic contractions. Contraction frequency changes over time during inflammatory arthritis in mice,25,38,59,73 and could prove to be an important biomarker or outcome measure in human disease.44,45,83 In the NIR-ICG image of the forearm from the same subject (obtained in the same session as the hand image), the lymphatic vessels that drain the web spaces flank the cephalic (yellow arrow) and basilic (red arrow) veins across the mid-forearm and the antecubital fossa. The graph (a still image from Supplementary video 6) presents quantification of the cephalic lymphatic vessel contraction frequency at the antecubital fossa, achieved through region of interest analysis.
Lymphatic-modulating treatments for RA
The findings in murine models of arthritis coupled with imaging findings in patients with RA strongly implicate the synovial lymphatic system in the events that promote exacerbation of arthritis. The next important challenge is to address therapeutic strategies to restore lymphatic flow (Table 1). Therapeutic options — both FDA-approved RA therapies and novel lymphatic-modulating interventions — should be considered. It is fascinating to note that despite the efficacy of our current targeted biologics, the mechanisms that promote resolution of synovitis remain enigmatic. Studies have focused on unique pathways that are interrupted by anti-TNF agents 127–129 and antibody-independent effects of anti-CD20 B cell depletion therapy.130,131 In particular, the marked reduction in synovial macrophage numbers following anti-TNF therapy does not result from apoptosis,132,133 or altered monocyte influx into the synovium.134 Rather, anti-TNF therapy restores lymphatic vessel contractions, attributed to lymphatic vessel repair and the restoration of contractions promotes leukocyte migration and egress from inflamed joints, as determined by transmission electron microscopy.83
Table 1.
Potential lymphatic-modulating therapies and their mechanisms of action
| Therapy | Target | Mechanism of action | Potential adverse effects | Ref |
|---|---|---|---|---|
| VEGF-C gene therapy | VEGFR-3 | Increases lymphangiogenesis | Unknown | 137,138 |
| TNF antagonists | TNFR | Reduces inflammation to restore lymphatic vessel contraction | Skin reaction at injection site; risk of infection (including tuberculosis) and cancer (including skin and lymphoma) | 83 |
| B cell depletion therapy | CD20 | Unclogs lymphatic sinuses | Mucocutaneous reactions (Stevens–Johnson syndrome, infections (rarely, progressive multifocal leukoencephalopathy) | 79,84 |
| L-NIL | iNOS | Reduces nitric oxide that keeps vessels dilated | Unknown, possible hypertension | 25,144,146 |
| PDE5 inhibitors | PDE5 | Dilates lymphoid vessels and sinuses to encourage lymphatic rerouting and B cell removal, respectively | Flushing, headache, visual disturbance | Unpublished data (Bouta et al, unpublished) |
| Ferulic acid | VEGFR-3 | Increases VEGF-C production Reduces inflammation and restore lymphatic contraction Reduces nitric oxide production by LECs |
Unknown | 25,141,142 |
Abbreviations: iNOS, inducible nitric oxide synthase; LEC, lymphatic endothelial cell; L-NIL, L-N6-(1-iminoethyl)lysine 5-tetrazole-amide; PDE5, phosphodiesterase 5; TNFR, TNF receptor; VEGF-C, vascular endothelial growth factor C; VEGFR, vascular endothelial growth factor receptor.
The pathways leading to joint inflammation and damage in the TNF-Tg murine arthritis model are lymphocyte-independent,135 providing an ideal setting to assess the antibody-independent efficacy of B cell depletion.124 With this in mind, the results of a study in which B cells were depleted in TNF-Tg mice before and after the onset of arthritis demonstrated that anti-CD20 prophylactic treatment prevented knee flare,79 and therapeutic treatment of flaring mice with collapsed popliteal lymph nodes ameliorated inflammatory-erosive arthritis.73 Moreover, longitudinal NIR-ICG imaging and immunofluorescent intravital microscopy before and after anti-CD20 treatment demonstrated that B cell depletion restores passive lymphatic flow and leukocyte migration in lymphatic vessels without restoring contractions.73 Given that anti-CD20 treatment removes Bin cells from the lymphatic sinuses in draining lymph nodes,73 we conclude that B cell depletion maintains lymph node expansion by preventing Bin cell influx, and ameliorates arthritic flare by unclogging draining lymphatic vessels and restoring passive lymphatic flow. However, it should be noted that, although these B cells are polyclonal and do not seem to have the ability to express pro-inflammatory cytokines,79 they can reduce inflammation or induce lymphangiogenesis by alternative mechanisms, as has been described elsewhere.136
Promoting lymphangiogenesis and drainage
Lymphangiogenesis is a compensatory response to joint inflammation, and inhibition of lymphatic vessel formation during arthritic progression considerably exacerbates disease.77 Therefore, an intervention for RA that enhances lymphatic drainage from affected joints has therapeutic potential. An initial proof-of-concept study examining this potential found that injection of VEGF-C adeno-associated virus into inflamed murine joints reduced synovitis, bone erosion, cartilage loss and the number of infiltrating macrophages.137 Interestingly, a 2014 study demonstrated that VEGF-C–VEGFR-3 signalling also inhibits macrophage activation via regulation of the Toll-like receptor 4–nuclear factor-κB pathway.138 Thus, VEGF-C might serve a dual therapeutic function in RA by improving lymphatic vessel function and inhibiting inflammation. Although gene therapies for arthritis have proven to have major clinical challenges,139 it is of interest that a clinical trial of VEGF-C gene therapy for lymphoedema following mastectomy are currently ongoing140.
Traditional Chinese medicines appeal to many patients, on the basis of their perceived safety and efficacy, coupled with the high costs and attendant risks associated with newer therapies. Indeed, traditional Chinese medicine is a mainstay therapy for arthritis worldwide.141 To test if traditional Chinese medicines have direct effects on lymphatic dysfunction, TNF-Tg mice with established arthritis were treated with Du-Huo-Ji-Sheng-Tang (DHJST)142 and its purified active compound ferulic acid25 for 3 months. The treated mice showed reduced joint inflammation and bone and cartilage erosion.25 Of particular relevance, DHJST and ferulic acid promoted lymphatic drainage from affected joints to draining lymph nodes while restoring lymphatic vessel contraction.25,142 Mechanistically, ferulic acid increased expression levels of contraction-related genes in LMCs, and inhibited TNF-induced NO production by LECs.25 DHJST and ferulic acid also increased the lymphatic thoracic duct formation in zebrafish.142 Another traditional Chinese medicine, panaxnotoginseng, promoted LEC migration, tube formation, and VEGF-C expression.143 Collectively, these findings suggest that traditional Chinese medicines regulate lymphatic vessel draining function by affecting LECs and LMCs, and might have DMARD activity.
Targeting lymphatic function
As described above, NO is a central regulator of lymphatic vessel contractions, and excessive iNOS-derived NO inhibits lymphatic vessel contractions in inflamed tissues (Figure 1E). Thus, there is a strong rationale for selective iNOS inhibition as a novel RA therapy. Indeed, a selective iNOS inhibitor (GW274150) was evaluated in a phase IIA clinical trial for early RA, and showed a trend towards reduction in synovial thickness (33%; P=0.072) and synovial vascularity (42%; P=0.075) compared with placebo at 28 days as shown by PDUS.144 However, our preclinical data suggest that expanding lymphatics would predominate in early RA (Figure 2), and therefore that selective iNOS inhibition would have greater effects on advanced RA (collapsed phase), although this remains speculative. To test this hypothesis in TNF-Tg mice, we evaluated the effects of L-N6-(1-iminoethyl)lysine 5-tetrazole-amide (L-NIL), a moderately selective inhibitor of iNOS that does not affect eNOS activity145 and reduces adjuvant-induced arthritis in rats.146 Remarkably, we found that a single injection of L-NIL into the footpad of TNF-Tg mice with collapsed lymph nodes significantly increased dye uptake and restored lymphatic contractions within 300 s of administration, whereas injection of saline had no effect.25 In another cohort of TNF-Tg mice, treatment with L-NIL, but not with saline or with the non-specific NOS inhibitor Nω-nitro-L-arginine methyl ester (L-NAME), significantly increased lymphatic contraction in TNF-Tg mice, demonstrating that the recovery of lymphatic function was attributable to iNOS inhibition. Furthermore, no changes in lymphatic contractions were observed in wild-type mice treated with L-NIL, L-NAME or saline.25 These proof-of-concept studies indicate that agents that selectively inhibit iNOS or iNOS-mediated downstream signals could represent new therapies for RA by regulating lymphatic vessel function.
Drug screens in wild-type mice for lymphatic-modulating drugs via NIR-ICG imaging revealed that a short-acting phosphodiesterase 5 (PDE5) inhibitor (sildenafil) induces lymphatic rerouting (see Supplementary video 7) versus placebo control (see Supplementary Video 1). This finding suggests a novel alternative intervention for collapsed lymphatics, in which dysfunctional lymphatic vessels can be circumvented by dilation of collateral lymphatic vessels; this approach warrants investigation in animal models of inflammatory arthritis. PDE5 in vascular smooth muscle cells hydrolyzes cGMP and inhibits vessel relaxation. Therefore, we hypothesize that PDE5 inhibitors, such as sildenafil and tadalafil, promote LMC relaxation and could therefore reduce lymphatic contraction (Figure 1E). Future studies are necessary to validate these preliminary findings and confirm the mechanism of action of PDE5 in lymphatics.
Lymphatic drug targets in other disease states
The lymphatic system is now a major research focus owing to the central importance of lymphatic transit in homeostasis and evidence for its involvement in many disease processes, including Alzheimer disease, glaucoma, hypertension, myocardial infarction, lymphoedema, chronic bronchitis, and cancer.147–152 Although this research is still in its early stages, it has catalyzed the need to understand basic lymphatic physiology (Figure 1) and potential treatments targeting lymphatics.
Secondary lymphoedema is a prevalent conventional lymphatic disease, but effective therapies are not available. It often occurs when lymph nodes are removed during cancer therapy and can lead to impaired lymphatic transport. Therefore, treatments that increase lymphangiogenesis show promise. As mentioned above, VEGF-C gene therapy is being studied in a phase I clinical trial140 in patients with lymph node transplant and breast cancer-associated lymphoedema. 153–156 In a study published in 2017, bestatin, a leukotriene A4 hydrolase antagonist, alleviated experimental lymphoedema by improving lymphatic anatomy and promoting lymphangiogensis;157 this drug is currently under evaluation in a phase II clinical trial in patients with secondary lymphoedema of the lower limb158. Similarly, investigators have set out to improve lymphangiogenesis in other models with lymphatic insufficiencies. Treatment with VEGF-C reduced intraocular pressure and restored heart function after myocardial infarction in mouse models.150,159
Lymphangiogenesis can also be pathological. The cornea is avascular, but during cornea transplants there is often both angiogenesis and lymphangiogenesis, which is considered a poor prognostic factor.160 In preclinical models, blocking lymphangiogenesis in a cornea graph improved the survival of the transplant.161,162 In this case, the regenerating lymphatics in cornea grafts deliver immune infiltrates that orchestrate graft rejection.163
It is now well-established that lymphatic dysfunction is an important factor in many disease states, and advantages in imaging have allowed for the functional assessment of lymphatics, both preclinically and clinically. Preclinically, lymphatic contraction is inhibited in obesity164 and inflammation.44 Clinically, Dercum disease (adiposis dolorosa)165, chronic venous insufficiency166, and lymphoedema167 demonstrate lymphatic contraction deficiencies. Moreover, in intestinal inflammation, a model of inflammatory bowel disease, lymphatic contraction can be restored with glibenclamide, which targets potassium channels located on LMCs.168
The preponderance of research is centered on understanding how lymphatics respond to a disease state or condition such as inflammation, whereas other investigations focus on how lymphatic dysfunction can drive disease. The most notable example of lymphatic dysfunction driving pathology and dysfunction is primary lymphoedema, which arises from a genetic mutation associated with loss of function in some component of the lymphatic vessel (such as the valves169 or VEGFR-3170). However, preclinical work has shown that, at least in murine models, lymphatic malformations can drive consequences other than lymphoedema. For instance, mice with haploinsufficiency in a gene essential for induction of the LEC phenotype (Prox1) were found to be a model for both lymphatic dysfunction and adult-onset obesity. Prox1+/− mice have tortuous and leaky lymphatic vessels and manifest increased fat stores in lymphatic-rich regions; furthermore, lymph collected from Prox1+/− mice promoted adipogenesis in vitro.171 Similarly, Chy mice that are Vegfc haploinsuffiecent have lymphatic insufficiency, increased cutaneous fat deposition, chylous ascities and lymphoedema.172
Conclusions
Although lymphatics have long been recognized as important in the pathogenesis of inflammatory arthritis, this area of research has only in the last 10 years been enabled by longitudinal imaging as described above. A high priority is investigation of the synovial lymphatic system, which originated from research aimed at elucidating the nature of the waxing and waning joint pathology characteristic of relapsing flare in RA.130,131 Mounting evidence demonstrates that lymphatic dysfunction exacerbates arthritic flare and disease progression in RA. Murine models have demonstrated that dramatic changes in lymphatic vessel function occur during the pathogenesis of inflammatory arthritis; these changes include an initial compensatory expanding phase in which the lymph node expands in size and lymphatic contraction and clearance are proficient, followed by a collapsed phase in which lymph nodes decrease in volume and there is a loss of the intrinsic lymphatic contraction. This dichotomy is mirrored by joint inflammation, where knee inflammation is not apparent until the collapsed phase, suggesting that targeting lymphatic function could be a potential treatment option and a promising area for future research.
Initial studies in patients demonstrate that alterations in the lymph nodes and lymphatic vessel function occur in RA. Moreover, near-infrared imaging can quantify lymphatic vessel function in patients, and permits identification of those who are likely to respond to specific treatments. Thus, we anticipate that therapies targeting lymphatic function will prove efficacious in RA.
Supplementary Material
Supplementary video 1: NIR-ICG imaging of lymphatic functioning in a healthy mouse. ICG was injected into the footpad of a WT mouse, and was imaged by NIR imaging to allow visualization of the lymphatic vessels. A 10 minute video session taken 40 minutes after ICG injection is shown at 20× real time. Note that the ICG travels from the injection site in the footpad (bottom) to the popletial lymph node (top) in two lymphatic vessels that contract with a normal frequency (once per mintute).
Supplementary video 2: NIR-ICG imaging of lymphatic functioning of a TNF-Tg mouse in the “expansion” phase. At 3-months of age, TNF-Tg mice have inflammatory arthiritis in their ankle joints, and expanding popletial lymph nodes as determined by PD-US. Lymphatic drainage in the lower limb of a 3-month-old TNF-Tg mouse is demonstrated by NIR-ICG imaging. This 10 minute video was taken 60 minutes after ICG injection, and is shown at 20× real time. Note the consistent contractions (~1 per minute) thought the imaging period.
Supplementary video 3: NIR-ICG imaging of lymphatic dysfunctioning in a TNF-Tg mouse during the “collapsed” phase. ICG was injected into the footpad of an 8-month TNF-Tg mouse with a collapsed popliteal lymph node, which was phenotyped by PD-US. NIR-ICG imaging was performed to demonstrate lymphatic dysfunction in the lower limb, which suffers from advanced inflammatory arthritis. This 10 minute video was taken 60 minutes after ICG injection, and is shown at 20× real time. Note the complete absence of lymphatic vessel contractions, and lack of popliteal lymph node signal enhancement, thought the imaging period.
Supplementary video 4: Injection of ICG into the web spaces of a healthy volunteer, and indentification of lymphatic vessels adjacent to veins in the hands. ICG was injected into the web spaces of a heathly human volunteer. The video shows the injection procedure and the ICG entering the lymphatic system directly adjacent to the major veins of the dorsal hand (video shown at 4× real time). Note the dramatic uptake of ICG in lymphatic vessels efferent to the 1st and 2nd web spaces, releative to ICG uptake in the 3rd and 4th webspace, potentially owing to differences in interstial pressure from the injection volume and/or proximatity to the intial lymphatic bed. The video also shows that manipulation of the webspace during removal of exceess iodine at the injection site increase interstial pressure, pushing ICG into the lymphatics as well (4th web space).
Supplementary video 5: Quantification of cephalic lymphatic vessel contrations at the wrist. ICG was injected into the web spaces of a heathly human volunteer. Approximately 15 mins later, NIR imaging was performed to quantify lymphatic vessel contrations. This 10 minute video (20× real time) shows lymphatic drainage in the vessels of the dorsal hand. To quantify cephalic lymphatic vessel contractions, a region of interest (ROI) is defined (green box), and the signal intensity within the ROI is quantified in real time (graph of mean signal intensity over time). Note how the dye moves as a bolus to collection points (presumed valves), and then moves proximally after a contraction.
Supplementary video 6: Quantification of the cephalic lymphatic vessel at the antecubital fossa. Approximately 40 mins after after injection of ICG in to the web spaces of a healthy human volunteer, NIR imaging is performed to quantify lymphatic contractions in the forearm. This 10 minute video (20× real time) shows lymphatic activity in the vessels of the antecubital fossa. ROI quantification of contraction frequency of the cephalic lymphatic vessel was also performed. Note how the dye moves as a bolus as it crosses the antecubital fossa.
Supplementary video 7: Sildenafil (a short acting PDE5 inhibitor) increases ICG uptake into collateral lymphatic vessels in a WT mouse. ICG was injected into the footpad of a WT healthy mouse, and NIR imaging was performed to assess lymphatic drainage after intraperitoneal administration of sildenafil (12 mg/kg). The video (shown at 20× real time) was taken 40 minutes after ICG injection, and 20 minutes after the sildenafil injection. Note the ICG filled collateral lymphatic vessels following sildenafil injection, indicating lymphatic rerouting.
Key points.
Dramatic changes in lymphatic vessel contraction and in the lymph nodes that drain inflamed joints are associated with disease progression and response to therapy in murine models of rheumatoid arthritis (RA)
During mild to moderate experimental arthritis, lymphatic vessels and nodes that drain the joint undergo an initial ‘expansion’ phase that facilitates efficient lymphatic clearance and lymphatic vessel contractions
In preclinical models, the expansion phase is followed by a ‘collapsed’ phase, in which B cells in the draining lymph node translocate from the follicles to lymphatic sinuses and the lymph node collapses
The collapsed phase is characterized by lymphatic vessel structural damage, loss of contraction, and reduction in lymphatic clearance
Pilot clinical studies indicate that alterations in lymph node volume and/or lymphatic flow could serve as biomarkers of treatment response with the potential to predict RA flare
Several lymphatic system-modulating therapies show promise in preclinical models of inflammatory arthritis and RA
Acknowledgements
E.M.B. and R.D.B. are supported by a training grant from the NIH (T32 AR053459). H.R. is supported by an NIH grant (K08 AR067885). L.X. is supported by NIH grants (AR069789 and AR063650), a University of Rochester CTSA award (UL1 TR000042), a grant from the National Natural Science Foundation of China (grant 81220108027), and a grant from the Lymphatic Malformation Institute. R.W.W. is supported by an NIH grant (AR061307). C.T.R. is supported by grants from the NIH (R01 AR056702 and R01 AR069000). E.M.S. is supported by grants from the NIH (P30 AR069655 and R01 AR056702).
Footnotes
Competing interests
C.T.R. declares that he has received consulting fees and research support from UCB Pharmaceuticals. E.M.B., R.W.W., L.X. and E.M.S. declare that they have applied for patents related to the content of the manuscript. R.D.B. and H.R. declare no competing interests.
References
- 1.Firestein GS Evolving concepts of rheumatoid arthritis. Nature 423, 356–361 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Rudan I et al. Prevalence of rheumatoid arthritis in low- and middle-income countries: A systematic review and analysis. J Glob Health 5, 010409, doi: 10.7189/jogh.05.010409 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinblatt ME et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis and rheumatism 48, 35–45, doi: 10.1002/art.10697 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Weinblatt ME et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. The New England journal of medicine 340, 253–259, doi: 10.1056/nejm199901283400401 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Firestein GS The disease formerly known as rheumatoid arthritis. Arthritis Res Ther 16, 114, doi: 10.1186/ar4593 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pratt AG & Isaacs JD Seronegative rheumatoid arthritis: pathogenetic and therapeutic aspects. Best Pract Res Clin Rheumatol 28, 651–659, doi: 10.1016/j.berh.2014.10.016 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Zawieja D Lymphatic biology and the microcirculation: past, present and future. Microcirculation (New York, N.Y. : 1994) 12, 141–150, doi: 10.1080/10739680590900003 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Skandalakis JE, Skandalakis LJ & Skandalakis PN Anatomy of the lymphatics. Surg Oncol Clin N Am 16, 1–16, doi: 10.1016/j.soc.2006.10.006 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Aspelund A, Robciuc MR, Karaman S, Makinen T & Alitalo K Lymphatic System in Cardiovascular Medicine. Circ Res 118, 515–530, doi: 10.1161/CIRCRESAHA.115.306544 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty S, Davis MJ & Muthuchamy M Emerging trends in the pathophysiology of lymphatic contractile function. Semin Cell Dev Biol 38, 55–66, doi: 10.1016/j.semcdb.2015.01.005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao LC, Baluk P, Srinivasan RS, Oliver G & McDonald DM Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am J Pathol 180, 2561–2575, doi: 10.1016/j.ajpath.2012.02.019 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trzewik J, Mallipattu SK, Artmann GM, Delano FA & Schmid-Schonbein GW Evidence for a second valve system in lymphatics: endothelial microvalves. FASEB J 15, 1711–1717 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Collin HB The ultrastructure of conjunctival lymphatic anchoring filaments. Exp Eye Res 8, 102–105 (1969). [DOI] [PubMed] [Google Scholar]
- 14.Baluk P et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 204, 2349–2362, doi: 10.1084/jem.20062596 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breslin JW Mechanical forces and lymphatic transport. Microvasc Res 96, 46–54, doi: 10.1016/j.mvr.2014.07.013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiig H & Swartz MA Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev 92, 1005–1060, doi: 10.1152/physrev.00037.2011 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Olszewski WL & Engeset A Intrinsic contractility of leg lymphatics in man. Preliminary communication. Lymphology 12, 81–84 (1979). [PubMed] [Google Scholar]
- 18.Olszewski WL & Engeset A Intrinsic contractility of prenodal lymph vessels and lymph flow in human leg. Am J Physiol 239, H775–783 (1980). [DOI] [PubMed] [Google Scholar]
- 19.Armenio S, Cetta F, Tanzini G & Guercia C Spontaneous contractility in the human lymph vessels. Lymphology 14, 173–178 (1981). [PubMed] [Google Scholar]
- 20.Muthuchamy M, Gashev A, Boswell N, Dawson N & Zawieja D Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J 17, 920–922, doi: 10.1096/fj.02-0626fje 02-0626fje [pii] (2003). [DOI] [PubMed] [Google Scholar]
- 21.Davis MJ, Davis AM, Ku CW & Gashev AA Myogenic constriction and dilation of isolated lymphatic vessels. Am J Physiol Heart Circ Physiol 296, H293–302, doi: 10.1152/ajpheart.01040.2008 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W et al. Inhibition of myosin light chain phosphorylation decreases rat mesenteric lymphatic contractile activity. Am J Physiol Heart Circ Physiol 297, H726–734, doi: 10.1152/ajpheart.00312.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito M, Nakano T, Erdodi F & Hartshorne DJ Myosin phosphatase: structure, regulation and function. Mol Cell Biochem 259, 197–209 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Roizes S & von der Weid PY Distinct roles of L- and T-type voltage-dependent Ca2+ channels in regulation of lymphatic vessel contractile activity. J Physiol 592, 5409–5427, doi: 10.1113/jphysiol.2014.280347 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang Q et al. Lymphatic endothelial cells efferent to inflamed joints produce iNOS and inhibit lymphatic vessel contraction and drainage in TNF-induced arthritis in mice. Arthritis Res Ther 18, 62, doi: 10.1186/s13075-016-0963-8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munn LL Mechanobiology of lymphatic contractions. Semin Cell Dev Biol 38, 67–74, doi: 10.1016/j.semcdb.2015.01.010 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Koning JJ & Mebius RE Interdependence of stromal and immune cells for lymph node function. Trends in immunology 33, 264–270, doi: 10.1016/j.it.2011.10.006 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Ding Y, Xu J & Bromberg JS Regulatory T cell migration during an immune response. Trends in immunology 33, 174–180, doi: 10.1016/j.it.2012.01.002 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Germain RN, Robey EA & Cahalan MD A decade of imaging cellular motility and interaction dynamics in the immune system. Science (New York, N.Y.) 336, 1676–1681, doi: 10.1126/science.1221063 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller SN & Germain RN Stromal cell contributions to the homeostasis and functionality of the immune system. Nature reviews. Immunology 9, 618–629, doi: 10.1038/nri2588 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alitalo K The lymphatic vasculature in disease. Nature medicine 17, 1371–1380, doi: 10.1038/nm.2545 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Iwami D, Brinkman CC & Bromberg JS Vascular Endothelial Growth Factor C/Vascular Endothelial Growth Factor Receptor 3 Signaling Regulates Chemokine Gradients and Lymphocyte Migration From Tissues to Lymphatics. Transplantation 99, 668–677, doi: 10.1097/Tp.0000000000000561 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abadie V et al. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood 106, 1843–1850, doi: 10.1182/blood-2005-03-1281 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Cromer W et al. Colonic Insult Impairs Lymph Flow, Increases Cellular Content of the Lymph, Alters Local Lymphatic Microenvironment, and Leads to Sustained Inflammation in the Rat Ileum. Inflammatory bowel diseases 21, 1553–1563, doi: 10.1097/mib.0000000000000402 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pflicke H & Sixt M Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med 206, 2925–2935, doi: 10.1084/jem.20091739 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Randolph GJ, Angeli V & Swartz MA Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nature reviews. Immunology 5, 617–628, doi: 10.1038/nri1670 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Thomas SN et al. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. Journal of immunology (Baltimore, Md. : 1950) 189, 2181–2190, doi: 10.4049/jimmunol.1103545 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouta EM et al. Brief Report: Treatment of Tumor Necrosis Factor-Transgenic Mice With Anti-Tumor Necrosis Factor Restores Lymphatic Contractions, Repairs Lymphatic Vessels, and May Increase Monocyte/Macrophage Egress. Arthritis Rheumatol 69, 1187–1193, doi: 10.1002/art.40047 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng W, Aspelund A & Alitalo K Lymphangiogenic factors, mechanisms, and applications. J Clin Invest 124, 878–887, doi: 10.1172/JCI71603 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim H, Kataru RP & Koh GY Inflammation-associated lymphangiogenesis: a double-edged sword? J Clin Invest 124, 936–942, doi: 10.1172/JCI71607 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji RC Macrophages are important mediators of either tumor- or inflammation-induced lymphangiogenesis. Cell Mol Life Sci 69, 897–914, doi: 10.1007/s00018-011-0848-6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogata F et al. Excess Lymphangiogenesis Cooperatively Induced by Macrophages and CD4(+) T Cells Drives the Pathogenesis of Lymphedema. J Invest Dermatol 136, 706–714, doi: 10.1016/j.jid.2015.12.001 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Johnson LA & Jackson DG Inflammation-induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin-mediated dendritic cell transmigration. Int Immunol 22, 839–849, doi: 10.1093/intimm/dxq435 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Liao S et al. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci U S A 108, 18784–18789, doi: 10.1073/pnas.1116152108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aldrich MB & Sevick-Muraca EM Cytokines are systemic effectors of lymphatic function in acute inflammation. Cytokine 64, 362–369, doi: 10.1016/j.cyto.2013.05.015 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y et al. The pro-inflammatory cytokine TNF-alpha inhibits lymphatic pumping via activation of the NF-kappaB-iNOS signaling pathway. Microcirculation (New York, N.Y. : 1994) 24, doi: 10.1111/micc.12364 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von der Weid PY & Muthuchamy M Regulatory mechanisms in lymphatic vessel contraction under normal and inflammatory conditions. Pathophysiology : the official journal of the International Society for Pathophysiology/ISP 17, 263–276, doi: 10.1016/j.pathophys.2009.10.005 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Liao S & von der Weid PY Inflammation-induced lymphangiogenesis and lymphatic dysfunction. Angiogenesis 17, 325–334, doi: 10.1007/s10456-014-9416-7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Becker F et al. Lymphatic dysregulation in intestinal inflammation: new insights into inflammatory bowel disease pathomechanisms. Lymphology 47, 3–27 (2014). [PubMed] [Google Scholar]
- 50.Gashev AA, Davis MJ, Delp MD & Zawieja DC Regional variations of contractile activity in isolated rat lymphatics. Microcirculation (New York, N.Y. : 1994) 11, 477–492, doi: 10.1080/10739680490476033 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Scallan JP et al. Independent and interactive effects of preload and afterload on the pump function of the isolated lymphangion. Am J Physiol Heart Circ Physiol 303, H809–824, doi: 10.1152/ajpheart.01098.2011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koller A, Mizuno R & Kaley G Flow reduces the amplitude and increases the frequency of lymphatic vasomotion: role of endothelial prostanoids. Am J Physiol 277, R1683–1689 (1999). [DOI] [PubMed] [Google Scholar]
- 53.Davis MJ, Davis AM, Lane MM, Ku CW & Gashev AA Rate-sensitive contractile responses of lymphatic vessels to circumferential stretch. J Physiol 587, 165–182, doi: 10.1113/jphysiol.2008.162438 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis MJ et al. Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. Am J Physiol Heart Circ Physiol 303, H795–808, doi: 10.1152/ajpheart.01097.2011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scallan JP, Wolpers JH, Muthuchamy M, Zawieja DC, Gashev AA, Davis MJ. Independent and interactive effects of preload and afterload on the pump function of the isolated lymphangion. Am J Physiol Heart Circ Physiol 303, H809–824 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lynch PM, Delano FA & Schmid-Schonbein GW The primary valves in the initial lymphatics during inflammation. Lymphatic research and biology 5, 3–10, doi: 10.1089/lrb.2007.5102 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Kajiya K et al. Promotion of lymphatic integrity by angiopoietin-1/Tie2 signaling during inflammation. Am J Pathol 180, 1273–1282, doi: 10.1016/j.ajpath.2011.11.008 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Savetsky IL et al. Lymphatic Function Regulates Contact Hypersensitivity Dermatitis in Obesity. J Invest Dermatol 135, 2742–2752, doi: 10.1038/jid.2015.283 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Q, Wood R, Schwarz EM, Wang YJ & Xing L Near-infrared lymphatic imaging demonstrates the dynamics of lymph flow and lymphangiogenesis during the acute versus chronic phases of arthritis in mice. Arthritis and rheumatism 62, 1881–1889, doi: 10.1002/art.27464 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meier TO et al. Increased permeability of cutaneous lymphatic capillaries and enhanced blood flow in psoriatic plaques. Dermatology (Basel, Switzerland) 227, 118–125, doi: 10.1159/000351878 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Kuan EL et al. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue DCs. Journal of immunology (Baltimore, Md. : 1950) 194, 5200–5210, doi: 10.4049/jimmunol.1500221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ivanov S et al. CCR7 and IRF4-dependent dendritic cells regulate lymphatic collecting vessel permeability. J Clin Invest 126, 1581–1591, doi: 10.1172/jci84518 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scallan JP, Hill MA & Davis MJ Lymphatic vascular integrity is disrupted in type 2 diabetes due to impaired nitric oxide signalling. Cardiovascular research 107, 89–97, doi: 10.1093/cvr/cvv117 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.da Fonseca DM et al. Microbiota-dependent sequelae of acute infection compromise tissue-specific immunity. Cell 163, 354–366, doi: 10.1016/j.cell.2015.08.030 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kawai Y, Kaidoh M, Yokoyama Y & Ohhashi T Pivotal roles of lymphatic endothelial cell layers in the permeability to hydrophilic substances through collecting lymph vessel walls: effects of inflammatory cytokines. Lymphatic research and biology 12, 124–135, doi: 10.1089/lrb.2014.0002 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Kakei Y, Akashi M, Shigeta T, Hasegawa T & Komori T Alteration of cell-cell junctions in cultured human lymphatic endothelial cells with inflammatory cytokine stimulation. Lymphatic research and biology 12, 136–143, doi: 10.1089/lrb.2013.0035 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van den Berg WB Animal models of arthritis. What have we learned? J Rheumatol Suppl 72, 7–9 (2005). [PubMed] [Google Scholar]
- 68.Kouskoff V et al. Organ-specific disease provoked by systemic autoimmunity. Cell 87, 811–822 (1996). [DOI] [PubMed] [Google Scholar]
- 69.Keffer J et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J 10, 4025–4031 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Proulx ST et al. Longitudinal assessment of synovial, lymph node, and bone volumes in inflammatory arthritis in mice by in vivo magnetic resonance imaging and microfocal computed tomography. Arthritis and rheumatism 56, 4024–4037 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bouta EM et al. Validation of power Doppler versus contrast-enhanced magnetic resonance imaging quantification of joint inflammation in murine inflammatory arthritis. J Bone Miner Res 30, 690–694, doi: 10.1002/jbmr.2392 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bouta EM et al. Power Doppler Ultrasound Phenotyping of Expanding versus Collapsed Popliteal Lymph Nodes in Murine Inflammatory Arthritis. PloS one 8, e73766, doi: 10.1371/journal.pone.0073766 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J et al. Efficacy of B cell depletion therapy for murine joint arthritis flare is associated with increased lymphatic flow. Arthritis and rheumatism 65, 130–138, doi: 10.1002/art.37709 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li J, Zhou Q Wood R Kuzin I Bottaro A Ritchlin CT Xing L Schwarz EM. CD23+/CD21hi B cell translocation and ipsilateral lymph node collapse is associated with asymmetric arthritic flare in TNF-Tg mice. Arthritis Res Ther 13, R138, doi:ar3452 [pii] 10.1186/ar3452 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Proulx ST et al. MRI and quantification of draining lymph node function in inflammatory arthritis. Ann N Y Acad Sci 1117, 106–123 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Proulx ST et al. Elucidating bone marrow edema and myelopoiesis in murine arthritis using contrast-enhanced magnetic resonance imaging. Arthritis and rheumatism 58, 2019–2029, doi: 10.1002/art.23546 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo R et al. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis and rheumatism 60, 2666–2676, doi: 10.1002/art.24764 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Q et al. Increased lymphangiogenesis in joints of mice with inflammatory arthritis. Arthritis Res Ther 9, R118 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J et al. Expanded CD23(+)/CD21(hi) B cells in inflamed lymph nodes are associated with the onset of inflammatory-erosive arthritis in TNF-transgenic mice and are targets of anti-CD20 therapy. Journal of immunology (Baltimore, Md. : 1950) 184, 6142–6150, doi:jimmunol.0903489 [pii] 10.4049/jimmunol.0903489 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuzin II et al. Increased numbers of CD23(+) CD21(hi) Bin-like B cells in human reactive and rheumatoid arthritis lymph nodes. Eur J Immunol 46, 1752–1757, doi: 10.1002/eji.201546266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bouta EM et al. In vivo Quantification of Lymph Viscosity and Pressure in Lymphatic Vessels and Draining Lymph Nodes of Arthritic Joints in Mice. J Physiol 592, 1213–1223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moshkani S et al. CD23+ CD21(high) CD1d(high) B cells in inflamed lymph nodes are a locally differentiated population with increased antigen capture and activation potential. Journal of immunology (Baltimore, Md. : 1950) 188, 5944–5953, doi: 10.4049/jimmunol.1103071 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bouta EM et al. Treatment of TNF-Tg Mice with Anti-TNF Restores Lymphatic Contraction, Repairs Lymphatic Vessels, and May Increase Monocyte/Macrophage Egress. Arthritis Rheumatol, doi: 10.1002/art.40047 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li J et al. Efficacy of B cell depletion therapy for murine joint arthritis flare is associated with increased lymphatic flow. Arthritis and rheumatism 65, 130–138, doi: 10.1002/art.37709 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li J et al. CD23+/CD21hi B cell translocation and ipsilateral lymph node collapse is associated with asymmetric arthritic flare in TNF-Tg mice. Arthritis Res Ther 13, R138, doi:ar3452 [pii] 10.1186/ar3452 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bouta EM et al. The role of the lymphatic system in inflammatory-erosive arthritis. Semin Cell Dev Biol 38, 90–97, doi: 10.1016/j.semcdb.2015.01.001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Young A & Koduri G Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol 21, 907–927, doi: 10.1016/j.berh.2007.05.007 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Robertson MD, Hart FD, White WF, Nuki G & Boardman PL Rheumatoid lymphadenopathy. Ann Rheum Dis 27, 253–260 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suami H, Pan WR & Taylor GI Changes in the lymph structure of the upper limb after axillary dissection: radiographic and anatomical study in a human cadaver. Plast Reconstr Surg 120, 982–991, doi: 10.1097/01.prs.0000277995.25009.3e (2007). [DOI] [PubMed] [Google Scholar]
- 90.Suami H, Taylor GI & Pan WR The lymphatic territories of the upper limb: anatomical study and clinical implications. Plast Reconstr Surg 119, 1813–1822, doi: 10.1097/01.prs.0000246516.64780.61 (2007). [DOI] [PubMed] [Google Scholar]
- 91.van de Stadt LA et al. The value of ultrasonography in predicting arthritis in auto-antibody positive arthralgia patients: a prospective cohort study. Arthritis Res Ther 12, R98, doi: 10.1186/ar3028 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rahimi H et al. Relationship Between Lymph Node Volume and Pain Following Certolizumab Therapy for Rheumatoid Arthritis Flare: A Pilot Study. Clin Med Insights Arthritis Musculoskelet Disord 9, 203–208, doi: 10.4137/CMAMD.S40237 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Foltz V et al. Power Doppler ultrasound, but not low-field magnetic resonance imaging, predicts relapse and radiographic disease progression in rheumatoid arthritis patients with low levels of disease activity. Arthritis and rheumatism 64, 67–76, doi: 10.1002/art.33312 (2012). [DOI] [PubMed] [Google Scholar]
- 94.Kiely PD, Bland JM, Joseph AE, Mortimer PS & Bourke BE Upper limb lymphatic function in inflammatory arthritis. J Rheumatol 22, 214–217 (1995). [PubMed] [Google Scholar]
- 95.Rahimi H et al. Lymphatic imaging to assess rheumatoid flare: mechanistic insights and biomarker potential. Arthritis Res Ther 18, 194, doi: 10.1186/s13075-016-1092-0 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Benaglio F et al. The draining lymph node in rheumatoid arthritis: current concepts and research perspectives. Biomed Res Int 2015, 420251, doi: 10.1155/2015/420251 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manzo A et al. Power Doppler ultrasonographic assessment of the joint-draining lymph node complex in rheumatoid arthritis: a prospective, proof-of-concept study on treatment with tumor necrosis factor inhibitors. Arthritis Res Ther 18, 242, doi: 10.1186/s13075-016-1142-7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Manzo A et al. Subclinical remodelling of draining lymph node structure in early and established rheumatoid arthritis assessed by power Doppler ultrasonography. Rheumatology (Oxford) 50, 1395–1400, doi:ker076 [pii] 10.1093/rheumatology/ker076 (2011). [DOI] [PubMed] [Google Scholar]
- 99.Bingham CO 3rd et al. Developing a standardized definition for disease “flare” in rheumatoid arthritis (OMERACT 9 Special Interest Group). J Rheumatol 36, 2335–2341, doi: 10.3899/jrheum.090369 (2009). [DOI] [PubMed] [Google Scholar]
- 100.Alten R et al. Developing a construct to evaluate flares in rheumatoid arthritis: a conceptual report of the OMERACT RA Flare Definition Working Group. J Rheumatol 38, 1745–1750, doi: 10.3899/jrheum.110400 (2011). [DOI] [PubMed] [Google Scholar]
- 101.Hewlett S et al. ‘I’m hurting, I want to kill myself’: rheumatoid arthritis flare is more than a high joint count--an international patient perspective on flare where medical help is sought. Rheumatology (Oxford) 51, 69–76, doi: 10.1093/rheumatology/keq455 (2012). [DOI] [PubMed] [Google Scholar]
- 102.Bartlett SJ et al. Identifying core domains to assess flare in rheumatoid arthritis: an OMERACT international patient and provider combined Delphi consensus. Ann Rheum Dis 71, 1855–1860, doi: 10.1136/annrheumdis-2011-201201 (2012). [DOI] [PubMed] [Google Scholar]
- 103.Bykerk VP et al. Establishing a core domain set to measure rheumatoid arthritis flares: report of the OMERACT 11 RA flare Workshop. J Rheumatol 41, 799–809, doi: 10.3899/jrheum.131252 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bartlett SJ et al. Feasibility and Domain Validation of Rheumatoid Arthritis (RA) Flare Core Domain Set: Report of the OMERACT 2014 RA Flare Group Plenary. J Rheumatol 42, 2185–2189, doi: 10.3899/jrheum.141169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bykerk VP et al. Identifying flares in rheumatoid arthritis: reliability and construct validation of the OMERACT RA Flare Core Domain Set. RMD Open 2, e000225, doi: 10.1136/rmdopen-2015-000225 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bartlett SJ et al. Identifying core domains to assess flare in rheumatoid arthritis: an OMERACT international patient and provider combined Delphi consensus. Ann Rheum Dis 71, 1855–1860, doi: 10.1136/annrheumdis-2011-201201 (2012). [DOI] [PubMed] [Google Scholar]
- 107.Naredo E et al. Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis and rheumatism 57, 116–124, doi: 10.1002/art.22461 (2007). [DOI] [PubMed] [Google Scholar]
- 108.Szkudlarek M, Wakefield RJ, Backhaus M & Terslev L The discriminatory capacity of ultrasound in rheumatoid arthritis: active vs inactive, early vs advanced, and more. Rheumatology (Oxford) 51 Suppl 7, vii6–9, doi: 10.1093/rheumatology/kes334 (2012). [DOI] [PubMed] [Google Scholar]
- 109.Patil P & Dasgupta B Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculoskelet Dis 4, 341–355, doi: 10.1177/1759720X12442112 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tan YK, Ostergaard M & Conaghan PG Imaging tools in rheumatoid arthritis: ultrasound vs magnetic resonance imaging. Rheumatology (Oxford) 51 Suppl 7, vii36–42, doi: 10.1093/rheumatology/kes329 (2012). [DOI] [PubMed] [Google Scholar]
- 111.Ostergaard M, Pedersen SJ & Dohn UM Imaging in rheumatoid arthritis--status and recent advances for magnetic resonance imaging, ultrasonography, computed tomography and conventional radiography. Best Pract Res Clin Rheumatol 22, 1019–1044, doi: 10.1016/j.berh.2008.09.014 (2008). [DOI] [PubMed] [Google Scholar]
- 112.Saleem B et al. Can flare be predicted in DMARD treated RA patients in remission, and is it important? A cohort study. Ann Rheum Dis 71, 1316–1321, doi: 10.1136/annrheumdis-2011-200548 (2012). [DOI] [PubMed] [Google Scholar]
- 113.Bellis E et al. Ultrasound-detected tenosynovitis independently associates with patient-reported flare in patients with rheumatoid arthritis in clinical remission: results from the observational study STARTER of the Italian Society for Rheumatology. Rheumatology 55, 1826–1836, doi: 10.1093/rheumatology/kew258 (2016). [DOI] [PubMed] [Google Scholar]
- 114.Berthelot JM et al. A tool to identify recent or present rheumatoid arthritis flare from both patient and physician perspectives: The ‘FLARE’ instrument. Ann Rheum Dis 71, 1110–1116, doi: 10.1136/ard.2011.150656 (2012). [DOI] [PubMed] [Google Scholar]
- 115.Fautrel B et al. Validation of FLARE-RA, a Self-Administered Tool to Detect Recent or Current Rheumatoid Arthritis Flare. Arthritis Rheumatol 69, 309–319, doi: 10.1002/art.39850 (2017). [DOI] [PubMed] [Google Scholar]
- 116.Konig H, Sieper J & Wolf KJ Rheumatoid-Arthritis - Evaluation of Hypervascular and Fibrous Pannus with Dynamic Mr Imaging Enhanced with Gd-Dtpa. Radiology 176, 473–477 (1990). [DOI] [PubMed] [Google Scholar]
- 117.Gaffney K, Cookson J, Blake D, Coumbe A & Blades S Quantification of Rheumatoid Synovitis by Magnetic-Resonance-Imaging. Arthritis and rheumatism 38, 1610–1617, doi:DOI 10.1002/art.1780381113 (1995). [DOI] [PubMed] [Google Scholar]
- 118.Tamai K, Yamato M, Yamaguchi T & Ohno W Dynamic Magnetic-Resonance-Imaging for the Evaluation of Synovitis in Patients with Rheumatoid-Arthritis. Arthritis and rheumatism 37, 1151–1157, doi:DOI 10.1002/art.1780370807 (1994). [DOI] [PubMed] [Google Scholar]
- 119.Bjorkengren AG, Geborek P, Rydholm U, Holtas S & Petterson H Mr Imaging of the Knee in Acute Rheumatoid-Arthritis - Synovial Uptake of Gadolinium-Dota. Am J Roentgenol 155, 329–332 (1990). [DOI] [PubMed] [Google Scholar]
- 120.Nusman CM et al. Dynamic contrast-enhanced magnetic resonance imaging can play a role in predicting flare in juvenile idiopathic arthritis. Eur J Radiol 88, 77–81, doi: 10.1016/j.ejrad.2017.01.003 (2017). [DOI] [PubMed] [Google Scholar]
- 121.Bremander ABI, Petersson IF & Roos E Validation of the rheumatoid arthritis outcome score (RAOS) - For the lower extremity. Ann Rheum Dis 62, 364–364 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rasmussen JC, Tan IC, Marshall MV, Fife CE & Sevick-Muraca EM Lymphatic imaging in humans with near-infrared fluorescence. Curr Opin Biotech 20, 74–82, doi: 10.1016/j.copbio.2009.01.009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rasmussen JC et al. Near-infrared fluorescence imaging techniques for non-invasive lymphatic architectural and functional analysis. J Nucl Med 52, 671–671 (2011). [Google Scholar]
- 124.Tan IC et al. Assessment of Lymphatic Contractile Function After Manual Lymphatic Drainage Using Near-Infrared Fluorescence Imaging. Arch Phys Med Rehab 92, 756–764, doi: 10.1016/j.apmr.2010.12.027 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rasmussen JC et al. Human Lymphatic Architecture and Dynamic Transport Imaged Using Near-infrared Fluorescence. Transl Oncol 3, 362–U346, doi: 10.1593/tlo.10190 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.NCT02680067: NIR Fluorescence Imaging of Lymphatic Transport Using ICG (NIR-ICG), <https://clinicaltrials.gov/ct2/show/NCT02680067 > (
- 127.Kameda H et al. Etanercept (ETN) with methotrexate (MTX) is better than ETN monotherapy in patients with active rheumatoid arthritis despite MTX therapy: a randomized trial. Modern rheumatology / the Japan Rheumatism Association 20, 531–538, doi: 10.1007/s10165-010-0324-4 (2010). [DOI] [PubMed] [Google Scholar]
- 128.Benucci M et al. Efficacy and safety of leflunomide or methotrexate plus subcutaneous tumour necrosis factor-alpha blocking agents in rheumatoid arthritis. International journal of immunopathology and pharmacology 24, 269–274 (2011). [DOI] [PubMed] [Google Scholar]
- 129.Nam JL et al. A randomised controlled trial of etanercept and methotrexate to induce remission in early inflammatory arthritis: the EMPIRE trial. Ann Rheum Dis 73, 1027–1036, doi: 10.1136/annrheumdis-2013-204882 (2014). [DOI] [PubMed] [Google Scholar]
- 130.Cohen SB et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis and rheumatism 54, 2793–2806 (2006). [DOI] [PubMed] [Google Scholar]
- 131.Rosengren S et al. Elevated autoantibody content in rheumatoid arthritis synovia with lymphoid aggregates and the effect of rituximab. Arthritis Res Ther 10, R105, doi:ar2497 [pii] 10.1186/ar2497 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smeets TJ, Kraan MC, van Loon ME & Tak PP Tumor necrosis factor alpha blockade reduces the synovial cell infiltrate early after initiation of treatment, but apparently not by induction of apoptosis in synovial tissue. Arthritis and rheumatism 48, 2155–2162, doi: 10.1002/art.11098 (2003). [DOI] [PubMed] [Google Scholar]
- 133.Wijbrandts CA et al. Analysis of apoptosis in peripheral blood and synovial tissue very early after initiation of infliximab treatment in rheumatoid arthritis patients. Arthritis and rheumatism 58, 3330–3339, doi: 10.1002/art.23989 (2008). [DOI] [PubMed] [Google Scholar]
- 134.Herenius MM et al. Monocyte migration to the synovium in rheumatoid arthritis patients treated with adalimumab. Ann Rheum Dis 70, 1160–1162, doi: 10.1136/ard.2010.141549 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F & Kollias G Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10, 387–398, doi:S1074-7613(00)80038-2 [pii] (1999). [DOI] [PubMed] [Google Scholar]
- 136.Angeli V et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 24, 203–215, doi: 10.1016/j.immuni.2006.01.003 (2006). [DOI] [PubMed] [Google Scholar]
- 137.Zhou Q et al. Vascular endothelial growth factor C attenuates joint damage in chronic inflammatory arthritis by accelerating local lymphatic drainage in mice. Arthritis and rheumatism 63, 2318–2328, doi: 10.1002/art.30421 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang Y et al. Activation of vascular endothelial growth factor receptor-3 in macrophages restrains TLR4-NF-kappaB signaling and protects against endotoxin shock. Immunity 40, 501–514, doi: 10.1016/j.immuni.2014.01.013 (2014). [DOI] [PubMed] [Google Scholar]
- 139.Evans CH et al. Gene therapy for rheumatoid arthritis. Expert Opin Biol Ther 1, 971–978. (2001). [DOI] [PubMed] [Google Scholar]
- 140.NCT02994771: A Phase I Study With Lymfactin® in the Treatment of Patients With Secondary Lymphedema, <https://clinicaltrials.gov/ct2/show/NCT02994771> (
- 141.Liang QQ, Shi Q, Wood RW, Xing LP & Wang YJ Peri-articular lymphatic system and “Bi” theory of Chinese medicine in the pathogenesis and treatment of arthritis. Chinese journal of integrative medicine 21, 648–655, doi: 10.1007/s11655-015-2305-0 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen Y et al. Du-Huo-Ji-Sheng-Tang Attenuates Inflammation of TNF-Tg Mice Related to Promoting Lymphatic Drainage Function. Evidence-based complementary and alternative medicine : eCAM 2016, 7067691, doi: 10.1155/2016/7067691 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li J et al. Total saponins of panaxnotoginseng promotes lymphangiogenesis by activation VEGF-C expression of lymphatic endothelial cells. Journal of ethnopharmacology 193, 293–302, doi: 10.1016/j.jep.2016.08.032 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Seymour M et al. Ultrasonographic measures of synovitis in an early phase clinical trial: a double-blind, randomised, placebo and comparator controlled phase IIa trial of GW274150 (a selective inducible nitric oxide synthase inhibitor) in rheumatoid arthritis. Clin Exp Rheumatol 30, 254–261, doi:4709 [pii] (2012). [PubMed] [Google Scholar]
- 145.Vitecek J, Lojek A, Valacchi G & Kubala L Arginine-based inhibitors of nitric oxide synthase: therapeutic potential and challenges. Mediators of inflammation 2012, 318087, doi: 10.1155/2012/318087 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Connor JR et al. Suppression of adjuvant-induced arthritis by selective inhibition of inducible nitric oxide synthase. European journal of pharmacology 273, 15–24 (1995). [DOI] [PubMed] [Google Scholar]
- 147.Iliff JJ et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Science translational medicine 4, 147ra111, doi: 10.1126/scitranslmed.3003748 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aspelund A et al. The Schlemm’s canal is a VEGF-C/VEGFR-3–responsive lymphatic-like vessel. The Journal of Clinical Investigation 124, 3975–3986, doi: 10.1172/JCI75395 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wiig H et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. The Journal of Clinical Investigation 123, 2803–2815, doi: 10.1172/JCI60113 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Klotz L et al. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 522, 62–67, doi: 10.1038/nature14483 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hoshida T et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res 66, 8065–8075 (2006). [DOI] [PubMed] [Google Scholar]
- 152.Baluk P et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest 115, 247–257, doi: 10.1172/jci22037 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Visuri MT et al. VEGF-C and VEGF-C156S in the pro-lymphangiogenic growth factor therapy of lymphedema: a large animal study. Angiogenesis 18, 313–326, doi: 10.1007/s10456-015-9469-2 (2015). [DOI] [PubMed] [Google Scholar]
- 154.Tervala TV et al. Growth factor therapy and lymph node graft for lymphedema. The Journal of surgical research 196, 200–207, doi: 10.1016/j.jss.2015.02.031 (2015). [DOI] [PubMed] [Google Scholar]
- 155.Saaristo A et al. Lymphangiogenic gene therapy with minimal blood vascular side effects. J Exp Med 196, 719–730 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tammela T et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nature medicine 13, 1458–1466, doi: 10.1038/nm1689 (2007). [DOI] [PubMed] [Google Scholar]
- 157.Tian W et al. Leukotriene B4 antagonism ameliorates experimental lymphedema. Science translational medicine 9, doi: 10.1126/scitranslmed.aal3920 (2017). [DOI] [PubMed] [Google Scholar]
- 158.NCT02700529: Ubenimex in Adult Patients With Lymphedema of The Lower Limb (ULTRA), <https://clinicaltrials.gov/ct2/show/NCT02700529> (
- 159.Aspelund A et al. The Schlemm’s canal is a VEGF-C/VEGFR-3–responsive. 124, 3975–3986, doi: 10.1172/jci75395 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cursiefen C et al. Lymphatic vessels in vascularized human corneas: immunohistochemical investigation using LYVE-1 and podoplanin. Investigative ophthalmology & visual science 43, 2127–2135 (2002). [PubMed] [Google Scholar]
- 161.Dietrich T et al. Cutting Edge: Lymphatic Vessels, Not Blood Vessels, Primarily Mediate Immune Rejections After Transplantation. Journal of immunology (Baltimore, Md. : 1950) 184, 535–539, doi: 10.4049/jimmunol.0903180 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen WS et al. Pathological lymphangiogenesis is modulated by galectin-8-dependent crosstalk between podoplanin and integrin-associated VEGFR-3. Nature communications 7, 11302, doi: 10.1038/ncomms11302 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Steven P, Bock F, Huttmann G & Cursiefen C Intravital two-photon microscopy of immune cell dynamics in corneal lymphatic vessels. PloS one 6, e26253, doi: 10.1371/journal.pone.0026253 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Blum KS et al. Chronic high-fat diet impairs collecting lymphatic vessel function in mice. PloS one 9, e94713, doi: 10.1371/journal.pone.0094713 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rasmussen JC et al. An abnormal lymphatic phenotype is associated with subcutaneous adipose tissue deposits in Dercum’s disease. Obesity (Silver Spring, Md.) 22, 2186–2192, doi: 10.1002/oby.20836 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rasmussen JC et al. Lymphatic transport in patients with chronic venous insufficiency and venous leg ulcers following sequential pneumatic compression. Journal of vascular surgery. Venous and lymphatic disorders 4, 9–17, doi: 10.1016/j.jvsv.2015.06.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Aldrich MB et al. Lymphatic abnormalities in the normal contralateral arms of subjects with breast cancer-related lymphedema as assessed by near-infrared fluorescent imaging. Biomedical optics express 3, 1256–1265, doi: 10.1364/boe.3.001256 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mathias R & von der Weid PY Involvement of the NO-cGMP-K(ATP) channel pathway in the mesenteric lymphatic pump dysfunction observed in the guinea pig model of TNBS-induced ileitis. American journal of physiology. Gastrointestinal and liver physiology 304, G623–634, doi: 10.1152/ajpgi.00392.2012 (2013). [DOI] [PubMed] [Google Scholar]
- 169.Tavian D et al. FOXC2 disease-mutations identified in lymphedema-distichiasis patients cause both loss and gain of protein function. Oncotarget 7, 54228–54239, doi: 10.18632/oncotarget.9797 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Butler C, Osterberg C, Horvai A & Breyer B Milroy’s disease and scrotal lymphoedema: pathological insight. BMJ case reports 2016, doi: 10.1136/bcr-2016-215396 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Harvey NL et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nature genetics 37, 1072–1081, doi: 10.1038/ng1642 (2005). [DOI] [PubMed] [Google Scholar]
- 172.Karkkainen MJ et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci U S A 98, 12677–12682, doi: 10.1073/pnas.221449198 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Suami H Lymphosome concept: Anatomical study of the lymphatic system. J Surg Oncol 115, 13–17, doi: 10.1002/jso.24332 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary video 1: NIR-ICG imaging of lymphatic functioning in a healthy mouse. ICG was injected into the footpad of a WT mouse, and was imaged by NIR imaging to allow visualization of the lymphatic vessels. A 10 minute video session taken 40 minutes after ICG injection is shown at 20× real time. Note that the ICG travels from the injection site in the footpad (bottom) to the popletial lymph node (top) in two lymphatic vessels that contract with a normal frequency (once per mintute).
Supplementary video 2: NIR-ICG imaging of lymphatic functioning of a TNF-Tg mouse in the “expansion” phase. At 3-months of age, TNF-Tg mice have inflammatory arthiritis in their ankle joints, and expanding popletial lymph nodes as determined by PD-US. Lymphatic drainage in the lower limb of a 3-month-old TNF-Tg mouse is demonstrated by NIR-ICG imaging. This 10 minute video was taken 60 minutes after ICG injection, and is shown at 20× real time. Note the consistent contractions (~1 per minute) thought the imaging period.
Supplementary video 3: NIR-ICG imaging of lymphatic dysfunctioning in a TNF-Tg mouse during the “collapsed” phase. ICG was injected into the footpad of an 8-month TNF-Tg mouse with a collapsed popliteal lymph node, which was phenotyped by PD-US. NIR-ICG imaging was performed to demonstrate lymphatic dysfunction in the lower limb, which suffers from advanced inflammatory arthritis. This 10 minute video was taken 60 minutes after ICG injection, and is shown at 20× real time. Note the complete absence of lymphatic vessel contractions, and lack of popliteal lymph node signal enhancement, thought the imaging period.
Supplementary video 4: Injection of ICG into the web spaces of a healthy volunteer, and indentification of lymphatic vessels adjacent to veins in the hands. ICG was injected into the web spaces of a heathly human volunteer. The video shows the injection procedure and the ICG entering the lymphatic system directly adjacent to the major veins of the dorsal hand (video shown at 4× real time). Note the dramatic uptake of ICG in lymphatic vessels efferent to the 1st and 2nd web spaces, releative to ICG uptake in the 3rd and 4th webspace, potentially owing to differences in interstial pressure from the injection volume and/or proximatity to the intial lymphatic bed. The video also shows that manipulation of the webspace during removal of exceess iodine at the injection site increase interstial pressure, pushing ICG into the lymphatics as well (4th web space).
Supplementary video 5: Quantification of cephalic lymphatic vessel contrations at the wrist. ICG was injected into the web spaces of a heathly human volunteer. Approximately 15 mins later, NIR imaging was performed to quantify lymphatic vessel contrations. This 10 minute video (20× real time) shows lymphatic drainage in the vessels of the dorsal hand. To quantify cephalic lymphatic vessel contractions, a region of interest (ROI) is defined (green box), and the signal intensity within the ROI is quantified in real time (graph of mean signal intensity over time). Note how the dye moves as a bolus to collection points (presumed valves), and then moves proximally after a contraction.
Supplementary video 6: Quantification of the cephalic lymphatic vessel at the antecubital fossa. Approximately 40 mins after after injection of ICG in to the web spaces of a healthy human volunteer, NIR imaging is performed to quantify lymphatic contractions in the forearm. This 10 minute video (20× real time) shows lymphatic activity in the vessels of the antecubital fossa. ROI quantification of contraction frequency of the cephalic lymphatic vessel was also performed. Note how the dye moves as a bolus as it crosses the antecubital fossa.
Supplementary video 7: Sildenafil (a short acting PDE5 inhibitor) increases ICG uptake into collateral lymphatic vessels in a WT mouse. ICG was injected into the footpad of a WT healthy mouse, and NIR imaging was performed to assess lymphatic drainage after intraperitoneal administration of sildenafil (12 mg/kg). The video (shown at 20× real time) was taken 40 minutes after ICG injection, and 20 minutes after the sildenafil injection. Note the ICG filled collateral lymphatic vessels following sildenafil injection, indicating lymphatic rerouting.


