Abstract
Strategies that enhance the host antitumor immune response promise to revolutionize cancer therapy. Optimally mobilizing the immune system will likely require a multi-pronged approach to overcome the resistance developed by tumors to therapy. Recently, it has become recognized that doxorubicin can contribute to re-establishing host antitumor immunity through the generation of immunogenic cell death. However, the potential for delivery strategies to further enhance the immunological effects of doxorubicin has not been adequately examined. We report herein that Chimeric Polypeptide Doxorubicin (CP-Dox), a nanoparticle formulation of doxorubicin, enhances antitumor immunity. Compared to free doxorubicin, a single intravenous (IV) administration of CP-Dox at the maximum tolerated dose increases the infiltration of leukocytes into the tumor, slowing tumor growth and preventing metastasis in poorly immunogenic 4T1 mammary carcinoma. We demonstrate that the full efficacy of CP-Dox is dependent on CD8+ T cells and IFN-γ. CP-dox treatment also repolarized intratumoral myeloid cells towards an antitumor phenotype. These findings demonstrate that a nanoparticle drug is distinct from the free drug in its ability to productively stimulate antitumor immunity. Our study strongly argues for the use of antitumor immunotherapies combined with nanoparticle-packaged chemotherapy
Keywords: Immunotherapy, Chemotherapy, Doxorubicin, Recombinant polypeptide, Nanoparticle, Polymer conjugate
1. Introduction
Cytotoxic chemotherapy is a cornerstone of cancer treatment, but it is hindered by its narrow therapeutic window imposed by poor tumor accumulation and dose limiting side effects. To address these limitations, chemotherapy can be re-packaged as nanoparticles [1]. As tumors have a porous vasculature, 10–100 nm sized nanoparticles passively accumulate in tumors because of the enhanced permeability and retention effect [2]. Nanoparticle delivery can also reduce side effects by redistributing drug accumulation away from critical organs such as the heart. This can permit administration of larger doses than is possible with free drug [3,4]. As nanoparticle approaches attain clinical adoption and are used in combination with immunotherapeutic strategies, there is a need to elucidate how repackaging chemotherapy alters the interaction between chemotherapy and the host antitumor immune response.
Cytotoxic chemotherapy was historically considered immunosuppressive because it can cause bone marrow suppression and a subsequent reduction in leukocyte count. However, a mounting body of evidence has shown that some cytotoxic chemotherapies can stimulate an antitumor immune response [5,6]. By simply reducing the number of cancer cells, cytotoxic chemotherapy can interfere with tumor-derived immunosuppressive signaling and create an environment conducive to a more effective immunological response [7,8]. Cytotoxic drugs can also exert immunomodulatory effects directly on leukocyte subsets [9–11]. Finally, some cytotoxic drugs, including doxorubicin, induce immunogenic cell death (ICD), whereby inflammatory signals are generated by dying tumor cells [12,13]. However, these effects have largely been observed using highly immunogenic tumors, or direct intratumoral injection of drug [6,13,14]. There is, therefore, a need to study nanoparticle conjugates of chemotherapy using clinically relevant routes of drug administration in poorly immunogenic tumor models.
We have previously developed a nanoparticle delivery system for doxorubicin (Dox), named chimeric polypeptide-doxorubicin (CP-Dox) [15], in which multiple copies of the drug are conjugated to one end of the CP via an acid-labile bond. Drug conjugation triggers the self-assembly of near monodisperse nanoparticles ~50 nm diameter. CP-Dox and similarly synthesized CP-paclitaxel [16] nanoparticles show significantly greater efficacy than free drug in multiple tumor models in different anatomic sites, and CP-Dox nanoparticles also delay the dissemination of metastases [17]. The dramatic improvement in CP-Dox efficacy led us to speculate that increased direct tumor cytotoxicity was not the sole mechanism for enhanced activity, and we hypothesized that nanoparticle formulation may improve antitumor immunity. Here, we report a comprehensive investigation of the host antitumor immune response after treatment with a chemotherapy-loaded nanoparticle. Consistent with our hypothesis, we found that CD8+ cells and IFN-γ were necessary for the full efficacy of CP-Dox, whereas their depletion had no effect in mice treated with freely dissolved doxorubicin. Our results show that the host antitumor immune response is stimulated after CP-Dox treatment, demonstrating the potential of combining nanoparticle delivery strategies with immunotherapy to improve the treatment of cancer.
2. Materials and methods
2.1. Cell culture
4T1-luciferase murine mammary carcinoma cells (4T1-luc) were provided by Prof. Mark Dewhirst at Duke University Medical Center. Lewis Lung carcinoma LL/2-Luc-M38 (LLC) cells were purchased from Caliper Life Sciences. Neither cell line is listed in the Database of Cross-Contaminated or Misidentified Cell Lines. Cells were passaged for < 5 generations before use in animal experiments. Cell lines were tested and found to be free of mycoplasma. 4T1-luc and LLC-luc were grown in DMEM (Sigma-Aldrich, St. Louis, MO) supplemented with 10% FBS, 1 mM sodium pyruvate, and 4 mM glutamine, in a 37 °C, humidified, 5% CO2 environment.
2.2. Animal studies
All animal experiments were performed in accordance with protocols approved by the Duke Institutional Animal Care and Use Committee (IACUC). BALB/c mice (Charles River, Female, 6–10 weeks old) were inoculated with 8 × 105 4T1-luciferase cells in the 4th mammary fat pad. Albino BL6 mice (Charles River, Female, 6–10 weeks old) were shaved and inoculated subcutaneously on the flank with 1 × 106 LLC-luc cells. For all inoculations, cells were suspended in serum-free DMEM at a concentration appropriate for a 50 μL injection. CP-Dox was synthesized as described in the supplementary information. Mice were treated on day 8 (post-inoculation) with free Dox or CP-Dox at the maximum tolerated dose (5 mg/kg and 20 mg/kg, respectively). In metastasis prevention studies, tumors were surgically resected on day 15. Mice were sacrificed if they appeared moribund or lost > 15% of their baseline body weight, or if the tumor volumes exceeded 2000 mm3. Tumor volumes were calculated using the formula Volume (mm3) = length * width2/2. Mice were randomized to treatment groups using the list randomizer from random.org. For CBC analysis, 100 μL of blood was drawn from mice into an EDTA coated tube (Sarstedt, Newton, NC). Samples were run on an Idexx Procyte (Idexx Operation, Inc., Memphis, TN).
2.3. Depletion studies
Mice were administered depleting antibodies or appropriate isotype control antibodies intraperitoneally (IP) for each experiment as follows: CD8 Depletion: 250 μg of anti-CD8a clone 2.43 (BioXCell, Lebanon, NH), starting day 6 and weekly thereafter. CD4 Depletion: 250 μg of anti-CD4 clone GK1.5 (BioXCell), starting day 6 then weekly thereafter. BALB/c NK cell depletion: 20 μL of anti-asialo GM1 rabbit serum (Wako Chemicals, Richmond, VA) or control rabbit serum, starting day 6, repeated every 4 days for a total of 4 injections. BL/6 NK cell depletion: 200 μg of anti-NK1.1 clone PK-136 (BioXCell) starting day 6, repeated every 4 days for a total of 4 injections. IFN-γ depletion: 100 μg of anti-IFN- γ clone R4–6A2 (BioXCell), on days 7, 9, 15 and 21. After repeated antibody injections, some mice developed a fatal anaphylactic reaction, which correlated with tumor burden. These mice were censored from survival curves since they did not meet the experimental endpoint, and antibody treatments were discontinued for the remaining mice. When more than half of the mice in a treatment group died or were sacrificed, the group was censored from the tumor regression curves to avoid skewing the mean.
2.4. Flow cytometry
Tumors were mechanically dissociated and then enzymatically degraded for 60 min at 37 °C in HBSS buffer containing 5 mg/mL Collagenase Type I Gibco, Grand Island, (NY) and 0.2 mg/mL DNAase I (Roche, Indianapolis, IN) supplemented with 5% FBS. The solution was diluted in PBS and passed through 70 μm strainers. Cells were then pelleted by centrifugation and resuspended in ACK red cell lysis buffer (Quality Biological, Gaithersburg, MD) for 2 min, after which the solution was diluted with PBS. Cells were pelleted and counted by Trypan blue exclusion. One million cells were used for antibody staining. LIVE/DEAD Fixable Aqua Dead Cell Stain (Invitrogen, Grand Island, NY) or Zombie Live/Dead Aqua stain (Biolegend, San Diego, CA) was applied for 30 min. Cells were then blocked (5% rat serum, 5% mouse serum, 1% CD16/32 (clone 93, eBioscience, San Diego, CA)) in FACS buffer (PBS with 3% FBS and 30 uM EDTA) for 30 min. Cells were then stained antibodies for 30 min, washed 2× with PBS, and then fixed with 0.4% paraformaldehyde in PBS. Antibody clone and fluorophore information can be found in the Supplementary information.
2.5. Cytokine and chemokine analysis
Tumors were homogenized in lysis buffer (20 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 0.05% Tween-20, 20–201 protease inhibitor cocktail (Roche, Indianapolis, IN)) and analyzed for protein content with a BCA assay (ThermoFisher, Waltham, MA). Samples were diluted to 1 mg/mL. 20 μL of blood was drawn into EDTA tubes for plasma analysis. Cytokine and chemokine analysis was performed on tumor and plasma samples using a Milliplex Kit (EMD Millipore, Billerica, MA) according to the manufacturer’s instructions. One outlier was removed for CP-Dox tumor samples for IL-6 level and Free Dox for IL-4 level (p < 0.05, Grubbs). One outlier mouse was removed from CP-Dox plasma chemokine analysis due to hemolysis. Overall data trends and conclusions drawn were unaffected.
2.6. Statistical analysis
Statistical analyses were performed using Prism 6 (GraphPad Software). Tumor growth curves and grouped bar graphs were analyzed by two-way ANOVA or one-way ANOVA where applicable, followed by Tukey-Kramer (Tukey’s) when global tests achieved significance. Event-time plots were made using Kaplan-Meier technique and analyzed using the log-rank test or for fraction of long-term survival achieved by Fischer’s Exact Test. Error bars are +/− standard error of the mean. * indicates p < 0.05, which was used as the cutoff for statistical significance.
3. Results
3.1. Functional T cells are required for the full efficacy of CP-dox
To test the immunomodulatory properties of doxorubicin, we used the widely studied 4T1-luc mammary carcinoma model. Inoculation with irradiated 4T1 cells confers no protection against subsequent challenge with live cells, indicating its poor immunogenicity as a cancer cell line [18]. We compared the drug’s efficacy in BALB/c nu/nu mice, which lack functional T cells, to its efficacy in immunocompetent BALB/c mice. On day 0, mice were injected orthotopically in the fourth mammary fat pad with 4T1-luc. Eight days later, mice were treated with CP-Dox or freely dissolved doxorubicin (Free Dox) at their maximum tolerated dose (MTD: 20 mg/kg and 5 mg/kg, respectively) or vehicle control (PBS). In both strains of mice, CP-Dox was more effective at reducing primary tumor growth than Free Dox or PBS treatment (Fig. 1A, p < 0.05, Tukey), whereas Free Dox had no significant effect compared to PBS. CP-Dox also significantly improved overall survival in both strains compared to Free Dox and PBS, but survival of CP-Dox treated mice was significantly improved in the immunocompetent strain (p < 0.05, log-rank) (Fig. 1B). This suggests that T cells contribute to the efficacy of CP-Dox.
Fig. 1.
Functional T cells are required for the full efficacy of CP-Dox. Immunocompetent BALB/c mice (dashed lines) or Nude (BALB/c (nu/nu), solid lines) mice lacking functional T cells were inoculated with 4T1 mammary carcinoma in the mammary fat pad. (A) Primary tumor volume and (B) Survival (n = 7 CP-Dox, 7 Free Dox, 6 PBS for Nude and BALB/c groups in D,E). Tumor growth curves analyzed by two-way ANOVA and Tukey’s post hoc, survival by log-rank. *p < 0.05.
3.2. CP-dox normalizes hematopoiesis in tumor-bearing mice
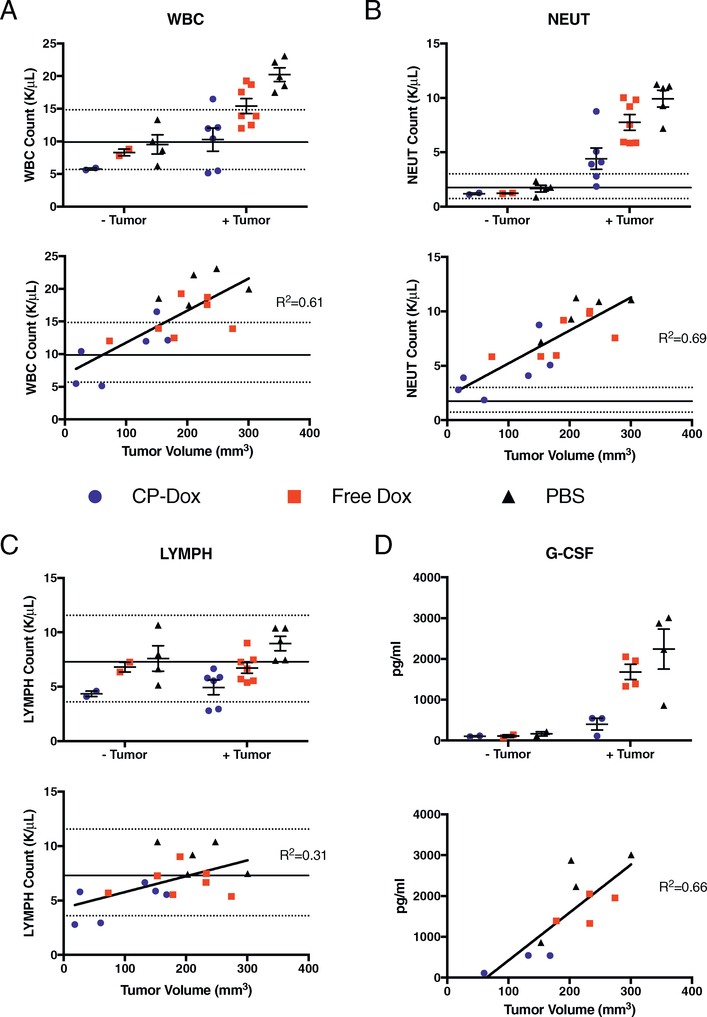
We next performed complete blood counts (CBC) and cytokine/chemokine analysis on BALB/c mice with and without 4T1 tumors after CP-Dox treatment to address the potential for myelosuppression. Red blood cell count, hemoglobin level, and platelet counts were within normal limits regardless of tumor presence or drug treatment. (Supplementary Fig. 1A–C). Although CP-Dox decreased the white blood cell count (WBC), Neutrophil count (NEUT) and Lymphocyte count (LYMPH) of non-tumor bearing mice, the mean values remained within normal limits (Fig. 2A–C). Implantation of mice with 4T1 tumors increased the WBC compared to healthy mice, largely driven by an increase in neutrophil count (Fig. 2A–B), This was consistent with our observation of the expansion of CD11b+ / Ly6G+ cells in the spleen and blood, and with other studies demonstrating that 4T1 induces immunosuppressive myeloid cells driven by G-CSF production [19,20]. Importantly, CP-Dox treatment normalized WBC, NEUT, and plasma G-CSF levels, in a pattern that correlated with tumor burden (Fig. 2A, B, D). Mice with 4T1 tumors displayed increased levels of IL-5 and IL-6, cytokines that promote a Th2 response (Supplementary Fig. 2A). In contrast, IL-12 p40, a Th1 cytokine, was reduced (Supplementary Fig. 2B), suggesting that 4T1 tumors promote Th2 responses. These phenomena were not significantly modified by drug treatment.
Fig. 2.
CP-Dox normalizes hematopoiesis in tumor-bearing mice. BALB/c mice were inoculated orthotophically with 4T1 mammary carcinoma and treated 8days post-inoculation IV with the maximum tolerated dose of Free Dox or CP-Dox (5 mg/kg and 20 mg/kg, respectively) or vehicle control (PBS). On day 15 post-inoculation, blood was collected and CBC or cytokine analysis was performed on tumor-bearing and non-tumor bearing mice (which had also undergone the same drug treatment 7 days prior) to quantify and compare (A) white blood cell count (WBC), (B) neutrophil count (NEUT), (C) lymphocyte count (LYMPH) and (D) granulocyte-colony stimulating factor (G-CSF). Note: Lines indicate mean (solid) and 95% confidence interval limits (dashed) reported by Charles River for female BALB/c mice.
3.3. CP-dox increases intratumoral cell death and leukocyte infiltration
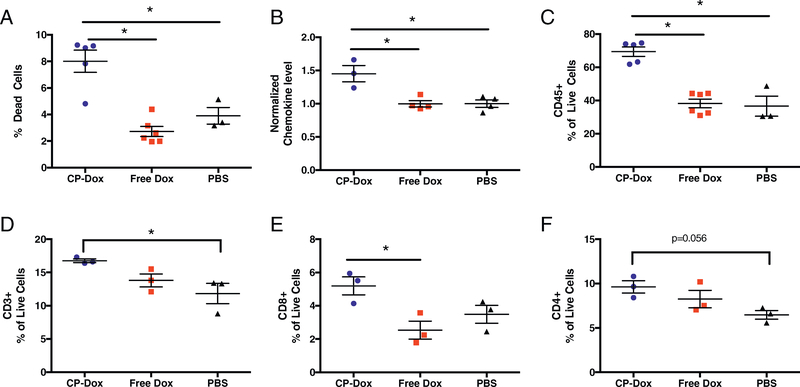
We next hypothesized that intratumoral leukocyte infiltration and cytokine signaling are altered by CP-Dox treatment, thus we analyzed 4T1 tumors by flow cytometry or multiplexed cytokine/chemokine assay. CP-Dox induced larger amounts of cell death compared to Free Dox and PBS (p < 0.05, Tukey’s, Fig. 3A). We next determined the intratumoral levels of seven different leukocyte recruiting chemokines (CCL2–5, CXCL1, 2, 10). We noted a general trend towards higher levels of each chemokine in CP-Dox treated tumors (Supplementary Fig. 3). To quantitatively confirm this observation, the levels of each chemokine were normalized to the mean level of each cytokine in PBS treated mice, and the normalized values were then averaged by drug treatment. This PBS-normalized average of chemokine levels was significantly increased in CP-Dox treated mice (p < 0.05, ANOVA, Fig. 3B). These changes correlated with a significant increase in leukocyte invasion and accumulation within tumors treated with CP-Dox (p < 0.05, Tukey’s, Fig. 3C), so that more than two thirds of the live cells in a CP-Dox treated tumor were leukocytes.
Fig. 3.
CP-Dox treatment increases tumor cell death and intratumoral leukocyte infiltration. One week after treatment of 4T1 mammary carcinoma with CP-Dox, Free Dox, or PBS, tumors were processed to a single-cell suspension and examined by flow cytometry or homogenized and analyzed for chemokine levels. (A) Percentage of dead cells (n = 5 CP-Dox, 6 Free Dox, 3 PBS). (B) Normalized and averaged values for 7 chemokines (raw data found in Supplementary Fig. 1) (n = 3 CP-Dox, 4 Free Dox, 4 PBS). (C) Leukocyte (CD45+) as a percentage of Live cells (n = 5 CP-Dox, 6 Free Dox, 3 PBS). (D-F) CD45+ cells were then gated for quantification of (D) T cells (CD3+), then (E) CD8+ and (F) CD4+ cells as a percentage of live cells (n = 3 CP-Dox, 3 Free Dox, 3 PBS for D-F). Data analyzed by ANOVA and Tukey’s post-hoc. *p < 0.05.
To dissect the identity of tumor infiltrating leukocytes, CD45+ cells were then gated by flow cytometry on the pan-T cell marker CD3 and quantified as a percentage of live cells in the tumor (Fig. 3D). CP-Dox increased the percentage of T cells in the tumor compared to PBS (p < 0.05, ANOVA, Tukey’s). CD8+ cells made up a larger percentage of the live cells within the tumor after CP-Dox treatment in comparison to Free Dox (p < 0.05, ANOVA, Tukey’s, Fig. 3E). There was also a trend towards increased helper T cells —defined as CD4+ T cells— in CP-Dox treated mice compared to PBS (p = 0.056, ANOVA, Tukey’s, Fig. 3F). Overall these results demonstrate that CP-Dox mobilizes the recruitment of lymphocytes involved in the adaptive immune system.
3.4. CD8+ cells, but not CD4+ cells or NK cells, are required for full efficacy of CP-dox in 4 T1
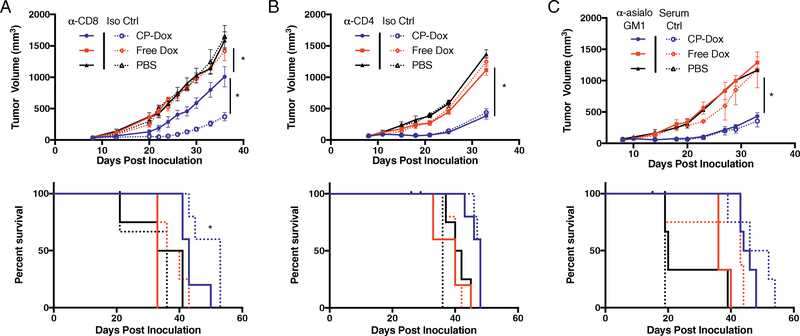
To determine which T cell subsets contribute to the efficacy of CP-Dox, we examined the effect of antibody-mediated depletion of CD8+ or CD4+ cells on the treatment response of 4T1 tumors. CD8 depletion drastically reduced the efficacy of CP-Dox (p < 0.05, Tukey’s, log-rank, Fig. 4A), but conversely had no effect on tumor growth or survival in Free Dox or PBS treated mice. In contrast, CD4 depletion had no significant effect on the tumor growth or survival in any of the drug treatment groups (Fig. 4B). These results suggest that the activity of CD8+ T cells accounts for the majority of the difference in efficacy of CP-Dox in nude versus immunocompetent mice. In fact, the growth of tumors was remarkably similar in nude and CD8-depleted mice after treatment with CP-Dox, as each group had a tumor volume of approximately 600 mm3 on Day 30 (Figs. 1A and 4A). In contrast, NK cells depletion had no significant effect on primary tumor growth or survival in any of the drug treatment groups (Fig. 4C). Because CD8+ cells, but not NK cells, were implicated in CP-Dox mediated tumor regression, we next investigated MHCI expression, which is necessary for T-cell recognition of cancer cells.
Fig. 4.
CD8+ T cells, but not CD4+ T cells or NK cells, are required for full efficacy of CP-Dox. Mice were inoculated with 4T1 and treated with drug and administered depleting antibodies as described in the methods section. Primary tumor growth (top) and survival (bottom) in the setting of (A), CD8 depletion (n = 5:5 CP-Dox, 4:4 Free Dox, 4:3 PBS for α-CD8:Iso Ctrl) (B) CD4 depletion (n = 5 CP-Dox, 5 Free Dox, 4 PBS for α-CD8 and Iso Ctrl), and (C) NK cell depletion(n = 6:5 CP-Dox, 4:4 Free Dox, 3:3 PBS for α-asialo GM1:Serum Ctrl). Tumor growth curves analyzed by two-way ANOVA and Tukey’s post hoc. Survival data analyzed by log-rank. *p < 0.05.
As 4T1 cells were found to express substantial levels of MHCI in vitro (Supplementary Fig. 5). Therefore, we selected a Lewis Lung Carcinoma model (LLC or LL/2) to examine the impact of depleted MHCI expression to further explore the roles of CD8+ versus NK cells in the antitumor effect of CP-Dox. LLC is syngeneic to C57Bl/6 mice and expresses undetectable levels of MHCI in vitro (Supplementary Fig. 4, middle). CD8 depletion had no overall effect on primary LLC tumor volume (p > 0.05, two-way ANOVA, Supplementary Fig. 5A). Combined with primary tumor resection on Day 15, an increased survival trend was seen in isotype control mice for the Free Dox and PBS groups, but CP-Dox mice survival was unaffected (p > 0.05, log-rank, Supplementary Fig. 5A). NK depletion lead to an overall increase in tumor volume independent of treatment group (p < 0.05, two-way ANOVA) and decreased the survival of CP-Dox treated mice (p < 0.05, log-rank, Supplementary Fig. 5B). These results suggest that the antitumor activity of CD8+ T cells induced by CP-Dox requires a baseline level of MHCI expression that is necessary for CD8+ T cells to recognize tumor cells.
3.5. CP-dox treatment increases the intratumoral ratio of Th1 to Th2 cytokines and requires IFN-γ for full efficacy
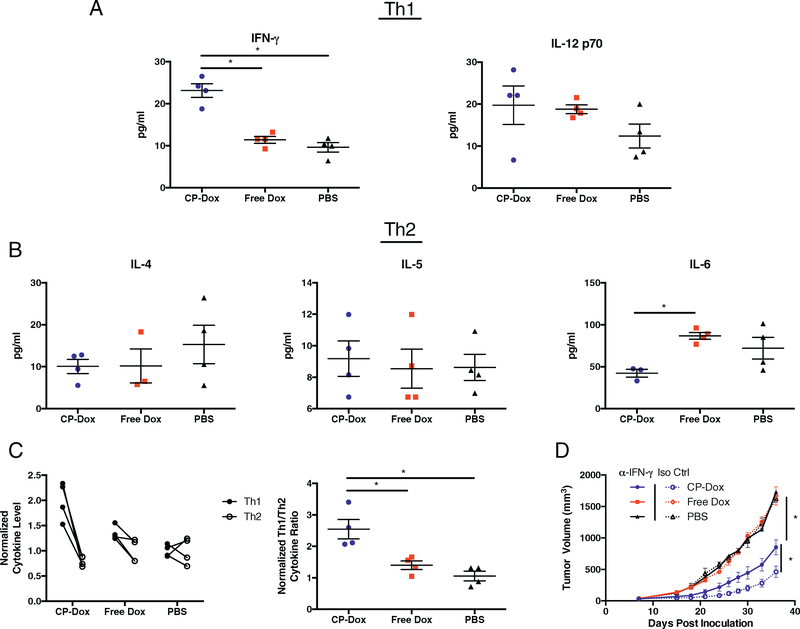
We hypothesized that the CD8+ T cell response stimulated by CP-Dox would correlate with an increase in Th1 cytokines that are critical for coordinating a cytotoxic T cell-mediated immune response. Tumors were therefore homogenized and assessed for cytokine levels by a multiplex bead assay. The levels of the 14 cytokines that were present within the detectable range are shown in Supplementary Fig. 6. In particular, we were interested in two Th1 cytokines (IFN-γ, IL-12 p70) and three Th2 cytokines (IL-4, IL-5, and IL-6). Although often classified as a pro-inflammatory cytokine, IL-6 has been implicated in pro-tumoral myeloid polarization [21] and in the enhancement of Th2 polarization [22,23], so IL-6 was included in the Th2 cytokine analysis. CP-Dox treatment significantly increased intratumoral IFN-γ levels (p < 0.05, ANOVA, Tukey’s), and there was a trend towards increased IL-12 p70 for both CP-Dox and Free Dox compared to PBS (Fig. 5A). IL-4 and IL-5 levels were similar across treatment groups, however CP-Dox significantly decreased IL-6 levels compared to Free Dox (p < 0.05, ANOVA, Tukey’s, Fig. 5B). To assess the overall effect of drug treatment on Th1 and Th2 cytokines, the level for each cytokine was normalized to the mean of PBS-treated mice and averaged. In CP-Dox treated mice, Th1 cytokines were approximately 2-fold higher, while Th2 cytokines were present at about 80% of the level of PBS treated mice (Fig. 5C, left), resulting in a significant increase in the ratio of Th1 to Th2 cytokines (p < 0.05, ANOVA, Tukey’s, Fig. 5C, right). To examine the mechanistic role of IFN-γ in the efficacy of CP-Dox, mice were treated with IFN-γ depleting antibodies, which significantly reduced the efficacy of CP-Dox (p < 0.05, Student’s t-test), while having no effect on PBS or Free Dox treated mice (Fig. 5D). These results demonstrate that CP-Dox increases intratumoral IFN-γ levels, and that this increase is critical for the full efficacy of CP-Dox.
Fig. 5.
CP-Dox treatment increases the intratumoral ratio of Th1 to Th2 cytokines and requires IFN-γ for full efficacy. Mice were inoculated with 4T1 and treated with drug as described earlier. Tumors were homogenized and analyzed for cytokine levels. (A) Th1 cytokine levels: IFN-γ, left and IL-12 p70, right (n = 4/group) (B) Th2 cytokine levels: IL-4, left (n = 4/group), IL-5, middle (n = 4/group), and IL-6, right (n = 3 CP-Dox, 4 Free Dox, 4 PBS). (C) Normalized and averaged Th1 vs. Th2 cytokine levels by drug treatment (left) and Th1/Th2 ratio (right) (n = 4/group). (D) Primary tumor growth in the setting of IFN-γ depleting antibody on days 7, 9, 15 and 21 (n = 5:5 CP-Dox, 4:4 Free Dox, 4:3 PBS for α-IFN-γ:Iso Ctrl). Data for bar graphs analyzed by ANOVA and Tukey’s post hoc, tumor growth curves analyzed by two-way ANOVA and Tukey’s post hoc. *p < 0.05.
3.6. CP-dox alters the phenotype of infiltrating myeloid cells
Based on our findings that CP-Dox significantly remodeled the cytokine milieu within tumors, we hypothesized that the phenotype of highly plastic myeloid cells in tumors may be similarly altered. To examine the myeloid cell infiltrate, 4T1 tumors were subjected to the gating analysis outlined in Supplementary Fig. 7. CP-Dox showed a trend towards fewer Ly6G+ cells (p = 0.10, ANOVA, Supplementary Fig. 8A), a subset consistently associated with suppressing the antitumor immune response [24]. The remaining myeloid cells (CD45+/CD11b+/Ly6G-), were further gated to detect Ly6Chi/MHCII- monocytes (Supplementary Fig. 7, bottom right), a subset containing immature myeloid cells such as inflammatory monocytes capable of differentiating into other myeloid subsets [25,26]. CP-Dox treated tumors contained a significantly higher percentage of these cells (p < 0.05, Tukey’s, Supplementary Fig. 8B).
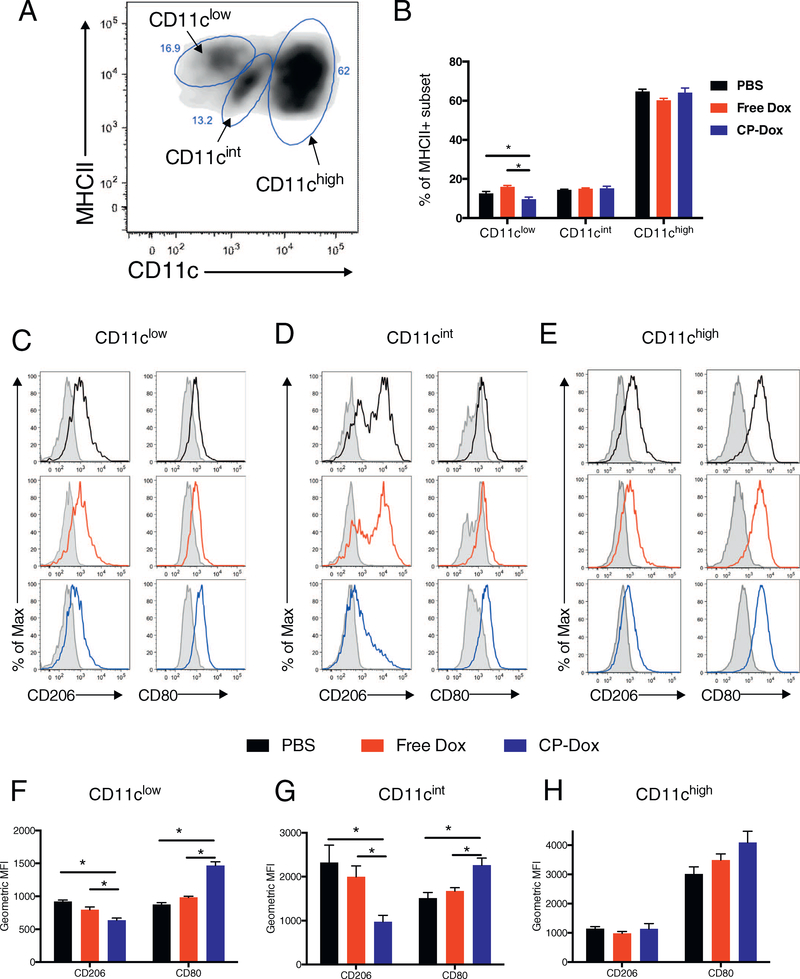
After this gating process, the remainder of the cells were CD11b+/MHCII+, a group of cells often described as tumor-associated macrophages (TAMs). Interestingly, when these cells were plotted against CD11c (Integrin alpha x) and IA/IE (MHCII) expression, three distinct sub-populations emerged based on differential CD11c expression, (Fig. 6A, named CD11clow, int, and high). As a percentage of total MHCII+ cells, there was a lower number of CD11clow cells in CP-Dox treated mice compared to free Dox or PBS (9.6% vs. 16.0% and 12.6% respectively, p < 0.05, Tukey, Fig. 6B). The other two subsets (CD11chigh, int) had similar percentages regardless of treatment. To explore the phenotypes of these cells, we applied the commonly used M1/M2 spectrum that ranges from classically activated antitumor phenotypes (type M1), defined by high expression of the cell surface marker CD80 (B7, T-cell co-stimulatory ligand) to the alternatively activated, pro-tumor phenotypes (type M2) defined by high expression of CD206 (mannose receptor) [25,27,28]. Histograms revealed that the CD11clow subset expressed relatively low levels of both CD206 and CD80 in PBS and Free Dox treated mice (Fig. 6C). Interestingly, CP-Dox induced down-regulation of CD206 concurrent with up-regulation of CD80 in the CD11clow subset (p < 0.05, Tukey) (Fig. 6F). The CD11cint subset of cells expressed high levels of CD206 in PBS and Free Dox treated tumors in a bimodal pattern, while in CP-Dox treated tumors, only the population with lower expression of CD206 remained (Fig. 6D). Additionally, this CD11cint subset of cells displayed a clear up-regulation of CD80 in CP-Dox treated tumors (Fig. 6D). Quantification of these shifts for the CD11cint group confirmed decreased expression of CD206 and increased expression of CD80 for the CP-Dox treated tumors (p < 0.05, Tukey) (Fig. 6G). For the CD11chigh subset, PBS-treated mice expressed relatively low levels of CD206 but very high levels of CD80 (Fig. 6E), and neither treatment with Free Dox or CP-Dox significantly altered phenotypic marker expression (Fig. 6H). Overall, these results suggest that CP-Dox treatment skews the mononuclear phagocyte infiltrate towards a more antitumor, M1 phenotype, which is consistent with the higher levels of Th1 signaling.
Fig. 6.
Treatment with CP-Dox alters the phenotype of mononuclear phagocytes in 4T1 mammary carcinoma. Mice were inoculated with 4T1 mammary carcinoma and treated with drug as described earlier. One week after drug treatment, cells were processed to a single cell suspension and analyzed by flow cytometry. (A) Flow cytometry plot showing CD45+/ CD11b + / Ly6G− / IA/IE+ myeloid cells (TAMs) for a PBS-treated mouse, displayed as CD11c vs. IA/IE (MHCII), revealing three subsets of cells based on their CD11c expression. (B) Breakdown of each subset as a percentage of IA/IE+ cells for each treatment group (n = 5 CP-Dox, 5 Free Dox, 3 PBS). (C–E) Flow cytometry histograms for the CD11clow, CD11cint and CD11chigh subsets, respectively for CD206 and CD80 expression for treatment with PBS (black), free Dox (red) or CP-Dox (blue). (F–H) Quantification of CD206 and CD80 expression for the (F) CD11clow, (G) CD11cint, and (H) CD11chigh subset for different treatments (n = 5 CP-Dox, 5 Free Dox, 3 PBS). (*p < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
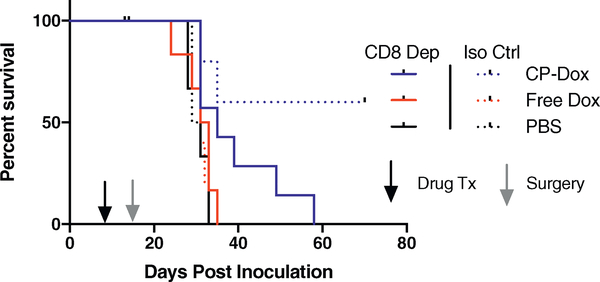
3.7. CP-dox prevention of metastasis is CD8+ T cell dependent in a surgical model of 4T1 mammary carcinoma
Drug delivery studies often focus solely on efficacy against primary tumors, despite the fact that metastasis causes the majority of cancer deaths. Previously we demonstrated that CP-Dox in combination with surgery delays the dissemination of 4T1 cells from the primary tumor, leading to cure in approximately 60% of mice [17]. To determine the role CD8+ cells play in CP-Dox’s ability to interfere with metastasis, CD8 depletion was included in addition to drug treatment and primary tumor resection. CP-Dox treatment prolonged the metastasis free survival of mice in both the CD8 depleted (p < 0.05, log-rank, Fig. 7) and the isotype control groups (p < 0.05, log-rank). CD8 depletion had no effect on the survival of mice in the PBS and Free Dox treatment groups. However, in the CD8 depleted mice treated with CP-Dox, all mice succumbed to metastatic disease by day 60 as confirmed by luciferase imaging and post-mortem examination, resulting in a significantly lower survival rate (0% vs. 60%, p < 0.05, Fisher’s Exact). These results show that CP-Dox requires the presence of CD8+ cells to prevent metastasis and achieve a long-term cure in combination with surgery.
Fig. 7.
CP-Dox prevention of metastasis is CD8+ T cell dependent in a surgical model of 4T1 mammary carcinoma. Metastasis free survival of mice inoculated with 4T1 and treated with CD8 depleting antibody or isotype control along with drug treatment and primary tumor resection (n = 7:5 CP-Dox, 6:5 Free Dox, 4:3 PBS for α-CD8:Iso Ctrl). CD8 depletion had no effect on the survival of Free Dox or PBS mice, but in CP-Dox treated mice, no mice survived to the end of the experiment in the setting of CD8 depletion (p < 0.05, Fisher’s Exact).
4. Discussion
We have shown in this study that a single intravenous injection of a nanoparticle formulation of doxorubicin dramatically alters the host antitumor immune response, stimulating CD8+ T cells to limit tumor growth and prevent metastasis in immunocompetent mice with 4T1 mammary carcinoma. We have also shown that CP-Dox increases the ratio of Th1 to Th2 cytokines in the tumor and that IFN-γ depletion reduces the efficacy of CP-Dox. Our data extends the work of many other groups who have elucidated the intricate signals mediating the host antitumor immune response and its involvement in the efficacy of chemotherapy. The critical role of IFN-γ and CD8+ T cells in the efficacy of intratumorally injected doxorubicin was recently demonstrated in carcinogenically induced tumors [6]. Similarly, a recent study showed that Doxil and doxorubicin were more effective in immunocompetent mice and demonstrated synergy with checkpoint blockade in CT26 colon carcinoma [29]. This study is particularly relevant to our work because Doxil also employs a nanoparticle delivery system, a PEGylated liposome. However, our current work is distinct from prior studies by demonstrating the immunomodulatory effects of doxorubicin in mice treated with a nanoparticle delivery system in poorly immunogenic 4T1 mammary carcinoma. Here we define immunogenicity of a cell line based on whether pre-treatment with irradiated cells confers a survival advantage to mice after subsequent live cell challenge, which is effective in CT26 [13,30], but not 4T1 [18,31].
Despite the poor immunogenicity of 4T1, there is evidence that 4T1 harbors mutations capable of recognition by the immune system [32,33], albeit fewer than CT26 [34]. Furthermore, 4T1 expresses MHCI, which should render the neo-antigens detectable. Nonetheless, we clearly demonstrate that CD8 depletion, or even growth in athymic nude mice, does not affect 4T1 tumor growth rate in PBS treated mice. This suggests that immunosuppressive signals may neutralize the host antitumor immune response to 4T1. Based on our data, it appears that CP-Dox alters the tumor microenvironment, reducing tumor-derived signaling, and activates a latent host antitumor immune response.
The immunomodulatory effects of CP-Dox also included an increase in the levels of intratumoral chemokines, which in turn led to a dramatic increase in the number of tumor-infiltrating leukocytes. CCL2–5 and CXCL10, which all trended towards induction with CP-Dox, have been tied to CD8+ T cell recruitment [35]. Interestingly, the increase in chemokine levels in CP-Dox treated tumors more specifically driven by CXCL10 and CCL5. These chemokines are ligands for the receptors CXCR3 and CCR5, which are markers of Th1 cells and suggest the preferential recruitment of Th1 cells after CP-Dox treatment [36,37]. Furthermore, these chemokines have been shown to be produced by activated neutrophils, monocytes, and Th1-polarized dendritic cells, suggesting a multi-faceted alteration in the immune landscape within the tumor [38–40].
Fundamentally, the increased expression of these chemokines is likely via CP-Dox’s induction of IFN-γ, the canonical Th1 cytokine. IFN-γ drives a variety of antitumor responses, including the promotion of antigen presentation and cytotoxic T cell activity [41]. IFN-γ has been shown to be produced by cells undergoing immunogenic cell death, as well as by Th1 CD4 T cells, CD8 cells, and activated myeloid cells [42–45]. Promoting Th1 immune responses and generating a CD8+ T cell response are central goals of tumor immunotherapy, making CP-Dox a promising complementary treatment in immunotherapy regimens [46–48].
In addition to T cell immunomodulators, there has been considerable effort dedicated to strategies to repolarize pro-tumor M2 cells into the M1 subset that supports an effective antitumor immune response [27,28,49]. Consistent with prior work with 4T1, we identified a significant myeloid cell infiltrate [50]. We characterized this myeloid infiltrate and tumor associated macrophages (TAMs) and identified a group of cells that were highly skewed towards the pro-tumor M2 phenotype (herein referred to as CD11cint, a subset of the CD11b+, MHCII+, Ly6G− cells) based on high levels of CD206 and low levels of CD80 expression [51,52]. While expression of mannose receptor complex (CD206) itself does not directly promote tumor growth, its expression is induced by the same genetic programs that promote tissue remodeling, angiogenesis, and immunosuppressive cytokines. Furthermore, the expression of CD206 is downregulated by IFN-γ [52]. CD206hi cells have been shown to promote the motility and metastasis of cancer cells [53,54]. CP-dox treatment resulted in phenotypic repolarization of these cells in our study, consistent with increased Th1 signaling in the tumor, and may account for the enhanced cell-mediated immunity in CP-Dox treated tumors. Overall, our studies contribute to the growing evidence that effective anticancer therapies can productively manipulate the phenotype of infiltrating myeloid cells [55].
In conclusion, our data show that the adoption of nanoparticle delivery strategy for doxorubicin can trigger potent antitumor immunomodulatory effects upon systemic administration, likely through increased delivery of drug to the tumor to reach the threshold required to reveal the immunomodulatory properties of the drug. If the host immune response can be recruited by nanoparticle delivery strategies, they may achieve a level of efficacy beyond that which would be predicted by a simple linear model relating efficacy and intratumoral drug levels. This study strongly argues for the use of nanoparticle delivery systems in future combinations of chemotherapy and immunotherapy [56,57].
Supplementary Material
Acknowledgments
We acknowledge the following Duke facilities for their support: Duke Cancer Institute Optical Molecular Imaging and Analysis Shared Resource for Luciferase Imaging; Duke Human Vaccine Institute Flow Cytometry Facility; Cancer Center Flow Cytometry Facility; Duke Cell Culture Facility; Division of Laboratory Animal Resources- Veterinary Diagnostic Laboratory, particularly Susie Currie-Mastin, Cara Richardson and Miriam Coltrane for CBC studies. The authors thank David Murdoch (Duke University) and Ramsey McIntire (MilliporeSigma) for assistance in cytokine/chemokine analysis, and Kelli Luginbuhl (Duke University) for a critical reading of the manuscript.
Financial support
This work was supported by a grant from the National Institute of Health (R01 EB000188) to A.C. and MSTP Training grant (T32 GM007171) for E.M.
Footnotes
Disclosure of Potential Conflicts of Interest
A.C. has a financial interest in PhaseBio Pharmaceuticals, which has licensed the ELP technology from Duke University.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jconrel.2017.11.021.
References
- [1].Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R, Nanocarriers as an emerging platform for cancer therapy, Nat. Nanotechnol 2 (2007) 751–760, 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- [2].Maeda H, Wu J, Sawa T, Matsumura Y, Hori K, Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review, J. Control. Release 65 (2000) 271–284, 10.1016/S0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- [3].Lammers T, Kiessling F, Hennink W, Storm G, Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress, J. Control. Release 161 (2012) 175–187, 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- [4].Cho K, Wang X, Nie S, Chen ZG, Shin DM, Therapeutic nanoparticles for drug delivery in cancer, Clin. Cancer Res 14 (2008) 1310–1316, 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- [5].Menard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F, Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity, Cancer Immunol. Immunother 57 (2008) 1579–1587, 10.1007/s00262-008-0505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ, Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors, Cancer Res. 71 (2011) 4809–4820, 10.1158/0008-5472.CAN-11-0753. [DOI] [PubMed] [Google Scholar]
- [7].Kang TH, Mao CP, Lee SY, Chen A, Lee JH, Kim TW, Alvarez RD, Roden RB, Pardoll D, Hung CF, Wu TC, Chemotherapy acts as an adjuvant to convert the tumor microenvironment into a highly permissive state for vaccination-induced antitumor immunity, Cancer Res. 73 (2013) 2493–2504, 10.1158/0008-5472.CAN-12-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G, Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance, Immunity 39 (2013) 74–88, 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- [9].Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G, Immunological aspects of cancer chemotherapy, Nat. Rev. Immunol 8 (2007) 59–73, 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- [10].Ujhazy P, Zaleskis G, Mihich E, Ehrke MJ, Berleth ES, Doxorubicin induces specific immune functions and cytokine expression in peritoneal cells, Cancer Immunol. Immunother 52 (2003) 463–472, 10.1007/s00262-003-0391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shurin GV, Tourkova IL, Kaneno R, Shurin MR, Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism, J. Immunol 183 (2009) (1950) 137–144 Baltimore,MD 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kroemer G, Galluzzi L, Kepp O, Zitvogel L, Immunogenic cell death in cancer therapy, Annu. Rev. Immunol 31 (2012) 51–72, 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- [13].Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G, Calreticulin exposure dictates the immunogenicity of cancer cell death, Nat. Med 13 (2007) 54–61, 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- [14].Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, Martins I, Schlemmer F, Michaud M, Kepp O, Sukkurwala AQ, Menger L, Vacchelli E, Droin N, Galluzzi L, Krzysiek R, Gordon S, Taylor PR, Van Endert P, Solary E, Smyth MJ, Zitvogel L, Kroemer G, Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells, Immunity 38 (2013) 729–741, 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- [15].MacKay JA, Chen M, McDaniel JR, Liu W, Simnick AJ, Chilkoti A, Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection, Nat. Mater 8 (2009) 993–999, 10.1038/nmat2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bhattacharyya J, Bellucci JJ, Weitzhandler I, McDaniel JR, Spasojevic I, Li X, Lin CC, Chi JT, Chilkoti A, A paclitaxel-loaded recombinant polypeptide nanoparticle outperforms Abraxane in multiple murine cancer models, Nat. Commun 6 (2015) 7939, 10.1038/ncomms8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mastria EM, Chen M, McDaniel JR, Li X, Hyun J, Dewhirst MW, Chilkoti A, Doxorubicin-conjugated polypeptide nanoparticles inhibit metastasis in two murine models of carcinoma, J. Control. Release 208 (2015) 52–58, 10.1016/j.jconrel.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pulaski BA, Ostrand-Rosenberg S, Mouse 4T1 breast tumor model, Curr. Protoc. Immunol, edited by Coligan John E. … [et al. ], Chapter 20 (2001) (Unit 20 22). 10.1002/0471142735.im2002s39. [DOI] [PubMed] [Google Scholar]
- [19].Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, Katsanis E, Larmonier N, Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer, Cancer Res. 74 (2014) 104–118, 10.1158/0008-5472.CAN-13-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].DuPre SA, Hunter KW Jr., Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: association with tumor-derived growth factors, Exp. Mol. Pathol 82 (2007) 12–24, 10.1016/j.yexmp.2006.06.007. [DOI] [PubMed] [Google Scholar]
- [21].Fernando MR, Reyes JL, Iannuzzi J, Leung G, McKay DM, The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages, PLoS One 9 (2014) e94188, 10.1371/journal.pone.0094188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Diehl S, Rincon M, The two faces of IL-6 on Th1/Th2 differentiation, Mol. Immunol 39 (2002) 531–536. [DOI] [PubMed] [Google Scholar]
- [23].Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA, Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells, J. Exp. Med 185 (1997) 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ostrand-Rosenberg S, Sinha P, Myeloid-derived suppressor cells: linking inflammation and cancer, J. Immunol 182 (2009) (1950) 4499–4506 (Baltimore, MD), 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, Van Ginderachter JA, Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes, Cancer Res. 70 (2010) 5728–5739, 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- [26].Bonecchi R, Savino B, Pesant M, Locati M, Cell lineage commitment and tumor microenvironment as determinants for tumor-associated myelomonocytic cells plasticity, Tumor Microenvironment and Myelomonocytic Cell, 2012, pp. 1–14. [Google Scholar]
- [27].Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, Costa S, Vinckier S, Dresselaer T, Akerud P, De Mol M, Salomaki H, Phillipson M, Wyns S, Larsson E, Buysschaert I, Botling J, Himmelreich U, Van Ginderachter JA, De Palma M, Dewerchin M, Claesson-Welsh L, Carmeliet P, HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF, Cancer Cell 19 (2011) 31–44, 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- [28].Buhtoiarov I, Sondel P, Wigginton J, Buhtoiarova T, Yanke E, Mahvi D, Rakhmilevich A, Anti-tumour synergy of cytotoxic chemotherapy and anti-CD40 plus CpG-ODN immunotherapy through repolarization of tumour-associated macrophages, Immunology 132 (2011) 226–239, 10.1111/j.1365-2567.2010.03357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rios-Doria J, Durham N, Wetzel L, Rothstein R, Chesebrough J, Holoweckyj N, Zhao W, Leow CC, Hollingsworth R, Doxil synergizes with cancer immunotherapies to enhance antitumor responses in syngeneic mouse models, Neoplasia 17 (2015) 661–670, 10.1016/j.neo.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC, Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity, Proc. Natl. Acad. Sci. U. S. A 90 (1993) 3539–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ravindranathan S, Smith SG, Nguyen K, Zaharoff DA, Colony stimulating factors secreted by irradiated autologous tumor cell vaccines inhibit immunity, J. Immunother. Cancer 3 (2015) P448, 10.1186/2051-1426-3-S2-P448. [DOI] [Google Scholar]
- [32].Lechner MG, Karimi SS, Barry-Holson K, Angell TE, Murphy KA, Church CH, Ohlfest JR, Hu P, Epstein AL, Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy, J. Immunother 36 (2013) 477–489, 10.1097/01.cji.0000436722.46675.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kreiter S, Vormehr M, van de Roemer N, Diken M, Lower M, Diekmann J, Boegel S, Schrors B, Vascotto F, Castle JC, Tadmor AD, Schoenberger SP, Huber C, Tureci O, Sahin U, Mutant MHC class II epitopes drive therapeutic immune responses to cancer, Nature 520 (2015) 692–696, 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, Diaz LA Jr., Papadopoulos N, Kinzler KW, Vogelstein B, Zhou S, Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 11774–11779, 10.1073/pnas.1410626111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF, Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment, Cancer Res. 69 (2009) 3077–3085, 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F, Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s, J. Exp. Med 187 (1998) 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chow MT, Luster AD, Chemokines in cancer, Cancer Immunol. Res 2 (2014) 1125–1131, 10.1158/2326-6066.CIR-14-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lebre MC, Burwell T, Vieira PL, Lora J, Coyle AJ, Kapsenberg ML, Clausen BE, De Jong EC, Differential expression of inflammatory chemokines by Th1- and Th2-cell promoting dendritic cells: a role for different mature dendritic cell populations in attracting appropriate effector cells to peripheral sites of inflammation, Immunol. Cell Biol 83 (2005) 525–535, 10.1111/j.1440-1711.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- [39].Liu M, Guo S, Stiles JK, The emerging role of CXCL10 in cancer (review), Oncol. Lett 2 (2011) 583–589, 10.3892/ol.2011.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C, Fend L, Hannani D, Aymeric L, Ma Y, Niso-Santano M, Kepp O, Schultze JL, Tuting T, Belardelli F, Bracci L, La Sorsa V, Ziccheddu G, Sestili P, Urbani F, Delorenzi M, Lacroix-Triki M, Quidville V, Conforti R, Spano JP, Pusztai L, Poirier-Colame V, Delaloge S, Penault-Llorca F, Ladoire S, Arnould L, Cyrta J, Dessoliers MC, Eggermont A, Bianchi ME, Pittet M, Engblom C, Pfirschke C, Preville X, Uze G, Schreiber RD, Chow MT, Smyth MJ, Proietti E, Andre F, Kroemer G, Zitvogel L, Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy, Nat. Med 20 (2014) 1301–1309, 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- [41].DeNardo DG, Andreu P, Coussens LM, Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity, Cancer Metastasis Rev. 29 (2010) 309–316, 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P, Tumour-associated macrophages as treatment targets in oncology, Nat. Rev. Clin. Oncol 14 (2017) 399–416, 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gebremeskel S, Johnston B, Concepts and mechanisms underlying chemotherapy induced immunogenic cell death: impact on clinical studies and considerations for combined therapies, Oncotarget 6 (2015) 41600–41619, 10.18632/oncotarget.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].de Araujo-Souza PS, Hanschke SC, Viola JP, Epigenetic control of interferon-gamma expression in CD8 T cells, J Immunol Res 2015 (2015) 849573, 10.1155/2015/849573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pandiyan P, Hegel JK, Krueger M, Quandt D, Brunner-Weinzierl MC, High IFN-gamma production of individual CD8 T lymphocytes is controlled by CD152 (CTLA-4), J. Immunol 178 (2007) 2132–2140. [DOI] [PubMed] [Google Scholar]
- [46].Knutson KL, Disis ML, Tumor antigen-specific T helper cells in cancer immunity and immunotherapy, Cancer Immunol. Immunother 54 (2005) 721–728, 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kennedy R, Celis E, Multiple roles for CD4+ T cells in anti-tumor immune responses, Immunol. Rev 222 (2008) 129–144, 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- [48].Burkholder B, Huang RY, Burgess R, Luo S, Jones VS, Zhang W, Lv ZQ, Gao CY, Wang BL, Zhang YM, Huang RP, Tumor-induced perturbations of cytokines and immune cell networks, Biochim. Biophys. Acta 1845 (2014) 182–201, 10.1016/j.bbcan.2014.01.004. [DOI] [PubMed] [Google Scholar]
- [49].Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP, Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection, Cancer Res. 65 (2005) 3437–3446, 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- [50].DuPre SA, Redelman D, Hunter KW Jr., The mouse mammary carcinoma 4T1: characterization of the cellular landscape of primary tumours and metastatic tumour foci, Int. J. Exp. Pathol 88 (2007) 351–360, 10.1111/j.1365-2613.2007.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gabrilovich DI, Ostrand-Rosenberg S, Bronte V, Coordinated regulation of myeloid cells by tumours, Nat. Rev. Immunol. 12 (2012) 253–268, 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Squadrito ML, Pucci F, Magri L, Moi D, Gilfillan GD, Ranghetti A, Casazza A, Mazzone M, Lyle R, Naldini L, De Palma M, miR-511–3p modulates genetic programs of tumor-associated macrophages, Cell Rep. 1 (2012) 141–154, 10.1016/j.celrep.2011.12.005. [DOI] [PubMed] [Google Scholar]
- [53].Tsujikawa T, Yaguchi T, Ohmura G, Ohta S, Kobayashi A, Kawamura N, Fujita T, Nakano H, Shimada T, Takahashi T, Nakao R, Yanagisawa A, Hisa Y, Kawakami Y, Autocrine and paracrine loops between cancer cells and macrophages promote lymph node metastasis via CCR4/CCL22 in head and neck squamous cell carcinoma, Int. J. Cancer 132 (2013) 2755–2766, 10.1002/ijc.27966. [DOI] [PubMed] [Google Scholar]
- [54].Zhang Y, Sime W, Juhas M, Sjolander A, Crosstalk between colon cancer cells and macrophages via inflammatory mediators and CD47 promotes tumour cell migration, Eur. J. Cancer 49 (2013) 3320–3334, 10.1016/j.ejca.2013.06.005. [DOI] [PubMed] [Google Scholar]
- [55].Mantovani A, Allavena P, The interaction of anticancer therapies with tumor-associated macrophages, J. Exp. Med 212 (2015) 435–445, 10.1084/jem.20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Da Silva CG, Rueda F, Lowik CW, Ossendorp F, Cruz LJ, Combinatorial prospects of nano-targeted chemoimmunotherapy, Biomaterials 83 (2016) 308–320, 10.1016/j.biomaterials.2016.01.006. [DOI] [PubMed] [Google Scholar]
- [57].Sheen MR, Lizotte PH, Toraya-Brown S, Fiering S, Stimulating antitumor immunity with nanoparticles, Wiley Interdisc. Rev. Nanomed. Nanobiotechnol 6 (2014) 496–505, 10.1002/wnan.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.