Abstract
In this issue of Molecular Cell, Cho et al. (2019) identify a mechanism by which the mitochondrial division machinery provides selective pressure to identify dysfunctional organelles through the coordinated action of DRP1, Zip1, and Zn2+ transport into mitochondria.
Within a cell, every mitochondrion coordinates a variety of cellular processes, including the Krebs cycle in the matrix, the establishment of a proton gradient (ΔΨM) across the inner mitochondrial membrane (IMM) to drive oxidative phosphorylation of ADP to ATP, and a unique environment at the outer mitochondrial membrane (OMM), which mediates calcium signals, anti-viral responses, and cellular commitment to apoptosis (Nunnari and Suomalainen, 2012). Despite the impact and complexity of mitochondria, our understanding of the molecular mechanisms that mediate organelle surveillance and mitochondrial network quality control still remain quite limited (Lin and Haynes, 2016).
We know that organelle surveillance centers almost exclusively on the cytoplasmic detection of decreased ΔΨM due to its requirement for both mitochondrial protein import and utilization of the mitochondrial genome. Decreased ΔΨM leads to the stabilization of PTEN-induced putative protein kinase (PINK1) at the OMM and subsequent activation of E3 ubiquitin ligases(e.g., Parkin and Arih1), which recruit additional factors to target a failing mitochondrion for degradation via mitophagy (Palikaras et al., 2018). Generally speaking, small mitochondria can be targeted for mitophagy, but how does a cell identify and select regions within a mitochondrial network exhibiting overt ΔΨM issues or, potentially, sectors within the mitochondrial network with decreased capability to repair a compromised proton gradient? Here, Cho et al. (2019) identify a mechanism by which the mitochondrial division machinery provides selective pressure to identify dysfunctional organelles through Zn2+ transport into mitochondria (Figure 1).
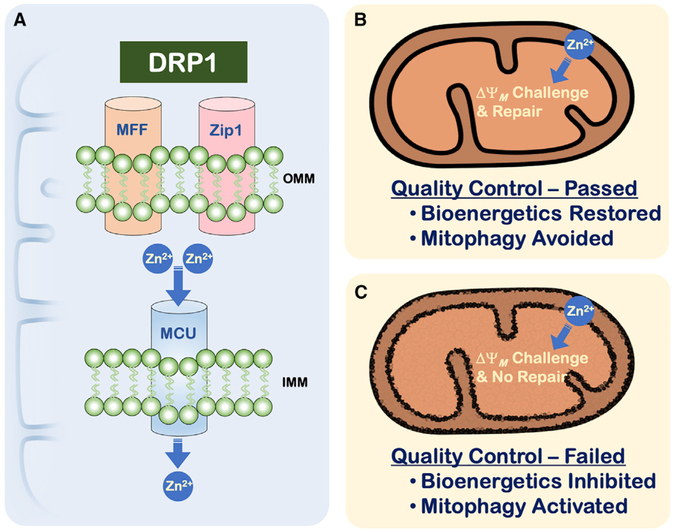
Figure 1. The DRP1-Zip1 Interaction Initiates a Mitochondrial Quality Control Surveillance Program.
(A) DRP1 re-localizes from the cytosol to the OMM by binding to a docking protein, MFF. Once at the OMM, DRP1 associates with Zip1, which promotes the entry of Zn2+ into the matrix via a MCU-dependent manner. The mechanism by which DRP1 activates Zip1 to stimulate MCU activity remains unclear. There are two proposed outcomes from this pathway (B and C).
(B) Upon Zn2+ entry into mitochondria, components of the electron transport chain are inhibited, resulting in decreased ΔΨM. Normal mitochondrial function (e.g., responsive changes to nuclear-mitochondrial signaling to re-establish ΔΨM) allows for the rapid recovery of ΔΨM and protection against mitophagy.
(C) Alternatively, Zn2+-induced inhibition of ΔΨM is not repaired, and the mitochondrion is destined for mitophagy. This mechanism may identify distinct organelles or regions of the mitochondrial network that fail to recover from bioenergetic stress due to aberrations in mitochondrial gene expression, coordinated nuclear-mitochondrial signaling, or compromised electron transport chain assembly.
Previous literature suggests that the mitochondrial dynamics machinery participates in organelle quality control, as pro-division proteins are essential to initiate selective mitophagy. Dynamin-related protein 1 (DRP1), a large GTPase required for mitochondrial division, and its OMM receptor, mitochondrial fragmentation factor (MFF), coordinate to assemble a scission machinery at the sites of mitochondrial division. Curiously, DRP1 was shown to decrease ΔΨM (Bras et al., 2007), while the suppression of mitophagy prevented mitochondrial division (Yu et al., 2011)—two observations that provided Cho et al. with experimental evidence that ΔΨM, mitochondrial dynamics, and mitophagy may be mechanistically linked by DRP1.
To explore this possibility, the authors performed time-lapse imaging to follow changes in ΔΨM during mitochondrial division and observed that the translocation of DRP1 caused a local decrease in ΔΨM, and this preceded mitochondrial division. The decrease in ΔΨM occurred nearly a minute prior to division, and while mitochondrial targeting of DRP1 was essential, its GTPase activity did not appear requisite. Using an unbiased approach, the authors identified a new DRP1-interacting protein: Zip1, a Zn2+ transporter (Bowers and Srai, 2018) that co-localizes with DRP1, MFF, and sites of mitochondrial division. While the GTPase function of DRP1 was not essential for reductions in ΔΨM, DRP1’s GTPase domain was required for binding to Zip1, most likely through the cytosolic amino terminus of Zip1. Through complementary gain-of-function and loss-of-function experiments, Cho et al. described that DRP1-mediated loss in ΔΨM is mediated through Zip1 interactions; but what links DRP1-mediated mitochondrial division, Zip1, and loss of ΔΨM?
As the DRP1-MFF-Zip1 complex is localized to the OMM, an IMM constituent was a likely requirement to signal within mitochondria, leading to ΔΨM reduction. Indeed, the authors demonstrated that Zip1 interacted with the mitochondrial calcium uniporter (MCU), an oligomeric protein capable of transporting ions like Ca2+ and Zn2+ into mitochondria (Pallafacchina et al., 2018). Moreover, RNAi and pharmacological inhibition of MCU were sufficient to abolish DRP1-medated reduction in ΔΨM, and RNAi against Zip1 and Zn2+ chelation prevented DRP1-mediated Zn2+ increases and ΔΨM reduction, respectively. Based on previous literature that mitochondrial Zn2+ uptake irreversibly inhibited components of the electron transport chain (ETC) and depolarized mitochondria (Faxén et al., 2006; Sharpley and Hirst, 2006), the authors propose a mechanism by which DRP1 is recruited to the OMM via MFF, leading to Zip1 interactions that enable Zn2+ flux into mitochondria via MCU and subsequent inhibition of mitochondrial respiration to decrease ΔΨM.
Of course, every round of mitochondrial division does not lead to the selective targeting of mitochondria for elimination, so where does the above mechanism impact on cell biology and, potentially, human disease? The authors position their observations in multiple contexts. First, a DRP1-mediated decrease in ΔΨM may be a means to examine if a recently divided mitochondrion can overcome Zn2+-mediated inhibition of the ETC, presumably through the rapid assembly of new ETC components suggestive of responsive mitochondrial transcription, translation, and assembly machineries within the matrix and IMM. Second, using an amino-terminal fragment of Zip1 (Zip11−28) that blocked DRP1 from binding Zip1 at the OMM, the authors demonstrated that reducing the DRP1-Zip1 interaction prevented hyperglycemia-induced reductions in ΔΨM, Parkin recruitment to mitochondria, and subsequent mitophagy. Together, these observations suggest that the DRP1-Zip1 interaction may be an essential component of mitochondrial network surveillance to ensure highly functional and/or repairable mitochondria remain after division, and this mechanism is likely responsive to metabolic perturbations that lead to mitophagy.
The study by Cho et al. also reveals several intriguing aspects of mitochondrial biology that require fresh insights and investigations to better appreciate their broader impact. For example, data showing that the GTPase function of DRP1 was not required for either interactions with Zip1 or decreased ΔΨM suggest alterations in mitochondrial function and mitochondrial division are separate processes. Given the numerous studies on DRP1 GTPase mutants and their role in apoptosis, redox, and energy metabolism, re-examination of the causes and consequences of Zn2+ influx is critical. Furthermore, the mechanism by which DRP1 activates Zn2+ influx via Zip1-MCU is unknown. As the physiological role of Zn2+ is to regulate both other metal ions and thousands of proteins, a rather significant fraction of total mitochondrial function could be influenced by DRP1-mediated Zip1-MCU activation. What is certain is that we need to continue thinking about and exploring the mechanisms of mitochondrial quality control and how this essential process is coordinated by not only proteins, membranes, organelle homeostasis, and ΔΨM, but also alterations in ion homeostasis.
REFERENCES
- Bowers K, and Srai SKS (2018). The trafficking of metal ion transporters of the Zrt- and Irt-like protein family. Traffic 19, 813–822. [DOI] [PubMed] [Google Scholar]
- Bras M, Yuste VJ, Roué G, Barbier S, Sancho P, Virely C, Rubio M, Baudet S, Esquerda JE, Merle-Béral H, et al. (2007). Drp1 mediates caspase-independent type III cell death in normal and leukemic cells. Mol. Cell. Biol 27, 7073–7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HM, Ryu JR, Jo Y, Seo TW, Choi YN, Kim JH, Chung JM, Cho B, Kang HC, Yu SW, et al. (2019). Drp1-Zip1 interaction regulates mitochondrial quality surveillance system. Mol. Cell 73, this issue, 364–376. [DOI] [PubMed] [Google Scholar]
- Faxén K, Salomonsson L, Adelroth P, and Brzezinski P (2006). Inhibition of proton pumping by zinc ions during specific reaction steps in cyto-chrome c oxidase. Biochim. Biophys. Acta 1757, 388–394. [DOI] [PubMed] [Google Scholar]
- Lin YF, and Haynes CM (2016). Metabolism and the UPR(mt). Mol. Cell 61, 677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnari J, and Suomalainen A (2012). Mitochondria: in sickness and in health. Cell 148, 1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palikaras K, Lionaki E, and Tavernarakis N (2018). Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol 20, 1013–1022. [DOI] [PubMed] [Google Scholar]
- Pallafacchina G, Zanin S, and Rizzuto R (2018). Recent advances in the molecular mechanism of mitochondrial calcium uptake. F1000Res. Published online November 28, 2018. 10.12688/f1000research.15723.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpley MS, and Hirst J (2006). The inhibition of mitochondrial complex I (NADH:ubiquinone oxidoreductase) by Zn2+. J. Biol. Chem 281, 34803–34809. [DOI] [PubMed] [Google Scholar]
- Yu W, Sun Y, Guo S, and Lu B (2011). The PINK1/Parkin pathway regulates mitochondrial dynamics and function in mammalian hippocampal and dopaminergic neurons. Hum. Mol. Genet 20, 3227–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]